Chiral Recognition Mechanism of 2,13-Bis(hydroxymethyl)-[7]thiaheterohelicene on Ag(111) Investigated by STM and MD Simulation
Abstract
1. Introduction
2. Results and Discussion
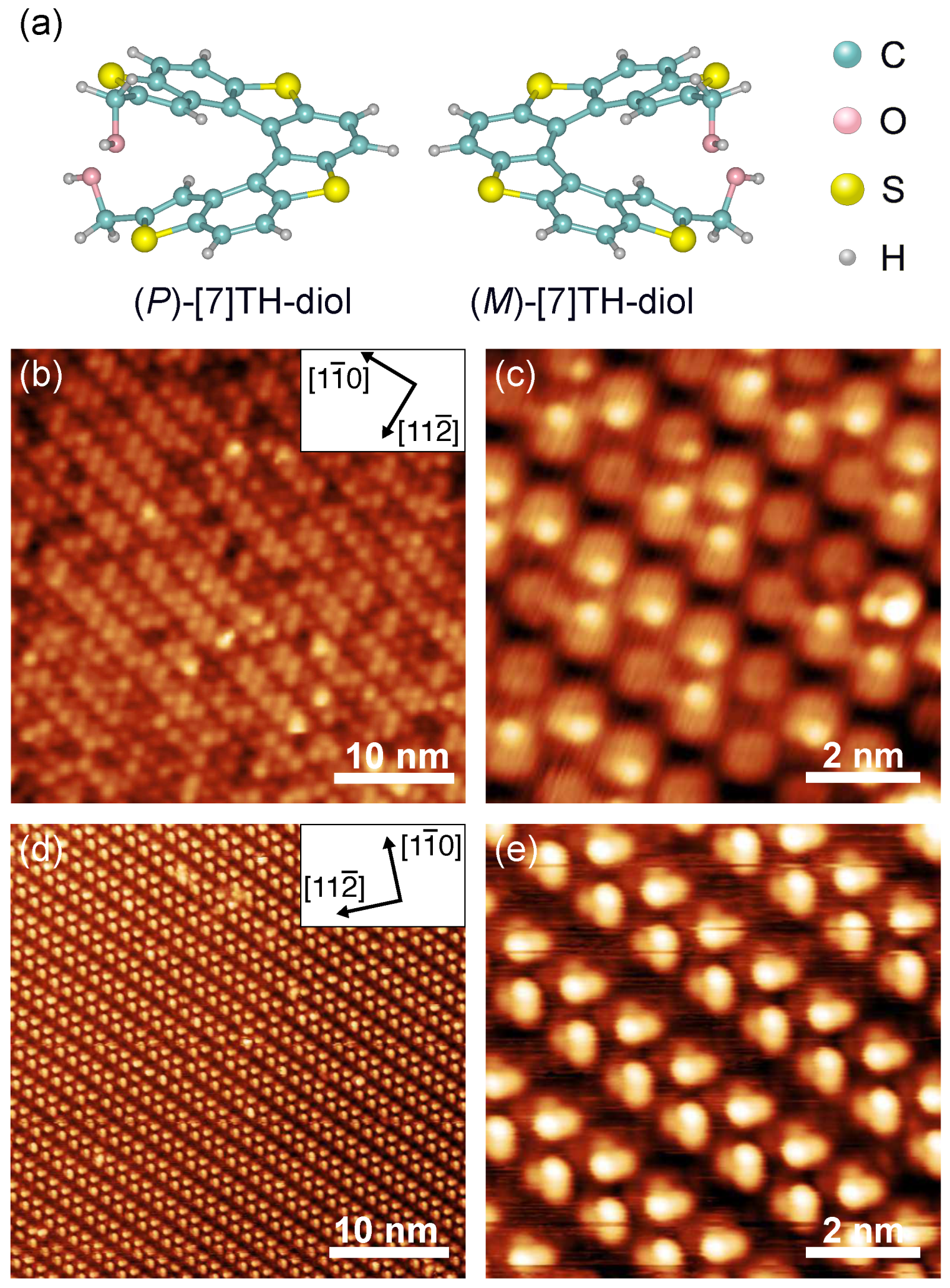
2.1. STM Observation of Self-Assembled Structures of [7]TH-diol on Ag(111)
2.2. MD Simulation of [7]TH-diol
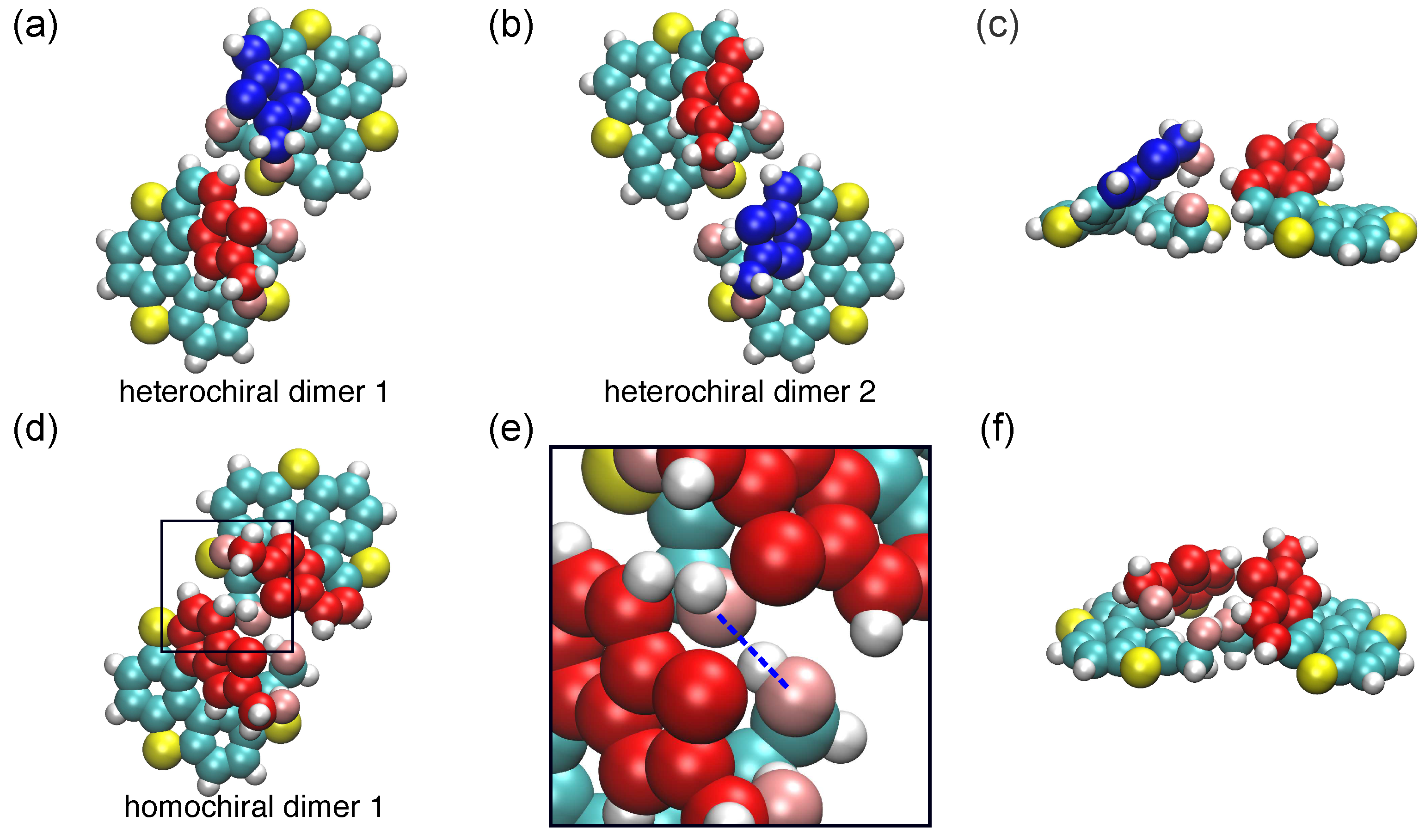
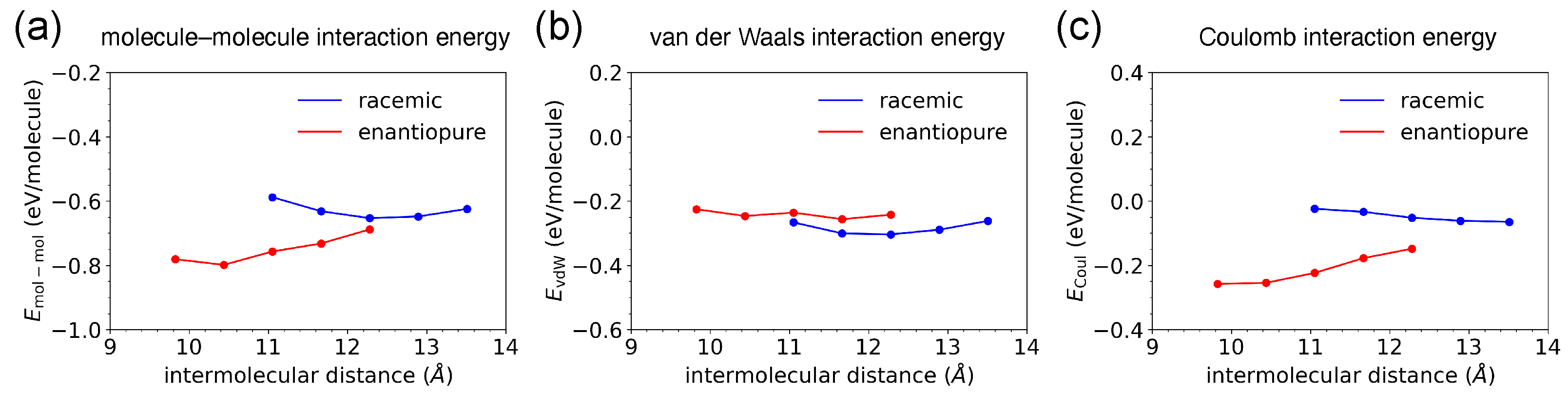
2.2.1. Dimer Conformations
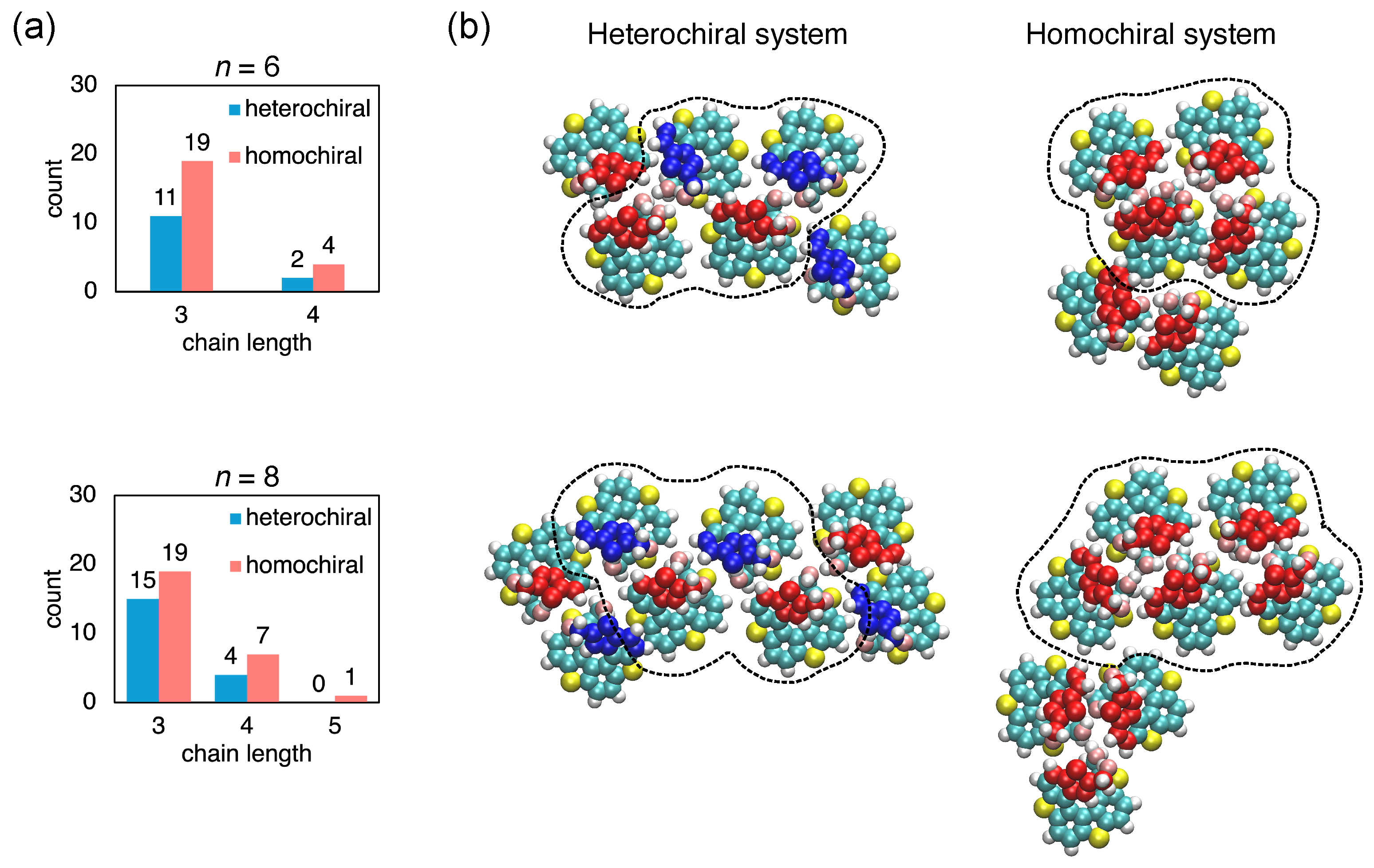
2.2.2. Formation of Chain-like Structures
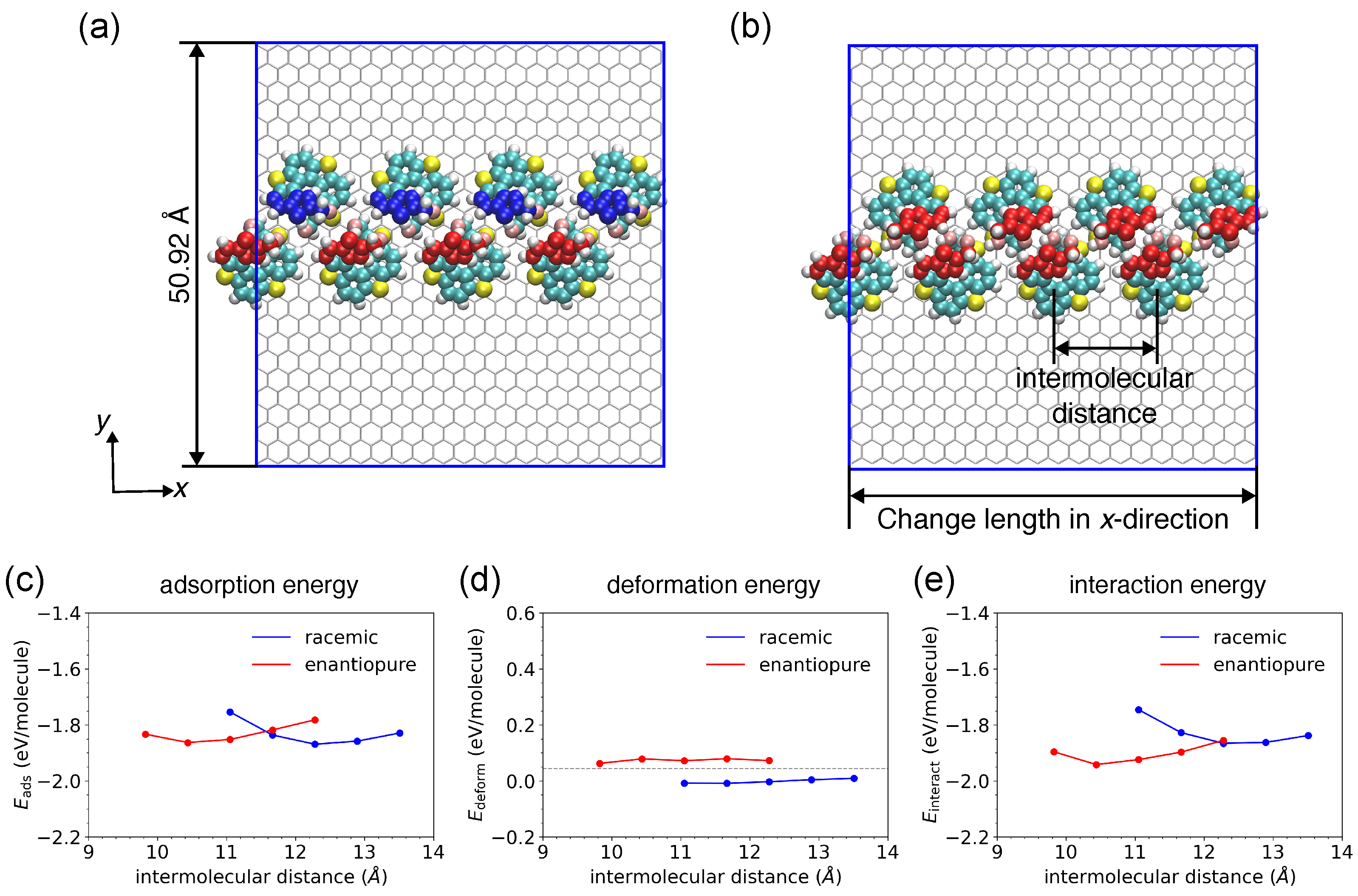
2.2.3. Extended Molecular Chain Models
2.2.4. Effect of Different Partial Charge Distributions
2.3. Role of Functional Groups in Molecular Arrangements
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| rac | racemic |
| [7]TH | [7]thiaheterohelicene |
| [7]TH-diol | 2,13-bis(hydroxymethyl)-[7]thiaheterohelicene |
| [7]CH | [7]carbohelicene |
| STM | scanning tunneling microscopy |
| ML | monolayer |
| MD | molecular dynamics |
| DFT | density functional theory |
| XPS | X-ray photoelectron spectroscopy |
References
- Proctor, R.S.; Colgan, A.C.; Phipps, R.J. Exploiting attractive non-covalent interactions for the enantioselective catalysis of reactions involving radical intermediates. Nat. Chem. 2020, 12, 990–1004. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Liu, J.; Ramesar, N.S.; Heinz, H.; Xu, L.; Xu, C.; Kotov, N.A. Single- and multi-component chiral supraparticles as modular enantioselective catalysts. Nat. Commun. 2019, 10, 4826. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zhang, L.; Wang, T. Supramolecular chirality in self-assembled systems. Chem. Rev. 2015, 115, 7304–7397. [Google Scholar] [CrossRef] [PubMed]
