Interkingdom Biofilms Are Affected by Non-Antibiotic Strategies: In Vitro Study in Lubbock Chronic Wound Biofilm Model
Abstract
1. Introduction
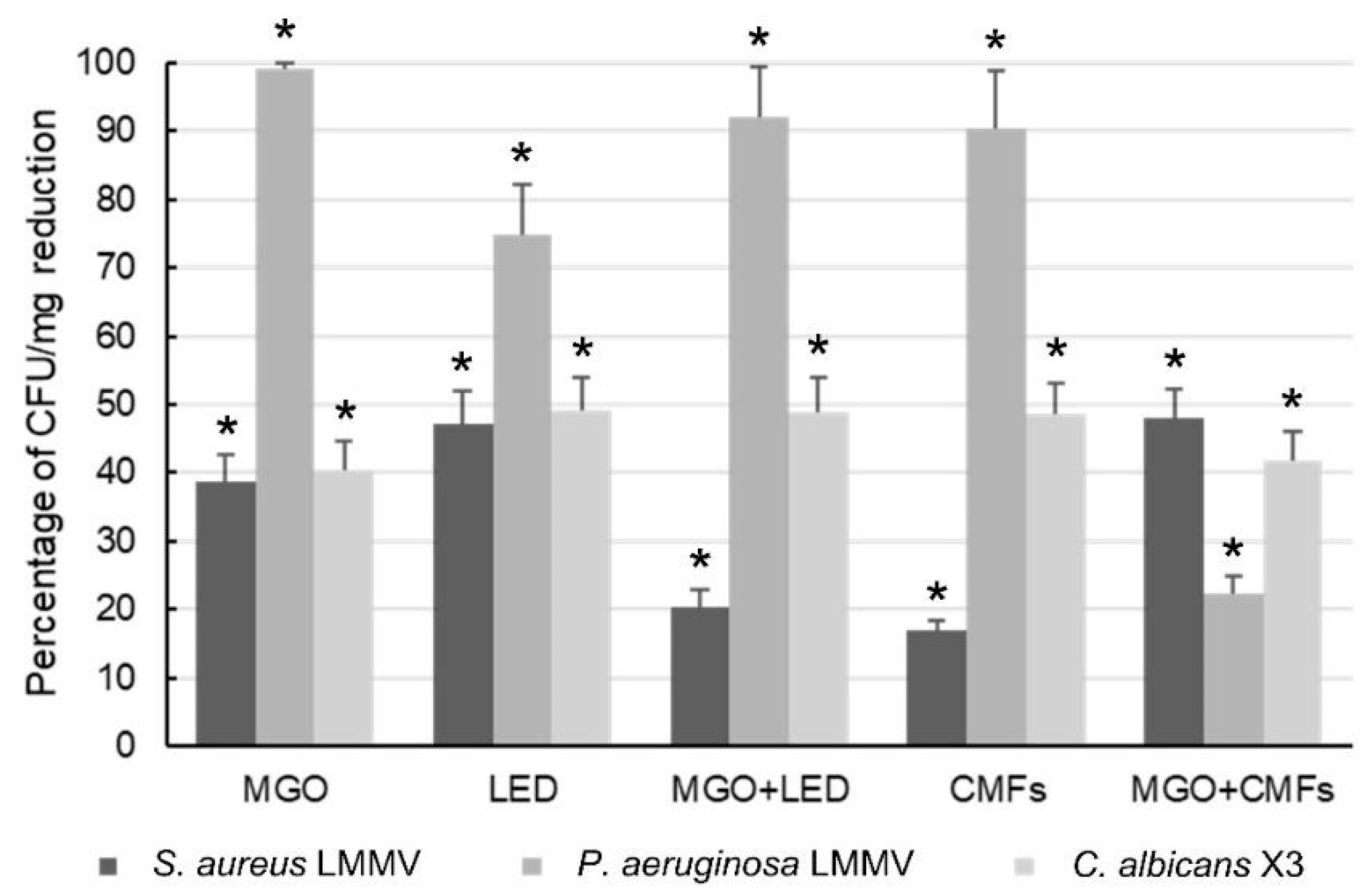
