Myocardial Infarction in Murine Models of Obesity and Related Metabolic Disorders: The Role of Inflammation
Abstract
1. Introduction
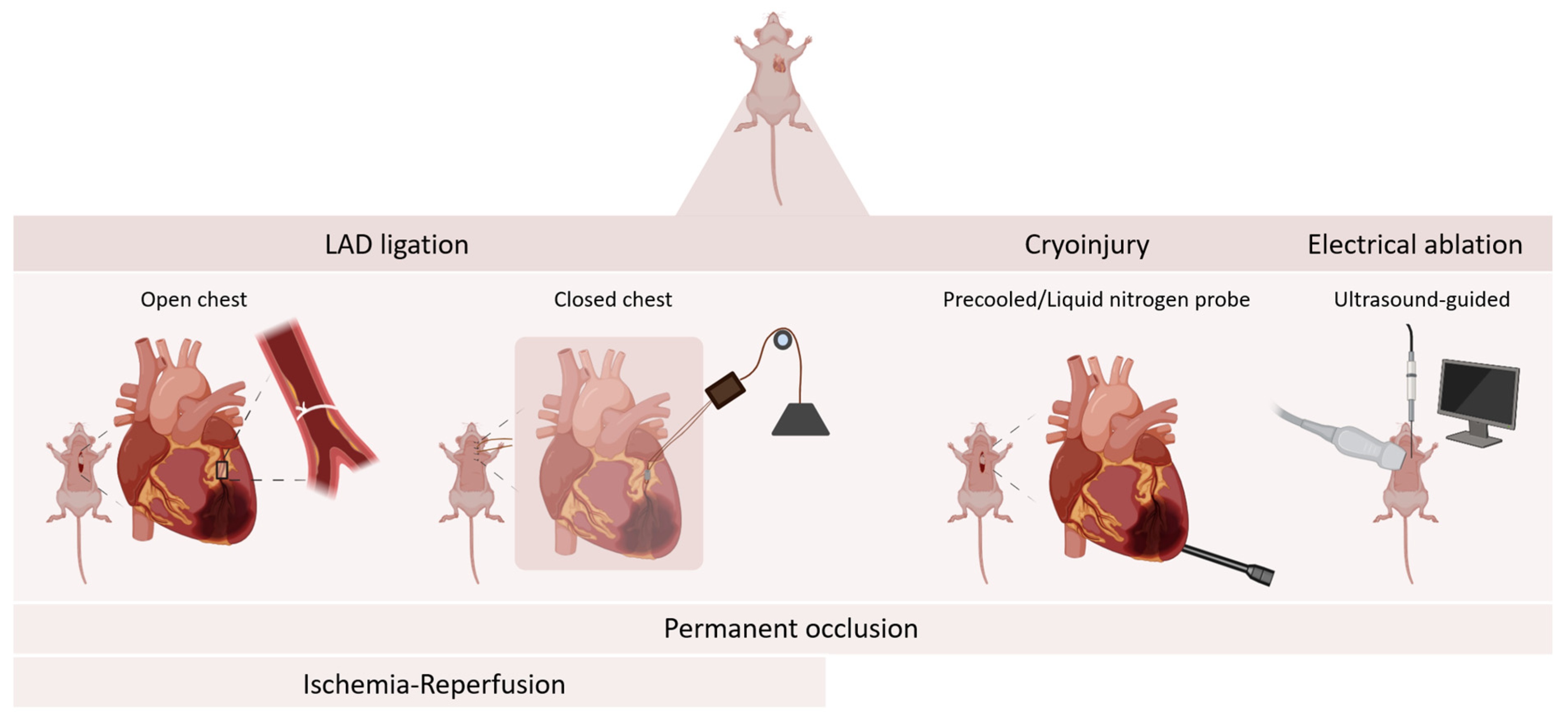
2. Mouse Models of Myocardial Infarction
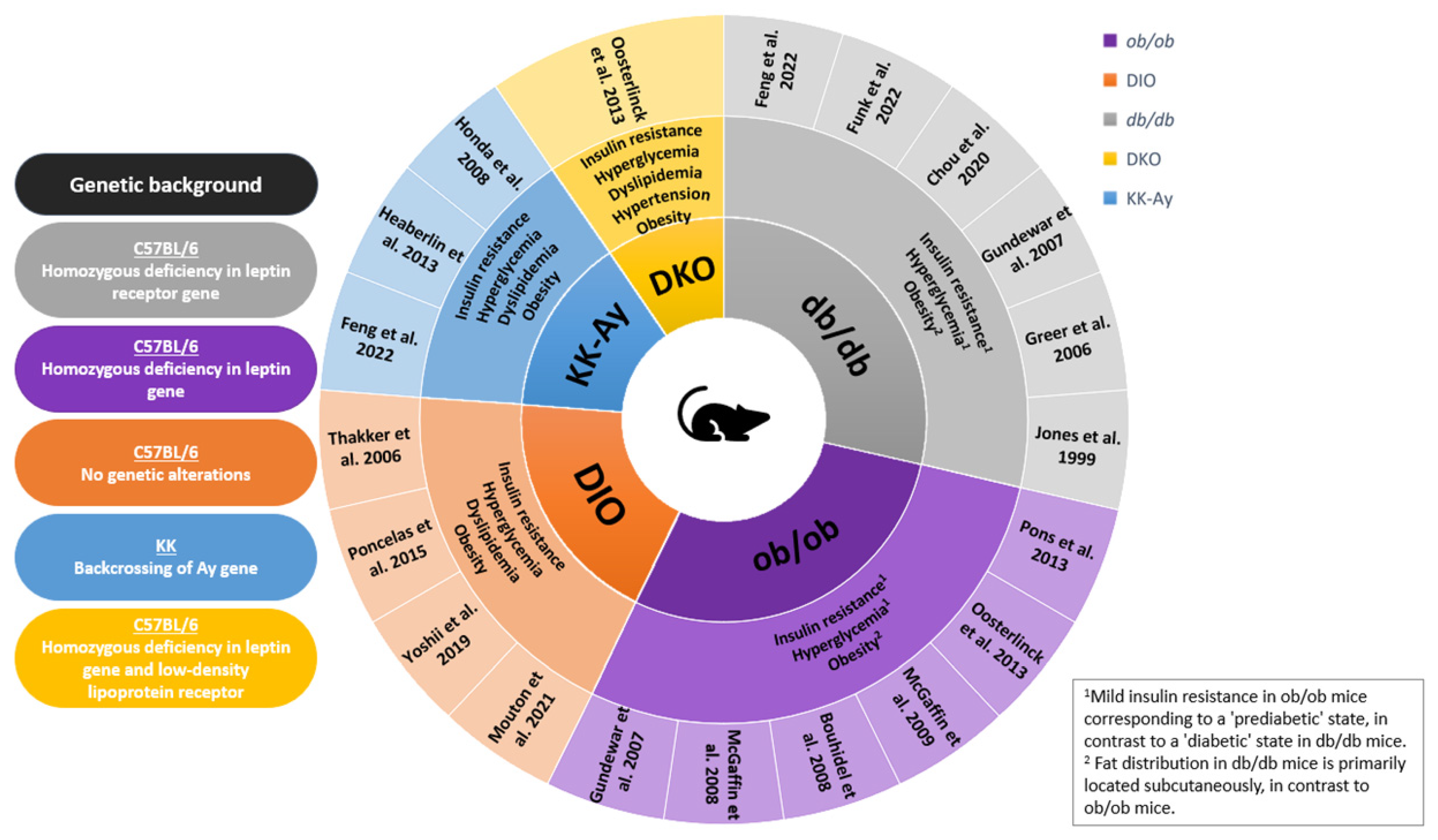
3. Myocardial Infarction in Murine Models of Obesity and Related Metabolic Disorders
| Paper | Mouse Model | Outcome | Sex | Total Number | Age | PL or I/R | Groups & Size | Weight (g) | Infarct Size-Timepoint | Cardiac Function | Survival | Inflammatory/Immune Markers |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| McGaffin et al., 2008 * [66] | ob/ob | MI increases leptin receptor activation & leptin repletion in ob/ob improves outcome | male | n = 110 | 5–6 weeks | PL | WT ad libitum (n = 16) WT ad libitum sham (n = 10) Ob ad libitum (n = 18) Ob ad libitum sham (n = 11) Ob food restrict (n = 15) Ob food restrict (n = 9) Ob leptin replete (n = 13) | 28.1 ± 0.5 51.8 ± 0.5 27.5 ± 0.5 27.5 ± 0.5 | 6.16 ± 0.07 mm2 6.19 ± 0.10 mm2 6.17 ± 0.15 mm2 6.25 ± 0.18 mm2 (short axis, 4 weeks PI) | ↓ ↓↓ ↓↓ ↓ (4 weeks PI) | 75% 44% 46% 69% | STAT3 ↑, timp1, hsp70 ↑ STAT3 =, timp1 =, hsp70 = STAT3 =, timp1 =, hsp70 = STAT3 ↑, timp1 ↑, hsp70 ↑ |
| McGaffin et al., 2009 * [65] | ob/ob | Leptin signaling after post-MI activates anti-apoptotic genes through STAT-3, reducing caspase-3 activity, limiting cardiac apoptosis | male | n = 16 | 5–6 weeks | PL | WT Ob ad libitum Ob food restrict Ob leptin replete | 28.1 ± 0.5 51.8 ± 0.5 27.5 ± 0.5 27.5 ± 0.6 | 6.16 ± 0.07 mm2 6.19 ± 0.10 mm2 6.17 ± 0.15 mm2 6.25 ± 0.18 mm2 (short axis, 4 weeks PI) | ↓ ↓↓ ↓↓ ↓ (4 weeks PI) | 75% 44% 46% 69% | Apoptosis ↑, CD45+ cells ↑ Apoptosis ↑↑↑, CD45+ cells ↑↑↑ Apoptosis ↑↑↑, CD45+ cells ↑↑↑ Apoptosis ↑, CD45+ cells ↑ |
| Oosterlinck et al., 2013 [58] | ob/ob DKO (MS) | Protection by IPostC against I/R injury remains but might be reduced in ob/ob mice and DKO mice | male & female | n = 80 | 24 weeks | I/R 30 min I | WT (n = 12) WT + IPostC (n = 6) WT sham (n = 6) ob/ob (n = 10) ob/ob + IPostC (n = 10) ob/ob sham (n = 8) DKO (n = 6) DKO + IPostC (n = 6) DKO sham (n = 6) | 27 ± 1 63 ± 1 60 ± 2 | 52% 31% 58% 44% 54% 41% (AN/AAR, 60 min PI) | ↓↓ * ↓ * ↓↓ ↓ ↓↓ * ↓ * (60 min PI) +1&10 weeks * | 100% 100% / / 17% 67% | / |
| Bouhidel et al., 2008 [53] | ob/ob | IPostC protection against I/R injury is lost in ob/ob mice, likely due to reduced RISK pathway activation | male | n = 33 | 8–10 weeks | I/R 30 min I | WT (n = 9) WT + IPostC (n = 9) ob/ob (n = 8) ob/ob + IPostC (n = 7) | 25.5 ± 0.7 23.4 ± 0.3 48.2 ± 1.2 46.0 ± 1.9 | 44 ± 3% 40 ± 3% 41 ± 4% 27 ± 2% (%AAR, 24 h PI) | / | / | P-Akt, P-ERK1/2, P-p70S6K1, P-AMPK ↑ P-Akt, P-ERK1/2, P-p70S6K1, P-AMPK ↑↑↑ P-Akt, P-ERK1/2, P-p70S6K1, P-AMPK ↑↑ P-Akt, P- ERK1/2, P-p70S6K1, P-AMPK ↑↑ |
| Pons et al., 2013 [64] | ob/ob | Treadmill exercise induces cardioprotection against MI and restores pro-survival signaling pathways | male | n = 33 | 5–10 weeks | I/R 30 min I | WT (n = 7) WT + exercise (n = 9) ob/ob (n = 8) ob/ob + excercise (n = 9) | 25.0 ± 0.6 24.2 ± 1.0 42.8 ± 0.6 44.8 ± 0.7 | 43 ± 3% 17 ± 2% 58 ± 3% 19 ± 1% (%AAR, 24 h PI) | / | / | P-Akt, P- ERK1/2, P-p70S6K1, P-AMPK, P-GSK3β, Ca2+ ↑ P-Akt, P- ERK1/2, P-p70S6K1, P-AMPK, P-GSK3β, Ca2+ ↑↑ P-Akt, P- ERK1/2, P-p70S6K1, P-AMPK, P-GSK3β, Ca2+ ↑ P-Akt, P- ERK1/2, P-p70S6K1, P-AMPK, P-GSK3β, Ca2+ ↑↑ |
| Gundewar et al., 2007 [62] | ob/ob db/db | Cytoprotective effect of TMS treatment upon I/R is lost in ob/ob and db/db | date not reported | n = 106 | 8–10 weeks | I/R 30 min I | WT (n = 13) WT + TMS (n = 30) ob/ob (n = 12) ob/ob + TMS (n = 23) db/db (n = 11) db/db + TMS (n = 17) | 26 ± 1 48 ± 1 44 ± 1 | 50.83 ± 1.89% 17.32 ±2.11% 53.41 ± 4.41% 34.24 ± 3.54%, ~59% (from graph) ~53% (from graph) (%AAR, 24 h PI) | ↓↓ ↓ / / / / (72 h PI) | / | PKC-δ translocation to mitochondria ↑↑ PKC-δ translocation to mitochondria ↑ / / PKC-δ translocation to mitochondria ↑↑ PKC-δ translocation to mitochondria ↑↑ |
| Thakker et al., 2006 [55] | DIO | DIO mice showed increased inflammation and impaired healing, associated with adverse remodeling | male & female | n = 32 | 30–32 weeks | I/R 1 h I | WT male (n = 8) WT female (n = 8) DIO male (n = 8) DIO female (n = 8) | 27.45 ± 1.26 23.54 ± 0.57 46.41 ± 2.42 36.82 ± 0.87 | (data on collagen deposition and amount of fibrosis provided) (7 days PI) | ↓ (7 days PI) | 79.3% 67.8% | MIP-1α, MIP-1β, MIP2, MCP-1, IP-10 ↑ IL-6, IL-10, Osteopontin, TGF-β1, TGF-β3 ↑ MIP-1α, MIP-1β, MIP2, MCP-1, IP-10 ↑↑ IL-6, IL-10, Osteopontin, TGF-β1, TGF-β3 ↑↑ |
| Mouton et al., 2021 [67] | DIO | Obesity lowers survival but improves cardiac function and metabolism in surviving normotensive mice | male & female | n = 90 | 12–24 weeks | PL | WT male (n = 9) WT female (n = 8) DIO male (n = 16) DIO female (n = 13) DIO male + HTN (n = 24) DIO female + HTN (n = 20) | / | ~51% ~40% ~42% ~41% ~49% ~39% (from graph, 7 days PI) | ↓↓ ↓↓ ↓ ↓ ↓↓↓ ↓↓↓ (7 days PI) | 89% 75% 56% 54% 29% 35% | P-Akt, P-ACC, P-PDH, P-AMPK, PPAR-gamma, PGC-1α = P-Akt, P-ACC, P-PDH, P-AMPK, PPAR-gamma, PGC-1α = P-Akt ↑↑, P-ACC ↑↑, P-PDH =, P-AMPK ↑, PPAR-gamma ↑, PGC-1α = P-Akt ↑, P-ACC ↑, P-PDH =, P-AMPK =, PPAR-gamma =, PGC-1α = P-Akt =, P-ACC ↑, P-PDH =, P-AMPK =, PPAR-gamma =, PGC-1α = P-Akt =, P-ACC =, P-PDH =, P-AMPK =, PPAR-gamma =, PGC-1α = |
