The Role of the Gut Microbiome in Type 2 Diabetes Mellitus
Abstract
1. Introduction
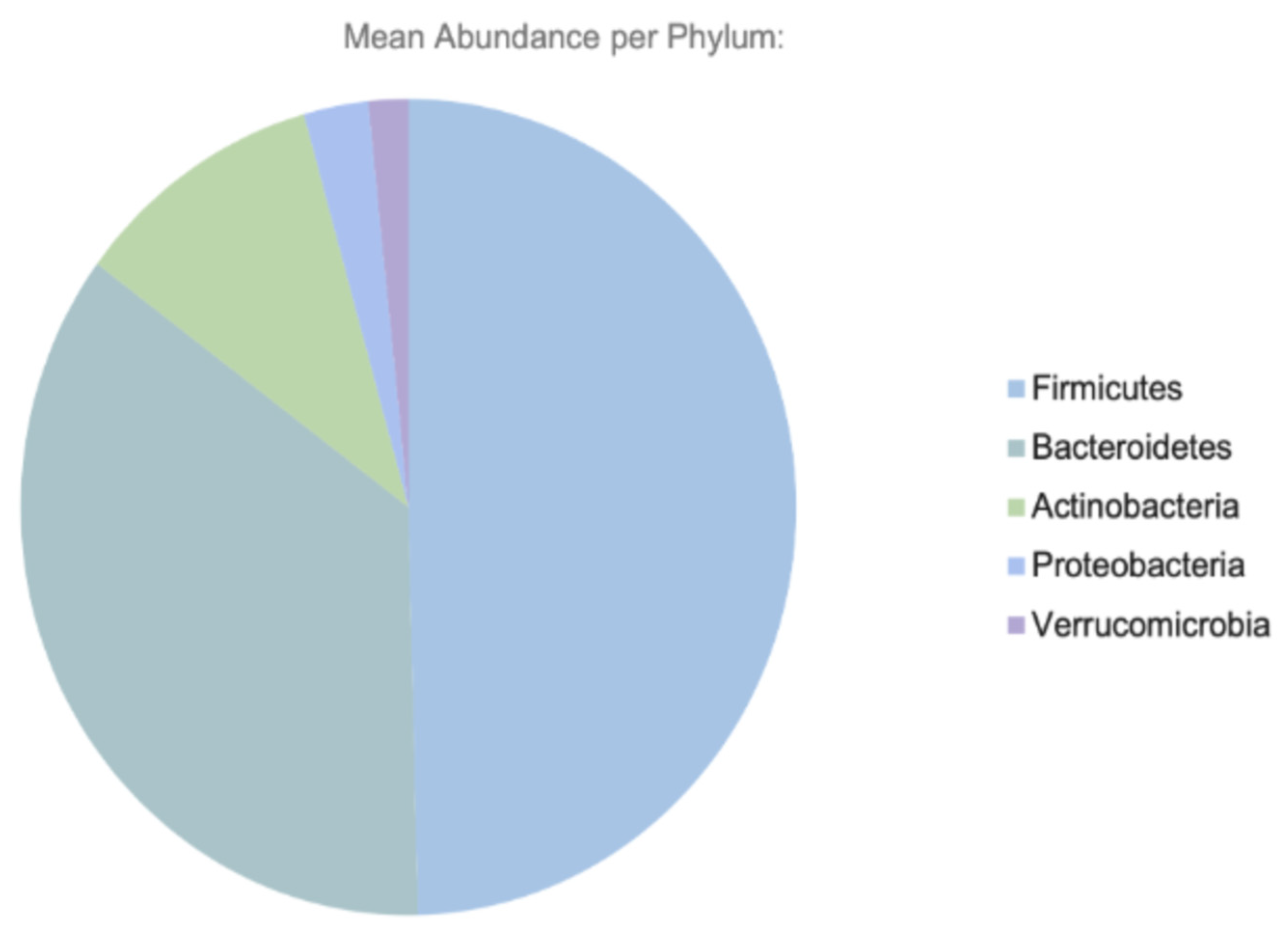
2. Gut Microbiome: Composition and Function
3. Discussion: Pathophysiological Link Between the Gut Microbiome and Type 2 Diabetes
3.1. Mechanisms of Microbiome-Induced Insulin Resistance
3.2. Influence on Glucose and Lipid Metabolism
3.3. Effects on Bile Acid Metabolism and Incretin Hormones
3.4. Microbiome-Derived Metabolites: SCFAs, BCAAs, and Bile Acids
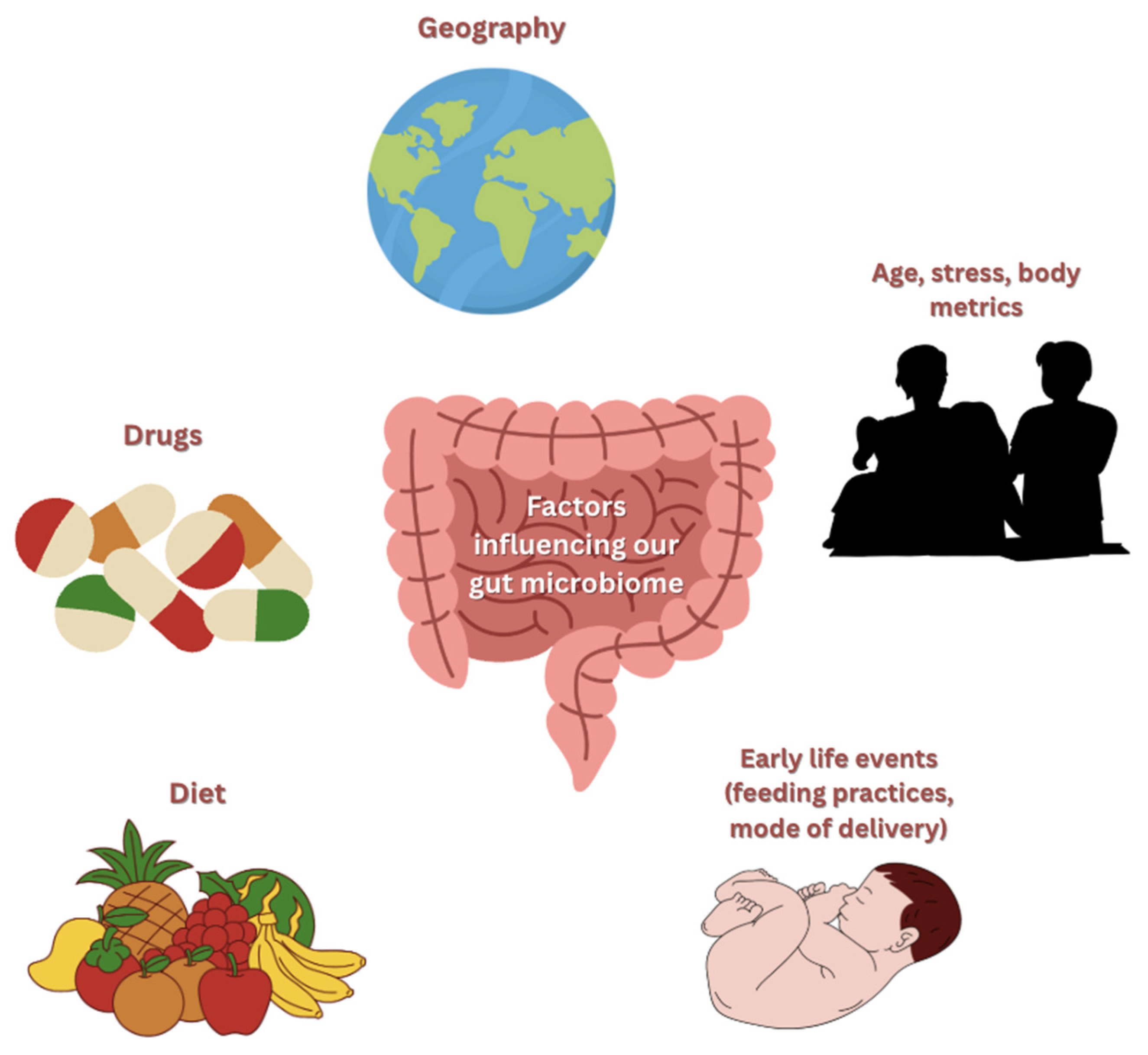
4. Factors Affecting the Microbiome in T2DM
4.1. Diet and Nutrition
4.2. Antibiotics and Medication
5. Dietary Interventions
5.1. Fibers
5.2. Mediterranean Diets
6. Fecal Microbiome Transplantation (FMT)
7. Prebiotics, Probiotics, and Postbiotics
8. Pharmacological Modulation
8.1. Metformin
8.2. Glucagon-like Peptide 1 (GLP-1) Agonists
8.3. Alpha Glucosidase Inhibitors (AGIs)
9. Future Therapeutic Directions
9.1. Next-Generation Probiotics
9.2. Microbiome Editing
10. Current Gaps and Future Research Directions
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Al Bander, Z.; Nitert, M.D.; Mousa, A.; Naderpoor, N. The Gut Microbiota and Inflammation: An Overview. Int. J. Environ. Res. Public Health 2020, 17, 7618. [Google Scholar] [CrossRef]
- Thursby, E.; Juge, N. Introduction to the human gut microbiota. Biochem. J. 2017, 474, 1823–1836. [Google Scholar] [CrossRef]
- Neish, A.S. Microbes in Gastrointestinal Health and Disease. Gastroenterology 2009, 136, 65–80. [Google Scholar] [CrossRef] [PubMed]
- Bäckhed, F.; Ley, R.E.; Sonnenburg, J.L.; Peterson, D.A.; Gordon, J.I. Host-Bacterial Mutualism in the Human Intestine. Science 2005, 307, 1915–1920. [Google Scholar] [CrossRef] [PubMed]
- De Vos, W.M.; Tilg, H.; Van Hul, M.; Cani, P.D. Gut microbiome and health: Mechanistic insights. Gut 2022, 71, 1020–1032. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Bays, D.J.; Savage, H.P.; Bäumler, A.J. The human gut microbiome in health and disease: Time for a new chapter? Infect. Immun. 2024, 92, e00302-24. [Google Scholar] [CrossRef]
- Hou, K.; Wu, Z.X.; Chen, X.Y.; Wang, J.Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J.; et al. Microbiota in health and diseases. Signal. Transduct. Target. Ther. 2022, 7, 135. [Google Scholar] [CrossRef]
- Bull, M.J.; Plummer, N.T. Part 1: The Human Gut Microbiome in Health and Disease. Integr. Med. Encinitas Calif. 2014, 13, 17–22. [Google Scholar]
- Cunningham, A.L.; Stephens, J.W.; Harris, D.A. Gut microbiota influence in type 2 diabetes mellitus (T2DM). Gut Pathog. 2021, 13, 50. [Google Scholar] [CrossRef]
- Zhang, P. Influence of Foods and Nutrition on the Gut Microbiome and Implications for Intestinal Health. Int. J. Mol. Sci. 2022, 23, 9588. [Google Scholar] [CrossRef]
- Shreiner, A.B.; Kao, J.Y.; Young, V.B. The gut microbiome in health and in disease. Curr. Opin. Gastroenterol. 2015, 31, 69–75. [Google Scholar] [CrossRef]
- Ursell, L.K.; Haiser, H.J.; Van Treuren, W.; Garg, N.; Reddivari, L.; Vanamala, J.; Dorrestein, P.C.; Turnbaugh, P.J.; Knight, R. The Intestinal Metabolome: An Intersection Between Microbiota and Host. Gastroenterology 2014, 146, 1470–1476. [Google Scholar] [CrossRef] [PubMed]
- Jandhyala, S.M. Role of the normal gut microbiota. World. J. Gastroenterol. 2015, 21, 8787. [Google Scholar] [CrossRef]
- Valdes, A.M.; Walter, J.; Segal, E.; Spector, T.D. Role of the gut microbiota in nutrition and health. BMJ 2018, 361, k2179. [Google Scholar] [CrossRef]
- Goodrich, J.K.; Waters, J.L.; Poole, A.C.; Sutter, J.L.; Koren, O.; Blekhman, R.; Beaumont, M.; Van Treuren, W.; Knight, R.; Bell, J.T.; et al. Human Genetics Shape the Gut Microbiome. Cell 2014, 159, 789–799. [Google Scholar] [CrossRef]
- Cresci, G.A.; Bawden, E. Gut Microbiome: What We Do and Don’t Know. Nutr. Clin. Pract. 2015, 30, 734–746. [Google Scholar] [CrossRef]
- Alagiakrishnan, K.; Morgadinho, J.; Halverson, T. Approach to the diagnosis and management of dysbiosis. Front. Nutr. 2024, 11, 1330903. [Google Scholar] [CrossRef] [PubMed]
