Photodynamic Inactivation Enhances Antibiotic Efficacy Without Affecting Drug Stability: Insights into Photosensitizer–Antibiotic Combination Therapies
Abstract
1. Introduction
2. Results and Discussion
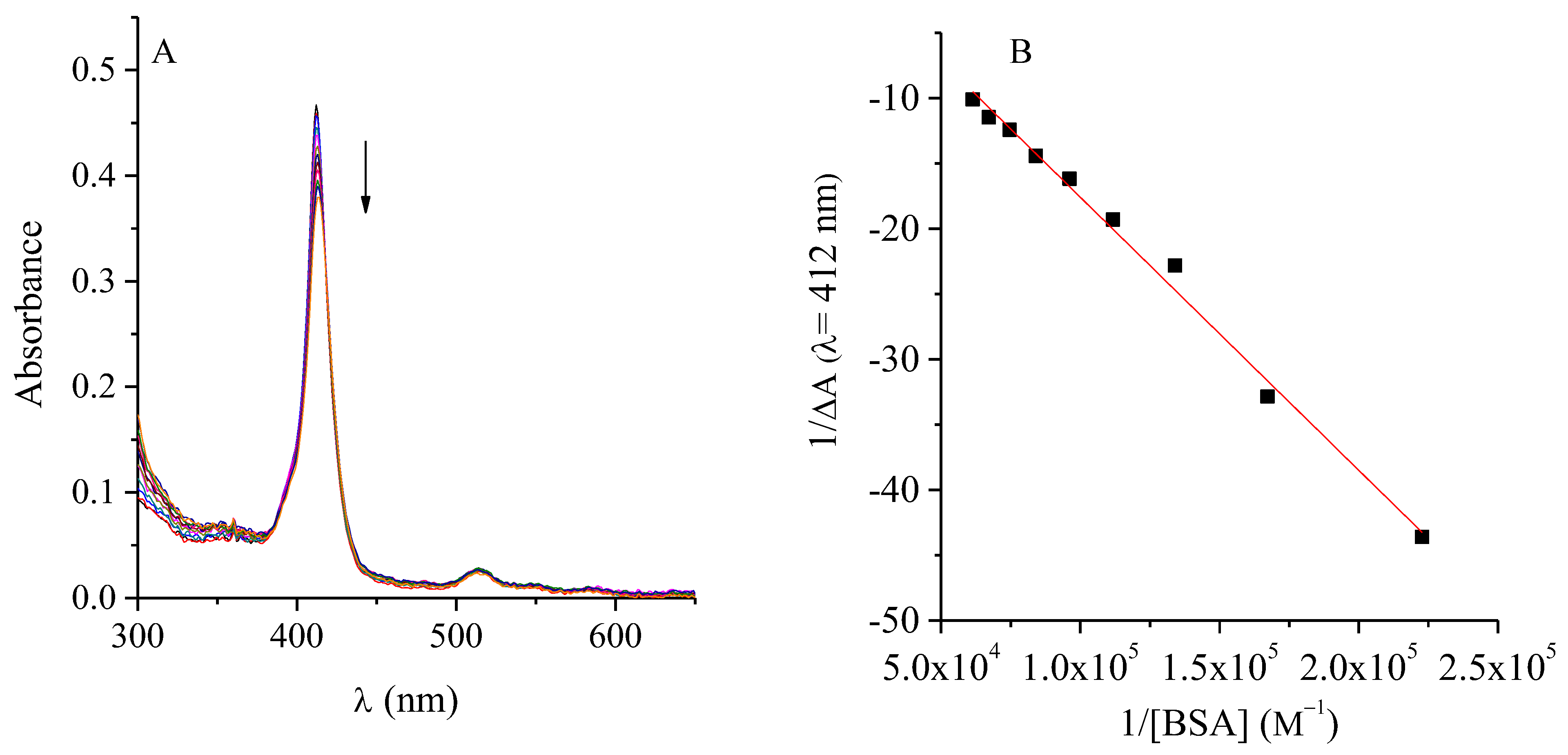
2.1. Interaction Between TMAP4+ and ATBs
2.2. MIC and MBC Determination
2.3. Determination of MIC and MBC of PS + ATB at a Fixed Concentration of PS
2.4. Determination of MIC and MBC of PS + ATB at Different Concentrations of PS
2.5. Interaction Between TMAP4+ and BSA

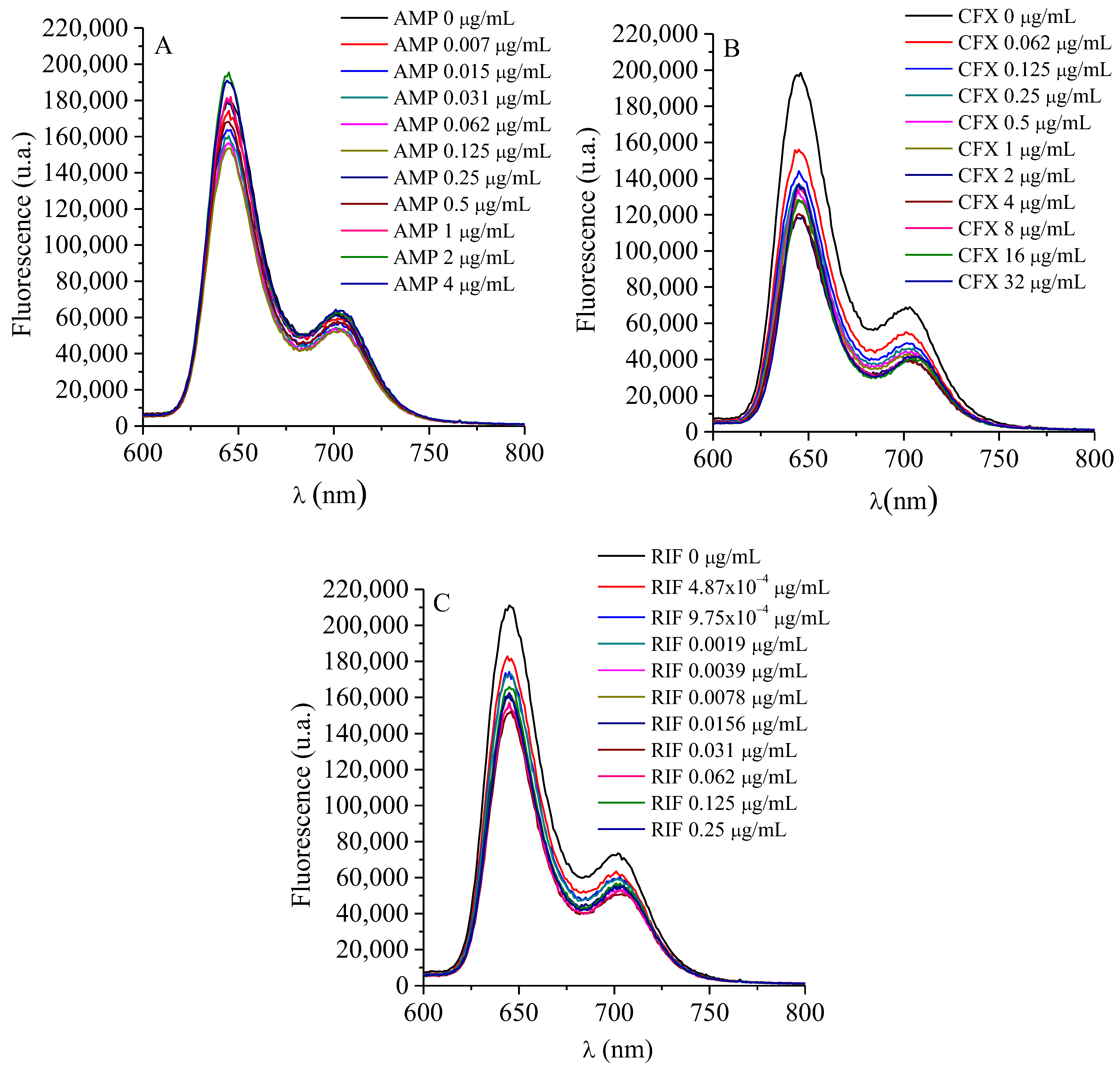
2.6. TMAP4+ and ATBs Growth Curves
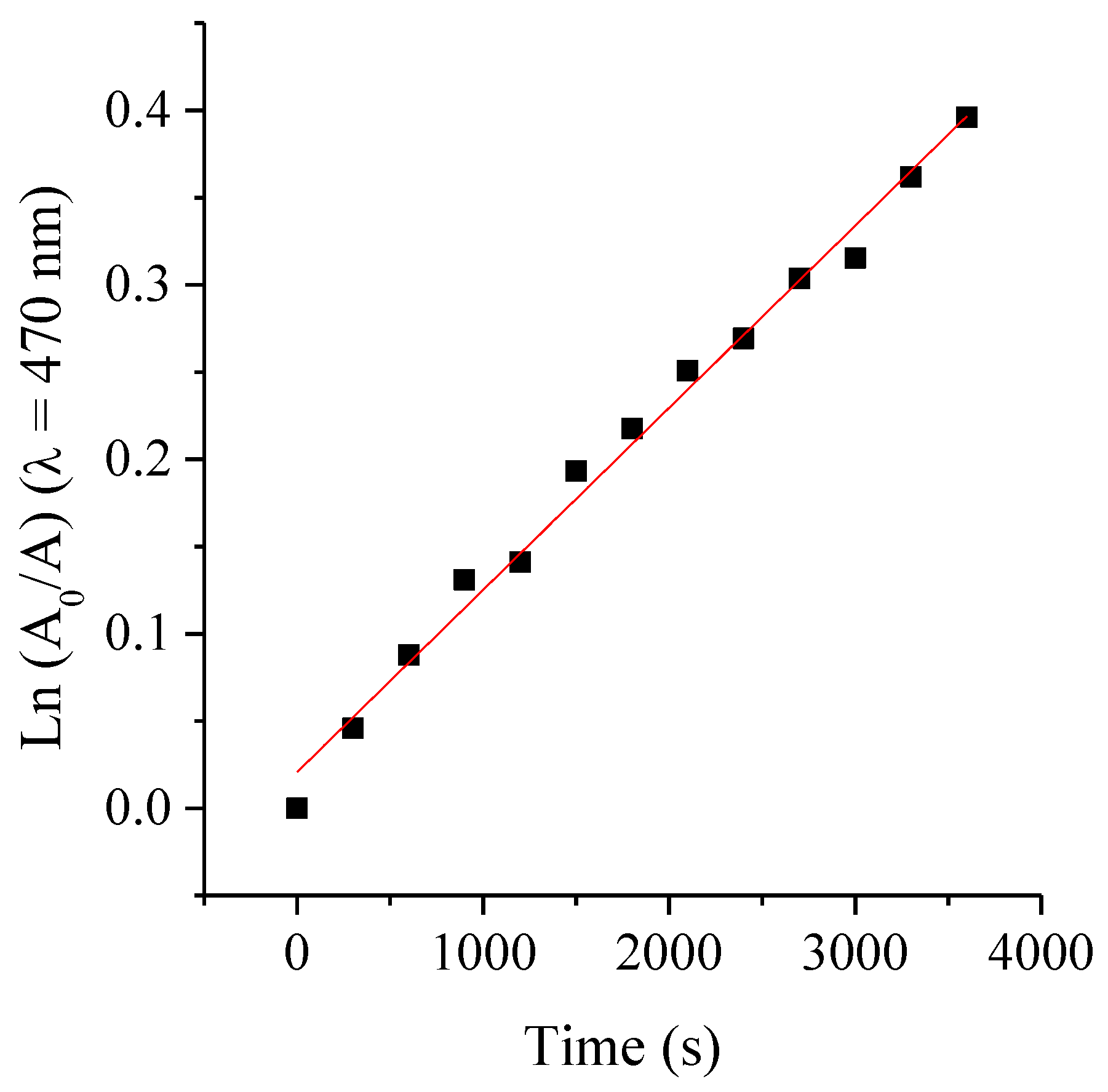
2.7. Photostability of ATBs
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AMP | Ampicillin |
| ATB | Antibiotic |
| BSA | bovine serum albumin |
| CFU | Colony-forming units |
| CFX | Cephalexin |
| DMF | N,N-dimethylformamide |
| DMSO | Dimethyl sulfoxide |
| F | Fluorescence intensity |
| F0 | Initial Fluorescence |
| MBC | Minimum bactericidal concentration |
| MIC | Minimum inhibitory concentration |
| PBS | Phosphate-buffered saline |
| PDI | Photodynamic inactivation |
| PS | Photosensitizer |
| RIF | Rifampicin |
| ROS | Reactive oxygen species |
| TMAP4+ | 5,10,15,20-tetra(4-N,N,N-trimethylammoniophenyl)porphyrin |
| TSB | Tryptic soy broth |
References
- Band, V.I.; Weiss, D.S. Heteroresistance A cause of unexplained antibiotic treatment failure? PLoS Pathog. 2019, 15, e1007726. [Google Scholar] [CrossRef]
- Sommer, M.O.A.; Munck, C.; Toft-Kehler, R.V.; Andersson, D.I. Prediction of antibiotic resistance: Time for a new preclinical paradigm? Nat. Rev. Microbiol. 2017, 15, 689–696. [Google Scholar] [CrossRef]
- Tanu, R.; Chaudhary, A.A.; Prakash, G.; Yasmeen, N.; Ali, M.A.M.; Raza, N.; Sharma, P.K.; Kumar, A.; Yadav, T.; Kumar, V. Exploring the potential of photodynamic therapy in overcoming multidrug resistance: Mechanisms, synergies, and clinical advancements in infectious diseases. Front. Cell Infect. Microbiol. 2025, 15, 1624036. [Google Scholar] [CrossRef]
