Multi-Pathway Mechanisms of Engeletin in Ischemic Stroke: A Comprehensive Study Based on Network Pharmacology, Machine Learning, and Immune Infiltration Analysis
Abstract
1. Introduction
2. Results
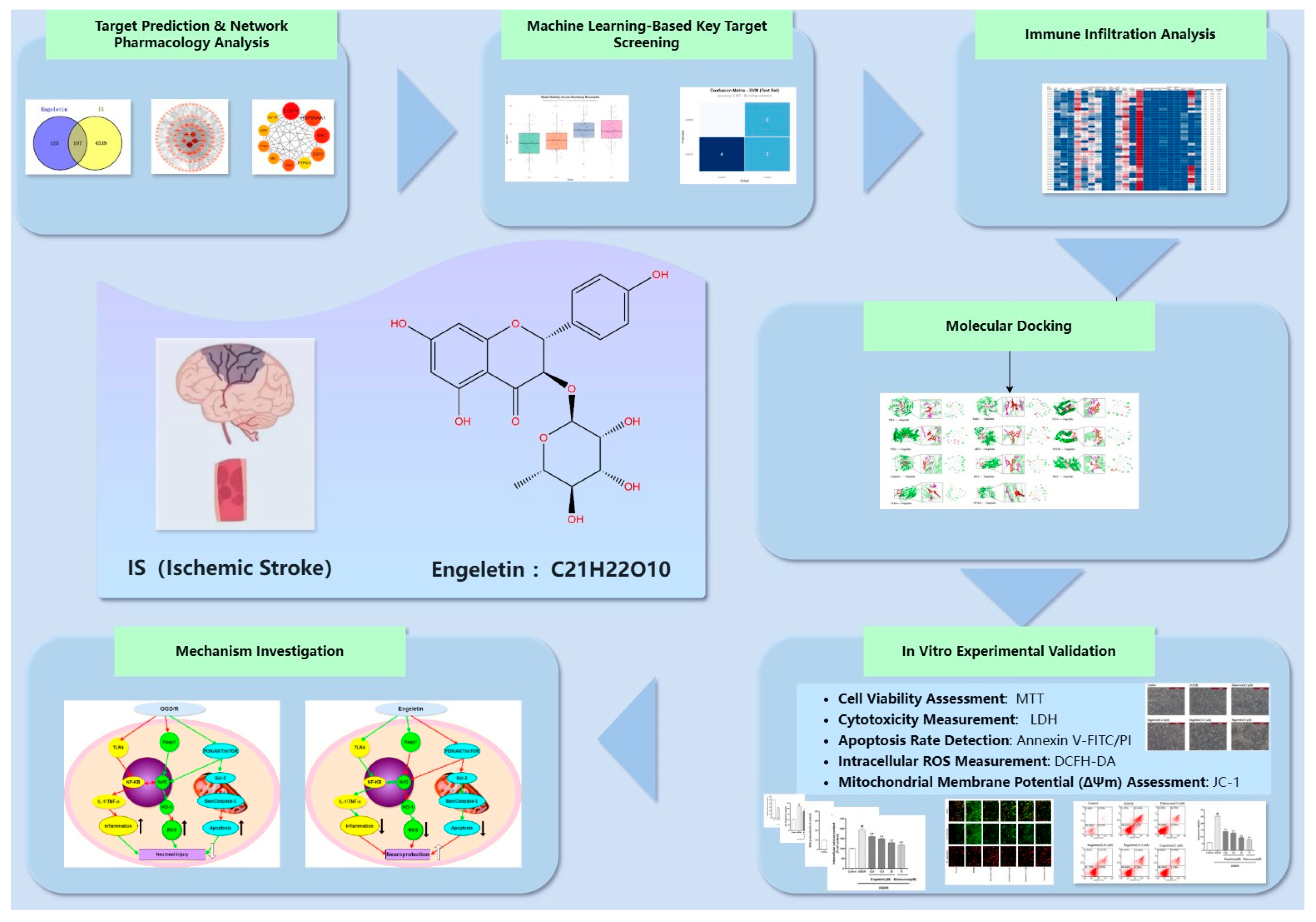
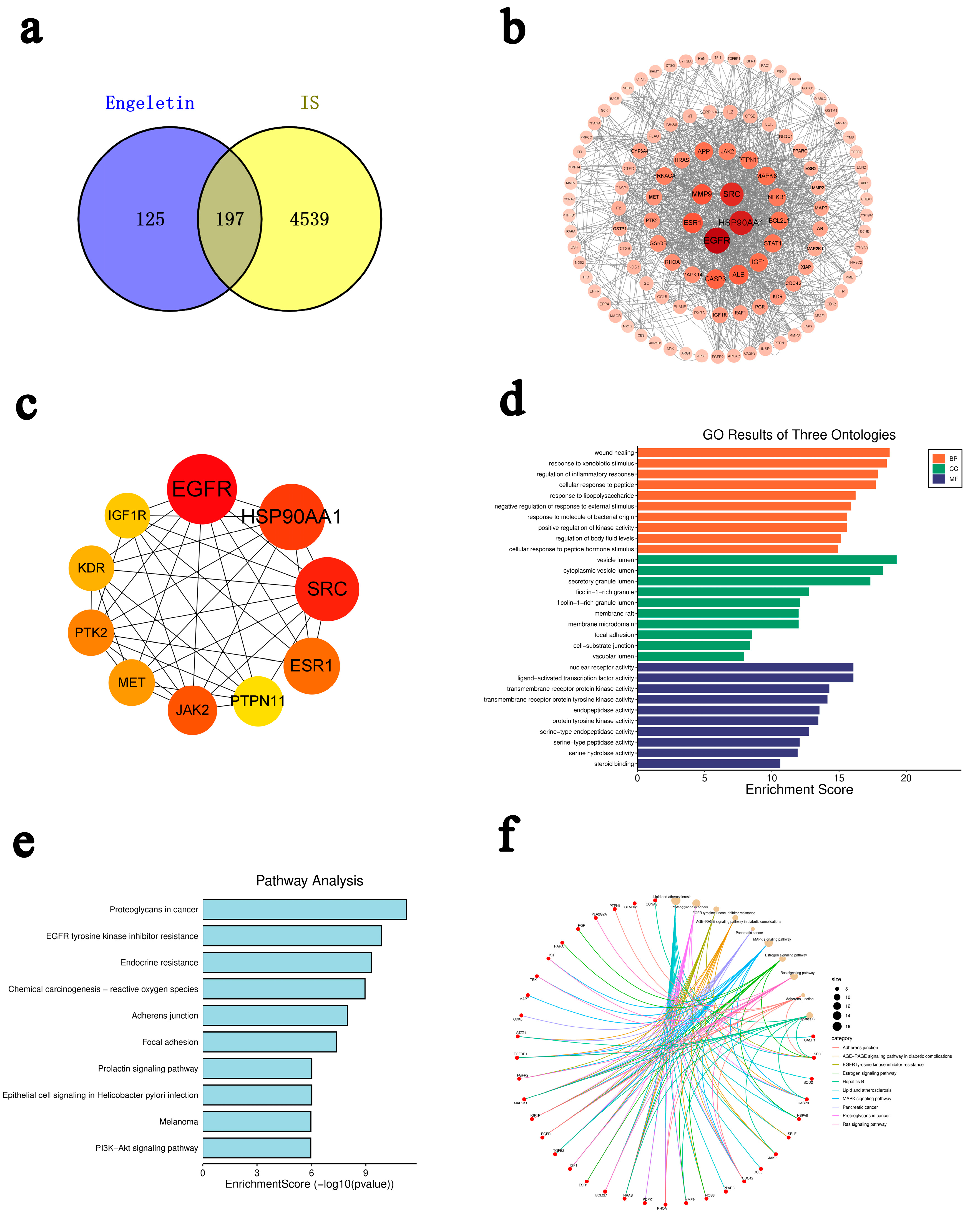
2.1. Network Pharmacology Prediction and Enrichment Analysis of Engeletin in IS
2.2. Multi-Model Machine Learning Prediction and Enrichment Analysis
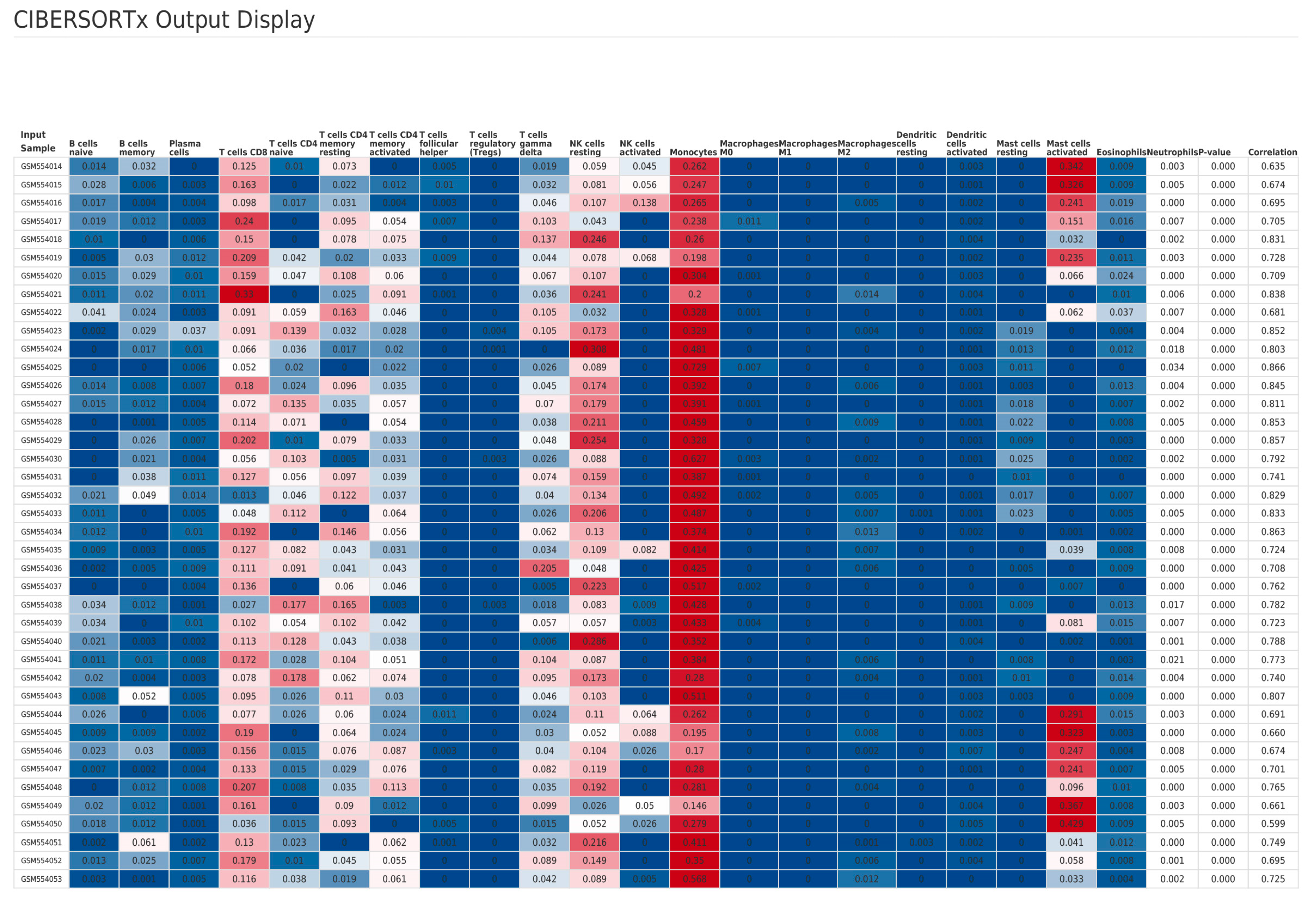
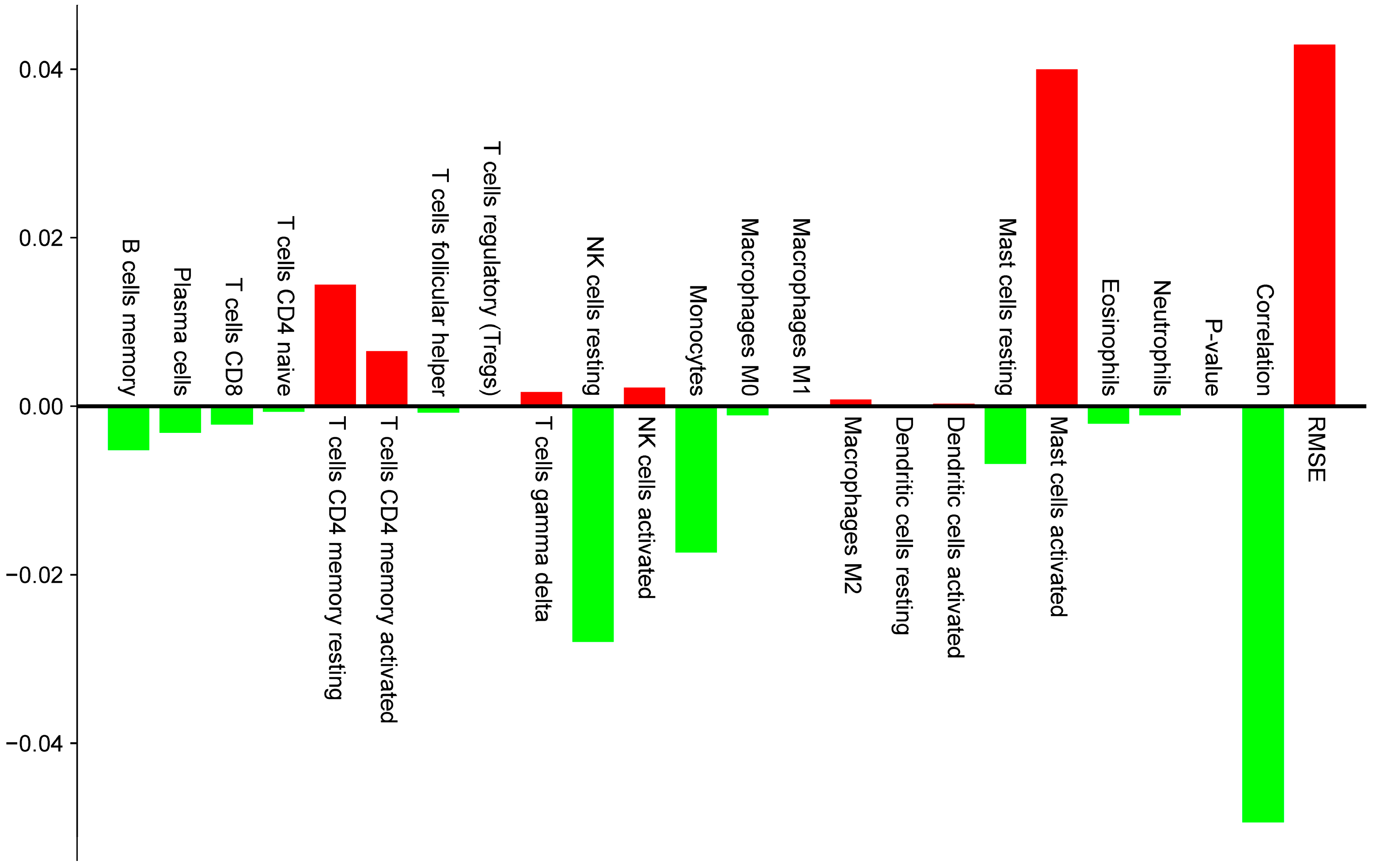
2.3. Immune Infiltration Analysis Based on Clinical Data
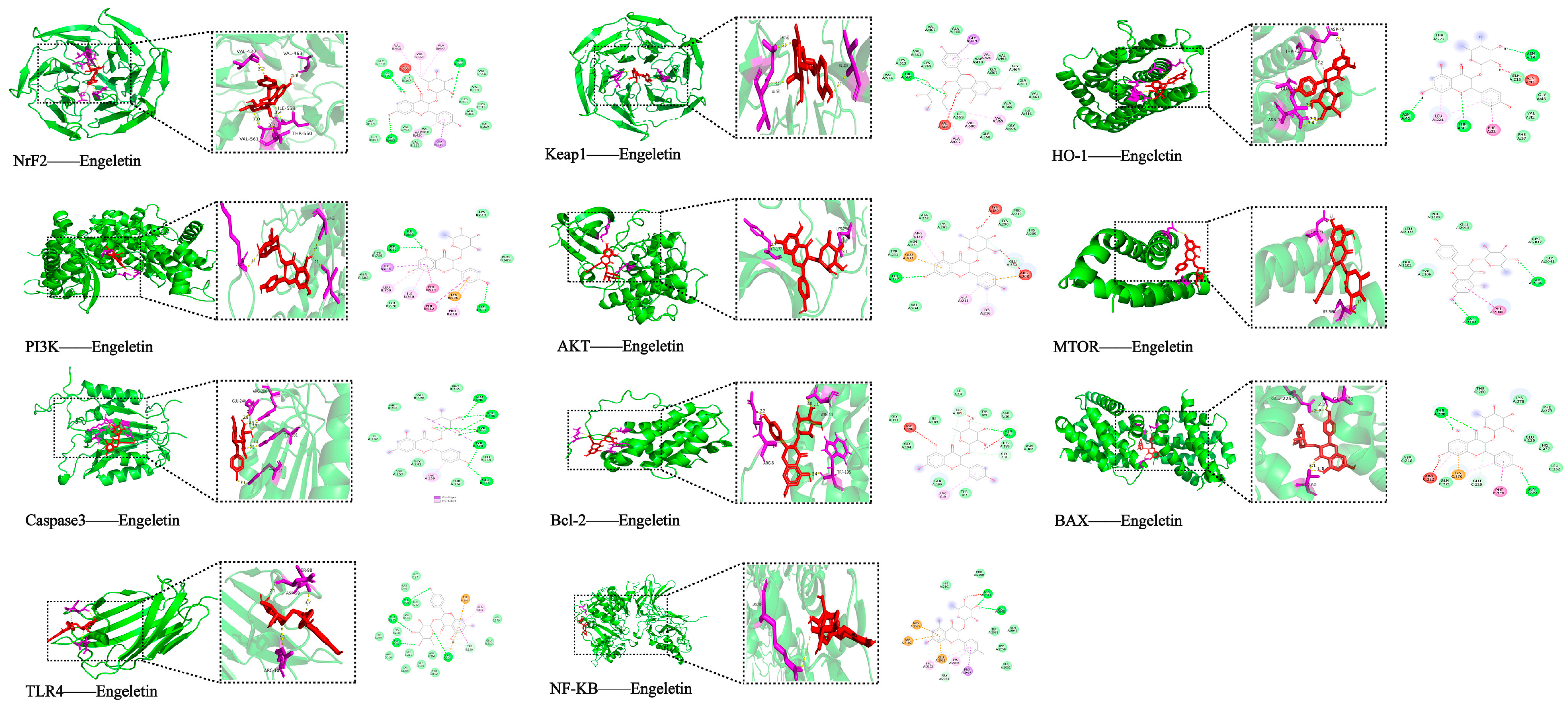
2.4. Molecular Docking with Predicted Pathway Proteins
2.5. Neuroprotective Effect of Engeletin in the OGD/R Model
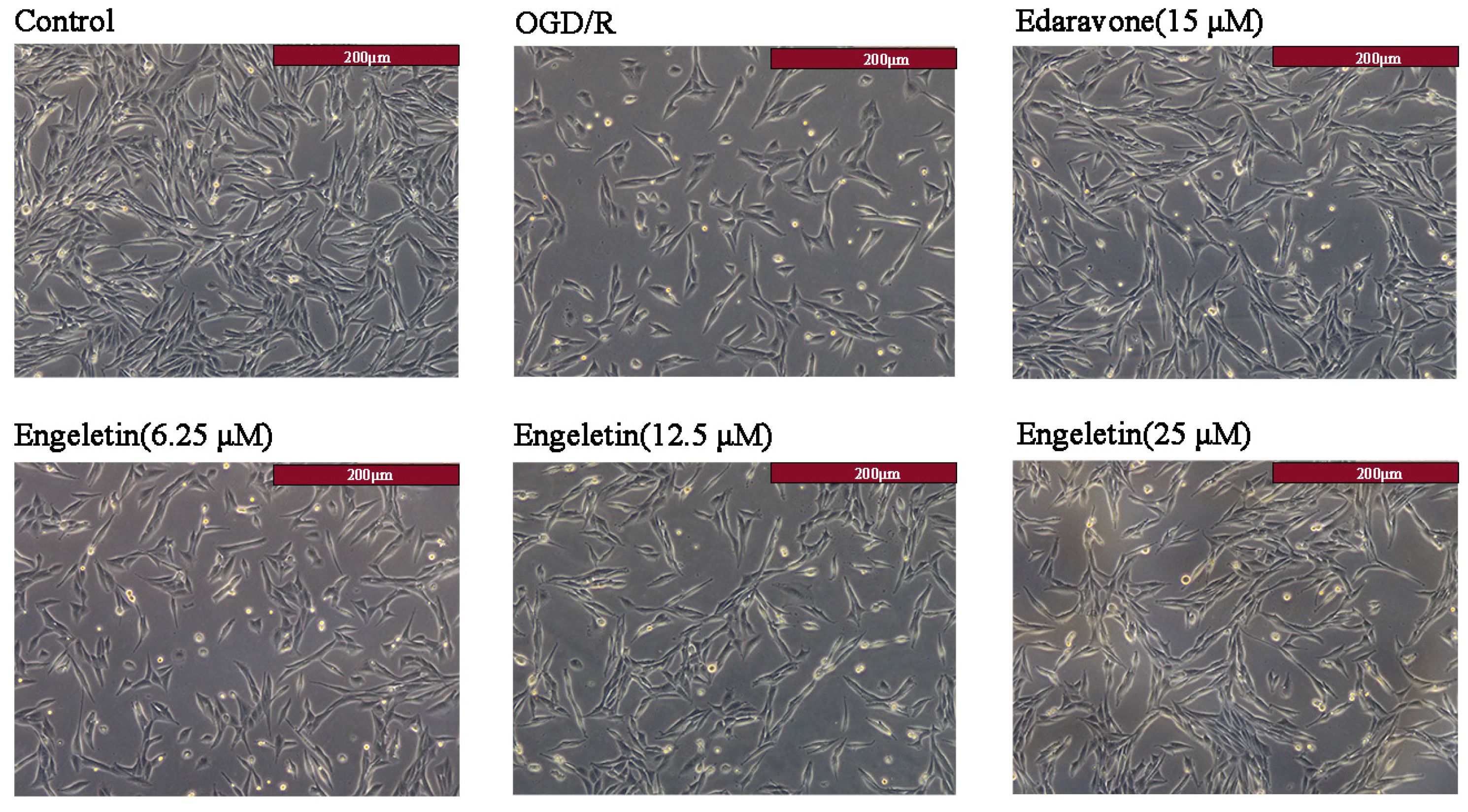
2.5.1. Morphological Changes in PC12 Cells
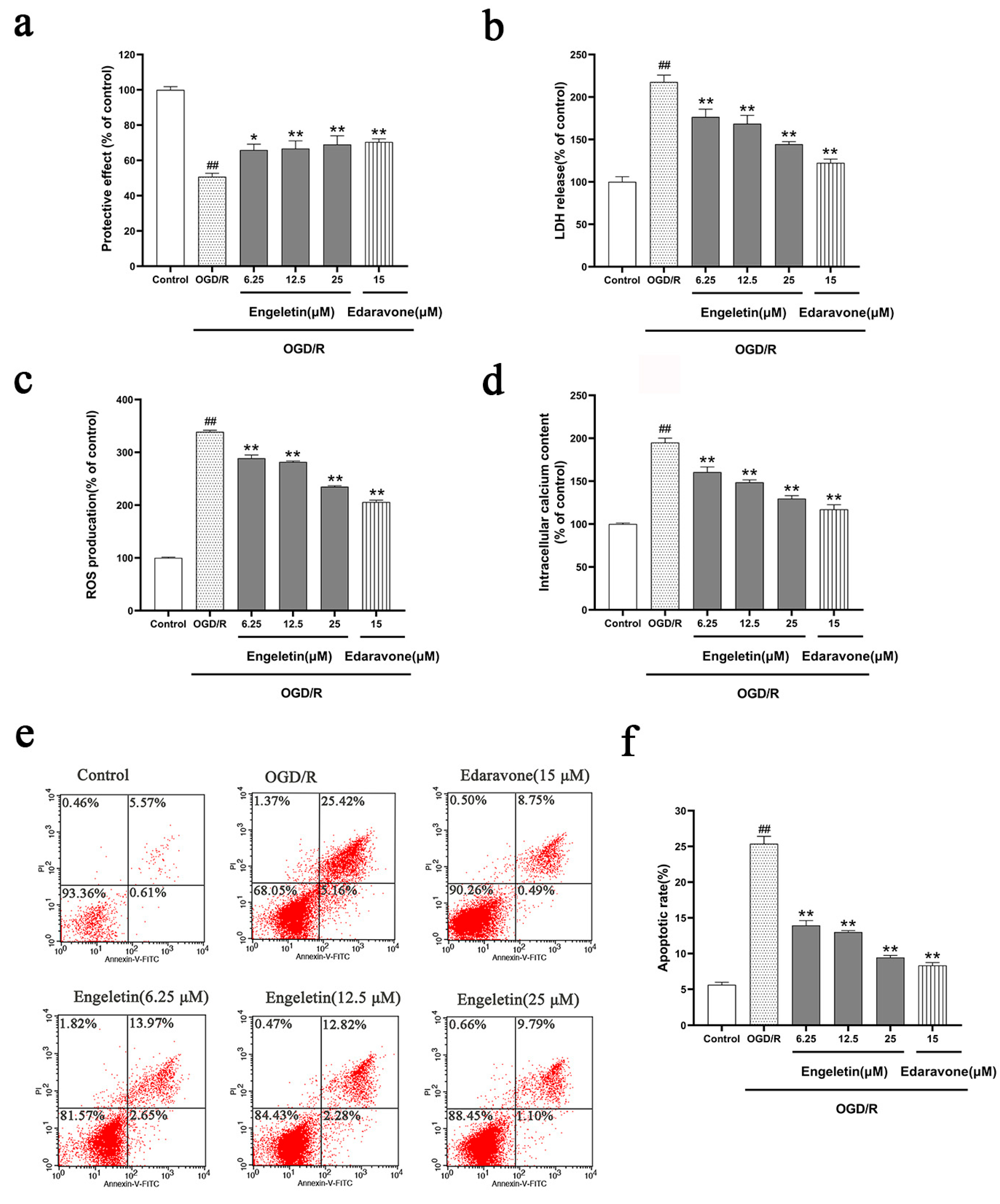
2.5.2. Comprehensive Protective Effects of Engeletin in the OGD/R Model
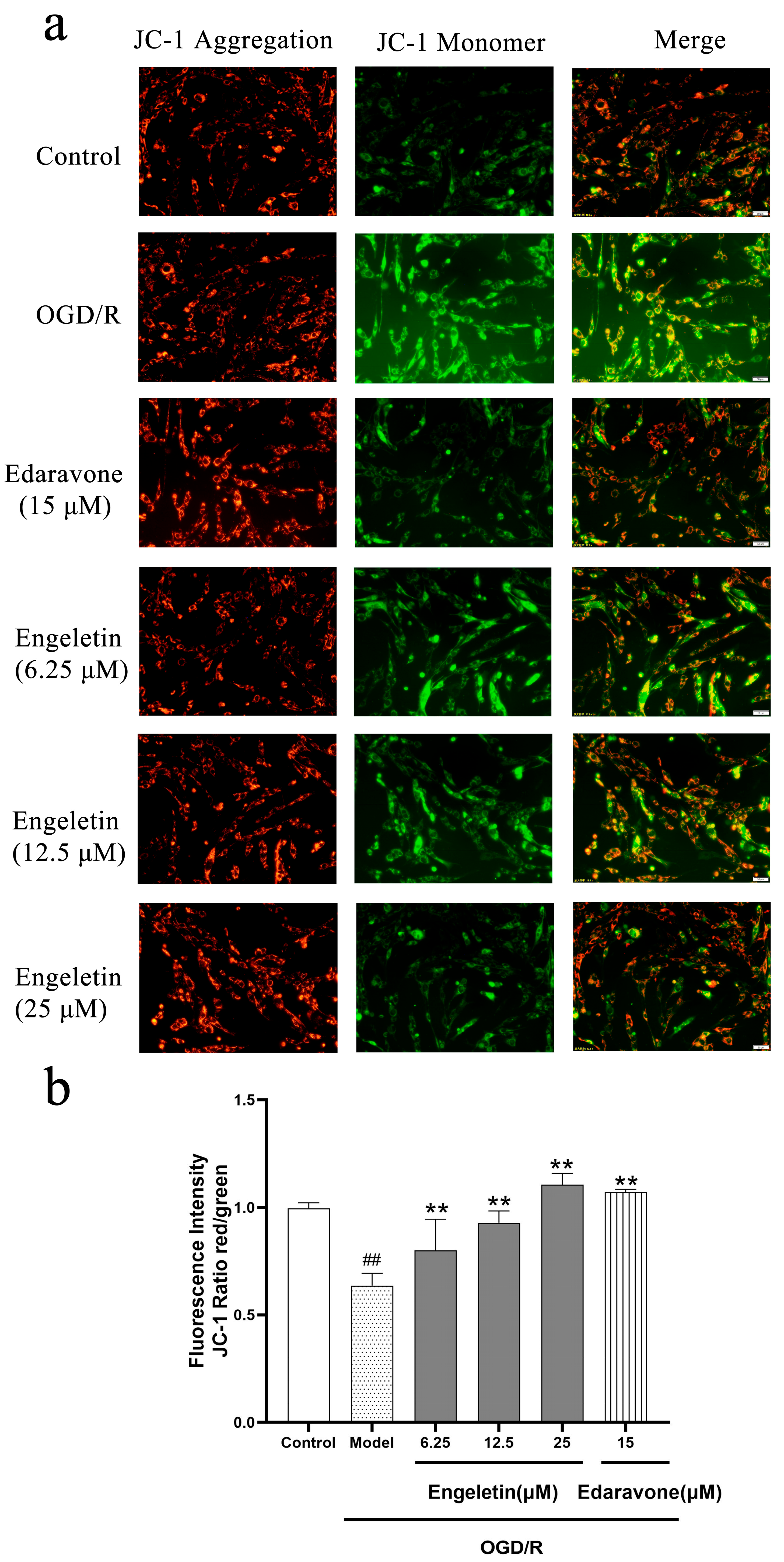
2.5.3. Engeletin Attenuates OGD/R-Induced Mitocghondrial Membrane Potential Loss
2.6. Experimental Validation of Predicted Target Proteins
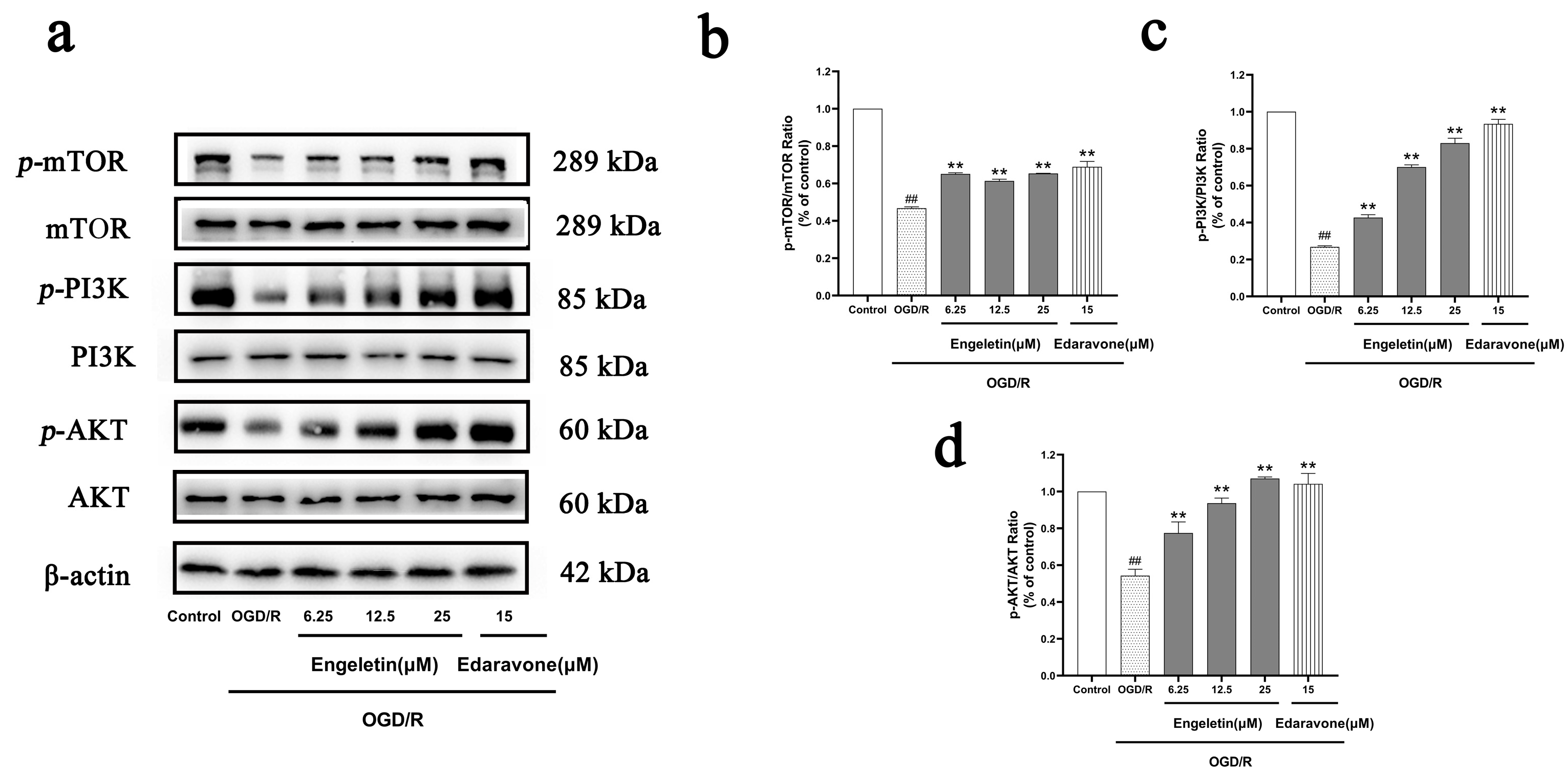
2.6.1. Engeletin Activates the PI3K/Akt/mTOR Signaling Pathway Under OGD/R Conditions
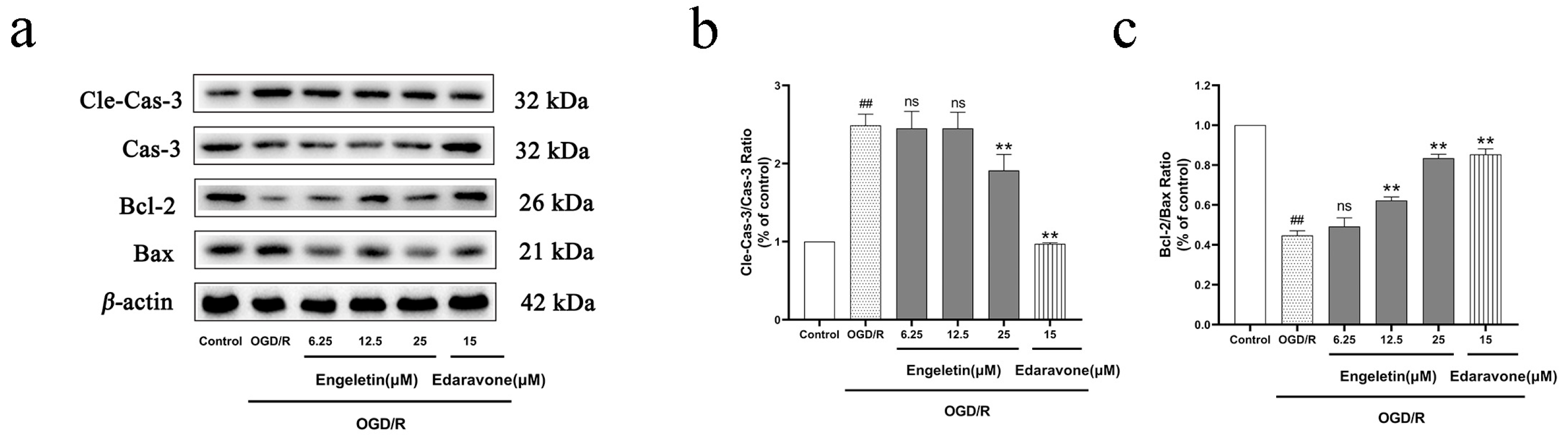
2.6.2. Engeletin Inhibits OGD/R-Induced Mitochondrial-Pathway-Mediated Apoptosis
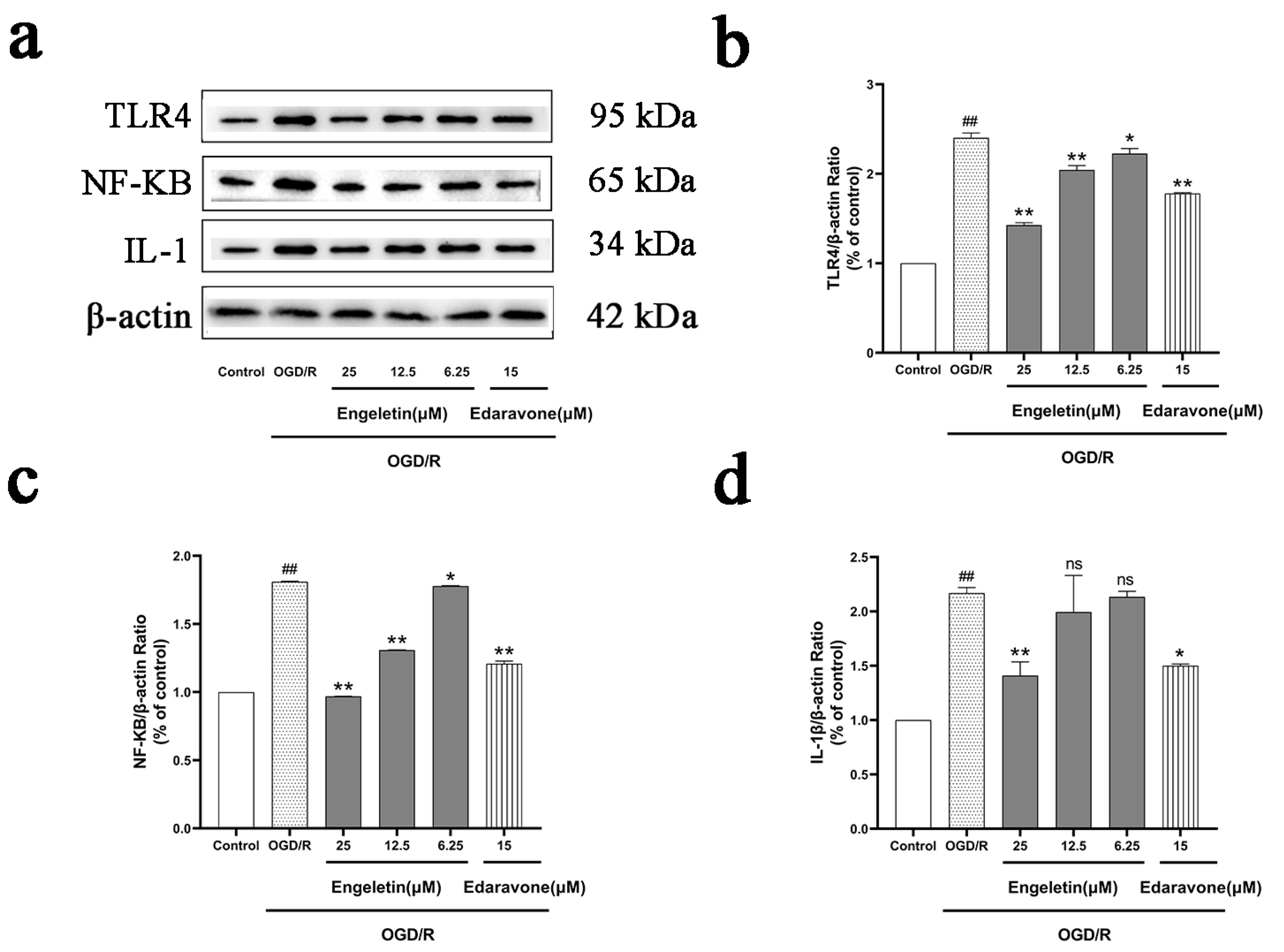
2.6.3. Engeletin Inhibits the TLR4/NF-κB Inflammatory Signaling Pathway Under OGD/R Conditions
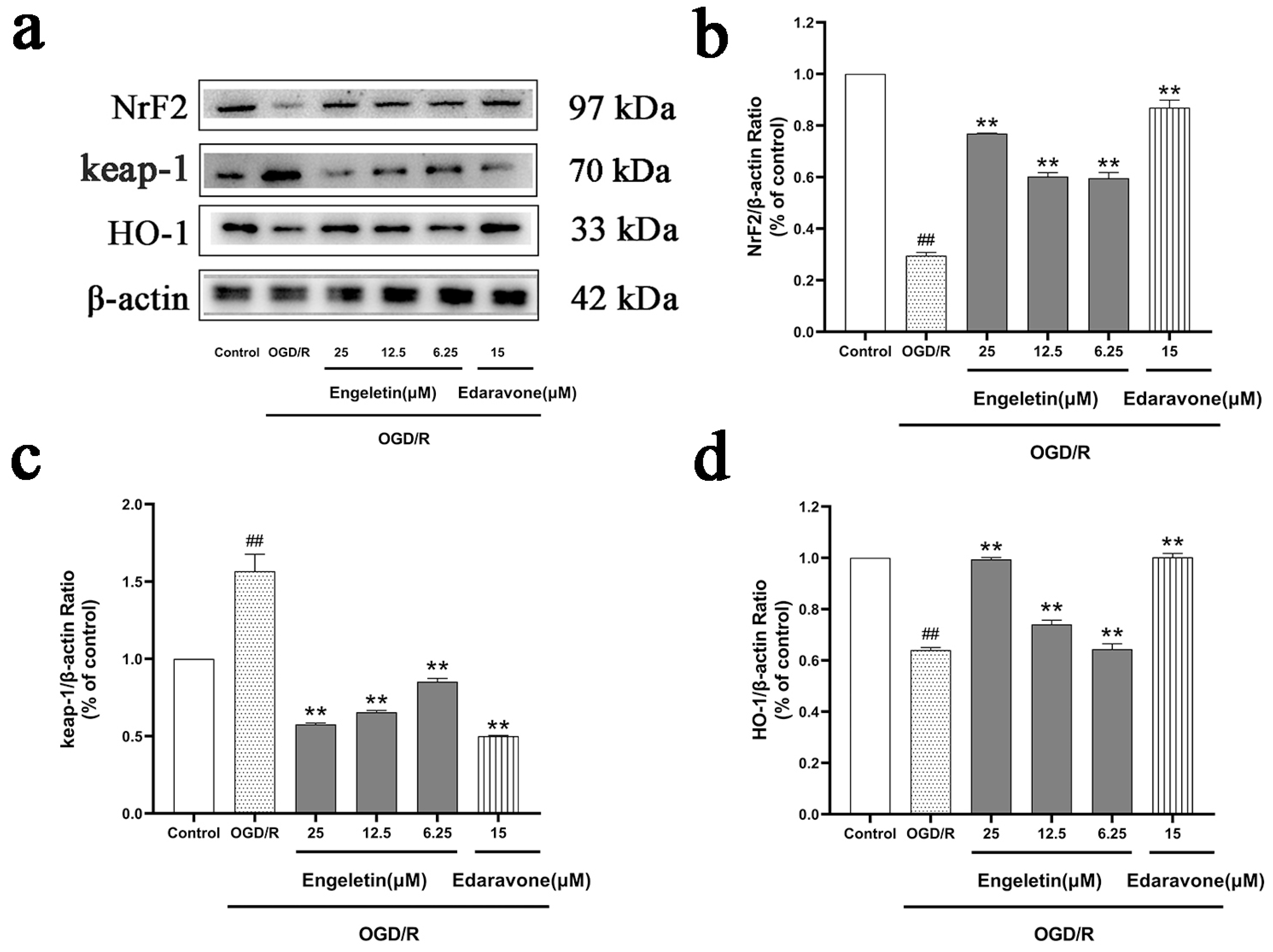
2.6.4. Engeletin Activates the NRF2/KEAP1/HO-1 Antioxidant Pathway Under OGD/R Conditions
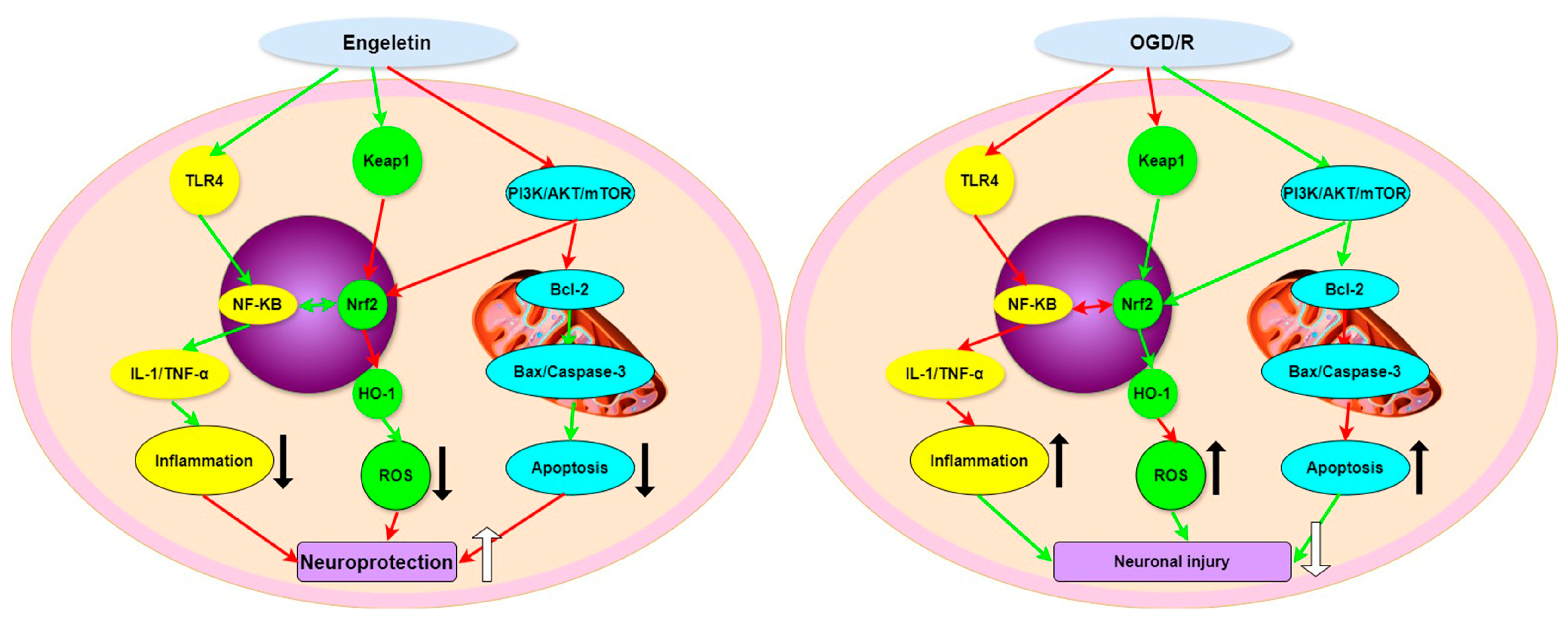
3. Discussion
4. Materials and Methods
4.1. Target Identification and Network Pharmacology Analysis
4.2. Multi-Model Machine-Learning-Based Target Prioritization
4.3. Immune Infiltration Analysis
4.4. Molecular Docking Analysis
4.5. In Vitro Experimental Procedures
4.5.1. Reagents and Antibodies
4.5.2. In Vitro Evaluation of Engeletin’s Neuroprotective Effects
4.5.3. Morphological Observation
4.5.4. MTT Method to Measure Cellular Activity
4.5.5. LDH Release Assay
4.5.6. Measurement of [Ca2+]i
4.5.7. Reactive Oxygen Species (ROS) Level Detection
4.5.8. Measurement of Mitochondrial Membrane Potential (MMP)
4.5.9. Apoptosis Rate Detection
4.6. Western Blotting
4.7. Statistics Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mosconi, M.G.; Paciaroni, M. Treatments in ischemic stroke: Current and future. Eur. Neurol. 2022, 85, 349–366. [Google Scholar] [CrossRef] [PubMed]
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Anderson, C.A.; Arora, P.; Avery, C.L.; Baker-Smith, C.M.; Beaton, A.Z.; Boehme, A.K.; Buxton, A.E.; et al. Heart disease and stroke statistics—2023 update: A report from the American Heart Association. Circulation 2023, 147, e93. [Google Scholar] [CrossRef]
