Vitamin D and Intrauterine Growth Restriction (IUGR)
Abstract
1. Introduction
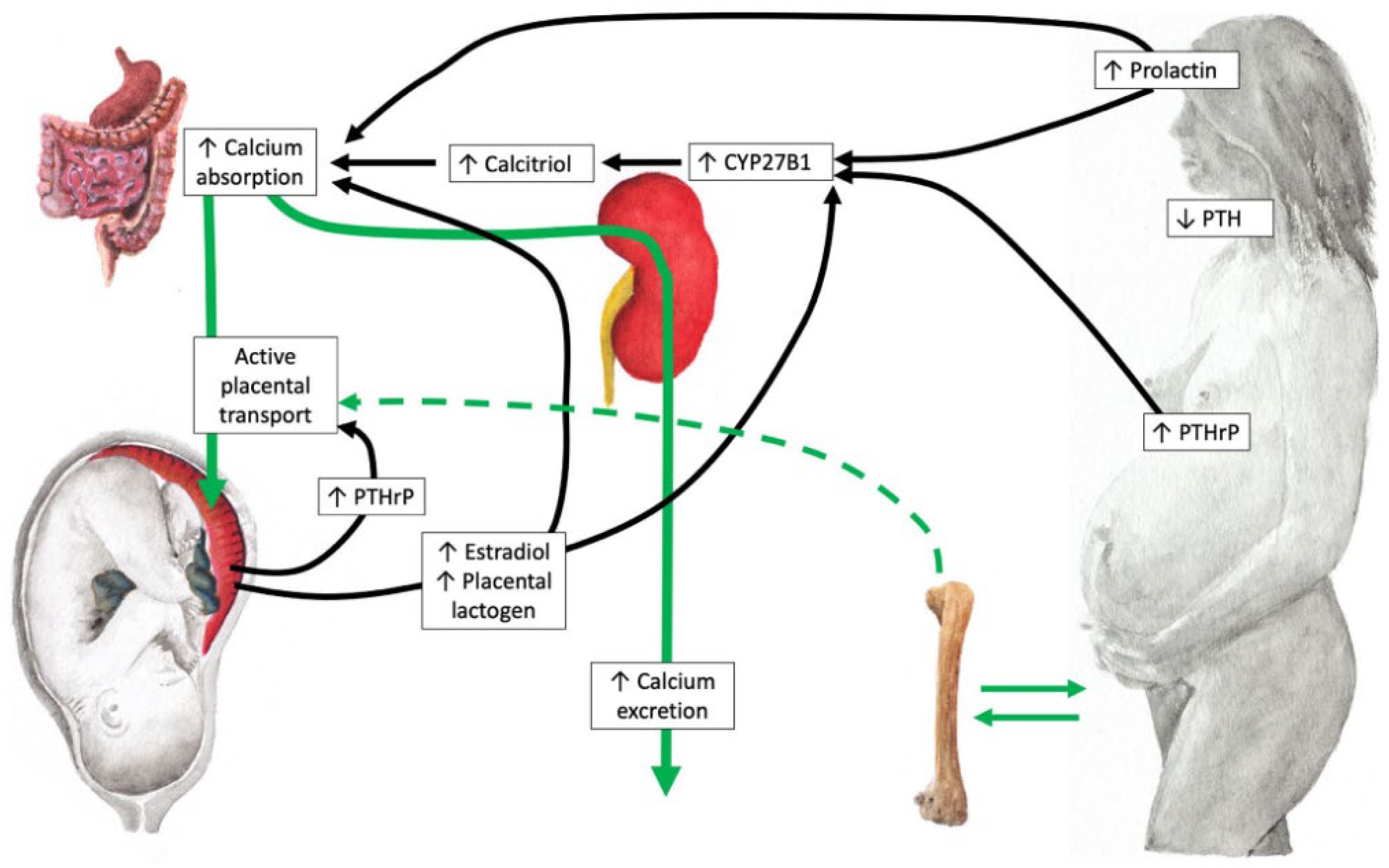
2. Vitamin D Metabolism and Adaptive Changes During Pregnancy
3. Prevalence of Vitamin D Deficiency in the Pregnant Women
4. Relationship Between Maternal and Neonatal Vitamin D Levels
5. Maternal Vitamin D Status and Risk of Intrauterine Growth Restriction
6. Effects of Vitamin D Supplementation During Pregnancy on Birth Size
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| CYP27B1 | enzyme 1α-hydroxylase |
| CYP2R1 | enzyme cholecalciferol-25-hydroxylase |
| FDA | U.S. Food and Drug Administration (FDA) |
| FGF23 | fibroblast growth factor |
| IL | interleukin |
| LBW | low birth weight |
| PMCA 1-4 | plasma membrane calcium-dependent ATPases |
| PTH | parathyroid hormone |
| PTH-rP | PTH-related peptide |
| RCTs | randomized controlled trials |
| SGA | small for gestational age |
| TNF-α | tumor necrosis factor-alfa |
| VDBP | vitamin D-binding protein |
| VDR | vitamin D receptor |
| YAP | Yes-associated protein |
References
- Wacker, M.; Holick, M.F. Sunlight and Vitamin D: A global perspective for health. Derm.-Endocrinol. 2013, 5, 51–108. [Google Scholar] [CrossRef]
- Hossein-Nezhad, A.; Holick, M.F. Vitamin D for health: A global perspective. Mayo Clin. Proc. 2013, 88, 720–755. [Google Scholar] [CrossRef]
- Sotunde, O.F.; Laliberte, A.; Weiler, H.A. Maternal risk factors and newborn infant vitamin D status: A scoping literature review. Nutr. Res. 2019, 63, 1–20. [Google Scholar] [CrossRef]
- Palacios, C.; Gonzalez, L. Is vitamin D deficiency a major global public health problem? J. Steroid. Biochem. Mol. Biol. 2014, 144, 138–145. [Google Scholar] [CrossRef]
- Cashman, K.D.; Sheehy, T.; O’Neill, C.M. Is vitamin D deficiency a public health concern for low middle income countries? A systematic literature review. Eur. J. Nutr. 2019, 58, 433–453. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. The vitamin D deficiency pandemic: Approaches for diagnosis, treatment and prevention. Rev. Endocr. Metab. Disord. 2017, 18, 153–165. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, S.; Tuo, L.; Zhai, Q.; Cui, J.; Chen, D.; Xu, D. Relationship between Maternal Vitamin D Levels and Adverse Outcomes. Nutrients 2022, 14, 4230. [Google Scholar] [CrossRef] [PubMed]
- Roth, D.E.; Abrams, S.A.; Aloia, J.; Bergeron, G.; Bourassa, M.W.; Brown, K.H.; Calvo, M.S.; Cashman, K.D.; Combs, G.; De-Regil, L.M.; et al. Global prevalence and disease burden of vitamin D deficiency: A roadmap for action in low- and middle-income countries. Ann. N. Y. Acad. Sci. 2018, 1430, 44–49. [Google Scholar] [CrossRef]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Evaluation, Treatment, and Prevention of Vitamin D Deficiency: An Endocrine Society Clinical Practice Guideline. Med. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef]
- Giustina, A.; Bilezikian, J.P.; Adler, R.A.; Banfi, G.; Bikle, D.D.; Binkley, N.C.; Bollerslev, J.; Bouillon, R.; Brandi, M.L.; Casanueva, F.F.; et al. Consensus statement on vitamin D status assessment and supplementation: Why, when, and how. Endocr. Rev. 2024, 45, 625–654. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.S.; Hewison, M. Extrarenal expression of the 25-hydroxyvitamin D-1-hydroxylase. Arch. Biochem. Biophys. 2012, 523, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Miliku, K.; Vinkhuyzen, A.; Blanken, L.M.; McGrath, J.J.; Eyles, D.W.; Burne, T.H.; Hofman, A.; Tiemeier, H.; Steegers, E.A.; Gaillard, R.; et al. Maternal vitamin D concentrations during pregnancy, feal growth patterns and risk of adverse birth outcome. Am. J. Clin. Nutr. 2016, 103, 1514–1522. [Google Scholar] [CrossRef]