- Mathur, A.; Parihar, A.S.; Modi, S.; Kalra, A. Photodynamic therapy for ESKAPE pathogens: An emerging approach to combat antimicrobial resistance (AMR). Microb. Pathog. 2023, 183, 106307. [Google Scholar] [CrossRef]
- Youf, R.; Müller, M.; Balasini, A.; Thétiot, F.; Müller, M.; Hascoët, A.; Jonas, U.; Schönherr, H.; Lemercier, G.; Montier, T.; et al. Antimicrobial Photodynamic Therapy: Latest Developments with a Focus on Combinatory Strategies. Pharmaceutics 2021, 13, 1995. [Google Scholar] [CrossRef]
- Spesia, M.B.; Caminos, D.A.; Pons, P.; Durantini, E.N. Mechanistic insight of the photodynamic inactivation of Escherichia coli by a tetracationic zinc(II) phthalocyanine derivative. Photodiagn. Photodyn. Ther. 2009, 6, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Amendola, G.; Di Luca, M.; Sgarbossa, A. Natural Biomolecules and Light: Antimicrobial Photodynamic Strategies in the Fight Against Antibiotic Resistance. Int. J. Mol. Sci. 2025, 26, 7993. [Google Scholar] [CrossRef] [PubMed]
- Gsponer, N.S.; Spesia, M.B.; Durantini, E.N. Effects of divalent cations, EDTA and chitosan on the uptake and photoinactivation of Escherichia coli it should be in italicmediated by cationic and anionic porphyrins. Photodiagnosis Photodyn. Ther. 2015, 12, 67–75. [Google Scholar] [CrossRef]
- Caminos, D.A.; Spesia, M.B.; Durantini, E.N. Photodynamic inactivation of Escherichia coli by novel meso-substituted porphyrins by 4-(3-N,N,N-trimethylammoniumpropoxy)phenyl and 4-(trifluoromethyl)phenyl groups. Photochem. Photobiol. Sci. 2006, 5, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Soares, J.M.; Corrêa, T.Q.; Barrera Patiño, C.P.; Salgado Gonçalves, I.; dos Santos, G.G.; Gomes Guimarães, G.; Vieira de Lima, R.; Nunes Lima, V.; Corrêa, B.C.; de Souza Cappellini, T.C.; et al. Synergistic Paradigms in Infection Control: A Review on Photodynamic Therapy as an Adjunctive Strategy to Antibiotics. ACS Infect. Dis. 2025, 11, 2671–2691. [Google Scholar] [CrossRef]
