Vitamin E and Fatty Acid Intake and Cardiometabolic Multimorbidity Risk: The Mediating Role of Plasma Lipid Metabolites
Abstract
1. Introduction
2. Results
2.1. Baseline Characteristics of the Study Population
2.2. Characteristics of the Dietary Vitamins and Fatty Acid Intake
2.3. Associations of Dietary Vitamins and Fatty Acid Intake and CMM
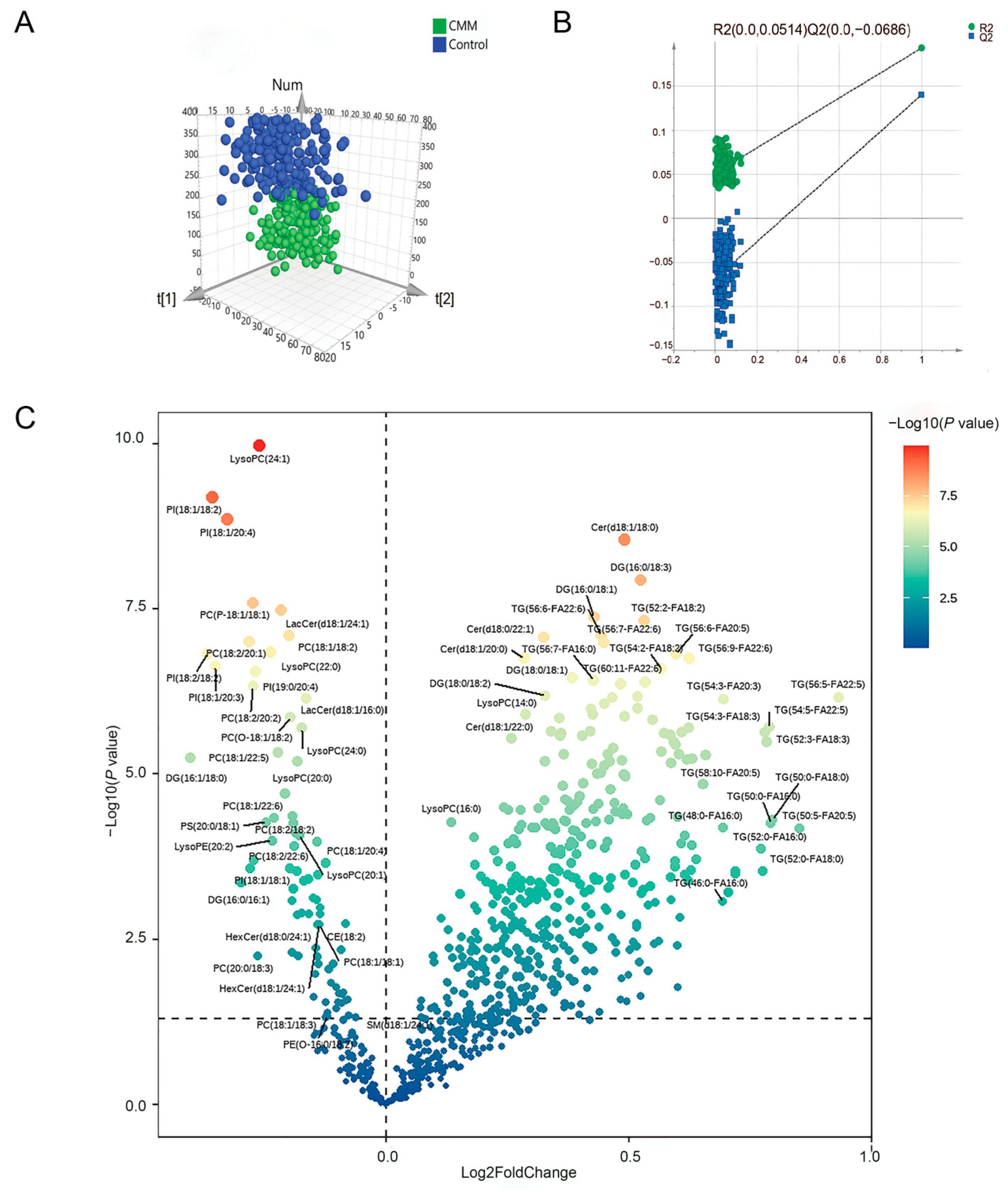
2.4. Lipid Metabolomics Analysis Results
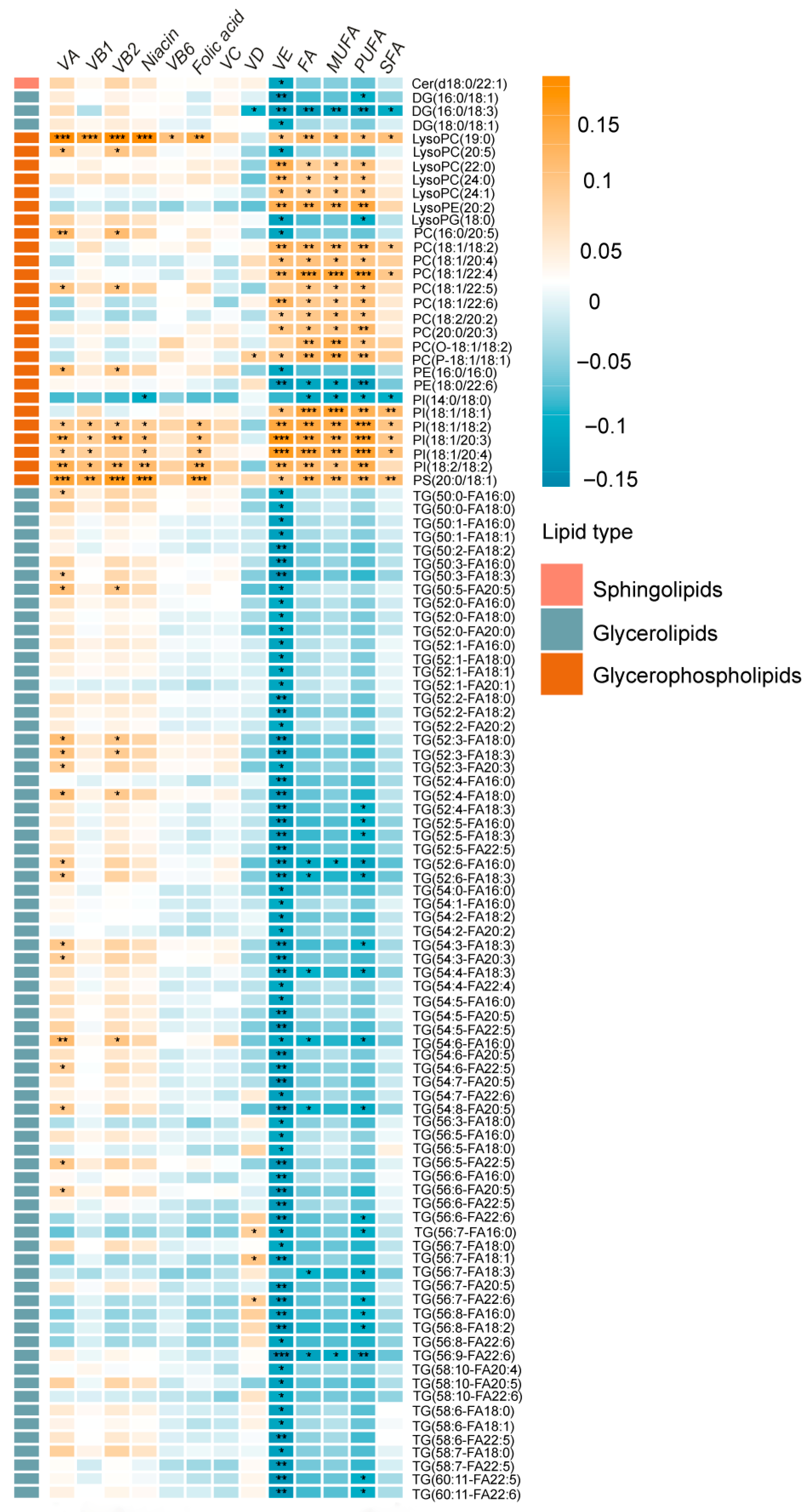
2.5. Associations of Dietary Vitamin and Fatty Acid Intake and Metabolites
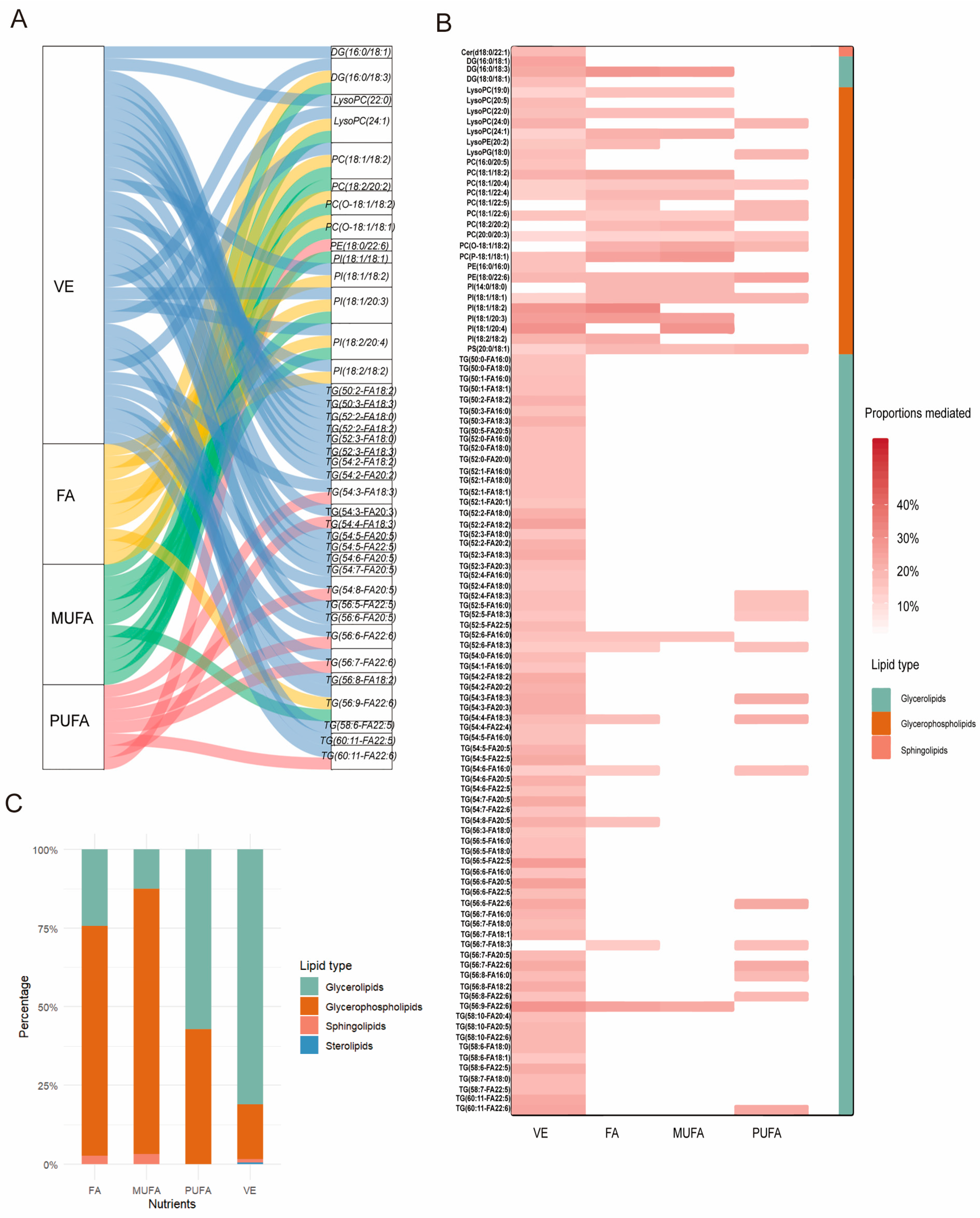
2.6. Metabolites as a Mediator Between Dietary Vitamin and Fatty Acid Intake and CMM
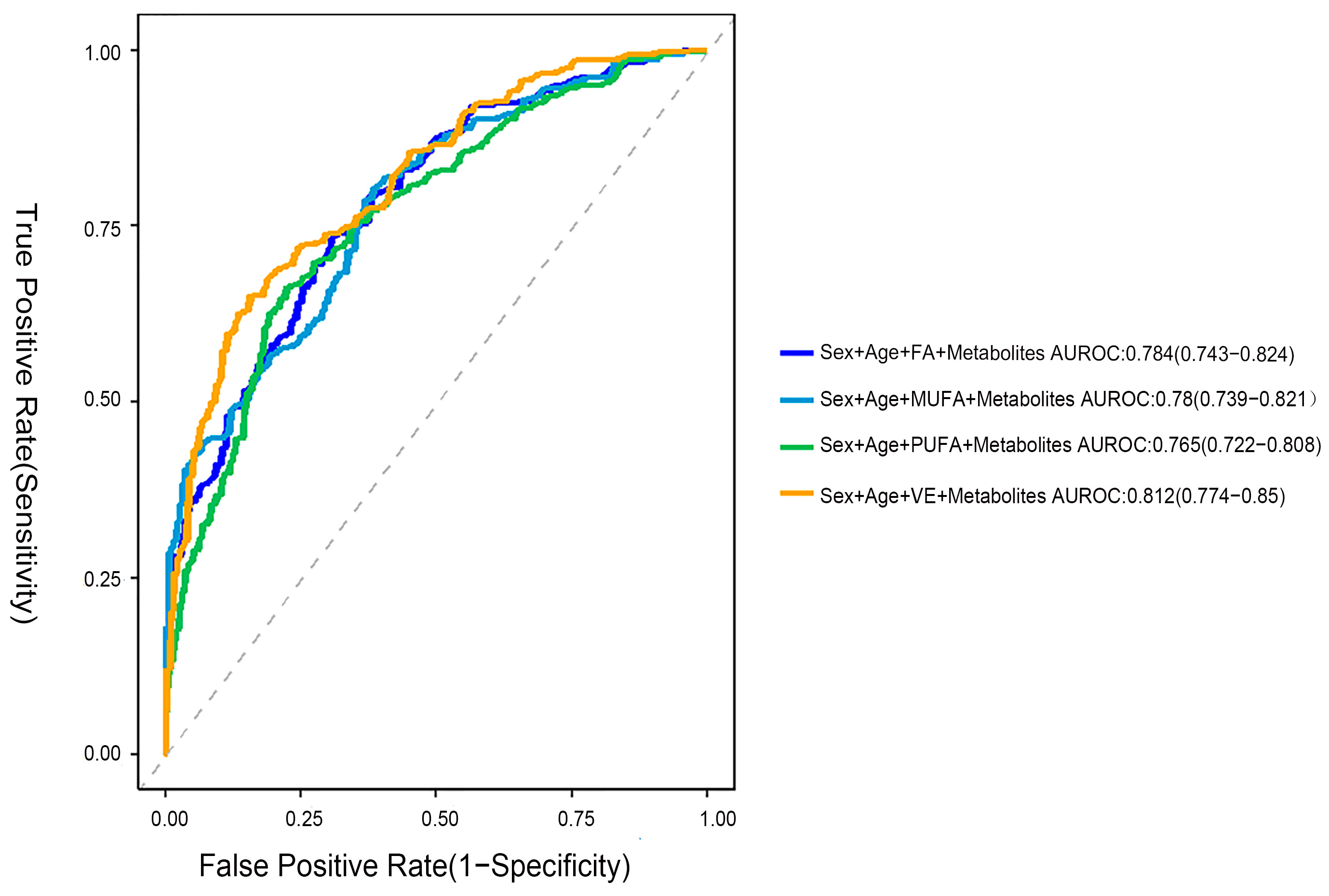
2.7. Using a Logistic Regression Model to Identify the Metabolic Characteristics of CMM
3. Discussion
4. Methods
4.1. Study Population
4.2. Diagnostic Criteria for CMM
4.3. Inclusion and Exclusion Criteria
4.4. Data Collection
4.4.1. Demographic Information
4.4.2. Assessment of Dietary Vitamins and Fatty Acid
4.4.3. Anthropometric Examination
4.4.4. Blood Collection
4.4.5. Biochemical Measurements
4.5. Plasma Lipid Metabolomics Analysis
4.5.1. Sample Preparation
4.5.2. Ultra-High-Performance Liquid Chromatography Coupled with Triple-Quadrupole Mass Spectrometry
4.5.3. Data Analysis
4.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Skou, S.T.; Mair, F.S.; Fortin, M.; Guthrie, B.; Nunes, B.P.; Miranda, J.J.; Boyd, C.M.; Pati, S.; Mtenga, S.; Smith, S.M. Multimorbidity. Nat. Rev. Dis. Primers 2022, 8, 48. [Google Scholar] [CrossRef]
- Emerging Risk Factors Collaboration; Di Angelantonio, E.; Kaptoge, S.; Wormser, D.; Willeit, P.; Butterworth, A.S.; Bansal, N.; O’Keeffe, L.M.; Gao, P.; Wood, A.M.; et al. Association of Cardiometabolic Multimorbidity with Mortality. JAMA 2015, 314, 52–60. [Google Scholar] [CrossRef]
- Prados-Torres, A.; Calderón-Larrañaga, A.; Hancco-Saavedra, J.; Poblador-Plou, B.; van den Akker, M. Multimorbidity Patterns: A Systematic Review. J. Clin. Epidemiol. 2014, 67, 254–266. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Moellering, D.R.; Garvey, W.T. The Progression of Cardiometabolic Disease: Validation of a New Cardiometabolic Disease Staging System Applicable to Obesity. Obesity 2014, 22, 110–118. [Google Scholar] [CrossRef]
- Han, Y.; Hu, Y.; Yu, C.; Sun, D.; Pang, Y.; Pei, P.; Yang, L.; Chen, Y.; Du, H.; Liu, J.; et al. Duration-Dependent Impact of Cardiometabolic Diseases and Multimorbidity on All-Cause and Cause-Specific Mortality: A Prospective Cohort Study of 0.5 Million Participants. Cardiovasc. Diabetol. 2023, 22, 135. [Google Scholar] [CrossRef]
