Recurrent CAPN3 p.Asp753Asn Variant Supports a Potential Dominant Calpainopathy with Variable Clinical Expressivity
Abstract
1. Introduction
2. Results
2.1. Subjects
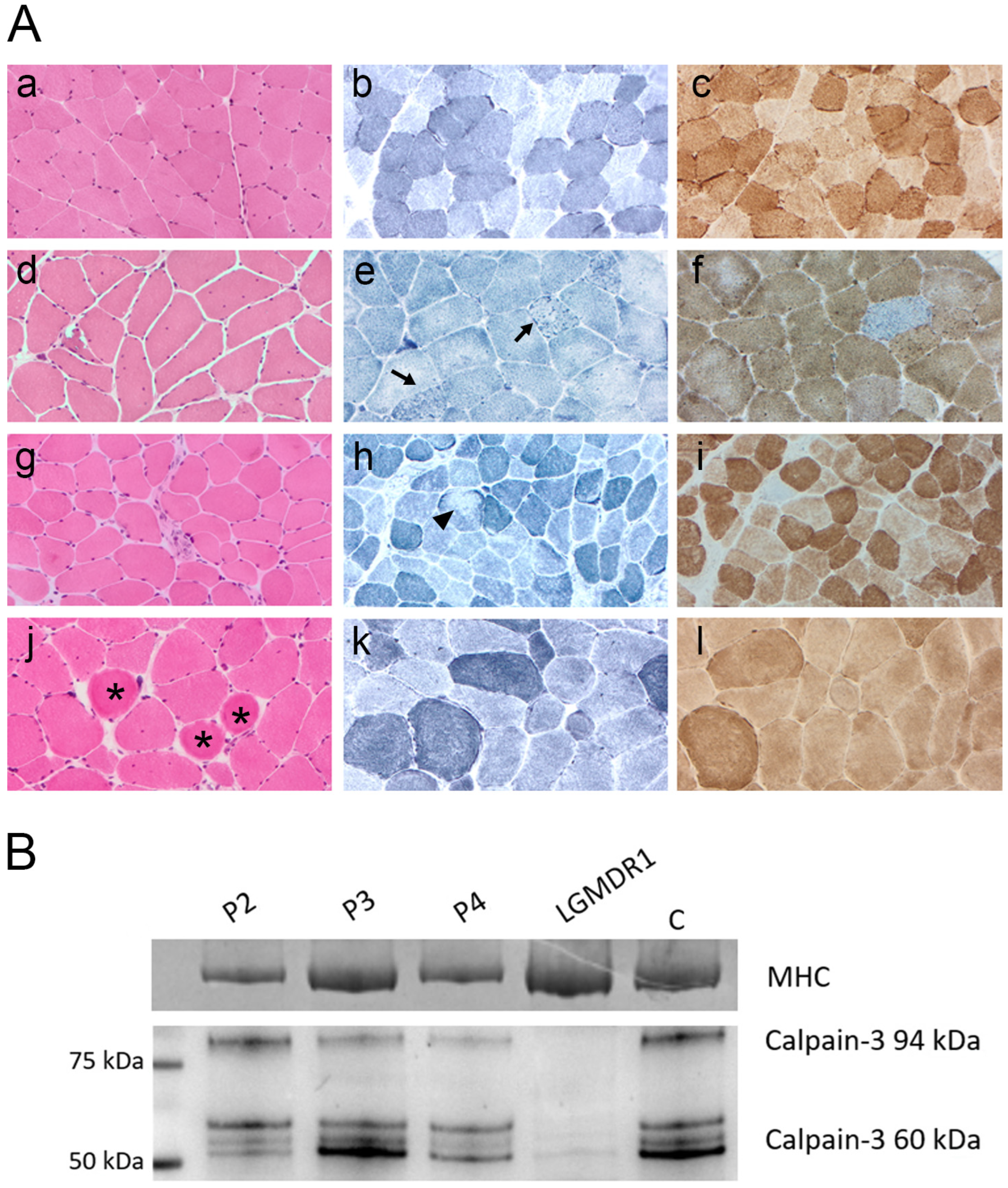
2.2. Clinical, Histopathological and Biochemical Features of p.Asp753Asn Patients
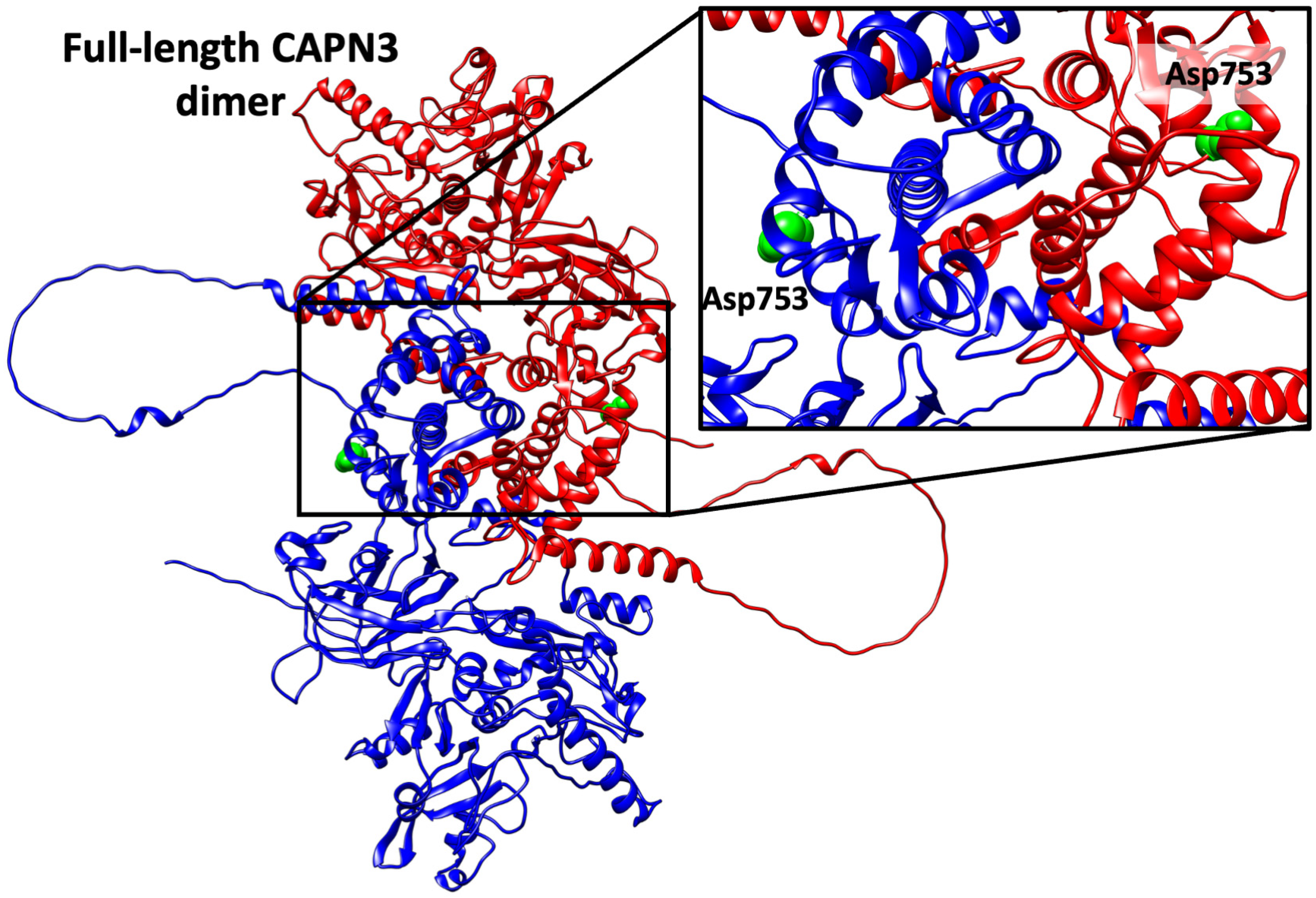
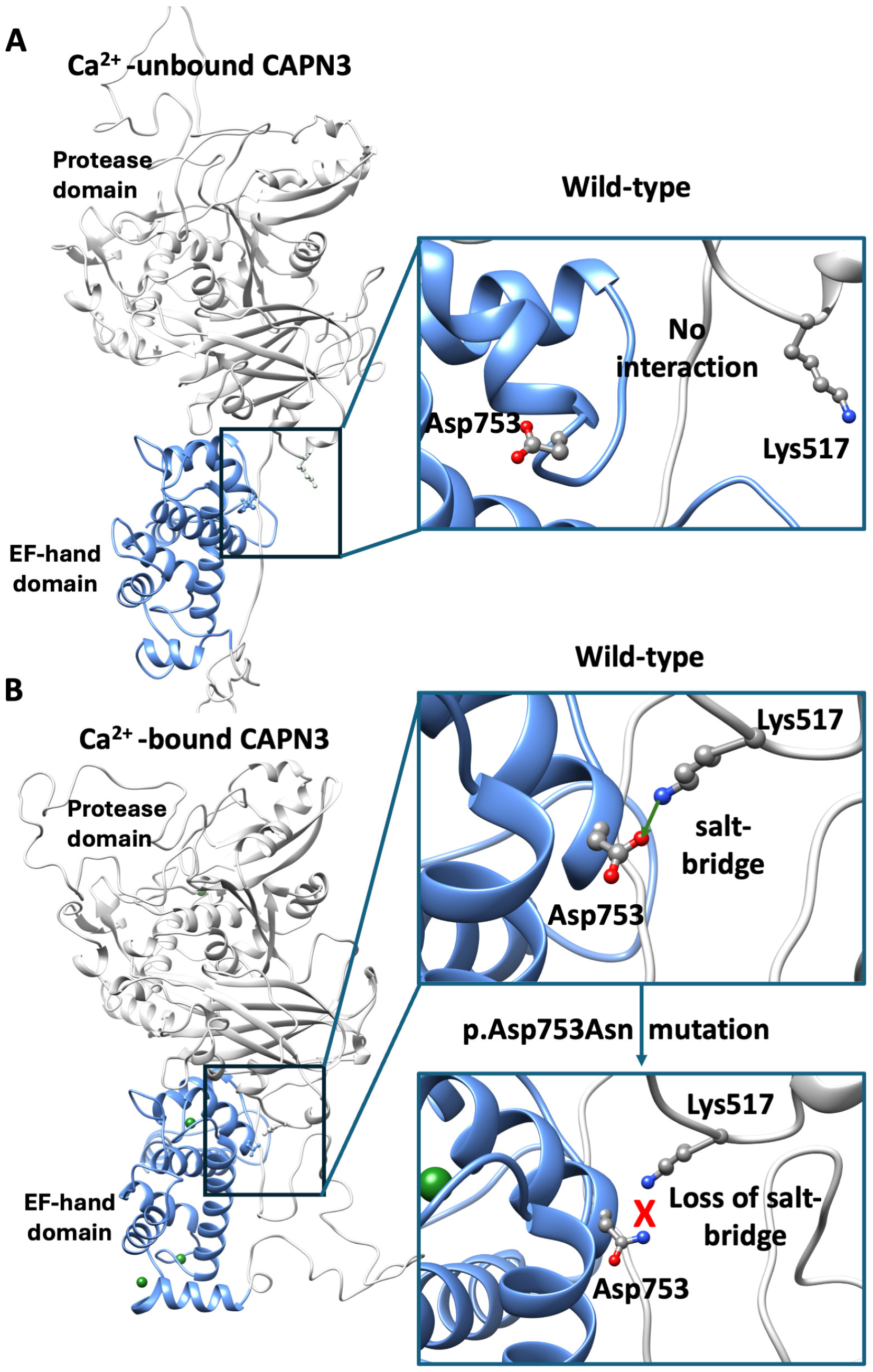
2.3. Structural Modeling of the p.Asp753Asn Variant
2.4. The p.Asp753Asn Variant in CAPN3 Is Recurrent Across Unrelated Individuals Worldwide
3. Discussion
4. Materials and Methods
4.1. Subjects and Literature Review
4.2. Sequencing Analysis
4.3. Calpain-3 Western Blot Analysis
4.4. Structural Modeling
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| LGMD | Limb-Girdle muscular dystrophy |
| WB | Western blot |
| WES | Whole exome sequencing |
| CK | Creatine kinase |
Appendix A
| Ref | No. PZ | No. in Study | Sex | Family History | Onset (Years) | Phenotype | Clinical Features | Severity | CK | WB Calpain-3 | Additional Mutations | Genetic Analysis |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| [26,38,40] | 4 | 52 | F | N | 3 | LGMD | LGMD | Moderate | / | Absent | No | SSCP, ARMS-PCR, SANGER HT-DHPLC |
| 71 | / | / | / | / | / | / | / | Normal | c.1355-6G4T | |||
| 48 | / | / | / | / | / | / | / | Reduced 5% | c.2242C4T (p.R748X) | |||
| 88 | / | / | / | LGMD | HyperCKmia | Mild | High | Normal | No | |||