- Wozniak, A.; Grinholc, M. Combined antimicrobial activity of photodynamic inactivation and antimicrobials—State of the art. Front. Microbiol. 2018, 9, 930. [Google Scholar] [CrossRef]
- Lima, L.M.; da Silva, B.N.; Barbosa, G.; Barreiro, E.J. β-lactam antibiotics: An overview from a medicinal chemistry perspective. Eur. J. Med. Chem. 2020, 208, 112829. [Google Scholar] [CrossRef] [PubMed]
- Sudzinová, P.; Šanderová, H.; Koval’, T.; Skálová, T.; Borah, N.; Hnilicová, J.; Kouba, T.; Dohnálek, J.; Krásný, L. What the Hel: Recent advances in understanding rifampicin resistance in bacteria. FEMS Microbiol. Rev. 2022, 47, fuac051. [Google Scholar] [CrossRef]
- Alves, E.; Faustino, M.A.F.; Neves, M.G.P.M.S.; Cunha, A.; Tome, J.; Almeida, A. An insight on bacterial cellular targets of photodynamic inactivation. Future Med. Chem. 2014, 6, 141–164. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Coradi Tonon, C.; Ashraf, S.; Hasan, T. Photodynamic and antibiotic therapy in combination against bacterial infections: Efficacy, determinants, mechanisms, and future perspectives. Adv. Drug Deliv. Rev. 2021, 177, 113941. [Google Scholar] [CrossRef]
- Pérez-Laguna, V.; García-Luque, I.; Ballesta, S.; Rezusta, A.; Gilaberte, Y. Photodynamic Therapy Combined with Antibiotics or Antifungals against Microorganisms That Cause Skin and Soft Tissue Infections: A Planktonic and Biofilm Approach to Overcome Resistances. Pharmaceuticals 2021, 14, 603. [Google Scholar] [CrossRef]
- Hsiao, K.-H.; Huang, C.-M.; Lee, Y.-H. Development of Rifampicin-Indocyanine Green-Loaded Perfluorocarbon Nanodroplets for Photo-Chemo-Probiotic Antimicrobial Therapy. Front. Pharmacol. 2018, 9, 1254. [Google Scholar] [CrossRef]