- Sang, Y.; Liu, M. Hierarchical self-assembly into chiral nanostructures. Chem. Sci. 2022, 13, 633–656. [Google Scholar] [CrossRef]
- Shen, B.; Kim, Y.; Lee, M. Supramolecular chiral 2D materials and emerging functions. Adv. Mater. 2020, 32, 1905669. [Google Scholar] [CrossRef]
- Zhao, W.L.; Li, M.; Lu, H.Y.; Chen, C.F. Advances in helicene derivatives with circularly polarized luminescence. Chem. Commun. 2019, 55, 13793–13803. [Google Scholar] [CrossRef]
- Sakunkaewkasem, S.; Petdum, A.; Panchan, W.; Sirirak, J.; Charoenpanich, A.; Sooksimuang, T.; Wanichacheva, N. Dual-Analyte Fluorescent Sensor Based on [5]Helicene Derivative with Super Large Stokes Shift for the Selective Determinations of Cu2+ or Zn2+ in Buffer Solutions and Its Application in a Living Cell. ACS Sens. 2018, 3, 1016–1023. [Google Scholar] [CrossRef]
- Stetsovych, O.; Švec, M.; Vacek, J.; Chocholoušová, J.V.; Janík, A.; Rybáek, J.; Kosmider, K.; Stará, I.G.; Jelínek, P.; Starý, I. From helical to planar chirality by on-surface chemistry. Nat. Chem. 2017, 9, 213–218. [Google Scholar] [CrossRef]
- Gingras, G. One hundred years of helicene chemistry. Part 3: Applications and properties of carbohelicenes. Chem. Soc. Rev. 2013, 42, 1051–1095. [Google Scholar] [CrossRef]
- Ernst, K.H. Stereochemical Recognition of Helicenes on Metal Surfaces. Accounts Chem. Res. 2016, 49, 1182–1190. [Google Scholar] [CrossRef]
- Ooe, H.; Yokoyama, T. On-surface polymerization reactions of dibrominated hexaphenylbenzene influenced by densely packed self-assembly. Phys. Chem. Chem. Phys. 2024, 26, 12939–12946. [Google Scholar] [CrossRef] [PubMed]