- Pathak, R.; Sharma, S.; Bhandari, M.; Nogai, L.; Mishra, R.; Saxena, A.; Reena, K.; Sharma, H. Neuroinflammation at the crossroads of metabolic and neurodegenerative diseases: Causes, consequences and interventions. J. Exp. Zool. India 2024, 27, 2. [Google Scholar]
- Yeo, L.L.L.; Paliwal, P.; Teoh, H.L.; Seet, R.C.; Chan, B.P.L.; Liang, S.; Venketasubramanian, N.; Rathakrishnan, R.; Ahmad, A.; Ng, K.W.P.; et al. Timing of recanalization after intravenous thrombolysis and functional outcomes after acute ischemic stroke. JAMA Neurol. 2013, 70, 353–358. [Google Scholar] [CrossRef]
- Giles, J.A.; Lee, J.M.; Dhar, R. Multi-Omics Approaches to Discovering Acute Stroke Injury and Recovery Mechanisms. In Stroke Genetics; Springer International Publishing: Cham, Switzerland, 2024; pp. 547–584. [Google Scholar]
- Chen, B.; Jin, W. A comprehensive review of stroke-related signaling pathways and treatment in western medicine and traditional Chinese medicine. Front. Neurosci. 2023, 17, 1200061. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Huang, R.; Chen, X.; Lei, Y. A review on the pharmacological aspects of engeletin as natural compound. Drug Des. Dev. Ther. 2023, 17, 3833–3843. [Google Scholar] [CrossRef]
- Patel, D.K. Medicinal importance, pharmacological activities, and analytical aspects of engeletin in medicine: Therapeutic benefit through scientific data analysis. Endocr. Metab. Immune Disord. Drug Targets 2023, 23, 273–282. [Google Scholar] [CrossRef]
- Wei, J.; Huang, L.; Li, D.; He, J.; Li, Y.; He, F.; Fang, W.; Wei, G. Total Flavonoids of Engelhardia roxburghiana Wall. Leaves Alleviated Foam Cells Formation through AKT/mTOR-Mediated Autophagy in the Progression of Atherosclerosis. Chem. Biodivers. 2021, 18, e2100308. [Google Scholar] [CrossRef]
- Liu, H.; Li, S.; Xu, Y.; Wang, X.; Ren, R.; Zhu, H.; Zhang, S. Engeletin protects against cerebral ischemia/reperfusion injury by modulating the VEGF/vasohibin and Ang-1/Tie-2 pathways. Braz. J. Med. Biol. Res. 2021, 54, e11028. [Google Scholar] [CrossRef] [PubMed]
- Bai, H.; Yin, H. Engeletin suppresses cervical carcinogenesis in vitro and in vivo by reducing NF-κB-dependent signaling. Biochem. Biophys. Res. Commun. 2020, 526, 497–504. [Google Scholar] [CrossRef]
- Zheng, B.; Zhou, X.; Pang, L.; Che, Y.; Qi, X. Dihydroquercetin Ameliorates Neuronal Ferroptosis in Rats After Subarachnoid Hemorrhage via the PI3K/AKT/Nrf2/HO-1 Pathway. J. Biochem. Mol. Toxicol. 2025, 39, e70099. [Google Scholar] [CrossRef]