- Jiang, Q. Natural Forms of Vitamin E: Metabolism, Antioxidant, and Anti-Inflammatory Activities and Their Role in Disease Prevention and Therapy. Free Radic. Biol. Med. 2014, 72, 76–90. [Google Scholar] [CrossRef]
- Wallert, M.; Schmölz, L.; Galli, F.; Birringer, M.; Lorkowski, S. Regulatory Metabolites of Vitamin E and Their Putative Relevance for Atherogenesis. Redox Biol. 2014, 2, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Taneera, J.; Yaseen, D.; Youssef, M.; Khalique, A.; Al Shehadat, O.S.; Mohammed, A.K.; Bustanji, Y.; Madkour, M.I.; El-Huneidi, W. Vitamin D Augments Insulin Secretion via Calcium Influx and Upregulation of Voltage Calcium Channels: Findings from INS-1 Cells and Human Islets. Mol. Cell. Endocrinol. 2025, 599, 112472. [Google Scholar] [CrossRef]
- Dikalov, S.; Panov, A.; Dikalova, A. Critical Role of Mitochondrial Fatty Acid Metabolism in Normal Cell Function and Pathological Conditions. Int. J. Mol. Sci. 2024, 25, 6498. [Google Scholar] [CrossRef]
- Lopaschuk, G.D.; Karwi, Q.G.; Tian, R.; Wende, A.R.; Abel, E.D. Cardiac Energy Metabolism in Heart Failure. Circ. Res. 2021, 128, 1487–1513. [Google Scholar] [CrossRef] [PubMed]
- Sellem, L.; Eichelmann, F.; Jackson, K.G.; Wittenbecher, C.; Schulze, M.B.; Lovegrove, J.A. Replacement of Dietary Saturated with Unsaturated Fatty Acids Is Associated with Beneficial Effects on Lipidome Metabolites: A Secondary Analysis of a Randomized Trial. Am. J. Clin. Nutr. 2023, 117, 1248–1261. [Google Scholar] [CrossRef]
- Dicks, L.M.T. How Important Are Fatty Acids in Human Health and Can They Be Used in Treating Diseases? Gut Microbes 2024, 16, 2420765. [Google Scholar] [CrossRef]
- Kotlyarov, S.; Kotlyarova, A. Involvement of Fatty Acids and Their Metabolites in the Development of Inflammation in Atherosclerosis. Int. J. Mol. Sci. 2022, 23, 1308. [Google Scholar] [CrossRef]
- Maki, K.C.; Dicklin, M.R.; Kirkpatrick, C.F. Saturated Fats and Cardiovascular Health: Current Evidence and Controversies. J. Clin. Lipidol. 2021, 15, 765–772. [Google Scholar] [CrossRef] [PubMed]
- An, P.; Wan, S.; Luo, Y.; Luo, J.; Zhang, X.; Zhou, S.; Xu, T.; He, J.; Mechanick, J.I.; Wu, W.-C.; et al. Micronutrient Supplementation to Reduce Cardiovascular Risk. J. Am. Coll. Cardiol. 2022, 80, 2269–2285. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Geng, T.; Huang, T.; Zhao, Q. Fish Oil Supplementation and Insulin Sensitivity: A Systematic Review and Meta-Analysis. Lipids Health Dis. 2017, 16, 131. [Google Scholar] [CrossRef]
