Rose Bengal-Incorporated Supramolecular Gels as a Topical Platform for Localized Antimicrobial Photodynamic Therapy
Abstract
1. Introduction
2. Results and Discussion
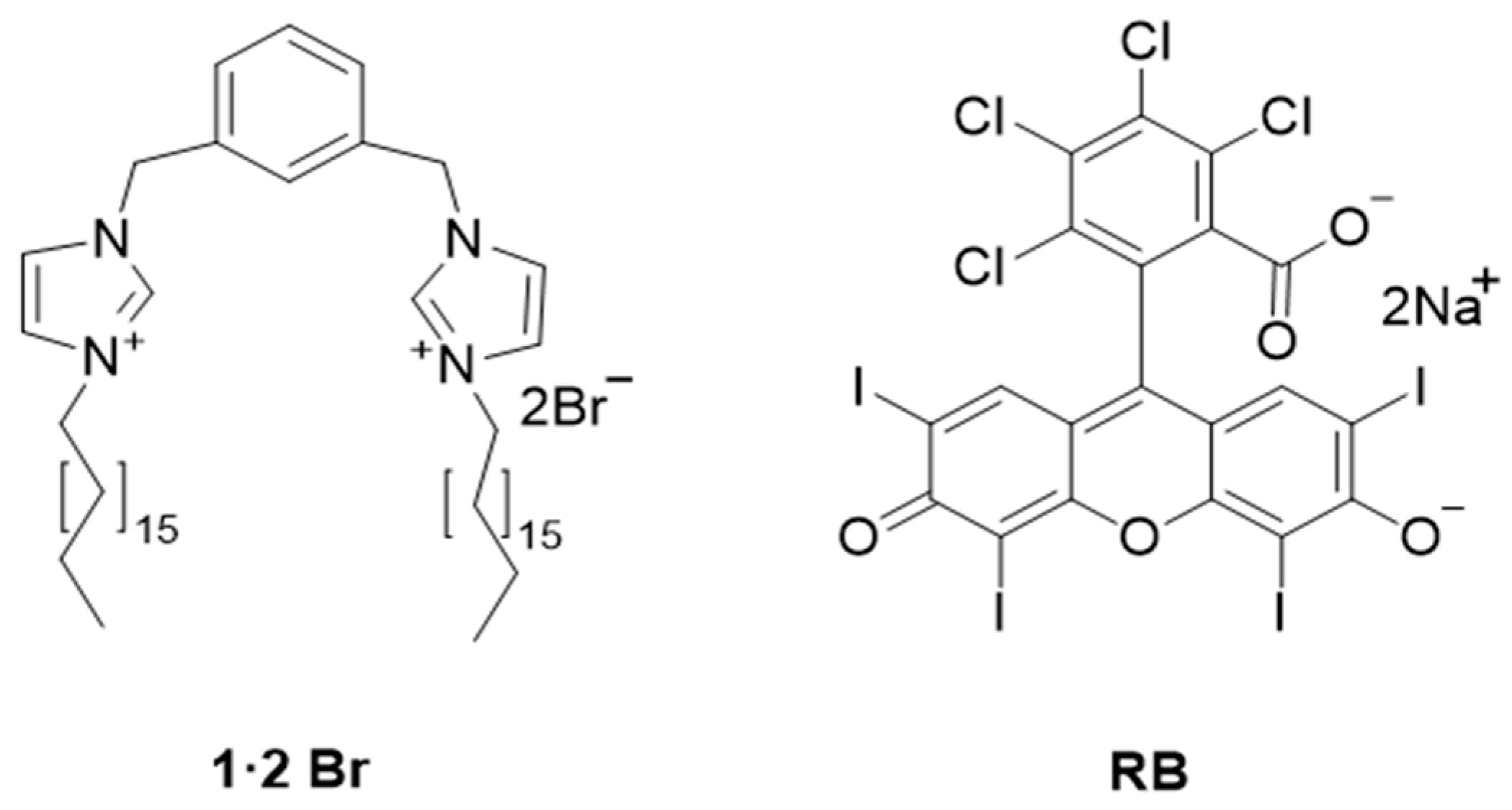
2.1. Self-Assembly of Gels
2.2. Gels Characterization
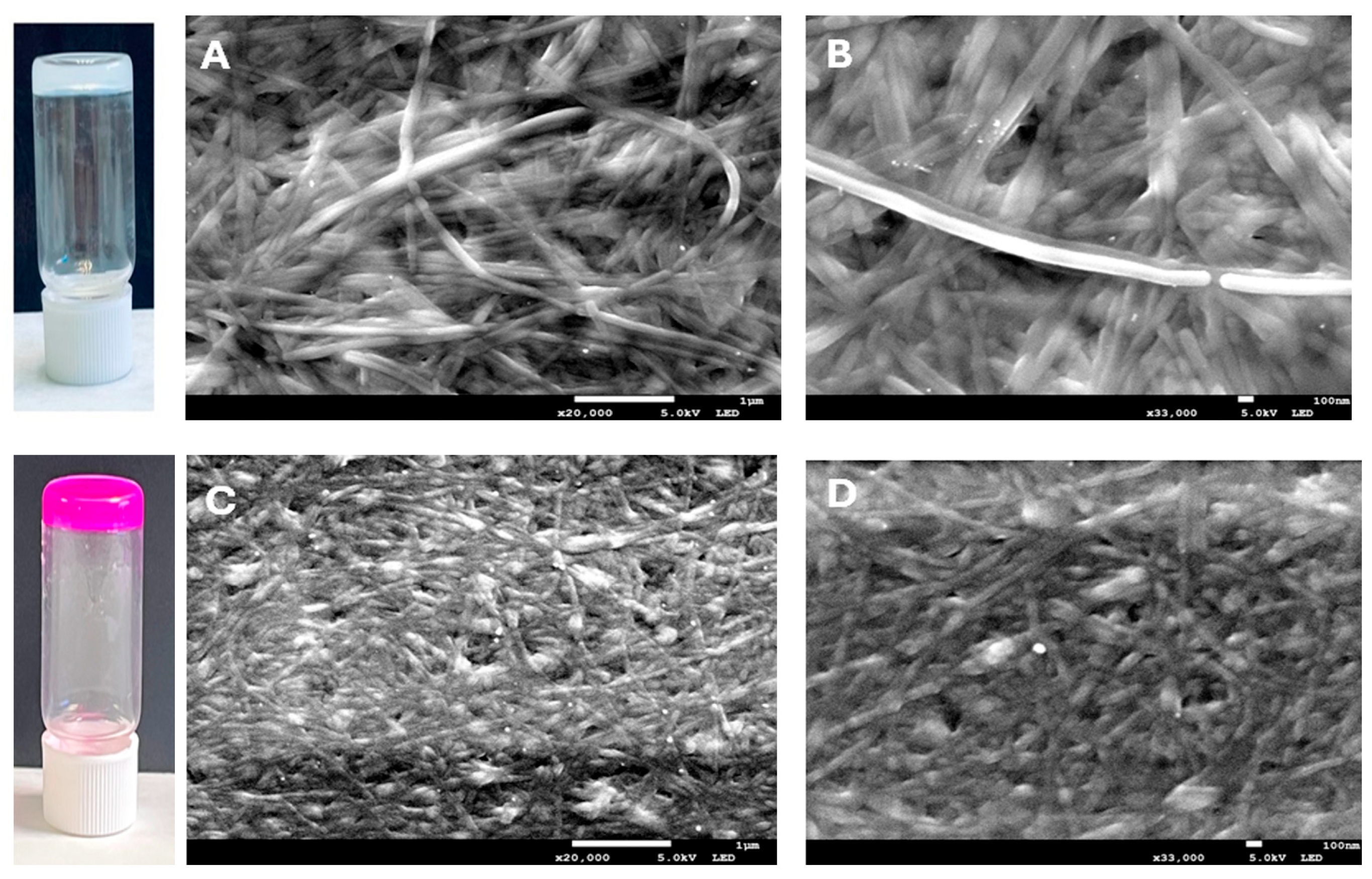
2.2.1. Morphological Analysis
2.2.2. Determination of pH and Density
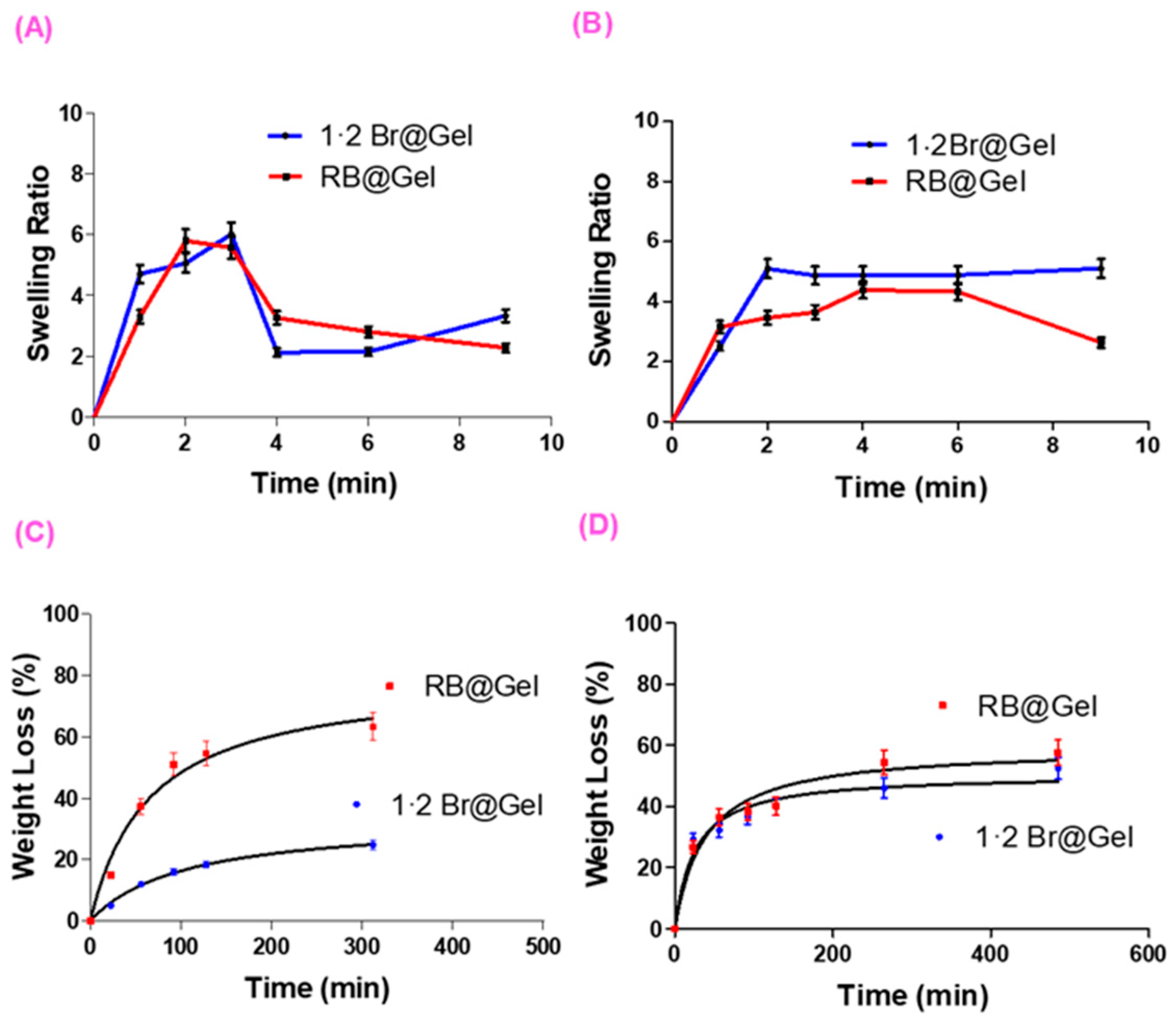
2.2.3. Swelling and Degradation Tests
2.2.4. Extensibility and Rheological Tests
2.2.5. Release Studies of RB from RB@Gel
2.2.6. Hen’s Egg Test–Chorioallantoic Membrane (HET-CAM) Irritancy Test
2.3. Ex Vivo Skin Permeation Studies of RB@Gel
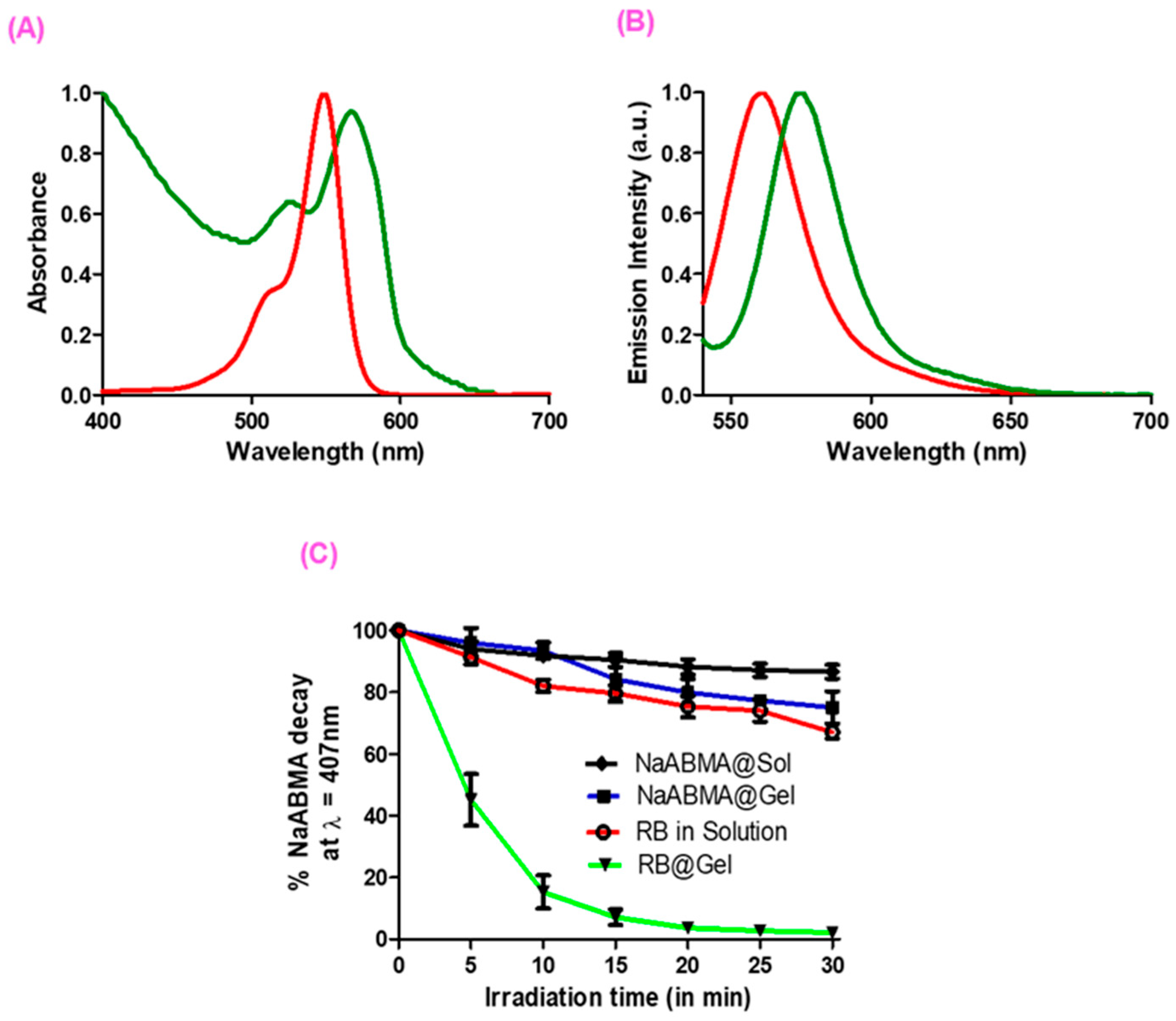
2.4. Optical Properties and SO Production of RB in Solution and in RB@Gel
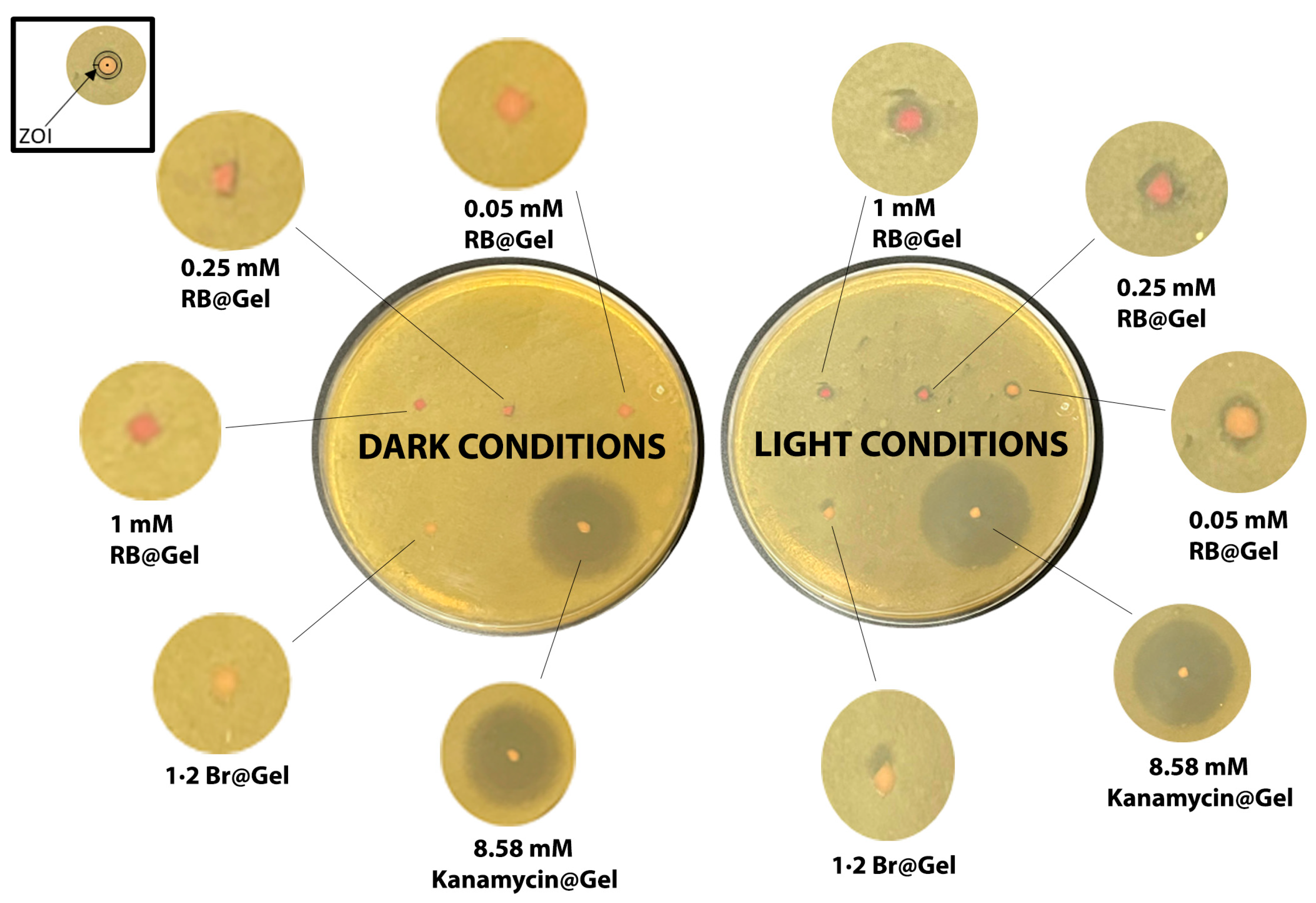
2.5. Antibacterial Studies
3. Materials and Methods
3.1. Materials
3.2. AI Usage Statement
3.3. Gels Preparation
3.4. Physicochemical Characterization
3.4.1. Morphological Characterization
3.4.2. pH and Density
3.4.3. Swelling Test
3.4.4. Degradation Test
3.4.5. Extensibility Test
3.4.6. Rheological Measurements
3.4.7. Release Studies
Dialysis Membrane Method
Direct-Surface Contact Method
Analytical Procedure for Release Studies
3.4.8. HET-CAM Irritancy Test
3.5. Ex Vivo Skin Permeation Studies
Analytical Procedure for Ex Vivo Skin Permeation Studies
3.6. Optical Properties and SO Production
3.7. Antimicrobial PDT Efficacy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abrahamse, H.; Hamblin, M.R. New Photosensitizers for Photodynamic Therapy. Biochem. J. 2016, 473, 347–364. [Google Scholar] [CrossRef] [PubMed]
- Sepulveda-Beltran, P.A.; Levine, H.; Altamirano, D.S.; Martinez, J.D.; Durkee, H.; Mintz, K.; Leblanc, R.; Tóthová, J.D.; Miller, D.; Parel, J.-M.; et al. Rose Bengal Photodynamic Antimicrobial Therapy: A Review of the Intermediate-Term Clinical and Surgical Outcomes. Am. J. Ophthalmol. 2022, 243, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Buck, S.T.G.; Bettanin, F.; Orestes, E.; Homem-de-Mello, P.; Imasato, H.; Viana, R.B.; Perussi, J.R.; da Silva, A.B.F. Photodynamic Efficiency of Xanthene Dyes and Their Phototoxicity against a Carcinoma Cell Line: A Computational and Experimental Study. J. Chem. 2017, 2017, 7365263. [Google Scholar] [CrossRef]
- Maker, A.V.; Prabhakar, B.; Pardiwala, K. The Potential of Intralesional Rose Bengal to Stimulate T-Cell Mediated Anti-Tumor Responses. J. Clin. Cell. Immunol. 2015, 6, 343. [Google Scholar] [CrossRef]