- Bera, A.; Henkel, S.; Mieres-Perez, J.; Andargie Tsegaw, Y.; Sanchez-Garcia, E.; Sander, W.; Morgenstern, K. Surface Diffusion Aided by a Chirality Change of Self-Assembled Oligomers under 2D Confinement. Angew. Chem. Int. Ed. 2022, 61, e202212245. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Zhang, Y.; Liu, X.; Qian, Y.; Ouyang, Z.; Kong, H. From Short- to Long-Range Chiral Recognition on Surfaces: Chiral Assembly and Synthesis. Small 2024, 20, 2307171. [Google Scholar] [CrossRef] [PubMed]
- Telychko, M.; Wang, L.; Hsu, C.H.; Li, G.; Peng, X.; Song, S.; Su, J.; Chuang, F.C.; Wu, J.; Wong, M.W.; et al. Tailoring long-range superlattice chirality in molecular self-assemblies: Via weak fluorine-mediated interactions. Phys. Chem. Chem. Phys. 2021, 23, 21489–21495. [Google Scholar] [CrossRef]
- Kong, H.; Qian, Y.; Liu, X.; Wan, X.; Amirjalayer, S.; Fuchs, H. Long-Range Chirality Recognition of a Polar Molecule on Au(111). Angew. Chem. 2020, 132, 188–192. [Google Scholar] [CrossRef]
- Parschau, M.; Fasel, R.; Ernst, K.H. Coverage and enantiomeric excess dependent enantiomorphism in two-dimensional molecular crystal. Cryst. Growth Des. 2008, 8, 1890–1896. [Google Scholar] [CrossRef]
- Seibel, J.; Parschau, M.; Ernst, K.H. Two-dimensional crystallization of enantiopure and racemic heptahelicene on Ag(111) and Au(111). J. Phys. Chem. C 2014, 118, 29135–29141. [Google Scholar] [CrossRef]
- Seibel, J.; Parschau, M.; Ernst, K.H. From Homo-chiral Clusters to Racemate Crystals: Viable Nuclei in 2D Chiral Crystallization. J. Am. Chem. Soc. 2015, 137, 7970–7973. [Google Scholar] [CrossRef]
- Krukowski, P.; Hattori, T.; Okada, M.; Piskorski, M.; Lutsyk, I.; Saito, A.; Osuga, H.; Kuwahara, Y. Study of stereochemical crystallization of racemic mixtures of [5] and [7]thiaheterohelicene molecules on Ag(111) surface by scanning tunneling microscopy and Raman scattering spectroscopy. Appl. Surf. Sci. 2022, 589, 152860. [Google Scholar] [CrossRef]
