Natural Ageing-Related Alterations of Biological Markers in Maize Seeds Under Ex-Situ Conservation
Abstract
1. Introduction
2. Results
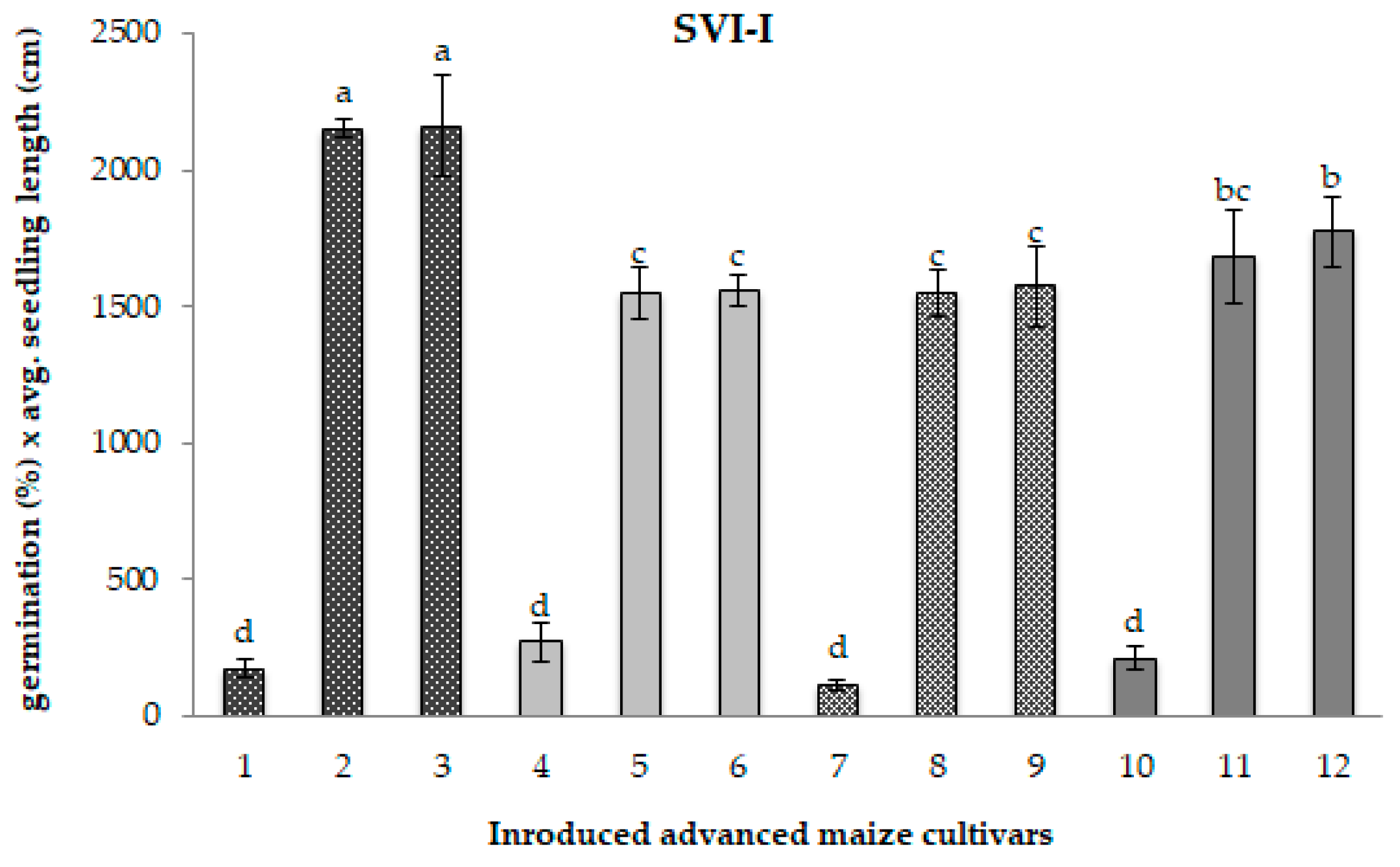
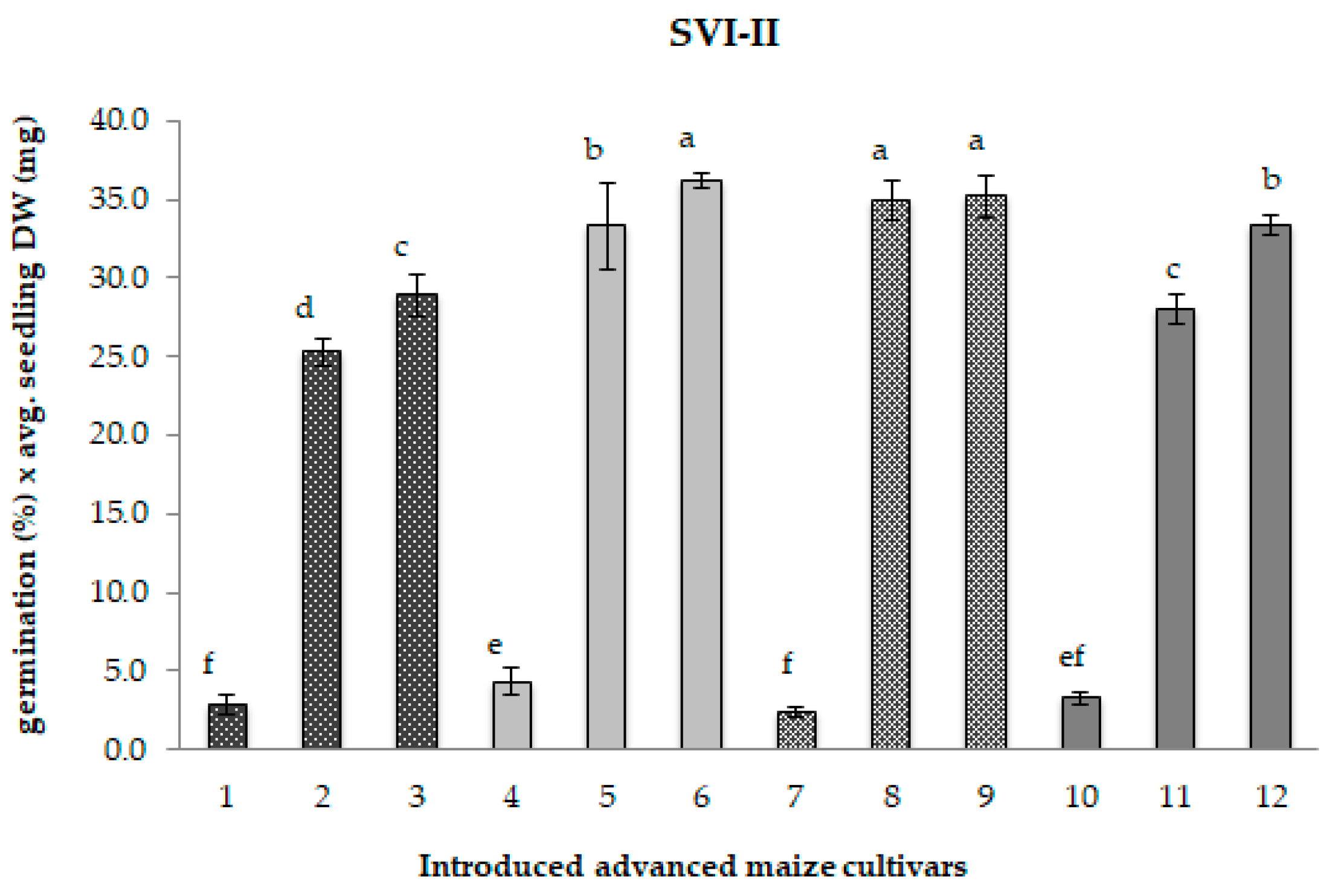
2.1. Seed Germination and Seedling Vigour
2.2. Biochemical Markers
2.2.1. Analysis of Total Protein and Protein Fractions
2.2.2. Analysis of Reducing and Non-Reducing Sugars
2.2.3. Analysis of Soluble-Free and Insoluble-Bound Phenolics
2.2.4. Analysis of Tocopherols and Carotenoids
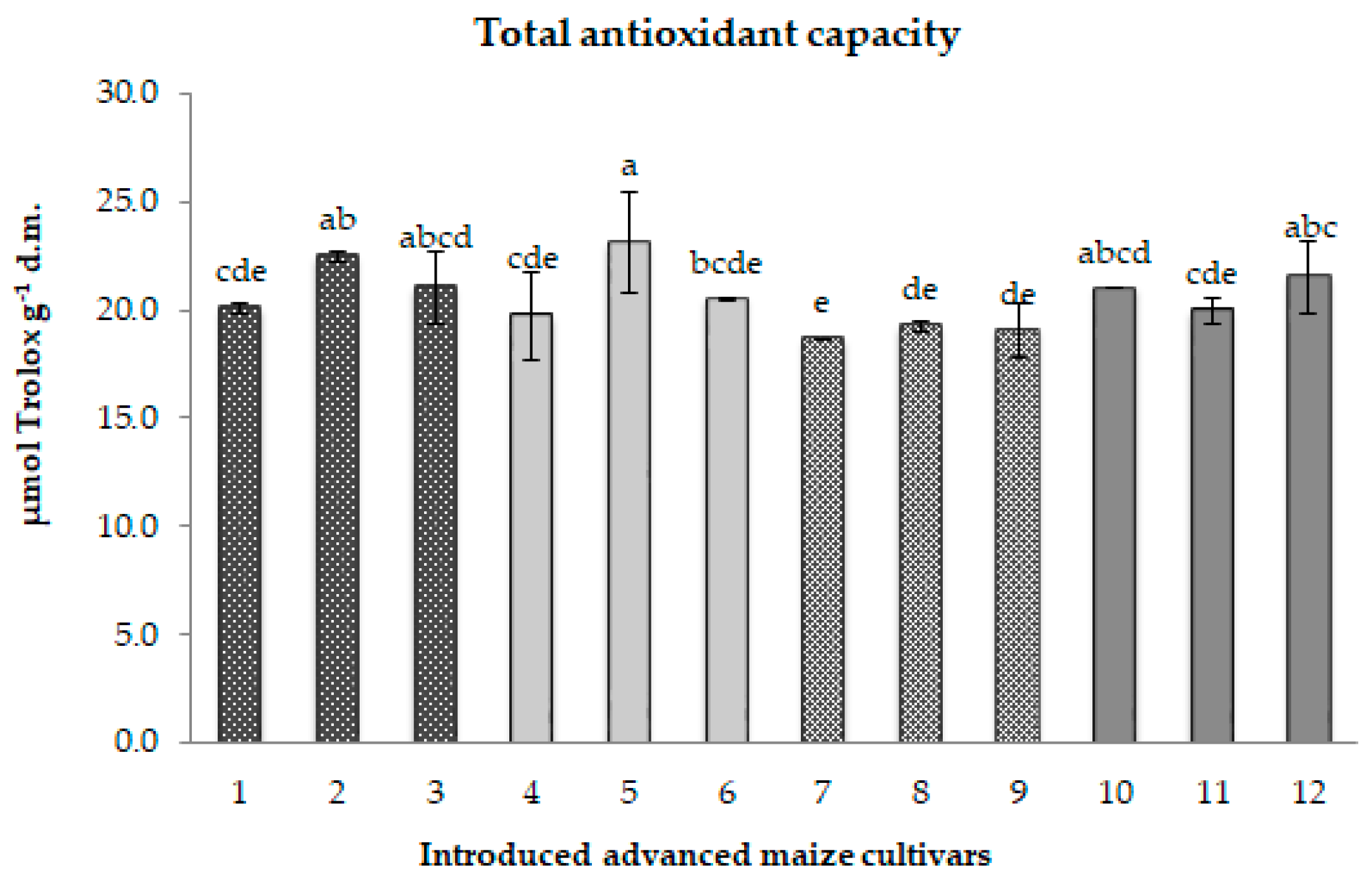
2.2.5. Analysis of the Total Antioxidant Capacity
2.3. Correlations
3. Discussion
3.1. Seed Germination and Seedling Vigour
3.2. Biochemical Markers
3.2.1. Analysis of Total Protein and Protein Fractions
3.2.2. Analysis of Reducing and Non-Reducing Sugars
3.2.3. Analysis of Soluble-Free and Insoluble-Bound Phenolics
3.2.4. Analysis of Tocopherols and Carotenoids
3.2.5. Analysis of Total Antioxidant Capacity
3.3. Correlations
3.3.1. The Total Protein, Protein Fractions and Seedling Growth Parameters
3.3.2. The Reducing and Non-Reducing Sugars and Seedling Growth Parameters
3.3.3. The Soluble-Free, and Insoluble-Bound Phenolics and Seedling Growth Parameters
3.3.4. The Tocopherols and Carotenoids and Seedling Growth Parameters
4. Materials and Methods
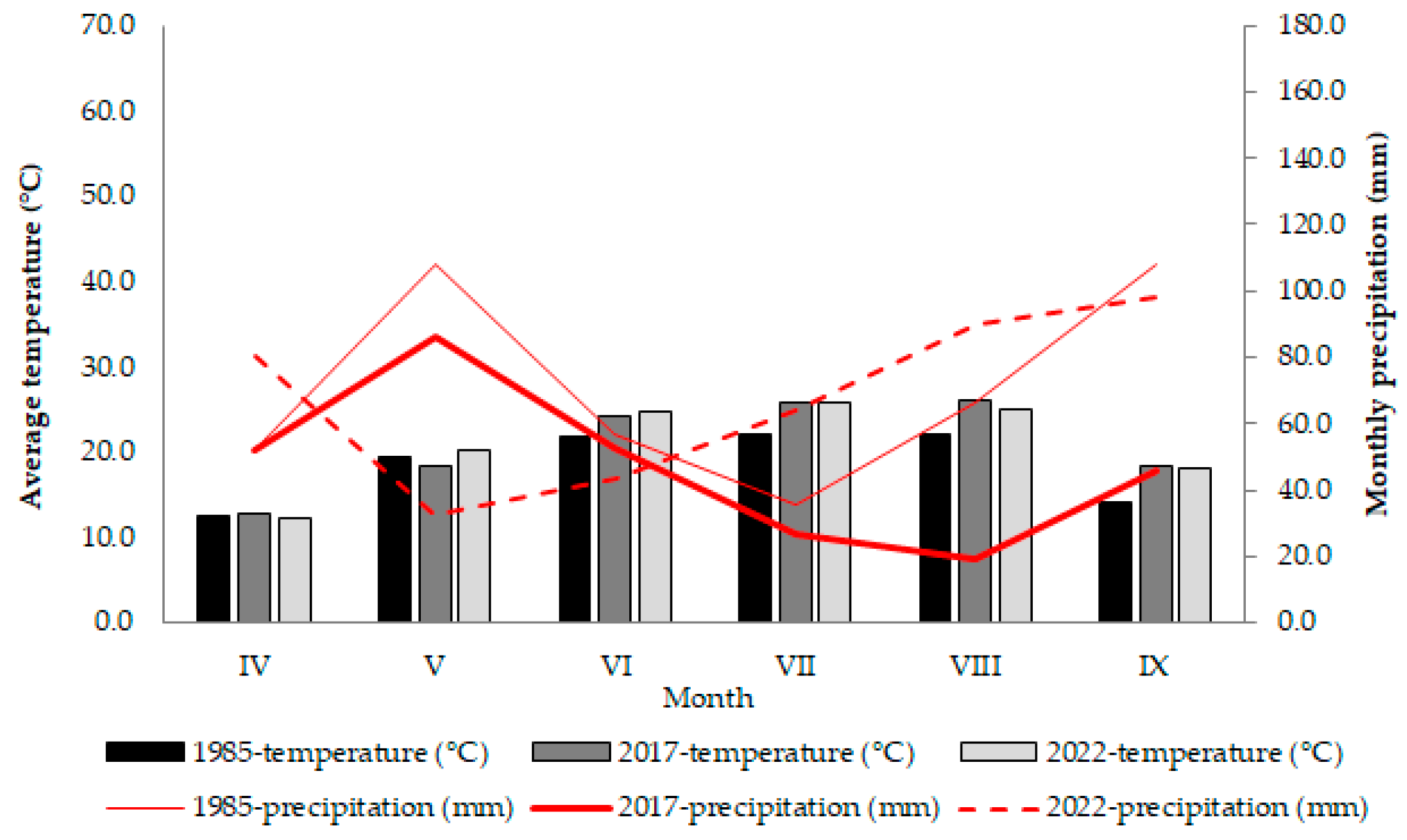
4.1. Plant Material
4.2. Assays for Seed Germination and Seedling Vigour
4.3. Biochemical Markers
4.3.1. Sample Preparation and Chemicals Used for the Analyses
4.3.2. Analysis of Total Protein
4.3.3. Analysis of Protein Fractions
4.3.4. Analysis of Sugars
4.3.5. Extraction of Soluble-Free and Insoluble-Bound Phenolic Compounds
4.3.6. Analysis of Total Phenolic Content
4.3.7. Analysis of Phenolic Acids
4.3.8. Analysis of Tocopherols and Carotenoids
4.3.9. Analysis of Total Antioxidant Capacity
4.3.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ROS | Reactive Oxygen Species |
| MRIZP | Maize Research Institute Zemun Polje |
| SVI | Seedling Vigour Index |
| GAE | Gallic Acid Equivalent |
| TAC | Total Antioxidant Capacity |
| SSPs | Seed Storage Proteins |
| RNS | Reactive Nitrogen Species |
| ISTA | International Seed Testing Association |
| HPLC | High-Performance Liquid Chromatography |
References
- Balconi, C.; Galaretto, A.; Malvar, R.A.; Nicolas, S.D.; Redaelli, R.; Andjelkovic, V.; Revilla, P.; Bauland, C.; Gouesnard, B.; Butron, A.; et al. Genetic and Phenotypic Evaluation of European Maize Landraces as a Tool for Conservation and Valorization of Agrobiodiversity. Biology 2024, 13, 454. [Google Scholar] [CrossRef]
- De Vitis, M.; Hay, F.R.; Dickie, J.B.; Trivedi, C.; Choi, J.; Fiegener, R. Seed Storage: Maintaining Seed Viability and Vigor for Restoration Use. Restor. Ecol. 2020, 28, S249–S255. [Google Scholar] [CrossRef]
- Trusjak, M.; Plitta-Michalak, B.P.; Michalak, M. Choosing the Right Path for the Successful Storage of Seeds. Plants 2023, 12, 72. [Google Scholar] [CrossRef]
- Leprince, O.; Pellizzaro, A.; Berriri, S.; Buitink, J. Late seed maturation: Drying without dying. J. Exp. Bot. 2017, 68, 827–841. [Google Scholar] [CrossRef]
- Buitink, J.; Leprince, O. Intracellular glasses and seed survival in the dry state. Comptes Rendus Biol. 2008, 331, 788–795. [Google Scholar] [CrossRef]
- Ballesteros, D.; Pritchard, H.W.; Walters, C. Dry architecture: Towards the understanding of the variation of longevity in desiccation-tolerant germplasm. Seed Sci. Res. 2020, 30, 142–155. [Google Scholar] [CrossRef]
- Matilla, A.J. The Orthodox Dry Seeds Are Alive: A Clear Example of Desiccation Tolerance. Plants 2022, 11, 20. [Google Scholar] [CrossRef]
- Hay, F.R.; Sershen, D. New technologies to improve the ex situ conservation of plant genetic resources. In Plant Genetic Resources; Dulloo, M.E., Ed.; Burleigh Dodds Science Publishing: London, UK, 2021; pp. 185–216. [Google Scholar] [CrossRef]
- Corbineau, F. The Effects of Storage Conditions on Seed Deterioration and Ageing: How to Improve Seed Longevity. Seeds 2024, 3, 56–75. [Google Scholar] [CrossRef]
- Roberts, E.H. Predicting the storage life of seeds. Seed Sci. Technol. 1973, 1, 499–514. [Google Scholar]
- Walters, C.; Wheeler, L.M.; Grotenhuis, J.M. Longevity of Seeds in a Gene-Bank: Species Characteristics. Seed Sci. Res. 2005, 15, 1–20. [Google Scholar] [CrossRef]
- Soldberg, S.Ø.; Yndgaard, F.; Andreasen, C.; Von Bothmer, R.; Loskutov, I.G.; Asdal, A. Long-term storage and longevity of orthodox seeds: A systematic review. Front. Plant Sci. 2020, 11, 1007. [Google Scholar] [CrossRef]
- Hay, F.R.; Rezaei, S.; Buitink, J. Seed moisture isotherms, sorption models and longevity. Front. Plant Sci. 2022, 13, 891913. [Google Scholar] [CrossRef] [PubMed]
- Nadarajan, J.; Walters, C.; Pritchard, H.W.; Ballesteros, D.; Colville, L. Seed longevity—The evolution of knowledge and a conceptual framework. Plants 2023, 12, 471. [Google Scholar] [CrossRef]
- Hampton, J.G. What is seed quality? Seed Sci. Technol. 2002, 30, 1–10. [Google Scholar]