- Fiscaletti, M.; Stewart, P.; Munns, C.F. The importance of vitamin D in maternal and child health: A global perspective. Public Health Rev. 2017, 38, 19. [Google Scholar] [CrossRef] [PubMed]
- Sarma, D.; Saikia, U.K.; Das, D.V. Fetal skeletal size and growth are relevant biometric markers in vitamin D deficient mothers: A North East India prospective cohort study. Indian J. Endocrinol. Metab. 2018, 22, 212–216. [Google Scholar] [CrossRef] [PubMed]
- Tamblyn, J.A.; Hewison, M.; Wagner, C.L.; Bulmer, J.N.; Kilby, M.D. Immunological role of vitamin D at the maternal-fetal interface. J. Endocrinol. 2015, 224, R107–R121. [Google Scholar] [CrossRef]
- Saraf, R.; Morton, S.M.B.; Camargo, C.A.; Grant, C.C. Global summary of maternal and newborn vitamin D status—A systematic review. Matern. Child Nutr. 2016, 12, 647–668. [Google Scholar] [CrossRef]
- van der Pligt, P.; Willcox, J.; Szymlek-Gay, E.A.; Murray, E.; Worsley, A.; Daly, R.M. Associations of maternal vitamin D deficiency with pregnancy and neonatal complications in developing countries: A systematic review. Nutrients 2018, 10, 640. [Google Scholar] [CrossRef]
- Amrein, K.; Scherkl, M.; Hoffmann, M.; Neuwersch-Sommeregger, S.; Köstenberger, M.; Tmava Berisha, A.; Martucci, G.; Pilz, S.; Malle, O. Vitamin D deficiency 2.0: An update on the current status worldwide. Eur. J. Clin. Nutr. 2020, 74, 1498–1513. [Google Scholar] [CrossRef]
- Christoph, P.; Challande, P.; Raio, L.; Surbek, D. High prevalence of severe vitamin D deficiency during the first trimester in pregnant women in Switzerland and its potential contributions to adverse outcomes in the pregnancy. Swiss Med. Wkly. 2020, 150, w20238. [Google Scholar] [CrossRef]
- Wei, S.; Qi, H.; Luo, Z.; Fraser, W. Maternal vitamin D status and adverse pregnancy outcomes: A systematic review and meta-analysis. J. Matern. Fetal Neonatal Med. 2013, 26, 889–899. [Google Scholar] [CrossRef]
- Aghajafari, F.; Nagulesapillai, T.; Ronksley, P.; Tough, S.; O’Beirne, M.; Rabi, D. Association between maternal serum 25-hydroxyvitamin D level and pregnancy and neonatal outcomes: Systematic review and meta-analysis of observational studies. BMJ 2013, 346, f1169. [Google Scholar] [CrossRef]
- Palermo, N.E.; Holick, M.F. Vitamin D, bone health, and other health benefits in pediatric patients. J. Pediatr. Rehabil. Med. 2014, 7, 179–192. [Google Scholar] [CrossRef] [PubMed]
- Eckhardt, C.L.; Gernand, A.D.; Roth, D.E.; Bodnar, L.M. Maternal vitamin D status and infant anthropometry in a US multicentre cohort study. Ann. Hum. Biol. 2015, 42, 215–222. [Google Scholar] [CrossRef]
- Olmos-Ortiz, A.; Avila, E.; Duland-Carbajal, M.; Díaz, L. Regulation of calcitriol byosinthesis and activity: Focus on gestational vitamin D deficiency and adverse pregnancy outcomes. Nutrients 2015, 7, 443–480. [Google Scholar] [CrossRef]
- Hou, W.; Yan, X.T.; Bai, C.M.; Zhang, X.V.; Hui, L.Y.; Yu, X.W. Decreased serum vitamin D levels in early spontaneous pregnancy loss. Eur. J. Clin. Nutr. 2016, 70, 1004–1008. [Google Scholar] [CrossRef]