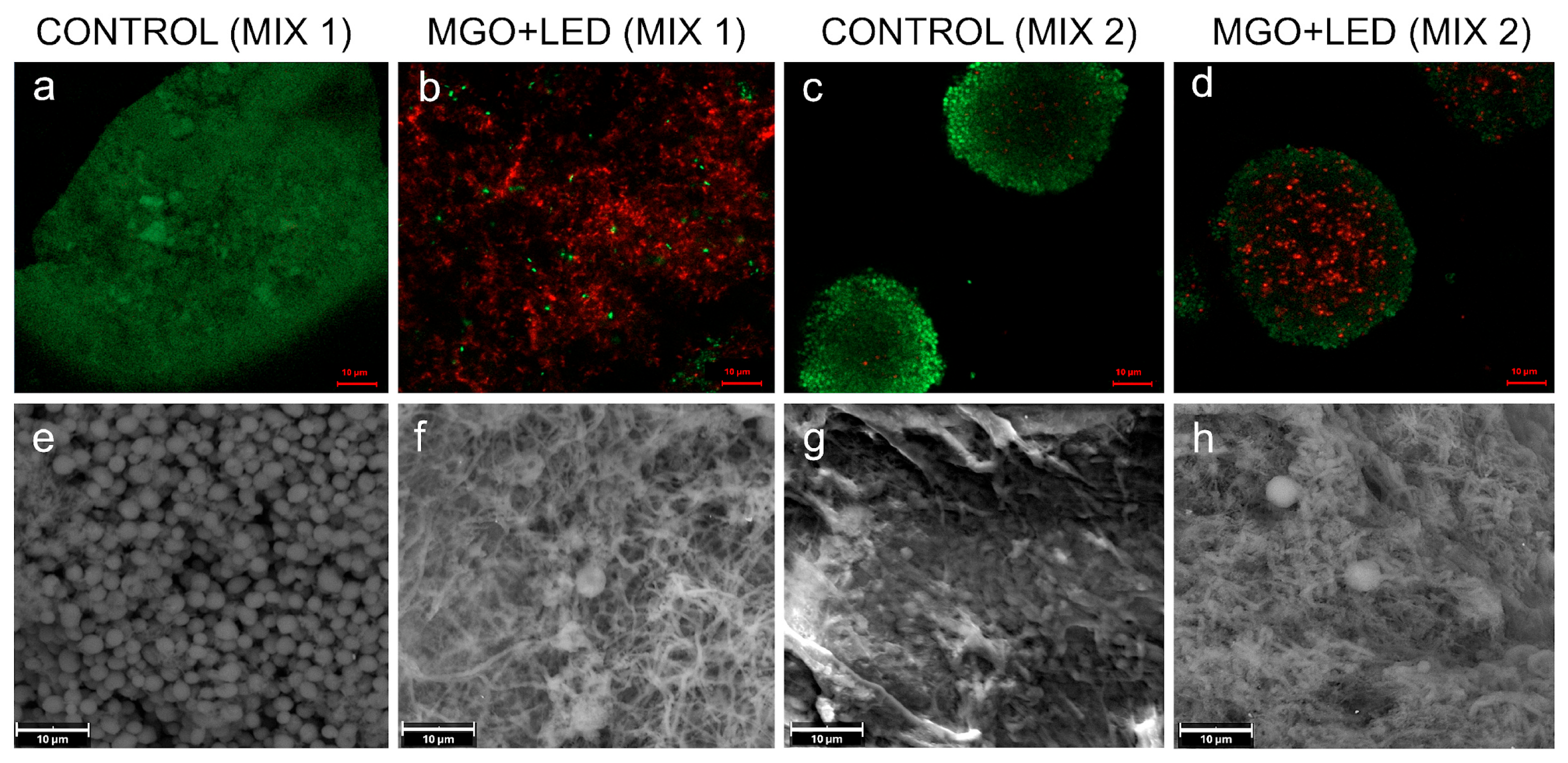
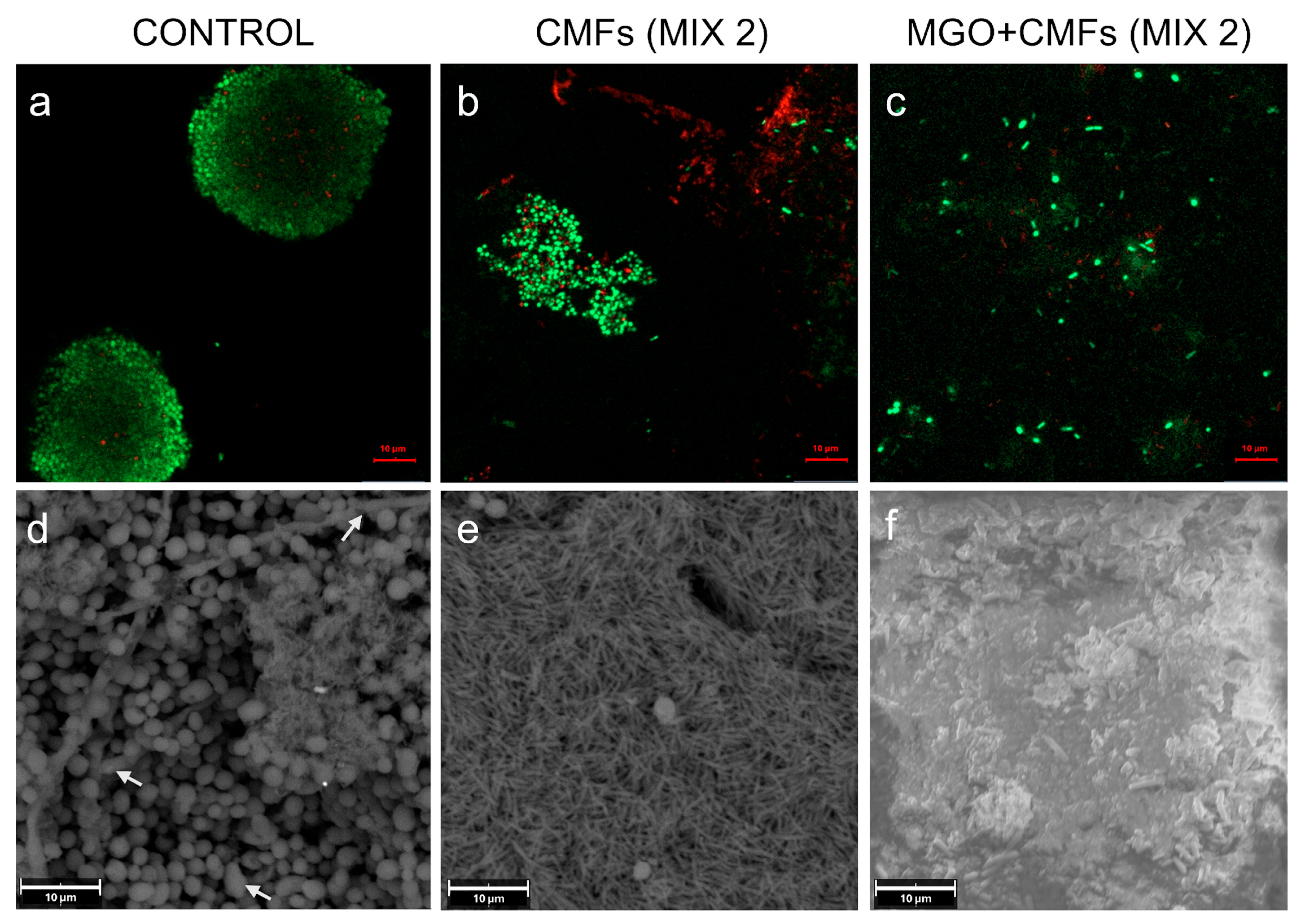
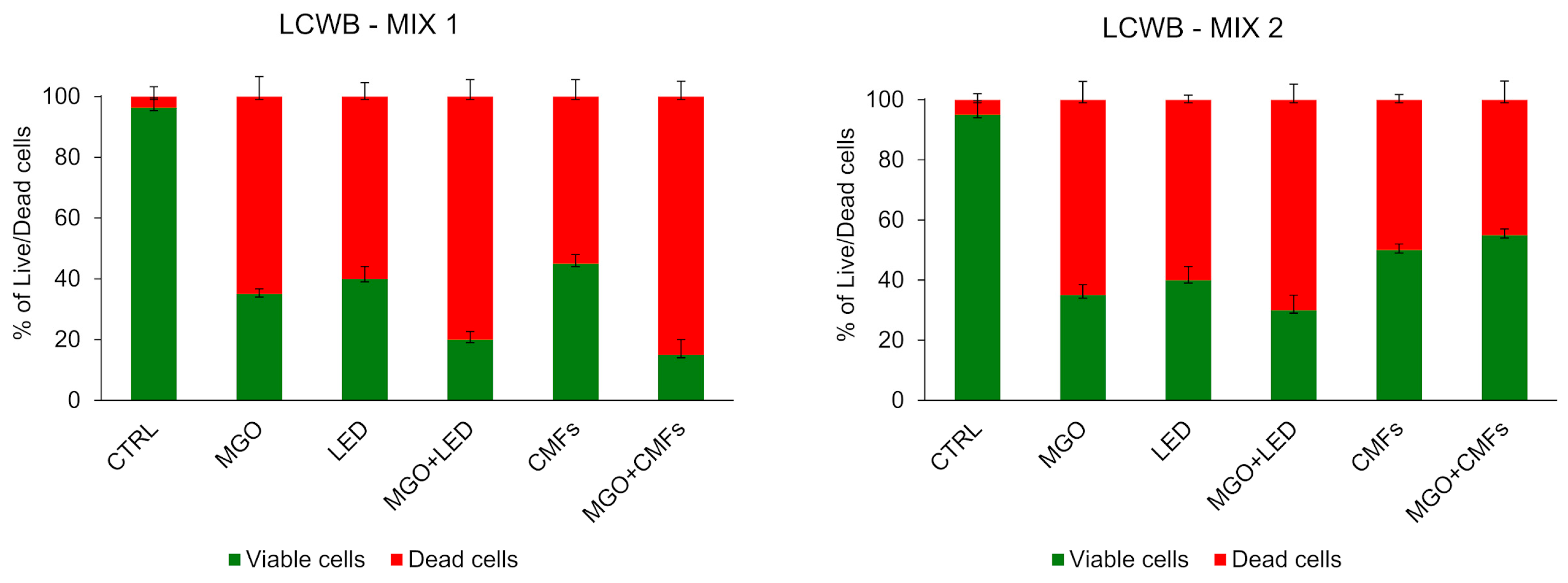
2. Results
3. Discussion
4. Materials and Methods
4.1. Methylglyoxal (MGO) Aqueous Dispersion
4.2. Light-Emitting Diode (LED) Device
4.3. Complex Magnetic Fields Source (CMFs)
4.4. Microbial Cultures
4.5. Lubbock Chronic Wound Biofilm (LCWB) Model Assay
- -
- MIX 1: S. aureus PECHA 10, P. aeruginosa PECHA 4 and C. albicans X3.
- -
- MIX 2: S. aureus LMMV, P. aeruginosa LMMV and C. albicans X3.