- Zhu, T.; Wang, L.; Wang, L.P.; Wan, Q. Therapeutic targets of neuroprotection and neurorestoration in ischemic stroke: Applications for natural compounds from medicinal herbs. Biomed. Pharmacother. 2022, 148, 112719. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Ni, X.; Cui, H.; Shu, C.; Peng, Y.; Liu, J.; Li, Y. Engeletin attenuates the inflammatory response via inhibiting TLR4-NFκB signaling pathway in Crohn’s disease-like colitis. J. Ethnopharmacol. 2025, 336, 118733. [Google Scholar] [CrossRef]
- Huang, L.; Bian, M.; Lu, S.; Wang, J.; Yu, J.; Jiang, L.; Zhang, J. Engeletin alleviates erastin-induced oxidative stress and protects against ferroptosis via Nrf2/Keap1 pathway in bone marrow mesenchymal stem cells. Tissue Cell 2023, 82, 102040. [Google Scholar] [CrossRef] [PubMed]
- Nidai Ozes, O.; Mayo, L.D.; Gustin, J.A.; Pfeffer, S.R.; Pfeffer, L.M.; Donner, D.B. NF-κB activation by tumour necrosis factor requires the Akt serine–threonine kinase. Nature 1999, 401, 82–85. [Google Scholar] [CrossRef]
- Liu, G.H.; Qu, J.; Shen, X. NF-κB/p65 antagonizes Nrf2-ARE pathway by depriving CBP from Nrf2 and facilitating recruitment of HDAC3 to MafK. Biochim. Biophys. Acta Mol. Cell Res. 2008, 1783, 713–727. [Google Scholar] [CrossRef]
- Huang, H.F.; Zeng, Z.; Wang, K.H.; Zhang, H.Y.; Wang, S.; Zhou, W.X.; Wang, Z.B.; Xu, W.G.; Duan, J. Heme oxygenase-1 protects rat liver against warm ischemia/reperfusion injury via TLR2/TLR4-triggered signaling pathways. World J. Gastroenterol. 2015, 21, 2937. [Google Scholar] [CrossRef] [PubMed]
- Farina, M.; Vieira, L.E.; Buttari, B.; Profumo, E.; Saso, L. The Nrf2 pathway in ischemic stroke: A review. Molecules 2021, 26, 5001. [Google Scholar] [CrossRef]
- Wang, Y.R.; Zhang, X.N.; Meng, F.G.; Zeng, T. Targeting macrophage polarization by Nrf2 agonists for treating various xenobiotics-induced toxic responses. Toxicol. Mech. Methods 2021, 31, 334–342. [Google Scholar] [CrossRef]
- Zeng, H.; Yang, K.; Cloer, C.; Neale, G.; Vogel, P.; Chi, H. mTORC1 couples immune signals and metabolic programming to establish Treg-cell function. Nature 2013, 499, 485–490. [Google Scholar] [CrossRef]
- Vergadi, E.; Ieronymaki, E.; Lyroni, K.; Vaporidi, K.; Tsatsanis, C. Akt signaling pathway in macrophage activation and M1/M2 polarization. J. Immunol. 2017, 198, 1006–1014. [Google Scholar] [CrossRef]
- Yoshida, H.; Yanai, H.; Namiki, Y.; Fukatsu-Sasaki, K.; Furutani, N.; Tada, N. Neuroprotective effects of edaravone: A novel free radical scavenger in cerebrovascular injury. CNS Drug Rev. 2006, 12, 9–20. [Google Scholar] [CrossRef]