| Poncelas et al., 2015 [69] | DIO | DIO attenuates postinfarct myocardial remodeling and dysfunction in adult B6D2F1 mice | male | n = 52 | 26–30 weeks | I/R 45 min I | WT (n = 16) WT sham (n = 10) DIO (n = 16) DIO sham (n = 10) | 37.8 ± 1.7 48.3 ± 0.1 | 34.06 ± 9.35% 15.57 ± 4.63% (area of fibrosis) | ↓↓ ↓ (7 and 28 days PI) | 92.4% (general survival) | P-Akt, P-GSK3β ↑ P-Akt, P-GSK3β ↑↑ |
| Yoshii et al., 2019 [68] | DIO | SGLT1 contributes to cardioprotection during the acute phase of I/R injury via enhanced glucose transport | male | n = 28 | 8–20 weeks | I/R 30 min I | WT (n = 6) WT + plorizin (SGLTi) (n = 6) DIO (n = 8) DIO + plorizin (SGLTi) (n = 8) | ~31 ~46 (from graph) | 32.3% ± 2.2% 52.5% ± 3.5% 60.2% ± 1.5% 71.8% ± 4.0% (MI area/ventricular area, 40 min PI) | ↓ ↓↓ ↓↓ ↓↓↓ (40 min PI) | / | GLUT-4 ↑↑, SGLT1 ↑ GLUT-4 ↑, SGLT1 ↑ |
| Greer et al., 2006 [63] | db/db | Varying durations of myocardial I/R in db/db mice influence survival and cardiac function (heart failure) | male | n = 134 | 10 weeks | I/R 30, 45 or 60 min I | WT − 30’ I (n = 12) WT − 45’ I (n = 16) db/db − 30’ I (n = 55) db/db − 45’ I (n = 34) db/db − 60’ I (n = 17) | 24 ± 0.4 49 ± 0.4 | \ | = = = ↓↓ / (28 days PI) | 100% 88% 71% 53% 18% | / |
| Jones et al., 1999 [56] | db/db | Reperfusion injury is worse in db/db mice, likely due to PMN-driven inflammation, as CD18 neutralization reduces infarct size, but independent of P-selectin | male | n = 42 | date not reported | I/R 30 min I | WT (n = 15) db/db (n = 14) db/db + RB40.34 (n = 6) db/db + GAME46 (n = 7) | / | 27.2 ± 3.1 % 56.3 ± 2.8 % 47.2 ± 9.4 % 34.4 ± 8.1% (%AAR, 2 h PI) | / | 88% 58% | Myocardial neutrophil (PMN) ↑ Myocardial neutrophil (PMN) ↑↑↑ Myocardial neutrophil (PMN) ↑↑ Myocardial neutrophil (PMN) ↑ |
| Chou et al., 2020 [61] | db/db | Ranolazine improves Ca(i) dynamics and conduction inhomogeneity in I/R injury | female | n = 29 | 23–30 weeks | I/R 15 min I | db/db (n = 7) db/db + Ranolazine (n = 7) db/+ (n = 8) db/+ + Ranolazine (n = 7) | 55.0 ± 7.8 59.6 ± 12.0 30.1 ± 3.9 31.9 ± 4.2 | / | / | / | / |
| Funk et al., 2022 [60] | db/db | Sarcomere function in the remote zone is impaired after I/R due to failed compensatory mechanisms and worsened calcium handling | male | n = 32 | 10–12 weeks | I/R 60 min I | db/+ (n = 10) db/+ + I/R (n = 6) db/db (n = 10) db/db + I/R (n = 6) | 27.5 ± 1.1 46.0 ± 2.0 | 37 ± 2% 36 ± 2 % (Ischemic area LV free wall) (24 h PI) | = ↓ = ↓↓ (24 h PI) | P-ERK1/2 =, P-PKCα =, P-PLN(m)(T17) =, Col3a1 = P- ERK1/2 =, P-PKCα ↑, P-PLN(m)(T17) ↑↑, Col3a1 = P- ERK1/2 =, P-PKCα ↑, P-PLN(m)(T17) =, Col3a1 ↑↑↑ P- ERK1/2 ↑, P-PKCα =, P-PLN(m)(T17) ↑, Col3a1 ↑↑↑ | |
| Heaberlin et al., 2013 [57] | Kkay | Kkay mice have a reduced post-MI survival but improved cardiac function through reduced inflammation, ECM accumulation, and neovascularization in the infarct region | male & female | n = 49 | 24–32 weeks | PL | WT (n = 7) WT + MI (n = 10) Kkay (n = 10) Kkay + MI (n = 22) | ~25 ~23 ~31.5 ~25 (from graph) | 50 ± 4% 49 ± 2% | ↓↓ ↓ (7 days PI) | 70% 45% | Macrophages ↑↑, CD40 ↑↑,eotaxin ↑, EGF ↓↓, MDC ↑↑ myoglobin ↓↓, SGOT ↓↓, Oncostatin M, VWF ↓↓ Macrophages ↑, CD40 =, eotaxin ↑↑, EGF ↓↓↓, MDC =, myoglobin ↓, SGOT =, Oncostatin M =, VWF ↓ |
| Feng et al., 2022 [59] | db/db Kkay | Mitsugumin 53 (MG53) protects diabetic hearts from I/R injury and ameliorates diet-induced cardiometabolic damage | male & female | n = 90 | 10 weeks | I/R 30 min I | db/+ + hSa (n = 8) db/+ + rhMG53-WT (n = 10) db/+ + rhMG53-C14A (n = 10) db/db + hSa (n = 10) db/db + rhMG53-WT (n = 12) db/db + rhMG53-C14A (n = 12) Kkay + hSa (n = 9) Kkay + rhMG53-WT (n = 10) Kkay + rhMG53-C14A (n = 9) | / | 36.7% <14% <14% ~47% ~35% ~23% / / / | ↓↓ ↓ ↓ ↓↓ ↓↓↓ ↓ (24 h PI) | / / / 79% 48% 80%4 / / / | \ |
| Honda et al., 2008 [70] | Kkay | Metabolic disorders exacerbate I/R injury as a result of overexpression of inflammatory mediators, and this effect might be improved by the anti-inflammatory effects of the thiazolidinedione, pioglitazone | male | n = 44 | 8–10 weeks | I/R 40 min I | WT (n = 10) WT + vehicle (n = 6) WT + Pioglitazone (n = 6) Kkay (n = 10) Kkay + vehicle (n = 6) Kkay + Pioglitazone (n = 6) | 38.1 ± 1.3 21.4 ± 0.5 34.3 ± 0.8 20.2 ± 0.3 34.8 ± 0.7 20.6 ± 0.3 | 19.6 ± 2.5% 17.9 ± 1.4% 16.7 ± 2.6% 45.3 ± 2.7% 36.3 ± 3.4% 14.8 ± 4.6% (%AAR, 3 days PI) | / | / | Gr-1-+ granulocytes ↑, FA-11+macrophages ↑↑, MCP-1, KC, MIP-2, TNF-α, IL-10, MMP-9, TIMP-1, thioredoxin-1 ↑ / / Gr-1-+ granulocytes ↑↑↑, FA-11+macrophages ↑↑↑ MCP-1, KC, MIP-2, TNF-α, IL-10, MMP-9, TIMP-1, thioredoxin-1 ↑↑↑ Gr-1-+ granulocytes ↑↑, FA-11+macrophages ↑↑↑ MCP-1, KC, MIP-2, TNF-α, IL-10, MMP-9, TIMP-1, thioredoxin-1 ↑↑ Gr-1-+ granulocytes ↑, FA-11+macrophages ↑↑ MCP-1, KC, MIP-2, TNF-α, IL-10, MMP-9, TIMP-1, thioredoxin-1 ↑ |