- Ossola, R.; Jönsson, O.M.; Moor, K.; McNeill, K. Singlet Oxygen Quantum Yields in Environmental Waters. Chem. Rev. 2021, 121, 4100–4146. [Google Scholar] [CrossRef]
- Demartis, S.; Obinu, A.; Gavini, E.; Giunchedi, P.; Rassu, G. Nanotechnology-Based Rose Bengal: A Broad-Spectrum Biomedical Tool. Dye. Pigment. 2021, 188, 109236. [Google Scholar] [CrossRef]
- Youf, R.; Müller, M.; Balasini, A.; Thétiot, F.; Müller, M.; Hascoët, A.; Jonas, U.; Schönherr, H.; Lemercier, G.; Montier, T.; et al. Antimicrobial Photodynamic Therapy: Latest Developments with a Focus on Combinatory Strategies. Pharmaceutics 2021, 13, 1995. [Google Scholar] [CrossRef]
- Liu, Y.; Qin, R.; Zaat, S.A.J.; Breukink, E.; Heger, M. Antibacterial Photodynamic Therapy: Overview of a Promising Approach to Fight Antibiotic-Resistant Bacterial Infections. J. Clin. Transl. Res. 2015, 1, 140–167. [Google Scholar]
- Zhu, Z.; Chen, H.; Tang, H.; Liu, Q.; Deng, G.; Li, S.; Luo, J.; Liu, J. Aggregation-Induced Photodegradability-Based Molecules for Tumor-Targeting Controllable Cancer Therapy Guided by Radio/NIR-II/PA Trimodal Imaging. Sens. Actuators B Chem. 2025, 449, 139123. [Google Scholar] [CrossRef]
- Liu, J.; Chen, H.; Yang, Y.; Wang, Q.; Zhang, S.; Zhao, B.; Li, Z.; Yang, G.; Deng, G. Aggregation-Induced Type I&II Photosensitivity and Photodegradability-Based Molecular Backbones for Synergistic Antibacterial and Cancer Phototherapy via Photodynamic and Photothermal Therapies. Mater. Horizons 2023, 10, 3791–3796. [Google Scholar] [CrossRef]
- Wainwright, M.; Maisch, T.; Nonell, S.; Plaetzer, K.; Almeida, A.; Tegos, G.P.; Hamblin, M.R. Photoantimicrobials; Are We Afraid of the Light? Lancet Infect. Dis. 2017, 17, e49–e55. [Google Scholar] [CrossRef] [PubMed]
- Aslam, B.; Wang, W.; Arshad, M.I.; Khurshid, M.; Muzammil, S.; Rasool, M.H.; Nisar, M.A.; Alvi, R.F.; Aslam, M.A.; Qamar, M.U.; et al. Antibiotic Resistance: A Rundown of a Global Crisis. Infect. Drug Resist. 2018, 11, 1645–1658. [Google Scholar] [CrossRef]
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Girotti, A.W.; Gollnick, S.O.; Hahn, S.M.; Hamblin, M.R.; Juzeniene, A.; Kessel, D.; et al. Photodynamic Therapy of Cancer: An Update. CA. Cancer J. Clin. 2011, 61, 250–281. [Google Scholar] [CrossRef]
- Prestinaci, F.; Pezzotti, P.; Pantosti, A. Antimicrobial Resistance: A Global Multifaceted Phenomenon. Pathog. Glob. Health 2015, 109, 309–318. [Google Scholar] [CrossRef]
- Hamblin, M.R. Antimicrobial Photodynamic Inactivation: A Bright New Technique to Kill Resistant Microbes. Curr. Opin. Microbiol. 2016, 33, 67–73. [Google Scholar] [CrossRef]