- Chen, J.; Yang, C.; Xu, X.; Yang, Y.; Xu, B. The effect of focal cerebral ischemia-reperfusion injury on TLR4 and NF-κB signaling pathway. Exp. Ther. Med. 2018, 15, 897–903. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.; Yang, S.; Chu, Y.H.; Zhang, H.; Pang, X.W.; Chen, L.; Zhou, L.Q.; Chen, M.; Tian, D.S.; Wang, W. Signaling pathways involved in ischemic stroke: Molecular mechanisms and therapeutic interventions. Signal Transduct. Target. Ther. 2022, 7, 215. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.D. Mechanistic studies of the Nrf2-Keap1 signaling pathway. Drug Metab. Rev. 2006, 38, 769–789. [Google Scholar] [CrossRef]
- Wang, M.; Wang, Y.; Zhang, P.; Gu, C.; Zhao, X.; Gong, X.; Yang, X.; Pan, J.; Xi, Y. Neutrophil-like cell membrane-coated metal-organic frameworks for siRNA delivery targeting NOX4 to alleviate oxidative stress in acute ischemic injury. Acta Biomater. 2025, 196, 487–505. [Google Scholar] [CrossRef] [PubMed]
- Dhanasekaran, A.; Kotamraju, S.; Karunakaran, C.; Kalivendi, S.V.; Thomas, S.; Joseph, J.; Kalyanaraman, B. Mitochondria superoxide dismutase mimetic inhibits peroxide-induced oxidative damage and apoptosis: Role of mitochondrial superoxide. Free Radic. Biol. Med. 2005, 39, 567–583. [Google Scholar] [CrossRef]
- Knox, C.; Wilson, M.; Klinger, C.M.; Franklin, M.; Oler, E.; Wilson, A.; Pon, A.; Cox, J.; Chin, N.E.; Strawbridge, S.A.; et al. DrugBank 6.0: The DrugBank knowledgebase for 2024. Nucleic Acids Res. 2024, 52, D1265–D1275. [Google Scholar] [CrossRef]
- Meng, X.; Xu, R.; Wang, H.; Zhu, J.; Ye, J.; Luo, C. Validation of machine learning application for the identification of lipid metabolism-associated diagnostic model in ischemic stroke. Int. J. Clin. Exp. Pathol. 2025, 18, 63. [Google Scholar] [CrossRef]
- Newman, A.M.; Liu, C.L.; Green, M.R.; Gentles, A.J.; Feng, W.; Xu, Y.; Hoang, C.D.; Diehn, M.; Alizadeh, A.A. Robust enumeration of cell subsets from tissue expression profiles. Nat. Methods 2015, 12, 453–457. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Xu, M.; Wang, Y.; Xie, F.; Zhang, G.; Qin, X. Nrf2—A promising therapeutic target for defensing against oxidative stress in stroke. Mol. Neurobiol. 2017, 54, 6006–6017. [Google Scholar] [CrossRef]
- Manning, B.D.; Toker, A. AKT/PKB signaling: Navigating the network. Cell 2017, 169, 381–405. [Google Scholar] [CrossRef]
- Chen, J.; Ji, Z.; Wu, D.; Wei, S.; Zhu, W.; Peng, G.; Hu, M.; Zhao, Y.; Wu, H. MYBL2 promotes cell proliferation and inhibits cell apoptosis via PI3K/AKT and BCL2/BAX/Cleaved-caspase-3 signaling pathway in gastric cancer cells. Sci. Rep. 2025, 15, 9148. [Google Scholar] [CrossRef]
- Dan, H.C.; Cooper, M.J.; Cogswell, P.C.; Duncan, J.A.; Ting, J.P.-Y.; Baldwin, A.S. Akt-dependent regulation of NF-κB is controlled by mTOR and Raptor in association with IKK. Genes. Dev. 2008, 22, 1490–1500. [Google Scholar] [CrossRef]