- Agarwal, S.; Kovilam, O.; Agrawal, D.K. Vitamin D and its impact on maternal-fetal outcomes in pregnancy: A critical review. Crit. Rev. Food Sci. Nutr. 2018, 58, 755–769. [Google Scholar] [CrossRef] [PubMed]
- Palacios, C.; Kostiuk, L.K.; Peña-Rosas, J.P. Vitamin D supplementation for women during pregnancy. Cochrane Database Syst. Rev. 2019, 7, CD008873. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.D.; Pang, T.T.; Li, P.S.; Zhou, Z.X.; Lin, D.X.; Fan, D.Z.; Guo, X.L.; Liu, Z.P. Early pregnancy vitamin D and the risk of adverse maternal and infant outcomes: A retrospective cohort study. BMC Pregnancy Childbirth 2020, 20, 465. [Google Scholar] [CrossRef]
- Arshad, R.; Sameen, A.; Murtaza, M.A.; Sharif, H.R.; Haq, I.-U.; Dawood, S.; Ahmed, Z.; Nemat, A.; Manzoor, M.F. Impact of vitamin D on maternal and fetal health: A review. Food Sci. Nutr. 2022, 10, 3230–3240. [Google Scholar] [CrossRef]
- Beck, C.; Blue, N.R.; Silver, R.M.; Na, M.; Grobman, W.A.; Steller, J.; Parry, S.; Scifres, C.; Gernand, A.D. Maternal vitamin D status, fetal growth patterns, and adverse pregnancy outcomes in a multisite prospective pregnancy cohort. Am. J. Clin. Nutr. 2025, 121, 376–384. [Google Scholar] [CrossRef]
- Hii, C.S.; Ferrante, A. The Non-Genomic Actions of Vitamin D. Nutrients 2016, 8, 135. [Google Scholar] [CrossRef]
- Marino, R.; Misra, M. Extra-Skeletal Effects of Vitamin D. Nutrients 2019, 11, 1460. [Google Scholar] [CrossRef]
- Botelho, J.; Machado, V.; Proença, L.; Delgado, A.S.; Mendes, J.J. Vitamin D deficiency and oral health: A comprehensive review. Nutrients 2020, 12, 1471. [Google Scholar] [CrossRef]
- Zmijewski, M.A. Nongenomic Activities of Vitamin D. Nutrients 2022, 14, 5104. [Google Scholar] [CrossRef]
- Park, C.Y.; Shin, S.; Han, S.N. Multifaceted Roles of Vitamin D for Diabetes: From Immunomodulatory Functions to Metabolic Regulations. Nutrients 2024, 16, 3185. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, C.S. Maternal mineral and bone metabolism during pregnancy, lactation, and post-weaning recovery. Physiol. Rev. 2016, 96, 449–547. [Google Scholar] [CrossRef] [PubMed]
- Ryan, B.A.; Kovacs, C.S. Calciotropic and phosphotropic hormones in fetal and neonatal bone development. Semin. Fetal Neonatal Med. 2020, 25, 101062. [Google Scholar] [CrossRef] [PubMed]
- Ryan, B.A.; Kovacs, C.S. Maternal and fetal vitamin D and their in mineral homeostasis and fetal bone development. J. Endocrinol. Investig. 2021, 44, 643–659. [Google Scholar] [CrossRef]
- Bikle, D.D.; Schwartz, J. Vitamin D binding protein, total and free vitamin D levels in different physiological and pathophysiological conditions. Front. Endocrinol. 2019, 10, 317. [Google Scholar] [CrossRef]
- Noyola-Martinez, N.; Diaz, L.; Avila, E.; Halhali, A.; Larrea, F.; Barrera, D. Calcitriol downregulates TNF-alpha and IL-6 expression in cultured placental cells from preeclamptic women. Cytokine 2013, 61, 245–250. [Google Scholar] [CrossRef]
- Rosen, Y.; Daich, J.; Soliman, I.; Brathwaite, E.; Shoenfeld, Y. Vitamin D and autoimmunity. Scand. J. Rheumatol. 2016, 45, 439–447. [Google Scholar] [CrossRef]
- Cyprian, F.; Lefkou, E.; Varoudi, K.; Girardi, G. Immunomodulatory Effects of Vitamin D in Pregnancy and Beyond. Front. Immunol. 2019, 10, 2739. [Google Scholar] [CrossRef] [PubMed]
