Feasibility of Patient-Derived 3D Gastrointestinal Stromal Tumour Models as Alternatives for In Vivo Mouse Models
Abstract
1. Introduction
2. Results
2.1. 2D vs. 3D Cell Lines
2.2. GIST Organoids
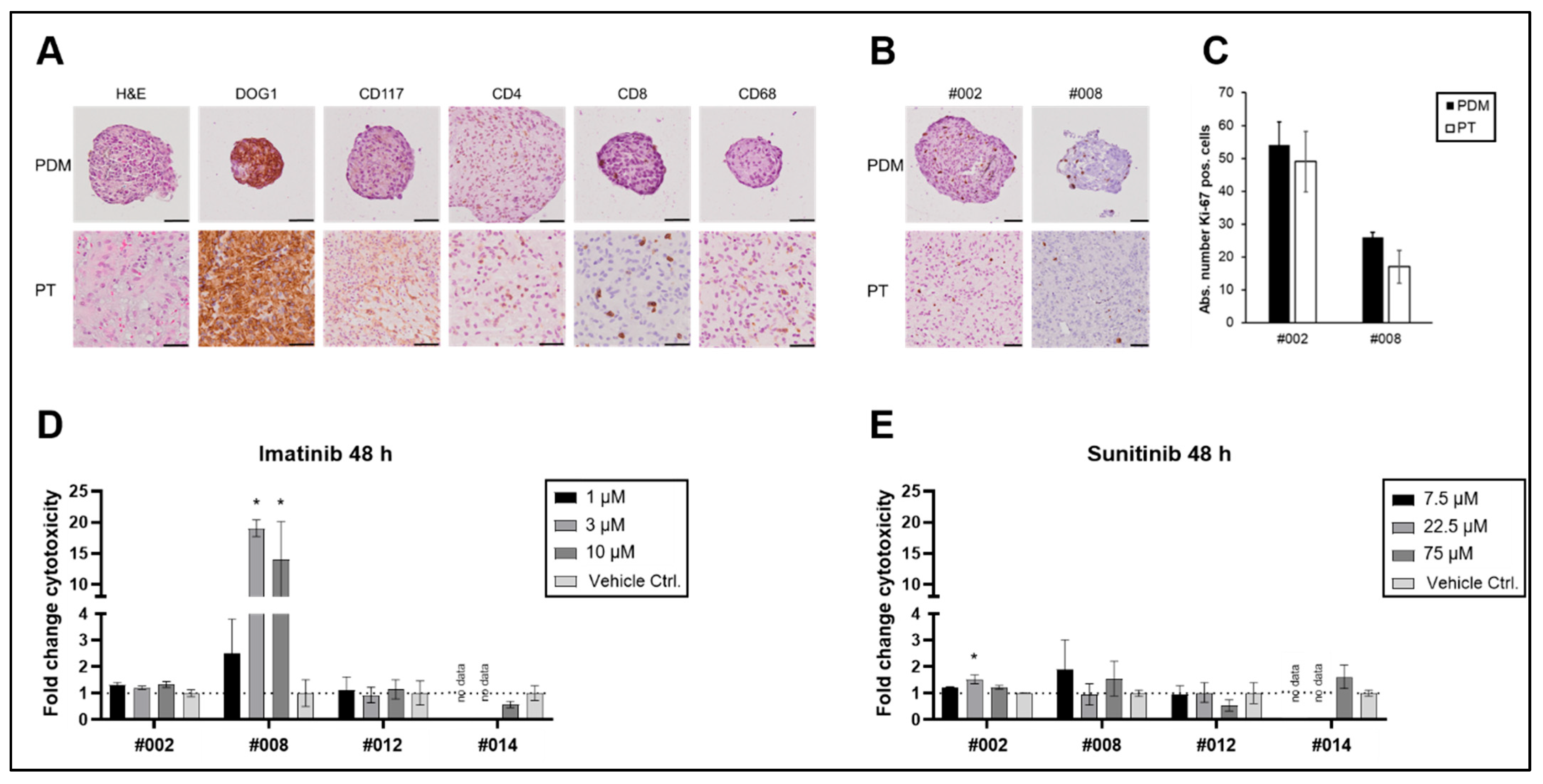
2.3. GIST Microtumours (PDMs)
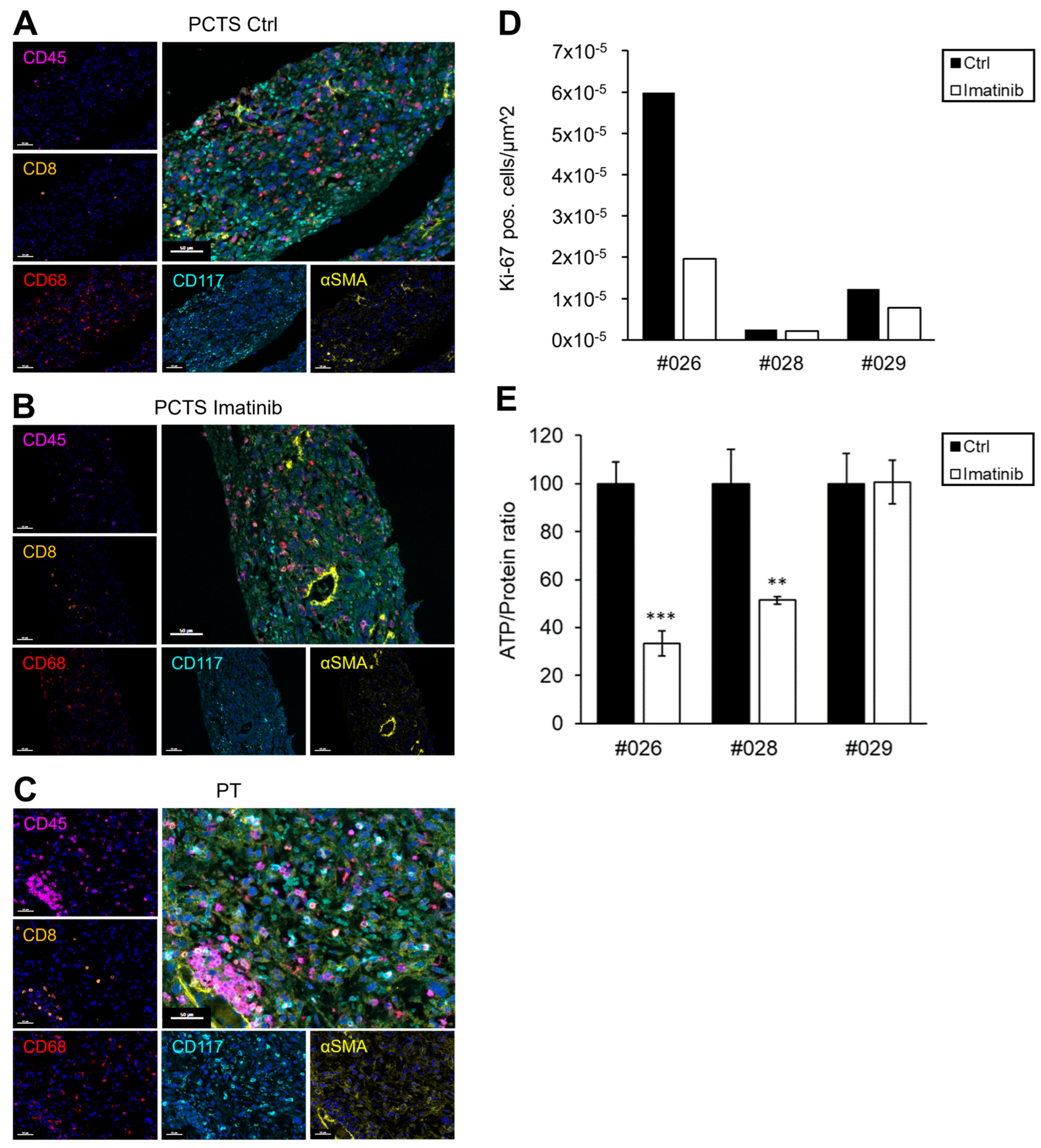
2.4. GIST Slice Cultures (PCTS)
3. Discussion
4. Materials and Methods
4.1. GIST Cell Culture (2D and 3D)
4.2. Organoid Culture
4.3. Generation and Processing of Patient-Derived Microtumours (PDMs) from Residual Fresh GIST Tissue
4.4. Precision-Cut Tumour Slice (PCTS) Generation
4.5. TKI Treatment
4.6. Viability Assays
4.7. Immunohistochemical (IHC) Staining
4.8. Quantification of Proliferation
4.9. Multiplex Immunofluorescence (mIF) Staining
4.10. Mutation Analysis
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| GIST | gastrointestinal stromal tumours |
| TKI | tyrosine kinase inhibitors |
| PDM | patient-derived microtumours |
| PCTS | precision-cut tumour slices |
| PDGFRA | platelet-derived growth factor |
References
- Klug, L.R.; Khosroyani, H.M.; Kent, J.D.; Heinrich, M.C. New Treatment Strategies for Advanced-Stage Gastrointestinal Stromal Tumours. Nat. Rev. Clin. Oncol. 2022, 19, 328–341. [Google Scholar] [CrossRef]
- Blay, J.-Y.; Kang, Y.-K.; Nishida, T.; von Mehren, M. Gastrointestinal Stromal Tumours. Nat. Rev. Dis. Primers 2021, 7, 22. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, M.C.; Rankin, C.; Blanke, C.D.; Demetri, G.D.; Borden, E.C.; Ryan, C.W.; von Mehren, M.; Blackstein, M.E.; Priebat, D.A.; Tap, W.D.; et al. Correlation of Long-Term Results of Imatinib in Advanced Gastrointestinal Stromal Tumors With Next-Generation Sequencing Results: Analysis of Phase 3 SWOG Intergroup Trial S0033. JAMA Oncol. 2017, 3, 944–952, Erratum in JAMA Oncol. 2017, 3, 1002. [Google Scholar] [CrossRef]
- Casali, P.G.; Zalcberg, J.; Le Cesne, A.; Reichardt, P.; Blay, J.-Y.; Lindner, L.H.; Judson, I.R.; Schöffski, P.; Leyvraz, S.; Italiano, A.; et al. Ten-Year Progression-Free and Overall Survival in Patients With Unresectable or Metastatic GI Stromal Tumors: Long-Term Analysis of the European Organisation for Research and Treatment of Cancer, Italian Sarcoma Group, and Australasian Gastrointestinal Trials Group Intergroup Phase III Randomized Trial on Imatinib at Two Dose Levels. J. Clin. Oncol. 2017, 35, 1713–1720. [Google Scholar] [CrossRef]
- Nishida, T.; Blay, J.-Y.; Hirota, S.; Kitagawa, Y.; Kang, Y.-K. The Standard Diagnosis, Treatment, and Follow-up of Gastrointestinal Stromal Tumors Based on Guidelines. Gastric Cancer 2016, 19, 3–14. [Google Scholar] [CrossRef]