3.1. Ob/Ob Mice
3.2. DIO Mice
3.3. Db/Db Mice
3.4. KKAy and DKO Mice
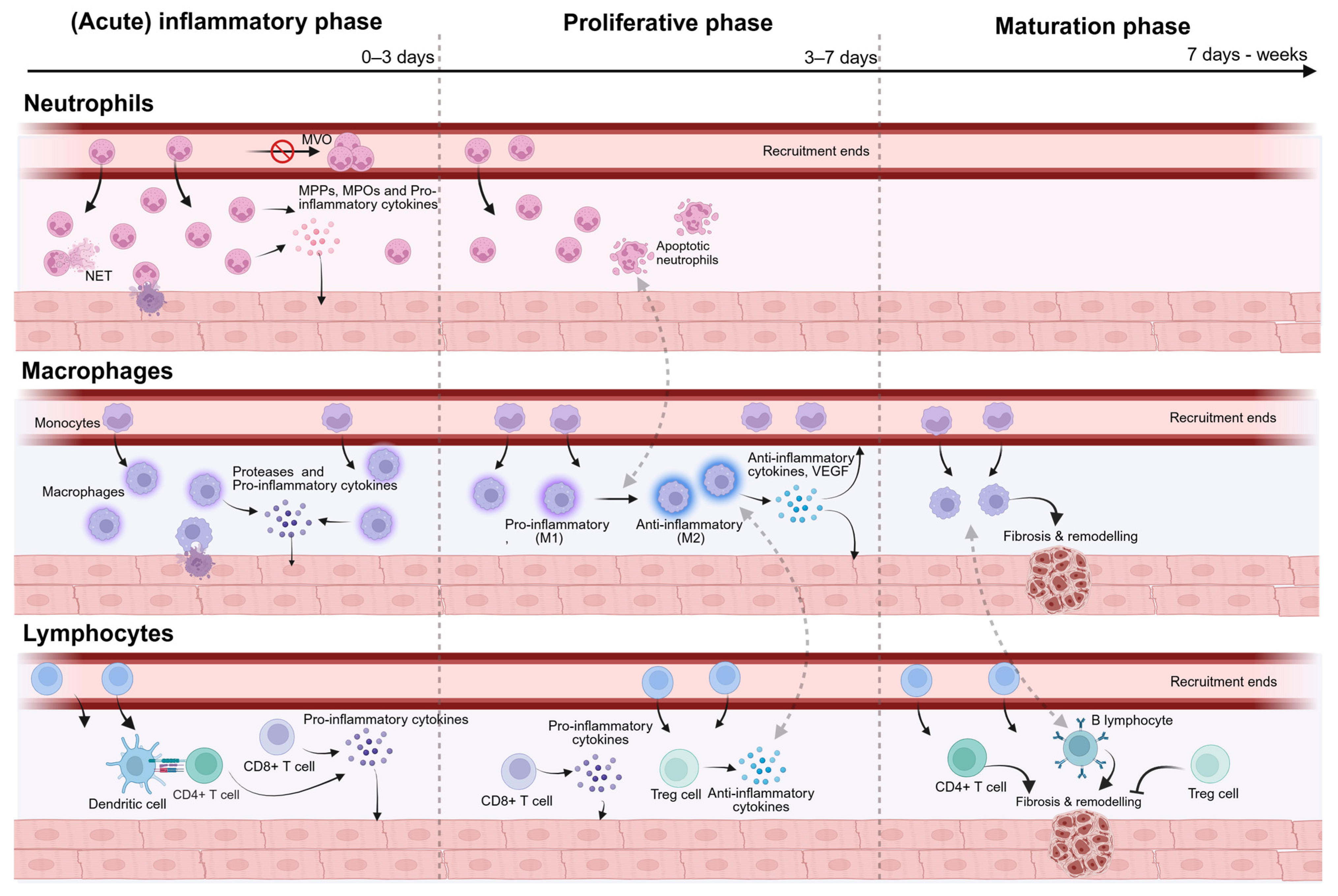
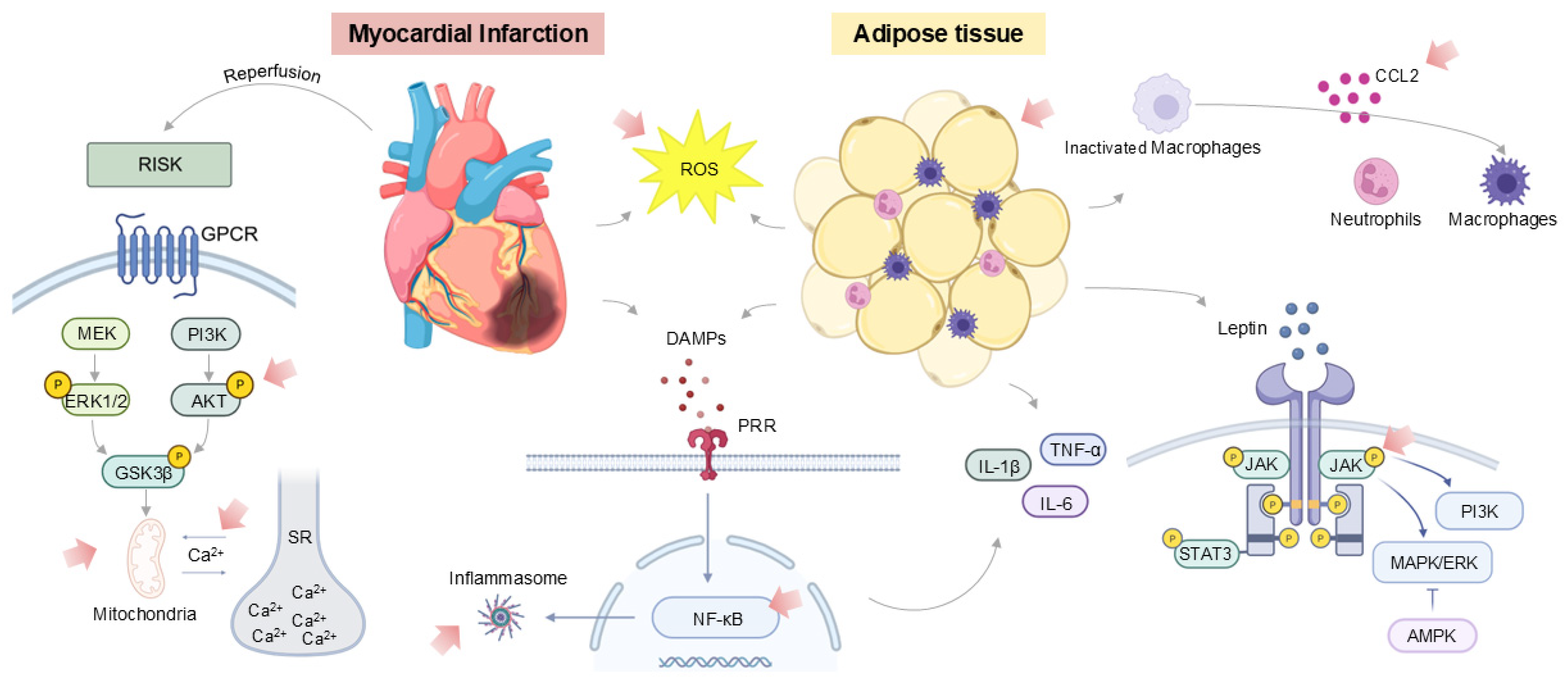
4. Inflammatory Profiles of Obese and Diabetic Mouse Models
5. Targeting Inflammation Following Myocardial Infarction
6. Imaging Inflammation
7. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CVD | Cardiovascular Disease |
| DAMPs | Damage-Associated Molecular Patterns |
| DIO | Diet-Induced Obese |
| IpostC | Ischemic Postconditioning |
| I/R | Ischemia–Reperfusion |
| LAD | Left Anterior Descending Artery |
| LV | Left Ventricle |
| MI | Myocardial Infarction |
| PCI | Percutaneous Coronary Intervention |
| RISK | Reperfusion Injury Salvage Kinase |
| WT | Wild-Type |
References
- Salari, N.; Morddarvanjoghi, F.; Abdolmaleki, A.; Rasoulpoor, S.; Khaleghi, A.A.; Hezarkhani, L.A.; Shohaimi, S.; Mohammadi, M. The global prevalence of myocardial infarction: A systematic review and meta-analysis. BMC Cardiovasc. Disord. 2023, 23, 206. [Google Scholar] [CrossRef]
- Laforgia, P.L.; Auguadro, C.; Bronzato, S.; Durante, A. The Reduction of Mortality in Acute Myocardial Infarction: From Bed Rest to Future Directions. Int. J. Prev. Med. 2022, 13, 56. [Google Scholar] [CrossRef]
- Algoet, M.; Janssens, S.; Himmelreich, U.; Gsell, W.; Pusovnik, M.; Eynde, J.V.D.; Oosterlinck, W. Myocardial ischemia–reperfusion injury and the influence of inflammation. Trends Cardiovasc. Med. 2023, 33, 357–366. [Google Scholar] [CrossRef]
- Elgendy, I.Y.; Mahtta, D.; Pepine, C.J. Medical Therapy for Heart Failure Caused by Ischemic Heart Disease. Circ. Res. 2019, 124, 1520–1535. [Google Scholar] [CrossRef]
- Ibanez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.L.P.; Crea, F.; Goudevenos, J.A.; Halvorsen, S.; et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur. Heart J. 2018, 39, 119–177. [Google Scholar] [CrossRef] [PubMed]
- Francisco, J.; Del Re, D.P. Inflammation in Myocardial Ischemia/Reperfusion Injury: Underlying Mechanisms and Therapeutic Potential. Antioxidants 2023, 12, 1944. [Google Scholar] [CrossRef] [PubMed]
- Veltman, D.; Wu, M.; Pokreisz, P.; Claus, P.; Gillijns, H.; Caluwé, E.; Vanhaverbeke, M.; Gsell, W.; Himmelreich, U.; Sinnaeve, P.R.; et al. Clec4e-Receptor Signaling in Myocardial Repair After Ischemia–Reperfusion Injury. JACC Basic Transl. Sci. 2021, 6, 631–646. [Google Scholar] [CrossRef]
- Zhang, R.Y.K.; Cochran, B.J.; Thomas, S.R.; Rye, K. Impact of Reperfusion on Temporal Immune Cell Dynamics After Myocardial Infarction. J. Am. Heart Assoc. 2023, 12, e027600. [Google Scholar] [CrossRef]
- Frangogiannis, N.G.; Smith, C.W.; Entman, M.L. The inflammatory response in myocardial infarction. Cardiovasc. Res. 2002, 53, 31–47. [Google Scholar] [CrossRef]
- Adrie, C.; Shin, S.A.; Monchi, M. Ischemia–Reperfusion Syndrome. In Inflammation: From Molecular and Cellular Mechanisms to the Clinic; Cavaillon, J.M., Ed.; Wiley-VCH: Weinheim, Germany, 2017; pp. 1313–1328. [Google Scholar]
- Zhang, H.; Hu, H.; Zhai, C.; Jing, L.; Tian, H. Cardioprotective Strategies After Ischemia–Reperfusion Injury. Am. J. Cardiovasc. Drugs 2023, 24, 5–18. [Google Scholar] [CrossRef]
- Li, T.; Yan, Z.; Fan, Y.; Fan, X.; Li, A.; Qi, Z.; Zhang, J. Cardiac repair after myocardial infarction: A two-sided role of inflammation-mediated. Front. Cardiovasc. Med. 2023, 9, 1077290. [Google Scholar] [CrossRef]
- Matter, M.A.; Paneni, F.; Libby, P.; Frantz, S.; Stähli, B.E.; Templin, C.; Mengozzi, A.; Wang, Y.-J.; Kündig, T.M.; Räber, L.; et al. Inflammation in acute myocardial infarction: The good, the bad and the ugly. Eur. Heart J. 2023, 45, 89–103. [Google Scholar] [CrossRef] [PubMed]
- Rohm, T.V.; Meier, D.T.; Olefsky, J.M.; Donath, M.Y. Inflammation in obesity, diabetes, and related disorders. Immunity 2022, 55, 31–55. [Google Scholar] [CrossRef]
- Powell-Wiley, T.M.; Poirier, P.; Burke, L.E.; Després, J.-P.; Gordon-Larsen, P.; Lavie, C.J.; Lear, S.A.; Ndumele, C.E.; Neeland, I.J.; Sanders, P.; et al. Obesity and Cardiovascular Disease: A Scientific Statement from the American Heart Association. Circulation 2021, 143, e984–e1010. [Google Scholar] [CrossRef]
- Thomsen, M.; Nordestgaard, B.G. Myocardial Infarction and Ischemic Heart Disease in Overweight and Obesity with and Without Metabolic Syndrome. JAMA Intern. Med. 2014, 174, 15–22. [Google Scholar] [CrossRef]
- Lassale, C.; Tzoulaki, I.; Moons, K.G.M.; Sweeting, M.; Boer, J.; Johnson, L.; Huerta, J.M.; Agnoli, C.; Freisling, H.; Weiderpass, E.; et al. Separate and combined associations of obesity and metabolic health with coronary heart disease: A pan-European case-cohort analysis. Eur. Heart J. 2018, 39, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Piché, M.-E.; Tchernof, A.; Després, J.-P. Obesity Phenotypes, Diabetes, and Cardiovascular Diseases. Circ. Res. 2020, 126, 1477–1500, Erratum in Circ. Res. 2020, 127, e107. https://doi: 10.1161/RES.0000000000000421. [Google Scholar] [CrossRef] [PubMed]
