Antimicrobial Activity Versus Virulence Potential of Hyaluronic Acid: Balancing Advantages and Disadvantages
Abstract
1. Introduction
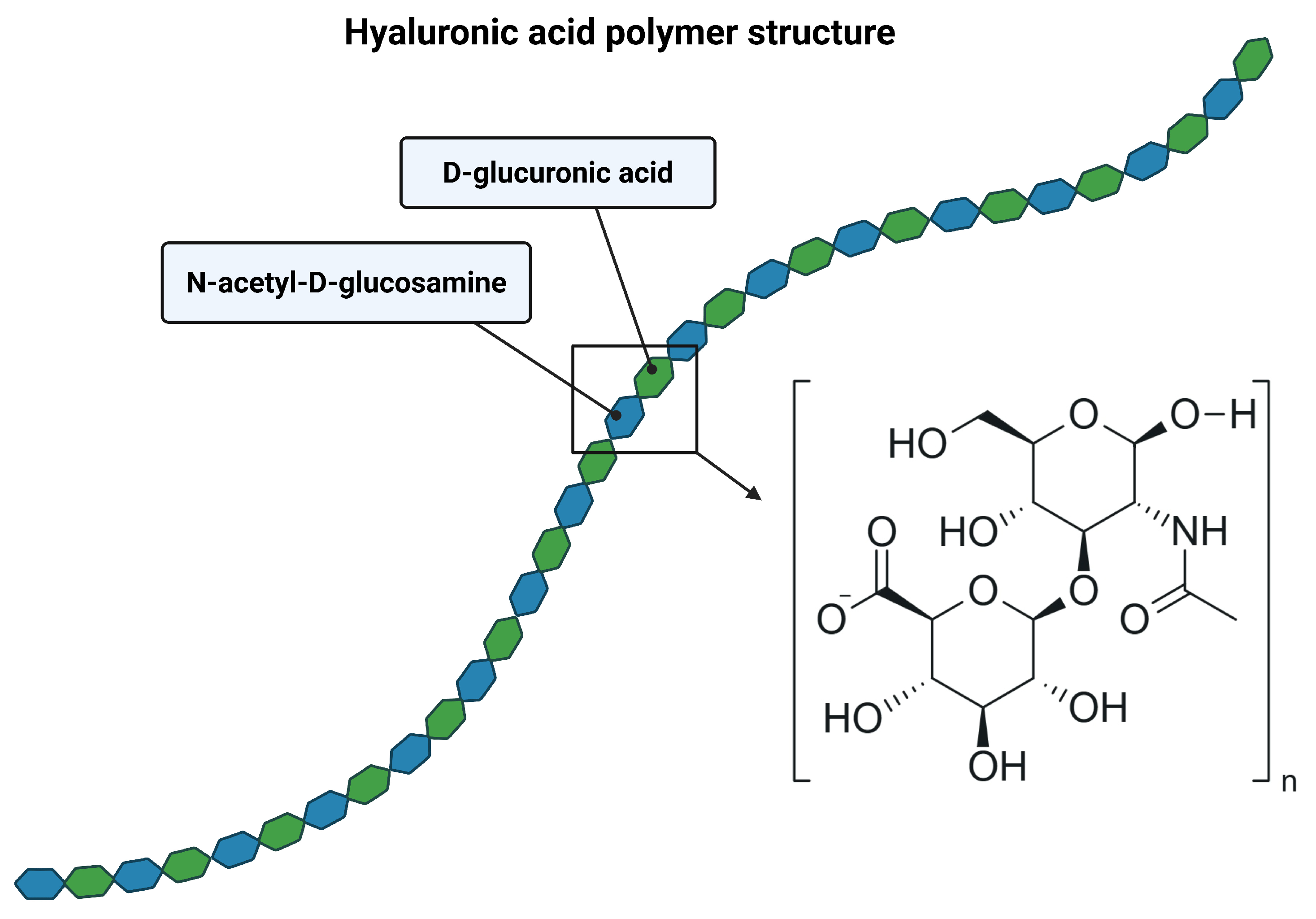
2. Structure of Hyaluronic Acid
Receptors for Hyaluronic Acid
3. Synthesis of Hyaluronic Acid
3.1. Hyaluronic Acid Synthesis in Vertebrates
3.2. Degradation of Hyaluronic Acid in Vertebrates
3.3. Hyaluronic Acid Synthesis in Microorganisms
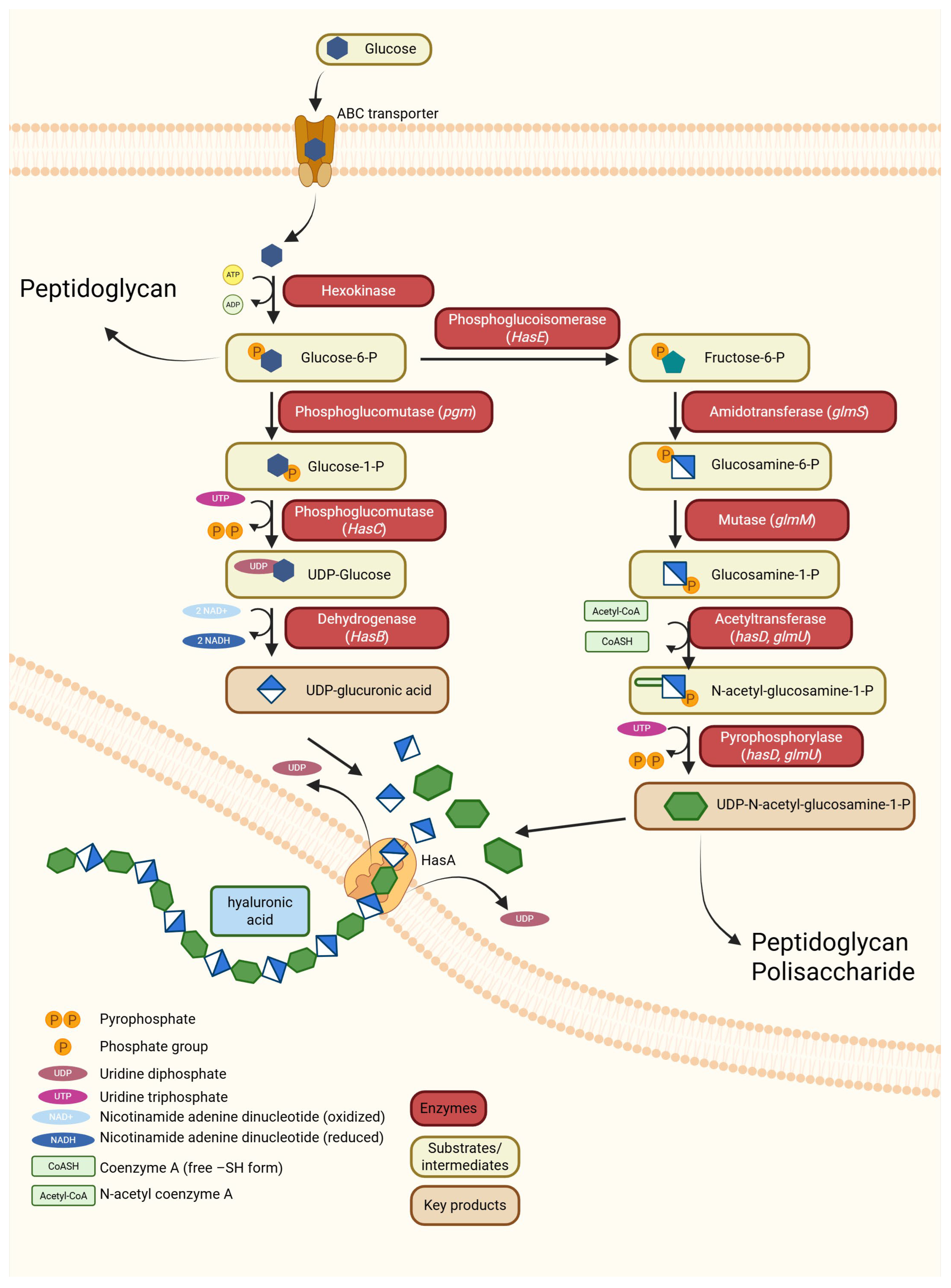
3.3.1. Hyaluronic Acid Synthesis Among Bacteria
3.3.2. Hyaluronic Acid Synthesis in Fungi
Native Fungal Producers
Heterologous Expression
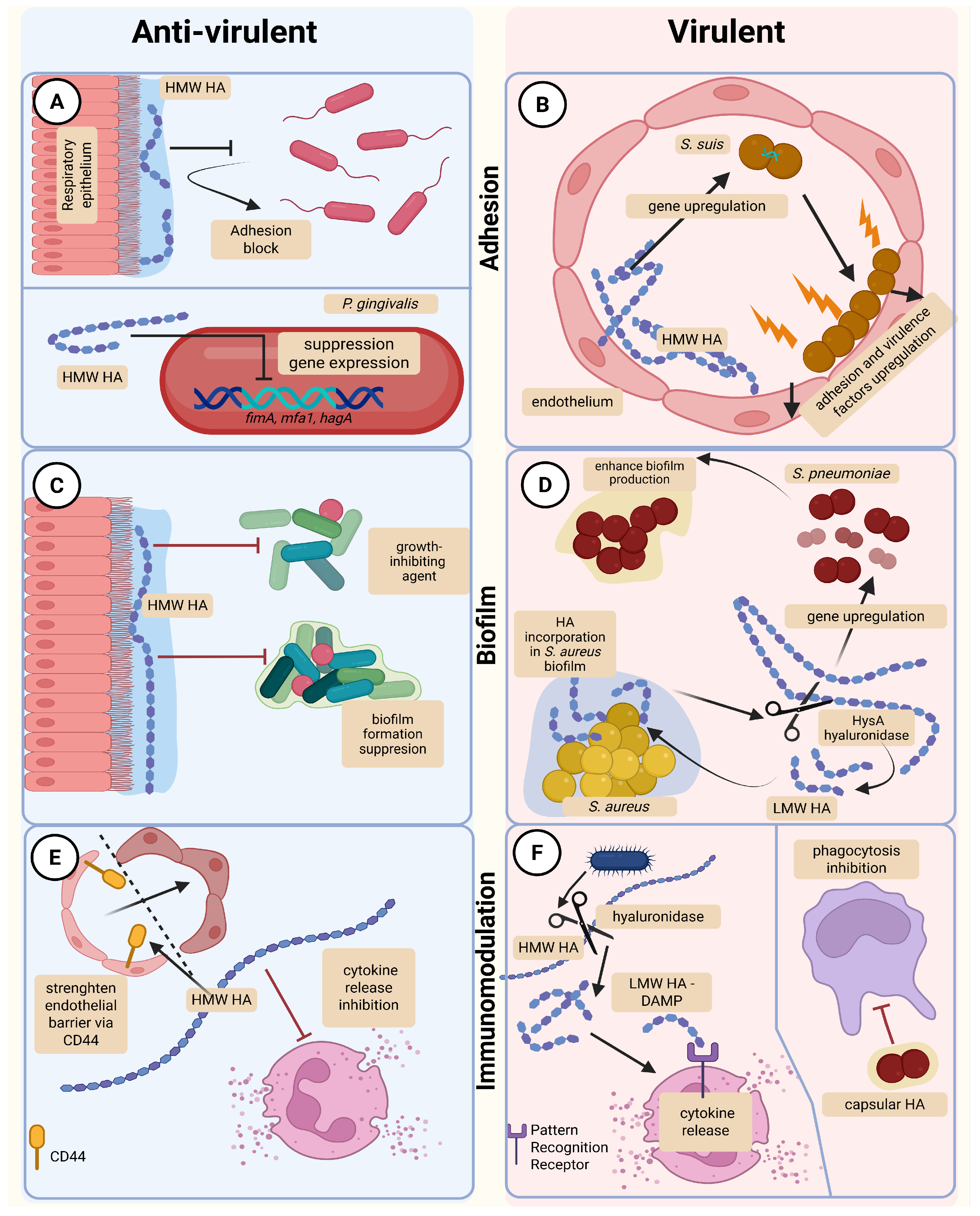
4. Microbial Hyaluronic Acid and Hyaluronic Acid-Degrading Enzymes as Potentiators of Microbial Virulence
4.1. Bacterial Enzymes Degrading Host Hyaluronic Acid
4.2. Bacterial Hyaluronic Acid in Biofilm Formation
4.3. Bacterial Hyaluronic Acid in Immune Evasion
5. Anti-Microbial and Anti-Virulent Activity of Hyaluronic Acid
5.1. The Direct Antimicrobial Activity of Hyaluronic Acid
5.2. The Indirect Immunomodulatory Antimicrobial Activity of Hyaluronic Acid
6. Hyaluronic Acid in Medical Practice
7. Potential Complications Resulting from the Use of Hyaluronic Acid
7.1. General Safety Profile of Hyaluronic Acid
7.2. Allergic Reactions and Contaminants
7.3. Early and Late Procedure-Related Complications
7.4. Biofilm-Associated Infections and Their Microbiology
7.5. Diagnostic Challenges in Biofilm-Related Infections
7.6. Contraindications and Patient Selection
7.7. Management of Suspected Infectious or Biofilm-Related Complications
7.8. Prevention Strategies
7.9. Novel HA Conjugates and Advanced Biomaterials
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HA | hyaluronic acid, hyaluronan |
| GAGs | glycosaminoglycans |
| CaMKKβ | calcium-dependent kinases |
| AMPKα | AMP-activated protein kinase |
| ULK1 | autophagy regulatory proteins |
| RHAMM | receptor for HA-mediated motility |
| LYVE-1 | lymphatic vessel endothelial HA receptor 1 |
| TSG-6 | TNF-stimulated gene 6 |
| ECM | extracellular matrix |
| HasA, HAS | hyaluronan synthase |
| HYAL | hyaluronidase |
| ROS | reactive oxygen species |
| T6SS | type VI secretion system |
| LMW HA | low-molecular-weight hyaluronic acid |
| DAMP | danger-associated molecular pattern |
| HysA | hyaluronidase |
| PRRs | phagocytic cell receptors |
| PAMPs | pathogen associated molecular patterns |
| CS | chondroitin sulfate |
| HMW HA | high-molecular-weight hyaluronic acid |
| TLR2 | toll-like receptor 2 |
| TLR4 | toll-like receptor 4 |
| MMP9 | metalloproteinase 9 |
| HBD2 | human β-defensin-2 |
| TEWL | trans epidermal water loss |
References
- Volpi, N.; Maccari, F. Purification and Characterization of Hyaluronic Acid from the Mollusc Bivalve Mytilus galloprovincialis. Biochimie 2003, 85, 619–625. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, J.D.; Carvalho, L.S.; Gomes, A.M.V.; Queiroz, L.R.; Magalhães, B.S.; Parachin, N.S. Genetic Basis for Hyper Production of Hyaluronic Acid in Natural and Engineered Microorganisms. Microb. Cell Fact. 2016, 15, 119. [Google Scholar] [CrossRef]
- DeAngelis, P.L. Hyaluronan Synthases: Fascinating Glycosyltransferases from Vertebrates, Bacterial Pathogens, and Algal Viruses. Cell Mol. Life Sci. 1999, 56, 670–682. [Google Scholar] [CrossRef]
- DeAngelis, P.L.; Jing, W.; Drake, R.R.; Achyuthan, A.M. Identification and Molecular Cloning of a Unique Hyaluronan Synthase from Pasteurella multocida. J. Biol. Chem. 1998, 273, 8454–8458. [Google Scholar] [CrossRef]
- Kogan, G.; Šoltés, L.; Stern, R.; Gemeiner, P. Hyaluronic Acid: A Natural Biopolymer with a Broad Range of Biomedical and Industrial Applications. Biotechnol. Lett. 2006, 29, 17–25. [Google Scholar] [CrossRef]
