Bone Marker Proteins at Baseline and After Insulin-Induced Hypoglycaemia in Type 2 Diabetes
Abstract
1. Introduction
2. Results
2.1. Demographic and Biochemical Characteristics of Study Participants
2.2. Linear Mixed Model for Repeated Measures for Each Bone Marker Protein Between T2D and Controls at Baseline
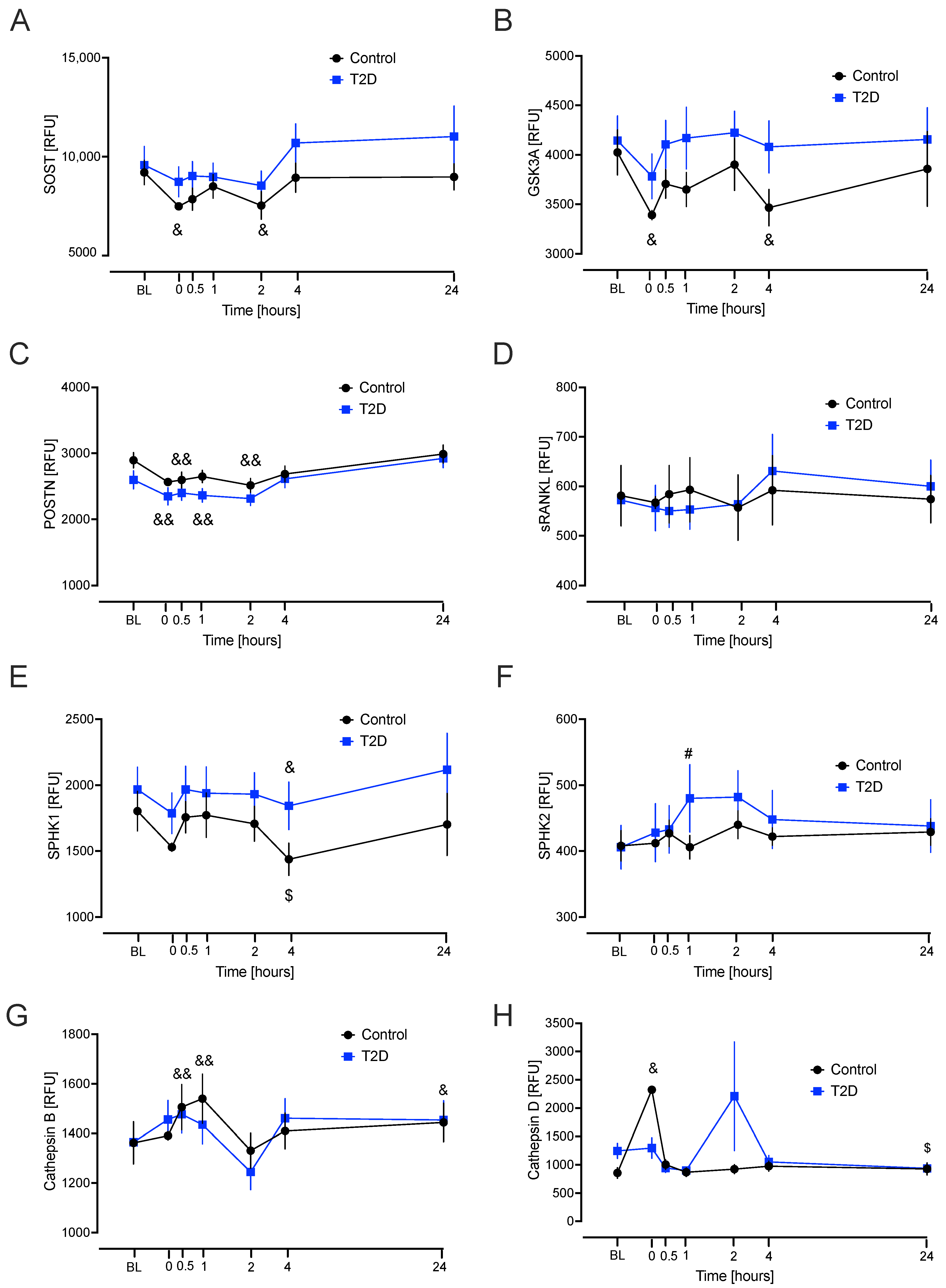
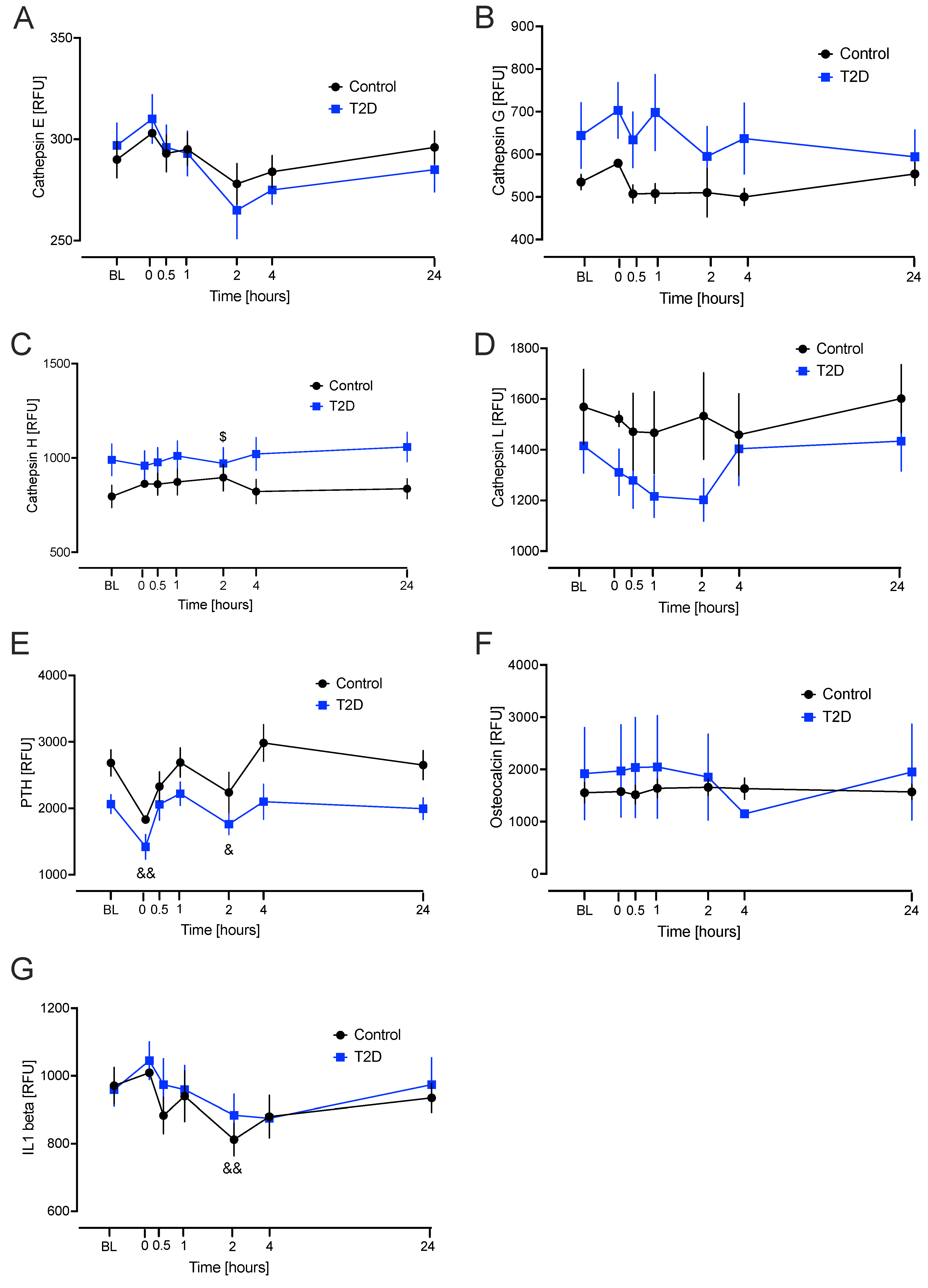
2.3. Linear Mixed Model for Repeated Measures for Each Bone Marker Protein from Baseline to the Hypoglycaemia Time Points
3. Discussion
4. Materials and Methods
4.1. Study Design
4.2. Biochemical Markers
4.3. Insulin Infusion
4.4. SOMAscan Assay
4.5. Statistics
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pietschmann, P.; Patsch, J.; Schernthaner, G. Diabetes and Bone. Horm. Metab. Res. 2010, 42, 763–768. [Google Scholar] [CrossRef]
- Schwartz, A.V. Impact of Diabetes and Its Treatment on Bone. Clin. Rev. Bone Miner. Metab. 2009, 7, 249–260. [Google Scholar] [CrossRef]
- Compston, J. Type 2 Diabetes Mellitus and Bone. J. Intern. Med. 2018, 283, 140–153. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Wei, F.L.; Lang, Y.; Liu, Y.C. Diabetes Mellitus and Risk of Hip Fractures: A Meta-Analysis. Osteoporos. Int. 2015, 27, 219–228. [Google Scholar] [CrossRef]
- Bai, J.; Gao, Q.; Wang, C.; Dai, J. Diabetes mellitus and risk of low-energy fracture: A meta-analysis. Aging Clin. Exp. Res. 2020, 32, 2173–2186. [Google Scholar] [CrossRef]
- Sheu, A.; Greenfield, J.R.; White, C.P.; Center, J.R. Contributors to impaired bone health in type 2 diabetes. Trends Endocrinol. Metab. 2023, 34, 34–48. [Google Scholar] [CrossRef]
- Sharma, P.; Sharma, R.K.; Gaur, K. Understanding the impact of diabetes on bone health: A clinical review. Metab. Open 2024, 24, 100330. [Google Scholar] [CrossRef]