- Lempesis, I.G.; Georgakopoulou, V.E. Physiopathological mechanisms related to inflammation in obesity and type 2 diabetes mellitus. World J. Exp. Med. 2023, 13, 7–16. [Google Scholar] [CrossRef]
- Battineni, G.; Sagaro, G.G.; Chintalapudi, N.; Amenta, F.; Tomassoni, D.; Tayebati, S.K. Impact of Obesity-Induced Inflammation on Cardiovascular Diseases (CVD). Int. J. Mol. Sci. 2021, 22, 4798. [Google Scholar] [CrossRef]
- Antonopoulos, A.S.; Papastamos, C.; Cokkinos, D.V.; Tsioufis, K.; Tousoulis, D. Epicardial Adipose Tissue in Myocardial Disease: From Physiology to Heart Failure Phenotypes. Curr. Probl. Cardiol. 2023, 48, 101841. [Google Scholar] [CrossRef]
- Kruszewska, J.; Cudnoch-Jedrzejewska, A.; Czarzasta, K. Remodeling and Fibrosis of the Cardiac Muscle in the Course of Obesity—Pathogenesis and Involvement of the Extracellular Matrix. Int. J. Mol. Sci. 2022, 23, 4195. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.; Li, Q.; Zhou, H.; Sun, L.; Lin, C.; Jin, Y.; Wang, D.; Guo, G. The role of major immune cells in myocardial infarction. Front. Immunol. 2023, 13, 1084460. [Google Scholar] [CrossRef] [PubMed]
- Lavie, C.J.; McAuley, P.A.; Church, T.S.; Milani, R.V.; Blair, S.N. Obesity and cardiovascular diseases: Implications regarding fitness, fatness, and severity in the obesity paradox. J. Am. Coll. Cardiol. 2014, 63, 1345–1354. [Google Scholar] [CrossRef]
- Kanic, V.; Vollrath, M.; Frank, B.; Kanic, Z. An obesity paradox in patients with myocardial infarction undergoing percutaneous intervention. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 127–136. [Google Scholar] [CrossRef]
- Ndrepepa, G.; Kastrati, A. Obesity paradox after percutaneous coronary intervention-Closing in on the truth behind the phe-nomenon. EuroIntervention 2020, 15, 1120–1122. [Google Scholar] [CrossRef]
- Aggrawal, K.; Gupta, V.; Singh, B.; Medatwal, R.; Singh, S.; Jain, P.; Jain, R. Exploring the obesity parADOX: A multisystem review. Am. J. Med. Sci. 2025, 370, 315–320. [Google Scholar] [CrossRef]
- Butt, J.H.; Petrie, M.C.; Jhund, P.S.; Sattar, N.; Desai, A.S.; Køber, L.; Rouleau, J.L.; Swedberg, K.; Zile, M.R.; Solomon, S.D.; et al. Anthropometric measures and adverse outcomes in heart failure with reduced ejection fraction: Revisiting the obesity paradox. Eur. Heart J. 2023, 44, 1136–1153. [Google Scholar] [CrossRef]
- Chang, V.W.; Langa, K.M.; Weir, D.; Iwashyna, T.J. The obesity paradox and incident cardiovascular disease: A population-based study. PLoS ONE 2017, 12, e0188636. [Google Scholar] [CrossRef]
- De Schutter, A.; Kachur, S.; Lavie, C.J.; Boddepalli, R.S.; Patel, D.A.; Milani, R.V. The impact of inflammation on the obesity paradox in coronary heart disease. Int. J. Obes. 2016, 40, 1730–1735. [Google Scholar] [CrossRef]
- Jia, T.; Wang, C.; Han, Z.; Wang, X.; Ding, M.; Wang, Q. Experimental Rodent Models of Cardiovascular Diseases. Front. Cardiovasc. Med. 2020, 7, 588075. [Google Scholar] [CrossRef]
- Martin, T.P.; Macdonald, E.A.; Ali, A.; Elbassioni, M.; O’toole, D.; Zaeri, A.A.I.; Nicklin, S.A.; Gray, S.A.; Loughrey, G.M. Preclinical models of myocardial infarction: From mechanism to translation. Br. J. Pharmacol. 2022, 179, 770–791. [Google Scholar] [CrossRef]
- Heusch, G. Myocardial ischaemia–reperfusion injury and cardioprotection in perspective. Nat. Rev. Cardiol. 2020, 17, 773–789. [Google Scholar] [CrossRef]
- Alfaddagh, A.; Martin, S.S.; Leucker, T.M.; Michos, E.D.; Blaha, M.J.; Lowenstein, C.J.; Jones, S.R.; Toth, P.P. Inflammation and cardiovascular disease: From mechanisms to therapeutics. Am. J. Prev. Cardiol. 2020, 4, 100130. [Google Scholar] [CrossRef]
- De Villiers, C.; Riley, P.R. Mouse models of myocardial infarction: Comparing permanent ligation and ischaemia-reperfusion. Dis. Model. Mech. 2020, 13, dmm046565. [Google Scholar] [CrossRef]
- Kane, J.J.; Murphy, M.L.; Bisset, J.K.; Desoyza, N.; Doherty, J.E.; Straub, K.D. Mitochondrial function, oxygen extraction, epicardial S-T segment changes and tritiated digoxin distribution after reperfusion of ischemic myocardium. Am. J. Cardiol. 1975, 36, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Bresnahan, G.F.; Roberts, R.; Shell, W.E.; Ross, J.; Sobel, B.E. Deleterious effects due to hemorrhage after myocardial reperfusion. Am. J. Cardiol. 1974, 33, 82–86. [Google Scholar] [CrossRef]
- Kloner, R.A.; Ganote, C.E.; Jennings, R.B. The “No-Reflow” Phenomenon after Temporary Coronary Occlusion in the Dog. J. Clin. Invest. 1974, 54, 1496–1508. [Google Scholar] [CrossRef] [PubMed]
- Saÿen, J.J.; Sheldon, W.F.; Horwitz, O.; Kuo, P.T.; Peirce, G.; Zinsser, H.F.; Mead, J. Studies of coronary disease in the experimental animal. Ii. Polarographic determinations of local oxygen availability in the dog’s left ventricle during coronary occlusion and pure oxygen breathing. J. Clin. Investig. 1951, 30, 932–940. [Google Scholar] [CrossRef]