- Demetri, G.D.; Reichardt, P.; Kang, Y.-K.; Blay, J.-Y.; Rutkowski, P.; Gelderblom, H.; Hohenberger, P.; Leahy, M.; von Mehren, M.; Joensuu, H.; et al. Efficacy and Safety of Regorafenib for Advanced Gastrointestinal Stromal Tumours after Failure of Imatinib and Sunitinib (GRID): An International, Multicentre, Randomised, Placebo-Controlled, Phase 3 Trial. Lancet 2013, 381, 295–302. [Google Scholar] [CrossRef]
- Demetri, G.D.; van Oosterom, A.T.; Garrett, C.R.; Blackstein, M.E.; Shah, M.H.; Verweij, J.; McArthur, G.; Judson, I.R.; Heinrich, M.C.; Morgan, J.A.; et al. Efficacy and Safety of Sunitinib in Patients with Advanced Gastrointestinal Stromal Tumour after Failure of Imatinib: A Randomised Controlled Trial. Lancet 2006, 368, 1329–1338. [Google Scholar] [CrossRef]
- Søreide, K.; Sandvik, O.M.; Søreide, J.A.; Giljaca, V.; Jureckova, A.; Bulusu, V.R. Global Epidemiology of Gastrointestinal Stromal Tumours (GIST): A Systematic Review of Population-Based Cohort Studies. Cancer Epidemiol. 2016, 40, 39–46. [Google Scholar] [CrossRef]
- Lee, D.M.; Sun, A.; Patil, S.S.; Liu, L.; Rao, A.V.; Trent, P.T.; Ali, A.A.; Liu, C.; Rausch, J.L.; Presutti, L.D.; et al. Targeting the Translational Machinery in Gastrointestinal Stromal Tumors (GIST): A New Therapeutic Vulnerability. Sci. Rep. 2022, 12, 8275. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Zhang, X.; Chen, Q.; Rao, X.; Qiu, E.; Wu, G.; Lin, Y.; Zeng, Z.; Zheng, B.; Li, Z.; et al. Patient-Derived Organoid Facilitating Personalized Medicine in Gastrointestinal Stromal Tumor With Liver Metastasis: A Case Report. Front. Oncol. 2022, 12, 920762. [Google Scholar] [CrossRef] [PubMed]
- Anderle, N.; Schäfer-Ruoff, F.; Staebler, A.; Kersten, N.; Koch, A.; Önder, C.; Keller, A.-L.; Liebscher, S.; Hartkopf, A.; Hahn, M.; et al. Breast Cancer Patient-Derived Microtumors Resemble Tumor Heterogeneity and Enable Protein-Based Stratification and Functional Validation of Individualized Drug Treatment. J. Exp. Clin. Cancer Res. 2023, 42, 210. [Google Scholar] [CrossRef]
- Anderle, N.; Koch, A.; Gierke, B.; Keller, A.-L.; Staebler, A.; Hartkopf, A.; Brucker, S.Y.; Pawlak, M.; Schenke-Layland, K.; Schmees, C. A Platform of Patient-Derived Microtumors Identifies Individual Treatment Responses and Therapeutic Vulnerabilities in Ovarian Cancer. Cancers 2022, 14, 2895. [Google Scholar] [CrossRef] [PubMed]
- Walter, B.; Hirsch, S.; Kuhlburger, L.; Stahl, A.; Schnabel, L.; Wisser, S.; Haeusser, L.A.; Tsiami, F.; Plöger, S.; Aghaallaei, N.; et al. Functionally-Instructed Modifiers of Response to ATR Inhibition in Experimental Glioma. J. Exp. Clin. Cancer Res. 2024, 43, 77. [Google Scholar] [CrossRef]
- Erne, E.; Anderle, N.; Schmees, C.; Stenzl, A. Patient-derived microtumors: Potential for therapeutic response prediction-a case study. Urologie 2022, 61, 739–744. [Google Scholar] [CrossRef]