- Abatangelo, G.; Vindigni, V.; Avruscio, G.; Pandis, L.; Brun, P. Hyaluronic Acid: Redefining Its Role. Cells 2020, 9, 1743. [Google Scholar] [CrossRef]
- DeAngelis, P.L.; Zimmer, J. Hyaluronan Synthases; Mechanisms, Myths, & Mysteries of Three Types of Unique Bifunctional Glycosyltransferases. Glycobiology 2023, 33, 1117–1127. [Google Scholar] [CrossRef]
- Garantziotis, S.; Savani, R.C. Hyaluronan Biology: A Complex Balancing Act of Structure, Function, Location and Context. Matrix Biol. 2019, 78–79, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Iaconisi, G.N.; Lunetti, P.; Gallo, N.; Cappello, A.R.; Fiermonte, G.; Dolce, V.; Capobianco, L. Hyaluronic Acid: A Powerful Biomolecule with Wide-Ranging Applications—A Comprehensive Review. Int. J. Mol. Sci. 2023, 24, 10296. [Google Scholar] [CrossRef] [PubMed]
- Valachová, K.; Hassan, M.E.; Šoltés, L. Hyaluronan: Sources, Structure, Features and Applications. Molecules 2024, 29, 739. [Google Scholar] [CrossRef] [PubMed]
- Serra, M.; Casas, A.; Toubarro, D.; Barros, A.N.; Teixeira, J.A. Microbial Hyaluronic Acid Production: A Review. Molecules 2023, 28, 2084. [Google Scholar] [CrossRef] [PubMed]
- Meyer, K.; Palmer, J.W. The Polysaccharide of the Vitreous Humor. J. Biol. Chem. 1934, 107, 629–634. [Google Scholar] [CrossRef]
- Girish, K.S.; Kemparaju, K. The Magic Glue Hyaluronan and Its Eraser Hyaluronidase: A Biological Overview. Life Sci. 2007, 80, 1921–1943. [Google Scholar] [CrossRef] [PubMed]
- Knopf-Marques, H.; Pravda, M.; Wolfova, L.; Velebny, V.; Schaaf, P.; Vrana, N.E.; Lavalle, P. Hyaluronic Acid and Its Derivatives in Coating and Delivery Systems: Applications in Tissue Engineering, Regenerative Medicine and Immunomodulation. Adv. Healthc. Mater. 2016, 5, 2841–2855. [Google Scholar] [CrossRef]
- Lierova, A.; Kasparova, J.; Filipova, A.; Cizkova, J.; Pekarova, L.; Korecka, L.; Mannova, N.; Bilkova, Z.; Sinkorova, Z. Hyaluronic Acid: Known for Almost a Century, but Still in Vogue. Pharmaceutics 2022, 14, 838. [Google Scholar] [CrossRef]
- Zhang, X.; Zhong, Y.; Miao, Z.; Yang, Q. Hyaluronic Acid Promotes Hepatocellular Carcinoma Proliferation by Upregulating CD44 Expression and Enhancing Glucose Metabolism Flux. Int. Immunopharmacol. 2025, 147, 114035. [Google Scholar] [CrossRef]
- Rügheimer, L.; Williams, J.C.; Evan, A.P.; Lingeman, J.E.; McAteer, J.A. Hyaluronan: A Matrix Component. In Proceedings of the AIP Conference Proceedings, Indianapolis, IN, USA, 17–18 April 2008; AIP: New York, NY, USA, 2008; pp. 126–132. [Google Scholar]
- Kablik, J.; Monheit, G.D.; Yu, L.; Chang, G.; Gershkovich, J. Comparative Physical Properties of Hyaluronic Acid Dermal Fillers. Dermatol. Surg. 2009, 35, 302–312. [Google Scholar] [CrossRef]
- Sobolewski, K. Structure of HA Polymer. 2025. Available online: https://BioRender.com/b69xsda (accessed on 25 November 2025).
- Jeerawattanawart, S.; Hansakon, A.; Alberts, R.; Zhang, Y.; Angkasekwinai, P. Transcriptomic Profiling of Lung Alveolar Macrophages Reveals Distinct Contribution of Sterol Metabolism in Macrophage Response to Cryptococcus gattii Infection. PLoS ONE 2025, 20, e0333090. [Google Scholar] [CrossRef]
- Itano, N.; Kimata, K. Mammalian Hyaluronan Synthases. IUBMB Life 2002, 54, 195–199. [Google Scholar] [CrossRef]
- Fallacara, A.; Baldini, E.; Manfredini, S.; Vertuani, S. Hyaluronic Acid in the Third Millennium. Polymers 2018, 10, 701. [Google Scholar] [CrossRef]