- Hampton, J.G.; Boelt, B.; Rolston, M.P.; Chastain, T.G. Effects of elevated CO2 and temperature on seed quality. J. Agric. Sci. 2013, 151, 154–162. [Google Scholar] [CrossRef]
- Zani, D.; Müller, J.V. Climatic control of seed longevity of Silene during the post-zygotic phase: Do seeds from warm, dry climates possess higher maturity and desiccation tolerance than seeds from cold, wet climates? Ecol. Res. 2017, 32, 983–994. [Google Scholar] [CrossRef]
- Mondoni, A.; Orsenigo, S.; Donà, M.; Balestrazzi, A.; Probert, R.J.; Hay, F.R.; Petraglia, A.; Abeli, T. Environmentally induced transgenerational changes in seed longevity: Maternal and genetic influence. Ann. Bot. 2014, 113, 1257–1263. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, P.A.; Fritsche-Neto, R.; Andrade, M.C.; Petroli, C.D.; Burgueño, J.; Galli, G.; Willcox, M.C.; Sonder, K.; Vidal-Martínez, V.A.; Sifuentes-Ibarra, E.; et al. Introgression of maize diversity for drought tolerance: Subtropical maize landraces as source of new positive variants. Front. Plant Sci. 2021, 12, 691211. [Google Scholar] [CrossRef]
- Walters, C.; Ballesteros, D.; Vertucci, V.A. Structural mechanics of seed deterioration: Standing the test of time. Plant Sci. 2010, 179, 565–573. [Google Scholar] [CrossRef]
- Probert, R.J.; Daws, M.I.; Hay, F.R. Ecological correlates of ex situ seed longevity: A comparative study on 195 species. Ann. Bot. 2009, 104, 57–69. [Google Scholar] [CrossRef]
- Gianella, M.; Balestrazzi, A.; Pagano, A.; Müller, J.V.; Kyratzis, A.C.; Kikodze, D.; Canella, M.; Mondoni, A.; Rossi, G.; Guzzon, F. Heteromorphic seeds of wheat wild relatives show germination niche differentiation. Plant Biol. 2020, 22, 191–202. [Google Scholar] [CrossRef] [PubMed]
- McDonald, M.B. Seed deterioration: Physiology, repair and assessment. Seed Sci. Technol. 1999, 27, 177–237. [Google Scholar]
- Ebone, L.A.; Caverzan, A.; Chavarria, G. Physiologic alterations in orthodox seeds due to deterioration processes. Plant Physiol. Biochem. 2019, 145, 34–42. [Google Scholar] [CrossRef]
- Fu, Y.B.; Ahmed, Z.; Diederichsen, A. Towards a better monitoring of seed ageing under ex situ seed conservation. Conserv. Physiol. 2015, 3, cov026. [Google Scholar] [CrossRef]
- Oenel, A.; Fekete, A.; Krischke, M.; Faul, S.C.; Gresser, G.; Havaux, M.; Mueller, M.J.; Berger, S. Enzymatic and non–enzymatic mechanisms contribute to lipid oxidation during seed aging. Plant Cell Physiol. 2017, 58, 925–933. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Xue, H.; Pritchard, H.W.; Wang, X. Changes in the mitochondrial protein profile due to ROS eruption during ageing of elm (Ulmus pumila L.) seeds. Plant Physiol. Biochem. 2017, 114, 72–87. [Google Scholar] [CrossRef]
- Murthy, U.M.N.; Kumar, P.P.; Sun, W.Q. Mechanisms of seed ageing under different storage conditions for Vigna radiata (L.) Wilczek: Lipid peroxidation, sugar hydrolysis, Maillard reactions and their relationship to glass state transition. J. Exp. Bot. 2003, 54, 1057–1067. [Google Scholar] [CrossRef] [PubMed]
- Fleming, M.; Hill, L.M.; Walters, C. The kinetics of ageing in dry-stored seeds: A comparison of viability loss and RNA degradation in unique legacy seed collections. Ann. Bot. 2019, 123, 1133–1146. [Google Scholar] [CrossRef] [PubMed]
- Pournik, S.; Abbasi-Rostami, M.; Sadeghipour, H.R.; Ghaderi-Far, F. True lipases beside phospholipases contribute to walnut kernel viability loss during controlled deterioration and natural aging. Environ. Exp. Bot. 2019, 164, 71–83. [Google Scholar] [CrossRef]
- Waszczak, C.; Carmody, M.; Kangasjärvi, J. Reactive oxygen species in plant signaling. Annu. Rev. Plant Biol. 2018, 69, 209–236. [Google Scholar] [CrossRef] [PubMed]
- Rajjou, L.; Lovigny, Y.; Groot, S.P.; Belghazi, M.; Job, C.; Job, D. Proteome–wide characterization of seed aging in Arabidopsis: A comparison between artificial and natural aging protocols. Plant Physiol. 2008, 148, 620–641. [Google Scholar] [CrossRef]
- Hay, F.R.; de Guzman, F.; Ellis, D.; Makahiya, H.; Borromeo, T.; Sackville Hamilton, N.R. Viability of Oryza sativa L. seeds stored under genebank conditions for up to 30 years. Genet. Resour. Crop Evol. 2013, 60, 275–296. [Google Scholar] [CrossRef]
- Mondoni, A.; Orsenigo, S.; Müller, J.V.; Carlsson-Graner, U.; Jiménez Alfaro, B.; Abeli, T. Seed dormancy and longevity in subarctic and alpine populations of Silene suecica. Alp. Bot. 2018, 128, 71–81. [Google Scholar] [CrossRef]
- van Treuren, R.; Bas, N.; Kodde, J.; Groot, S.P.C.; Kik, C. Rapid loss of seed viability in ex situ conserved wheat and barley at 4 °C as compared to −20 °C storage. Conserv. Physiol. 2018, 6, coy033. [Google Scholar] [CrossRef] [PubMed]
- Desheva, G. The longevity of crop seeds stored under long-term condition in the National Gene Bank of Bulgaria. Agriculture 2016, 62, 90–100. [Google Scholar] [CrossRef]
- Commission on Genetic Resources for Food. The Second Report on the State of the World’s Plant Genetic Resources for Food and Agriculture; FAO: Rome, Italy, 2010; Available online: www.fao.org/docrep/013/i1500e/i1500e.pdf (accessed on 9 May 2023).