- Przystal, J.M.; Becker, H.; Canjuga, D.; Tsiami, F.; Anderle, N.; Keller, A.-L.; Pohl, A.; Ries, C.H.; Schmittnaegel, M.; Korinetska, N.; et al. Targeting CSF1R Alone or in Combination with PD1 in Experimental Glioma. Cancers 2021, 13, 2400. [Google Scholar] [CrossRef]
- Walter, B.; Canjuga, D.; Yüz, S.G.; Ghosh, M.; Bozko, P.; Przystal, J.M.; Govindarajan, P.; Anderle, N.; Keller, A.-L.; Tatagiba, M.; et al. Argyrin F Treatment-Induced Vulnerabilities Lead to a Novel Combination Therapy in Experimental Glioma. Adv. Ther. 2021, 4, 2100078. [Google Scholar] [CrossRef]
- Murray, M.; Hatcher, H.; Jessop, F.; Williams, D.; Carroll, N.; Bulusu, R.; Judson, I. Treatment of Wild-Type Gastrointestinal Stromal Tumor (WT-GIST) with Imatinib and Sunitinib. Pediatr. Blood Cancer 2008, 50, 386–388. [Google Scholar] [CrossRef]
- Miettinen, M.; Paal, E.; Lasota, J.; Sobin, L.H. Gastrointestinal Glomus Tumors: A Clinicopathologic, Immunohistochemical, and Molecular Genetic Study of 32 Cases. Am. J. Surg. Pathol. 2002, 26, 301–311. [Google Scholar] [CrossRef]
- Bauer, S.; Yu, L.K.; Demetri, G.D.; Fletcher, J.A. Heat Shock Protein 90 Inhibition in Imatinib-Resistant Gastrointestinal Stromal Tumor. Cancer Res. 2006, 66, 9153–9161. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, T.; Sonobe, H.; Toyonaga, S.; Yamasaki, I.; Shuin, T.; Takano, A.; Araki, K.; Akimaru, K.; Yuri, K. Conventional and Molecular Cytogenetic Characterization of a New Human Cell Line, GIST-T1, Established from Gastrointestinal Stromal Tumor. Lab. Investig. 2002, 82, 663–665. [Google Scholar] [CrossRef] [PubMed]
- Tuveson, D.A.; Willis, N.A.; Jacks, T.; Griffin, J.D.; Singer, S.; Fletcher, C.D.; Fletcher, J.A.; Demetri, G.D. STI571 Inactivation of the Gastrointestinal Stromal Tumor C-KIT Oncoprotein: Biological and Clinical Implications. Oncogene 2001, 20, 5054–5058. [Google Scholar] [CrossRef]
- Gebreyohannes, Y.K.; Wozniak, A.; Zhai, M.-E.; Wellens, J.; Cornillie, J.; Vanleeuw, U.; Evans, E.; Gardino, A.K.; Lengauer, C.; Debiec-Rychter, M.; et al. Robust Activity of Avapritinib, Potent and Highly Selective Inhibitor of Mutated KIT, in Patient-Derived Xenograft Models of Gastrointestinal Stromal Tumors. Clin. Cancer Res. 2019, 25, 609–618. [Google Scholar] [CrossRef] [PubMed]
- Rubin, B.P.; Antonescu, C.R.; Scott-Browne, J.P.; Comstock, M.L.; Gu, Y.; Tanas, M.R.; Ware, C.B.; Woodell, J. A Knock-in Mouse Model of Gastrointestinal Stromal Tumor Harboring Kit K641E. Cancer Res. 2005, 65, 6631–6639. [Google Scholar] [CrossRef] [PubMed]