- Zhang, Y.-H.; Sun, X.-T.; Guo, R.-F.; Feng, G.-Y.; Gao, H.-L.; Zhong, M.-L.; Tian, L.-W.; Qiu, Z.-Y.; Cui, Y.-W.; Li, J.-Y.; et al. AβPP-Tau-HAS1 Axis Trigger HAS1-Related Nuclear Speckles and Gene Transcription in Alzheimer’s Disease. Matrix Biol. 2024, 129, 29–43. [Google Scholar] [CrossRef]
- Zamboni, F.; Wong, C.K.; Collins, M.N. Hyaluronic Acid Association with Bacterial, Fungal and Viral Infections: Can Hyaluronic Acid Be Used as an Antimicrobial Polymer for Biomedical and Pharmaceutical Applications? Bioact. Mater. 2023, 19, 458–473. [Google Scholar] [CrossRef]
- Weigel, P.H. Hyaluronan Synthase: The Mechanism of Initiation at the Reducing End and a Pendulum Model for Polysaccharide Translocation to the Cell Exterior. Int. J. Cell Biol. 2015, 2015, 367579. [Google Scholar] [CrossRef]
- Wessels, M.R. Capsular Polysaccharide of Group A Streptococcus. Microbiol. Spectr. 2019, 7, 1–12. [Google Scholar] [CrossRef]
- Blackburn, M.R.; Hubbard, C.; Kiessling, V.; Bi, Y.; Kloss, B.; Tamm, L.K.; Zimmer, J. Distinct Reaction Mechanisms for Hyaluronan Biosynthesis in Different Kingdoms of Life. Glycobiology 2018, 28, 108–121. [Google Scholar] [CrossRef] [PubMed]
- Pasomboon, P.; Chumnanpuen, P.; E-kobon, T. Comparison of Hyaluronic Acid Biosynthetic Genes From Different Strains of Pasteurella multocida. Bioinform. Biol. Insights 2021, 15, 1–14. [Google Scholar] [CrossRef]
- van der Vlist, J.; Loos, K. Transferases in Polymer Chemistry. In Enzymatic Polymerisation; Springer: Berlin/Heidelberg, Germany, 2010; pp. 21–54. [Google Scholar]
- Tsepilov, R.N.; Beloded, A.V. Hyaluronic Acid—An “Old” Molecule with “New” Functions: Biosynthesis and Depolymerization of Hyaluronic Acid in Bacteria and Vertebrate Tissues Including during Carcinogenesis. Biochemistry 2015, 80, 1093–1108. [Google Scholar] [CrossRef] [PubMed]
- Csoka, A.B.; Frost, G.I.; Stern, R. The Six Hyaluronidase-like Genes in the Human and Mouse Genomes. Matrix Biol. 2001, 20, 499–508. [Google Scholar] [CrossRef]
- Bao, X.; Ran, J.; Kong, C.; Wan, Z.; Wang, J.; Yu, T.; Ruan, S.; Ding, W.; Xia, L.; Zhang, D. Pan-Cancer Analysis Reveals the Potential of Hyaluronate Synthase as Therapeutic Targets in Human Tumors. Heliyon 2023, 9, e19112. [Google Scholar] [CrossRef] [PubMed]
- Stern, R.; Jedrzejas, M.J. Hyaluronidases: Their Genomics, Structures, and Mechanisms of Action. Chem. Rev. 2006, 106, 818–839. [Google Scholar] [CrossRef]
- Heldin, P.; Lin, C.-Y.; Kolliopoulos, C.; Chen, Y.-H.; Skandalis, S.S. Regulation of Hyaluronan Biosynthesis and Clinical Impact of Excessive Hyaluronan Production. Matrix Biol. 2019, 78–79, 100–117. [Google Scholar] [CrossRef]
- Adamia, S.; Maxwell, C.; Pilarski, L. Hyaluronan and Hyaluronan Synthases: Potential Therapeutic Targets in Cancer. Curr. Drug Target.-Cardiovasc. Hematol. Disord. 2005, 5, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Tian, X.; Lu, J.Y.; Boit, K.; Ablaeva, J.; Zakusilo, F.T.; Emmrich, S.; Firsanov, D.; Rydkina, E.; Biashad, S.A.; et al. Increased Hyaluronan by Naked Mole-Rat Has2 Improves Healthspan in Mice. Nature 2023, 621, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Sánchez-Rivera, F.J.; He, L.; Basnet, H.; Chen, F.X.; Spina, E.; Li, L.; Torner, C.; Chan, J.E.; Yarlagadda, D.V.K.; et al. TGF-β and RAS Jointly Unmask Primed Enhancers to Drive Metastasis. Cell 2024, 187, 6182–6199.e29. [Google Scholar] [CrossRef]
- Fink, S.P.; Triggs-Raine, B. Genetic Deficiencies of Hyaluronan Degradation. Cells 2024, 13, 1203. [Google Scholar] [CrossRef]
- Šoltés, L.; Mendichi, R.; Kogan, G.; Schiller, J.; Stankovská, M.; Arnhold, J. Degradative Action of Reactive Oxygen Species on Hyaluronan. Biomacromolecules 2006, 7, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Cherr, G.N.; Yudin, A.I.; Overstreet, J.W. The Dual Functions of GPI-Anchored PH-20: Hyaluronidase and Intracellular Signaling. Matrix Biol. 2001, 20, 515–525. [Google Scholar] [CrossRef]