- DeGruttola, A.K.; Low, D.; Mizoguchi, A.; Mizoguchi, E. Current Understanding of Dysbiosis in Disease in Human and Animal Models. Inflamm. Bowel Dis. 2016, 22, 1137–1150. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, F.B.; Cisewski, J.A.; Xu, J.; Anderson, R.N. Provisional Mortality Data—United States, 2022. MMWR Morb. Mortal. Wkly. Rep. 2023, 72, 488–492. [Google Scholar] [CrossRef]
- Karlsson, F.H.; Fåk, F.; Nookaew, I.; Tremaroli, V.; Fagerberg, B.; Petranovic, D.; Bäckhed, F.; Nielsen, J. Symptomatic atherosclerosis is associated with an altered gut metagenome. Nat. Commun. 2012, 3, 1245. [Google Scholar] [CrossRef]
- Arthur, J.C.; Perez-Chanona, E.; Mühlbauer, M.; Tomkovich, S.; Uronis, J.M.; Fan, T.J.; Campbell, B.J.; Abujamel, T.; Dogan, B.; Rogers, A.B.; et al. Intestinal Inflammation Targets Cancer-Inducing Activity of the Microbiota. Science 2012, 338, 120–123. [Google Scholar] [CrossRef]
- Qin, J.; Li, Y.; Cai, Z.; Li, S.; Zhu, J.; Zhang, F.; Liang, S.; Zhang, W.; Guan, Y.; Shen, D.; et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 2012, 490, 55–60. [Google Scholar] [CrossRef]
- Wu, S.; Rhee, K.J.; Albesiano, E.; Rabizadeh, S.; Wu, X.; Yen, H.R.; Huso, D.L.; Brancati, F.L.; Wick, E.; McAllister, F.; et al. A human colonic commensal promotes colon tumorigenesis via activation of T helper type 17 T cell responses. Nat. Med. 2009, 15, 1016–1022. [Google Scholar] [CrossRef]
- Galicia-Garcia, U.; Benito-Vicente, A.; Jebari, S.; Larrea-Sebal, A.; Siddiqi, H.; Uribe, K.B.; Ostolaza, H.; Martín, C. Pathophysiology of Type 2 Diabetes Mellitus. Int. J. Mol. Sci. 2020, 21, 6275. [Google Scholar] [CrossRef]
- Kalyani, R.R.; Neumiller, J.J.; Maruthur, N.M.; Wexler, D.J. Diagnosis and Treatment of Type 2 Diabetes in Adults: A Review. JAMA 2025, 334, 11. [Google Scholar] [CrossRef]
- Ong, K.L.; Stafford, L.K.; McLaughlin, S.A.; Boyko, E.J.; Vollset, S.E.; Smith, A.E.; Dalton, B.E.; Duprey, J.; Cruz, J.A.; Hagins, H.; et al. Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: A systematic analysis for the Global Burden of Disease Study 2021. Lancet 2023, 402, 203–234, Erratum in: Lancet 2023, 402, 1132; Erratum in: Lancet 2025, 405, 202. [Google Scholar] [CrossRef] [PubMed]
- Schellenberg, E.S.; Dryden, D.M.; Vandermeer, B.; Ha, C.; Korownyk, C. Lifestyle Interventions for Patients with and at Risk for Type 2 Diabetes: A Systematic Review and Meta-analysis. Ann. Intern. Med. 2013, 159, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.B.; Manson, J.E.; Stampfer, M.J.; Colditz, G.; Liu, S.; Solomon, C.G.; Willett, W.C. Diet, Lifestyle, and the Risk of Type 2 Diabetes Mellitus in Women. N. Engl. J. Med. 2001, 345, 790–797. [Google Scholar] [CrossRef]
- Goyal, R.; Singhal, M.; Jialal, I. Type 2 Diabetes. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: http://www.ncbi.nlm.nih.gov/books/NBK513253/ (accessed on 6 August 2025).
- Dash, N.R.; Al Bataineh, M.T.; Alili, R.; Al Safar, H.; Alkhayyal, N.; Prifti, E.; Zucker, J.-D.; Belda, E.; Clément, K. Functional alterations and predictive capacity of gut microbiome in type 2 diabetes. Sci. Rep. 2023, 13, 22386. [Google Scholar] [CrossRef]
- Sharma, S.; Tripathi, P. Gut microbiome and type 2 diabetes: Where we are and where to go? J. Nutr. Biochem. 2019, 63, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Ma, K.L.; Ruan, X.Z. Dysbiosis of Gut Microbiota Contributes to the Development of Diabetes Mellitus. Infect. Microbes. Dis. 2019, 1, 43–48. [Google Scholar] [CrossRef]
- Baars, D.P.; Fondevila, M.F.; Meijnikman, A.S.; Nieuwdorp, M. The central role of the gut microbiota in the pathophysiology and management of type 2 diabetes. Cell. Host. Microbe. 2024, 32, 1280–1300. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Sun, B.; Yu, D.; Zhu, C. Gut Microbiota: An Important Player in Type 2 Diabetes Mellitus. Front. Cell. Infect. Microbiol. 2022, 12, 834485. [Google Scholar] [CrossRef] [PubMed]
- Kashtanova, D.A.; Tkacheva, O.N.; Doudinskaya, E.N.; Strazhesko, I.D.; Kotovskaya, Y.V.; Popenko, A.S.; Tyakht, A.V.; Alexeev, D.G. Gut Microbiota in Patients with Different Metabolic Statuses: Moscow Study. Microorganisms 2018, 6, 98. [Google Scholar] [CrossRef]