- Jiang, J.; Lv, X.; Cheng, H.; Yang, D.; Xu, W.; Hu, Y.; Song, Y.; Zeng, G. Type I Photodynamic Antimicrobial Therapy: Principles, Progress, and Future Perspectives. Acta Biomater. 2024, 177, 1–19. [Google Scholar] [CrossRef]
- Patil, P.B.; Datir, S.K.; Saudagar, R.B. A Review on Topical Gels as Drug Delivery System. J. Drug Deliv. Ther. 2019, 9, 989–994. [Google Scholar] [CrossRef]
- Khullar, R.; Kumar, D.; Seth, N.; Saini, S. Formulation and Evaluation of Mefenamic Acid Gel for Topical Delivery. Saudi Pharm. J. 2012, 20, 63–67. [Google Scholar] [CrossRef]
- Monk, E.J.M.; Jones, T.P.W.; Bongomin, F.; Kibone, W.; Nsubuga, Y.; Ssewante, N.; Muleya, I.; Nsenga, L.; Rao, V.B.; van Zandvoort, K. Antimicrobial Resistance in Bacterial Wound, Skin, Soft Tissue and Surgical Site Infections in Central, Eastern, Southern and Western Africa: A Systematic Review and Meta-Analysis. PLoS Glob. Public Health 2024, 4, e0003077, Erratum in PLoS Glob. Public Health 2025, 5, e0004608. [Google Scholar] [CrossRef]
- Abdipour Mehrian, S.R.; Noushadi, F.; Pourasghar, Y.; Farkarian, A.; Meftah, E.; Homayounifar, F.; Amanati, A. Prevalence, Microbiology, and Antimicrobial Susceptibility Profile of Bacterial Skin and Soft Tissue Infections in Pediatric Patients with Malignancies at a Referral Teaching Hospital in Shiraz, Iran. BMC Infect. Dis. 2025, 25, 707. [Google Scholar] [CrossRef]
- Ranjan, A.; Shaik, S.; Nandanwar, N.; Hussain, A.; Tiwari, S.K.; Semmler, T.; Jadhav, S.; Wieler, L.H.; Alam, M.; Colwell, R.R.; et al. Comparative Genomics of Escherichia coli Isolated from Skin and Soft Tissue and Other Extraintestinal Infections. MBio 2017, 8, e01070-17. [Google Scholar] [CrossRef] [PubMed]
- Živa, P.; Kristina, E.; Marija, G.; Darja, Ž.-B.; Marjanca, S.E. Virulence Potential of Escherichia coli Isolates from Skin and Soft Tissue Infections. J. Clin. Microbiol. 2009, 47, 1811–1817. [Google Scholar] [CrossRef]
- Janny, S.; Bert, F.; Dondero, F.; Nicolas Chanoine, M.-H.; Belghiti, J.; Mantz, J.; Paugam-Burtz, C. Fatal Escherichia coli Skin and Soft Tissue Infections in Liver Transplant Recipients: Report of Three Cases. Transpl. Infect. Dis. 2013, 15, E49–E53. [Google Scholar] [CrossRef]
- Ioannou, P.; Tsagkaraki, E.; Athanasaki, A.; Tsioutis, C.; Gikas, A. Gram-Negative Bacteria as Emerging Pathogens Affecting Mortality in Skin and Soft Tissue Infections. Hippokratia 2018, 22, 23–28. [Google Scholar]
- Ungaro, R.; Mikulska, M. The Skin and Soft Tissue Infections in Hematological Patients. Curr. Opin. Infect. Dis. 2020, 33, 101–109. [Google Scholar] [CrossRef]