- Lepperdinger, G.; Strobl, B.; Kreil, G. HYAL2, a Human Gene Expressed in Many Cells, Encodes a Lysosomal Hyaluronidase with a Novel Type of Specificity. J. Biol. Chem. 1998, 273, 22466–22470. [Google Scholar] [CrossRef]
- Monzon, M.E.; Fregien, N.; Schmid, N.; Falcon, N.S.; Campos, M.; Casalino-Matsuda, S.M.; Forteza, R.M. Reactive Oxygen Species and Hyaluronidase 2 Regulate Airway Epithelial Hyaluronan Fragmentation. J. Biol. Chem. 2010, 285, 26126–26134. [Google Scholar] [CrossRef]
- Stern, R.; Kogan, G.; Jedrzejas, M.J.; Šoltés, L. The Many Ways to Cleave Hyaluronan. Biotechnol. Adv. 2007, 25, 537–557. [Google Scholar] [CrossRef]
- Sprott, H.; Fleck, C. Hyaluronic Acid in Rheumatology. Pharmaceutics 2023, 15, 2247. [Google Scholar] [CrossRef]
- Smallman, T.R.; Williams, G.C.; Harper, M.; Boyce, J.D. Genome-Wide Investigation of Pasteurella Multocida Identifies the Stringent Response as a Negative Regulator of Hyaluronic Acid Capsule Production. Microbiol. Spectr. 2022, 10, e00195-22. [Google Scholar] [CrossRef]
- Yadav, M.K.; Chae, S.-W.; Park, K.; Song, J.-J. Hyaluronic Acid Derived from Other Streptococci Supports Streptococcus pneumoniae In Vitro Biofilm Formation. Biomed. Res. Int. 2013, 2013, 690217. [Google Scholar] [CrossRef] [PubMed]
- Hurst, J.R.; Shannon, B.A.; Craig, H.C.; Rishi, A.; Tuffs, S.W.; McCormick, J.K. The Streptococcus Pyogenes Hyaluronic Acid Capsule Promotes Experimental Nasal and Skin Infection by Preventing Neutrophil-Mediated Clearance. PLoS Pathog. 2022, 18, e1011013. [Google Scholar] [CrossRef]
- Gunasekaran, V.; Gowdhaman, D.; Ponnusami, V. Role of Membrane Proteins in Bacterial Synthesis of Hyaluronic Acid and Their Potential in Industrial Production. Int. J. Biol. Macromol. 2020, 164, 1916–1926. [Google Scholar] [CrossRef]
- Midgley, A.C.; Bowen, T. Analysis of Human Hyaluronan Synthase Gene Transcriptional Regulation and Downstream Hyaluronan Cell Surface Receptor Mobility in Myofibroblast Differentiation. In Glycosaminoglycans: Chemistry and Biology; Springer: New York, NY, USA, 2022; pp. 453–468. [Google Scholar]
- Romanò, C.L.; Vecchi, E.D.; Bortolin, M.; Morelli, I.; Drago, L. Hyaluronic Acid and Its Composites as a Local Antimicrobial/Antiadhesive Barrier. J. Bone Jt. Infect. 2017, 2, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Sobolewski, K. Biosynthesis Pathway of Hyaluronic Acid in Bacteria. 2025. Available online: https://BioRender.com/isoncbg (accessed on 25 November 2025).
- Jong, A.; Wu, C.-H.; Chen, H.-M.; Luo, F.; Kwon-Chung, K.J.; Chang, Y.C.; LaMunyon, C.W.; Plaas, A.; Huang, S.-H. Identification and Characterization of CPS1 as a Hyaluronic Acid Synthase Contributing to the Pathogenesis of Cryptococcus neoformans Infection. Eukaryot. Cell 2007, 6, 1486–1496. [Google Scholar] [CrossRef] [PubMed]
- Bobkova, L.; Smirnou, D.; Krcmar, M.; Kulhanek, J.; Hermannova, M.; Franke, L.; Velebny, V. Discovery and Characteristic of Hyaluronidases from Filamentous Fungi. Curr. Biotechnol. 2018, 7, 2–9. [Google Scholar] [CrossRef]
- Jamal, M.; Ahmad, W.; Andleeb, S.; Jalil, F.; Imran, M.; Nawaz, M.A.; Hussain, T.; Ali, M.; Rafiq, M.; Kamil, M.A. Bacterial Biofilm and Associated Infections. J. Chin. Med. Assoc. 2018, 81, 7–11. [Google Scholar] [CrossRef]
- Sobolewski, K. Effects of High- and Low-Molecular-Weight Hyaluronic Acid on Bacterial Virulence and Host Defense. 2025. Available online: https://BioRender.com/iqmhy1r (accessed on 25 November 2025).
