Role of ERβ in Triple-Negative Breast Cancer Associated with p53 and Androgen Receptor
Abstract
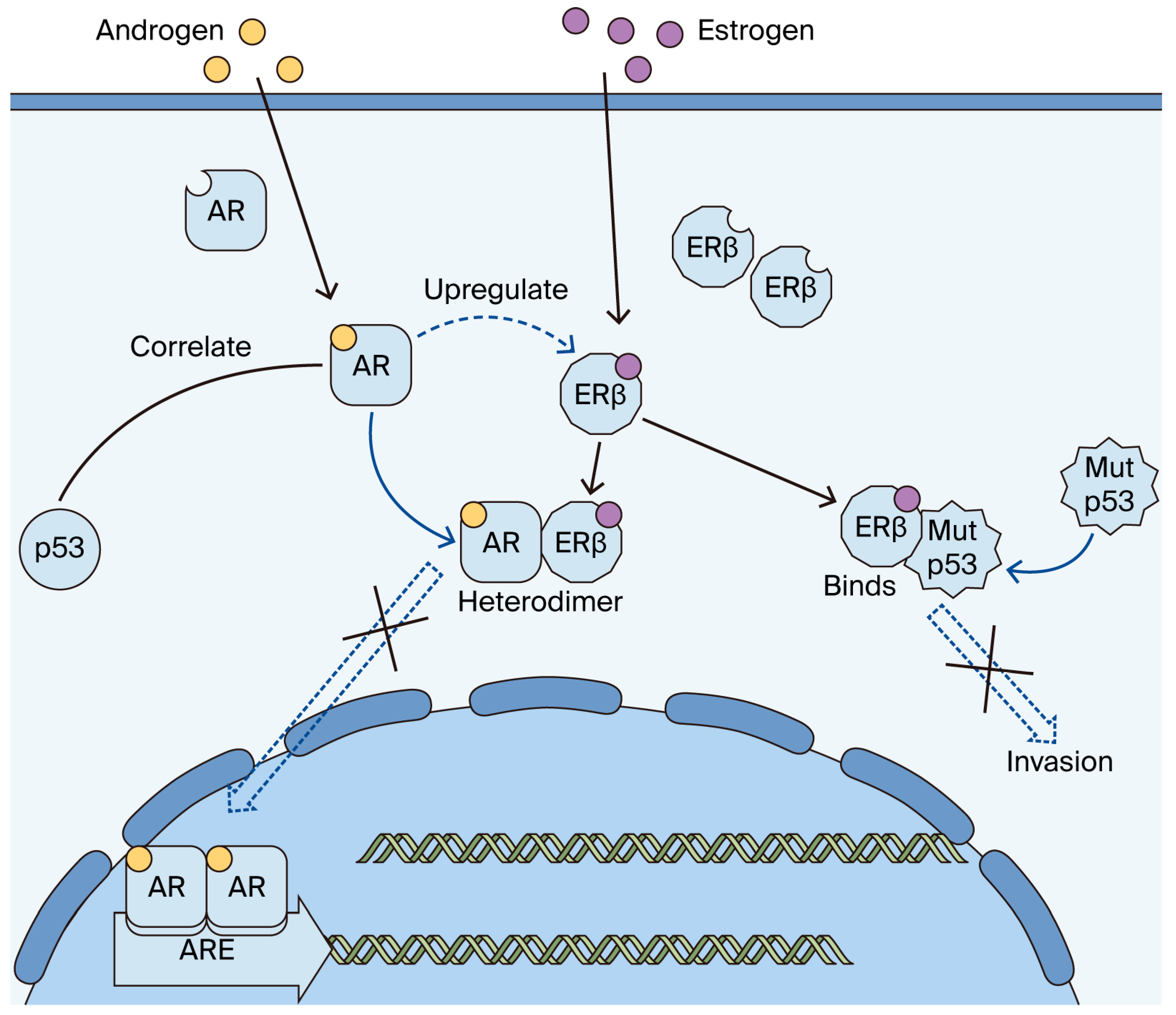
1. Introduction
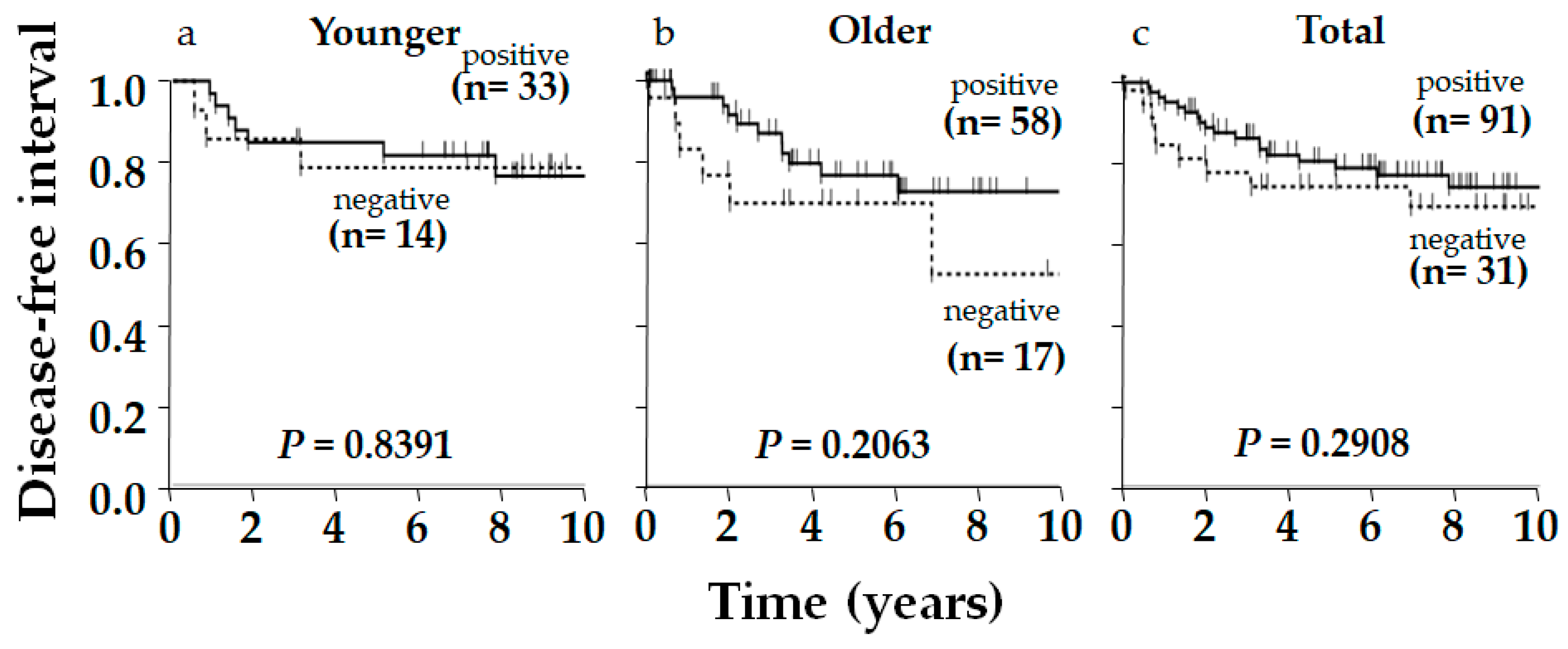
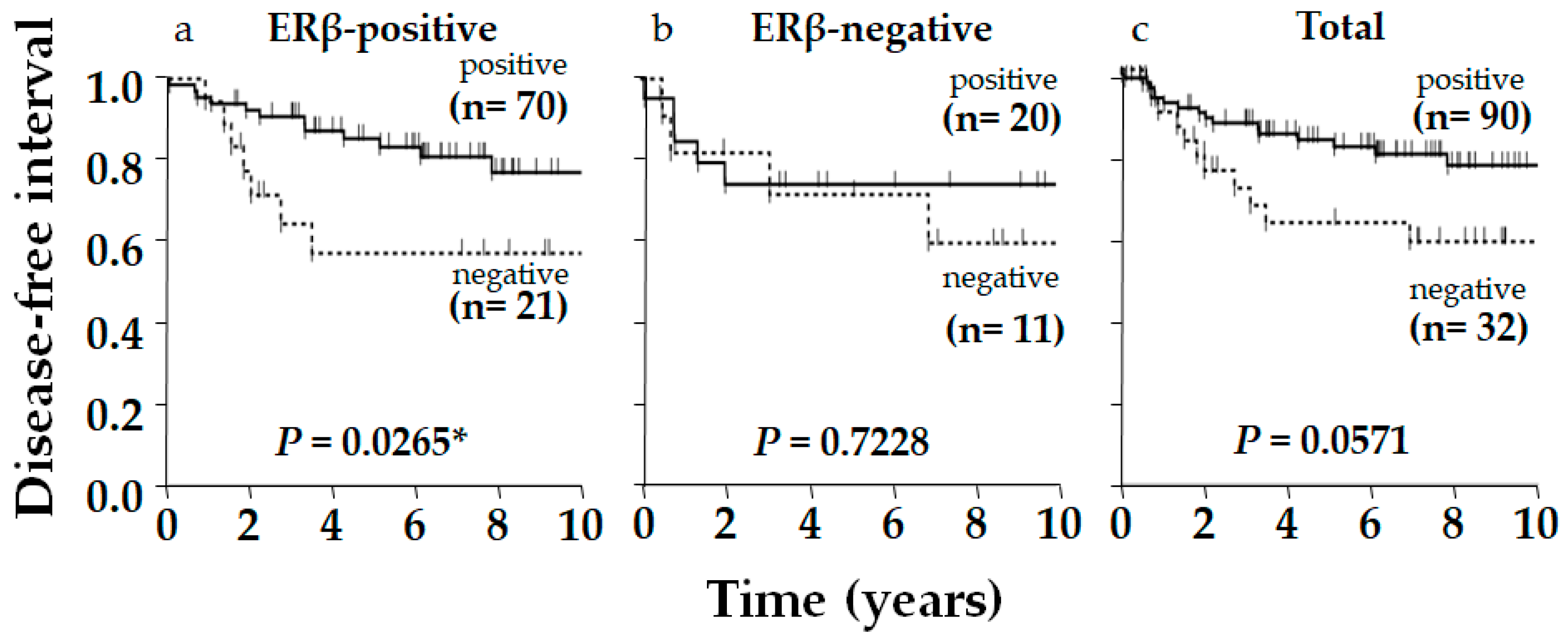
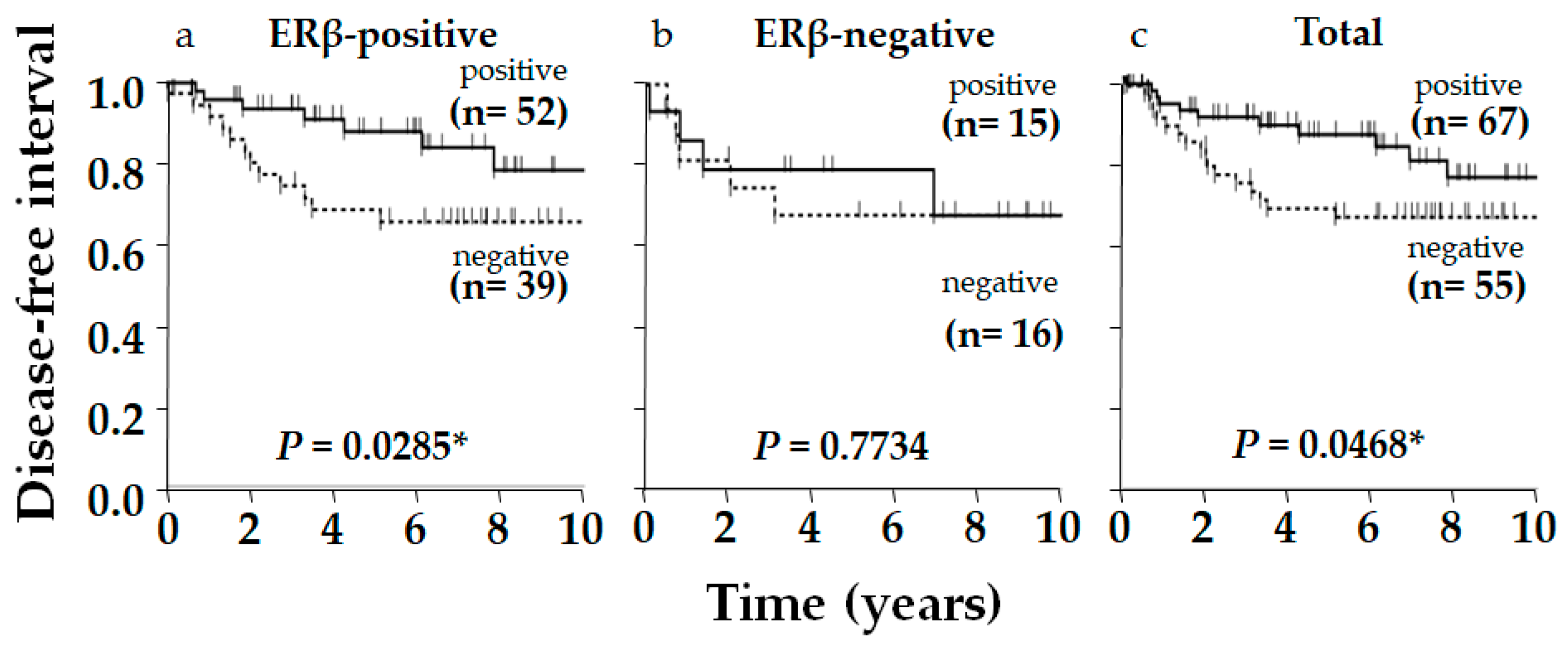
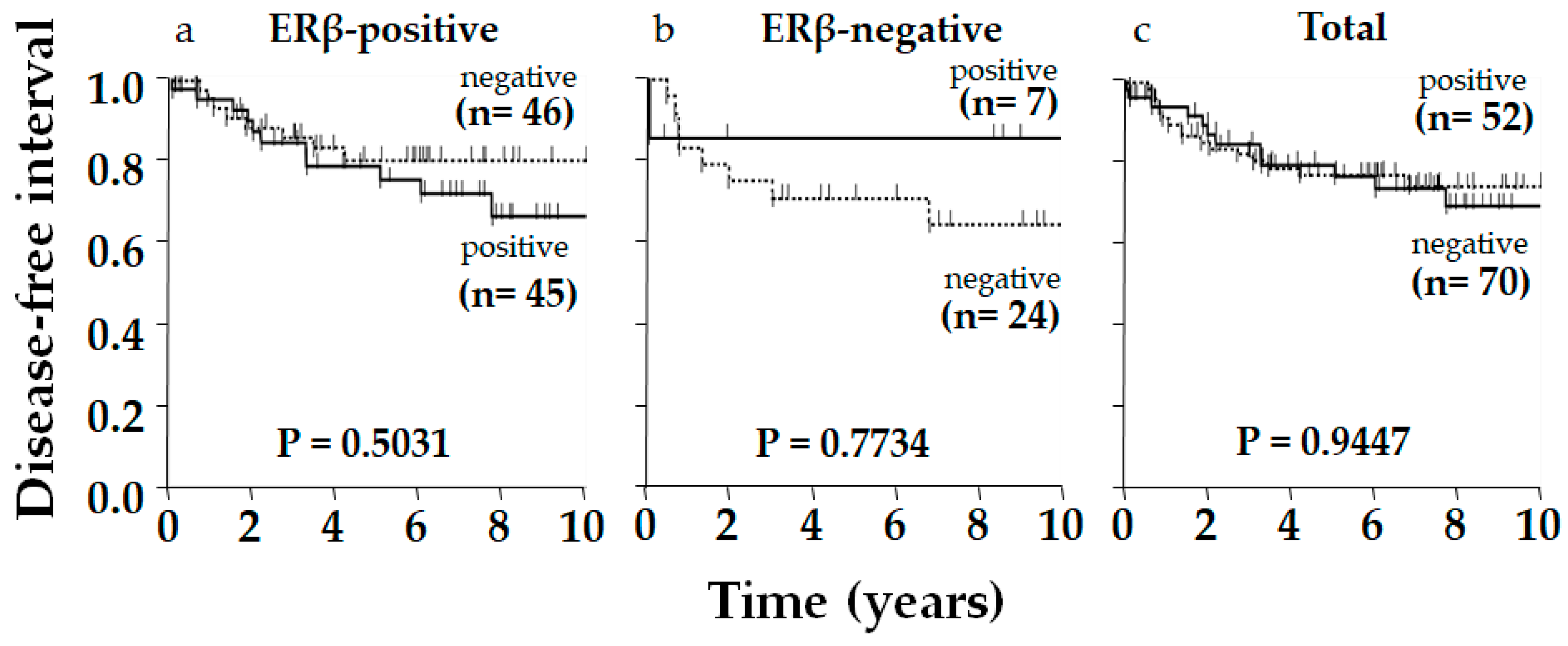
2. Results
3. Discussion
4. Materials and Methods
4.1. Subjects
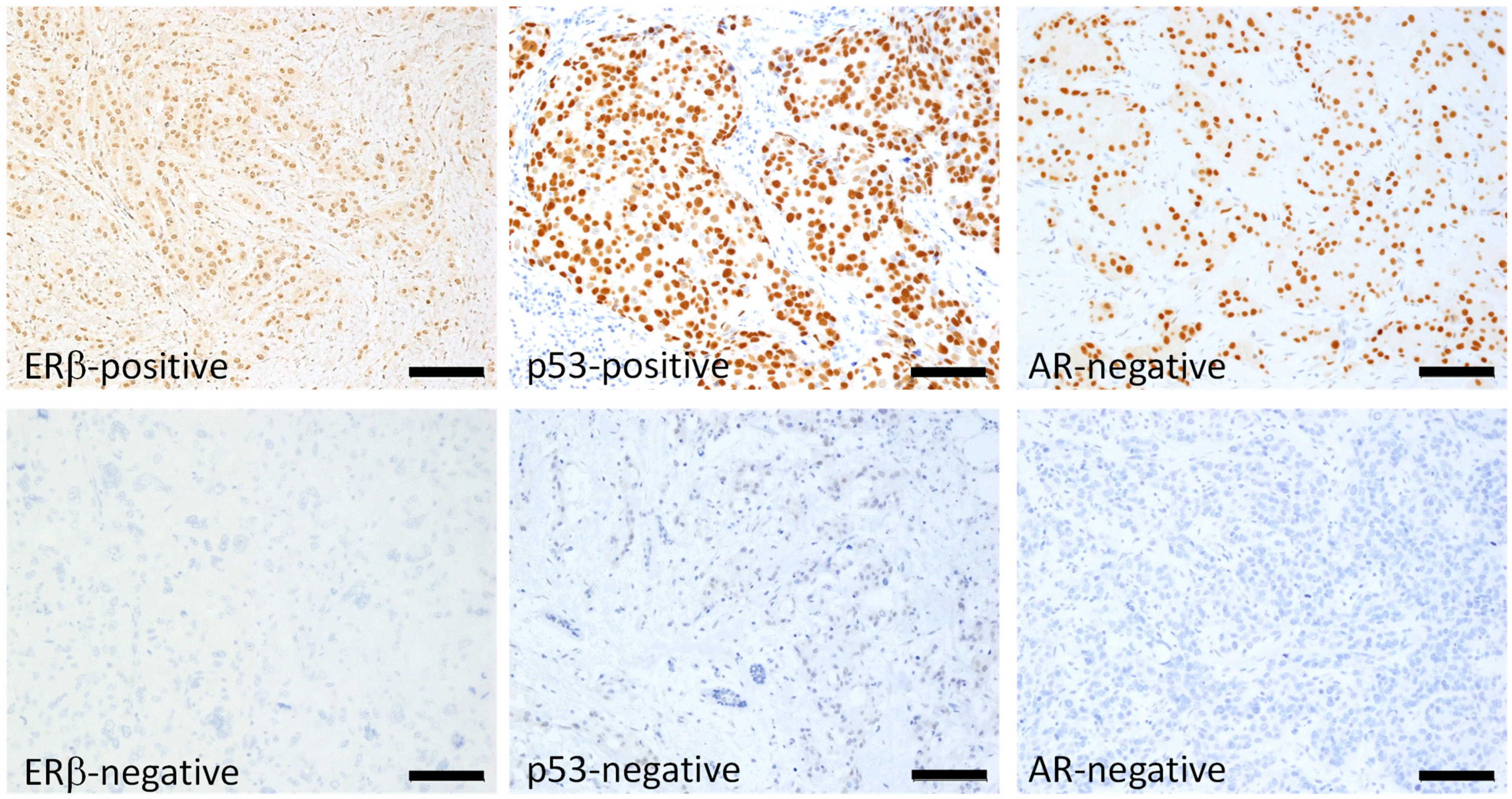
4.2. Immunohistochemistry
4.3. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AR | androgen receptor |
| ERα | estrogen receptor-α |
| ERβ | estrogen receptor-β |
| GPER | G protein-coupled estrogen receptor |
| HER2 | human epidermal growth factor receptor-2 |
| PgR | progesterone receptor |
| TNBC | triple-negative breast cancer(s) |
References
- Denkert, C.; Liedtke, C.; Tutt, A.; von Minckwitz, G. Molecular Alterations in Triple-Negative Breast Cancer-the Road to New Treatment Strategies. Lancet 2017, 389, 2430–2442. [Google Scholar] [CrossRef]
- Fu, X.; Yin, H.; Chen, X.; Yao, J.; Ma, Y.; Song, M.; Xu, H.; Yu, Q.; Du, S.; Qi, Y.; et al. Three Rounds of Stability-Guided Optimization and Systematical Evaluation of Oncolytic Peptide LTX-315. J. Med. Chem. 2024, 67, 3885–3908. [Google Scholar] [CrossRef]
- Treeck, O.; Schüler-Toprak, S.; Ortmann, O. Estrogen Actions in Triple-Negative Breast Cancer. Cells 2020, 9, 2358. [Google Scholar] [CrossRef] [PubMed]
- Shaaban, A.M.; O’neill, P.A.; Davies, M.P.A.; Sibson, R.; West, C.R.; Smith, P.H.; Foster, C.S. Declining Estrogen Receptor-beta Expression Defines Malignant Progression of Human Breast Neoplasia. Am. J. Pathol. 2003, 27, 1502–1512. [Google Scholar] [CrossRef] [PubMed]
- Grober, O.M.V.; Mutarelli, M.; Giurato, G.; Ravo, M.; Cicatiello, L.; De Filippo, M.R.; Ferraro, L.; Nassa, G.; Papa, M.F.; Paris, O.; et al. Global Analysis of Estrogen Receptor Beta Binding to Breast Cancer Cell Genome Reveals an Extensive Interplay with Estrogen Receptor Alpha for Target Gene Regulation. BMC Genom. 2011, 12, 36. [Google Scholar] [CrossRef]