- Kreutzer, F.P.; Meinecke, A.; Schmidt, K.; Fiedler, J.; Thum, T. Alternative strategies in cardiac preclinical research and new clinical trial formats. Cardiovasc. Res. 2022, 118, 746–762. [Google Scholar] [CrossRef] [PubMed]
- Camacho, P.; Fan, H.; Liu, Z.; He, J.-Q. Small mammalian animal models of heart disease. Am. J. Cardiovasc. Dis. 2016, 6, 70–80. [Google Scholar]
- Yeap, X.Y.; Dehn, S.; Adelman, J.; Lipsitz, J.; Thorp, E.B. Quantitation of acute necrosis after experimental myocardial infarction. Methods Mol. Biol. 2013, 1004, 115–133. [Google Scholar]
- Stone, G.W.; Selker, H.P.; Thiele, H.; Patel, M.R.; Udelson, J.E.; Ohman, E.M.; Maehara, A.; Eitel, I.; Granger, C.B.; Jenkins, P.L.; et al. Relationship Between Infarct Size and Outcomes Following Primary PCI: Patient-Level ASnalysis From 10 Randomized Trials. J. Am. Coll. Cardiol. 2016, 67, 1674–1683. [Google Scholar] [CrossRef]
- Heusch, G.; Gersh, B.J. The pathophysiology of acute myocardial infarction and strategies of protection beyond reperfusion: A continual challenge. Eur. Heart J. 2017, 38, 774–784. [Google Scholar] [CrossRef] [PubMed]
- Lindsey, M.L.; Brunt, K.R.; Kirk, J.A.; Kleinbongard, P.; Calvert, J.W.; Brás, L.E.; DeLeon-Pennell, K.Y.; Del Re, D.P.; Frangogiannis, N.G.; Frantz, S.; et al. Guidelines for in vivo mouse models of myocardial infarction. Am. J. Physiol. Heart Circ. Physiol. 2021, 321, H1056–H1073. [Google Scholar] [CrossRef] [PubMed]
- Lindsey, M.L.; Bolli, R.; Canty, J.M., Jr.; Du, X.-J.; Frangogiannis, N.G.; Frantz, S.; Gourdie, R.G.; Holmes, J.W.; Jones, S.P.; Kloner, R.A.; et al. Guidelines for experimental models of myocardial ischemia and infarction. Am. J. Physiol. Heart Circ. Physiol. 2018, 314, H812–H838. [Google Scholar] [CrossRef]
- Peet, C.; Ivetic, A.; Bromage, D.I.; Shah, A.M. Cardiac monocytes and macrophages after myocardial infarction. Cardiovasc. Res. 2021, 116, 1101–1112. [Google Scholar] [CrossRef]
- Heusch, G. Molecular basis of cardioprotection signal transduction in ischemic pre-, post-, and remote conditioning. Circ. Res. 2015, 116, 674–699. [Google Scholar] [CrossRef] [PubMed]
- Bromage, D.I.; Pickard, J.M.J.; Rossello, X.; Ziff, O.J.; Burke, N.; Yellon, D.M.; Davidson, S.M. Remote ischaemic conditioning reduces infarct size in animal in vivo models of ischaemia-reperfusion injury: A systematic review and meta-analysis. Cardiovasc. Res. 2017, 113, 288–297. [Google Scholar]
- Kim, S.C.; Boehm, O.; Meyer, R.; Hoeft, A.; Knüfermann, P.; Baumgarten, G.A. murine closed-chest model of myocardial ischemia and reperfusion. J. Vis. Exp. 2012, 65, e3896. [Google Scholar]
- Algoet, M.; Pusovnik, M.; Gillijns, H.; Mestdagh, S.; Billiau, J.; Artoos, I.; Gsell, W.; Janssens, S.P.; Himmelreich, U.; Oosterlinck, W. Remotely Triggered LAD Occlusion Using a Balloon Catheter in Spontaneously Breathing Mice. J. Vis. Exp. 2024, 205, e66386. [Google Scholar] [CrossRef]
- Sicklinger, F.; Zhang, Y.; Lavine, K.J.; Simon, N.; Bucher, V.; Jugold, M.; Lehmann, L.; Konstandin, M.H.; Katus, H.A.; Leuschner, F. A Minimal-Invasive Approach for Standardized Induction of Myocardial Infarction in Mice. Circ. Res. 2020, 127, 1214–1216. [Google Scholar] [CrossRef]
- Bouhidel, O.; Pons, S.; Souktani, R.; Zini, R.; Berdeaux, A.; Ghaleh, B. Myocardial ischemic postconditioning against ischemia–reperfusion is impaired in ob/ob mice. Am. J. Physiol. Heart Circ. Physiol. 2008, 295, H1580–H1586. [Google Scholar] [CrossRef]
- Shin, H.S.; Shin, H.H.; Shudo, Y. Current Status and Limitations of Myocardial Infarction Large Animal Models in Cardiovascular Translational Research. Front. Bioeng. Biotechnol. 2021, 9, 673683. [Google Scholar] [CrossRef] [PubMed]
- Thakker, G.D.; Frangogiannis, N.G.; Bujak, M.; Zymek, P.; Gaubatz, J.W.; Reddy, A.K.; Taffet, G.; Michael, L.H.; Entman, M.L.; Ballantyne, C.M. Effects of diet-induced obesity on inflammation and remodeling after myocardial infarction. Am. J. Physiol. Heart Circ. Physiol. 2006, 291, 2504–2514. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.P.; Girod, W.G.; Granger, D.N.; Palazzo, A.J.; Lefer, D.J. Reperfusion injury is not affected by blockade of P-selectin in the diabetic mouse heart. Am. J. Physiol. Circ. Physiol. 1999, 277, H763–H769. [Google Scholar] [CrossRef]
- Heaberlin, J.R.; Ma, Y.; Zhang, J.; Ahuja, S.S.; Lindsey, M.L.; Halade, G.V. Obese and diabetic KKAy mice show increased mortality but improved cardiac function following myocardial infarction. Cardiovasc. Pathol. 2013, 22, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Oosterlinck, W.; Dresselaers, T.; Geldhof, V.; Nevelsteen, I.; Janssens, S.; Himmelreich, U.; Herijgers, P. Diabetes mellitus and the metabolic syndrome do not abolish, but might reduce, the cardioprotective effect of ischemic postconditioning. J. Thorac. Cardiovasc. Surg. 2013, 145, 1595–1602. [Google Scholar] [CrossRef]
- Feng, H.; Shen, H.; Robeson, M.; Wu, Y.; Wu, H.; Chen, G.; Zhang, S.; Xie, P.; Jin, L.; He, Y.; et al. MG53 E3 Ligase–Dead Mutant Protects Diabetic Hearts from Acute Ischemic/Reperfusion Injury and Ameliorates Diet-Induced Cardiometabolic Damage. Diabetes 2022, 71, 298–314. [Google Scholar] [CrossRef]