- Mogire, R.M.; Mutua, A.; Kimita, W.; Kamau, A.; Bejon, P.; Pettifor, J.M.; Adeyemo, A.; Williams, T.N.; Atkinson, S.H. Prevalence of vitamin D deficiency in Africa: A systematic review and meta-analysis. Lancet Glob. Health 2020, 8, e134–e142. [Google Scholar] [CrossRef]
- Li, H.; Ma, J.; Huang, R.; Wen, Y.; Liu, G.; Xuan, M.; Yang, L.; Yang, J.; Song, L. Prevalence of vitamin D deficiency in the pregnant women: An observational study in Shanghai, China Arch. Public. Health 2020, 78, 31. [Google Scholar] [CrossRef]
- Ravinder, S.S.; Padmavathi, R.; Maheshkumar, K.; Mohankumar, M.; Maruthy, K.N.; Sankar, S.; Balakrishnan, K. Prevalence of vitamin D deficiency among South Indian pregnant women. J. Family Med. Prim. Care 2022, 11, 2884–2889. [Google Scholar] [CrossRef]
- Amelia, C.Z.; Gwan, C.H.; Qi, T.S.; Seng, J.T.C. Prevalence of vitamin D insufficiency in early pregnancies—A Singapore study. PLoS ONE 2024, 19, e0300063. [Google Scholar] [CrossRef]
- Octavius, G.S.; Daleni, V.A.; Angeline, G.; Virliani, C. A systematic review and meta-analysis of prevalence of vitamin D deficiency among Indonesian pregnant women: A public health emergency. AJOG Glob. Rep. 2023, 3, 100189. [Google Scholar] [CrossRef]
- Woo, J.; Guffey, T.; Dailey, R.; Misra, D.; Giurgescu, C. Vitamin D Status as an Important Predictor of Preterm Birth in a Cohort of Black Women. Nutrients 2023, 15, 4637. [Google Scholar] [CrossRef]
- Stoica, A.B.; Săsăran, M.O.; Suciu, L.M.; Hutanu, A.; Mărginean, C. Vitamin D Status in Roma Mothers and Newborns: Socioeconomic Factors and Impact on Neonatal Outcome. Nutrients 2024, 16, 4361. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, S.; Bansal, U.; Rathoria, E.; Rathoria, R.; Ahuja, R.; Agarwa, A. Association Between Neonatal and Maternal Vitamin D Levels at Birth. Cureus 2024, 16, e72261. [Google Scholar] [CrossRef] [PubMed]
- Kokkinari, A.; Dagla, M.; Antoniou, E.; Lykeridou, A.; Kyrkou, G.; Bagianos, K.; Iatrakis, G. The Correlation between Maternal and Neonatal Vit D (25(OH)D) Levels in Greece: A Cross-Sectional Study. Clin. Pract. 2024, 14, 749–764. [Google Scholar] [CrossRef] [PubMed]
- Dragomir, R.E.; Toader, D.O.; Gheoca Mutu, D.E.; Dogaru, I.A.; Răducu, L.; Tomescu, L.C.; Moleriu, L.C.; Bordianu, A.; Petre, I.; Stănculescu, R. Consequences of Maternal Vitamin D Deficiency on Newborn Health. Life 2024, 14, 714. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Shobhane, H.; Tiwari, K.; Agarwal, S. To Study the Correlation of Maternal Serum Vitamin D Levels and Infant Serum Vitamin D Levels With Infant Birth Weight: A Single-Centre Experience From the Bundelkhand Region, India. Cureus 2024, 16, e68696. [Google Scholar] [CrossRef]
- O’Callaghan, K.M.; Nowak, K.G.; Dalrymple, K.V.; Poston, L.; Rigutto-Farebrother, J.; Quotah, O.F.; White, S.L.; Flynn, A.C.; UPBEAT Consortium. Vitamin D status of pregnant women with obesity in the UK and its association with pregnancy outcomes: A secondary analysis of the UK Pregnancies Better Eating and Activity Trial (UPBEAT) study. Br. J. Nutr. 2024, 132, 40–49. [Google Scholar] [CrossRef]
- Reverzani, C.; Zaake, D.; Nansubuga, F.; Ssempewo, H.; Manirakiza, L.; Kayiira, A.; Tumwine, G. Prevalence of vitamin D deficiency and its association with adverse obstetric outcomes among pregnant women in Uganda: A cross- sectional study. BMJ Open 2025, 15, e089504. [Google Scholar] [CrossRef]