- Treeck, O.; Juhasz-Boess, I.; Lattrich, C.; Horn, F.; Goerse, R.; Ortmann, O. Effects of Exon-Deleted Estrogen Receptor Β Transcript Variants on Growth, Apoptosis and Gene Expression of Human Breast Cancer Cell Lines. Breast Cancer Res. Treat. 2007, 110, 507–520. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Li, Y.; Dong, Y.; Liang, Y.; Qu, H.; Qi, D.; Lu, Y.; Jin, X.; Guo, Y.; Jia, Y.; et al. Estrogen Receptor Β Inhibits Breast Cancer Cells Migration and Invasion through CLDN6-Mediated Autophagy. J. Exp. Clin. Cancer Res. 2019, 38, 354. [Google Scholar] [CrossRef]
- Prossnitz, E.R.; Arterburn, J.B.; Smith, H.O.; Oprea, T.I.; Sklar, L.A.; Hathaway, H.J. Estrogen Signaling through the Transmembrane G Protein–Coupled Receptor GPR30. Annu. Rev. Physiol. 2008, 70, 165–190. [Google Scholar] [CrossRef]
- Sartorius, C.A.; Hanna, C.T.; Gril, B.; Cruz, H.; Serkova, N.J.; Huber, K.M.; Kabos, P.; Schedin, T.B.; Borges, V.F.; Steeg, P.S.; et al. Estrogen Promotes the Brain Metastatic Colonization of Triple Negative Breast Cancer Cells Via an Astrocyte-Mediated Paracrine Mechanism. Oncogene 2016, 35, 2881–2892. [Google Scholar] [CrossRef]
- Schüler-Toprak, S.; Häring, J.; Inwald, E.C.; Moehle, C.; Ortmann, O.; Treeck, O. Agonists and Knockdown of Estrogen Receptor Β Differentially Affect Invasion of Triple-Negative Breast Cancer Cells in Vitro. BMC Cancer 2016, 16, 951. [Google Scholar] [CrossRef]
- Hinsche, O.; Girgert, R.; Emons, G.; Gründker, C. Estrogen Receptor Β Selective Agonists Reduce Invasiveness of Triple-Negative Breast Cancer Cells. Int. J. Oncol. 2014, 46, 878–884. [Google Scholar] [CrossRef]