- Sommer, G.; Agosti, V.; Ehlers, I.; Rossi, F.; Corbacioglu, S.; Farkas, J.; Moore, M.; Manova, K.; Antonescu, C.R.; Besmer, P. Gastrointestinal Stromal Tumors in a Mouse Model by Targeted Mutation of the Kit Receptor Tyrosine Kinase. Proc. Natl. Acad. Sci. USA 2003, 100, 6706–6711. [Google Scholar] [CrossRef]
- Kondo, J.; Huh, W.J.; Franklin, J.L.; Heinrich, M.C.; Rubin, B.P.; Coffey, R.J. A Smooth Muscle-Derived, Braf-Driven Mouse Model of Gastrointestinal Stromal Tumor (GIST): Evidence for an Alternative GIST Cell-of-Origin. J. Pathol. 2020, 252, 441–450. [Google Scholar] [CrossRef]
- Blakely, A.M.; Glod, J.W.; Wedekind Malone, M.F. Taming the Wild-Type Gastrointestinal Stromal Tumor: Improved Tissue Culture. Clin. Cancer Res. 2022, 28, 3–4. [Google Scholar] [CrossRef]
- Edris, B.; Willingham, S.B.; Weiskopf, K.; Volkmer, A.K.; Volkmer, J.-P.; Mühlenberg, T.; Montgomery, K.D.; Contreras-Trujillo, H.; Czechowicz, A.; Fletcher, J.A.; et al. Anti-KIT Monoclonal Antibody Inhibits Imatinib-Resistant Gastrointestinal Stromal Tumor Growth. Proc. Natl. Acad. Sci. USA 2013, 110, 3501–3506. [Google Scholar] [CrossRef] [PubMed]
- Zook, P.; Pathak, H.B.; Belinsky, M.G.; Gersz, L.; Devarajan, K.; Zhou, Y.; Godwin, A.K.; von Mehren, M.; Rink, L. Combination of Imatinib Mesylate and AKT Inhibitor Provides Synergistic Effects in Preclinical Study of Gastrointestinal Stromal Tumor. Clin. Cancer Res. 2017, 23, 171–180. [Google Scholar] [CrossRef]
- Debiec-Rychter, M.; Sciot, R.; Le Cesne, A.; Schlemmer, M.; Hohenberger, P.; van Oosterom, A.T.; Blay, J.-Y.; Leyvraz, S.; Stul, M.; Casali, P.G.; et al. KIT Mutations and Dose Selection for Imatinib in Patients with Advanced Gastrointestinal Stromal Tumours. Eur. J. Cancer 2006, 42, 1093–1103. [Google Scholar] [CrossRef]
- Heinrich, M.C.; Corless, C.L.; Demetri, G.D.; Blanke, C.D.; von Mehren, M.; Joensuu, H.; McGreevey, L.S.; Chen, C.-J.; Van den Abbeele, A.D.; Druker, B.J.; et al. Kinase Mutations and Imatinib Response in Patients with Metastatic Gastrointestinal Stromal Tumor. J. Clin. Oncol. 2003, 21, 4342–4349. [Google Scholar] [CrossRef]
- Koch, J.; Mönch, D.; Maaß, A.; Gromoll, C.; Hehr, T.; Leibold, T.; Schlitt, H.J.; Dahlke, M.-H.; Renner, P. Three Dimensional Cultivation Increases Chemo- and Radioresistance of Colorectal Cancer Cell Lines. PLoS ONE 2021, 16, e0244513. [Google Scholar] [CrossRef]
- Fontoura, J.C.; Viezzer, C.; dos Santos, F.G.; Ligabue, R.A.; Weinlich, R.; Puga, R.D.; Antonow, D.; Severino, P.; Bonorino, C. Comparison of 2D and 3D Cell Culture Models for Cell Growth, Gene Expression and Drug Resistance. Mater. Sci. Eng. C 2020, 107, 110264. [Google Scholar] [CrossRef]
- Minami, F.; Sasaki, N.; Shichi, Y.; Gomi, F.; Michishita, M.; Ohkusu-Tsukada, K.; Toyoda, M.; Takahashi, K.; Ishiwata, T. Morphofunctional Analysis of Human Pancreatic Cancer Cell Lines in 2- and 3-Dimensional Cultures. Sci. Rep. 2021, 11, 6775. [Google Scholar] [CrossRef]