- Bauerfeld, C.P.; Rastogi, R.; Pirockinaite, G.; Lee, I.; Hüttemann, M.; Monks, B.; Birnbaum, M.J.; Franchi, L.; Nuñez, G.; Samavati, L. TLR4-mediated AKT activation is MyD88/TRIF dependent and critical for induction of oxidative phosphorylation and mitochondrial transcription factor A in murine macrophages. J. Immunol. 2012, 188, 2847–2857. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Song, Y.; Wang, Y.; Zhou, H.; Chen, L.; Zhan, Y.; Li, T.; Xie, G.; Wu, H. Biological role of mitochondrial TLR4-mediated NF-κB signaling pathway in central nervous system injury. Cell Biochem. Funct. 2024, 42, e4056. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, X.; Li, J.J. The role of NF-κB in the regulation of cell stress responses. Int. Immunopharmacol. 2002, 2, 1509–1520. [Google Scholar] [CrossRef]
- Qian, Y.; Zhang, Y.; Chen, Y.; Wang, X.; Liu, L.; Yu, H.; Wu, Z.; Gui, L.; He, X.; Huang, L.; et al. Linarin attenuates hyperuricemic nephropathy by modulating Nrf2/Keap1 and TLR4/NF-κB signaling pathways: Linarin attenuates hyperuricemic nephropathy. Phytomedicine 2025, 139, 156440. [Google Scholar] [CrossRef]
- Mohamed, S.S.; Rasheed, N.O.A.; Ibrahim, W.W.; Shiha, N.A. Targeting toll-like receptor 4/nuclear factor-κB and Nrf2/heme oxygenase-1 crosstalk via trimetazidine alleviates lipopolysaccharide-induced depressive-like behaviors in mice. J. Neuroimmune Pharmacol. 2024, 19, 50. [Google Scholar] [CrossRef] [PubMed]
- Park, C.; Cha, H.J.; Lee, H.; Kim, G.Y.; Choi, Y.H. The regulation of the TLR4/NF-κB and Nrf2/HO-1 signaling pathways is involved in the inhibition of lipopolysaccharide-induced inflammation and oxidative reactions by morroniside in RAW 264. 7 macrophages. Arch. Biochem. Biophys. 2021, 706, 108926. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissTargetPrediction: Updated data and new features for efficient prediction of protein targets of small molecules. Nucleic Acids Res. 2019, 47, W357–W364. [Google Scholar] [CrossRef]
- Piñero, J.; Bravo, À.; Queralt-Rosinach, N.; Gutiérrez-Sacristán, A.; Deu-Pons, J.; Centeno, E.; García-García, J.; Sanz, F.; Furlong, L.I. DisGeNET: A comprehensive platform integrating information on human disease-associated genes and variants. Nucleic Acids Res. 2016, 44, gkw943. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Nastou, K.; Koutrouli, M.; Kirsch, R.; Mehryary, F.; Hachilif, R.; Hu, D.; Peluso, M.E.; Huang, Q.; Fang, T.; et al. The STRING database in 2025: Protein networks with directionality of regulation. Nucleic Acids Res. 2025, 53, D730–D737. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009, 4, 44–57. [Google Scholar] [CrossRef]
- Wang, S.; Li, X.; Bi, Y.; Yan, C.; Chen, Y. The impact of inflammation and iron metabolism on gene expression alterations in ischemic stroke: A bioinformatics approach. Sci. Rep. 2025, 15, 15233. [Google Scholar] [CrossRef]