| [25] | 1 | / | / | / | 40 | Not suggestive of LGMD | / | Benign evolution | / | / | No | SSCP, Sanger |
| [27] | 1 | 18 | F | N | 25 | Mild proximal weakness | Difficulty climbing stairs | Moderate | 140 U/L | / | No | Sanger |
| [28] | 1 | XXXV | F | N | 37 | LGMD | / | Slow progression | 10–13× | 94 kDa Reduced 45.1%, 30 kDa Normal | No | Sanger |
| [29] | 2 | 19 | / | N | 40 | LGMD | Pelvic girdle onset, Mild facial involvement | Mild, slow/moderate progression | 2× | Normal | No | Sanger |
| 20 | / | N | 55 | LGMD | Pelvic girdle onset | Mild, slow/moderate progression | 1.5× | Normal | No | |||
| [17] | 2 | C21 | / | / | / | / | / | / | / | / | No | NGS panel |
| C36 | / | / | / | / | / | / | / | / | No | |||
| [30] | 1 | P19 | M | / | 39 | Asymptomatic | HyperCKmia | Asymptomatic | 2000 U/L | / | c.1395-1397del (p.Leu465_Glu466del); c.1453A>G (p.Met485Val) | NGS panel |
| [33] | 2 | / | / | / | / | / | / | / | / | / | N/A | WES |
| [31] | 10 | / | / | / | / | Proximal weakness | / | / | / | / | N/A | NGS panel |
| [32] | 1 | P8 | M | / | / | / | / | / | Normal | / | No | NGS panel, Sanger, MLPA |
| [34] | 1 | A13 | F | N | 30 | Pseudometabolic | Paternal inheritance, Myalgia, fatigability | Mild | 6× | Normal | g.42350479A>G; g.42354194C>T | WES |