- Yusof, H.A.; Desa, M.N.; Masri, S.N.; Malina, O.; Jamal, F. Hyaluronatelyase Production by Streptococcus Pneumoniae Isolated from Patients and Carriers. Trop. Biomed. 2015, 32, 413–418. [Google Scholar]
- Rehan, A.M.; Othman, M.I.E.; Amin, N.M.M.; Shahdan, I.A.; Yusof@Hanafi, H.A. PCR-based Construction of CodY Gene Deletion Mutants from Streptococcus pneumoniae. Int. J. Emerg. Trends Eng. Res. 2020, 8, 98–107. [Google Scholar] [CrossRef]
- Kumon, T.; Oiki, S.; Hashimoto, W. Molecular Identification of Hyaluronate Lyase, Not Hyaluronidase, as an Intrinsic Hyaluronan-Degrading Enzyme in Clostridium Perfringens Strain ATCC 13124. Sci. Rep. 2024, 14, 24266. [Google Scholar] [CrossRef] [PubMed]
- Haas, B.; Vaillancourt, K.; Bonifait, L.; Gottschalk, M.; Grenier, D. Hyaluronate Lyase Activity of Streptococcus Suis Serotype 2 and Modulatory Effects of Hyaluronic Acid on the Bacterium’s Virulence Properties. BMC Res. Notes 2015, 8, 722. [Google Scholar] [CrossRef] [PubMed]
- Wojtovich, A.P.; Berry, B.J.; Galkin, A. Redox Signaling Through Compartmentalization of Reactive Oxygen Species: Implications for Health and Disease. Antioxid. Redox Signal. 2019, 31, 591–593. [Google Scholar] [CrossRef]
- Eble, J.A.; de Rezende, F.F. Redox-Relevant Aspects of the Extracellular Matrix and Its Cellular Contacts via Integrins. Antioxid. Redox Signal 2014, 20, 1977–1993. [Google Scholar] [CrossRef]
- Meitzler, J.L.; Antony, S.; Wu, Y.; Juhasz, A.; Liu, H.; Jiang, G.; Lu, J.; Roy, K.; Doroshow, J.H. NADPH Oxidases: A Perspective on Reactive Oxygen Species Production in Tumor Biology. Antioxid. Redox Signal. 2014, 20, 2873–2889. [Google Scholar] [CrossRef] [PubMed]
- Myint, P.; Deeble, D.; Beaumont, P.; Blake, S.; Phillips, G. The Reactivity of Various Free Radicals with Hyaluronic Acid: Steady-State and Pulse Radiolysis Studies. Biochim. Biophys. Acta (BBA)-Gen. Subj. 1987, 925, 194–202. [Google Scholar] [CrossRef]
- Rees, M.D.; Hawkins, C.L.; Davies, M.J. Hypochlorite and Superoxide Radicals Can Act Synergistically to Induce Fragmentation of Hyaluronan and Chondroitin Sulphates. Biochem. J. 2004, 381, 175–184. [Google Scholar] [CrossRef]
- Sokolowska, M.; Chen, L.-Y.; Eberlein, M.; Martinez-Anton, A.; Liu, Y.; Alsaaty, S.; Qi, H.-Y.; Logun, C.; Horton, M.; Shelhamer, J.H. Low Molecular Weight Hyaluronan Activates Cytosolic Phospholipase A2α and Eicosanoid Production in Monocytes and Macrophages. J. Biol. Chem. 2014, 289, 4470–4488. [Google Scholar] [CrossRef]
- Lee, C.-H.; Choi, E.Y. Macrophages and Inflammation. J. Rheum. Dis. 2018, 25, 11. [Google Scholar] [CrossRef]
- Li, Z.; Nagy, N.; Kaber, G.; Marshall, P.L.; Haddock, N.L.; Meyer, E.; Vernon, R.R.; Butte, M.J.; Chaudhuri, O.; Wight, T.N.; et al. Hyaluronan Accumulates in Inflamed Lymph Nodes and Promotes B-Cell Activation. Proteoglycan Res. 2025, 3, e70031. [Google Scholar] [CrossRef]
- Ibberson, C.B.; Parlet, C.P.; Kwiecinski, J.; Crosby, H.A.; Meyerholz, D.K.; Horswill, A.R. Hyaluronan Modulation Impacts Staphylococcus Aureus Biofilm Infection. Infect. Immun. 2016, 84, 1917–1929. [Google Scholar] [CrossRef]
- Rahman, M.A.; Amirkhani, A.; Chowdhury, D.; Vickery, K.; Hu, H. Comparison of the Proteome of Staphylococcus aureus Planktonic Culture and 3-Day Biofilm Reveals Potential Role of Key Proteins in Biofilm. Hygiene 2024, 4, 238–257. [Google Scholar] [CrossRef]
- Suaidan, M.A.; Mohd Amin, N.M.; Enche Othman, M.I.; Mohd Nayian, N.A.; Abu Mansor, A.Z.; Ahmay Yusof, H. Hyaluronidase Involvement in Streptococcus pneumoniae Biofilm Activity. ASM Sci. J. 2024, 19, 1–7. [Google Scholar] [CrossRef]
- Zaragoza, O. Basic Principles of the Virulence of Cryptococcus. Virulence 2019, 10, 490–501. [Google Scholar] [CrossRef]
- Fehervari, Z. Evading Hyaluronan Danger. Nat. Immunol. 2016, 17, 121. [Google Scholar] [CrossRef]
- Santambrogio, L.; Clement, C.; Osan, J.; Becerra, A.; Mota, I.; Nanaware, P.; Franco, A.; Stern, L. Molecular Mimicry in Pathogen Immune Evasion. J. Immunol. 2024, 212, 1438–4569. [Google Scholar] [CrossRef]
- Mondino, S.; Schmidt, S.; Buchrieser, C. Molecular Mimicry: A Paradigm of Host-Microbe Coevolution Illustrated by Legionella. mBio 2020, 11, e01201-20. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Morita, N.; Shinkura, R. Role of Mucosal IgA Antibodies as Novel Therapies to Enhance Mucosal Barriers. Semin. Immunopathol. 2025, 47, 1. [Google Scholar] [CrossRef] [PubMed]
- Wayan Teguh Wibawan, I.; Pasaribu, F.H.; Utama, I.H.; Abdulmawjood, A.; Lämmler, C. The Role of Hyaluronic Acid Capsular Material of Streptococcus equi Subsp. Zooepidemicus in Mediating Adherence to HeLa Cells and in Resisting Phagocytosis. Res. Vet. Sci. 1999, 67, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Kass, E.H.; Seastone, C.V. The role of the mucoid polysaccharide (hyaluronic acid) in the virulence of group A hemolytic streptococci. J. Exp. Med. 1944, 79, 319–330. [Google Scholar] [CrossRef]
- Dale, J.B.; Washburn, R.G.; Marques, M.B.; Wessels, M.R. Hyaluronate Capsule and Surface M Protein in Resistance to Opsonization of Group A Streptococci. Infect. Immun. 1996, 64, 1495–1501. [Google Scholar] [CrossRef] [PubMed]
- Moses, A.E.; Wessels, M.R.; Zalcman, K.; Albertí, S.; Natanson-Yaron, S.; Menes, T.; Hanski, E. Relative Contributions of Hyaluronic Acid Capsule and M Protein to Virulence in a Mucoid Strain of the Group A Streptococcus. Infect. Immun. 1997, 65, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Lin, L.; Zang, D.; Guo, X.; Liu, M. Designing Bioinspired Anti-Biofouling Surfaces Based on a Superwettability Strategy. Small 2017, 13, 1503334. [Google Scholar] [CrossRef]
- Xia, Y.; Adibnia, V.; Shan, C.; Huang, R.; Qi, W.; He, Z.; Xie, G.; Olszewski, M.; De Crescenzo, G.; Matyjaszewski, K.; et al. Synergy between Zwitterionic Polymers and Hyaluronic Acid Enhances Antifouling Performance. Langmuir 2019, 35, 15535–15542. [Google Scholar] [CrossRef] [PubMed]