- Saccone, G.; Buonomo, G.; Guerra, S.; Gentile, D.; Di Spiezio Sardo, A. Prevalence of Hypovitaminosis D in Pregnancy and Potential Benefits of Oral Supplementation. Am. J. Perinatol. 2025, 42, 1421–1424. [Google Scholar] [CrossRef]
- Gironés Soriano, R.J.; Victoria Gomis, C.; Llop Furquet, G.; Simón García, V.; Rubio Igual, M.; Garrido Navarro, C.; Ballester Carbonell, J.; Fuster Molina, D.; Martínez Massa, A.; Smith Ballester, S.A.; et al. Prevalence of hypovitaminosis D in pregnant women in the Hospital of Sagunto area (Valencia): PrevitaD Study. Rev. Esp. Salud Publica 2025, 99, e202508044. [Google Scholar]
- Karras, S.N.; Shah, I.; Petroczi, A.; Goulis, D.G.; Bili, H.; Papadopoulou, F.; Harizopoulou, V.; Tarlatzis, B.C.; Naughton, D.P. An observational study reveals that neonatal vitamin D is primarily determined by maternal contributions: Implications of a new assay on the roles of vitamin D forms. Nutr. J. 2013, 12, 77. [Google Scholar] [CrossRef]
- Arora, S.; Goel, P.; Chawla, D.; Huria, A.; Arya, A. Vitamin D Status in Mothers and Their Newborns and Its Association with Pregnancy Outcomes: Experience from a Tertiary Care Center in Northern India. J. Obstet. Gynecol. India 2018, 68, 389–393. [Google Scholar] [CrossRef]
- Esmeraldo, C.U.; Martins, M.E.P.; Maia, E.R.; Leite, J.L.A.; Ramos, J.L.S.; Gonçalves, J., Jr.; Neta, C.M.; Suano-Souza, F.I.; Sarni, R.O. Vitamin D in Term Newborns: Relation with Maternal Concentrations and Birth Weight. Ann. Nutr. Metab. 2019, 75, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Rabbani, S.; Afaq, S.; Fazid, S.; Khattak, M.I.; Yousafzai, Y.M.; Habib, S.H.; Lowe, N.; Ul-Haq, Z. Correlation between maternal and neonatal blood Vitamin D level: Study from Pakistan. Matern. Child Nutr. 2021, 17, e13028. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.C.; Huang, J.P. Potential benefits of vitamin D supplementation on pregnancy. J. Formos Med. Assoc. 2023, 122, 557–563. [Google Scholar] [CrossRef]
- Leffelaar, E.R.; Vrijkotte, T.G.M.; van Eijsden, M. Maternal early pregnancy vitamin D status in relation to fetal and neonatal growth: Results of the multi-ethnic Amsterdam Born Children and their Development cohort. Br. J. Nutr. 2010, 104, 108–117. [Google Scholar] [CrossRef]
- Pérez-López, F.R.; Pasupuleti, V.; Mezones-Holguin, E.; Benites-Zapata, V.A.; Thota, P.; Deshpande, A.; Hernandez, A.V. Effect of vitamin D supplementation during pregnancy on maternal and neonatal outcomes: A systematic review and meta-analysis of randomized controlled trials. Fertil. Steril. 2015, 103, 1278–1288. [Google Scholar] [CrossRef] [PubMed]
- Tous, M.; Villalobos, M.; Iglesias-Vázquez, L.; Fernández-Barrés, S.; Arija, V. Vitamin D status during pregnancy and offspring outcomes: A systematic review and meta-analysis of observational studies. Eur. J. Clin. Nutr. 2020, 74, 36–53. [Google Scholar] [CrossRef]
- Deepa, R.; Schayck, O.C.P.V.; Babu, G.R. Low levels of Vitamin D during pregnancy associated with gestational diabetes mellitus and low birth weight: Results from the MAASTHI birth cohort. Front. Nutr. 2024, 11, 1352617. [Google Scholar] [CrossRef]
- Chien, M.C.; Huang, C.Y.; Wang, J.H.; Shih, C.L.; Wu, P. Effects of vitamin D in pregnancy on maternal and offspring health-related outcomes: An umbrella review of systematic review and meta-analyses. Nutr. Diabetes 2024, 14, 35. [Google Scholar] [CrossRef] [PubMed]