- Finch--Savage, W.E.; Bassel, G.W. Seed vigour and crop establishment: Extending performance beyond adaptation. J. Exp. Bot. 2016, 67, 567–591. [Google Scholar] [CrossRef]
- Pirredda, M.; Fañanás-Pueyo, I.; Oñate-Sánchez, L.; Mira, S. Seed Longevity and Ageing: A Review on Physiological and Genetic Factors with an Emphasis on Hormonal Regulation. Plants 2024, 13, 41. [Google Scholar] [CrossRef]
- Ҫakmak, T.; Atıcı, Ö.; Ağar, G. The natural aging-related biochemical changes in the seeds of two legume varieties stored for 40 years. Acta Agric. Scand. Sect. B Plant Soil Sci. 2010, 60, 353–360. [Google Scholar] [CrossRef]
- Ҫakmak, T.; Atıcı, Ö.; Ağar, G.; Sunar, S. Natural aging-related biochemical changes in alfalfa (Medicago Sativa L.) seeds stored for 42 years. Int. Res. J. Plant Sci. 2010, 1, 1–6. [Google Scholar]
- Solberg, S.Ø.; Brodal, G.; von Bothmer, R.; Meen, E.; Yndgaard, F.; Andreasen, C.; Asdal, Å. Seed Germination after 30 Years Storage in Permafrost. Plants 2020, 9, 579. [Google Scholar] [CrossRef]
- Šerá, B. Methodological contribution on seed germination and seedling initial growth tests in wild plants. Not. Bot. Horti Agrobot. 2023, 51, 13164. [Google Scholar] [CrossRef]
- Adetunji, A.E.; Adetunji, T.L.; Varghese, B.; Sershen; Pammenter, N.W. Oxidative Stress, Ageing and Methods of Seed Invigoration: An Overview and Perspectives. Agronomy 2021, 11, 2369. [Google Scholar] [CrossRef]
- Waterworth, W.M.; West, C.E. Genome damage accumulated in seed aging leads to plant genome instability and growth inhibition. Biochem. J. 2023, 480, 461–470. [Google Scholar] [CrossRef]
- Kannababu, N.; Nanjundappa, S.; Narayanan, N.; Vetriventhan, M.; Venkateswarlu, R.; Kanta Das, I.; Srikanth, A.; Viswanath, A.; Singh, S.; Malipatil, R.; et al. Role of functional genes for seed vigor related traits through genome-wide association mapping in finger millet (Eleusine coracana L. Gaertn.). Sci. Rep. 2025, 15, 5569. [Google Scholar] [CrossRef] [PubMed]
- Bhandhari, U.; Gajurel, A.; Khadka, B.; Thapa, I.; Chand, I.; Bhatta, D.; Poudel, A.; Pandey, M.; Shrestha, S.; Shrestha, J. Morpho-physiological and biochemical response of rice (Oryza sativa L.) to drought stress: A review. Heliyon 2023, 9, e13744. [Google Scholar] [CrossRef] [PubMed]
- Kravic, N.; Babic, V.; Vukadinovic, J.; Ristic, D.; Dragicevic, V.; Mladenovic Drinic, S.; Andjelkovic, V. Alteration of Metabolites Accumulation in Maize Inbreds Leaf Tissue under Long-Term Water Deficit. Biology 2021, 10, 694. [Google Scholar] [CrossRef]
- Lee, J.-S.; Hay, F.R. Variation in Seed Metabolites between Two Indica Rice Accessions Differing in Seed Longevity. Plants 2020, 9, 1237. [Google Scholar] [CrossRef]
- Mira, S.; Pirredda, M.; Martín-Sánchez, M.; Marchessi, J.E.; Martín, C. DNA Methylation and Integrity in Aged Seeds and Regenerated Plants. Seed Sci. Res. 2020, 30, 92–100. [Google Scholar] [CrossRef]
- Kurek, K.; Plitta-Michalak, B.; Ratajczak, E. Reactive Oxygen Species as Potential Drivers of the Seed Aging Process. Plants 2019, 8, 174. [Google Scholar] [CrossRef]
- Kravić, N.; Anđelković, V.; Hadži-Tašković-Šukalović, V.; Vuletić, M. Antioxidant activity in seeds of maize genotypes with different percentage of exotic germplasm. Genet.-Belgr. 2009, 41, 56–63. [Google Scholar] [CrossRef]
- Nickas, J.; N’Danikou, S.; Shango, A.J.; Kilasi, N. Physio-biochemical response of vegetable seeds to ageing: A systematic review. Plant Physiol. Biochem. 2025, 228, 110223. [Google Scholar] [CrossRef]
- Sano, N.; Rajjou, L.; North, H.M.; Debeaujon, I.; Marion-Poll, A.; Seo, M. Staying Alive: Molecular Aspects of Seed Longevity. Plant Cell Physiol. 2016, 57, 660–674. [Google Scholar] [CrossRef]
- Arc, E.; Galland, M.; Cueff, G.; Godin, B.; Lounifi, I.; Job, D.; Rajjou, L. Reboot the system thanks to protein post-translational modifications and proteome diversity: How quiescent seeds restart their metabolism to prepare seedling establishment. Proteomics 2011, 11, 1606–1618. [Google Scholar] [CrossRef]
- Nguyen, T.P.; Cueff, G.; Hegedus, D.D.; Rajjou, L.; Bentsink, L. A role for seed storage proteins in Arabidopsis seed longevity. J. Exp. Bot. 2015, 66, 6399–6413. [Google Scholar] [CrossRef] [PubMed]
- Devi, V.; Sethi, M.; Kaur, C.; Singh, V.; Kumar, R.; Chaudhary, D.P. Temporal profile of amino acids and protein fractions in the developing kernel of maize germplasm. Sci Rep. 2024, 14, 27161. [Google Scholar] [CrossRef]
- Company, T.; Soriano, P.; Estrelles, E.; Mayoral, O. Seed bank longevity and germination ecology of invasive and native grass species from Mediterranean wetlands. Folia Geobot. 2019, 54, 151–161. [Google Scholar] [CrossRef]
- Hourston, J.E.; Pérez, M.; Gawthrop, F.; Richards, M.; Steinbrecher, T.; Leubner-Metzger, G. The effects of high oxygen partial pressure on vegetable Allium seeds with a short shelf-life. Planta 2020, 251, 9. [Google Scholar] [CrossRef]
- Li, W.; Niu, Y.; Zheng, Y.; Wang, Z. Oxygen Species-Dependent Regulation on Seed Dormancy, Germination, and Deterioration in Crops. Front. Plant Sci. 2022, 13, 826809. [Google Scholar] [CrossRef] [PubMed]
- Lounifi, I.; Arc, E.; Molassiotis, A.; Job, D.; Rajjou, L.; Tanou, G. Interplay between protein carbonylation and nitrosylation in plants. Proteomics 2013, 13, 568–578. [Google Scholar] [CrossRef]
- Boucelha, L.; Abrous-Belbachir, O.; Djebbar, R. Is protein carbonylation a biomarker of seed priming and ageing? Funct. Plant Biol. 2021, 48, 611–623. [Google Scholar] [CrossRef]
- Smakowska, E.; Czarna, M.; Janska, H. Mitochondrial ATP-dependent proteases in protection against accumulation of carbonylated proteins. Mitochondrion 2014, 19, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Satour, P.; Youssef, C.; Chatelain, E.; Vu, B.L.; Teulat, B.; Job, C.; Job, D.; Montrichard, F. Patterns of protein carbonylation during Medicago truncatula seed maturation. Plant Cell Environ. 2018, 41, 2183–2194. [Google Scholar] [CrossRef]
- Biswas, M.S.; Terada, R.; Mano, J.I. Inactivation of carbonyl-detoxifying enzymes by H2O2 is a trigger to increase carbonyl load for initiating programmed cell death in plants. Antioxidants 2020, 9, 141. [Google Scholar] [CrossRef]
- Yin, G.; Xin, X.; Fu, S.; An, M.; Wu, S.; Chen, X.; Zhang, J.; He, J.; Whelan, J.; Lu, X. Proteomic and carbonylation profile analysis at the critical node of seed ageing in Oryza sativa. Sci. Rep. 2017, 7, 40611. [Google Scholar] [CrossRef]
- Chen, B.; Yin, G.; Whelan, J.; Zhang, Z.; Xin, X.; He, J.; Chen, X.; Zhang, J.; Zhou, J.; Lu, X. Composition of mitochondrial complex I during the critical node of seed aging in Oryza sativa. J. Plant Physiol. 2019, 236, 7–14. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, Y.; Sun, J.; Meng, J.; Tao, J. Deterioration of orthodox seeds during ageing: Influencing factors, physiological alterations and the role of reactive oxygen species. Plant Physiol. Biochem. 2021, 158, 475–485. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Gao, H.; Wang, Y.; Zhang, L.; Jia, J.; Ma, H. Storage Time Affects the Viability, Longevity, and Germination of Eriochloa villosa (Thunb.) Kunth Seeds. Sustainability 2023, 15, 8576. [Google Scholar] [CrossRef]
- Xing, W.; Li, Y.; Zhou, L.; Hong, H.; Liu, Y.; Luo, S.; Zou, J.; Zhao, Y.; Yang, Y.; Xu, Z.; et al. Deciphering Seed Deterioration: Molecular Insights and Priming Strategies for Revitalizing Aged Seeds. Plants 2025, 14, 1730. [Google Scholar] [CrossRef]
- Yazdanpanah, F.; Maurino, V.G.; Mettler-Altmann, T.; Buijs, G.; Bailly, M.; Jashni, M.K.; Willems, L.; Sergeeva, L.I.; Rajjou, L.; Hilhorst, H.W.M.; et al. NADP-MALIC ENZYME 1 Affects germination after seed storage in Arabidopsis thaliana. Plant Cell Physiol. 2019, 60, 318–328. [Google Scholar] [CrossRef]
- Golovina, E.A.; Wolkers, W.F.; Hoekstra, F.A. Long-term stability of protein secondary structure in dry seeds. Comp. Biochem. Physiol. Part A Physiol. 1997, 117, 343–348. [Google Scholar] [CrossRef]
- Choudhary, P.; Pramitha, L.; Aggarwal, P.R.; Rana, S.; Vetriventhan, M.; Muthamilarasan, M. Biotechnological interventions for improving the seed longevity in cereal crops: Progress and prospects. Crit. Rev. Biotechnol. 2022, 43, 309–325. [Google Scholar] [CrossRef] [PubMed]