4.6. Effect of MGO, LED and CMFs Alone and in Combination on LCWB Model
4.7. Scanning Electron Microscopy (SEM) Analysis
4.8. Confocal Laser Scanning Microscopy (CLSM) Analysis
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CFU/mg | Colony-Forming Unit per milligram |
| CLSM | Confocal Laser Scanning Microscopy |
| CMFs | Complex Electromagnetic Fields |
| EPS | Extracellular Polymeric Substances |
| LCWB | Lubbock Chronic Wound Biofilm |
| LED | Light-Emitting Diodes |
| MGO | Methylglyoxal |
| SEM | Scanning Electron Microscopy |
References
- Diban, F.; Di Lodovico, S.; Di Fermo, P.; D’Ercole, S.; D’Arcangelo, S.; Di Giulio, M.; Cellini, L. Biofilms in Chronic wound infections: Innovative antimicrobial approaches using the In vitro Lubbock Chronic Wound Biofilm model. Int. J. Mol. Sci. 2023, 24, 1004. [Google Scholar] [CrossRef]
- Liu, H.Y.; Prentice, E.L.; Webber, M.A. Mechanisms of antimicrobial resistance in biofilms. Npj Antimicrob. Resist. 2024, 2, 27. [Google Scholar] [CrossRef]
- Gondil, V.S.; Subhadra, B. Biofilms and their role on diseases. BMC Microbiol. 2023, 23, 203. [Google Scholar] [CrossRef] [PubMed]
- Peters, B.M.; Jabra-Rizk, M.A.; Scheper, M.A.; Leid, J.G.; Costerton, J.W.; Shirtliff, M.E. Microbial interactions and differential protein expression in Staphylococcus aureus–Candida albicans dual-species biofilms. FEMS Immunol. Med. Microbiol. 2010, 59, 493–503. [Google Scholar] [CrossRef]
- Peters, B.M.; Ovchinnikova, E.S.; Krom, B.P.; Schlecht, L.M.; Zhou, H.; Hoyer, L.L.; Busscher, H.J.; Van Der Mei, H.C.; Jabra-Rizk, M.A.; Shirtliff, M.E. Staphylococcus aureus adherence to Candida albicans hyphae is mediated by the hyphal adhesin Als3p. Microbiology 2012, 158, 2975–2986. [Google Scholar] [CrossRef]
- Kong, E.F.; Tsui, C.; Kucharíková, S.; Van Dijck, P.; Jabra-Rizk, M.A. Modulation of Staphylococcus aureus Response to Antimicrobials by the Candida albicans Quorum Sensing Molecule Farnesol. Antimicrob. Agents Chemother. 2017, 61, 10–1128. [Google Scholar] [CrossRef]
- Krause, J.; Geginat, G.; Tammer, I. Prostaglandin E2 from Candida albicans Stimulates the Growth of Staphylococcus aureus in Mixed Biofilms. PLoS ONE 2015, 10, e0135404. [Google Scholar] [CrossRef]
- Hoffman, L.R.; Déziel, E.; D’Argenio, D.A.; Lépine, F.; Emerson, J.; McNamara, S.; Gibson, R.L.; Ramsey, B.W.; Miller, S.I. Selection for Staphylococcus aureus small-colony variants due to growth in the presence of Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 2006, 103, 19890–19895. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.T.; Oglesby-Sherrouse, A.G. Interactions between Pseudomonas aeruginosa and Staphylococcus aureus during co-cultivations and polymicrobial infections. Appl. Microbiol. Biotechnol. 2016, 100, 6141–6148. [Google Scholar] [CrossRef] [PubMed]
- Kahl, L.J.; Stremmel, N.; Esparza-Mora, M.A.; Wheatley, R.M.; MacLean, R.C.; Ralser, M. Interkingdom interactions between Pseudomonas aeruginosa and Candida albicans affect clinical outcomes and antimicrobial responses. Curr. Opin. Microbiol. 2023, 75, 102368. [Google Scholar] [CrossRef]