- Takagi, T.; Naito, Y.; Kashiwagi, S.; Uchiyama, K.; Mizushima, K.; Kamada, K.; Ishikawa, T.; Inoue, R.; Okuda, K.; Tsujimoto, Y.; et al. Changes in the Gut Microbiota are Associated with Hypertension, Hyperlipidemia, and Type 2 Diabetes Mellitus in Japanese Subjects. Nutrients 2020, 12, 2996. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.C.; Chien, Y.W.; Yang, S.C. The alteration of gut microbiota in newly diagnosed type 2 diabetic patients. Nutrition 2019, 63–64, 51–56. [Google Scholar] [CrossRef]
- He, Y.; Wu, W.; Zheng, H.M.; Li, P.; McDonald, D.; Sheng, H.F.; Chen, M.-X.; Chen, Z.-H.; Ji, G.-Y.; Zheng, Z.-D.; et al. Regional variation limits applications of healthy gut microbiome reference ranges and disease models. Nat. Med. 2018, 24, 1532–1535, Erratum in: Nat Med. 2018, 24, 1940. [Google Scholar] [CrossRef]
- Chong, S.; Lin, M.; Chong, D.; Jensen, S.; Lau, N.S. A systematic review on gut microbiota in type 2 diabetes mellitus. Front. Endocrinol. 2025, 15, 1486793. [Google Scholar] [CrossRef]
- Crudele, L.; Gadaleta, R.M.; Cariello, M.; Moschetta, A. Gut microbiota in the pathogenesis and therapeutic approaches of diabetes. eBioMedicine 2023, 97, 104821. [Google Scholar] [CrossRef]
- Xu, J.; Gordon, J.I. Honor thy symbionts. Proc. Natl. Acad. Sci. USA 2003, 100, 10452–10459. [Google Scholar] [CrossRef]
- Goodman, A.L.; Gordon, J.I. Our Unindicted Coconspirators: Human Metabolism from a Microbial Perspective. Cell. Metab. 2010, 12, 111–116. [Google Scholar] [CrossRef]
- Ghosh, S.; Pramanik, S. Structural diversity, functional aspects and future therapeutic applications of human gut microbiome. Arch. Microbiol. 2021, 203, 5281–5308. [Google Scholar] [CrossRef]
- Kho, Z.Y.; Lal, S.K. The Human Gut Microbiome—A Potential Controller of Wellness and Disease. Front. Microbiol. 2018, 9, 1835. [Google Scholar] [CrossRef]
- Thomas, C.M.; Hong, T.; Van Pijkeren, J.P.; Hemarajata, P.; Trinh, D.V.; Hu, W.; Britton, R.A.; Kalkum, M.; Versalovic, J. Histamine Derived from Probiotic Lactobacillus reuteri Suppresses TNF via Modulation of PKA and ERK Signaling. PLoS ONE 2012, 7, e31951. [Google Scholar] [CrossRef] [PubMed]
- Bansal, T.; Alaniz, R.C.; Wood, T.K.; Jayaraman, A. The bacterial signal indole increases epithelial-cell tight-junction resistance and attenuates indicators of inflammation. Proc. Natl. Acad. Sci. USA 2010, 107, 228–233. [Google Scholar] [CrossRef]
- Hrncir, T. Gut Microbiota Dysbiosis: Triggers, Consequences, Diagnostic and Therapeutic Options. Microorganisms 2022, 10, 578. [Google Scholar] [CrossRef] [PubMed]
- Kozyrskyj, A.L.; Bahreinian, S.; Azad, M.B. Early life exposures: Impact on asthma and allergic disease. Curr. Opin. Allergy Clin. Immunol. 2011, 11, 400–406. [Google Scholar] [CrossRef]
- Stiemsma, L.; Reynolds, L.; Turvey, S.; Finlay, B. The hygiene hypothesis: Current perspectives and future therapies. ImmunoTargets Ther. 2015, 4, 143–157. [Google Scholar] [CrossRef]
- Walker, R.L.; Vlamakis, H.; Lee, J.W.J.; Besse, L.A.; Xanthakis, V.; Vasan, R.S.; Shaw, S.Y.; Xavier, R.J. Population study of the gut microbiome: Associations with diet, lifestyle, and cardiometabolic disease. Genome Med. 2021, 13, 188. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Portlock, T.; Le Chatelier, E.; Garcia-Guevara, F.; Clasen, F.; Oñate, F.P.; Pons, N.; Begum, N.; Harzandi, A.; Proffitt, C.; et al. Global compositional and functional states of the human gut microbiome in health and disease. Genome Res. 2024, 34, 967–978. [Google Scholar] [CrossRef]
- Kim, N.; Ma, J.; Kim, W.; Kim, J.; Belenky, P.; Lee, I. Genome-resolved metagenomics: A game changer for microbiome medicine. Exp. Mol. Med. 2024, 56, 1501–1512. [Google Scholar] [CrossRef] [PubMed]
- Bradley, E.; Haran, J. The human gut microbiome and aging. Gut Microbes 2024, 16, 2359677. [Google Scholar] [CrossRef]
- Mikkelsen, K.H.; Allin, K.H.; Knop, F.K. Effect of antibiotics on gut microbiota, glucose metabolism and body weight regulation: A review of the literature. Diabetes Obes. Metab. 2016, 18, 444–453. [Google Scholar] [CrossRef]
- Dowling, L.R.; Strazzari, M.R.; Keely, S.; Kaiko, G.E. Enteric nervous system and intestinal epithelial regulation of the gut-brain axis. J. Allergy Clin. Immunol. 2022, 150, 513–522. [Google Scholar] [CrossRef]