| [35] | 3 | 29 | M | / | 21 | ataxia | / | / | / | / | No, carrying other causative variants in other genes | Custom Target Capture NGS panel |
| 32 | M | / | 2 | / | Difficulty climbing stairs | / | High | / | No, carrying causative variants in other genes | |||
| 35 | M | / | 1 | / | Hypotonia | / | 400 U/L | / | No | |||
| [36] | 1 | U12 | M | / | 3 | Asymptomatic | HyperCKmia | Asymptomatic | High | / | Not found | NGS panel, Sanger |
| [39] | 1 | 4 | F | / | 40 | LGMD | Scapular winging, waddling gait, severe upper and lower girlde weakness | Severe | 1169 UI/L | / | c.967G>T (p.Glu323*), c.1401_1403delGGA (p.Glu467del) | Sanger |
References
- Sorimachi, H.; Imajoh-Ohmi, S.; Emori, Y.; Kawasaki, H.; Ohno, S.; Minami, Y.; Suzuki, K. Molecular cloning of a novel mammalian calcium-dependent protease distinct from both m- and mu-types. Specific expression of the mRNA in skeletal muscle. J. Biol. Chem. 1989, 264, 20106–20111. [Google Scholar] [CrossRef]
- Beckmann, J.S.; Richard, I.; Hillaire, D.; Broux, O.; Antignac, C.; Bois, E.; Cann, H.; Cottingham, R.W., Jr.; Feingold, N.; Feingold, J.; et al. A gene for limb-girdle muscular dystrophy maps to chromosome 15 by linkage. Comptes Rendus Acad. Sci. III 1991, 312, 141–148. [Google Scholar]
- Richard, I.; Broux, O.; Allamand, V.; Fougerousse, F.; Chiannilkulchai, N.; Bourg, N.; Brenguier, L.; Devaud, C.; Pasturaud, P.; Roudaut, C.; et al. Mutations in the proteolytic enzyme calpain 3 cause limb-girdle muscular dystrophy type 2A. Cell 1995, 81, 27–40. [Google Scholar] [CrossRef]