- Catalfamo, D.L.; Britten, T.M.; Storch, D.L.; Calderon, N.L.; Sorenson, H.L.; Wallet, S.M. Hyperglycemia induced and intrinsic alterations in type 2 diabetes-derived osteoclast function. Oral Dis. 2013, 19, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Pierce, J.; Begun, D.; Westendorf, J.; McGee-Lawrence, M. Defining osteoblast and adipocyte lineages in the bone marrow. Bone 2019, 118, 2–7. [Google Scholar] [CrossRef]
- Almutlaq, N.; Neyman, A.; DiMeglio, L.A. Are Diabetes Microvascular Complications Risk Factors for Fragility Fracture? Curr. Opin. Endocrinol. Diabetes Obes. 2021, 28, 354–359. [Google Scholar] [CrossRef] [PubMed]
- Douglas, I.; Evans, S.; Pocock, S.J.; Smeeth, L. The Risk of Fractures Associated With Thiazolidinediones: A Self-Controlled Case-Series Study. PLoS Med. 2009, 6, e1000154. [Google Scholar] [CrossRef] [PubMed]
- Betteridge, D.J. Thiazolidinediones and Fracture Risk in Patients With Type 2 Diabetes. Diabet. Med. 2011, 28, 759–771. [Google Scholar] [CrossRef]
- Freire, L.B.; Brasil-Neto, J.P.; da Silva, M.L.; Miranda, M.G.C.; de Mattos Cruz, L.; Martins, W.R.; da Silva Paz, L.P. Risk factors for falls in older adults with diabetes mellitus: Systematic review and meta-analysis. BMC Geriatr. 2024, 24, 201. [Google Scholar] [CrossRef]
- Hidayat, K.; Du, X.; Wu, M.; Shi, B.M. The Use of Metformin, Insulin, Sulphonylureas, and Thiazolidinediones and the Risk of Fracture: Systematic Review and Meta-analysis of Observational Studies. Obes. Rev. 2019, 20, 1494–1503. [Google Scholar] [CrossRef] [PubMed]
- Napoli, N.; Chandran, M.; Pierroz, D.D.; Abrahamsen, B.; Schwartz, A.V.; Ferrari, S.L. Mechanisms of diabetes mellitus-induced bone fragility. Nat. Rev. Endocrinol. 2017, 13, 208–219. [Google Scholar] [CrossRef]
- Clowes, J.A.; Robinson, R.T.; Heller, S.R.; Eastell, R.; Blumsohn, A. Acute changes of bone turnover and PTH induced by insulin and glucose: Euglycemic and hypoglycemic hyperinsulinemic clamp studies. J. Clin. Endocrinol. Metab. 2002, 87, 3324–3329. [Google Scholar] [CrossRef] [PubMed]