- Mukhopadhyay, U.K.; Oturkar, C.C.; Adams, C.; Wickramasekera, N.; Bansal, S.; Medisetty, R.; Miller, A.; Swetzig, W.M.; Silwal-Pandit, L.; Børresen-Dale, A.; et al. TP53 Status as a Determinant of Pro- vs Anti-Tumorigenic Effects of Estrogen Receptor-Beta in Breast Cancer. J. Natl. Cancer Inst. 2019, 111, 1202–1215. [Google Scholar] [CrossRef]
- Honma, N.; Matsuda, Y.; Mikami, T. Carcinogenesis of Triple-Negative Breast Cancer and Sex Steroid Hormones. Cancers 2021, 13, 2588. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, S.; Klimov, S.; Mittal, K.; Krishnamurti, U.; Li, X.; Oprea-Ilies, G.; Wetherilt, C.; Riaz, A.; Aleskandarany, M.A.; Green, A.R.; et al. Prognostic Role of Androgen Receptor in Triple Negative Breast Cancer: A Multi-Institutional Study. Cancers 2019, 11, 995. [Google Scholar] [CrossRef] [PubMed]
- Gasparini, P.; Fassan, M.; Cascione, L.; Guler, G.; Balci, S.; Irkkan, C.; Paisie, C.; Lovat, F.; Morrison, C.; Zhang, J.; et al. Androgen Receptor Status is a Prognostic Marker in Non-Basal Triple Negative Breast Cancers and Determines Novel Therapeutic Options. PLoS ONE 2014, 9, e88525. [Google Scholar] [CrossRef]
- Choi, J.E.; Kang, S.H.; Lee, S.J.; Bae, Y.K. Androgen Receptor Expression Predicts Decreased Survival in Early Stage Triple-Negative Breast Cancer. Ann. Surg. Oncol. 2014, 22, 82–89. [Google Scholar] [CrossRef]
- Mishra, A.; Mishra, S.K.; Sharanappa, V.; Krishnani, N.; Kumari, N.; Agarwal, G. Incidence and Prognostic Significance of Androgen Receptors (AR) in Indian Triple-Negative Breast Cancer (TNBC). Indian J. Surg. Oncol. 2024, 15, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Honma, N.; Ogata, H.; Yamada, A.; Matsuda, Y.; Kontani, K.; Miyashita, M.; Arai, T.; Sasaki, E.; Shibuya, K.; Mikami, T.; et al. Clinicopathological Characteristics and Prognostic Marker of Triple-Negative Breast Cancer in Older Women. Hum. Pathol. 2021, 111, 10–20. [Google Scholar] [CrossRef]