- Kowalska-Krochmal, B.; Dudek-Wicher, R. The minimum inhibitory concentration of antibiotics: Methods, interpretation, clinical relevance. Pathogens 2021, 10, 165. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically (CLSI Standard M07), 12th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2024. [Google Scholar]
- Dastgheyb, S.S.; Eckmann, D.M.; Composto, R.J.; Hickok, N.J. Photo-activated porphyrin in combination with antibiotics: Therapies against Staphylococci. J. Photochem. Photobiol. B Biol. 2013, 129, 27–35. [Google Scholar] [CrossRef]
- Spesia, M.B.; Durantini, E.N. Evolution of phthalocyanine structures as photodynamic agents for bacteria inactivation. Chem. Rec. 2022, 22, e202100292. [Google Scholar] [CrossRef]
- Branco, T.M.; Valerio, N.C.; Rodrigues Jesus, V.I.; Dias, C.J.; Neves, M.G.P.M.S.; Faustino, M.A.F.; Almeida, A. Single and combined effects of photodynamic therapy and antibiotics to inactivate Staphylococcus aureus on skin. Photodiagn. Photodyn. Ther. 2018, 21, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Iluz, N.; Maor, Y.; Keller, N.; Malik, Z. The synergistic effect of PDT and oxacillin on clinical isolates of Staphylococcus aureus. Lasers Surg. Med. 2018, 50, 535–551. [Google Scholar] [CrossRef] [PubMed]
- Spesia, M.B.; Rovera, M.; Durantini, E.N. Photodynamic inactivation of Escherichia coli and Streptococcus mitis by cationic zinc(II) phthalocyanines in media with blood derivatives. Eur. J. Med. Chem. 2010, 45, 2198–2205. [Google Scholar] [CrossRef]
- Morshnev, P.K.; Kustov, A.V.; Drondel, E.A.; Khlydeev, I.I.; Abramova, O.B.; Yaroslavtseva-Isaeva, E.B.; Lyalyakina, E.V.; Koifman, M.O.; Berezin, D.B. The interaction of chlorin photosensitizers for photodynamic therapy with blood transport proteins. J. Mol. Liq. 2023, 390, 123116. [Google Scholar] [CrossRef]
- Santamarina, S.C.; Heredia, D.A.; Durantini, A.M.; Durantini, E.N. Antimicrobial Photosensitizing Material Based on Conjugated Zn(II) Porphyrins. Antibiotics 2022, 11, 91. [Google Scholar] [CrossRef]