- Bernal-Lugo, I.; Leopold, A.C. Changes in Soluble Carbohydrates during Seed Storage. Plant Physiol. 1992, 98, 1207–1210. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Qin, A.; Gao, Y.; Ma, S.; Liu, Z.; Zhao, B.; Ning, D.; Zhang, K.; Gong, W.; Sun, M.; et al. Effects of water deficit at different stages on growth and ear quality of waxy maize. Front. Plant Sci. 2023, 14, 1069551. [Google Scholar] [CrossRef]
- Murthy, U.M.; Sun, W.Q. Protein modification by Amadori and Maillard reactions during seed storage: Roles of sugar hydrolysis and lipid peroxidation. J. Exp. Bot. 2000, 51, 1221–1228. [Google Scholar] [CrossRef][Green Version]
- He, D.; Han, C.; Yao, J.; Shen, S.; Yang, P. Constructing the metabolic and regulatory pathways in germinating rice seeds through proteomic approach. Proteomics 2011, 11, 2693–2713. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Zang, Y.; Zhang, X.; Shang, S.; Xue, S.; Chen, J.; Tang, X. Insights into the regulation of energy metabolism during the seed-to-seedling transition in marine angiosperm Zostera marina L.: Integrated metabolomic and transcriptomic analysis. Front. Plant Sci. 2023, 14, 1130292. [Google Scholar] [CrossRef]
- He, H.; Gao, H.; Xue, X.; Ren, J.; Chen, X.; Niu, B. Variation of sugar compounds in Phoebe chekiangensis seeds during natural desiccation. PLoS ONE 2024, 19, e0299669. [Google Scholar] [CrossRef]
- Bilova, T.; Lukasheva, E.; Brauch, D.; Greifenhagen, U.; Paudel, G.; Tarakhovskaya, E.; Frolova, N.; Mittasch, J.; Balcke, G.U.; Tissier, A.; et al. A snapshot of the plant glycated proteome: Structural, functional and mechanistic aspects. J. Biol. Chem. 2016, 291, 7621–7636. [Google Scholar] [CrossRef]
- Bechtold, U.; Rabbani, N.; Mullineaux, P.M.; Thornalley, P.J. Quantitative measurement of specific biomarkers for protein oxidation, nitration and glycation in Arabidopsis leaves. Plant J. 2009, 59, 661–671. [Google Scholar] [CrossRef]
- Paudel, G.; Bilova, T.; Schmidt, R.; Greifenhagen, U.; Berger, R.; Tarakhovskaya, E.; Stöckhardt, S.; Balcke, G.U.; Humbeck, K.; Brandt, W.; et al. Osmotic stress is accompanied by protein glycation in Arabidopsis thaliana. J. Exp. Bot. 2016, 67, 6283–6295. [Google Scholar] [CrossRef]
- Frolov, A.; Bilova, T.; Paudel, G.; Berger, R.; Balcke, G.U.; Birkemeyer, C.; Wessjohann, L.A. Early responses of mature Arabidopsis thaliana plants to reduced water potential in the agar-based polyethylene glycol infusion drought model. J. Plant Physiol. 2017, 208, 70–83. [Google Scholar] [CrossRef]
- Zhou, W.; Chen, F.; Zhao, S.; Yang, C.; Meng, Y.; Shuai, H.; Luo, X.; Dai, Y.; Yin, H.; Du, J.; et al. DA-6 promotes germination and seedling establishment from aged soybean seeds by mediating fatty acid metabolism and glycometabolism. J. Exp. Bot. 2019, 70, 101–114. [Google Scholar] [CrossRef] [PubMed]
- Rolland, F.; Baena-Gonzalez, E.; Sheen, J. Sugar sensing and signalling in plants: Conserved and novel mechanisms. Annu. Rev. Plant Biol. 2006, 57, 675–709. [Google Scholar] [CrossRef] [PubMed]
- Shumilina, J.; Kusnetsova, A.; Tsarev, A.; Janse van Rensburg, H.C.; Medvedev, S.; Demidchik, V.; Van den Ende, W.; Frolov, A. Glycation of Plant Proteins: Regulatory Roles and Interplay with Sugar Signalling? Int. J. Mol. Sci. 2019, 20, 2366. [Google Scholar] [CrossRef]
- Jiang, F.L.; Bo, L.P.; Xu, J.J.; Wu, Z. Changes in respiration and structure of non-heading Chinese cabbage seeds during gradual artificial aging. Sci. Hortic. 2018, 238, 14–22. [Google Scholar] [CrossRef]
- Zhou, L.; Lu, L.; Chen, C.; Zhou, T.; Wu, Q.; Wen, F.; Chen, J.; Pritchard, H.W.; Peng, C.; Pei, J.; et al. Comparative changes in sugars and lipids show evidence of a critical node for regeneration in safflower seeds during aging. Front. Plant Sci. 2022, 13, 1020478. [Google Scholar] [CrossRef]
- Martinez-Maldonado, F.E.; Miranda-Lasprilla, D.; Magnitskiy, S.; Melgarejo, L.M. Germination, protein contents and soluble carbohydrates during storage of sugar apple seeds (Annona squamosa L.). J. Appl. Bot. Food Qual. 2015, 88, 308–314. [Google Scholar] [CrossRef]
- Thomas, A.; Beena, R. Sucrose Metabolism in Plants under Drought Stress Condition: A Review. Indian J. Agric. Res. 2021, 58, 943. [Google Scholar] [CrossRef]
- Sehgal, A.; Sita, K.; Kumar, J.; Kumar, S.; Singh, S.; Siddique, K.H.; Nayyar, H. Effects of drought, heat and their interaction on the growth, yield and photosynthetic function of lentil (Lens culinaris Medikus) genotypes varying in heat and drought sensitivity. Front. Plant Sci. 2017, 8, 1776. [Google Scholar] [CrossRef]
- Du, Y.; Zhao, Q.; Chen, L.; Yao, X.; Zhang, H.; Wu, J.; Xie, F. Effect of Drought Stress during Soybean R2–R6 Growth Stages on Sucrose Metabolism in Leaf and Seed. Int. J. Mol. Sci. 2020, 21, 618. [Google Scholar] [CrossRef]
- Pinheiro, C.; Rodrigues, A.P.; de Carvalho, I.S.; Chaves, M.M.; Ricardo, C.P. Sugar metabolism in developing lupin seeds is affected by a short-term water deficit. J. Exp. Bot. 2005, 56, 2705–2712. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Jensen, C.R.; Andersen, M.N. Drought stress effect on carbohydrate concentration in soybean leaves and pods during early reproductive development: Its implication in altering pod set. Field Crop Res. 2004, 86, 1–13. [Google Scholar] [CrossRef]
- Feregrino-Pérez, A.A.; Mercado-Luna, A.; Murillo-Cárdenas, C.A.; González-Santos, R.; Chávez-Servín, J.L.; Vargas-Madriz, A.F.; Luna-Sánchez, E. Polyphenolic Compounds and Antioxidant Capacity in Native Maize of the Sierra Gorda of Querétaro. Agronomy 2024, 14, 142. [Google Scholar] [CrossRef]
- Satya Srii, V.; Nagarajappa, N. Impact of accelerated aging on seed quality, seed coat physical structure and antioxidant enzyme activity of Maize (Zea mays L.). PeerJ 2024, 12, e17988. [Google Scholar] [CrossRef]
- Lattanzio, V. Relationship of Phenolic Metabolism to Growth in Plant and Cell Cultures Under Stress. In Plant Cell and Tissue Differentiation and Secondary Metabolites: Fundamentals and Applications, 1st ed.; Ramawat, K.G., Ekiert, H.M., Goyal, S., Eds.; Reference Series in Phytochemistry; Springer: Cham, Switzerland, 2021; Volume 2, pp. 837–868. [Google Scholar] [CrossRef]
- Krebs, S.A.; Schummer, M.L. A review of plant phenolics and endozoochory. Ecol Evol. 2024, 14, e70255. [Google Scholar] [CrossRef]
- Chakhchar, A.; Aissam, S.; El Modafar, C. Quantitative and Qualitative Study of Phenolic Compounds Involved in Germination Inhibition of Wheat under Water Deficit. Technol. Investig. 2016, 7, 86–95. [Google Scholar] [CrossRef][Green Version]
- Kumar, N.; Goel, N. Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnol. Rep. 2019, 24, e00370. [Google Scholar] [CrossRef]
- Sharma, A.; Shahzad, B.; Rehman, A.; Bhardwaj, R.; Landi, M.; Zheng, B. Response of Phenylpropanoid Pathway and the Role of Polyphenols in Plants under Abiotic Stress. Molecules 2019, 24, 2452. [Google Scholar] [CrossRef]
- Rajashekar, C. Dual Role of Plant Phenolic Compounds as Antioxidants and Prooxidants. Am. J. Plant Sci. 2023, 14, 15–28. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Mahmud, J.A.; Fujita, M.; Fotopoulos, V. Reactive oxygen species and antioxidant defense in plants under abiotic stress: Revisiting the crucial role of a universal defense regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef]
- Kumar, N.; Pruthi, V. Potential applications of ferulic acid from natural sources. Biotechnol. Rep. 2014, 4, 86–93. [Google Scholar] [CrossRef]
- Le Gall, H.; Philippe, F.; Domon, J.-M.; Gillet, F.; Pelloux, J.; Rayon, C. Cell Wall Metabolism in Response to Abiotic Stress. Plants 2015, 4, 112–166. [Google Scholar] [CrossRef]
- Shahidi, F.; Hossain, A. Importance of Insoluble-Bound Phenolics to the Antioxidant Potential Is Dictated by Source Material. Antioxidants 2023, 12, 203. [Google Scholar] [CrossRef]
- Chandrasekara, A.; Shahidi, F. Content of insoluble bound phenolics in millets and their contribution to antioxidant capacity. J. Agric. Food Chem. 2010, 58, 6706–6714. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekara, A.; Shahidi, F. Inhibitory activities of soluble and bound millet seed phenolics on free radicals and reactive oxygen species. J. Agric. Food Chem. 2011, 59, 428–436. [Google Scholar] [CrossRef]
- García-Lara, S. Maize hydroxycinnamic acids: Unveiling their role in stress resilience and human health. Front. Nutr. 2024, 11, 1322904. [Google Scholar] [CrossRef]