- Micha, R.; Peñalvo, J.L.; Cudhea, F.; Imamura, F.; Rehm, C.D.; Mozaffarian, D. Association between Dietary Factors and Mortality from Heart Disease, Stroke, and Type 2 Diabetes in the United States. JAMA 2017, 317, 912–924. [Google Scholar] [CrossRef]
- Hu, F.B. Metabolic Profiling of Diabetes: From Black-Box Epidemiology to Systems Epidemiology. Clin. Chem. 2011, 57, 1224–1226. [Google Scholar] [CrossRef] [PubMed]
- Ji, Q.; Jiang, X.; Wang, M.; Xin, Z.; Zhang, W.; Qu, J.; Liu, G.-H. Multimodal Omics Approaches to Aging and Age-Related Diseases. Phenomics 2024, 4, 56–71. [Google Scholar] [CrossRef]
- Lee, C.-H.; Chan, R.S.M.; Wan, H.Y.L.; Woo, Y.-C.; Cheung, C.Y.Y.; Fong, C.H.Y.; Cheung, B.M.Y.; Lam, T.-H.; Janus, E.; Woo, J.; et al. Dietary Intake of Anti-Oxidant Vitamins a, C, and E Is Inversely Associated with Adverse Cardiovascular Outcomes in Chinese-a 22-Years Population-Based Prospective Study. Nutrients 2018, 10, 1664. [Google Scholar] [CrossRef]
- Ziegler, M.; Wallert, M.; Lorkowski, S.; Peter, K. Cardiovascular and Metabolic Protection by Vitamin E: A Matter of Treatment Strategy? Antioxidants 2020, 9, 935. [Google Scholar] [CrossRef]
- Kuwabara, A.; Nakade, M.; Tamai, H.; Tsuboyama-Kasaoka, N.; Tanaka, K. The Association between Vitamin E Intake and Hypertension: Results from the Re-Analysis of the National Health and Nutrition Survey. J. Nutr. Sci. Vitaminol. 2014, 60, 239–245. [Google Scholar] [CrossRef]
- Ricciarelli, R.; Zingg, J.M.; Azzi, A. Vitamin E Reduces the Uptake of Oxidized LDL by Inhibiting CD36 Scavenger Receptor Expression in Cultured Aortic Smooth Muscle Cells. Circulation 2000, 102, 82–87. [Google Scholar] [CrossRef]
- Huang, Z.-G.; Liang, C.; Han, S.-F.; Wu, Z.-G. Vitamin E Ameliorates Ox-LDL-Induced Foam Cells Formation through Modulating the Activities of Oxidative Stress-Induced NF-κB Pathway. Mol. Cell. Biochem. 2012, 363, 11–19. [Google Scholar] [CrossRef]
- Sesso, H.D.; Buring, J.E.; Christen, W.G.; Kurth, T.; Belanger, C.; MacFadyen, J.; Bubes, V.; Manson, J.E.; Glynn, R.J.; Gaziano, J.M. Vitamins E and C in the Prevention of Cardiovascular Disease in Men: The Physicians’ Health Study II Randomized Controlled Trial. JAMA 2008, 300, 2123–2133. [Google Scholar] [CrossRef]
- Asbaghi, O.; Nazarian, B.; Yousefi, M.; Anjom-Shoae, J.; Rasekhi, H.; Sadeghi, O. Effect of Vitamin E Intake on Glycemic Control and Insulin Resistance in Diabetic Patients: An Updated Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutr. J. 2023, 22, 10. [Google Scholar] [CrossRef] [PubMed]