- Funk, F.; Kronenbitter, A.; Isić, M.; Flocke, V.; Gorreßen, S.; Semmler, D.; Brinkmann, M.; Beck, K.; Steinhoff, O.; Srivastava, T.; et al. Diabetes disturbs functional adaptation of the remote myocardium after ischemia/reperfusion. J. Mol. Cell. Cardiol. 2022, 173, 47–60. [Google Scholar] [CrossRef]
- Chou, C.C.; Lee, H.L.; Chang, G.J.; Wo, H.T.; Yen, T.H.; Wen, M.S.; Chu, Y.; Liu, H.T.; Chang, P.C. Mechanisms of ranolazine pretreatment in preventing ventricular tachyarrhythmias in diabetic db/db mice with acute regional ischemia–reperfusion injury. Sci. Rep. 2020, 10, 20032. [Google Scholar] [CrossRef]
- Gundewar, S.; Calvert, J.W.; Elrod, J.W.; Lefer, D.J. Cytoprotective effects of N,N,N-trimethylsphingosine during ischemia–reperfusion injury are lost in the setting of obesity and diabetes. Am. J. Physiol. Circ. Physiol. 2007, 293, H2462–H2471. [Google Scholar] [CrossRef] [PubMed]
- Greer, J.J.M.; Ware, D.P.; Lefer, D.J. Myocardial infarction and heart failure in the db/db diabetic mouse. Am. J. Physiol.-Heart Circ. Physiol. 2006, 290, H146–H153. [Google Scholar] [CrossRef]
- Pons, S.; Martin, V.; Portal, L.; Zini, R.; Morin, D.; Berdeaux, A.; Ghaleh, B. Regular treadmill exercise restores cardioprotective signaling pathways in obese mice independently from improvement in associated co-morbidities. J. Mol. Cell. Cardiol. 2013, 54, 82–89. [Google Scholar] [CrossRef]
- McGaffin, K.R.; Zou, B.; McTiernan, C.F.; O’dOnnell, C.P. Leptin attenuates cardiac apoptosis after chronic ischaemic injury. Cardiovasc. Res. 2009, 83, 313–324. [Google Scholar] [CrossRef]
- McGaffin, K.R.; Sun, C.-K.; Rager, J.J.; Romano, L.C.; Zou, B.; Mathier, M.A.; O’Doherty, R.M.; McTiernan, C.F.; O’Donnell, C.P. Leptin signalling reduces the severity of cardiac dysfunction and remodelling after chronic ischaemic injury. Cardiovasc. Res. 2008, 77, 54–63. [Google Scholar] [CrossRef]
- Mouton, A.J.; Flynn, E.R.; Moak, S.P.; Li, X.; da Silva, A.A.; Wang, Z.; Carmo, J.M.D.; Hall, M.E.; Hall, J.E. Interaction of Obesity and Hypertension on Cardiac Metabolic Remodeling and Survival Following Myocardial Infarction. J. Am. Heart Assoc. 2021, 10, e018212. [Google Scholar] [CrossRef]
- Yoshii, A.; Nagoshi, T.; Kashiwagi, Y.; Kimura, H.; Tanaka, Y.; Oi, Y.; Ito, K.; Yoshino, T.; Tanaka, T.D.; Yoshimura, M. Cardiac ischemia–reperfusion injury under insulin-resistant conditions: SGLT1 but not SGLT2 plays a compensatory protective role in diet-induced obesity. Cardiovasc. Diabetol. 2019, 18, 85. [Google Scholar] [CrossRef]
- Poncelas, M.; Inserte, J.; Vilardosa, Ú.; Rodriguez-Sinovas, A.; Bañeras, J.; Simó, R.; Garcia-Dorado, D. Obesity induced by high fat diet attenuates postinfarct myocardial remodeling and dysfunction in adult B6D2F1 mice. J. Mol. Cell. Cardiol. 2015, 84, 154–161. [Google Scholar] [CrossRef]
- Honda, T.; Kaikita, K.; Tsujita, K.; Hayasaki, T.; Matsukawa, M.; Fuchigami, S.; Sugiyama, S.; Sakashita, N.; Ogawa, H.; Takeya, M. Pioglitazone, a peroxisome proliferator-activated receptor-γ agonist, attenuates myocardial ischemia–reperfusion injury in mice with metabolic disorders. J. Mol. Cell. Cardiol. 2008, 44, 915–926. [Google Scholar] [CrossRef]
- Inserte, J.; Aluja, D.; Barba, I.; Ruiz-Meana, M.; Miró, E.; Poncelas, M.; Vilardosa, Ú.; Castellano, J.; Garcia-Dorado, D. High-fat diet improves tolerance to myocardial ischemia by delaying normalization of intracellular PH at reperfusion. J. Mol. Cell. Cardiol. 2019, 133, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Liu, Y.; Li, Y.; Xu, F.; Liu, Y. Type 2 Diabetes and Myocardial Infarction: Recent Clinical Evidence and Perspective. Front. Cardiovasc. Med. 2021, 8, 644189. [Google Scholar] [CrossRef]
- Ferdinandy, P.; Hausenloy, D.J.; Heusch, G.; Baxter, G.F.; Schulz, R. Interaction of Risk Factors, Comorbidities, and Comedications with Ischemia/Reperfusion Injury and Cardioprotection by Preconditioning, Postconditioning, and Remote Conditioning. Pharmacol. Rev. 2014, 66, 1142–1174. [Google Scholar] [CrossRef]
- Riehle, C.; Bauersachs, J. Of mice and men: Models and mechanisms of diabetic cardiomyopathy. Basic Res. Cardiol. 2019, 114, 2. [Google Scholar] [CrossRef]
- The Jackson Laboratory [Internet]. KK.Cg-Ay/J.; The Jackson Laboratory: Bar Harbor, MN, USA, 2024; Available online: https://www.jax.org/strain/002468 (accessed on 14 March 2024).
- Lee, S.E.; Jang, I.S.; Park, J.S.; Lee, J.H.; Lee, S.Y.; Baek, S.Y.; Lee, S.H.; Lee, H. Systemic immunity of obese-diabetes model (db/db) mice. Mol. Cell. Toxicol. 2010, 6, 143–149. [Google Scholar] [CrossRef]
- Hornung, F.; Rogal, J.; Loskill, P.; Löffler, B.; Deinhardt-Emmer, S. The Inflammatory Profile of Obesity and the Role on Pulmonary Bacterial and Viral Infections. Int. J. Mol. Sci. 2021, 22, 3456. [Google Scholar] [CrossRef] [PubMed]