- Kuhn, M. Building predictive models in R using the caret package. J. Stat. Softw. 2008, 28, 1–26. [Google Scholar] [CrossRef]
- Ono, K.; Fong, D.; Gao, C.; Churas, C.; Pillich, R.; Lenkiewicz, J.; Pratt, D.; Pico, A.R.; Hanspers, K.; Xin, Y.; et al. Cytoscape Web: Bringing network biology to the browser. Nucleic Acids Res. 2025, 53, gkaf365. [Google Scholar] [CrossRef] [PubMed]
- Kleijn, S.; Langereis, J.D.; Leentjens, J.; Kox, M.; Netea, M.G.; Koenderman, L.; Ferwerda, G.; Pickkers, P.; Hermans, P.W.M. IFN-γ-stimulated neutrophils suppress lymphocyte proliferation through expression of PD-L1. PLoS ONE 2013, 8, e72249. [Google Scholar] [CrossRef]
- Chen, B.; Khodadoust, M.S.; Liu, C.L.; Newman, A.M.; Alizadeh, A.A. Profiling tumor infiltrating immune cells with CIBERSORT. In Cancer Systems Biology: Methods and Protocols; Humana: New York, NY, USA, 2018; pp. 243–259. [Google Scholar]
- Ru, J.; Li, P.; Wang, J.; Zhou, W.; Li, B.; Huang, C.; Li, P.; Guo, Z.; Tao, W.; Yang, Y.; et al. TCMSP: A database of systems pharmacology for drug discovery from herbal medicines. J. Cheminform. 2014, 6, 13. [Google Scholar] [CrossRef]
- Yuan, S.; Chan, H.C.S.; Hu, Z. Using PyMOL as a platform for computational drug design. WIREs Comput. Mol. Sci. 2017, 7, e1298. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, X.; Gan, J.; Chen, S.; Xiao, Z.-X.; Cao, Y. Improved protein–ligand blind docking by integrating cavity detection, docking and homologous template fitting. Nucleic Acids Res. 2022, 50, W159–W164. [Google Scholar] [CrossRef]
- Sun, Y.; Zhu, W.; Zhou, S.; Wang, Z.; Chen, X.; Jia, L. Exploring the model of PC12 apoptosis induced by OGSD/R through in vitro experiments. Oncotarget 2017, 8, 90176. [Google Scholar] [CrossRef] [PubMed]
- Vermes, I.; Haanen, C.; Steffens-Nakken, H.; Reutellingsperger, C. A novel assay for apoptosis flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled annexin V. J. Immunol. Methods 1995, 184, 39–47. [Google Scholar] [CrossRef] [PubMed]
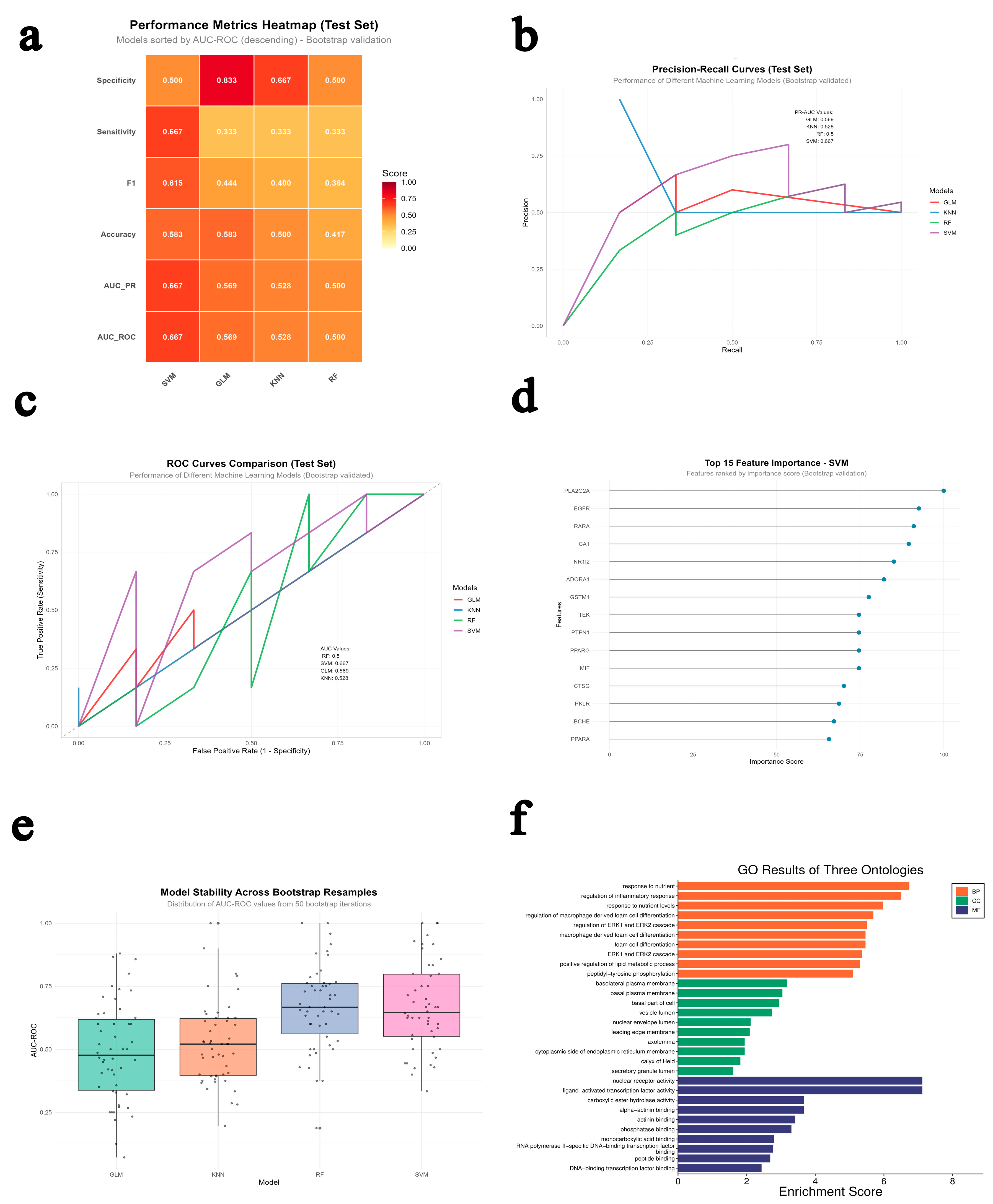
| Model | Data_Type | Accuracy | Sensitivity | Specificity | Precision | F1 | AUC_ROC | AUC_PR |
|---|---|---|---|---|---|---|---|---|
| RF | Test | 0.4167 | 0.3333 | 0.5000 | 0.4000 | 0.3636 | 0.5000 | 0.5000 |
| SVM | Test | 0.5833 | 0.6667 | 0.5000 | 0.5714 | 0.6154 | 0.6667 | 0.6667 |
| GLM | Test | 0.5833 | 0.3333 | 0.8333 | 0.6667 | 0.4444 | 0.5694 | 0.5694 |
| KNN | Test | 0.5000 | 0.3333 | 0.6667 | 0.5000 | 0.4000 | 0.5278 | 0.5278 |
| Rank | Pathway | KEGG ID | Main Associated System | Related Function Overview |
|---|---|---|---|---|
| 1 | PI3K-Akt signaling pathway | hsa04151 | PI3K-Akt-mTOR/BCL2 | It primarily regulates cell growth, metabolism, and anti-apoptosis by activating Akt to upregulate mTOR and BCL2 signaling. |