- Gassl, V.; Aberle, M.R.; Boonen, B.; Vaes, R.D.W.; Olde Damink, S.W.M.; Rensen, S.S. Chemosensitivity of 3D Pancreatic Cancer Organoids Is Not Affected by Transformation to 2D Culture or Switch to Physiological Culture Medium. Cancers 2022, 14, 5617. [Google Scholar] [CrossRef] [PubMed]
- Fabro, F.; Kannegieter, N.M.; de Graaf, E.L.; Queiroz, K.; Lamfers, M.L.M.; Ressa, A.; Leenstra, S. Novel Kinome Profiling Technology Reveals Drug Treatment Is Patient and 2D/3D Model Dependent in Glioblastoma. Front. Oncol. 2022, 12, 1012236. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Stange, D.E.; Ferrante, M.; Vries, R.G.J.; Van Es, J.H.; Van den Brink, S.; Van Houdt, W.J.; Pronk, A.; Van Gorp, J.; Siersema, P.D.; et al. Long-Term Expansion of Epithelial Organoids from Human Colon, Adenoma, Adenocarcinoma, and Barrett’s Epithelium. Gastroenterology 2011, 141, 1762–1772. [Google Scholar] [CrossRef]
- Zhao, Z.; Chen, X.; Dowbaj, A.M.; Sljukic, A.; Bratlie, K.; Lin, L.; Fong, E.L.S.; Balachander, G.M.; Chen, Z.; Soragni, A.; et al. Organoids. Nat. Rev. Methods Primers 2022, 2, 94. [Google Scholar] [CrossRef]
- Chandra, L.; Borcherding, D.C.; Kingsbury, D.; Atherly, T.; Ambrosini, Y.M.; Bourgois-Mochel, A.; Yuan, W.; Kimber, M.; Qi, Y.; Wang, Q.; et al. Derivation of Adult Canine Intestinal Organoids for Translational Research in Gastroenterology. BMC Biol. 2019, 17, 33. [Google Scholar] [CrossRef] [PubMed]
- Derouet, M.F.; Allen, J.; Wilson, G.W.; Ng, C.; Radulovich, N.; Kalimuthu, S.; Tsao, M.-S.; Darling, G.E.; Yeung, J.C. Towards Personalized Induction Therapy for Esophageal Adenocarcinoma: Organoids Derived from Endoscopic Biopsy Recapitulate the Pre-Treatment Tumor. Sci. Rep. 2020, 10, 14514. [Google Scholar] [CrossRef]
- Wallisch, S.; Neef, S.K.; Denzinger, L.; Mönch, D.; Koch, J.; Marzi, J.; Mürdter, T.; Janssen, N. Protocol for Establishing a Coculture with Fibroblasts and Colorectal Cancer Organoids. STAR Protoc. 2023, 4, 102481. [Google Scholar] [CrossRef]
- Dong, M.; Böpple, K.; Thiel, J.; Winkler, B.; Liang, C.; Schueler, J.; Davies, E.J.; Barry, S.T.; Metsalu, T.; Mürdter, T.E.; et al. Perfusion Air Culture of Precision-Cut Tumor Slices: An Ex Vivo System to Evaluate Individual Drug Response under Controlled Culture Conditions. Cells 2023, 12, 807. [Google Scholar] [CrossRef]
- Rossi, F.; Ehlers, I.; Agosti, V.; Socci, N.D.; Viale, A.; Sommer, G.; Yozgat, Y.; Manova, K.; Antonescu, C.R.; Besmer, P. Oncogenic Kit Signaling and Therapeutic Intervention in a Mouse Model of Gastrointestinal Stromal Tumor. Proc. Natl. Acad. Sci. USA 2006, 103, 12843–12848. [Google Scholar] [CrossRef] [PubMed]