- Minamiyama, Y.; Takemura, S.; Bito, Y.; Shinkawa, H.; Tsukioka, T.; Nakahira, A.; Suehiro, S.; Okada, S. Supplementation of Alpha-Tocopherol Improves Cardiovascular Risk Factors via the Insulin Signalling Pathway and Reduction of Mitochondrial Reactive Oxygen Species in Type II Diabetic Rats. Free Radic. Res. 2008, 42, 261–271. [Google Scholar] [CrossRef]
- Lampousi, A.-M.; Lundberg, T.; Löfvenborg, J.E.; Carlsson, S. Vitamins C, E, and β-Carotene and Risk of Type 2 Diabetes: A Systematic Review and Meta-Analysis. Adv. Nutr. 2024, 15, 100211. [Google Scholar] [CrossRef] [PubMed]
- Sears, B.; Perry, M. The Role of Fatty Acids in Insulin Resistance. Lipids Health Dis. 2015, 14, 121. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Leng, X.; Liu, S.; Zeng, Z.; Huang, F.; Huang, R.; Zou, Y.; Xu, Y. Association between Dietary Intake of Omega-3 Polyunsaturated Fatty Acids and All-Cause and Cardiovascular Mortality among Hypertensive Adults: Results from NHANES 1999–2018. Clin. Nutr. 2023, 42, 2434–2442. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, L.; Wang, D.; Yan, N.; Li, C.; Wu, M.; Wang, F.; Mi, B.; Chen, F.; Jia, W.; et al. Omega-3 Polyunsaturated Fatty Acid Biomarkers and Risk of Type 2 Diabetes, Cardiovascular Disease, Cancer, and Mortality. Clin. Nutr. 2022, 41, 1798–1807. [Google Scholar] [CrossRef]
- Wang, D.D.; Li, Y.; Chiuve, S.E.; Stampfer, M.J.; Manson, J.E.; Rimm, E.B.; Willett, W.C.; Hu, F.B. Association of Specific Dietary Fats with Total and Cause-Specific Mortality. JAMA Intern. Med. 2016, 176, 1134–1145. [Google Scholar] [CrossRef]
- Farvid, M.S.; Ding, M.; Pan, A.; Sun, Q.; Chiuve, S.E.; Steffen, L.M.; Willett, W.C.; Hu, F.B. Dietary Linoleic Acid and Risk of Coronary Heart Disease: A Systematic Review and Meta-Analysis of Prospective Cohort Studies. Circulation 2014, 130, 1568–1578. [Google Scholar] [CrossRef] [PubMed]
- Guasch-Ferré, M.; Babio, N.; Martínez-González, M.A.; Corella, D.; Ros, E.; Martín-Peláez, S.; Estruch, R.; Arós, F.; Gómez-Gracia, E.; Fiol, M.; et al. Dietary Fat Intake and Risk of Cardiovascular Disease and All-Cause Mortality in a Population at High Risk of Cardiovascular Disease. Am. J. Clin. Nutr. 2015, 102, 1563–1573. [Google Scholar] [CrossRef] [PubMed]
- Palomer, X.; Pizarro-Delgado, J.; Barroso, E.; Vázquez-Carrera, M. Palmitic and Oleic Acid: The Yin and Yang of Fatty Acids in Type 2 Diabetes Mellitus. Trends Endocrinol. Metab. 2018, 29, 178–190. [Google Scholar] [CrossRef]