- Kachroo, S.; Kawabata, H.; Colilla, S.; Shi, L.; Zhao, Y.; Mukherjee, J.; Iloeje, U.; Fonseca, V. Association between hypoglycemia and fall-related events in type 2 diabetes mellitus: Analysis of a U.S. commercial database. J. Manag. Care Spec. Pharm. 2015, 21, 243–253. [Google Scholar] [CrossRef]
- Hidayat, K.; Fang, Q.-L.; Shi, B.-M.; Qin, L.-Q. Influence of glycemic control and hypoglycemia on the risk of fracture in patients with diabetes mellitus: A systematic review and meta-analysis of observational studies. Osteoporos. Int. 2021, 32, 1693–1704. [Google Scholar] [CrossRef]
- Xing, B.; Yu, J.; Zhang, H.; Li, Y. RANKL inhibition: A new target of treating diabetes mellitus? Ther. Adv. Endocrinol. Metab. 2023, 14, 20420188231170754. [Google Scholar] [CrossRef]
- Ferrari, S.; Akesson, K.; Al-Daghri, N.; Biver, E.; Chandran, M.; Chevalley, T.; Josse, R.; Kendler, D.; Lane, N.; Makras, P. Bone microstructure and TBS in diabetes: What have we learned? A narrative review. Osteoporos. Int. 2025, 36, 1115–1128. [Google Scholar] [CrossRef]
- Masuhara, M.; Sato, T.; Hada, N.; Hakeda, Y. Protective protein/cathepsin A down-regulates osteoclastogenesis by associating with and degrading NF-kappaB p50/p65. J. Bone Miner. Metab. 2009, 27, 46–56. [Google Scholar] [CrossRef]
- Frørup, C.; Jensen, M.H.; Haupt-Jorgensen, M.; Buschard, K.; Størling, J.; Pociot, F.; Fløyel, T. Elevated Cathepsin S Serum Levels in New-Onset Type 1 Diabetes and Autoantibody-Positive Siblings. Diabetes 2024, 73, 1278–1284. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, Y.; Kaleta, J.; Brömme, D. The role of cathepsins in osteoporosis and arthritis: Rationale for the design of new therapeutics. Adv. Drug Deliv. Rev. 2005, 57, 973–993. [Google Scholar] [CrossRef]
- Dera, A.A.; Ranganath, L.; Barraclough, R.; Vinjamuri, S.; Hamill, S.; Barraclough, D.L. Cathepsin Z as a novel potential biomarker for osteoporosis. Sci. Rep. 2019, 9, 9752. [Google Scholar] [CrossRef] [PubMed]