- You, Z.; Mei, H.; Zhang, Y.; Song, D.; Zhang, Y.; Liu, C. The effect of vitamin D deficiency during pregnancy on adverse birth outcomes in neonates: A systematic review and meta-analysis. Front. Pediatr. 2024, 12, 1399615. [Google Scholar] [CrossRef]
- Monier, I.; Baptiste, A.; Tsatsaris, V.; Senat, M.V.; Jani, J.; Jouannic, J.M.; Winer, N.; Elie, C.; Souberbielle, J.C.; Zeitlin, J.; et al. First Trimester Maternal Vitamin D Status and Risks of Preterm Birth and Small-For-Gestational Age. Nutrients 2019, 11, 3042. [Google Scholar] [CrossRef]
- Pérez-Castillo, I.M.; Rivero-Blanco, T.; León-Ríos, X.A.; Expósito-Ruiz, M.; López-Criado, M.S.; Aguilar-Cordero, M.J. Associations of Vitamin D Deficiency, Parathyroid hormone, Calcium, and Phosphorus with Perinatal. Adverse Outcomes. A Prospective Cohort Study. Nutrients 2020, 12, 3279. [Google Scholar] [CrossRef]
- Marçal, V.M.G.; Sousa, F.L.P.; Daher, S.; Grohmann, R.M.; Peixoto, A.B.; Araujo Júnior, E.; Nardozza, L.M.M. The Assessment of Vitamin D Levels in Pregnant Women is not Associated to Fetal Growth Restriction: A Cross Sectional Study. Rev. Bras. Ginecol. Obstet. 2021, 43, 743–748. [Google Scholar] [CrossRef]
- Chan, S.Y.; Susarla, R.; Canovas, D.; Vasilopoulou, E.; Ohizua, O.; McCabe, C.J.; Hewison, M.; Kilby, M.D. Vitamin D promotes human extravillous trophoblast invasion in vitro. Placenta 2015, 36, 403–409. [Google Scholar] [CrossRef]
- Ganguly, A.; Tamblyn, J.A.; Finn-Sell, S.; Chan, S.Y.; Westwood, M.; Gupta, J.; Kilby, M.D.; Gross, S.R.; Hewison, M. Vitamin D. the placenta and early pregnancy: Effects on trophoblast function. J. Endocrinol. 2018, 236, R93–R103. [Google Scholar] [CrossRef] [PubMed]
- Mansur, J.L.; Oliveri, B.; Giacoia, E.; Fusaro, D.; Costanzo, P.R. Vitamin D: Before, during and after Pregnancy: Effect on Neonates and Children. Nutrients 2022, 14, 1900. [Google Scholar] [CrossRef] [PubMed]
- Kiely, M.E.; Zhang, J.Y.; Kinsella, M.; Khashan, A.S.; Kenny, L.C. Vitamin D status is associated with uteroplacental dysfunction indicated by pre-eclampsia and small-for-gestational-age birth in a large prospective pregnancy cohort in Ireland with low vitamin D status. Am. J. Clin. Nutr. 2016, 104, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.H.; Liu, Z.B.; Ma, L.; Zhang, Z.C.; Fu, L.; Yu, Z.; Chen, W.; Song, Y.P.; Wang, P.; Wang, H.; et al. Gestational vitamin D deficiency causes placental insufficiency and fetal intrauterine growth restriction partially through inducing placental inflammation. J. Steroid. Biochem. Mol. Biol. 2020, 203, 105733. [Google Scholar] [CrossRef]
- Wang, J.; Qiu, F.; Zhao, Y.; Gu, S.; Wang, J.; Zhang, H. Exploration of fetal growth restriction induced by vitamin D deficiency in rats via Hippo-YAP signaling pathway. Placenta 2022, 128, 91–99. [Google Scholar] [CrossRef]
- Francis, E.C.; Hinkle, S.N.; Song, Y.; Rawal, S.; Donnelly, S.R.; Zhu, Y.; Chen, L.; Zhang, C. Longitudinal Maternal Vitamin D Status during Pregnancy Is Associated with Neonatal Anthropometric Measures. Nutrients 2018, 10, 1631. [Google Scholar] [CrossRef] [PubMed]
- Bärebring, L.; Bullarbo, M.; Glantz, A.; Hulthén, L.; Ellis, J.; Jagner, Å.; Schoenmakers, I.; Winkvist, A.; Augustin, H. Trajectory of vitamin D status during pregnancy in relation to neonatal birth size and fetal survival: A prospective cohort study. BMC Pregnancy Childbirth 2018, 18, 51. [Google Scholar] [CrossRef]