- Tidjani Alou, M.; Lagier, J.C.; Raoult, D. Diet influence on the gut microbiota and dysbiosis related to nutritional disorders. Hum. Microbiome J. 2016, 1, 3–11. [Google Scholar] [CrossRef]
- Patangia, D.V.; Anthony Ryan, C.; Dempsey, E.; Paul Ross, R.; Stanton, C. Impact of antibiotics on the human microbiome and consequences for host health. MicrobiologyOpen 2022, 11, e1260. [Google Scholar] [CrossRef] [PubMed]
- Larsen, O.F.A.; Van Der Grint, M.; Wiegers, C.; Van De Burgwal, L.H.M. The Gut Microbiota: Master of Puppets Connecting the Epidemiology of Infectious, Autoimmune, and Metabolic Disease. Front. Microbiol. 2022, 13, 902106. [Google Scholar] [CrossRef]
- Chen, Z.; Radjabzadeh, D.; Chen, L.; Kurilshikov, A.; Kavousi, M.; Ahmadizar, F.; Ikram, M.A.; Uitterlinden, A.G.; Zhernakova, A.; Fu, J.; et al. Association of Insulin Resistance and Type 2 Diabetes with Gut Microbial Diversity: A Microbiome-Wide Analysis from Population Studies. JAMA Netw. Open 2021, 4, e2118811. [Google Scholar] [CrossRef]
- Smith, P.M.; Howitt, M.R.; Panikov, N.; Michaud, M.; Gallini, C.A.; Bohlooly, Y.M.; Glickman, J.N.; Garrett, W.S. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 2013, 341, 569–573. [Google Scholar] [CrossRef]
- Wu, J.; Yang, K.; Fan, H.; Wei, M.; Xiong, Q. Targeting the gut microbiota and its metabolites for type 2 diabetes mellitus. Front. Endocrinol. 2023, 14, 1114424. [Google Scholar] [CrossRef] [PubMed]
- Sikalidis, A.K.; Maykish, A. The Gut Microbiome and Type 2 Diabetes Mellitus: Discussing A Complex Relationship. Biomedicines 2020, 8, 8. [Google Scholar] [CrossRef] [PubMed]
- den Besten, G.; van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.J.; Bakker, B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar] [CrossRef]
- Sadagopan, A.; Mahmoud, A.; Begg, M.; Tarhuni, M.; Fotso, M.; Gonzalez, N.A.; Sanivarapu, R.R.; Osman, U.; Kumar, A.L.; Mohammed, L.; et al. Understanding the Role of the Gut Microbiome in Diabetes and Therapeutics Targeting Leaky Gut: A Systematic Review. Cureus 2023, 15, e41559. [Google Scholar] [CrossRef]
- Dehghan, P.; Pourghassem Gargari, B.; Asghari Jafar-Abadi, M. Oligofructose-enriched inulin improves some inflammatory markers and metabolic endotoxemia in women with type 2 diabetes mellitus: A randomized controlled clinical trial. Nutrition 2014, 30, 418–423. [Google Scholar] [CrossRef]
- Zhu, T.; Goodarzi, M.O. Metabolites Linking the Gut Microbiome with Risk for Type 2 Diabetes. Curr. Nutr. Rep. 2020, 9, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Kootte, R.S.; Levin, E.; Salojärvi, J.; Smits, L.P.; Hartstra, A.V.; Udayappan, S.D.; Hermes, G.; Bouter, K.E.; Koopen, A.M.; Holst, J.J.; et al. Improvement of Insulin Sensitivity after Lean Donor Feces in Metabolic Syndrome Is Driven by Baseline Intestinal Microbiota Composition. Cell Metab. 2017, 26, 611–619.e6. [Google Scholar] [CrossRef] [PubMed]
- Ellingsgaard, H.; Hauselmann, I.; Schuler, B.; Habib, A.M.; Baggio, L.L.; Meier, D.T.; Eppler, E.; Bouzakri, K.; Wueest, S.; Muller, Y.D.; et al. Interleukin-6 enhances insulin secretion by increasing glucagon-like peptide-1 secretion from L cells and alpha cells. Nat. Med. 2011, 17, 1481–1489. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, H.K.; Gudmundsdottir, V.; Nielsen, H.B.; Hyotylainen, T.; Nielsen, T.; Jensen, B.A.H.; Forslund, K.; Hildebrand, F.; Prifti, E.; Falony, G.; et al. Human gut microbes impact host serum metabolome and insulin sensitivity. Nature 2016, 535, 376–381. [Google Scholar] [CrossRef]
- Wang, Z.; Klipfell, E.; Bennett, B.J.; Koeth, R.; Levison, B.S.; DuGar, B.; Feldstein, A.E.; Britt, E.B.; Fu, X.; Chung, Y.-M.; et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 2011, 472, 57–63. [Google Scholar] [CrossRef]
- Gao, R.; Meng, X.; Xue, Y.; Mao, M.; Liu, Y.; Tian, X.; Sui, B.; Li, X.; Zhang, P. Bile acids-gut microbiota crosstalk contributes to the improvement of type 2 diabetes mellitus. Front. Pharmacol. 2022, 13, 1027212. [Google Scholar] [CrossRef]
- Angelini, G.; Russo, S.; Mingrone, G. Incretin hormones, obesity and gut microbiota. Peptides 2024, 178, 171216. [Google Scholar] [CrossRef] [PubMed]