- Trejo-Hernández, A.; Andrade-Domínguez, A.; Hernández, M.; Encarnación, S. Interspecies competition triggers virulence and mutability in Candida albicans–Pseudomonas aeruginosa mixed biofilms. ISME J. 2014, 8, 1974–1988. [Google Scholar] [CrossRef]
- Alves, P.M.; Al-Badi, E.; Withycombe, C.; Jones, P.M.; Purdy, K.J.; Maddocks, S.E. Interaction between Staphylococcus aureus and Pseudomonas aeruginosa is beneficial for colonisation and pathogenicity in a mixed biofilm. Pathog. Dis. 2018, 76, fty003. [Google Scholar] [CrossRef]
- Di Fermo, P.; Ciociola, T.; Di Lodovico, S.; D’Ercole, S.; Petrini, M.; Giovati, L.; Conti, S.; Di Giulio, M.; Cellini, L. Antimicrobial Peptide L18R Displays a Modulating Action against Inter-Kingdom Biofilms in the Lubbock Chronic Wound Biofilm Model. Microorganisms 2021, 9, 1779. [Google Scholar] [CrossRef] [PubMed]
- Gkoutzouvelidou, M.; Panos, G.; Xanthou, M.N.; Papachristoforou, A.; Giaouris, E. Comparing the Antimicrobial Actions of Greek Honeys from the Island of Lemnos and Manuka Honey from New Zealand against Clinically Important Bacteria. Foods 2021, 10, 1402. [Google Scholar] [CrossRef]
- Di Lodovico, S.; Diban, F.; Di Fermo, P.; Petrini, M.; Fontana, A.; Di Giulio, M.; Piattelli, A.; D’Ercole, S.; Cellini, L. Antimicrobial combined action of graphene oxide and light emitting diodes for chronic wound management. Int. J. Mol. Sci. 2022, 23, 6942. [Google Scholar] [CrossRef]
- D’Amico, E.; Di Lodovico, S.; Pierfelice, T.V.; Tripodi, D.; Piattelli, A.; Iezzi, G.; Petrini, M.; D’Ercole, S. What is the impact of antimicrobial photodynamic therapy on oral candidiasis? an in vitro study. Gels 2024, 10, 110. [Google Scholar] [CrossRef]
- D’Ercole, S.; Spoto, G.; Trentini, P.; Tripodi, D.; Petrini, M. In vitro inactivation of Enterococcus faecalis with a led device. J. Photochem. Photobiol. B Biol. 2016, 160, 172–177. [Google Scholar] [CrossRef]
- Han, Q.; Jiang, Y.; Brandt, B.W.; Yang, J.; Chen, Y.; Buijs, M.J.; Crielaard, W.; Cheng, L.; Deng, D. Regrowth of microcosm biofilms on titanium surfaces after various antimicrobial treatments. Front. Microbiol. 2019, 10, 2693. [Google Scholar] [CrossRef] [PubMed]
- Graves, N.; Phillips, C.J.; Harding, K. A narrative review of the epidemiology and economics of chronic wounds. Br. J. Dermatol. 2021, 187, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Diban, F.; Di Fermo, P.; Di Lodovico, S.; Petrini, M.; Pilato, S.; Fontana, A.; Pinti, M.; Di Giulio, M.; Lence, E.; González-Bello, C.; et al. Methylglyoxal Alone or Combined with Light-Emitting Diodes/Complex Electromagnetic Fields Represent an Effective Response to Microbial Chronic Wound Infections. Antibiotics 2025, 14, 396. [Google Scholar] [CrossRef]
- Di Lodovico, S.; Petrini, M.; D’Amico, E.; Di Fermo, P.; Diban, F.; D’Arcangelo, S.; Piattelli, A.; Cellini, L.; Iezzi, G.; Di Giulio, M.; et al. Complex magnetic fields represent an eco-sustainable technology to counteract the resistant Candida albicans growth without affecting the human gingival fibroblasts. Sci. Rep. 2023, 13, 22067. [Google Scholar] [CrossRef]