- Sung, J.M.; Jeng, T.L. Lipid peroxidation and peroxide-scavenging enzymes associated with accelerated aging of peanut seed. Physiol. Plant. 1994, 91, 51–55. [Google Scholar] [CrossRef]
- Dwiecki, K.; Siger, A.; Czubiński, J.; Nogala-Kałucka, M.; Lampart-Szczapa, E. The Interactions Between Rapeseed Lipoxygenase and Native Polyphenolic Compounds in a Model System. J. Am. Oil. Chem. Soc. 2012, 89, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Chen, W.; Xu, Z.; Chen, M.; Yu, D. Functions of WRKYs in plant growth and development. Trends Plant Sci. 2023, 28, 630–645. [Google Scholar] [CrossRef]
- Singh, S.; Koyama, H.; Bhati, K.K.; Alok, A. The biotechnological importance of the plant-specific NAC transcription factor family in crop improvement. J. Plant Res. 2021, 134, 475–495. [Google Scholar] [CrossRef]
- Gulcin, İ. Antioxidants: A comprehensive review. Arch. Toxicol. 2025, 99, 1893–1997. [Google Scholar] [CrossRef]
- Lee, J.S.; Kwak, J.; Yoon, M.R.; Lee, J.S.; Hay, F.R. Contrasting tocol ratios associated with seed longevity in rice variety groups. Seed Sci. Res. 2017, 27, 273–280. [Google Scholar] [CrossRef]
- Vinutha, T.; Bansal, N.; Kumari, K.; Prashat, G.R.; Sreevathsa, R.; Krishnan, V.; Kumari, S.; Dahuja, A.; Lal, S.K.; Sachdev, A.; et al. Comparative Analysis of Tocopherol Biosynthesis Genes and its Transcriptional Regulation in Soybean Seeds. J. Agric. Food Chem. 2017, 65, 11054–11064. [Google Scholar] [CrossRef]
- Fritsche, S.; Wang, X.; Jung, C. Recent Advances in our Understanding of Tocopherol Biosynthesis in Plants: An Overview of Key Genes, Functions, and Breeding of Vitamin E Improved Crops. Antioxidants 2017, 6, 99. [Google Scholar] [CrossRef]
- Sattler, S.E.; Cheng, Z.; DellaPenna, D. From Arabidopsis to agriculture: Engineering improved Vitamin E content in soybean. Trends Plant Sci. 2004, 9, 365–367. [Google Scholar] [CrossRef]
- Porfirova, S.; Bergmüller, E.; Tropf, S.; Lemke, R.; Dörmann, P. Isolation of an Arabidopsis mutant lacking vitamin E and identification of a cyclase essential for all tocopherol biosynthesis. Proc. Natl. Acad. Sci. USA 2002, 99, 12495–12500. [Google Scholar] [CrossRef] [PubMed]
- Laxa, M.; Liebthal, M.; Telman, W.; Chibani, K.; Dietz, K.-J. The Role of the Plant Antioxidant System in Drought Tolerance. Antioxidants 2019, 8, 94. [Google Scholar] [CrossRef]
- Kreszies, V.; Hoppe, N.; Gutbrod, K.; Dörmann, P. Regulation of tocopherol (vitamin E) biosynthesis by abscisic acid-dependent and -independent pathways during abiotic stress in Arabidopsis. Planta 2025, 261, 94. [Google Scholar] [CrossRef] [PubMed]
- Sattler, S.E.; Gilliland, L.U.; Magallanes-Lundback, M.; Pollard, M.; DellaPenna, D. Vitamin E is essential for seed longevity and for preventing lipid peroxidation during germination. Plant Cell 2004, 16, 1419–1432. [Google Scholar] [CrossRef]
- Falk, J.; Krauß, N.; Dähnhardt, D.; Krupinska, K. The senescence associated gene of barley encoding 4-hydroxyphenylpyruvate dioxygenase is expressed during oxidative stress. J. Plant Physiol. 2002, 159, 1245–1253. [Google Scholar] [CrossRef]
- Trono, D. Carotenoids in Cereal Food Crops: Composition and Retention throughout Grain Storage and Food Processing. Plants 2019, 8, 551. [Google Scholar] [CrossRef]
- Mordi, R.C.; Ademosun, O.T.; Ajanaku, C.O.; Olanrewaju, I.O.; Walton, J.C. Free Radical Mediated Oxidative Degradation of Carotenes and Xanthophylls. Molecules 2020, 25, 1038. [Google Scholar] [CrossRef]
- Liang, M.H.; He, Y.J.; Liu, D.M.; Jiang, J.G. Regulation of carotenoid degradation and production of apocarotenoids in natural and engineered organisms. Crit. Rev. Biotechnol. 2021, 41, 513–534. [Google Scholar] [CrossRef]
- Zheng, X.; Yang, Y.; Al-Babili, S. Exploring the Diversity and Regulation of Apocarotenoid Metabolic Pathways in Plants. Front. Plant Sci. 2021, 12, 787049. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Rao, S.; Wrightstone, E.; Sun, T.; Lui, A.C.W.; Welsch, R.; Li, L. Phytoene Synthase: The Key Rate-Limiting Enzyme of Carotenoid Biosynthesis in Plants. Front. Plant Sci. 2022, 13, 884720. [Google Scholar] [CrossRef] [PubMed]
- Howitt, C.A.; Pogson, B.J. Carotenoid accumulation and function in seeds and non-green tissues. Plant Cell Environ. 2006, 29, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; He, D.; Gupta, R.; Yang, P. Physiological and proteomic analyses on artificially aged Brassica napus seed. Front. Plant Sci. 2015, 6, 112. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Jorge, S.; Ha, S.H.; Magallanes-Lundback, M.; Gilliland, L.U.; Zhou, A.; Lipka, A.E.; Nguyen, Y.N.; Angelovici, R.; Lin, H.; Cepela, J.; et al. CAROTENOID CLEAVAGE DIOXYGENASE4 is a negative regulator of β-carotene content in Arabidopsis seeds. Plant Cell 2013, 25, 4812–4826. [Google Scholar] [CrossRef]
- Li, F.; Vallabhaneni, R.; Wurtzel, E.T. PSY3, a New Member of the Phytoene Synthase Gene Family Conserved in the Poaceae and Regulator of Abiotic Stress-Induced Root Carotenogenesis. Plant Physiol. 2008, 146, 1333–1345. [Google Scholar] [CrossRef]
- Sun, T.; Rao, S.; Zhou, X.; Li, L. Plant carotenoids: Recent advances and future perspectives. Mol. Hortic. 2022, 2, 3. [Google Scholar] [CrossRef]
- Niki, E.; Noguchi, N. Evaluation of antioxidant capacity. What capacity is being measured by which method? IUBMB Life 2000, 50, 323–329. [Google Scholar] [CrossRef]
- Apak, R.; Özyürek, M.; Güçlü, K.; Çapanoğlu, E. Antioxidant Activity/Capacity Measurement. 1. Classification, Physicochemical Principles, Mechanisms, and Electron Transfer (ET)-Based Assays. J. Agric. Food Chem. 2016, 64, 997–1027. [Google Scholar] [CrossRef]
- Serpen, A.; Gökmen, V.; Pellegrini, N.; Fogliano, V. Direct measurement of the total antioxidant capacity of cereal products. J. Cereal Sci. 2008, 48, 816–820. [Google Scholar] [CrossRef]
- Poljsak, B.; Šuput, D.; Milisav, I. Achieving the balance between ROS and antioxidants: When to use the synthetic antioxidants. Oxid. Med. Cell Longev. 2013, 2013, 956792. [Google Scholar] [CrossRef] [PubMed]
- Renard, J.; Niñoles, R.; Martínez-Almonacid, I.; Gayubas, B.; Mateos-Fernández, R.; Bissoli, G.; Bueso, E.; Serrano, R.; Gadea, J. Identification of Novel Seed Longevity Genes Related to Oxidative Stress and Seed Coat by Genome-wide Association Studies and Reverse Genetics. Plant Cell Environ. 2020, 43, 2523–2539. [Google Scholar] [CrossRef]
- Kranner, I.; Minibayeva, F.V.; Beckett, R.P.; Seal, C.E. What Is Stress? Concepts, Definitions and Applications in Seed Science. New Phytol. 2010, 188, 655–673. [Google Scholar] [CrossRef] [PubMed]
- Hay, P.C.; Ramsaur, T.; Smith, C.; Miernyk, J.A. Storage protein mobilization during germination and early seedling growth of Zea mays. Physiol. Plant. 1991, 81, 377–384. [Google Scholar] [CrossRef]
- Galland, M.; Huguet, R.; Arc, E.; Cueff, G.; Job, D.; Rajjou, L. Dynamic proteomics emphasizes the importance of selective mRNA translation and protein turnover during Arabidopsis seed germination. Mol. Cell. Proteom. 2014, 13, 252–268. [Google Scholar] [CrossRef]
- Oracz, K.; Stawska, M. Cellular Recycling of Proteins in Seed Dormancy Alleviation and Germination. Front. Plant Sci. 2016, 7, 1128. [Google Scholar] [CrossRef]
- Bailly, C.; El-Maarouf-Bouteau, H.; Corbineau, F. From intracellular signalling networks to cell death: The dual role of reactive oxygen species in seed physiology. Comptes Rendus Biol. 2008, 331, 806–814. [Google Scholar] [CrossRef]
- Dumont, S.; Rivoal, J. Consequences of Oxidative Stress on Plant Glycolytic and Respiratory Metabolism. Front. Plant Sci. 2019, 10, 166. [Google Scholar] [CrossRef] [PubMed]
- Li, B.B.; Zhang, S.B.; Lv, Y.Y.; Wei, S.; Hu, Y.S. Reactive oxygen species-induced protein carbonylation promotes deterioration of physiological activity of wheat seeds. PLoS ONE 2022, 17, e0263553. [Google Scholar] [CrossRef] [PubMed]
- Radha, B.N.; Channakeshava, B.C.; Hullur, N.; Pandurange Gowda, K.T.; Bhanuprakash, K.; Ramachandrappa, B.K.; Munirajappa, R. Effect of seed ageing on protein quality and quantity in maize. Int. J. Bioassays 2014, 3, 1708–1713. Available online: https://www.ijbio.com/archive/ijbio-volume-3-issue-1-year-2014.html (accessed on 11 November 2025).