- Ran, L.; Sirota, I.; Cao, Z.; Murphy, D.; Chen, Y.; Shukla, S.; Xie, Y.; Kaufmann, M.C.; Gao, D.; Zhu, S.; et al. Combined Inhibition of MAP Kinase and KIT Signaling Synergistically Destabilizes ETV1 and Suppresses GIST Tumour Growth. Cancer Discov. 2015, 5, 304–315. [Google Scholar] [CrossRef]
- Zhao, Y.; Weng, Z.; Zhou, X.; Xu, Z.; Cao, B.; Wang, B.; Li, J. Mesenchymal Stromal Cells Promote the Drug Resistance of Gastrointestinal Stromal Tumors by Activating the PI3K-AKT Pathway via TGF-Β2. J. Transl. Med. 2023, 21, 219. [Google Scholar] [CrossRef]
- Roulleaux Dugage, M.; Jones, R.L.; Trent, J.; Champiat, S.; Dumont, S. Beyond the Driver Mutation: Immunotherapies in Gastrointestinal Stromal Tumors. Front. Immunol. 2021, 12, 715727. [Google Scholar] [CrossRef] [PubMed]
- Etherington, M.S.; Hanna, A.N.; Medina, B.D.; Liu, M.; Tieniber, A.D.; Kwak, H.V.; Tardy, K.J.; Levin, L.; Do, K.J.; Rossi, F.; et al. Tyrosine Kinase Inhibition Activates Intratumoral Γδ T Cells in Gastrointestinal Stromal Tumor. Cancer Immunol. Res. 2024, 12, 107–119. [Google Scholar] [CrossRef]
- Vitiello, G.A.; Bowler, T.G.; Liu, M.; Medina, B.D.; Zhang, J.Q.; Param, N.J.; Loo, J.K.; Goldfeder, R.L.; Chibon, F.; Rossi, F.; et al. Differential Immune Profiles Distinguish the Mutational Subtypes of Gastrointestinal Stromal Tumor. J. Clin. Investig. 2019, 129, 1863–1877. [Google Scholar] [CrossRef] [PubMed]
- Indio, V.; Astolfi, A.; Urbini, M.; Nannini, M.; Pantaleo, M.A. Genetics and Treatment of Gastrointestinal Stromal Tumors with Immune Checkpoint Inhibitors: What Do We Know? Pharmacogenomics 2020, 21, 231–234. [Google Scholar] [CrossRef]
- Indio, V.; Astolfi, A.; Tarantino, G.; Urbini, M.; Patterson, J.; Nannini, M.; Saponara, M.; Gatto, L.; Santini, D.; do Valle, I.F.; et al. Integrated Molecular Characterization of Gastrointestinal Stromal Tumors (GIST) Harboring the Rare D842V Mutation in PDGFRA Gene. Int. J. Mol. Sci. 2018, 19, 732. [Google Scholar] [CrossRef]
- Nishioka, Y.; Aono, Y.; Sone, S. Role of Tyrosine Kinase Inhibitors in Tumor Immunology. Immunotherapy 2011, 3, 107–116. [Google Scholar] [CrossRef]
- Haubeiss, S.; Schmid, J.O.; Mürdter, T.E.; Sonnenberg, M.; Friedel, G.; van der Kuip, H.; Aulitzky, W.E. Dasatinib Reverses Cancer-Associated Fibroblasts (CAFs) from Primary Lung Carcinomas to a Phenotype Comparable to That of Normal Fibroblasts. Mol. Cancer 2010, 9, 168. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.; Tang, C.-M.; Banerjee, S.; Yebra, M.; Noh, S.; Burgoyne, A.M.; De la Torre, J.; Siena, M.D.; Liu, M.; Klug, L.R.; et al. Cancer-Associated Fibroblast Secretion of PDGFC Promotes Gastrointestinal Stromal Tumor Growth and Metastasis. Oncogene 2021, 40, 1957–1973. [Google Scholar] [CrossRef]