- Lee, J.H.; Parveen, A.; Do, M.H.; Kang, M.C.; Yumnam, S.; Kim, S.Y. Molecular mechanisms of methylglyoxal-induced aortic endothelial dysfunction in human vascular endothelial cells. Cell Death Dis. 2020, 11, 403. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Rodriguez-Niño, A.; Pastene, D.O.; Pallavi, P.; van den Born, J.; Bakker, S.J.L.; Krämer, B.K.; Yard, B.A. Methylglyoxal induces p53 activation and inhibits mTORC1 in human umbilical vein endothelial cells. Sci. Rep. 2021, 11, 8004. [Google Scholar] [CrossRef]
- Di Lodovico, S.; Bacchetti, T.; D’Ercole, S.; Covone, S.; Petrini, M.; Di Giulio, M.; Di Fermo, P.; Diban, F.; Ferretti, G.; Cellini, L. Complex Chronic Wound Biofilms Are Inhibited in vitro by the Natural Extract of Capparis spinose. Front. Microbiol. 2022, 13, 832919. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.; Tang, X.; Dong, W.; Sun, N.; Yuan, W. A Review of Biofilm Formation of Staphylococcus aureus and Its Regulation Mechanism. Antibiotics 2022, 12, 12. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira Mima, E.G.; Pavarina, A.C.; Dovigo, L.N.; Vergani, C.E.; De Souza Costa, C.A.; Kurachi, C.; Bagnato, V.S. Susceptibility of Candida albicans to photodynamic therapy in a murine model of oral candidosis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2010, 109, 392–401. [Google Scholar] [CrossRef]
- Carmello, J.C.; Jordão, C.C.; Dias, L.M.; De Sousa, T.V.; Oliveira, R.; Pavarina, A.C. Light-induced effects against Candida albicans and Staphylococcus aureus. Res. Soc. Dev. 2022, 11, e23111032600. [Google Scholar] [CrossRef]
- D’Ercole, S.; Di Fermo, P.; Di Giulio, M.; Di Lodovico, S.; Di Campli, E.; Scarano, A.; Tripodi, D.; Cellini, L.; Petrini, M. Near-infrared NIR irradiation and sodium hypochlorite: An efficacious association to counteract the Enterococcus faecalis biofilm in endodontic infections. J. Photochem. Photobiol. B 2020, 210, 111989. [Google Scholar] [CrossRef]
- Ismaili, A.; Meddings, J.B.; Ratnam, S.; Sherman, P.M. Modulation of host cell membrane fluidity: A novel mechanism for preventing bacterial adhesion. Am. J. Physiol. Gastrointest. Liver Physiol. 1999, 277, G201–G208. [Google Scholar] [CrossRef]
- Chakraborty, S.; Karmakar, K.; Chakravortty, D. Cells producing their own nemesis: Understanding methylglyoxal metabolism. IUBMB Life 2014, 66, 667–678. [Google Scholar] [CrossRef]
- Hayashi, K.; Fukushima, A.; Hayashi-Nishino, M.; Nishino, K. Effect of methylglyoxal on multidrug-resistant Pseudomonas aeruginosa. Front. Microbiol. 2014, 5, 180. [Google Scholar] [CrossRef]
- Petrini, M.; Spoto, G.; Scarano, A.; D’Arcangelo, C.; Tripodi, D.; Di Fermo, P.; D’Ercole, S. Near-Infrared LEDS Provide Persistent and Increasing Protection against E. Faecalis. J. Photochem. Photobiol. B Biol. 2019, 197, 111527. [Google Scholar] [CrossRef]
- Rima, M.; Villeneuve-Faure, C.; Pilloux, L.; Roques, C.; El Garah, F.; Makasheva, K. From Adhesion to Biofilms Formation and Resilience: Exploring the Impact of Silver Nanoparticles-Based Biomaterials on Pseudomonas Aeruginosa. Biofilm 2025, 9, 100267. [Google Scholar] [CrossRef]