- Salamone, D.; Rivellese, A.A.; Vetrani, C. The relationship between gut microbiota, short-chain fatty acids and type 2 diabetes mellitus: The possible role of dietary fibre. Acta Diabetol. 2021, 58, 1131–1138. [Google Scholar] [CrossRef]
- Özsoy, S.; Sultanoglu, N.; Sanlidag, T. The role of mediterranean diet and gut microbiota in type- diabetes mellitus associated with obesity (diabesity). J. Prev. Med. Hyg. 2022, 63 (Suppl. S3), E87. [Google Scholar]
- Xu, X.; Zhang, F.; Ren, J.; Zhang, H.; Jing, C.; Wei, M.; Jiang, Y.; Xie, H. Dietary intervention improves metabolic levels in patients with type 2 diabetes through the gut microbiota: A systematic review and meta-analysis. Front. Nutr. 2024, 10, 1243095, Erratum in: Front Nutr. 2024, 11, 1414687. [Google Scholar] [CrossRef]
- Menafra, D.; Proganò, M.; Tecce, N.; Pivonello, R.; Colao, A. Diet and gut microbiome: Impact of each factor and mutual interactions on prevention and treatment of type 1, type 2, and gestational diabetes mellitus. Hum. Nutr. Metab. 2024, 38, 200286. [Google Scholar] [CrossRef]
- Fu, J.; Zheng, Y.; Gao, Y.; Xu, W. Dietary Fiber Intake and Gut Microbiota in Human Health. Microorganisms 2022, 10, 2507. [Google Scholar] [CrossRef] [PubMed]
- Vich Vila, A.; Collij, V.; Sanna, S.; Sinha, T.; Imhann, F.; Bourgonje, A.R.; Mujagic, Z.; Jonkers, D.M.A.E.; Masclee, A.A.M.; Fu, J.; et al. Impact of commonly used drugs on the composition and metabolic function of the gut microbiota. Nat. Commun. 2020, 11, 362. [Google Scholar] [CrossRef]
- Wu, H.; Esteve, E.; Tremaroli, V.; Khan, M.T.; Caesar, R.; Mannerås-Holm, L.; Ståhlman, M.; Olsson, L.M.; Serino, M.; Planas-Fèlix, M.; et al. Metformin alters the gut microbiome of individuals with treatment-naive type 2 diabetes, contributing to the therapeutic effects of the drug. Nat. Med. 2017, 23, 850–858. [Google Scholar] [CrossRef]
- Kim, H.B.; Cho, Y.J.; Choi, S.S. Metformin increases gut multidrug resistance genes in type 2 diabetes, potentially linked to Escherichia coli. Sci. Rep. 2024, 14, 21480. [Google Scholar] [CrossRef]
- Yang, Q.; Liang, Q.; Balakrishnan, B.; Belobrajdic, D.P.; Feng, Q.J.; Zhang, W. Role of Dietary Nutrients in the Modulation of Gut Microbiota: A Narrative Review. Nutrients 2020, 12, 381. [Google Scholar] [CrossRef]
- Swann, O.G.; Kilpatrick, M.; Breslin, M.; Oddy, W.H. Dietary fiber and its associations with depression and inflammation. Nutr. Rev. 2020, 78, 394–411. [Google Scholar] [CrossRef]
- Soliman, G.A. Dietary Fiber, Atherosclerosis, and Cardiovascular Disease. Nutrients 2019, 11, 1155. [Google Scholar] [CrossRef] [PubMed]
- Titgemeyer, E.; Bourquin, L.; Fahey, G.; Garleb, K. Fermentability of various fiber sources by human fecal bacteria in vitro. Am. J. Clin. Nutr. 1991, 53, 1418–1424. [Google Scholar] [CrossRef]
- Portincasa, P.; Bonfrate, L.; Vacca, M.; De Angelis, M.; Farella, I.; Lanza, E.; Khalil, M.; Wang, D.Q.-H.; Sperandio, M.; Di Ciaula, A. Gut Microbiota and Short Chain Fatty Acids: Implications in Glucose Homeostasis. Int. J. Mol. Sci. 2022, 23, 1105. [Google Scholar] [CrossRef]
- Diniz Do Nascimento, L.; Moraes, A.A.B.D.; Costa, K.S.D.; Pereira Galúcio, J.M.; Taube, P.S.; Costa, C.M.L.; Cruz, J.N.; Andrade, E.H.D.A.; De Faria, L.J.G. Bioactive Natural Compounds and Antioxidant Activity of Essential Oils from Spice Plants: New Findings and Potential Applications. Biomolecules 2020, 10, 988. [Google Scholar] [CrossRef]
- Sobiecki, J.G.; Imamura, F.; Davis, C.R.; Sharp, S.J.; Koulman, A.; Hodgson, J.M.; Guevara, M.; Schulze, M.B.; Zheng, J.-S.; Agnoli, C.; et al. A nutritional biomarker score of the Mediterranean diet and incident type 2 diabetes: Integrated analysis of data from the MedLey randomised controlled trial and the EPIC-InterAct case-cohort study. Popkin BM, editor. PLoS Med. 2023, 20, e1004221. [Google Scholar] [CrossRef]
- Zhang, Q.; Hu, N. Effects of Metformin on the Gut Microbiota in Obesity and Type 2 Diabetes Mellitus. Diabetes Metab. Syndr. Obes. Targets Ther. 2020, 13, 5003–5014. [Google Scholar] [CrossRef]