- Abuin, E.; Aspée, A.; Lissi, E.; León, L. Binding of rose Bengal to bovine serum albumin. J. Chil. Chem. Soc. 2007, 52, 1196–1197. [Google Scholar] [CrossRef]
- Durmus, M.; Yaman, H.; Göl, C.; Ahsen, V.; Nyokong, T. Water-soluble quaternized mercaptopyridine-substituted zinc-phthalocyanines: Synthesis, photophysical, photochemical and bovine serum albumin binding properties. Dyes Pigm 2011, 91, 153–163. [Google Scholar] [CrossRef]
- Thomas, A.H.; Lorente, C.; Roitman, K.; Morales, M.M.; Dántola, M.L. Photosensitization of bovine serum albumin by pterin: A mechanistic study. J. Photochem. Photobiol. B Biol. 2013, 120, 52–58. [Google Scholar] [CrossRef]
- Cordero, P.V.; Ferreyra, D.D.; Pérez, M.E.; Alvarez, M.G.; Durantini, E.N. Photodynamic Effect of 5,10,15,20-Tetrakis [4-(3-N,N-dimethylaminopropoxy)phenyl]chlorin towards the Human Pathogen Candida albicans under Different Culture Conditions. Photochem 2021, 1, 505–522. [Google Scholar] [CrossRef]
- Nitzan, Y.; Balzam-Sudakevitz, A.; Ashkenazi, H. Eradication of Acinetobacter baumannii by photosensitized agents in vitro. J. Photochem. Photobiol. B 1998, 42, 211–218. [Google Scholar] [CrossRef]
- Spesia, M.B.; Durantini, E.N. Photosensitizers combination approach to enhance photodynamic inactivation of planktonic and biofilm bacteria. Photochem. Photobiol. Sci. 2023, 22, 2433–2444. [Google Scholar] [CrossRef] [PubMed]
- Wozniak, A.; Kruszewska, B.; Pieranski, M.K.; Rychłowski, M.; Grinholc, M. Antimicrobial Photodynamic Inactivation Affects the Antibiotic Susceptibility of Enterococcus spp. Clinical Isolates in Biofilm and Planktonic Cultures. Biomolecules 2021, 11, 693. [Google Scholar] [CrossRef]