- Mouna, K.; Akkari, H.; Faten, H.; Yosra, K.; Hichem, B.; Maha, M.; Abdelfatteh, Z.; Jamelledine, Z. Ecthyma Gangrenosum Caused by Escherichia coli in a Previously Healthy Girl. Pediatr. Dermatol. 2015, 32, e179–e180. [Google Scholar] [CrossRef]
- Patel, J.K.; Perez, O.A.; Viera, M.H.; Halem, M.; Berman, B. Ecthyma Gangrenosum Caused by Escherichia coli Bacteremia: A Case Report and Review of the Literature. Cutis 2009, 84, 261–267. [Google Scholar]
- Justine, B.N.; Mushi, M.F.; Silago, V.; Igembe, Z.; Muyombe, J.; Kishengena, P.P.; Michael, N.S.; Maganga, M.G.; Massenga, A.; Tegete, F.; et al. Antimicrobial Resistance Surveillance of Skin and Soft Tissue Infections: Hospital-Wide Bacterial Species and Antibiograms to Inform Management at a Zonal Tertiary Hospital in Mwanza, Tanzania. Infect. Drug Resist. 2025, 18, 791–802. [Google Scholar] [CrossRef]
- Almenara-Blasco, M.; Pérez-Laguna, V.; Navarro-Bielsa, A.; Gracia-Cazaña, T.; Gilaberte, Y. Antimicrobial Photodynamic Therapy for Dermatological Infections: Current Insights and Future Prospects. Front. Photobiol. 2024, 2, 1294511. [Google Scholar] [CrossRef]
- Bravo, A.R.; Fuentealba, F.A.; González, I.A.; Palavecino, C.E. Use of Antimicrobial Photodynamic Therapy to Inactivate Multidrug-Resistant Klebsiella pneumoniae: Scoping Review. Pharmaceutics 2024, 16, 1626. [Google Scholar] [CrossRef]
- Helal, D.A.; El-Rhman, D.A.; Abdel-Halim, S.A.; El-Nabarawi, M.A. Formulation and Evaluation of Fluconazole Topical Gel. Int. J. Pharm. Pharm. Sci. 2012, 4, 176–183. [Google Scholar]
- Calixto, G.M.F.; Bernegossi, J.; de Freitas, L.M.; Fontana, C.R.; Chorilli, M. Nanotechnology-Based Drug Delivery Systems for Photodynamic Therapy of Cancer: A Review. Molecules 2016, 21, 342. [Google Scholar] [CrossRef]
- Al-Jamal, A.N.; Al-Hussainy, A.F.; Mohammed, B.A.; Abbas, H.H.; Kadhim, I.M.; Ward, Z.H.; Mahapatra, D.K.; Joseph, T.M.; kianfar, E.; Thomas, S. Photodynamic Therapy (PDT) in Drug Delivery: Nano-Innovations Enhancing Treatment Outcomes. Health Sci. Rev. 2025, 14, 100218. [Google Scholar] [CrossRef]
- Anguluri, K.; Sharma, B.; Bagherpour, S.; Calpena, A.C.; Halbaut, L.; Amabilino, D.B.; Kaur, G.; Chaudhary, G.R.; Pérez-García, L. Supramolecular Gels for Antimicrobial Photodynamic Therapy against E. coli and S. aureus. Photodiagn. Photodyn. Ther. 2025, 52, 104529. [Google Scholar] [CrossRef]