- Julibert, A.; Bibiloni, M.d.M.; Bouzas, C.; Martínez-González, M.Á.; Salas-Salvadó, J.; Corella, D.; Zomeño, M.D.; Romaguera, D.; Vioque, J.; Alonso-Gómez, Á.M.; et al. Total and Subtypes of Dietary Fat Intake and Its Association with Components of the Metabolic Syndrome in a Mediterranean Population at High Cardiovascular Risk. Nutrients 2019, 11, 1493. [Google Scholar] [CrossRef]
- Alshehry, Z.H.; Mundra, P.A.; Barlow, C.K.; Mellett, N.A.; Wong, G.; McConville, M.J.; Simes, J.; Tonkin, A.M.; Sullivan, D.R.; Barnes, E.H.; et al. Plasma Lipidomic Profiles Improve on Traditional Risk Factors for the Prediction of Cardiovascular Events in Type 2 Diabetes Mellitus. Circulation 2016, 134, 1637–1650. [Google Scholar] [CrossRef]
- Havulinna, A.S.; Sysi-Aho, M.; Hilvo, M.; Kauhanen, D.; Hurme, R.; Ekroos, K.; Salomaa, V.; Laaksonen, R. Circulating Ceramides Predict Cardiovascular Outcomes in the Population-Based FINRISK 2002 Cohort. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 2424–2430. [Google Scholar] [CrossRef]
- Zhong, J.; Cheung, C.Y.Y.; Su, X.; Lee, C.-H.; Ru, Y.; Fong, C.H.Y.; Liu, Y.; Cheung, C.K.Y.; Lam, K.S.L.; Cai, Z.; et al. Specific Triacylglycerol, Diacylglycerol, and Lyso-Phosphatidylcholine Species for the Prediction of Type 2 Diabetes: A ~ 16-Year Prospective Study in Chinese. Cardiovasc. Diabetol. 2022, 21, 234. [Google Scholar] [CrossRef] [PubMed]
- Rhee, E.P.; Cheng, S.; Larson, M.G.; Walford, G.A.; Lewis, G.D.; McCabe, E.; Yang, E.; Farrell, L.; Fox, C.S.; O’Donnell, C.J.; et al. Lipid Profiling Identifies a Triacylglycerol Signature of Insulin Resistance and Improves Diabetes Prediction in Humans. J. Clin. Investig. 2011, 121, 1402–1411. [Google Scholar] [CrossRef]
- Eichelmann, F.; Sellem, L.; Wittenbecher, C.; Jäger, S.; Kuxhaus, O.; Prada, M.; Cuadrat, R.; Jackson, K.G.; Lovegrove, J.A.; Schulze, M.B. Deep Lipidomics in Human Plasma: Cardiometabolic Disease Risk and Effect of Dietary Fat Modulation. Circulation 2022, 146, 21–35. [Google Scholar] [CrossRef]
- Imamura, F.; Fretts, A.M.; Marklund, M.; Ardisson Korat, A.V.; Yang, W.-S.; Lankinen, M.; Qureshi, W.; Helmer, C.; Chen, T.-A.; Virtanen, J.K.; et al. Fatty Acids in the de Novo Lipogenesis Pathway and Incidence of Type 2 Diabetes: A Pooled Analysis of Prospective Cohort Studies. PLoS Med. 2020, 17, e1003102. [Google Scholar] [CrossRef]
- Stegemann, C.; Pechlaner, R.; Willeit, P.; Langley, S.R.; Mangino, M.; Mayr, U.; Menni, C.; Moayyeri, A.; Santer, P.; Rungger, G.; et al. Lipidomics Profiling and Risk of Cardiovascular Disease in the Prospective Population-Based Bruneck Study. Circulation 2014, 129, 1821–1831. [Google Scholar] [CrossRef]