- Drago, L.; Cappelletti, L.; De Vecchi, E.; Pignataro, L.; Torretta, S.; Mattina, R. Antiadhesive and Antibiofilm Activity of Hyaluronic Acid against Bacteria Responsible for Respiratory Tract Infections. APMIS 2014, 122, 1013–1019. [Google Scholar] [CrossRef]
- Ciani, O.; Arendsen, E.; Romancik, M.; Lunik, R.; Costantini, E.; Di Biase, M.; Morgia, G.; Fragalà, E.; Roman, T.; Bernat, M.; et al. Intravesical Administration of Combined Hyaluronic Acid (HA) and Chondroitin Sulfate (CS) for the Treatment of Female Recurrent Urinary Tract Infections: A European Multicentre Nested Case–Control Study. BMJ Open 2016, 6, e009669. [Google Scholar] [CrossRef]
- Parolin, C.; Abruzzo, A.; Giordani, B.; Oliver, J.C.; Marangoni, A.; Luppi, B.; Vitali, B. Anti-Candida Activity of Hyaluronic Acid Combined with Lactobacillus crispatus Lyophilised Supernatant: A New Antifungal Strategy. Antibiotics 2021, 10, 628. [Google Scholar] [CrossRef]
- Shukla, P.; Srivastava, P.; Mishra, A. On the Potential Activity of Hyaluronic Acid as an Antimicrobial Agent: Experimental and Computational Validations. Bioprocess. Biosyst. Eng. 2025, 48, 27–42. [Google Scholar] [CrossRef]
- Alharbi, M.S.; Alshehri, F.A. High Molecular Weight Hyaluronic Acid Reduces the Expression of Virulence Genes FimA, Mfa1, HagA, RgpA, and Kgp in the Oral Pathogen Porphyromonas gingivalis. Pharmaceutics 2022, 14, 1628. [Google Scholar] [CrossRef]
- Pirnazar, P.; Wolinsky, L.; Nachnani, S.; Haake, S.; Pilloni, A.; Bernard, G.W. Bacteriostatic Effects of Hyaluronic Acid. J. Periodontol. 1999, 70, 370–374. [Google Scholar] [CrossRef]
- Weindl, G.; Schaller, M.; Schäfer-Korting, M.; Korting, H.C. Hyaluronic Acid in the Treatment and Prevention of Skin Diseases: Molecular Biological, Pharmaceutical and Clinical Aspects. Ski. Pharmacol. Physiol. 2004, 17, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Petrey, A.C.; de la Motte, C.A. Hyaluronan, a Crucial Regulator of Inflammation. Front. Immunol. 2014, 5, 101. [Google Scholar] [CrossRef] [PubMed]
- Singleton, P.A.; Mirzapoiazova, T.; Guo, Y.; Sammani, S.; Mambetsariev, N.; Lennon, F.E.; Moreno-Vinasco, L.; Garcia, J.G.N. High-Molecular-Weight Hyaluronan Is a Novel Inhibitor of Pulmonary Vascular Leakiness. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2010, 299, L639–L651. [Google Scholar] [CrossRef]
- Kenji, T.; Tadashi, Y.; Takao, A.; Masahiro, H.; Noriko, M.; Masaki, S.; Itsuhiko, M. Inhibitory Effects of Hyaluronan on [14C]Arachidonic Acid Release from Labeled Human Synovial Fibroblasts. Jpn. J. Pharmacol. 1992, 60, 79–84. [Google Scholar] [CrossRef]
- Scheibner, K.A.; Lutz, M.A.; Boodoo, S.; Fenton, M.J.; Powell, J.D.; Horton, M.R. Hyaluronan Fragments Act as an Endogenous Danger Signal by Engaging TLR2. J. Immunol. 2006, 177, 1272–1281. [Google Scholar] [CrossRef] [PubMed]
- Gunasekaran, A.; Eckert, J.; Burge, K.; Zheng, W.; Yu, Z.; Kessler, S.; de la Motte, C.; Chaaban, H. Hyaluronan 35 KDa Enhances Epithelial Barrier Function and Protects against the Development of Murine Necrotizing Enterocolitis. Pediatr. Res. 2020, 87, 1177–1184. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, D.; Liang, J.; Meltzer, E.B.; Gray, A.; Miura, R.; Wogensen, L.; Yamaguchi, Y.; Noble, P.W. Severe Lung Fibrosis Requires an Invasive Fibroblast Phenotype Regulated by Hyaluronan and CD44. J. Exp. Med. 2011, 208, 1459–1471. [Google Scholar] [CrossRef]
- Hill, D.R.; Kessler, S.P.; Rho, H.K.; Cowman, M.K.; de la Motte, C.A. Specific-Sized Hyaluronan Fragments Promote Expression of Human β-Defensin 2 in Intestinal Epithelium. J. Biol. Chem. 2012, 287, 30610–30624. [Google Scholar] [CrossRef]
- Routsias, J.G.; Karagounis, P.; Parvulesku, G.; Legakis, N.J.; Tsakris, A. In Vitro Bactericidal Activity of Human β-Defensin 2 against Nosocomial Strains. Peptides 2010, 31, 1654–1660. [Google Scholar] [CrossRef]
- Parducho, K.R.; Beadell, B.; Ybarra, T.K.; Bush, M.; Escalera, E.; Trejos, A.T.; Chieng, A.; Mendez, M.; Anderson, C.; Park, H.; et al. The Antimicrobial Peptide Human Beta-Defensin 2 Inhibits Biofilm Production of Pseudomonas Aeruginosa Without Compromising Metabolic Activity. Front. Immunol. 2020, 11, 805. [Google Scholar] [CrossRef] [PubMed]
- Fusco, A.; Savio, V.; Cammarota, M.; Alfano, A.; Schiraldi, C.; Donnarumma, G. Beta-Defensin-2 and Beta-Defensin-3 Reduce Intestinal Damage Caused by Salmonella typhimurium Modulating the Expression of Cytokines and Enhancing the Probiotic Activity of Enterococcus faecium. J. Immunol. Res. 2017, 2017, 6976935. [Google Scholar] [CrossRef]
- Soto, E.; Espinoza, J.; Nien, J.K.; Kusanovic, J.P.; Erez, O.; Richani, K.; Santolaya-Forgas, J.; Romero, R. Human β-Defensin-2: A Natural Antimicrobial Peptide Present in Amniotic Fluid Participates in the Host Response to Microbial Invasion of the Amniotic Cavity. J. Matern.-Fetal Neonatal Med. 2007, 20, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Pavicic, T.; Gauglitz, G.G.; Lersch, P.; Schwach-Abdellaoui, K.; Malle, B.; Korting, H.C.; Farwick, M. Efficacy of Cream-Based Novel Formulations of Hyaluronic Acid of Different Molecular Weights in Anti-Wrinkle Treatment. J. Drugs Dermatol. 2011, 10, 990–1000. [Google Scholar]
- Farwick, M.; Gauglitz, G.; Pavicic, T.; Köhler, T.; Wegmann, M.; Schwach-Abdellaoui, K.; Malle, B.; Tarabin, V.; Schmitz, G.; Korting, H.C. Fifty-KDa Hyaluronic Acid Upregulates Some Epidermal Genes without Changing TNF-α Expression in Reconstituted Epidermis. Ski. Pharmacol. Physiol. 2011, 24, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.; Jones, S. Hyaluronic Acid: A Unique Topical Vehicle for the Localized Delivery of Drugs to the Skin. J. Eur. Acad. Dermatol. Venereol. 2005, 19, 308–318. [Google Scholar] [CrossRef]
- Rzany, B.; Cartier, H.; Kestemont, P.; Trevidic, P.; Sattler, G.; Kerrouche, N.; Dhuin, J.-C.; Ma, M.Y. Full-Face Rejuvenation Using a Range of Hyaluronic Acid Fillers: Efficacy, Safety, and Patient Satisfaction over 6 Months. Dermatol. Surg. 2012, 38, 1153–1161. [Google Scholar] [CrossRef]