- Rodrigues, M.; Calpena, A.C.; Amabilino, D.B.; Garduño-Ramírez, M.L.; Pérez-García, L. Supramolecular Gels Based on a Gemini Imidazolium Amphiphile as Molecular Material for Drug Delivery. J. Mater. Chem. B 2014, 2, 5419–5429. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, H.; Zou, Q.; Xing, R.; Jiao, T.; Yan, X. An Injectable Dipeptide–Fullerene Supramolecular Hydrogel for Photodynamic Antibacterial Therapy. J. Mater. Chem. B 2018, 6, 7335–7342. [Google Scholar] [CrossRef]
- Xie, Y.-Y.; Zhang, Y.-W.; Liu, X.-Z.; Ma, X.-F.; Qin, X.-T.; Jia, S.-R.; Zhong, C. Aggregation-Induced Emission-Active Amino Acid/Berberine Hydrogels with Enhanced Photodynamic Antibacterial and Anti-Biofilm Activity. Chem. Eng. J. 2021, 413, 127542. [Google Scholar] [CrossRef]
- Samperi, M.; Pérez-García, L.; Amabilino, D.B. Quantification of Energy of Activation to Supramolecular Nanofibre Formation Reveals Enthalpic and Entropic Effects and Morphological Consequence. Chem. Sci. 2019, 10, 10256–10266. [Google Scholar] [CrossRef]
- Samperi, M.; Limón, D.; Amabilino, D.B.; Pérez-García, L. Enhancing Singlet Oxygen Generation by Self-Assembly of a Porphyrin Entrapped in Supramolecular Fibers. Cell Rep. Phys. Sci. 2020, 1, 100030. [Google Scholar] [CrossRef]
- Guo, J.; Cao, Y.; Wu, Q.-Y.; Zhou, Y.-M.; Cao, Y.-H.; Cen, L.-S. Implications of PH and Ionic Environment in Chronic Diabetic Wounds: An Overlooked Perspective. Clin. Cosmet. Investig. Dermatol. 2024, 17, 2669–2686. [Google Scholar] [CrossRef]
- Brooks, S.G.; Mahmoud, R.H.; Lin, R.R.; Fluhr, J.W.; Yosipovitch, G. The Skin Acid Mantle: An Update on Skin PH. J. Investig. Dermatol. 2025, 145, 509–521. [Google Scholar] [CrossRef]
- Han, Z.; Yuan, M.; Liu, L.; Zhang, K.; Zhao, B.; He, B.; Liang, Y.; Li, F. PH-Responsive Wound Dressings: Advances and Prospects. Nanoscale Horiz. 2023, 8, 422–440. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, B.; Banerjee, A. Short-Peptide-Based Hydrogel: A Template for the in Situ Synthesis of Fluorescent Silver Nanoclusters by Using Sunlight. Chemistry 2010, 16, 13698–13705. [Google Scholar] [CrossRef] [PubMed]
- Terech, P.; Weiss, R.G. Low Molecular Mass Gelators of Organic Liquids and the Properties of Their Gels. Chem. Rev. 1997, 97, 3133–3160. [Google Scholar] [CrossRef]
- Moura, R.S.; Afonso, J.P.R.; Mello, D.A.C.P.G.; Palma, R.K.; Oliveira-Silva, I.; Oliveira, R.F.; Oliveira, D.A.A.P.; Santos, D.B.; Silva, C.H.M.; Guedes, O.A.; et al. Hydrogels Associated with Photodynamic Therapy Have Antimicrobial Effect against Staphylococcus aureus: A Systematic Review. Gels 2024, 10, 635. [Google Scholar] [CrossRef]
- Lim, J.Y.C.; Lin, Q.; Xue, K.; Loh, X.J. Recent Advances in Supramolecular Hydrogels for Biomedical Applications. Mater. Today Adv. 2019, 3, 100021. [Google Scholar] [CrossRef]