- Chakrabarti, A.; Eiden, M.; Morin-Rivron, D.; Christinat, N.; Monteiro, J.P.; Kaput, J.; Masoodi, M. Impact of Multi-Micronutrient Supplementation on Lipidemia of Children and Adolescents. Clin. Nutr. 2020, 39, 2211–2219. [Google Scholar] [CrossRef]
- Morze, J.; Wittenbecher, C.; Schwingshackl, L.; Danielewicz, A.; Rynkiewicz, A.; Hu, F.B.; Guasch-Ferré, M. Metabolomics and Type 2 Diabetes Risk: An Updated Systematic Review and Meta-Analysis of Prospective Cohort Studies. Diabetes Care 2022, 45, 1013–1024. [Google Scholar] [CrossRef]
- Chen, G.-C.; Chai, J.C.; Yu, B.; Michelotti, G.A.; Grove, M.L.; Fretts, A.M.; Daviglus, M.L.; Garcia-Bedoya, O.L.; Thyagarajan, B.; Schneiderman, N.; et al. Serum Sphingolipids and Incident Diabetes in a US Population with High Diabetes Burden: The Hispanic Community Health Study/Study of Latinos (HCHS/SOL). Am. J. Clin. Nutr. 2020, 112, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Barry, E.; Roberts, S.; Oke, J.; Vijayaraghavan, S.; Normansell, R.; Greenhalgh, T. Efficacy and Effectiveness of Screen and Treat Policies in Prevention of Type 2 Diabetes: Systematic Review and Meta-Analysis of Screening Tests and Interventions. BMJ 2017, 356, i6538. [Google Scholar] [CrossRef]
- Vidal-Petiot, E. Thresholds for Hypertension Definition, Treatment Initiation, and Treatment Targets: Recent Guidelines at a Glance. Circulation 2022, 146, 805–807. [Google Scholar] [CrossRef] [PubMed]
- Joseph, J.J.; Rajwani, A.; Roper, D.; Zhao, S.; Kline, D.; Odei, J.; Brock, G.; Echouffo-Tcheugui, J.B.; Kalyani, R.R.; Bertoni, A.G.; et al. Associations of Cardiometabolic Multimorbidity with All-Cause and Coronary Heart Disease Mortality among Black Adults in the Jackson Heart Study. JAMA Netw. Open 2022, 5, e2238361. [Google Scholar] [CrossRef] [PubMed]
- Yan, N.; Li, N.; Liu, W.; Li, X.; Liu, X.; Zhang, P.; Liu, C.; Li, J.; Qiu, J.; Zhang, Y.; et al. Validity and Reliability of a Semi-Quantitative Food Frequency Questionnaire in Groups at High Risk for Cardiovascular Diseases. Nutr. J. 2022, 21, 63. [Google Scholar] [CrossRef]
| Characteristics | CMM 1 | Healthy | p |
|---|---|---|---|
| (median (P25, P75)) | |||
| N | 200 | 200 | |
| Age, y | 66 (61, 69) | 65 (61, 69) | 0.795 |
| Sex, n (%) | |||
| Female | 117 (58.5%) | 117 (58.5%) | 1.000 |
| Male | 83 (41.5%) | 83 (41.5%) | |
| Nation, n (%) | |||
| the Han nationality | 97 (48.5%) | 93 (46.5%) | 0.689 |
| the Hui nationality | 103 (51.5%) | 107 (53.5%) | |
| Smoking, n (%) | 27 (13.5%) | 33 (16.5%) | 0.401 |
| Alcohol intake, n (%) | 16 (8%) | 30 (15%) | 0.028 |
| Marriage, n (%) | |||
| Married | 174 (87%) | 173 (86.5%) | 0.883 |