- Kimura, S.; Hattori, T.; Ye, C.; Okada, M.; Kondo, S.; Sakurama, Y.; Saito, A.; Krukowski, P.; Osuga, H.; Kuwahara, Y. STM/TERS observation of (M)-type diphenyl[7]thiaheterohelicene on Ag(111). Phys. Chem. Chem. Phys. 2024, 26, 7658–7663. [Google Scholar] [CrossRef]
- Chaunchaiyakul, S.; Krukowski, P.; Tsuzuki, T.; Minagawa, Y.; Akai-Kasaya, M.; Saito, A.; Osuga, H.; Kuwahara, Y. Self-Assembly Formation of M-Type Enantiomer of 2,13-Bis(hydroxymethyl)[7]-thiaheterohelicene Molecules on Au(111) Surface Investigated by STM/CITS. J. Phys. Chem. C 2015, 119, 21434–21442. [Google Scholar] [CrossRef]
- Fasel, R.; Parschau, M.; Ernst, K.H. Amplification of chirality in two-dimensional enantiomorphous lattices. Nature 2006, 439, 449–452. [Google Scholar] [CrossRef]
- Mairena, A.; Mendieta, J.I.; Stetsovych, O.; Terfort, A.; Stará, I.G.; Starý, I.; Jelínek, P.; Ernst, K.H. Hetero-chiral recognition among functionalized heptahelicenes on noble metal surfaces. Chem. Commun. 2019, 55, 10595–10598. [Google Scholar] [CrossRef]
- Balandina, T.; van der Meijden, M.W.; Ivasenko, O.; Cornil, D.; Cornil, J.; Lazzaroni, R.; Kellogg, R.M.; Feyter, S.D. Self-assembly of an asymmetrically functionalized [6]helicene at liquid/solid interfaces. Chem. Commun. 2013, 49, 2207–2209. [Google Scholar] [CrossRef] [PubMed]
- Ascolani, H.; Meijden, M.W.V.D.; Cristina, L.J.; Gayone, J.E.; Kellogg, R.M.; Fuhr, J.D.; Lingenfelder, M. Van der Waals interactions in the self-assembly of 5-amino[6]helicene on Cu(100) and Au(111). Chem. Commun. 2014, 50, 13907–13909. [Google Scholar] [CrossRef]