- Romagnolo, D.F.; Selmin, O.I. Mediterranean Diet and Prevention of Chronic Diseases. Nutr. Today 2017, 52, 208–222. [Google Scholar] [CrossRef]
- Choi, H.H.; Cho, Y.S. Fecal Microbiota Transplantation: Current Applications, Effectiveness, and Future Perspectives. Clin. Endosc. 2016, 49, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.O.; Gluck, M. Fecal Microbiota Transplantation: An Update on Clinical Practice. Clin. Endosc. 2019, 52, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.; Yacyshyn, B.R.; Yacyshyn, M.B. Gut microbiota and obesity: An opportunity to alter obesity through faecal microbiota transplant (FMT). Diabetes Obes. Metab. 2019, 21, 479–490. [Google Scholar] [CrossRef]
- Bibbò, S.; Settanni, C.R.; Porcari, S.; Bocchino, E.; Ianiro, G.; Cammarota, G.; Gasbarrini, A. Fecal Microbiota Transplantation: Screening and Selection to Choose the Optimal Donor. J. Clin. Med. 2020, 9, 1757. [Google Scholar] [CrossRef] [PubMed]
- Edelstein, C.; Daw, J.R.; Kassam, Z. Seeking safe stool: Canada needs a universal donor model. Can. Med. Assoc. J. 2016, 188, E431–E432. [Google Scholar] [CrossRef]
- De Angelis, M.; Ferrocino, I.; Calabrese, F.M.; De Filippis, F.; Cavallo, N.; Siragusa, S.; Rampelli, S.; Di Cagno, R.; Rantsiou, K.; Vannini, L.; et al. Diet influences the functions of the human intestinal microbiome. Sci. Rep. 2020, 10, 4247. [Google Scholar] [CrossRef]
- Kaur, I.P.; Chopra, K.; Saini, A. Probiotics: Potential pharmaceutical applications. Eur. J. Pharm. Sci. 2002, 15, 1–9. [Google Scholar] [CrossRef]
- Yao, K.; Zeng, L.; He, Q.; Wang, W.; Lei, J.; Zou, X. Effect of Probiotics on Glucose and Lipid Metabolism in Type 2 Diabetes Mellitus: A Meta-Analysis of 12 Randomized Controlled Trials. Med. Sci. Monit. 2017, 23, 3044–3053. [Google Scholar] [CrossRef]
- Dubey, V.P.; Kansagra, J.J.; Sureja, V.P.; Kheni, D.B. Efficacy of a Probiotic Combination on Glycemic Index and Insulin Resistance in Adults: A Systematic Review and Meta-Analysis. J. Diet. Suppl. 2025, 22, 641–663. [Google Scholar] [CrossRef]
- Everard, A.; Belzer, C.; Geurts, L.; Ouwerkerk, J.P.; Druart, C.; Bindels, L.B.; Guiot, Y.; Derrien, M.; Muccioli, G.G.; Delzenne, N.M.; et al. Cross-talk between Akkermansia. muciniphila and intestinal epithelium controls diet-induced obesity. Proc. Natl. Acad. Sci. USA 2013, 110, 9066–9071. [Google Scholar] [CrossRef] [PubMed]
- Sako, T.; Tanaka, R. Prebiotics|Functions. In Encyclopedia of Dairy Sciences; Elsevier: Amsterdam, The Netherlands, 2011; pp. 365–371. [Google Scholar]
- Iatcu, O.C.; Hamamah, S.; Covasa, M. Harnessing Prebiotics to Improve Type 2 Diabetes Outcomes. Nutrients 2024, 16, 3447. [Google Scholar] [CrossRef] [PubMed]
- Antony, M.A.; Chowdhury, A.; Edem, D.; Raj, R.; Nain, P.; Joglekar, M.; Verma, V.; Kant, R. Gut microbiome supplementation as therapy for metabolic syndrome. World J. Diabetes 2023, 14, 1502–1513. [Google Scholar] [CrossRef]
- Remely, M.; Hippe, B.; Zanner, J.; Aumueller, E.; Brath, H.G.; Haslberger, A. Gut Microbiota of Obese, Type 2 Diabetic Individuals is Enriched in Faecalibacterium prausnitzii, Akkermansia muciniphila and Peptostreptococcus anaerobius after Weight Loss. Endocr. Metab. Immune Disord. 2016, 16, 99–106. [Google Scholar] [CrossRef]
- Remely, M.; Hippe, B.; Geretschlaeger, I.; Stegmayer, S.; Hoefinger, I.; Haslberger, A. Increased gut microbiota diversity and abundance of Faecalibacterium prausnitzii and Akkermansia after fasting: A pilot study. Wien. Klin. Wochenschr. 2015, 127, 394–398. [Google Scholar] [CrossRef]
- Salminen, S.; Collado, M.C.; Endo, A.; Hill, C.; Lebeer, S.; Quigley, E.M.M.; Sanders, M.E.; Shamir, R.; Swann, J.R.; Szajewska, H.; et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 649–667, Erratum in: Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 671. Erratum in: Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 551. [Google Scholar] [CrossRef]
- Amiri, S.; Rezazadeh-Bari, M.; Alizadeh-Khaledabad, M.; Rezaei-Mokarram, R.; Sowti-Khiabani, M. Fermentation Optimization for Co-production of Postbiotics by Bifidobacterium lactis BB12 in Cheese Whey. Waste Biomass Valorization 2021, 12, 5869–5884. [Google Scholar] [CrossRef]