- Cho, Y.H.; Yoo, S.D. Signalling role of fructose mediated by FINS1/FBP in Arabidopsis thaliana. PLoS Genet. 2011, 7, e1001263. [Google Scholar] [CrossRef]
- Weitbrecht, K.; Muller, K.; Leubner-Metzger, G. First off the mark: Early seed germination. J. Exp. Bot. 2011, 62, 3289–3309. [Google Scholar] [CrossRef]
- Thakur, M.; Sharma, P.; Anand, A.; Pandita, V.K.; Bhatia, A.; Pushkar, S. Raffinose and Hexose Sugar Content During Germination Are Related to Infrared Thermal Fingerprints of Primed Onion (Allium cepa L.) Seeds. Front. Plant Sci. 2020, 11, 579037. [Google Scholar] [CrossRef]
- Rognoni, S.; Teng, S.; Arru, L.; Smeekens, S.C.M.; Perata, P. Sugar effects on early seedling development in Arabidopsis. Plant Growth Regul. 2007, 52, 217–228. [Google Scholar] [CrossRef]
- Dekkers, B.J.; Schuurmans, J.A.; Smeekens, S.C. Interaction between sugar and abscisic acid signalling during early seedling development in Arabidopsis. Plant Mol. Biol. 2008, 67, 151–167. [Google Scholar] [CrossRef]
- Obroucheva, N.V.; Lityagina, S.V.; Richter, A. Dynamics of carbohydrates in the embryo axes of horse chestnut seeds during their transition from dormancy to germination. Russ. J. Plant Physiol. 2006, 53, 768–778. [Google Scholar] [CrossRef]
- Rawat, S.S.; Laxmi, A. Sugar signals pedal the cell cycle! Front. Plant Sci. 2024, 15, 1354561. [Google Scholar] [CrossRef]
- Chen, Y.; Shirley Lin, S.; Duguid, S.; Paul Dribnenki, P.; Kenaschuk, E. Effect of sucrose concentration on elongation of shoots from flax anther culture. Plant Cell Tissue Organ Cult. 2003, 72, 181–183. [Google Scholar] [CrossRef]
- Corso, M.; Perreau, F.; Mouille, G.; Lepiniec, L. Specialized phenolic compounds in seeds: Structures, functions, and regulations. Plant Sci. 2020, 296, 110471. [Google Scholar] [CrossRef]
- Janovicek, K.J.; Vyn, T.J.; Voroney, R.P.; Allen, O.B. Early corn seedling growth response to phenolic acids. Can. J. Plant Sci. 1997, 77, 391–393. [Google Scholar] [CrossRef][Green Version]
- Buanafina, M.M.D.O.; Morris, P. The Impact of Cell Wall Feruloylation on Plant Growth, Responses to Environmental Stress, Plant Pathogens and Cell Wall Degradability. Agronomy 2022, 12, 1847. [Google Scholar] [CrossRef]
- Devi, S.R.; Prasad, M.N. Effect of ferulic acid on growth and hydrolytic enzyme activities of germinating maize seeds. J. Chem. Ecol. 1992, 18, 1981–1990. [Google Scholar] [CrossRef]
- Politycka, B.; Gmerek, J. Effects of ferulic and p-coumaric acids on the activity of hydrolytic enzymes and the growth of radicles in germinating seeds of cucumber and pea. Allelopath. J. 2008, 21, 227–238. [Google Scholar]
- Bewley, J.D.; Bradford, K.J.; Hilhorst, H.W.M.; Nonogaki, H. Germination. In Seeds: Physiology of Development, Germination and Dormancy, 3rd ed.; Bewley, J.D., Bradford, K.J., Hilhorst, H.W.M., Nonogaki, H., Eds.; Springer: New York, NY, USA, 2013; Volume 4, pp. 133–181. [Google Scholar] [CrossRef]
- Reigosa, M.J.; Souto, X.C.; González, L. Effect of phenolic compounds on the germination of six weeds species. Plant Growth Regul. 1999, 28, 83–88. [Google Scholar] [CrossRef]
- Shahid, N.; Ul-Haq, I.; Nayik, G.A.; Ramniwas, S.; Damto, T.; Ali Alharbi, S.; Ansari, M.J. Physicochemical and bioactive traits of black chickpea (Cicer arietinum) as affected by germination-induced modifications. Int. J. Food Prop. 2024, 27, 165–179. [Google Scholar] [CrossRef]
- Hernández-Miranda, J.; Reyes-Portillo, K.A.; García-Castro, A.; Ramírez-Moreno, E.; Román-Gutiérrez, A.D. Impacts of Phenolic Compounds and Their Benefits on Human Health: Germination. Metabolites 2025, 15, 425. [Google Scholar] [CrossRef]
- López-Malvar, A.; Main, O.; Guillaume, S.; Jacquemot, M.P.; Meunier, F.; Revilla, P.; Santiago, R.; Mechin, V.; Reymond, M. Genotype-dependent response to water deficit: Increases in maize cell wall digestibility occurs through reducing both p-coumaric acid and lignification of the rind. Front. Plant Sci. 2025, 16, 1571407. [Google Scholar] [CrossRef]
- Sathiyamoorthy, P. Identification of Vanillic and p-Coumaric Acid as Endogenous Inhibitors of Soybean Seeds and Their Inhibitory Effect on Germination. J. Plant Physiol. 1990, 136, 120–121. [Google Scholar] [CrossRef]
- Nkomo, M.; Gokul, A.; Keyster, M.; Klein, A. Exogenous p-Coumaric Acid Improves Salvia hispanica L. Seedling Shoot Growth. Plants 2019, 8, 546. [Google Scholar] [CrossRef]
- Iwasaki, M.; Paszkowski, J. Epigenetic memory in plants. EMBO J. 2014, 33, 1987–1998. [Google Scholar] [CrossRef]
- Steward, N.; Ito, M.; Yamaguchi, Y.; Koizumi, N.; Sano, H. Periodic DNA methylation in maize nucleosomes and demethylation by environmental stress. J. Biol. Chem. 2002, 277, 37741–37746. [Google Scholar] [CrossRef]
- Tsuji, H.; Saika, H.; Tsutsumi, N.; Hirai, A.; Nakazono, M. Dynamic and reversible changes in histone H3-Lys4 methylation and H3 acetylation occurring at submergence-inducible genes in rice. Plant Cell Physiol. 2006, 47, 995–1003. [Google Scholar] [CrossRef]
- Kim, J.M.; To, T.K.; Ishida, J.; Morosawa, T.; Kawashima, M.; Matsui, A.; Toyoda, T.; Kimura, H.; Shinozaki, K.; Seki, M. Alterations of lysine modifications on the histone H3 N-tail under drought stress conditions in Arabidopsis thaliana. Plant Cell Physiol. 2008, 49, 1580–1588. [Google Scholar] [CrossRef]
- Simić, A.; Sredojević, S.; Todorović, M.; Đukanović, L.; Radenović, C. Studies on the relationship between the content of total phenolics in exudates and germination ability of maize seed during accelerated aging. Seed Sci. Technol. 2004, 32, 213–218. [Google Scholar] [CrossRef]
- Sadiq, M.; Akram, N.A.; Ashraf, M.; Al-Qurainy, F.; Ahmad, P. Alpha-Tocopherol-Induced Regulation of Growth and Metabolism in Plants under Non-stress and Stress Conditions. J. Plant Growth Regul. 2019, 38, 1325–1340. [Google Scholar] [CrossRef]
- Wang, X.; Quinn, P.J. The location and function of vitamin E in membranes. Mol. Membr. Biol. 2000, 17, 143–156. [Google Scholar] [CrossRef] [PubMed]
- Tammela, P.; Salo-Väänänen, P.; Laakso, I.; Hopia, A.; Vuorela, H.; Nygren, M. Tocopherols, tocotrienols and fatty acids as indicators of natural ageing in Pinus sylvestris seeds. Scan. J. For. Res. 2005, 20, 378–384. [Google Scholar] [CrossRef]
- Desel, C.; Krupinska, K. The impact of tocochromanols on early seedling development and NO release. J. Plant Physiol. 2005, 162, 771–776. [Google Scholar] [CrossRef]
- Tavva, V.S.; Kim, Y.H.; Kagan, I.A.; Dinkins, R.D.; Kim, K.H.; Collins, G.B. Increased α-tocopherol content in soybean seed overexpressing the Perilla frutescens γ-tocopherol methyl transferase gene. Plant Cell Rep. 2007, 26, 61–70. [Google Scholar] [CrossRef]
- Voll, L.M.; Abbasi, A.R. Are there Specific In Vivo Roles for α- and γ-Tocopherol in Plants? Plant Signal. Behav. 2007, 2, 486–488. [Google Scholar] [CrossRef]
- Zhang, L.; Luo, Y.; Liu, B.; Zhang, L.; Zhang, W.; Chen, R.; Wang, L. Overexpression of the maize γ-tocopherol methyltransferase gene (ZmTMT) increases α-tocopherol content in transgenic Arabidopsis and maize seeds. Transgenic Res. 2020, 29, 95–104. [Google Scholar] [CrossRef]
- Omoarelojie, L.O.; Kulkarni, M.G.; Finnie, J.F.; Van Staden, J. Strigolactones and their crosstalk with other phytohormones. Ann. Bot. 2019, 124, 749–767. [Google Scholar] [CrossRef]
- Mazzoni-Putman, S.M.; Brumos, J.; Zhao, C.; Alonso, J.M.; Stepanova, A.N. Auxin Interactions with other Hormones in Plant Development. Cold Spring Harb. Perspect. Biol. 2021, 13, a039990. [Google Scholar] [CrossRef]
- Rosental, L.; Nonogaki, H.; Fait, A. Activation and regulation of primary metabolism during seed germination. Seed Sci. Res. 2014, 24, 1–15. [Google Scholar] [CrossRef]
- Stewart, J.L.; Maloof, J.N.; Nemhauser, J.L. PIF genes mediate the effect of sucrose on seedling growth dynamics. PLoS Genet. 2011, 8, e1002594. [Google Scholar] [CrossRef] [PubMed]
- Sommer, Â.S.; Medeiros Coelho, C.M.; Padilha, M.S.; Nerling, D. Hydrolytic enzyme activity (phytase and α-amylase) is decisive in the germination metabolism of maize seeds under salt stress. Ciênc. Agrotec. 2024, 48, e003224. [Google Scholar] [CrossRef]