- Elhadad, M.A.; Jonasson, C.; Huth, C.; Wilson, R.; Gieger, C.; Matias, P.; Grallert, H.; Graumann, J.; Gailus-Durner, V.; Rathmann, W.; et al. Deciphering the Plasma Proteome of Type 2 Diabetes. Diabetes 2020, 69, 2766–2778. [Google Scholar] [CrossRef]
- Duan, P.; Bonewald, L.F. The role of the wnt/β-catenin signaling pathway in formation and maintenance of bone and teeth. Int. J. Biochem. Cell Biol. 2016, 77 Pt A, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Pinzone, J.J.; Hall, B.M.; Thudi, N.K.; Vonau, M.; Qiang, Y.W.; Rosol, T.J.; Shaughnessy, J.D., Jr. The role of Dickkopf-1 in bone development, homeostasis, and disease. Blood 2009, 113, 517–525. [Google Scholar] [CrossRef]
- Lattanzio, S.; Santilli, F.; Liani, R.; Vazzana, N.; Ueland, T.; Di Fulvio, P.; Formoso, G.; Consoli, A.; Aukrust, P.; Davì, G. Circulating dickkopf-1 in diabetes mellitus: Association with platelet activation and effects of improved metabolic control and low-dose aspirin. J. Am. Heart Assoc. 2014, 3, e001000. [Google Scholar] [CrossRef]
- Guo, Y.C.; Yuan, Q. Fibroblast growth factor 23 and bone mineralisation. Int. J. Oral Sci. 2015, 7, 8–13. [Google Scholar] [CrossRef]
- Rhee, Y.; Bivi, N.; Farrow, E.; Lezcano, V.; Plotkin, L.I.; White, K.E.; Bellido, T. Parathyroid hormone receptor signaling in osteocytes increases the expression of fibroblast growth factor-23 in vitro and in vivo. Bone 2011, 49, 636–643. [Google Scholar] [CrossRef]
- Atkin, A.S.; Moin, A.S.M.; Nandakumar, M.; Al-Qaissi, A.; Sathyapalan, T.; Atkin, S.L.; Butler, A.E. Impact of severe hypoglycemia on the heat shock and related protein response. Sci. Rep. 2021, 11, 17057. [Google Scholar] [CrossRef] [PubMed]
- Starup-Linde, J.; Lykkeboe, S.; Handberg, A.; Vestergaard, P.; Høyem, P.; Fleischer, J.; Hansen, T.K.; Poulsen, P.L.; Laugesen, E. Glucose variability and low bone turnover in people with type 2 diabetes. Bone 2021, 153, 116159. [Google Scholar] [CrossRef]