- Chaunchaiyakul, S.; Setiadi, A.; Krukowski, P.; Catalan, F.C.I.; Akai-Kasaya, M.; Saito, A.; Hayazawa, N.; Kim, Y.; Osuga, H.; Kuwahara, Y. Nanoscale Dehydrogenation Observed by Tip-Enhanced Raman Spectroscopy. J. Phys. Chem. C 2017, 121, 18162–18168. [Google Scholar] [CrossRef]
- Mairena, A.; Zoppi, L.; Seibel, J.; Tröster, A.F.; Grenader, K.; Parschau, M.; Terfort, A.; Ernst, K.H. Hetero-chiral to Homo-chiral Transition in Pentahelicene 2D Crystallization Induced by Second-Layer Nucleation. ACS Nano 2017, 11, 865–871. [Google Scholar] [CrossRef]
- Han, Q.; Li, Z.; Sun, K.; Tao, M.L.; Shi, M.X.; Yang, D.X.; Xia, J.X.; Wan, J.J.; Wang, J.Z. Spontaneous chiral resolution of pentahelicene molecules on Cd(0001). Phys. Chem. Chem. Phys. 2022, 24, 10292–10296. [Google Scholar] [CrossRef]
- Tuca, E.; Paci, I. Structural analysis of helicene molecules adsorbed on symmetric surfaces. Phys. Chem. Chem. Phys. 2019, 21, 9189–9199. [Google Scholar] [CrossRef]
- Ye, C.; Hattori, T.; Hamamoto, Y.; Krukowski, P.; Inagaki, K.; Saito, A.; Osuga, H.; Morikawa, Y.; Kuwahara, Y. Chiral Recognition Mechanism of Two-Dimensional Self-Assembly Formed by [7]Thiaheterohelicene. J. Phys. Chem. C 2023, 127, 21305–21312. [Google Scholar] [CrossRef]
- Ruben, M.; Payer, D.; Landa, A.; Comisso, A.; Gattinoni, C.; Lin, N.; Collin, J.P.; Sauvage, J.P.; Vita, A.D.; Kern, K. 2D Supramolecular Assemblies of Benzene-1,3,5-triyl-tribenzoic Acid: Temperature-Induced Phase Transformations and Hierarchical Organization with Macrocyclic Molecules. J. Am. Chem. Soc. 2006, 128, 15644–15651. [Google Scholar] [CrossRef]
- Maleki, A.; Alavi, S.; Najafi, B. Molecular dynamics simulation study of adsorption and patterning of DNA bases on the Au(111) surface. J. Phys. Chem. C 2011, 115, 22484–22494. [Google Scholar] [CrossRef]