- Fu, Y.; Zheng, G.; Ma, L.; Li, J.; Hou, D.; Zhang, L.; Zeng, B.; Bi, Q.; Tan, J.; Yu, X.; et al. Metabolite accumulation contributes to differences in seed germination of water-saving and drought-resistance rice under dry direct seeding. BMC Plant Biol. 2025, 25, 1417. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.; Martinez, M.D.P.; Bouzid, M.; Balparda, M.; Schwarzländer, M.; Maurino, V.G. Regulation of plant glycolysis and the tricarboxylic acid cycle by posttranslational modifications. Plant J. 2025, 122, e70142. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.; Du, S.; Jiao, F.; Xi, M.; Wang, A.; Xu, H.; Jiao, Q.; Zhang, X.; Jiang, H.; Chen, J.; et al. The regulatory network behind maize seed germination: Effects of temperature, water, phytohormones, and nutrients. Crop J. 2021, 9, 718–724. [Google Scholar] [CrossRef]
- Limami, A.M.; Rouillon, C.; Glevarec, G.; Gallais, A.; Hirel, B. Genetic and physiological analysis of germination efficiency in maize in relation to nitrogen metabolism reveals the importance of cytosolic glutamine synthetase. Plant Physiol. 2002, 130, 1860–1870. [Google Scholar] [CrossRef][Green Version]
- Lin, F.; Manisseri, C.; Fagerström, A.; Peck, M.L.; Vega-Sánchez, M.E.; Williams, B.; Chiniquy, D.M.; Saha, P.; Pattathil, S.; Conlin, B.; et al. Cell Wall Composition and Candidate Biosynthesis Gene Expression during Rice Development. Plant Cell Physiol. 2016, 57, 2058–2075. [Google Scholar] [CrossRef]
- Shumaila, K.; Chandn, I.Z.; Tayyaba, R.; Sara, A.; Aimen, A.; Aiman, Z.; Bushra, A.; Mujahid, A. The significance of chlorophylls and carotenoids in enhancing seed tolerance to abiotic stress. Biol. Clin. Sci. Res. J. 2024, 2024, 1065. [Google Scholar] [CrossRef]
- Arif, M.A.R.; Afzal, I.; Börner, A. Genetic Aspects and Molecular Causes of Seed Longevity in Plants-A Review. Plants 2022, 11, 598. [Google Scholar] [CrossRef]
- FAO. Genebank Standards for Plant Genetic Resources for Food and Agriculture; Food and Agriculture Organization of the United Nations: Rome, Italy, 2014; ISBN 978-92-5-108262-1. [Google Scholar]
- International Seed Testing Association (ISTA). ISTA Handbook on Seedling Evaluation, 4th ed.; Don, R., Ducournau, S., Eds.; International Seed Testing Association: Bassersdorf, Switzerland, 2018; ISBN 978-3-906549-39-2. [Google Scholar]
- Kim, S.H.; Choe, Z.R.; Kang, J.H.; Copeland, L.O.; Elias, S.G. Multiple seed vigour indices to predict field emergence and performance of barley. Seed Sci. Technol. 1994, 22, 29–38. [Google Scholar]
- AOAC International; Cunniff, P. Official Methods of Analysis of AOAC International, 16th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 1995; pp. 1–771. [Google Scholar]
- Lookhart, G.; Bean, S. Separation and characterization of wheat protein fractions by high-performance capillary electrophoresis. Cereal Chem. 1995, 72, 527–532. [Google Scholar]
- Kocadağlı, T.; Žilić, S.; Göncüoglu Taş, N.; Vančetović, J.; Dodig, D.; Gökmen, V. Formatio of α-dicarbonyl compounds in cookies made from wheat, hull-less barley and colored corn and its relation with phenolic compounds, free amino acids and sugars. Eur. Food Res. Technol. 2016, 241, 51–60. [Google Scholar] [CrossRef]
- Žilić, S.; Dodig, D.; Basić, Z.; Vančetović, J.; Titan, P.; Đurić, N.; Tolimir, N. The potential of cereals for acrylamide formation in the food: A comparison of species and genotypes for free asparagine and sugars content. Food Addit. Contam. Part A 2017, 34, 705–713. [Google Scholar] [CrossRef]
- Antoine, C.; Peyron, S.; Lullien-Pellerin, V.; Abecassis, J.; Rouau, X. Wheat bran tissue fractionation using biochemical markers. J. Cereal Sci. 2004, 39, 387–393. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Žilić, S.; Serpen, A.; Akıllıoğlu, G.; Gökmen, V.; Vančetović, J. Phenolic compounds, carotenoids, anthocyanins and antioxidant capacity of colored maize (Zea mays L.) kernels. J. Agric. Food Chem. 2012, 60, 1224–1231. [Google Scholar] [CrossRef] [PubMed]
- Gliszczyńska-Świgło, A.; Sikorska, E. Simple reversed-phase liquid chromatography method for determination of tocopherols in edible plant oils. J. Chromatogr. A 2004, 1048, 195–198. [Google Scholar] [CrossRef] [PubMed]
- Rivera, S.; Canela, R. Influence of sample processing on the analysis of carotenoids in maize. Molecules 2012, 17, 11255–11268. [Google Scholar] [CrossRef]
- Vukadinović, J.; Srdić, J.; Kravić, N.; Mladenović Drinić, S.; Simić, M.; Brankov, M.; Dragičević, V. Assessment of Popcorn’s Bioactive Status in Response to Popping. Molecules 2024, 29, 807. [Google Scholar] [CrossRef]
| Sample | Root FW | Shoot FW | Seed Rest FW | Root DW | Shoot DW | Seed Rest DW | Root Length | Shoot Length | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| g | cm | |||||||||||||||
| 1 | 0.051 ± 0.20 | c | 0.076 ± 0.04 | d | 0.254 ± 0.12 | c | 0.005 ± 0.00 | cd | 0.007 ± 0.00 | bcde | 0.136 ± 0.06 | de | 5.83 ± 0.29 | d | 3.233 ± 0.23 | d |
| 2 | 0.267 ± 0.03 | a | 0.312 ± 0.011 | ab | 0.449 ± 0.19 | b | 0.023 ± 0.01 | ab | 0.025 ± 0.01 | a | 0.208 ± 0.09 | cd | 14.67 ± 0.12 | a | 7.133 ± 0.21 | a |
| 3 | 0.289 ± 0.00 | ab | 0.349 ± 0.14 | a | 0.540 ± 0.25 | ab | 0.026 ± 0.01 | a | 0.030 ± 0.01 | a | 0.243 ± 0.11 | bc | 14.83 ± 1.28 | a | 7.467 ± 0.29 | a |
| 4 | 0.078 ± 0.01 | c | 0.065 ± 0.03 | d | 0.241 ± 0.10 | c | 0.006 ± 0.00 | bcd | 0.007 ± 0.00 | cde | 0.137 ± 0.06 | de | 7.10 ± 0.35 | d | 2.400 ± 0.34 | e |
| 5 | 0.289 ± 0.04 | ab | 0.184 ± 0.08 | c | 0.536 ± 0.23 | ab | 0.023 ± 0.01 | ab | 0.018 ± 0.01 | abcde | 0.294 ± 0.12 | ab | 11.47 ± 0.91 | c | 4.133 ± 0.16 | c |
| 6 | 0.369 ± 0.01 | a | 0.227 ± 0.11 | c | 0.566 ± 0.24 | ab | 0.032 ± 0.02 | a | 0.024 ± 0.01 | ab | 0.311 ± 0.13 | ab | 11.77 ± 0.32 | bc | 4.033 ± 0.28 | c |
| 7 | 0.028 ± 0.04 | c | 0.031 ± 0.01 | d | 0.201 ± 0.08 | c | 0.003 ± 0.00 | d | 0.003 ± 0.00 | e | 0.117 ± 0.04 | e | 4.30 ± 0.29 | e | 1.667 ± 0.14 | f |
| 8 | 0.225 ± 0.08 | b | 0.212 ± 0.10 | c | 0.553 ± 0.25 | ab | 0.020 ± 0.01 | abc | 0.021 ± 0.01 | abcd | 0.311 ± 0.14 | ab | 11.83 ± 1.03 | bc | 4.067 ± 0.22 | c |
| 9 | 0.206 ± 0.11 | b | 0.221 ± 0.10 | c | 0.574 ± 0.29 | a | 0.020 ± 0.01 | abc | 0.023 ± 0.01 | abc | 0.329 ± 0.17 | a | 12.47 ± 1.16 | bc | 4.167 ± 0.05 | c |
| 10 | 0.043 ± 0.04 | c | 0.063 ± 0.09 | d | 0.237 ± 0.10 | c | 0.004 ± 0.00 | cd | 0.006 ± 0.00 | de | 0.132 ± 0.05 | de | 6.68 ± 0.58 | d | 2.867 ± 0.15 | d |
| 11 | 0.231 ± 0.11 | b | 0.229 ± 0.09 | c | 0.461 ± 0.20 | ab | 0.020 ± 0.01 | abc | 0.022 ± 0.01 | abcd | 0.248 ± 0.11 | bc | 12.57 ± 0.94 | bc | 4.800 ± 0.42 | b |
| 12 | 0.266 ± 0.04 | b | 0.249 ± 0.12 | bc | 0.545 ± 0.25 | ab | 0.025 ± 0.01 | a | 0.023 ± 0.01 | a | 0.298 ± 0.13 | ab | 13.07 ± 1.34 | b | 5.167 ± 0.29 | b |
| CV (%) | 28.15 | 23.20 | 17.05 | 27.90 | 22.87 | 17.33 | 7.68 | 6.05 | ||||||||
| LSD0.05 | 0.0928 | 0.0757 | 0.1197 | 0.0169 | 0.0169 | 0.0757 | 1.371 | 0.4383 | ||||||||
| Sample | Total Protein | Albumins | Globulins | Glutelins | α-Zeins | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| % d.m. | ||||||||||
| 1 | 12.90 ± 0.20 | b | 0.765 ± 0.02 | d | 1.225 ± 0.02 | e | 2.915 ± 0.02 | b | 2.915 ± 0.02 | f |
| 2 | 13.54 ± 0.03 | a | 0.655 ± 0.04 | ef | 1.255 ± 0.02 | d | 3.035 ± 0.05 | a | 2.815 ± 0.02 | g |
| 3 | 12.93 ± 0.00 | b | 1.090 ± 0.03 | a | 1.255 ± 0.01 | d | 2.755 ± 0.06 | c | 3.370 ± 0.03 | c |
| 4 | 11.79 ± 0.01 | fg | 0.515 ± 0.02 | g | 1.125 ± 0.01 | i | 2.420 ± 0.03 | de | 3.060 ± 0.06 | e |
| 5 | 12.38 ± 0.04 | d | 0.710 ± 0.03 | de | 1.085 ± 0.02 | j | 2.075 ± 0.04 | h | 3.270 ± 0.00 | d |
| 6 | 11.93 ± 0.01 | ef | 0.740 ± 0.01 | d | 1.150 ± 0.01 | g | 2.335 ± 0.02 | ef | 3.300 ± 0.01 | d |
| 7 | 11.70 ± 0.04 | g | 0.425 ± 0.04 | h | 1.280 ± 0.01 | a | 2.310 ± 0.01 | f | 2.860 ± 0.03 | fg |
| 8 | 12.06 ± 0.08 | e | 0.620 ± 0.01 | f | 1.260 ± 0.01 | c | 2.260 ± 0.03 | fg | 2.740 ± 0.06 | h |
| 9 | 12.37 ± 0.11 | d | 0.650 ± 0.03 | ef | 1.265 ± 0.01 | b | 2.440 ± 0.03 | d | 3.100 ± 0.01 | e |
| 10 | 12.04 ± 0.04 | e | 0.365 ± 0.04 | h | 1.015 ± 0.05 | k | 2.295 ± 0.06 | fg | 3.650 ± 0.01 | b |
| 11 | 12.80 ± 0.11 | bc | 0.930 ± 0.01 | b | 1.155 ± 0.02 | f | 1.885 ± 0.06 | i | 3.760 ± 0.04 | a |
| 12 | 12.65 ± 0.04 | c | 0.855 ± 0.01 | c | 1.140 ± 0.01 | h | 2.205 ± 0.08 | g | 3.670 ± 0.01 | b |
| CV (%) | 0.64 | 3.80 | 1.84 | 1.99 | 0.96 | |||||
| LSD0.05 | 0.1705 | 0.0696 | 0.0022 | 0.0984 | 0.0696 | |||||
| Sample | Glucose | Fructose | Sucrose | Maltose | ||||
|---|---|---|---|---|---|---|---|---|
| % d.m. | ||||||||
| 1 | 0.467 ± 0.001 | h | 0.188 ± 0.00 | h | 1.52 ± 0.04 | a | 1.089 ± 0.01 | bcd |
| 2 | 0.436 ± 0.00 | i | 0.231 ± 0.00 | g | 1.322 ± 0.02 | bcd | 0.856 ± 0.00 | efg |
| 3 | 0.692 ± 0.002 | b | 0.366 ± 0.01 | c | 1.072 ± 0.01 | g | 1.277 ± 0.02 | ab |
| 4 | 0.522 ± 0.00 | g | 0.269 ± 0.00 | e | 1.173 ± 0.01 | efg | 0.842 ± 0.00 | efg |