- Moin, A.S.M.; Al-Qaissi, A.; Sathyapalan, T.; Atkin, S.L.; Butler, A.E. Hypoglycaemia in type 2 diabetes exacerbates amyloid-related proteins associated with dementia. Diabetes Obes. Metab. 2021, 23, 338–349. [Google Scholar] [CrossRef] [PubMed]
- Suhre, K.; Arnold, M.; Bhagwat, A.M.; Cotton, R.J.; Engelke, R.; Raffler, J.; Sarwath, H.; Thareja, G.; Wahl, A.; DeLisle, R.K.; et al. Connecting genetic risk to disease end points through the human blood plasma proteome. Nat. Commun. 2017, 8, 14357. [Google Scholar] [CrossRef]
- Birkett, M.A.; Day, S.J. Internal pilot studies for estimating sample size. Stat. Med. 1994, 13, 2455–2463. [Google Scholar] [CrossRef] [PubMed]


| Baseline | T2D (n = 23) | Controls (n = 23) | p Value |
|---|---|---|---|
| Age (years) | 64 ± 8 | 60 ± 10 | <0.0001 |
| Sex (M/F) | 12/11 | 11/12 | 0.77 |
| Weight (kg) | 91 ± 11 | 80 ± 9 | <0.0001 |
| Height (cm) | 167 ± 14 | 169 ± 5 | 0.64 |
| BMI (kg/m2) | 32 ± 4 | 28 ± 3 | <0.0001 |
| Systolic BP (mmHg) | 132 ± 8 | 122 ± 8 | 0.001 |
| Diastolic BP (mmHg) | 81 ± 7 | 75 ± 6 | 0.003 |
| Duration of diabetes (years) | 4.5 ± 2.2 | N/A | |
| HbA1c (mmol/mol) | 51.2 ± 11.4 | 35.2 ± 2.2 | <0.0001 |
| HbA1c (%) | 6.8 ± 1.0 | 5.4 ± 0.2 | <0.0001 |
| Total cholesterol (mmol/L) | 4.2 ± 1.0 | 4.8 ± 0.8 | 0.01 |
| Triglyceride (mmol/L) | 1.7 ± 0.7 | 1.34 ± 0.6 | 0.06 |
| HDL cholesterol (mmol/L) | 1.1 ± 0.3 | 1.5 ± 0.4 | 0.001 |
| LDL cholesterol (mmol/L) | 2.2 ± 0.8 | 2.7 ± 0.87 | 0.05 |
| CRP (mg/L) | 3.0 ± 2.7 | 5.1 ± 10.3 | 0.33 |
| Control | T2D | p Value | |||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| Sclerostin | 9199 | 2963 | 9573 | 4506 | 0.63 |
| Dickkopf-related protein 1 | 18,151 | 8053 | 28,249 | 17,076 | 0.017 * |
| Glycogen synthase kinase-3 alpha/beta | 4024 | 1092 | 4143 | 1192 | 0.76 |
| Periostin | 2894 | 556 | 2596 | 655 | 0.08 |
| Tumor necrosis factor ligand superfamily member 11 | 580 | 291 | 571 | 235 | 0.91 |