- Hu, X.; Chen, W.; Ma, H.; Jiang, K. Androgen Receptor Expression Identifies Patient with Favorable Outcome in Operable Triple Negative Breast Cancer. Oncotarget 2017, 8, 56364–56374. [Google Scholar] [CrossRef] [PubMed]
- Honma, N.; Horii, R.; Iwase, T.; Saji, S.; Younes, M.; Takubo, K.; Matsuura, M.; Ito, Y.; Akiyama, F.; Sakamoto, G. Clinical Importance of Estrogen Receptor-Β Evaluation in Breast Cancer Patients Treated with Adjuvant Tamoxifen Therapy. J. Clin. Oncol. 2008, 26, 3727–3734. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, C.; Chen, K.; Tang, H.; Tang, J.; Song, C.; Xie, X. ERβ1 Inversely Correlates with PTEN/PI3K/AKT Pathway and Predicts a Favorable Prognosis in Triple-Negative Breast Cancer. Breast Cancer Res. Treat. 2015, 152, 255–269. [Google Scholar]
- Wimberly, H.; Han, G.; Pinnaduwage, D.; Murphy, L.C.; Yang, X.R.; Andrulis, I.L.; Sherman, M.; Figueroa, J.; Rimm, D.L. ERβ Splice Variant Expression in Four Large Cohorts of Human Breast Cancer Patient Tumors. Breast Cancer Res. Treat. 2020, 146, 657–667. [Google Scholar]
- Shanle, E.K.; Onitilo, A.A.; Huang, W.; Kim, K.; Zang, C.; Engel, J.M.; Xu, W.; Wisinski, K.B. Prognostic Significance of Full-Length Estrogen Receptor Beta Expression in Stage I-III Triple Negative Breast Cancer. Am. J. Transl. Res. 2015, 5, 1246–1259. [Google Scholar]
- Ito, K.; Honma, N.; Ogata, H.; Yamada, A.; Miyashita, M.; Arai, T.; Sasaki, E.; Shibuya, K.; Mikami, T.; Sawaki, M. Clinicopathological Importance of Bcl-2 and p53 in Postmenopausal Triple-negative Breast Carcinoma and Association with Age. Pathol. Int. 2024, 74, 574–582. [Google Scholar] [CrossRef]
- Allred, D.C.; Clark, G.M.; Elledge, R.; Fuqua, S.A.; Brown, R.W.; Chamness, G.C.; Osborne, C.K.; McGuire, W.L. Association of p53 Protein Expression with Tumor Cell Proliferation Rate and Clinical Outcome in Node-Negative Breast Cancer. J. Natl. Cancer Inst. 1993, 85, 200–206. [Google Scholar]