- Heredia, D.A.; Durantini, J.E.; Ferreyra, D.D.; Reynoso, E.; Gonzalez Lopez, E.J.; Durantini, A.M.; Milanesio, M.E.; Durantini, E.N. Charge density distribution effect in pyrrolidine-fused chlorins on microbial uptake and antimicrobial photoinactivation of microbial pathogens. J. Photochem. Photobiol. B Biol. 2021, 225, 112321. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, S.M.; Ullman, J.L.; Teel, A.L.; Watts, R.J. pH and temperature effects on the hydrolysis of three β-lactam antibiotics: Ampicillin, cefalotin and cefoxitin. Sci. Total Environ. 2014, 466–467, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Biondi, M.A.; Cacciari, R.D.; Sabini, M.C.; Spesia, M.B.; Biasutti, M.A.; Reynoso, E.; Montejano, H.A. Natural degradation of ceftriaxone promoted by direct UVB light in aqueous media. Mechanistic analysis and cytotoxic effects on a eukaryotic cell line and on bacteria. New J. Chem. 2023, 47, 17799–17809. [Google Scholar] [CrossRef]
- Lazzeri, D.; Rovera, M.; Pascual, L.; Durantini, E.N. Photodynamic Studies and Photoinactivation of Escherichia coli Using meso-Substituted Cationic Porphyrin Derivatives with Asymmetric Charge Distribution. Photochem. Photobiol. 2004, 80, 286–293. [Google Scholar]
- Merchat, M.; Spikes, G.; Bertoloni, G.; Jori, G. Studies on the mechanism of bacteria photosensitization by meso-substituted cationic porphyrins. J. Photochem. Photobiol. B Biol. 1996, 35, 149–157. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing (CLSI Supplement M100), 35th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2025. [Google Scholar]
- López, M.; Gsponer, N.S.; Heredia, D.A.; Durantini, E.N. Chlorin-based photodynamic antimicrobial glass surfaces for the eradication of Staphylococcus aureus. Surf. Interfaces 2025, 66, 106575. [Google Scholar] [CrossRef]



| Strains | MIC in Dark | MIC Post PDI | MBC in Dark | MBC Post PDI |
|---|---|---|---|---|
| S. aureus | 32 | 0.5 | 32 | 16 |
| E. coli | 32 | 32 | 32 | 32 |
| Strain | Antimicrobial Agents | MIC in Dark | MIC Post PDI | MBC in Dark | MBC Post PDI |
|---|---|---|---|---|---|
| S. aureus | AMP + TMAP4+ | 0.1250 + 0.5 | 0.0156 + 0.5 | 0.2500 + 0.5 | 0.1250 + 0.5 |