- Barolet, D. Light-emitting diodes (LEDs) in dermatology. Semin. Cutan. Med. Surg. 2008, 27, 227–238. [Google Scholar] [CrossRef]
- Ryu, J.H.; Park, J.; Kim, J.W.; Shin, Y.I.; Lee, S.D.; Oh, Y.; Kang, S.W. Exploring the effects of 630 nm wavelength of light-emitting diode irradiation on the proliferation and migration ability of human biceps tendon fibroblast cells. Clin. Orthop. Surg. 2023, 15, 166–174. [Google Scholar] [CrossRef]
- Tong, J.; Subbiah, S.K.; Rampal, S.; Ramasamy, R.; Wu, X.; You, Y.; Wang, J.; Mok, P.L. Effect of 660-nm LED photobiomodulation on the proliferation and chondrogenesis of meniscus-derived stem cells (MeSCs). Sci. Rep. 2024, 14, 19735. [Google Scholar] [CrossRef] [PubMed]
- De Santis, V.; Chen, X.L.; Laakso, I.; Hirata, A. An equivalent skin conductivity model for low-frequency magnetic field dosimetry. Biomed. Phys. Eng. Express 2015, 1, 015201. [Google Scholar] [CrossRef]
- Zanotti, F.; Trentini, M.; Zanolla, I.; Tiengo, E.; Mantarro, C.; Dalla Paola, L.; Tremoli, E.; Sambataro, M.; Sambado, L.; Picari, M.; et al. Playing with biophysics: How a symphony of different electromagnetic fields acts to reduce the inflammation in diabetic derived cells. Int. J. Mol. Sci. 2023, 24, 1754. [Google Scholar] [CrossRef] [PubMed]
- Geary, N. Understanding synergy. Am. J. Physiol. Endocrinol. Metab. 2013, 304, E237–E253. [Google Scholar] [CrossRef] [PubMed]
- Klinger-Strobel, M.; Ernst, J.; Lautenschläger, C.; Pletz, M.W.; Fischer, D.; Makarewicz, O. A blue fluorescent labeling technique utilizing micro- and nanoparticles for tracking in LIVE/DEAD® stained pathogenic biofilms of Staphylococcus aureus and Burkholderia cepacia. Int. J. Nanomed. 2016, 11, 575–583. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Fermo, P.; Diban, F.; Di Campli, E.; Cellini, L.; Pinti, M.; Di Giulio, M.; Petrini, M.; D’Ercole, S.; Di Lodovico, S. Interkingdom Biofilms Are Affected by Non-Antibiotic Strategies: In Vitro Study in Lubbock Chronic Wound Biofilm Model. Int. J. Mol. Sci. 2025, 26, 11658. https://doi.org/10.3390/ijms262311658
Di Fermo P, Diban F, Di Campli E, Cellini L, Pinti M, Di Giulio M, Petrini M, D’Ercole S, Di Lodovico S. Interkingdom Biofilms Are Affected by Non-Antibiotic Strategies: In Vitro Study in Lubbock Chronic Wound Biofilm Model. International Journal of Molecular Sciences. 2025; 26(23):11658. https://doi.org/10.3390/ijms262311658
Chicago/Turabian StyleDi Fermo, Paola, Firas Diban, Emanuela Di Campli, Luigina Cellini, Morena Pinti, Mara Di Giulio, Morena Petrini, Simonetta D’Ercole, and Silvia Di Lodovico. 2025. "Interkingdom Biofilms Are Affected by Non-Antibiotic Strategies: In Vitro Study in Lubbock Chronic Wound Biofilm Model" International Journal of Molecular Sciences 26, no. 23: 11658. https://doi.org/10.3390/ijms262311658
APA StyleDi Fermo, P., Diban, F., Di Campli, E., Cellini, L., Pinti, M., Di Giulio, M., Petrini, M., D’Ercole, S., & Di Lodovico, S. (2025). Interkingdom Biofilms Are Affected by Non-Antibiotic Strategies: In Vitro Study in Lubbock Chronic Wound Biofilm Model. International Journal of Molecular Sciences, 26(23), 11658. https://doi.org/10.3390/ijms262311658