- Green, D.R.; Kroemer, G. Cytoplasmic Functions of the Tumour Suppressor p53. Nature 2009, 458, 1127–1130. [Google Scholar] [CrossRef] [PubMed]
- Hao, Q.; Chen, J.; Liao, J.; Huang, Y.; Gan, Y.; Larisch, S.; Zeng, S.X.; Lu, H.; Zhou, X. p53 Induces ARTS to Promote Mitochondrial Apoptosis. Cell Death Dis. 2021, 12, 204. [Google Scholar] [CrossRef] [PubMed]
- Bertheau, P.; Lehmann-Che, J.; Varna, M.; Dumay, A.; Poirot, B.; Porcher, R.; Turpin, E.; Plassa, L.; de Roquancourt, A.; Bourstyn, E.; et al. P53 in Breast Cancer Subtypes and New Insights into Response to Chemotherapy. Breast 2013, 22 (Suppl. S2), 27. [Google Scholar] [CrossRef]
- Bado, I.; Nikolos, F.; Rajapaksa, G.; Gustafsson, J.; Thomas, C. ERβ Decreases the Invasiveness of Triple-Negative Breast Cancer Cells by Regulating Mutant p53 Oncogenic Function. Oncotarget 2016, 7, 13599–13611. [Google Scholar] [CrossRef]
- Oturkar, C.C.; Gandhi, N.; Rao, P.; Eng, K.H.; Miller, A.; Singh, P.K.; Zsiros, E.; Odunsi, K.O.; Das, G.M. Estrogen Receptor-Beta2 (ERβ2)–Mutant p53–FOXM1 Axis: A Novel Driver of Proliferation, Chemoresistance, and Disease Progression in High Grade Serous Ovarian Cancer (HGSOC). Cancers 2022, 14, 1120. [Google Scholar] [PubMed]
- Scarpetti, L.; Oturkar, C.C.; Juric, D.; Shellock, M.; Malvarosa, G.; Post, K.; Isakoff, S.; Wang, N.; Nahed, B.; Oh, K.; et al. Therapeutic Role of Tamoxifen for Triple-Negative Breast Cancer: Leveraging the Interaction between ERβ and Mutant p53. Oncologist 2023, 28, 358–363. [Google Scholar] [CrossRef]
- Song, W.; Tang, L.; Xu, Y.; Sun, Q.; Yang, F.; Guan, X. ERβ1 Inhibits Metastasis of Androgen Receptor-Positive Triple-Negative Breast Cancer by Suppressing ZEB1. J. Exp. Clin. Cancer Res. 2017, 36, 75. [Google Scholar] [CrossRef]