- Chen, Q.; Chu, Y.; Liu, R.; Lin, Y. Predictive value of Vitamin D levels in pregnant women on gestational length and neonatal weight in China: A population-based retrospective study. Reprod. Biol. Endocrinol. 2024, 22, 102. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Y.; Chen, X.; Zhang, X. Associations between prenatal sunshine exposure and birth outcomes in China. Sci. Total Environ. 2020, 713, 136472. [Google Scholar] [CrossRef]
- Harvey, N.C.; Holroyd, C.; Ntani, G.; Javaid, K.; Cooper, P.; Moon, R.; Cole, Z.; Tinati, T.; Godfrey, K.; Dennison, E.; et al. Vitamin D supplementation in pregnancy: A systematic review. Health Technol. Assess. 2014, 18, 1–190. [Google Scholar] [CrossRef]
- De-Regil, L.M.; Palacios, C.; Lombardo, L.K.; Peña-Rosas, J.P. Vitamin D supplementation for women during pregnancy. Sao Paulo Med. J. 2016, 134, 274–275. [Google Scholar] [CrossRef]
- Gallo, S.; McDermid, J.M.; Al-Nimr, R.I.; Hakeem, R.; Moreschi, J.M.; Pari-Keener, M.; Stahnke, B.; Papoutsakis, C.; Handu, D.; Cheng, F.W. Vitamin D supplementation during pregnancy: An evidence analysis center systematic review and meta-analysis. J. Acad. Nutr. Diet. 2020, 120, 898–924. [Google Scholar] [CrossRef]
- Nausheen, S.; Habib, A.; Bhura, M.; Rizvi, A.; Shaheen, F.; Begum, K.; Iqbal, J.; Ariff, S.; Shaikh, L.; Raza, S.S.; et al. Impact evaluation of the efficacy of different doses of vitamin D supplementation during pregnancy on pregnancy and birth outcomes: A randomised, controlled, dose comparison trial in Pakistan. BMJ Nutr. Prev. Health 2021, 4, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Hynes, C.; Jesurasa, A.; Evans, P.; Mitchell, C. Vitamin D supplementation for women before and during pregnancy: An update of the guidelines, evidence, and role of GPs and practice nurses. Br. J. Gen. Pract. 2017, 67, 423–424. [Google Scholar] [CrossRef] [PubMed]
- Ross, A.C.; Manson, J.E.; Abrams, S.A.; Aloia, J.F.; Brannon, P.M.; Clinton, S.K.; Durazo-Arvizu, R.A.; Gallagher, J.C.; Gallo, R.L.; Jones, G.; et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: What clinicians need to know. J. Clin. Endocrinol. Metab. 2011, 96, 53–58. [Google Scholar] [CrossRef]
- Dawodu, A.; Saadi, H.F.; Bekdache, G.; Javed, Y.; Altaye, M.; Hollis, B.W. Randomized controlled trial (RCT) of vitamin D supplementation in pregnancy in a population with endemic vitamin D deficiency. J. Clin. Endocrinol. Metab. 2013, 98, 2337–2346. [Google Scholar] [CrossRef]
- Holick, M.F. A call to action: Pregnant women indeed require vitamin D supplementation for better health outcomes. J. Clin. Endocrinol. Metab. 2019, 104, 13–15. [Google Scholar] [CrossRef] [PubMed]
- Litonjua, A.A.; Carey, V.J.; Laranjo, N.; Stubbs, B.J.; Mirzakhani, H.; O’Connor, G.T.; Sandel, M.; Beigelman, A.; Bacharier, L.B.; Zeiger, R.S.; et al. Six-year follow-up of a trial of antenatal vitamin D for asthma reduction. N. Engl. J. Med. 2020, 382, 525–533. [Google Scholar] [CrossRef]
- Ali, A.M.; Alobaid, A.; Malhis, T.N.; Khattab, A.F. Effect of vitamin D3 supplementation in pregnancy on risk of pre-eclampsia -Randomized controlled trial. Clin. Nutr. 2019, 38, 557–563. [Google Scholar] [CrossRef]
- Roth, D.E.; Leung, M.; Mesfin, E.; Qamar, H.; Watterworth, J.; Papp, E. Vitamin D supplementation during pregnancy: State of the evidence from a systematic review of randomised trials. BMJ 2017, 359, j5237. [Google Scholar] [CrossRef]