- Kinoshita, K.; Nakagawa, K.; Hamada, J.-I.; Hida, Y.; Tada, M.; Kondo, S.; Moriuchi, T. Imatinib Mesylate Inhibits the Proliferation-Stimulating Effect of Human Lung Cancer-Associated Stromal Fibroblasts on Lung Cancer Cells. Int. J. Oncol. 2010, 37, 869–877. [Google Scholar] [CrossRef]
- Dong, M.; Philippi, C.; Loretz, B.; Nafee, N.; Schaefer, U.F.; Friedel, G.; Ammon-Treiber, S.; Griese, E.-U.; Lehr, C.-M.; Klotz, U.; et al. Tissue Slice Model of Human Lung Cancer to Investigate Telomerase Inhibition by Nanoparticle Delivery of Antisense 2′-O-Methyl-RNA. Int. J. Pharm. 2011, 419, 33–42. [Google Scholar] [CrossRef]
- Hickman, J.A.; Graeser, R.; de Hoogt, R.; Vidic, S.; Brito, C.; Gutekunst, M.; van der Kuip, H. IMI PREDECT Consortium Three-Dimensional Models of Cancer for Pharmacology and Cancer Cell Biology: Capturing Tumor Complexity in Vitro/Ex Vivo. Biotechnol. J. 2014, 9, 1115–1128. [Google Scholar] [CrossRef]
- Davies, E.J.; Dong, M.; Gutekunst, M.; Närhi, K.; van Zoggel, H.J.A.A.; Blom, S.; Nagaraj, A.; Metsalu, T.; Oswald, E.; Erkens-Schulze, S.; et al. Capturing Complex Tumour Biology in vitro: Histological and Molecular Characterisation of Precision Cut Slices. Sci. Rep. 2015, 5, 17187. [Google Scholar] [CrossRef] [PubMed]
- Roife, D.; Dai, B.; Kang, Y.; Rios Perez, M.V.; Pratt, M.; Li, X.; Fleming, J.B. Ex Vivo Testing of Patient-Derived Xenografts Mirrors the Clinical Outcome of Patients with Pancreatic Ductal Adenocarcinoma. Clin. Cancer Res. 2016, 22, 6021–6030. [Google Scholar] [CrossRef] [PubMed]
- Neef, S.K.; Winter, S.; Hofmann, U.; Mürdter, T.E.; Schaeffeler, E.; Horn, H.; Buck, A.; Walch, A.; Hennenlotter, J.; Ott, G.; et al. Optimized Protocol for Metabolomic and Lipidomic Profiling in Formalin-Fixed Paraffin-Embedded Kidney Tissue by LC-MS. Anal. Chim. Acta 2020, 1134, 125–135. [Google Scholar] [CrossRef]
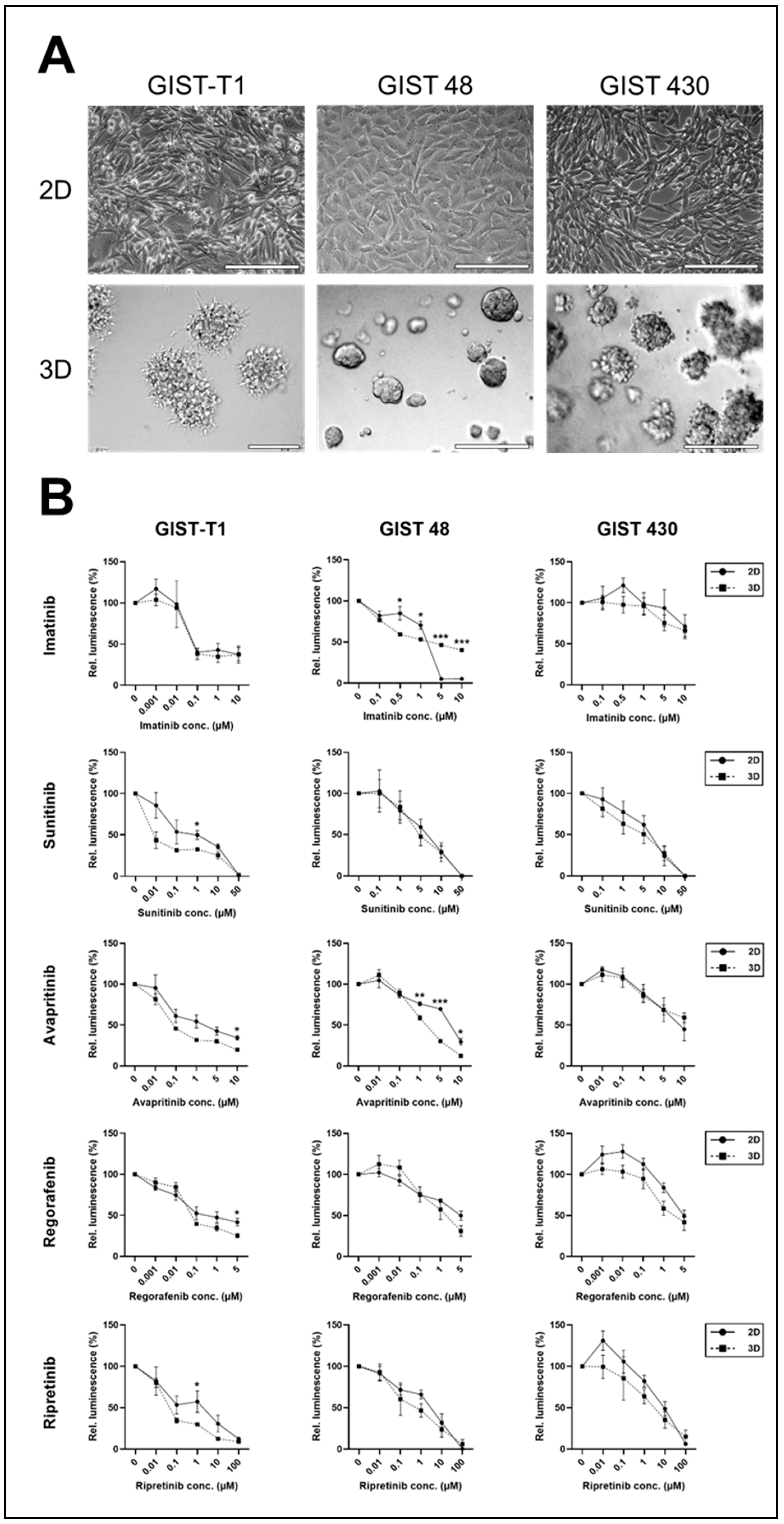
| Cell Line | c-KIT | PDGFRA | ||||
|---|---|---|---|---|---|---|
| Exon 9 | Exon 11 | Exon 13 | Exon 17 | Exon 18 | Exon 12 | |
| GIST-T1 | WT | V560_Y578del | K642E (homozygous) | WT | WT | WT |
| GIST 48 * | WT | V560D (homozygous) | WT | D820A | WT | WT |
| GIST 430 * | WT | V560_L576del | V654A | WT | WT | WT |
| Pat ID | Mutation Status | Ki-67 Index | Risk Stratification (Miettinen [18]) | Expected Clinical Response | TKI-Therapy After Resection | PDM Response | Match In Vitro vs. Expectation | Clinical Follow-Up (Months After Surgery) |
|---|---|---|---|---|---|---|---|---|
| #002 | PDGFRA exon 18: D842V | - | very low | imatinib-resistant | no TKI therapy recommended | - | yes | no relapse (27) |