- Dinić, M.; Lukić, J.; Djokić, J.; Milenković, M.; Strahinić, I.; Golić, N.; Begović, J. Lactobacillus fermentum Postbiotic-induced Autophagy as Potential Approach for Treatment of Acetaminophen Hepatotoxicity. Front. Microbiol. 2017, 8, 594. [Google Scholar] [CrossRef] [PubMed]
- Gofron, K.K.; Wasilewski, A.; Małgorzewicz, S. Effects of GLP-1 Analogues and Agonists on the Gut Microbiota: A Systematic Review. Nutrients 2025, 17, 1303. [Google Scholar] [CrossRef]
- Zhang, M.; Feng, R.; Yang, M.; Qian, C.; Wang, Z.; Liu, W.; Ma, J. Effects of metformin, acarbose, and sitagliptin monotherapy on gut microbiota in Zucker diabetic fatty rats. BMJ Open Diabetes Res. Care 2019, 7, e000717. [Google Scholar] [CrossRef]
- Huda, M.N.; Kim, M.; Bennett, B.J. Modulating the Microbiota as a Therapeutic Intervention for Type 2 Diabetes. Front. Endocrinol. 2021, 12, 632335. [Google Scholar] [CrossRef]
- Liu, W.; Luo, Z.; Zhou, J.; Sun, B. Gut Microbiota and Antidiabetic Drugs: Perspectives of Personalized Treatment in Type 2 Diabetes Mellitus. Front. Cell. Infect. Microbiol. 2022, 12, 853771. [Google Scholar] [CrossRef]
- Rittiphairoj, T.; Pongpirul, K.; Janchot, K.; Mueller, N.T.; Li, T. Probiotics Contribute to Glycemic Control in Patients with Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis. Adv. Nutr. 2021, 12, 722–734. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhang, Q.; Zhang, X.; Du, S.; Zhang, Y.; Wang, X.; Liu, Y.; Fang, B.; Chen, J.; Liu, R.; et al. Effects of synbiotics surpass probiotics alone in improving type 2 diabetes mellitus: A randomized, double-blind, placebo-controlled trial. Clin. Nutr. 2025, 44, 248–258. [Google Scholar] [CrossRef]
- Esposito, K.; Maiorino, M.I.; Ciotola, M.; Di Palo, C.; Scognamiglio, P.; Gicchino, M.; Petrizzo, M.; Saccomanno, F.; Beneduce, F.; Ceriello, A.; et al. Effects of a Mediterranean-Style Diet on the Need for Antihyperglycemic Drug Therapy in Patients with Newly Diagnosed Type 2 Diabetes: A Randomized Trial. Ann. Intern. Med. 2009, 151, 306–314. [Google Scholar] [CrossRef]
- Itsiopoulos, C.; Brazionis, L.; Kaimakamis, M.; Cameron, M.; Best, J.D.; O’Dea, K.; Rowley, K. Can the Mediterranean diet lower HbA1c in type 2 diabetes? Results from a randomized cross-over study. Nutr. Metab. Cardiovasc. Dis. 2011, 21, 740–747. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhang, F.; Ding, X.; Wu, G.; Lam, Y.Y.; Wang, X.; Fu, H.; Xue, X.; Lu, C.; Ma, J.; et al. Gut bacteria selectively promoted by dietary fibers alleviate type 2 diabetes. Science 2018, 359, 1151–1156. [Google Scholar] [CrossRef] [PubMed]
- Markowiak, P.; Śliżewska, K. Effects of Probiotics, Prebiotics, and Synbiotics on Human Health. Nutrients 2017, 9, 1021. [Google Scholar] [CrossRef]
- Zmora, N.; Suez, J.; Elinav, E. You are what you eat: Diet, health and the gut microbiota. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 35–56. [Google Scholar] [CrossRef]
- Tiwari, A.; Ika Krisnawati, D.; Susilowati, E.; Mutalik, C.; Kuo, T.R. Next-Generation Probiotics and Chronic Diseases: A Review of Current Research and Future Directions. J. Agric. Food. Chem. 2024, 72, 27679–27700. [Google Scholar] [CrossRef]
- O’Toole, P.W.; Claesson, M.J. Gut microbiota: Changes throughout the lifespan from infancy to elderly. Int. Dairy. J. 2010, 20, 281–291. [Google Scholar] [CrossRef]
- MetaHITConsortium; Qin, J.; Li, R.; Raes, J.; Arumugam, M.; Burgdorf, K.S.; Manichanh, C.; Nielsen, T.; Pons, N.; Levenez, F.; et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010, 464, 59–65. [Google Scholar] [CrossRef]
- Zhang, H.; Duan, Y.; Cai, F.; Cao, D.; Wang, L.; Qiao, Z.; Hong, Q.; Li, N.; Zheng, Y.; Su, M.; et al. Next-Generation Probiotics: Microflora Intervention to Human Diseases. BioMed. Res. Int. 2022, 2022, 5633403. [Google Scholar] [CrossRef]
- Mu, Y.; Zhang, C.; Li, T.; Jin, F.J.; Sung, Y.J.; Oh, H.M.; Lee, H.-G.; Jin, L. Development and Applications of CRISPR/Cas9-Based Genome Editing in Lactobacillus. Int. J. Mol. Sci. 2022, 23, 12852. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo-Cantabrana, C.; O’Flaherty, S.; Barrangou, R. CRISPR-based engineering of next-generation lactic acid bacteria. Curr. Opin. Microbiol. 2017, 37, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Qayyum, H.; Ishaq, Z.; Ali, A.; Kayani, M.U.R.; Huang, L. Genome-resolved metagenomics from short-read sequencing data in the era of artificial intelligence. Funct. Integr. Genom. 2025, 25, 124. [Google Scholar] [CrossRef] [PubMed]