- Adamowski, M.; Sharma, Y.; Molcan, T.; Wołodko, K.; Kelsey, G.; Galvão, A.M. Leptin signalling regulates transcriptional differences in granulosa cells from genetically obese mice but not the activation of NLRP3 inflammasome. Sci. Rep. 2024, 14, 8070. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Chandrasekera, P.C.; Pippin, J.J. Leptin and Leptin Receptor-Deficient Rodent Models: Relevance for Human Type 2 Diabetes. Curr. Diabetes Rev. 2014, 10, 131–145. [Google Scholar] [CrossRef] [PubMed]
- Frühbeck, G.; Catalán, V.; Rodríguez, A.; Ramírez, B.; Becerril, S.; Portincasa, P.; Gómez-Ambrosi, J. Normalization of adiponectin concentrations by leptin replacement in ob/ob mice is accompanied by reductions in systemic oxidative stress and inflammation. Sci. Rep. 2017, 7, 2752. [Google Scholar] [CrossRef]
- Fenton, J.I.; Nuñez, N.P.; Yakar, S.; Perkins, S.N.; Hord, N.G.; Hursting, S.D. Diet-induced adiposity alters the serum profile of inflammation in C57BL/6N mice as measured by antibody array. Diabetes Obes. Metab. 2009, 11, 343–354. [Google Scholar] [CrossRef]
- Kiran, S.; Rakib, A.; Kodidela, S.; Kumar, S.; Singh, U.P. High-Fat Diet-Induced Dysregulation of Immune Cells Correlates with Macrophage Phenotypes and Chronic Inflammation in Adipose Tissue. Cells 2022, 11, 1327. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, S.; Mochizuki, K.; Goda, T. Gene Expression of Inflammatory Cytokines in Peripheral Leukocytes in db/db Mice Rose with Progression of Diabetes. Biosci. Biotechnol. Biochem. 2010, 74, 1488–1490. [Google Scholar] [CrossRef][Green Version]
- Kammoun, H.; Allen, T.; Henstridge, D.; Barre, S.; Coll, R.; Lancaster, G.; Cron, L.; Reibe, S.; Chan, J.; Bensellam, M.; et al. Evidence against a role for NLRP3-driven islet inflammation in db/db mice. Mol. Metab. 2018, 10, 66–73. [Google Scholar] [CrossRef]
- Suriano, F.; Manca, C.; Flamand, N.; Depommier, C.; Van Hul, M.; Delzenne, N.M.; Silvestri, C.; Cani, P.D.; Di Marzo, V. Exploring the endocannabinoidome in genetically obese (ob/ob) and diabetic (db/db) mice: Links with inflammation and gut microbiota. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2022, 1867, 159056. [Google Scholar] [CrossRef]
- Suriano, F.; Vieira-Silva, S.; Falony, G.; Roumain, M.; Paquot, A.; Pelicaen, R.; Régnier, M.; Delzenne, N.M.; Raes, J.; Muccioli, G.G.; et al. Novel insights into the genetically obese (ob/ob) and diabetic (db/db) mice: Two sides of the same coin. Microbiome 2021, 9, 147. [Google Scholar] [CrossRef]
- Oppi, S.; Lüscher, T.F.; Stein, S. Mouse Models for Atherosclerosis Research—Which Is My Line? Front. Cardiovasc. Med. 2019, 6, 46. [Google Scholar] [CrossRef] [PubMed]
- Shioura, K.M.; Geenen, D.L.; Goldspink, P.H. Sex-related changes in cardiac function following myocardial infarction in mice. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2008, 295, R528–R534. [Google Scholar] [CrossRef]
- Pullen, A.B.; Kain, V.; Serhan, C.N.; Halade, G.V. Molecular and Cellular Differences in Cardiac Repair of Male and Female Mice. J. Am. Heart Assoc. 2020, 9, e015672. [Google Scholar] [CrossRef] [PubMed]
- Andreadou, I.; Cabrera-Fuentes, H.A.; Devaux, Y.; Frangogiannis, N.G.; Frantz, S.; Guzik, T.; Liehn, E.A.; Gomes, C.P.C.; Schulz, R.; Hausenloy, D.J. Immune cells as targets for cardioprotection: New players and novel therapeutic opportunities. Cardiovasc. Res. 2019, 115, 1117–1130. [Google Scholar] [CrossRef]
- Frangogiannis, N.G. The inflammatory response in myocardial injury, repair, and remodelling. Nat. Rev. Cardiol. 2014, 11, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Heusch, G. Myocardial ischemia/reperfusion: Translational pathophysiology of ischemic heart disease. Med 2024, 5, 10–31. [Google Scholar] [CrossRef]
- Wachsmuth, L.; Mensen, A.; Barca, C.; Wiart, M.; Tristão-Pereira, C.; Busato, A.; Waiczies, S.; Himmelreich, U.; Millward, J.M.; Reimann, H.M.; et al. Contribution of preclinical MRI to responsible animal research: Living up to the 3R principle. Magn. Reson. Mater. Phys. Biol. Med. 2021, 34, 469–474. [Google Scholar] [CrossRef]
- Kiessling, F.; Pichler, B.J.; Hauff, P. Small Animal Imaging: Basics and Practical Guide, 2nd ed.; Springer: Cham, Switzerland, 2017. [Google Scholar]
- Sosnovik, D.E.; Scherrer-Crosbie, M. Biomedical Imaging in Experimental Models of Cardiovascular Disease. Circ. Res. 2022, 130, 1851–1868. [Google Scholar] [CrossRef]
- Sinusas, A.J.; Bengel, F.; Nahrendorf, M.; Epstein, F.H.; Wu, J.C.; Villanueva, F.S.; Fayad, Z.A.; Gropler, R.J. Multimodality Cardiovascular Molecular Imaging, Part, I. Circ. Cardiovasc. Imaging 2008, 1, 244–256. [Google Scholar] [CrossRef]
- Vandoorne, K.; Nahrendorf, M. Multiparametric Imaging of Organ System Interfaces. Circ. Cardiovasc. Imaging 2017, 10, e005613. [Google Scholar] [CrossRef] [PubMed]
- Macritchie, N.; Noonan, J.; Tomasz, |.; Guzik, J.; Maffia, P. Molecular imaging of cardiovascular inflammation. Br. J. Pharmacol. 2021, 178, 4216–4245. [Google Scholar] [CrossRef]
- MacRitchie, N.; Frleta-Gilchrist, M.; Sugiyama, A.; Lawton, T.; McInnes, I.B.; Maffia, P. Molecular imaging of inflammation-Current and emerging technologies for diagnosis and treatment. Pharmacol. Ther. 2020, 211, 107550. [Google Scholar] [CrossRef]