| Fibroblast growth factor 23 | 544 | 299 | 420 | 50 | 0.026 * |
| Sphingosine kinase 1 | 1802 | 717 | 1967 | 808 | 0.51 |
| Sphingosine kinase 2 | 407 | 109 | 406 | 159 | 0.98 |
| Cathepsin A | 5571 | 1803 | 8772 | 4517 | 0.004 * |
| Cathepsin B | 1362 | 406 | 1364 | 33 | 0.99 |
| Cathepsin D | 857 | 447 | 1247 | 637 | 0.56 |
| Cathepsin E | 290 | 43 | 297 | 53 | 0.76 |
| Cathepsin G | 534 | 87 | 643 | 371 | 0.16 |
| Cathepsin H | 796 | 282 | 990 | 402 | 0.08 |
| Cathepsin L | 1569 | 711 | 1415 | 512 | 0.38 |
| Cathepsin S | 739 | 169 | 888 | 283 | 0.027 * |
| Cathepsin Z | 3083 | 668 | 3607 | 930 | 0.032 * |
| Parathyroid hormone | 2684 | 945 | 2064 | 682 | 0.06 |
| Osteocalcin | 1552 | 925 | 1919 | 4234 | 0.76 |
| Interleukin-1 beta | 971 | 257 | 959 | 236 | 0.92 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atkin, B.M.L.; Sathyapalan, T.; Dempsey, L.; Atkin, S.L.; Butler, A.E. Bone Marker Proteins at Baseline and After Insulin-Induced Hypoglycaemia in Type 2 Diabetes. Int. J. Mol. Sci. 2025, 26, 11432. https://doi.org/10.3390/ijms262311432
Atkin BML, Sathyapalan T, Dempsey L, Atkin SL, Butler AE. Bone Marker Proteins at Baseline and After Insulin-Induced Hypoglycaemia in Type 2 Diabetes. International Journal of Molecular Sciences. 2025; 26(23):11432. https://doi.org/10.3390/ijms262311432
Chicago/Turabian StyleAtkin, Benjamin M. L., Thozhukat Sathyapalan, Laura Dempsey, Stephen L. Atkin, and Alexandra E. Butler. 2025. "Bone Marker Proteins at Baseline and After Insulin-Induced Hypoglycaemia in Type 2 Diabetes" International Journal of Molecular Sciences 26, no. 23: 11432. https://doi.org/10.3390/ijms262311432
APA StyleAtkin, B. M. L., Sathyapalan, T., Dempsey, L., Atkin, S. L., & Butler, A. E. (2025). Bone Marker Proteins at Baseline and After Insulin-Induced Hypoglycaemia in Type 2 Diabetes. International Journal of Molecular Sciences, 26(23), 11432. https://doi.org/10.3390/ijms262311432