| RIF + TMAP4+ | 0.0156 + 0.5 | 0.0039 + 0.5 | 0.0625–0.0312 + 0.5 | 0.0312 + 0.5 | |
| E. coli | CFX + TMAP4+ | <0.1250 + 32 | <0.1250 + 32 | <0.1250 + 32 | <0.1250 + 32 |
| CFX + TMAP4+ | 4 + 16 | 4 + 16 | 16 + 16 | 8 + 16 |
| Strain | Antimicrobial Agents | MIC in Dark | MIC Post PDI | MBC in Dark | MBC Post PDI |
|---|---|---|---|---|---|
| S. aureus | AMP + TMAP4+ | 0.2500 + 2 | 0.0156–0.0039 + 0.1250–0.0039 | 0.2500 + 2 | 0.1250 + 1 |
| RIF + TMAP4+ | 0.0156–0.0078 + 1–0.5 | 0.0039–0.0009 + 0.2500–0.0625 | 0.0625–0.0312 + 4–2 | 0.0625–0.0312 + 4–2 | |
| E. coli | CFX + TMAP4+ | 2–1 + 8–4 | 2–1 + 8–4 | 8 + 32 | 8 + 32 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Acosta, R.B.; Durantini, E.N.; Spesia, M.B. Photodynamic Inactivation Enhances Antibiotic Efficacy Without Affecting Drug Stability: Insights into Photosensitizer–Antibiotic Combination Therapies. Int. J. Mol. Sci. 2025, 26, 11267. https://doi.org/10.3390/ijms262311267
Acosta RB, Durantini EN, Spesia MB. Photodynamic Inactivation Enhances Antibiotic Efficacy Without Affecting Drug Stability: Insights into Photosensitizer–Antibiotic Combination Therapies. International Journal of Molecular Sciences. 2025; 26(23):11267. https://doi.org/10.3390/ijms262311267
Chicago/Turabian StyleAcosta, Rocío B., Edgardo N. Durantini, and Mariana B. Spesia. 2025. "Photodynamic Inactivation Enhances Antibiotic Efficacy Without Affecting Drug Stability: Insights into Photosensitizer–Antibiotic Combination Therapies" International Journal of Molecular Sciences 26, no. 23: 11267. https://doi.org/10.3390/ijms262311267
APA StyleAcosta, R. B., Durantini, E. N., & Spesia, M. B. (2025). Photodynamic Inactivation Enhances Antibiotic Efficacy Without Affecting Drug Stability: Insights into Photosensitizer–Antibiotic Combination Therapies. International Journal of Molecular Sciences, 26(23), 11267. https://doi.org/10.3390/ijms262311267