| Metabolite | Host Target | Physiological Effect |
|---|---|---|
| SCFAs (acetate, butyrate, propionate) | FFAR2/FFAR3, colonocytes | Improves insulin sensitivity, enhances barrier integrity |
| BCAAs (leucine, isoleucine, valine) | Liver, skeletal muscle | Impairs insulin signaling, activates mTOR |
| Secondary bile acids | FXR, TGR5 | Modulates GLP-1, glucose, and lipid homeostasis |
| LPS | TLR4 | Triggers inflammation and insulin resistance |
| Intervention | Study Design/Population | Duration | Primary Outcomes | Microbiome Effect | References |
|---|---|---|---|---|---|
| Inulin-type fructans (FOS/inulin) | RCT in women with T2DM | 8 weeks | ↓ Fasting glucose, ↓ HbA1C, ↓ Endotoxemia (LPS) | ↑ Bifidobacterium, ↑ SCFAs | [65] |
| Probiotics (Lactobacillus/Bifidobacterium strains) | Meta-analysis of RCTs in T2DM (12 trials) | — | ↓ HbA1c, ↓ HOMA-IR; improved fasting glucose | ↑ gut diversity, ↓ inflammatory species | [112] |
| Synbiotics | RCTs in T2DM | 8–12 weeks | ↓ HOMA-IR, ↓ inflammation | Synergistic increase in SCFA producers | [113] |
| FMT (lean donor → recipients with metabolic syndrome) | Double-blind, controlled | 6 weeks | ↑ Peripheral insulin sensitivity; ↑ butyrate producers (Roseburia) | ↑ Roseburia, ↑ butyrate-producing taxa | [67] |
| Metformin (microbiome-linked effects) | Human cohorts/intervention | — | ↑ Akkermansia muciniphila, ↑ SCFA-producing taxa; improved glycemic control; GI intolerance varies by microbiome profile | ↑ Akkermansia, ↑ SCFAs | [79] |
| GLP-1 receptor agonists | Systematic review of animal + human studies | — | ↑ Microbial diversity; shifts in Akkermansia, Bacteroides, Ruminococcus | ↑ diversity; shifts in Akkermansia and Bacteroides | [108] |
| Dietary fiber/Mediterranean diet | Cohorts and interventions | 8–24 weeks | ↑ SCFAs; ↓ HbA1c; improved TG/HDL | ↑ SCFAs, ↑ microbial diversity | [114,115,116] |
| Probiotic Strain | Health Benefit |
|---|---|
| Lactobacillus acidophilus | Enhances the gut barrier, suppresses pathogens |
| Bifidobacterium longum | Anti-inflammatory, reduces gut permeability |
| Streptococcus thermophilus | Aids lactose digestion, modulates immunity |
| Saccharomyces boulardii | Prevents diarrhea, supports microbiota recovery |
| Escherichia coli Nissle 1917 | Competes with pathogens, maintains balance |
| Intervention | Mechanism of Action | Microbiota Impact |
|---|---|---|
| Prebiotics | Stimulate growth of beneficial bacteria | Increase SCFA producers |
| Probiotics | Introduce live beneficial microbes | Restore microbial diversity |
| FMT | Transplant fecal microbiota from healthy donor | Re-establish eubiosis |
| Dietary fiber | Substrate for microbial fermentation | Boosts butyrate production |
| Metformin | Modifies gut microbiota composition | Enriches Akkermansia and SCFA-producing bacteria |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mashal, R.; Al-Muhanna, A.; Khader, S.; Khudair, A.; Khudair, A.; Butler, A.E. The Role of the Gut Microbiome in Type 2 Diabetes Mellitus. Int. J. Mol. Sci. 2025, 26, 11412. https://doi.org/10.3390/ijms262311412
Mashal R, Al-Muhanna A, Khader S, Khudair A, Khudair A, Butler AE. The Role of the Gut Microbiome in Type 2 Diabetes Mellitus. International Journal of Molecular Sciences. 2025; 26(23):11412. https://doi.org/10.3390/ijms262311412
Chicago/Turabian StyleMashal, Rahaf, Amnah Al-Muhanna, Salma Khader, Aiman Khudair, Ahmed Khudair, and Alexandra E. Butler. 2025. "The Role of the Gut Microbiome in Type 2 Diabetes Mellitus" International Journal of Molecular Sciences 26, no. 23: 11412. https://doi.org/10.3390/ijms262311412
APA StyleMashal, R., Al-Muhanna, A., Khader, S., Khudair, A., Khudair, A., & Butler, A. E. (2025). The Role of the Gut Microbiome in Type 2 Diabetes Mellitus. International Journal of Molecular Sciences, 26(23), 11412. https://doi.org/10.3390/ijms262311412