| 2 | MAPK signaling pathway | hsa04010 | PI3K-Akt/NF-κB | MAPK can cross-activate the NF-κB and mTOR pathways, regulating cell inflammation and survival. |
| 3 | HIF-1 signaling pathway | hsa04066 | PI3K-Akt/NRF2 | Closely related to oxidative stress, stabilizes HIF-1α through PI3K/Akt and promotes antioxidant responses. |
| 4 | Ras signaling pathway | hsa04014 | PI3K-Akt/NF-κB | Activation of PI3K-Akt and MAPK jointly promotes growth and anti-apoptosis. |
| 5 | NF-κB signaling (via TLR4/NF-κB crosslink) | (hsa04620/04151) | TLR4/NF-κB | Core pathways of inflammatory response, regulating the expression of immune factors and cell survival. |
| 6 | JAK-STAT signaling pathway | hsa04630 | NF-κB/PI3K-Akt | Promotes the expression of anti-inflammatory cytokines while interacting with NF-κB. |
| 7 | AMPK signaling pathway | hsa04152 | NRF2/KEAP1/PI3K-Akt | Can activate NRF2 and inhibit mTOR, thereby enhancing antioxidant capacity and metabolic homeostasis. |
| 8 | FoxO signaling pathway | hsa04068 | PI3K-Akt/NRF2 | Akt inhibits FoxO transcription factors, which regulate the expression of antioxidant enzymes (such as SOD and CAT). |
| 9 | TNF/Toll-like receptor signaling pathway | hsa04620 | TLR4/NF-κB | A key upstream regulator of inflammation and cell death, linking immunity and oxidative stress responses. |
| 10 | Glutathione metabolism | hsa00480 | NRF2/KEAP1/HO-1 | The main antioxidant metabolic pathways regulated by NRF2 maintain cellular redox balance. |
| Protein | PDB ID | Ligands | Affinity (kcal/mol) | RMSD | Bonding Interaction |
|---|---|---|---|---|---|
| AKT | 1GZK | engeletin | −7.5 | 0.605 Å | Hydrophobic Interactions (LYS 216A, GLU230A, GLU433A) Hydroge nBondsInte Ractions (ARG208A, ASN233A, GLU433A, LYS285A) Salt Bridges (LYS285A, LYS290A) |
| BAX | BAX | engeletin | −8.3 | 0.607 Å | Hydrophobic Interactions (ARG22C, GLN221C, LYS276A, PHE273A, THR280A) Hydrogen Bonds Interactions (GLN229C, LYS276A, THR280A) |
| Bcl-2 | 1G5M | engeletin | −8.5 | 1.625 Å | Hydrophobic Interaction (ARG6A, ASN11A) Hydrogen Bond Interactions (TRP195A, ASN11A, ASP10A, TYR9A, ARG6A) |
| Caspase-3 | 1CP3 | engeletin | −7.1 | 0.610 Å | Hydrophobic Interaction (TYR329B, VAL390B) Hydrogen Bond Interactions (ARG286A, LYS259A, TYR331B) |
| foxo | 3CO6 | engeletin | −8.1 | 0.603 Å | Hydrophobic Interaction (VAL1188C, TRP1206C) Hydrogen Bond Interactions (GLU1185C, GLY1198C, LYS1195C) Salt Bridges (LYS1207C) |
| Hif1 | 1H2K | engeletin | −7.1 | 0.613 Å | Hydrophobic Interactions (PHE114A) Hydrogen Bond Interactions (ARG251A, LEU101A, LYS115A, TYR230A) |
| HO-1 | 1N3U | engeletin | −8.0 | 0.609 Å | Hydrophobic Interactions (PHE33A, ASN36A, ASP45A, LEU21A) Hydrogen Bond Interactions (ASN36A, ASP45A) |