| #008 | WT | <1% | intermediate | imatinib-resistant | no TKI therapy recommended | ++ | no, but case reports with successful therapy [17] | - |
| #012 | c-KIT exon 11: V559D | - | low (3a) | imatinib-sensitive | patient decision: no TKI therapy | - | no | no relapse (26) |
| #014 | c-KIT exon 11: W557R | 10% | low (3a) | imatinib-sensitive | individual decision: no TKI therapy | - | no | - |
| Pat ID | Mutation Status | Ki-67 Index | Risk Stratification (Miettinen [18]) | Expected Clinical Response | TKI-Therapy After Resection | PCTS Response (Ki-67/ATP Assay) | Match In Vitro vs. Expectation | Clinical Follow-Up (Months After Surgery) |
|---|---|---|---|---|---|---|---|---|
| #024 | c-KIT exon 11: 1648-4_1673del | <1% | - | imatinib-sensitive | permanent imatinib treatment | 17%/38% (due to lower concentration) | yes | no relapse (16) |
| #026 | c-KIT exon 9: A502_Y503dup | <5% | - | imatinib-sensitive | no TKI therapy recommended | 67%/67% | yes | - |
| #028 | PDGFRA exon 18: D842V | 3–4% | 3b | imatinib-resistant | no TKI therapy recommended | 16%/49% | yes | no relapse (7) |
| #029 | PDGFRA exon 18: D842V | <5% | 2 | imatinib-resistant | no TKI therapy recommended | 37%/0% | yes | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mönch, D.; Thiel, J.; Dong, M.; Maaß, A.; Wegner, E.; Binner, A.; Staiger, A.M.; Kurz, K.S.; Ott, G.; Renner, P.; et al. Feasibility of Patient-Derived 3D Gastrointestinal Stromal Tumour Models as Alternatives for In Vivo Mouse Models. Int. J. Mol. Sci. 2025, 26, 11456. https://doi.org/10.3390/ijms262311456
Mönch D, Thiel J, Dong M, Maaß A, Wegner E, Binner A, Staiger AM, Kurz KS, Ott G, Renner P, et al. Feasibility of Patient-Derived 3D Gastrointestinal Stromal Tumour Models as Alternatives for In Vivo Mouse Models. International Journal of Molecular Sciences. 2025; 26(23):11456. https://doi.org/10.3390/ijms262311456
Chicago/Turabian StyleMönch, Dina, Julia Thiel, Meng Dong, Annika Maaß, Eileen Wegner, Anna Binner, Annette M. Staiger, Katrin S. Kurz, German Ott, Philipp Renner, and et al. 2025. "Feasibility of Patient-Derived 3D Gastrointestinal Stromal Tumour Models as Alternatives for In Vivo Mouse Models" International Journal of Molecular Sciences 26, no. 23: 11456. https://doi.org/10.3390/ijms262311456
APA StyleMönch, D., Thiel, J., Dong, M., Maaß, A., Wegner, E., Binner, A., Staiger, A. M., Kurz, K. S., Ott, G., Renner, P., Leibold, T., Schmees, C., Mürdter, T. E., Schwab, M., Dahlke, M.-H., & Koch, J. (2025). Feasibility of Patient-Derived 3D Gastrointestinal Stromal Tumour Models as Alternatives for In Vivo Mouse Models. International Journal of Molecular Sciences, 26(23), 11456. https://doi.org/10.3390/ijms262311456