| Others | 26 (13%) | 27 (13.5%) | |
| Physical activity, n (%) | |||
| High | 84 (42%) | 89 (44.5%) | 0.703 |
| Low | 67 (33.5%) | 65 (32.5%) | |
| SBP (mmHg) | 145 (135.00, 158.25) | 133 (122.00, 146.50) | <0.001 |
| DBP (mmHg) | 88 (79.00, 96.00) | 80 (72, 88) | <0.001 |
| BMI (kg/m2) | 25.85 (24.10, 28.30) | 23.85 (22.00, 26.15) | <0.001 |
| FBG (mmol/L) | 7.13 (5.50, 9.70) | 5.30 (4.90, 5.77) | <0.001 |
| TC (mmol/L) | 4.91 (4.13, 5.62) | 4.80 (4.01, 5.34) | 0.254 |
| TG (mmol/L) | 1.72 (1.22, 2.67) | 1.31 (0.90, 1.98) | <0.001 |
| HDL-c (mmol/L) | 1.17 (0.95, 1.45) | 1.30 (1.12, 1.55) | <0.001 |
| LDL-c (mmol/L) | 2.93 (2.25, 3.62) | 2.91 (2.31, 3.44) | 0.872 |
| UA (μmol/L) | 281.5 (231.25, 349.00) | 270 (218.50, 325.00) | 0.053 |
| Comorbidity | |||
| Hypertension, n% | 182 (91%) | 0 (0%) | <0.001 |
| Diabetes, n% | 130 (65%) | 0 (0%) | <0.001 |
| AMI, n% | 34 (17%) | 0 (0%) | <0.001 |
| Angina, n% | 24 (12%) | 0 (0%) | <0.001 |
| Stroke, n% | 39 (19.5%) | 0 (0%) | <0.001 |
| Others, n% | 29 (14.5%) | 0 (0%) | <0.001 |
| Diabetes + Hypertension, n% | 118 (59%) | 0 (%) | <0.001 |
| Vitamins and Fatty Acids | CMM | Healthy | p |
|---|---|---|---|
| N = 200 | N = 200 | ||
| Median (P25, P75) | Median (P25, P75) | ||
| Energy (kcal/d) | 1876.86 (1454.26, 2268.92) | 1977.12 (1568.98, 2564.04) | 0.016 |
| VA (μgRAE/d) | 1992.01 (100.64, 9044.21) | 4568.60 (180.76, 9258.70) | 0.060 |
| VB1 (mg/d) | 0.72 (0.46, 0.97) | 0.79 (0.51, 1.10) | 0.028 |
| VB2 (mg/d) | 1.10 (0.39, 2.57) | 1.54 (0.45, 2.89) | 0.076 |
| Niacin (mg/d) | 13.05 (7.70, 23.33) | 16.66 (7.76, 27.26) | 0.041 |
| VB6 (mg/d) | 0.14 (0.09, 0.27) | 0.18 (0.09, 0.31) | 0.134 |
| Folic acid (μg/d) | 113.03 (65.40, 189.56) | 126.61 (76.07, 208.16) | 0.041 |
| VC (mg/d) | 39.13 (27.08, 73.09) | 54.76 (27.08, 86.67) | 0.033 |
| VD (μg/d) | 1.07 (0.87, 3.86) | 1.70(0.96, 6.87) | 0.072 |
| VE (mg/d) | 129.40 (81.49, 190.41) | 145.79 (100.18, 224.79) | 0.007 |
| FA (g/d) | 59.79 (41.87, 89.56) | 67.25 (44.62, 96.25) | 0.039 |
| SFA (g/d) | 10.36 (7.33, 14.77) | 11.19 (8.05, 17.89) | 0.036 |
| MUFA (g/d) | 23.14 (16.75, 33.79) | 25.98 (17.79, 35.86) | 0.033 |
| PUFA (g/d) | 24.06 (16.19, 35.63) | 26.46 (17.06, 37.76) | 0.069 |
| Factors | Model 1 1 | Model 2 2 | Mode 3 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| OR (95%CI) 4 | p value | FDR | OR (95%CI) 5 | p value | FDR | OR (95%CI) 6 | p value | FDR | |
| VA | 0.88 (0.73–1.06) | 0.175 | 0.207 | 0.89 (0.73–1.07) | 0.219 | 0.258 | 0.89 (0.72–1.09) | 0.253 | 0.299 |