- Cao, Y.; Keller, E.; Rowen, J.F.; Margraf, J.T.; Reuter, K.; Morgenstern, K. Self-Assembly of Monosized Cyclic Nanoarchitectures under Surface Confinement. ACS Nano 2025, 19, 21942–21949. [Google Scholar] [CrossRef]
- Vonau, F.; Aubel, D.; Bouteiller, L.; Reiter, G.; Simon, L. Cooperative Rearrangements Leading to Long Range Order in Monolayers of Supramolecular Polymers. Phys. Rev. Lett. 2007, 99, 086103. [Google Scholar] [CrossRef]
- Vonau, F.; Linares, M.; Isare, B.; Aubel, D.; Habar, M.; Bouteiller, L.; Reiter, G.; Geskin, V.; Zerbetto, F.; Lazzaroni, R.; et al. Branched Substituents Generate Improved Supramolecular Ordering in Physisorbed Molecular Assemblies. J. Phys. Chem. C 2009, 113, 4955–4959. [Google Scholar] [CrossRef]
- Nečas, D.; Klapetek, P. Gwyddion: An open-source software for SPM data analysis. Open Phys. 2012, 10, 181–188. [Google Scholar] [CrossRef]
- Tanaka, K.; Osuga, H.; Suzuki, H.; Shogase, Y.; Kitahara, Y. Synthesis, enzymic resolution and enantiomeric enhancement of bis (hydroxymethyl)[7] thiaheterohelicenes. J. Chem. Soc. Perkin Trans. 1 1998, 935–940. [Google Scholar] [CrossRef]
- Yamada, K.; Nakagawa, H.; Kawazura, H. Thermal Racemization of Thiaheterohelicenes. Bull. Chem. Soc. Jpn. 1986, 59, 2429–2432. [Google Scholar] [CrossRef]
- Lucht, K.; Schweer, P.; Cao, Y.; Mieres-Perez, J.; Ulrich, I.; Sanchez-Garcia, E.; Sander, W.; Morgenstern, K. On-Surface Alcohol Formation from a Carbene Precursor. J. Phys. Chem. C 2024, 128, 15347–15355. [Google Scholar] [CrossRef]
- Jethwa, S.J.; Kolsbjerg, E.L.; Vadapoo, S.R.; Cramer, J.L.; Lammich, L.; Gothelf, K.V.; Hammer, B.; Linderoth, T.R. Supramolecular Corrals on Surfaces Resulting from Aromatic Interactions of Nonplanar Triazoles. ACS Nano 2017, 11, 8302–8310. [Google Scholar] [CrossRef]