| IL-1 | 2ILA | engeletin | −8.5 | 0.616 Å | Hydrophobic Interactions (GLU11B) Hydrogen Bond Interactions (ARG9B, GLU10B, LYS27A) Salt Bridges (ARG9B) |
| keap1 | 1U6D | engeletin | −11.7 | 0.610 Å | Hydrophobic Interactions (VAL467A) Hydrogen Bond Interactions (ILE559A, VAL420A, VAL465A, VAL561A, VAL608A) |
| MTOR | 4DRI | engeletin | −7.6 | 0.611 Å | Hydrogen Bond Interactions (ARG111A, ASN92A, ILE61A, LYS87A, TYR58A) π-Stacking (PHE209A) |
| NF-KB | 1MDI | engeletin | −7.1 | 0.608 Å | Hydrophobic Interactions (PRO2307A, LYS2309A) Hydrogen Bond Interactions (ARG2043A, LYS2039A) Salt Bridges (ARG2305A) |
| NQ01 | 1D4A | engeletin | −9.8 | 0.605 Å | Hydrophobic Interactions (PRO102B GLU117D Hydrogen Bond Interactions (TRP105B, PHE106B, PHE120D) |
| nrf2 | 2FLU | engeletin | −9.9 | 0.609 Å | Hydrophobic Interactions (VAL420B, ILE559B) Hydrogen Bond Interactions (ILE559B, THR560B, VAL420B, VAL465B) |
| PI3K | 3IHY | engeletin | −8.0 | 0.606 Å | Hydrophobic Interactions (ILE634B, ILE760B, LEU750B) Hydrogen Bond Interactions (LYS613B, SER614B) |
| TLR4 | 2Z62 | engeletin | −8.6 | 0.604 Å | Hydrogen Bond Interactions (SER98D) Salt Bridges (ARG106D) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, H.; Wen, Y.; Yang, J.; Zhang, Y.; Jin, C.; Li, B.; Ai, Y.; Zheng, M.; Wen, B.; Song, K. Multi-Pathway Mechanisms of Engeletin in Ischemic Stroke: A Comprehensive Study Based on Network Pharmacology, Machine Learning, and Immune Infiltration Analysis. Int. J. Mol. Sci. 2025, 26, 11446. https://doi.org/10.3390/ijms262311446
Xue H, Wen Y, Yang J, Zhang Y, Jin C, Li B, Ai Y, Zheng M, Wen B, Song K. Multi-Pathway Mechanisms of Engeletin in Ischemic Stroke: A Comprehensive Study Based on Network Pharmacology, Machine Learning, and Immune Infiltration Analysis. International Journal of Molecular Sciences. 2025; 26(23):11446. https://doi.org/10.3390/ijms262311446
Chicago/Turabian StyleXue, Huiming, Yuchen Wen, Jiahui Yang, Yue Zhang, Chang Jin, Bing Li, Yongxing Ai, Meizhu Zheng, Boge Wen, and Kai Song. 2025. "Multi-Pathway Mechanisms of Engeletin in Ischemic Stroke: A Comprehensive Study Based on Network Pharmacology, Machine Learning, and Immune Infiltration Analysis" International Journal of Molecular Sciences 26, no. 23: 11446. https://doi.org/10.3390/ijms262311446
APA StyleXue, H., Wen, Y., Yang, J., Zhang, Y., Jin, C., Li, B., Ai, Y., Zheng, M., Wen, B., & Song, K. (2025). Multi-Pathway Mechanisms of Engeletin in Ischemic Stroke: A Comprehensive Study Based on Network Pharmacology, Machine Learning, and Immune Infiltration Analysis. International Journal of Molecular Sciences, 26(23), 11446. https://doi.org/10.3390/ijms262311446