| 5 | 0.644 ± 0.01 | d | 0.288 ± 0.00 | d | 1.343 ± 0.00 | bc | 1.113 ± 0.01 | bc |
| 6 | 0.697 ± 0.01 | b | 0.230 ± 0.00 | g | 1.203 ± 0.02 | def | 1.441 ± 0.34 | a |
| 7 | 0.656 ± 0.00 | c | 0.395 ± 0.00 | b | 1.088 ± 0.18 | fg | 0.671 ± 0.00 | g |
| 8 | 0.586 ± 0.00 | e | 0.250 ± 0.00 | f | 1.238 ± 0.01 | cde | 0.889 ± 0.01 | defg |
| 9 | 0.658 ± 0.05 | c | 0.184 ± 0.01 | h | 1.216 ± 0.01 | de | 0.956 ± 0.05 | cdef |
| 10 | 0.713 ± 0.00 | a | 0.439 ± 0.00 | a | 1.172 ± 0.00 | fg | 0.762 ± 0.01 | fg |
| 11 | 0.559 ± 0.00 | f | 0.182 ± 0.00 | h | 1.421 ± 0.00 | ab | 1.022 ± 0.00 | cde |
| 12 | 0.641 ± 0.02 | d | 0.253 ± 0.01 | f | 1.247 ± 0.02 | cde | 0.915 ± 0.03 | cdef |
| CV (%) | 2.77 | 1.65 | 4.41 | 10.25 | ||||
| LSD0.05 | 0.00696 | 0.0070 | 0.06028 | 0.2201 | ||||
| (a) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Sample | Total FPs | Free p-CouA | Free FA | Free CA | ||||
| μg GAE g−1 d.m. | μg g−1 d.m. | |||||||
| 1 | 219.71 ± 15.1 | e | 3.73 ± 0.07 | d | 1.69 ± 0.22 | bc | n.d. * | |
| 2 | 213.48 ± 15.2 | e | 2.00 ± 0.30 | g | 1.20 ± 0.04 | cdef | n.d. | |
| 3 | 211.37 ± 22.5 | e | 1.56 ± 0.30 | h | 1.04 ± 0.09 | defg | n.d. | |
| 4 | 319.28 ± 34.7 | b | 4.49 ± 0.00 | bc | 2.18 ± 0.04 | b | 2.56 ± 0.13 | b |
| 5 | 353.81 ± 28.7 | a | 4.40 ± 0.04 | c | 1.29 ± 0.17 | cde | 4.62 ± 0.26 | ab |
| 6 | 315.55 ± 18.0 | b | 3.75 ± 0.13 | d | 1.25 ± 0.22 | cde | 3.51 ± 0.49 | ab |
| 7 | 248.60 ± 12.1 | cd | 4.63 ± 0.09 | bc | 1.30 ± 0.17 | cde | n.d. | |
| 8 | 247.22 ± 19.7 | d | 3.21 ± 0.17 | e | 0.68 ± 0.09 | fg | n.d. | |
| 9 | 218.68 ± 19.4 | e | 4.69 ± 0.09 | b | 0.64 ± 0.04 | g | 3.83 ± 0.26 | ab |
| 10 | 259.75 ± 13.6 | cd | 5.05 ± 0.17 | a | 3.54 ± 0.04 | a | n.d. | |
| 11 | 268.32 ± 39.2 | c | 2.94 ± 0.17 | f | 1.56 ± 0.30 | cd | 3.86 ± 0.26 | ab |
| 12 | 267.45 ± 32.8 | c | 3.18 ± 0.21 | e | 0.76 ± 0.13 | efg | 5.34 ± 0.26 | a |
| CV (%) | 3.46 | 2.95 | 7.47 | 18.49 | ||||
| LSD0.05 | 19.95 | 0.2308 | 0.5480 | 2.544 | ||||
| (b) | ||||||||
| Sample | total IBPs | bound p-CouA | bound FA | bound CA | ||||
| μg GAE g−1 d.m. | μg g−1 d.m. | |||||||
| 1 | 2454.3 ± 138.2 | bc | 331.26 ± 3.64 | b | 2412.32 ± 11.81 | d | 1.88 ± 0.08 | d |
| 2 | 2744.4 ± 136.3 | a | 359.84 ± 6.86 | a | 2655.54 ± 6.45 | b | 2.53 ± 0.13 | a |
| 3 | 2486.3 ± 85.6 | b | 287.06 ± 6.47 | c | 2370.91 ± 1.09 | e | 2.56 ± 0.19 | a |
| 4 | 2340.9 ± 71.1 | bcd | 188.61 ± 6.90 | g | 2307.14 ± 10.84 | f | 1.54 ± 0.02 | e |
| 5 | 2446.5 ± 262.1 | bc | 260.48 ± 3.45 | d | 2746.20 ± 6.23 | a | 1.89 ± 0.08 | d |
| 6 | 2329.1 ± 185.5 | cd | 224.39 ± 5.41 | f | 2264.17 ± 2.80 | g | 1.62 ± 0.15 | e |
| 7 | 2157.9 ± 56.3 | e | 192.59 ± 10.44 | g | 2027.14 ± 12.84 | i | 1.89 ± 0.08 | d |
| 8 | 2200.3 ± 60.0 | de | 272.18 ± 3.35 | cd | 2115.23 ± 11.35 | h | 1.93 ± 0.14 | cd |
| 9 | 2169.3 ± 40.7 | e | 242.73 ± 10.74 | e | 1992.43 ± 4.01 | j | 2.11 ± 0.00 | bc |
| 10 | 2454.4 ± 26.2 | bc | 193.29 ± 8.76 | g | 2478.78 ± 11.78 | c | 2.17 ± 0.04 | b |
| 11 | 2278.7 ± 97.0 | de | 197.28 ± 6.14 | g | 2260.00 ± 16.48 | g | 1.64 ± 0.17 | e |
| 12 | 2245.6 ± 121.7 | de | 230.90 ± 2.89 | ef | 2120.10 ± 22.07 | h | 1.89 ± 0.13 | d |
| CV (%) | 2.85 | 2.79 | 0.40 | 4.53 | ||||
| LSD0.05 | 148.1 | 7.622 | 20.25 | 0.1969 | ||||
| Sample | α-T | β+γ-T | δ-T | β-Carotene | Lut+Zeaxanth | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| μg g−1 d.m. | ||||||||||
| 1 | 8.23 ± 0.46 | e | 10.18 ± 0.57 | j | 0.59 ± 0.03 | g | 0.35 ± 0.00 | i | 9.06 ± 0.12 | h |
| 2 | 11.48 ± 0.49 | b | 18.92 ± 0.81 | i | 1.10 ± 0.04 | f | 1.92 ± 0.06 | h | 16.64 ± 0.34 | f |
| 3 | 10.76 ± 0.42 | c | 22.38 ± 0.87 | h | 1.40 ± 0.05 | ef | 2.61 ± 0.07 | g | 21.52 ± 0.43 | d |
| 4 | 4.24 ± 0.21 | j | 39.93 ± 1.94 | ef | 0.43 ± 0.12 | g | 1.79 ± 0.05 | h | 4.97 ± 0.10 | k |
| 5 | 6.71 ± 0.28 | h | 48.19 ± 2.02 | c | 1.42 ± 0.10 | e | 9.78 ± 0.29 | d | 14.25 ± 0.23 | g |
| 6 | 4.29 ± 0.21 | j | 41.63 ± 2.17 | e | 2.02 ± 0.10 | bc | 16.87 ± 0.49 | a | 22.61 ± 0.43 | c |
| 7 | 5.25 ± 0.23 | i | 32.11 ± 1.46 | g | 1.59 ± 0.08 | de | 2.91 ± 0.08 | g | 6.43 ± 0.13 | i |
| 8 | 8.87 ± 0.38 | d | 39.36 ± 1.69 | f | 1.80 ± 0.06 | cd | 8.41 ± 0.25 | f | 17.41 ± 0.45 | e |
| 9 | 12.12 ± 0.54 | a | 39.40 ± 1.75 | f | 1.44 ± 0.06 | e | 15.58 ± 0.45 | b | 35.44 ± 0.54 | a |
| 10 | 5.09 ± 0.26 | i | 45.68 ± 2.33 | d | 2.32 ± 0.12 | b | 0.33 ± 0.04 | i | 5.83 ± 0.13 | j |
| 11 | 7.42 ± 0.40 | f | 54.64 ± 2.91 | b | 2.96 ± 0.16 | a | 8.81 ± 0.25 | e | 16.69 ± 0.29 | f |
| 12 | 7.12 ± 0.37 | g | 58.90 ± 3.12 | a | 2.86 ± 0.15 | a | 14.33 ± 0.42 | c | 32.01 ± 0.51 | b |
| CV (%) | 1.49 | 2.10 | 2.47 | 2.59 | 0.97 | |||||
| LSD0.05 | 0.2510 | 1.737 | 0.1797 | 0.6603 | 0.3617 | |||||
| Root Length | Shoot Length | SVI-I | SVI-II | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | CS1 | CS2 | Control | CS1 | CS2 | Control | CS1 | CS2 | Control | CS1 | CS2 | |
| total protein | 0.712 | 0.076 | 0.823 | 0.859 | 0.796 | 0.945 | 0.774 | −0.091 | 0.875 | −0.883 | −0.199 | −0.905 |
| glutelins | 0.558 | 0.017 | 0.726 | 0.743 | 0.639 | 0.852 | 0.633 | −0.045 | 0.823 | −0.713 | −0.077 | −0.501 |
| fructose | 0.705 | −0.186 | −0.359 | 0.953 | −0.446 | −0.301 | 0.887 | −0.217 | −0.275 | −0.878 | −0.208 | 0.592 |
| sucrose | −0.640 | 0.086 | 0.108 | −0.834 | 0.694 | 0.117 | −0.776 | −0.021 | 0.061 | 0.771 | −0.077 | −0.507 |
| total FPs | −0.453 | 0.551 | −0.667 | −0.576 | −0.335 | −0.590 | −0.482 | 0.649 | −0.639 | 0.521 | 0.643 | 0.420 |
| free p-CouA | −0.701 | 0.095 | −0.793 | −0.918 | −0.393 | −0.820 | −0.856 | 0.095 | −0.798 | 0.889 | 0.080 | 0.733 |
| total IBPs | 0.581 | 0.645 | 0.559 | 0.640 | 0.798 | 0.817 | 0.632 | 0.447 | 0.695 | −0.677 | 0.325 | −0.688 |
| bound p-CouA | 0.709 | −0.072 | 0.604 | 0.899 | 0.643 | 0.718 | 0.825 | −0.191 | 0.711 | −0.829 | −0.243 | −0.307 |
| bound FA | 0.394 | 0.784 | 0.240 | 0.674 | 0.890 | 0.402 | 0.602 | 0.541 | 0.346 | −0.569 | 0.351 | −0.284 |
| bound CA | 0.704 | −0.230 | 0.680 | 0.820 | 0.245 | 0.774 | 0.771 | −0.437 | 0.773 | −0.799 | −0.522 | −0.396 |
| β+γ-tocopherols | −0.408 | 0.361 | −0.696 | −0.594 | −0.354 | −0.784 | −0.501 | 0.380 | −0.783 | 0.529 | 0.347 | 0.398 |
| β-carotene | −0.767 | −0.551 | −0.848 | −0.971 | −0.925 | −0.942 | −0.896 | −0.323 | −0.916 | 0.930 | −0.150 | 0.686 |
| Sample | ID No. | Accession Name | Country of Origin | Year of the Last Regeneration |
|---|---|---|---|---|
| 1 | MRIZP13509 | Illinois composite | Mexico | 1985 |
| 2 | MRIZP13509 | Illinois composite | Mexico | 2017 |
| 3 | MRIZP13509 | Illinois composite | Mexico | 2022 |
| 4 | MRIZP13695 | BK × ETO-2007 × 00 | Argentina | 1985 |
| 5 | MRIZP13695 | BK × ETO-2007 × 00 | Argentina | 2017 |
| 6 | MRIZP13695 | BK × ETO-2007 × 00 | Argentina | 2022 |
| 7 | MRIZP13687 | BK × ETO-2004 × 08 | Argentina | 1985 |
| 8 | MRIZP13687 | BK × ETO-2004 × 08 | Argentina | 2017 |
| 9 | MRIZP13687 | BK × ETO-2004 × 08 | Argentina | 2022 |
| 10 | MRIZP13706 | BK × ETO-1995 × 07 | Argentina | 1985 |
| 11 | MRIZP13706 | BK × ETO-1995 × 07 | Argentina | 2017 |
| 12 | MRIZP13706 | BK × ETO-1995 × 07 | Argentina | 2022 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kravic, N.; Zilic, S.; Vukadinovic, J.; Petrovic, T.; Milivojevic, M.; Srdic, J.; Simic, M.; Mladenovic Drinic, S.; Andjelkovic, V. Natural Ageing-Related Alterations of Biological Markers in Maize Seeds Under Ex-Situ Conservation. Int. J. Mol. Sci. 2025, 26, 12124. https://doi.org/10.3390/ijms262412124
Kravic N, Zilic S, Vukadinovic J, Petrovic T, Milivojevic M, Srdic J, Simic M, Mladenovic Drinic S, Andjelkovic V. Natural Ageing-Related Alterations of Biological Markers in Maize Seeds Under Ex-Situ Conservation. International Journal of Molecular Sciences. 2025; 26(24):12124. https://doi.org/10.3390/ijms262412124
Chicago/Turabian StyleKravic, Natalija, Sladjana Zilic, Jelena Vukadinovic, Tanja Petrovic, Marija Milivojevic, Jelena Srdic, Marijana Simic, Snezana Mladenovic Drinic, and Violeta Andjelkovic. 2025. "Natural Ageing-Related Alterations of Biological Markers in Maize Seeds Under Ex-Situ Conservation" International Journal of Molecular Sciences 26, no. 24: 12124. https://doi.org/10.3390/ijms262412124
APA StyleKravic, N., Zilic, S., Vukadinovic, J., Petrovic, T., Milivojevic, M., Srdic, J., Simic, M., Mladenovic Drinic, S., & Andjelkovic, V. (2025). Natural Ageing-Related Alterations of Biological Markers in Maize Seeds Under Ex-Situ Conservation. International Journal of Molecular Sciences, 26(24), 12124. https://doi.org/10.3390/ijms262412124