- Thompson, A.P.; Aktulga, H.M.; Berger, R.; Bolintineanu, D.S.; Brown, W.M.; Crozier, P.S.; in ’t Veld, P.J.; Kohlmeyer, A.; Moore, S.G.; Nguyen, T.D.; et al. LAMMPS-a flexible simulation tool for particle-based materials modeling at the atomic, meso, and continuum scales. Comput. Phys. Commun. 2022, 271, 108171. [Google Scholar] [CrossRef]
- Wang, J.; Wolf, R.M.; Caldwell, J.W.; Kollman, P.A.; Case, D.A. Development and testing of a general amber force field. J. Comput. Chem. 2004, 25, 1157–1174. [Google Scholar] [CrossRef] [PubMed]
- Cornell, W.D.; Cieplak, P.; Bayly, C.I.; Gould, I.R.; Merz, K.M.; Ferguson, D.M.; Spellmeyer, D.C.; Fox, T.; Caldwell, J.W.; Kollman, P.A. A second generation force field for the simulation of proteins, nucleic acids, and organic molecules. J. Am. Chem. Soc. 1995, 117, 5179–5197. [Google Scholar] [CrossRef]
- Jakalian, A.; Jack, D.B.; Bayly, C.I. Fast, efficient generation of high-quality atomic charges. AM1-BCC model: II. Parameterization and validation. J. Comput. Chem. 2002, 23, 1623–1641. [Google Scholar] [CrossRef]
- Jakalian, A.; Bush, B.L.; Jack, D.B.; Bayly, C.I. Fast, Efficient Generation of High-Quality Atomic Charges. AM1-BCC Model: I. Method. J. Comput. Chem. 2000, 21, 132–146. [Google Scholar] [CrossRef]
- Andersen, H.C. Rattle: A “velocity” version of the shake algorithm for molecular dynamics calculations. J. Comput. Phys. 1983, 52, 24–34. [Google Scholar] [CrossRef]
- Nosé, S. A unified formulation of the constant temperature molecular dynamics methods. J. Chem. Phys. 1984, 81, 511–519. [Google Scholar] [CrossRef]