- Ozon, E.A.; Burloiu, A.M.; Musuc, A.M.; Manda, G.; Anuta, V.; Dinu-Pîrvu, C.E.; Lupuliasa, D.; Neagoe, I.V.; Anastasescu, M.; Socoteanu, R.P.; et al. Cellulose-Derived Gels for Topical Delivery: HPMC as a Functional Matrix for Porphyrinic Photosensitizers. Gels. 2025, 11, 824. [Google Scholar] [CrossRef]
- Liu, S.; Feng, Y.; Tan, Y.; Chen, J.; Yang, T.; Wang, X.; Li, L.; Wang, F.; Liang, H.; Zhong, J.-L.; et al. Photosensitizer-Loaded Hydrogels: A New Antibacterial Dressing. Wound Repair Regen. 2024, 32, 301–313. [Google Scholar] [CrossRef]
- Burloiu, A.M.; Ozon, E.A.; Musuc, A.M.; Anastasescu, M.; Socoteanu, R.P.; Atkinson, I.; Culita, D.C.; Anuta, V.; Popescu, I.A.; Lupuliasa, D.; et al. Porphyrin Photosensitizers into Polysaccharide-Based Biopolymer Hydrogels for Topical Photodynamic Therapy: Physicochemical and Pharmacotechnical Assessments. Gels 2024, 10, 499. [Google Scholar] [CrossRef]
- Glass, S.; Rüdiger, T.; Griebel, J.; Abel, B.; Schulze, A. Uptake and Release of Photosensitizers in a Hydrogel for Applications in Photodynamic Therapy: The Impact of Structural Parameters on Intrapolymer Transport Dynamics. RSC Adv. 2018, 8, 41624–41632. [Google Scholar] [CrossRef]
- Junqueira Garcia, M.T.; Pedralino Gonçalves, T.; São Félix Martins, É.; Silva Martins, T.; Carvalho de Abreu Fantini, M.; Regazi Minarini, P.R.; Costa Fernandez, S.; Cassone Salata, G.; Biagini Lopes, L. Improvement of Cutaneous Delivery of Methylene Blue by Liquid Crystals. Int. J. Pharm. 2018, 548, 454–465. [Google Scholar] [CrossRef]
- Goergen, N.; Wojcik, M.; Drescher, S.; Pinnapireddy, S.R.; Brüßler, J.; Bakowsky, U.; Jedelská, J. The Use of Artificial Gel Forming Bolalipids as Novel Formulations in Antimicrobial and Antifungal Therapy. Pharmaceutics 2019, 11, 307. [Google Scholar] [CrossRef]
- Chen, L.; Han, L.; Lian, G. Recent Advances in Predicting Skin Permeability of Hydrophilic Solutes. Adv. Drug Deliv. Rev. 2013, 65, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Tsuneda, T.; Taketsugu, T. Singlet Fission Initiating Organic Photosensitizations. Sci. Rep. 2024, 14, 829. [Google Scholar] [CrossRef]
- Sibrian-Vazquez, M.; Escobedo, J.O.; Lowry, M.; Fronczek, F.R.; Strongin, R.M. Field Effects Induce Bathochromic Shifts in Xanthene Dyes. J. Am. Chem. Soc. 2012, 134, 10502–10508. [Google Scholar] [CrossRef][Green Version]
- Alvarez-Lopez, C.; Cavazos-Elizondo, D.; Heyne, B.; Kochevar, I.E.; Aguirre-Soto, A. Nanocaging Rose Bengal to Inhibit Aggregation and Enhance Photo-Induced Oxygen Consumption†. Photochem. Photobiol. 2023, 99, 580–592. [Google Scholar] [CrossRef]