- Ulf, B. Impaired Reparative Processes in Particular Related to Hyaluronan in Various Cutaneous Disorders: A Structural Analysis. Ph.D. Thesis, Umeå University, Umeå, Sweden, 2004. [Google Scholar]
- Sudhakar, K.; Ji, S.M.; Kummara, M.R.; Han, S.S. Recent Progress on Hyaluronan-Based Products for Wound Healing Applications. Pharmaceutics 2022, 14, 2235. [Google Scholar] [CrossRef]
- Pecová, J.; Rohlíková, V.; Šmoldasová, M.; Marek, J. Clinical Efficacy of Hyaluronic Acid with Iodine in Hard-to-Heal Wounds. Pharmaceutics 2023, 15, 2268. [Google Scholar] [CrossRef]
- Yang, H.; Song, L.; Zou, Y.; Sun, D.; Wang, L.; Yu, Z.; Guo, J. Role of Hyaluronic Acids and Potential as Regenerative Biomaterials in Wound Healing. ACS Appl. Bio Mater. 2021, 4, 311–324. [Google Scholar] [CrossRef]
- Ding, X.; Zhu, Y.; Zuo, Y.; Wang, L.; Huang, Q.; Zhang, X.; Xu, H.; Liu, C. Functional Antibacterial Strategies of Natural Polysaccharides Chitosan, Hyaluronic Acid, and Alginate and Their Applications in Tissue Engineering: A Review. Int. J. Biol. Macromol. 2025, 328, 147609. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Zi, L.; Cen, Y.; You, C.; Tian, M. Copper Sulfide Nanoparticles-Incorporated Hyaluronic Acid Injectable Hydrogel With Enhanced Angiogenesis to Promote Wound Healing. Front. Bioeng. Biotechnol. 2020, 8, 417. [Google Scholar] [CrossRef] [PubMed]
- Sierra-Sánchez, Á.; Fernández-González, A.; Lizana-Moreno, A.; Espinosa-Ibáñez, O.; Martinez-Lopez, A.; Guerrero-Calvo, J.; Fernández-Porcel, N.; Ruiz-García, A.; Ordóñez-Luque, A.; Carriel, V.; et al. Hyaluronic Acid Biomaterial for Human Tissue-engineered Skin Substitutes: Preclinical Comparative in Vivo Study of Wound Healing. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 2414–2427. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Liu, Y.; Liu, X.; Guo, Y.; Liu, J.; Ding, J.; Zhang, Z.; Ni, X.; Chen, Y. Hyaluronic Acid-Modified and Verteporfin-Loaded Polylactic Acid Nanogels Promote Scarless Wound Healing by Accelerating Wound Re-Epithelialization and Controlling Scar Formation. J. Nanobiotechnol. 2023, 21, 241. [Google Scholar] [CrossRef]
- He, M.; Huang, Y.; Wang, J.; Chen, Z.; Xie, J.; Cui, Z.; Xu, D.; Zhang, X.; Yao, W. Advances in Polysaccharide-Based Antibacterial Materials. Int. J. Biol. Macromol. 2025, 308, 142598. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhao, Y.; Li, Z.; Xu, M.; Lu, Y.; Li, X. Puerarin-Containing Rhein-Crosslinked Tyramine-Modified Hyaluronic Acid Hydrogel for Antibacterial and Anti-Inflammatory Wound Dressings. Int. J. Biol. Macromol. 2024, 271, 132527. [Google Scholar] [CrossRef]
- Pei, J.; Palanisamy, C.P.; Alugoju, P.; Anthikapalli, N.V.A.; Natarajan, P.M.; Umapathy, V.R.; Swamikannu, B.; Jayaraman, S.; Rajagopal, P.; Poompradub, S. A Comprehensive Review on Bio-Based Materials for Chronic Diabetic Wounds. Molecules 2023, 28, 604. [Google Scholar] [CrossRef]
- Dalmedico, M.M.; Meier, M.J.; Felix, J.V.; Pott, F.S.; Petz, F.F.; Santos, M.C. Hyaluronic Acid Covers in Burn Treatment: A Systematic Review. Rev. Esc. Enferm. USP 2016, 50, 522–528. [Google Scholar] [CrossRef]
- Woodward, J.; Khan, T.; Martin, J. Facial Filler Complications. Facial Plast. Surg. Clin. N. Am. 2015, 23, 447–458. [Google Scholar] [CrossRef]
- Draelos, Z.D.; Diaz, I.; Namkoong, J.; Wu, J.; Boyd, T. Efficacy Evaluation of a Topical Hyaluronic Acid Serum in Facial Photoaging. Dermatol. Ther. 2021, 11, 1385–1394. [Google Scholar] [CrossRef]
- Grabowski, M.; Gmyrek, D.; Żurawska, M.; Trusek, A. Hyaluronic Acid: Production Strategies, Gel-Forming Properties, and Advances in Drug Delivery Systems. Gels 2025, 11, 424. [Google Scholar] [CrossRef]
- Gomes, C.; Silva, A.C.; Marques, A.C.; Sousa Lobo, J.; Amaral, M.H. Biotechnology Applied to Cosmetics and Aesthetic Medicines. Cosmetics 2020, 7, 33. [Google Scholar] [CrossRef]
- Rowland-Warmann, M. Hypersensitivity Reaction to Hyaluronic Acid Dermal Filler Following Novel Coronavirus Infection—A Case Report. J. Cosmet. Dermatol. 2021, 20, 1557–1562. [Google Scholar] [CrossRef] [PubMed]
- Simons, F.E.R.; Frew, A.J.; Ansotegui, I.J.; Bochner, B.S.; Golden, D.B.K.; Finkelman, F.D.; Leung, D.Y.M.; Lotvall, J.; Marone, G.; Metcalfe, D.D.; et al. Risk Assessment in Anaphylaxis: Current and Future Approaches. J. Allergy Clin. Immunol. 2007, 120, S2–S24. [Google Scholar] [CrossRef]
- Bitterman-Deutsch, O.; Kogan, L.; Nasser, F. Delayed Immune Mediated Adverse Effects to Hyaluronic Acid Fillers: Report of Five Cases and Review of the Literature. Dermatol. Rep. 2015, 7, 5851. [Google Scholar] [CrossRef]
- Cohen, J.L. Understanding, Avoiding, and Managing Dermal Filler Complications. Dermatol. Surg. 2008, 34, S92–S99. [Google Scholar] [CrossRef]
- Bailey, S.H.; Cohen, J.L.; Kenkel, J.M. Etiology, Prevention, and Treatment of Dermal Filler Complications. Aesthet. Surg. J. 2011, 31, 110–121. [Google Scholar] [CrossRef]
- Alhede, M.; Bjarnsholt, T. Are Biofilms Responsible for the Adverse Effects Experienced Following Soft-Tissue Fillers? Future Microbiol. 2014, 9, 931–933. [Google Scholar] [CrossRef] [PubMed]
- Signorini, M.; Liew, S.; Sundaram, H.; De Boulle, K.L.; Goodman, G.J.; Monheit, G.; Wu, Y.; Trindade de Almeida, A.R.; Swift, A.; Vieira Braz, A. Global Aesthetics Consensus: Avoidance and Management of Complications from Hyaluronic Acid Fillers—Evidence- and Opinion-Based Review and Consensus Recommendations. Plast. Reconstr. Surg. 2016, 137, 961e–971e. [Google Scholar] [CrossRef]
- Choi, W.Y.; Cho, H.W.; Lee, D.W. Complications of Injectable Soft Tissue Filler. Arch. Aesthetic Plast. Surg. 2015, 21, 1–6. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, Z.; Hong, W.; Chen, Y.; Zhou, Y.; Luo, S. Biofilm Formation Is a Risk Factor for Late and Delayed Complications of Filler Injection. Front. Microbiol. 2024, 14, 1297948. [Google Scholar] [CrossRef] [PubMed]