- Hoover, W.G. Canonical dynamics: Equilibrium phase-space distributions. Phys. Rev. A 1985, 31, 1695. [Google Scholar] [CrossRef]
- North, S.C.; Jorgensen, K.R.; Pricetolstoy, J.; Wilson, A.K. Population analysis and the effects of Gaussian basis set quality and quantum mechanical approach: Main group through heavy element species. Front. Chem. 2023, 11, 1152500. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, C.; Hattori, T.; Hamamoto, Y.; Krukowski, P.; Saito, A.; Osuga, H.; Morikawa, Y.; Kuwahara, Y. Chiral Recognition Mechanism of 2,13-Bis(hydroxymethyl)-[7]thiaheterohelicene on Ag(111) Investigated by STM and MD Simulation. Int. J. Mol. Sci. 2025, 26, 11458. https://doi.org/10.3390/ijms262311458
Ye C, Hattori T, Hamamoto Y, Krukowski P, Saito A, Osuga H, Morikawa Y, Kuwahara Y. Chiral Recognition Mechanism of 2,13-Bis(hydroxymethyl)-[7]thiaheterohelicene on Ag(111) Investigated by STM and MD Simulation. International Journal of Molecular Sciences. 2025; 26(23):11458. https://doi.org/10.3390/ijms262311458
Chicago/Turabian StyleYe, Changqing, Takuma Hattori, Yuji Hamamoto, Pawel Krukowski, Akira Saito, Hideji Osuga, Yoshitada Morikawa, and Yuji Kuwahara. 2025. "Chiral Recognition Mechanism of 2,13-Bis(hydroxymethyl)-[7]thiaheterohelicene on Ag(111) Investigated by STM and MD Simulation" International Journal of Molecular Sciences 26, no. 23: 11458. https://doi.org/10.3390/ijms262311458
APA StyleYe, C., Hattori, T., Hamamoto, Y., Krukowski, P., Saito, A., Osuga, H., Morikawa, Y., & Kuwahara, Y. (2025). Chiral Recognition Mechanism of 2,13-Bis(hydroxymethyl)-[7]thiaheterohelicene on Ag(111) Investigated by STM and MD Simulation. International Journal of Molecular Sciences, 26(23), 11458. https://doi.org/10.3390/ijms262311458