- Bruce, G.; Samperi, M.; Amabilino, D.B.; Duch, M.; Plaza, J.A.; Pérez-García, L. Singlet Oxygen Generation from Porphyrin-Functionalized Hexahedral Polysilicon Microparticles. J. Porphyr. Phthalocyanines 2019, 23, 223–233. [Google Scholar] [CrossRef]
- Redmond, R.W.; Kochevar, I.E. Spatially Resolved Cellular Responses to Singlet Oxygen. Photochem. Photobiol. 2006, 82, 1178–1186. [Google Scholar] [CrossRef]
- Przygoda, M.; Bartusik-Aebisher, D.; Dynarowicz, K.; Cieślar, G.; Kawczyk-Krupka, A.; Aebisher, D. Cellular Mechanisms of Singlet Oxygen in Photodynamic Therapy. Int. J. Mol. Sci. 2023, 24, 16890. [Google Scholar] [CrossRef]
- Wei, D.; Hamblin, M.R.; Wang, H.; Fekrazad, R.; Wang, C.; Wen, X. Rose Bengal Diacetate-Mediated Antimicrobial Photodynamic Inactivation: Potentiation by Potassium Iodide and Acceleration of Wound Healing in MRSA-Infected Diabetic Mice. BMC Microbiol. 2024, 24, 246. [Google Scholar] [CrossRef]
- Jiménez-Gastelum, G.R.; Villegas-Mercado, C.E.; Cota-Quintero, J.L.; Arzola-Rodríguez, S.I.; Ramos-Payán, R.; Bermúdez, M. Hydrogels Modulating the Microbiome: Therapies for Tissue Regeneration with Infection Control. Gels. 2025, 11, 584. [Google Scholar] [CrossRef]
- He, C.; Feng, P.; Hao, M.; Tang, Y.; Wu, X.; Cui, W.; Ma, J.; Ke, C. Nanomaterials in Antibacterial Photodynamic Therapy and Antibacterial Sonodynamic Therapy. Adv. Funct. Mater. 2024, 34, 2402588. [Google Scholar] [CrossRef]
- Casal-Dujat, L.; Rodrigues, M.; Yague, A.; Calpena, A.C.; Amabilino, D.B.; Gonzalez-Linares, J.; Borras, M.; Pérez-García, L. Gemini Imidazolium Amphiphiles for the Synthesis, Stabilization, and Drug Delivery from Gold Nanoparticles. Langmuir 2012, 28, 2368–2381. [Google Scholar] [CrossRef] [PubMed]
- Asad, M.I.; Khan, D.; Rehman, A.U.; Elaissari, A.; Ahmed, N. Development and In Vitro/In Vivo Evaluation of PH-Sensitive Polymeric Nanoparticles Loaded Hydrogel for the Management of Psoriasis. Nanomaterials 2021, 11, 3433. [Google Scholar] [CrossRef]
- Calpena, A.C.; Escribano, E.; San Martin, H.; Lauroba, J.; Obach, R.; Domenech, J. Influence of the Formulation on the in Vitro Transdermal Penetration of Sodium Diclofenac. Evaluation of the Topical and Systemic Anti-Inflammatory Activity in the Rat. Arzneim.-Forsch. 1999, 49, 1012–1017. [Google Scholar]