- Anestis, A.; Sarantis, P.; Theocharis, S.; Zoi, I.; Tryfonopoulos, D.; Korogiannos, A.; Koumarianou, A.; Xingi, E.; Thomaidou, D.; Kontos, M.; et al. Estrogen Receptor Beta Increases Sensitivity to Enzalutamide in Androgen Receptor-Positive Triple-Negative Breast Cancer. J. Cancer Res. Clin. Oncol. 2019, 145, 1221–1233. [Google Scholar] [CrossRef]
- Kuiper, G.G.; Lemmen, J.G.; Carlsson, B.; Corton, J.C.; Safe, S.H.; van der Saag, P.T.; van der Burg, B.; Gustafsson, J.A. Interaction of Estrogenic Chemicals and Phytoestrogens with Estrogen Receptor Beta. Endocrinology 1998, 139, 4252–4263. [Google Scholar] [CrossRef] [PubMed]
- Paruthiyil, S.; Parmar, H.; Kerekatte, V.; Cunha, G.R.; Firestone, G.L.; Leitman, D.C. Estrogen Receptor Beta Inhibits Human Breast Cancer Cell Proliferation and Tumor Formation by Causing a G2 Cell Cycle Arrest. Cancer Res. 2004, 64, 423–428. [Google Scholar] [CrossRef]
- Silwal-Pandit, L.; Vollan, H.K.M.; Chin, S.; Rueda, O.M.; Mckinney, S.; Osako, T.; Quigley, D.A.; Kristensen, V.N.; Aparicio, S.; Børresen-Dale, A.; et al. TP53 Mutation Spectrum in Breast Cancer is Subtype Specific and has Distinct Prognostic Relevance. Clin. Cancer Res. 2014, 20, 3569–3580, Erratum in Clin. Cancer Res. 2015, 21, 1502. [Google Scholar] [CrossRef]
- Hickey, T.E.; Robinson, J.L.L.; Carroll, J.S.; Tilley, W.D. Minireview: The Androgen Receptor in Breast Tissues: Growth Inhibitor, Tumor Suppressor, Oncogene? Mol. Endocrinol. 2012, 26, 1252–1267. [Google Scholar] [CrossRef]
- Ravaioli, S.; Maltoni, R.; Pasculli, B.; Parrella, P.; Giudetti, A.M.; Vergara, D.; Tumedei, M.M.; Pirini, F.; Bravaccini, S. Androgen Receptor in Breast Cancer: The “5W” Questions. Front. Endocrinol. 2022, 13, 977331. [Google Scholar] [CrossRef]
- Doane, A.S.; Danso, M.; Lal, P.; Donaton, M.; Zhang, L.; Hudis, C.; Gerald, W.L. An Estrogen Receptor-Negative Breast Cancer Subset Characterized by a Hormonally Regulated Transcriptional Program and Response to Androgen. Oncogene 2006, 25, 3994–4008. [Google Scholar] [CrossRef] [PubMed]