- Heo, G.S.; Diekmann, J.; Thackeray, J.T.; Liu, Y. Nuclear Methods for Immune Cell Imaging: Bridging Molecular Imaging and Individualized Medicine. Circ. Cardiovasc. Imaging 2023, 16, e014067. [Google Scholar] [CrossRef] [PubMed]
- Thackeray, J.T.; Bengel, F.M. Molecular Imaging of Myocardial Inflammation with Positron Emission Tomography Post-Ischemia: A Determinant of Subsequent Remodeling or Recovery. JACC Cardiovasc. Imaging 2018, 11, 1340–1355. [Google Scholar] [CrossRef]
- Thackeray, J.T.; Lavine, K.J.; Liu, Y. Imaging Inflammation Past, Present, and Future: Focus on Cardioimmunology. J. Nucl. Med. 2023, 64, 39S–48S. [Google Scholar] [CrossRef]
- Hess, A.; Derlin, T.; Koenig, T.; Diekmann, J.; Wittneben, A.; Wang, Y.; Wester, H.J.; Ross, T.L.; Wollert, K.C.; Bauersachs, J.; et al. Molecular imaging-guided repair after acute myocardial infarction by targeting the chemokine receptor CXCR4. Eur. Heart J. 2020, 41, 3564–3575. [Google Scholar] [CrossRef]
- Sosnovik, D.E.; Nahrendorf, M.; Weissleder, R. Molecular Magnetic Resonance Imaging in Cardiovascular Medicine. Circulation 2007, 115, 2076–2086. [Google Scholar] [CrossRef]
- Flögel, U.; Ding, Z.; Hardung, H.; Jander, S.; Reichmann, G.; Jacoby, C.; Schubert, R.; Schrader, J. In Vivo Monitoring of Inflammation After Cardiac and Cerebral Ischemia by Fluorine Magnetic Resonance Imaging. Circulation 2008, 118, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Bouvain, P.; Ding, Z.; Kadir, S.; Kleimann, P.; Kluge, N.; Tiren, Z.B.; Steckel, B.; Flocke, V.; Zalfen, R.; Petzsch, P.; et al. Non-invasive mapping of systemic neutrophil dynamics upon cardiovascular injury. Nat. Cardiovasc. Res. 2023, 2, 126–143. [Google Scholar] [CrossRef]
- Strijkers, G.J. Targeted Nanoparticles for Cardiovascular Molecular Imaging. Curr. Radiol. Rep. 2013, 1, 191–204. [Google Scholar] [CrossRef]
- Stirrat, C.G.; Newby, D.E.; Robson, J.M.J.; Jansen, M.A. The Use of Superparamagnetic Iron Oxide Nanoparticles to Assess Cardiac Inflammation. Curr. Cardiovasc. Imaging Rep. 2014, 7, 9263. [Google Scholar] [CrossRef]
- Tsampasian, V.; Merinopoulos, I.; Cameron, D.; Garg, P.; Vassiliou, V.S. Ultrasmall Superparamagnetic Particles of Iron Oxide and Cardiac Magnetic Resonance: Novel Imaging in Everyday Conditions. Appl. Sci. 2022, 12, 6913. [Google Scholar] [CrossRef]
- Merinopoulos, I.; Gunawardena, T.; Stirrat, C.; Cameron, D.; Eccleshall, S.C.; Dweck, M.R.; Newby, D.E.; Vassiliou, V.S. Diagnostic Applications of Ultrasmall Superparamagnetic Particles of Iron Oxide for Imaging Myocardial and Vascular Inflammation. JACC Cardiovasc. Imaging 2021, 14, 1249–1264. [Google Scholar] [CrossRef] [PubMed]
- Kazimierczyk, R.; Kaminski, K.A.; Nekolla, S.G. Cardiac PET/MRI: Recent Developments and Future Aspects. Semin. Nucl. Med. 2024, 54, 733–746. [Google Scholar] [CrossRef]
- Rischpler, C.; Siebermair, J.; Kessler, L.; Quick, H.H.; Umutlu, L.; Rassaf, T.; Antoch, G.; Herrmann, K.; Nensa, F. Cardiac PET/MRI: Current Clinical Status and Future Perspectives. Semin. Nucl. Med. 2020, 50, 260–269. [Google Scholar] [CrossRef]
- Strunk, M.; Heo, G.S.; Hess, A.; Luehmann, H.; Ross, T.L.; Gropler, R.J.; Bengel, F.M.; Liu, Y.; Thackeray, J.T. Toward Quantitative Multisite Preclinical Imaging Studies in Acute Myocardial Infarction: Evaluation of the Immune-Fibrosis Axis. J. Nucl. Med. 2024, 65, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Gsell, W.; Molinos, C.; Correcher, C.; Belderbos, S.; Wouters, J.; Junge, S.; Heidenreich, M.; Velde, G.V.; Rezaei, A.; Nuyts, J.; et al. Characterization of a preclinical PET insert in a 7 tesla MRI scanner: Beyond NEMA testing. Phys. Med. Biol. 2020, 65, 245016. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geerkens, L.; Janssens, S.; De Groote, S.; Pusovnik, M.; Oosterlinck, W.; Himmelreich, U. Myocardial Infarction in Murine Models of Obesity and Related Metabolic Disorders: The Role of Inflammation. Int. J. Mol. Sci. 2025, 26, 11663. https://doi.org/10.3390/ijms262311663
Geerkens L, Janssens S, De Groote S, Pusovnik M, Oosterlinck W, Himmelreich U. Myocardial Infarction in Murine Models of Obesity and Related Metabolic Disorders: The Role of Inflammation. International Journal of Molecular Sciences. 2025; 26(23):11663. https://doi.org/10.3390/ijms262311663
Chicago/Turabian StyleGeerkens, Lotte, Stefan Janssens, Senne De Groote, Matic Pusovnik, Wouter Oosterlinck, and Uwe Himmelreich. 2025. "Myocardial Infarction in Murine Models of Obesity and Related Metabolic Disorders: The Role of Inflammation" International Journal of Molecular Sciences 26, no. 23: 11663. https://doi.org/10.3390/ijms262311663
APA StyleGeerkens, L., Janssens, S., De Groote, S., Pusovnik, M., Oosterlinck, W., & Himmelreich, U. (2025). Myocardial Infarction in Murine Models of Obesity and Related Metabolic Disorders: The Role of Inflammation. International Journal of Molecular Sciences, 26(23), 11663. https://doi.org/10.3390/ijms262311663