- Bi, W.G.; Nuyt, A.M.; Weiler, H.; Leduc, L.; Santamaria, C.; Wei, S.Q. Association Between Vitamin D Supplementation During Pregnancy and Offspring Growth, Morbidity, and Mortality: A Systematic Review and Meta-analysis. JAMA Pediatr. 2018, 172, 635–645. [Google Scholar] [CrossRef]
- Maugeri, A.; Barchitta, M.; Blanco, I.; Agodi, A. Effects of Vitamin D Supplementation During Pregnancy on Birth Size: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 2019, 11, 442. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.B.; Kirby, J.K.; Sorensen, J.C.; Pollard, E.L.; Audhya, T. Evidence based recommendations for an optimal prenatal supplement for women in the US: Vitamins and related nutrients. Matern. Health Neonatol. Perinatol. 2022, 8, 4. [Google Scholar] [CrossRef] [PubMed]
- Abdulah, D.M.; Hasan, J.N.; Hasan, S.B. Effectiveness of Vitamin D supplementation in combination with calcium on risk of maternal and neonatal outcomes: A quasi-experimental clinical. Tzu. Chi. Med. J. 2023, 36, 175–187. [Google Scholar] [CrossRef] [PubMed]
- Motamed, S.; Nikooyeh, B.; Kashanian, M.; Hollis, B.W.; Neyestani, T.R. Efficacy of two different doses of oral vitamin D supplementation on inflammatory biomarkers and maternal and neonatal outcomes. Matern. Child Nutr. 2019, 15, e12867. [Google Scholar] [CrossRef]

| Vitamin D Status | Calcidiol Level (1 ng/mL Corresponds to 2.5 nmol/L) |
|---|---|
| Vitamin D deficiency | <20 ng/m (<50 nmol/L) |
| Vitamin D insufficiency | 20 to 29 ng/mL (51–74 nmol/L) |
| Vitamin D sufficiency | ≥30 ng/m (≥75 nmol/L) |
| Author and Year | Region/Country (Latitude) | Calcidiol < 20 ng/mL (%) |
|---|---|---|
| Woo et al., 2023 [48] | Orlando, FL, USA (28°32′ N) Dallas, TX, USA (32°46′ N) Detroit, MI, USA (42°19′ N) | 42 |
| Stoica et al., 2024 [49] | Târgu Mures, Romania (46°32′ N) | 74 |
| Jaiswal et al., 2024 [50] | Barabanki, India (26°55′ N) | 66 |
| Kokkinari et al., 2024 [51] | Athens, Greece (37°59′ N) | 58 |
| Dragomir et al., 2024 [52] | Bucharest, Romania (44°24′ N) | 73 |
| Singh et al., 2024 [53] | Jhansi, India (25°26′55″ N) | 55 |
| O’Callaghan et al., 2024 [54] | Zurich, Switzerland (47°22′ N) London, UK (51°30′ N) Cambridge, UK (52°12′ N)) Dublin, Ireland (53°20′ N) | 67 |
| Reverzani et al., 2025 [55] | Kampala, Uganda (0°18′ N) | 54 |
| Saccone et al., 2025 [56] | Naples, Italy (40°50′ N) | 56 |
| Gironés Soriano et al., 2025 [57] | Sagunto, Spain (39°40′ N) | 53 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Durá-Travé, T.; Gallinas-Victoriano, F. Vitamin D and Intrauterine Growth Restriction (IUGR). Int. J. Mol. Sci. 2025, 26, 11422. https://doi.org/10.3390/ijms262311422
Durá-Travé T, Gallinas-Victoriano F. Vitamin D and Intrauterine Growth Restriction (IUGR). International Journal of Molecular Sciences. 2025; 26(23):11422. https://doi.org/10.3390/ijms262311422
Chicago/Turabian StyleDurá-Travé, Teodoro, and Fidel Gallinas-Victoriano. 2025. "Vitamin D and Intrauterine Growth Restriction (IUGR)" International Journal of Molecular Sciences 26, no. 23: 11422. https://doi.org/10.3390/ijms262311422
APA StyleDurá-Travé, T., & Gallinas-Victoriano, F. (2025). Vitamin D and Intrauterine Growth Restriction (IUGR). International Journal of Molecular Sciences, 26(23), 11422. https://doi.org/10.3390/ijms262311422