- Rizza, P.; Barone, I.; Zito, D.; Giordano, F.; Lanzino, M.; De Amicis, F.; Mauro, L.; Sisci, D.; Catalano, S.; Dahlman, K.; et al. Estrogen Receptor Beta as a Novel Target of Androgen Receptor Action in Breast Cancer Cell Lines. Breast Cancer Res. 2014, 16, R21. [Google Scholar] [CrossRef] [PubMed]
- Crespo, B.; Illera, J.C.; Silvan, G.; Lopez-Plaza, P.; Herrera De La Muela, M.; De La Puente Yagüe, M.; Diaz Del Arco, C.; Illera, M.J.; Caceres, S. Androgen and Estrogen Β Receptor Expression Enhances Efficacy of Antihormonal Treatments in Triple-Negative Breast Cancer Cell Lines. Int. J. Mol. Sci. 2024, 25, 1471. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; He, X.; Yu, Q.; Eng, C. Androgen Receptor-Induced Tumor Suppressor, KLLN, Inhibits Breast Cancer Growth and Transcriptionally Activates p53/p73-Mediated Apoptosis in Breast Carcinomas. Human Mol. Genet. 2013, 22, 2263–2272. [Google Scholar] [CrossRef]
- Honma, N.; Saji, S.; Mikami, T.; Yoshimura, N.; Mori, S.; Saito, Y.; Murayama, S.; Harada, N. Estrogen-Related Factors in the Frontal Lobe of Alzheimer’s Disease Patients and Importance of Body Mass Index. Sci. Rep. 2017, 7, 726. [Google Scholar] [CrossRef] [PubMed]
- Honma, N.; Sakamoto, G.; Akiyama, F.; Esaki, Y.; Sawabe, M.; Arai, T.; Hosoi, T.; Harada, N.; Younes, M.; Takubo, K. Breast Carcinoma in Women over the Age of 85: Distinct Histological Pattern and Androgen, Oestrogen, and Progesterone Receptor Status. Histopathology 2003, 42, 120–127. [Google Scholar] [CrossRef] [PubMed]
| Factors | ERβ | |||||
|---|---|---|---|---|---|---|
| Positive | Negative | Pos % | p-Value a | |||
| Age (y) | Younger | 33 | 14 | 70 | 0.38 | NS |
| Older | 58 | 17 | 77 | |||
| Size (mm) | ≤20 | 37 | 8 | 82 | 0.128 | NS |
| 20< | 53 | 23 | 70 | |||
| Nodal stats | Positive | 31 | 16 | 66 | 0.142 | NS |
| Negative | 54 | 15 | 78 | |||
| pStage (I and II vs. III) | III | 15 | 10 | 60 | 0.060 | NS |