| VB1 | 0.81 (0.68–0.98) | 0.028 | 0.061 | 0.82 (0.68–0.99) | 0.042 | 0.090 | 0.82 (0.67–0.99) | 0.045 | 0.098 |
| VB2 | 0.87 (0.72–1.04) | 0.127 | 0.166 | 0.87 (0.72–1.06) | 0.164 | 0.214 | 0.87 (0.71–1.07) | 0.182 | 0.237 |
| Niacin | 0.83 (0.69–1.00) | 0.047 | 0.088 | 0.84 (0.69–1.01) | 0.067 | 0.124 | 0.83 (0.68–1.02) | 0.074 | 0.137 |
| VB6 | 0.92 (0.76–1.11) | 0.373 | 0.405 | 0.93 (0.77–1.12) | 0.413 | 0.447 | 0.92 (0.76–1.12) | 0.411 | 0.411 |
| Folic acid | 0.86 (0.72–1.04) | 0.115 | 0.165 | 0.87 (0.72–1.05) | 0.141 | 0.204 | 0.87 (0.71–1.06) | 0.154 | 0.223 |
| VC | 0.86 (0.71–1.03) | 0.104 | 0.165 | 0.86 (0.71–1.04) | 0.131 | 0.204 | 0.86 (0.70–1.05) | 0.136 | 0.222 |
| VD | 0.93 (0.7–1.12) | 0.436 | 0.436 | 0.93 (0.78–1.13) | 0.470 | 0.470 | 0.90 (0.74–1.09) | 0.276 | 0.299 |
| VE | 0.75 (0.61–0.90) | 0.002 | 0.032 | 0.74 (0.61–0.89) | 0.002 | 0.027 | 0.74 (0.61–0.90) | 0.002 | 0.031 |
| FA | 0.78 (0.65–0.94) | 0.008 | 0.038 | 0.78 (0.64–0.94) | 0.009 | 0.041 | 0.77 (0.64–0.93) | 0.008 | 0.037 |
| PUFA | 0.80 (0.66–0.96) | 0.016 | 0.045 | 0.79 (0.66–0.95) | 0.014 | 0.045 | 0.79 (0.66–0.96) | 0.015 | 0.049 |
| MUFA | 0.78 (0.65–0.94) | 0.009 | 0.038 | 0.78 (0.65–0.94) | 0.009 | 0.041 | 0.77 (0.64–0.94) | 0.009 | 0.037 |
| SFA | 0.80 (0.66–0.96) | 0.017 | 0.045 | 0.80 (0.66–0.97) | 0.023 | 0.059 | 0.79 (0.65–0.96) | 0.019 | 0.051 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Cen, Y.; Li, X.; Yang, B.; Xing, K.; Lian, L.; Yu, Y.; Zhao, Y. Vitamin E and Fatty Acid Intake and Cardiometabolic Multimorbidity Risk: The Mediating Role of Plasma Lipid Metabolites. Int. J. Mol. Sci. 2025, 26, 11477. https://doi.org/10.3390/ijms262311477
Zhang Y, Cen Y, Li X, Yang B, Xing K, Lian L, Yu Y, Zhao Y. Vitamin E and Fatty Acid Intake and Cardiometabolic Multimorbidity Risk: The Mediating Role of Plasma Lipid Metabolites. International Journal of Molecular Sciences. 2025; 26(23):11477. https://doi.org/10.3390/ijms262311477
Chicago/Turabian StyleZhang, Yannan, Yangyang Cen, Xiaoxia Li, Bowen Yang, Kaijun Xing, Linxi Lian, Yongjie Yu, and Yi Zhao. 2025. "Vitamin E and Fatty Acid Intake and Cardiometabolic Multimorbidity Risk: The Mediating Role of Plasma Lipid Metabolites" International Journal of Molecular Sciences 26, no. 23: 11477. https://doi.org/10.3390/ijms262311477
APA StyleZhang, Y., Cen, Y., Li, X., Yang, B., Xing, K., Lian, L., Yu, Y., & Zhao, Y. (2025). Vitamin E and Fatty Acid Intake and Cardiometabolic Multimorbidity Risk: The Mediating Role of Plasma Lipid Metabolites. International Journal of Molecular Sciences, 26(23), 11477. https://doi.org/10.3390/ijms262311477