- Mohammadi-Meyabadi, R.; Beirampour, N.; Garrós, N.; Alvarado, H.L.; Limón, D.; Silva-Abreu, M.; Calpena, A.C.; Mallandrich, M. Assessing the Solubility of Baricitinib and Drug Uptake in Different Tissues Using Absorption and Fluorescence Spectroscopies. Pharmaceutics 2022, 14, 2714. [Google Scholar] [CrossRef]
- de Araujo Lowndes Viera, L.M.; Silva, R.S.; da Silva, C.C.; Presgrave, O.A.F.; Boas, M.H.S.V. Comparison of the Different Protocols of the Hen’s Egg Test-Chorioallantoic Membrane (HET-CAM) by Evaluating the Eye Irritation Potential of Surfactants. Toxicol. Vitr. 2022, 78, 105255. [Google Scholar] [CrossRef]
- Reis Mansur, M.C.P.P.; Leitão, S.G.; Cerqueira-Coutinho, C.; Vermelho, A.B.; Silva, R.S.; Presgrave, O.A.F.; Leitão, Á.A.C.; Leitão, G.G.; Ricci-Júnior, E.; Santos, E.P. In Vitro and In Vivo Evaluation of Efficacy and Safety of Photoprotective Formulations Containing Antioxidant Extracts. Rev. Bras. Farmacogn. 2016, 26, 251–258. [Google Scholar] [CrossRef]
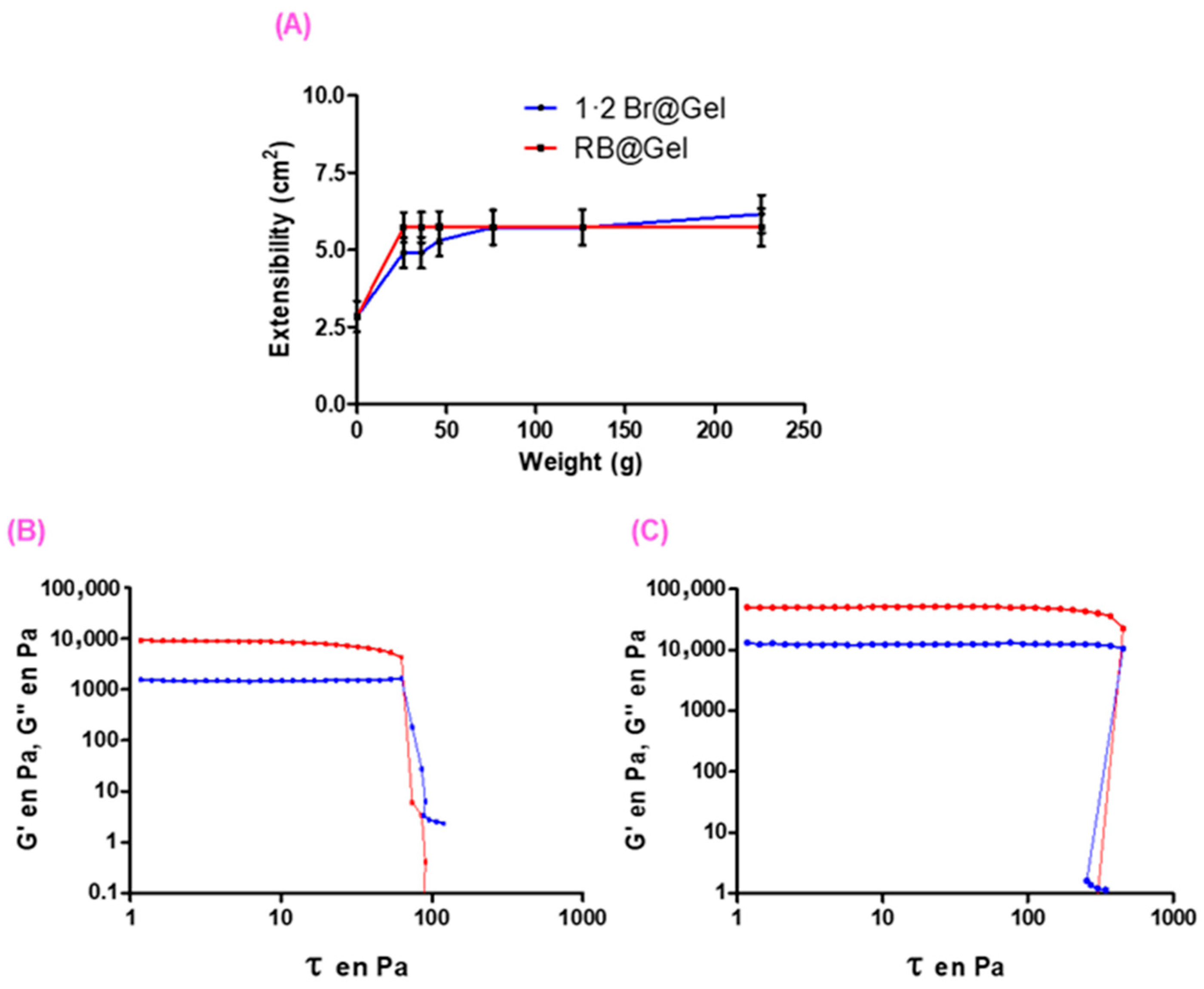

| Sample | G′ (Pa) [a] | G″ (Pa) [a] | Critical Stress (Pa) [b] |
|---|---|---|---|
| 1·2 Br@Gel | 5923 | 1124 | 63 |
| RB@Gel [c] | 58,394 | 13,395 | 423 |
| Irritation Score | Irritation Classification |
|---|---|
| 0–0.9 | Non-Irritant |
| 1–4.9 | Slight Irritant |
| 5–8.9 | Moderate Irritant |
| 9–21 | Severe Irritant |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anguluri, K.; Bagherpour, S.; Calpena, A.C.; Halbaut, L.; Espargaró, A.; Sabate, R.; Pérez-García, L. Rose Bengal-Incorporated Supramolecular Gels as a Topical Platform for Localized Antimicrobial Photodynamic Therapy. Int. J. Mol. Sci. 2025, 26, 11455. https://doi.org/10.3390/ijms262311455
Anguluri K, Bagherpour S, Calpena AC, Halbaut L, Espargaró A, Sabate R, Pérez-García L. Rose Bengal-Incorporated Supramolecular Gels as a Topical Platform for Localized Antimicrobial Photodynamic Therapy. International Journal of Molecular Sciences. 2025; 26(23):11455. https://doi.org/10.3390/ijms262311455
Chicago/Turabian StyleAnguluri, Kavya, Saman Bagherpour, Ana C. Calpena, Lyda Halbaut, Alba Espargaró, Raimon Sabate, and Lluïsa Pérez-García. 2025. "Rose Bengal-Incorporated Supramolecular Gels as a Topical Platform for Localized Antimicrobial Photodynamic Therapy" International Journal of Molecular Sciences 26, no. 23: 11455. https://doi.org/10.3390/ijms262311455
APA StyleAnguluri, K., Bagherpour, S., Calpena, A. C., Halbaut, L., Espargaró, A., Sabate, R., & Pérez-García, L. (2025). Rose Bengal-Incorporated Supramolecular Gels as a Topical Platform for Localized Antimicrobial Photodynamic Therapy. International Journal of Molecular Sciences, 26(23), 11455. https://doi.org/10.3390/ijms262311455