| I and II | 76 | 21 | 78 | |||
| Histological grade | III | 69 | 21 | 77 | 0.377 | NS |
| I and II | 22 | 10 | 69 | |||
| Ki-67 | Positive | 45 | 7 | 87 | 0.009 * | Positive Correlation |
| Negative | 46 | 24 | 66 | |||
| AR | Positive | 52 | 15 | 78 | 0.396 | NS |
| Negative | 39 | 16 | 71 | |||
| p53 | Positive | 70 | 20 | 78 | 0.175 | NS |
| Negative | 21 | 11 | 66 | |||
| p53 | |||||
|---|---|---|---|---|---|
| Positive | Negative | Pos % | p-Value a | ||
| AR-Positive | 55 | 12 | 82 | 0.0211 * | Positive Correlation |
| AR-Negative | 35 | 20 | 64 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ito, K.; Honma, N.; Ogata, H.; Yamada, A.; Miyashita, M.; Arai, T.; Sasaki, E.; Shibuya, K.; Mikami, T.; Sawaki, M. Role of ERβ in Triple-Negative Breast Cancer Associated with p53 and Androgen Receptor. Int. J. Mol. Sci. 2025, 26, 11459. https://doi.org/10.3390/ijms262311459
Ito K, Honma N, Ogata H, Yamada A, Miyashita M, Arai T, Sasaki E, Shibuya K, Mikami T, Sawaki M. Role of ERβ in Triple-Negative Breast Cancer Associated with p53 and Androgen Receptor. International Journal of Molecular Sciences. 2025; 26(23):11459. https://doi.org/10.3390/ijms262311459
Chicago/Turabian StyleIto, Kei, Naoko Honma, Hideaki Ogata, Akimitsu Yamada, Mika Miyashita, Tomio Arai, Eiichi Sasaki, Kazutoshi Shibuya, Tetuo Mikami, and Masataka Sawaki. 2025. "Role of ERβ in Triple-Negative Breast Cancer Associated with p53 and Androgen Receptor" International Journal of Molecular Sciences 26, no. 23: 11459. https://doi.org/10.3390/ijms262311459
APA StyleIto, K., Honma, N., Ogata, H., Yamada, A., Miyashita, M., Arai, T., Sasaki, E., Shibuya, K., Mikami, T., & Sawaki, M. (2025). Role of ERβ in Triple-Negative Breast Cancer Associated with p53 and Androgen Receptor. International Journal of Molecular Sciences, 26(23), 11459. https://doi.org/10.3390/ijms262311459