- Alhede, M.; Er, Ö.; Eickhardt, S.; Kragh, K.; Alhede, M.; Christensen, L.D.; Poulsen, S.S.; Givskov, M.; Christensen, L.H.; Høiby, N.; et al. Bacterial Biofilm Formation and Treatment in Soft Tissue Fillers. Pathog. Dis. 2014, 70, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Ardizzoni, A.; Neglia, R.G.; Baschieri, M.C.; Cermelli, C.; Caratozzolo, M.; Righi, E.; Palmieri, B.; Blasi, E. Influence of Hyaluronic Acid on Bacterial and Fungal Species, Including Clinically Relevant Opportunistic Pathogens. J. Mater. Sci. Mater. Med. 2011, 22, 2329–2338. [Google Scholar] [CrossRef]
- Qi, C.; Sun, Q.; Xiao, D.; Zhang, M.; Gao, S.; Guo, B.; Lin, Y. Tetrahedral Framework Nucleic Acids/Hyaluronic Acid-Methacrylic Anhydride Hybrid Hydrogel with Antimicrobial and Anti-Inflammatory Properties for Infected Wound Healing. Int. J. Oral. Sci. 2024, 16, 30. [Google Scholar] [CrossRef]
- Țăin (Anastasiu), A.-E.; Bîrcă, A.C.; Naulea, A.M.I.; Niculescu, A.-G.; Grumezescu, A.M.; Croitoru, G.-A. Alginate/PVA Hydrogel Incorporating HA-Liposomes and Aronia-Derived Silver Nanoparticles for Advanced Wound Management. Int. J. Mol. Sci. 2025, 26, 9203. [Google Scholar] [CrossRef] [PubMed]
- Saththianathan, M.; Johani, K.; Taylor, A.; Hu, H.; Vickery, K.; Callan, P.; Deva, A.K. The Role of Bacterial Biofilm in Adverse Soft-Tissue Filler Reactions: A Combined Laboratory and Clinical Study. Plast. Reconstr. Surg. 2017, 139, 613–621. [Google Scholar] [CrossRef]
- Marusza, W.; Romuald, O.; Janusz, S.; Szyller, K.; Ostrowski, T.; Gruber-Miazga, J.; Netsvyetayeva, I. The Impact of Lifestyle upon the Probability of Late Bacterial Infection after Soft-Tissue Filler Augmentation. Infect. Drug Resist. 2019, 12, 855–863. [Google Scholar] [CrossRef]
- Haneke, E. Adverse Effects of Fillers and Their Histopathology. Facial Plast. Surg. 2014, 30, 599–614. [Google Scholar] [CrossRef]
- Kim, J.H.; Ahn, D.K.; Jeong, H.S.; Suh, I.S. Treatment Algorithm of Complications after Filler Injection: Based on Wound Healing Process. J. Korean Med. Sci. 2014, 29, S176. [Google Scholar] [CrossRef]
- Dumitraşcu, D.I.; Georgescu, A. V The Management of Biofilm Formation after Hyaluronic Acid Gel Filler Injections: A Review. Clujul Med. 2013, 86, 192–195. [Google Scholar]
- Baranska-Rybak, W.; Lajo-Plaza, J.V.; Walker, L.; Alizadeh, N. Late-Onset Reactions after Hyaluronic Acid Dermal Fillers: A Consensus Recommendation on Etiology, Prevention and Management. Dermatol. Ther. 2024, 14, 1767–1785. [Google Scholar] [CrossRef]
- Ma, T.; Zhou, J.; Li, J.; Chen, Q. Hyaluronic Acid-Modified Liposomes for Ursolic Acid-Targeted Delivery Treat Lung Cancer Based on P53/ARTS-Mediated Mitochondrial Apoptosis. Iran. J. Pharm. Res. 2023, 22, e131758. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, Y.; Li, Y.; Kang, W.; Zhang, Y.; Xia, X.; Wang, W. Notch Pathway Deactivation Sensitizes Breast Cancer Stem Cells toward Chemotherapy Using NIR Light-Responsive Nanoparticles. ACS Appl. Mater. Interfaces 2025, 17, 430–444. [Google Scholar] [CrossRef] [PubMed]
- Sansanaphongpricha, K.; Sonthithai, P.; Kaewkong, P.; Thavornyutikarn, B.; Bamrungsap, S.; Kosorn, W.; Thinbanmai, T.; Saengkrit, N. Hyaluronic Acid-Coated Gold Nanorods Enhancing BMP-2 Peptide Delivery for Chondrogenesis. Nanotechnology 2020, 31, 435101. [Google Scholar] [CrossRef] [PubMed]
- O’Dwyer, J.; Murphy, R.; González-Vázquez, A.; Kovarova, L.; Pravda, M.; Velebny, V.; Heise, A.; Duffy, G.P.; Cryan, S.A. Translational Studies on the Potential of a VEGF Nanoparticle-Loaded Hyaluronic Acid Hydrogel. Pharmaceutics 2021, 13, 779. [Google Scholar] [CrossRef]
- Lee, Y.; Lim, S.; Kim, J.A.; Chun, Y.H.; Lee, H.J. Development of Thiol–Ene Reaction-Based HA Hydrogel with Sustained Release of EGF for Enhanced Skin Wound Healing. Biomacromolecules 2023, 24, 5342–5352. [Google Scholar] [CrossRef]
- Prebeg, T.; Perić Kačarević, Ž.; Matijašić, G. Hybrid Hydrogels for Bioink Development and Potential Use in Dental Tissue Engineering. Int. J. Dent. Biomater. Res. 2023, 1, 22–29. [Google Scholar] [CrossRef]
| Protein | Key Characteristics | Class | References |
|---|---|---|---|
| Human HAS1 |
| Class I membrane-integrated Hyaluronan synthase | DeAngelis & Zimmer (2023). [7] |
| Human HAS2 |
| Class I membrane-integrated GT-2 Hyaluronan synthase | DeAngelis & Zimmer (2023). [7] |
| Human HAS3 |
| Class I membrane-integrated Hyaluronan synthase | DeAngelis & Zimmer (2023). [7] |
| S. pyogenes HasA |
| Class I membrane-integrated GT-2 Hyaluronan synthase Reducing-end addition mechanism | Weigel (2015). [25] Wessels (2019). [26] |
| S. zooepidemicus HasA |
| Class I membrane-integrated GT-2 Hyaluronan synthase | de Oliveira et al. (2016). [2] Blackburn et al. (2018). [27] |
| P. multocida PmHAS |
| Class II dual-domain GT-2 Two separate catalytic modules Different from Class I mechanism | Pasomboon et al. (2021). [28] van der Vlist & Loos (2010). [29] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korzekwa, K.; Sobolewski, K.; Wiciejowska, M.; Augustyniak, D. Antimicrobial Activity Versus Virulence Potential of Hyaluronic Acid: Balancing Advantages and Disadvantages. Int. J. Mol. Sci. 2025, 26, 11549. https://doi.org/10.3390/ijms262311549
Korzekwa K, Sobolewski K, Wiciejowska M, Augustyniak D. Antimicrobial Activity Versus Virulence Potential of Hyaluronic Acid: Balancing Advantages and Disadvantages. International Journal of Molecular Sciences. 2025; 26(23):11549. https://doi.org/10.3390/ijms262311549
Chicago/Turabian StyleKorzekwa, Kamila, Kamil Sobolewski, Miriam Wiciejowska, and Daria Augustyniak. 2025. "Antimicrobial Activity Versus Virulence Potential of Hyaluronic Acid: Balancing Advantages and Disadvantages" International Journal of Molecular Sciences 26, no. 23: 11549. https://doi.org/10.3390/ijms262311549
APA StyleKorzekwa, K., Sobolewski, K., Wiciejowska, M., & Augustyniak, D. (2025). Antimicrobial Activity Versus Virulence Potential of Hyaluronic Acid: Balancing Advantages and Disadvantages. International Journal of Molecular Sciences, 26(23), 11549. https://doi.org/10.3390/ijms262311549