- Sorimachi, H.; Ohmi, S.; Emori, Y.; Kawasaki, H.; Saido, T.C.; Ohno, S.; Minami, Y.; Suzuki, K. A novel member of the calcium-dependent cysteine protease family. Biol. Chem. Hoppe Seyler 1990, 371, 171–176. [Google Scholar]
- Partha, S.K.; Ravulapalli, R.; Allingham, J.S.; Campbell, R.L.; Davies, P.L. Crystal structure of calpain-3 penta-EF-hand (PEF) domain—A homodimerized PEF family member with calcium bound at the fifth EF-hand. FEBS J. 2014, 281, 3138–3149. [Google Scholar] [CrossRef]
- Sorimachi, H.; Toyama-Sorimachi, N.; Saido, T.C.; Kawasaki, H.; Sugita, H.; Miyasaka, M.; Arahata, K.; Ishiura, S.; Suzuki, K. Muscle-specific calpain, p94, is degraded by autolysis immediately after translation, resulting in disappearance from muscle. J. Biol. Chem. 1993, 268, 10593–10605. [Google Scholar] [CrossRef]
- Kinbara, K.; Sorimachi, H.; Ishiura, S.; Suzuki, K. Skeletal muscle-specific calpain, p49: Structure and physiological function. Biochem. Pharmacol. 1998, 56, 415–420. [Google Scholar]
- Chen, L.; Tang, F.; Gao, H.; Zhang, X.; Li, X.; Xiao, D. CAPN3: A muscle-specific calpain with an important role in the pathogenesis of diseases (Review). Int. J. Mol. Med. 2021, 48, 203. [Google Scholar] [CrossRef]
- Hata, S.; Doi, N.; Shinkai-Ouchi, F.; Ono, Y. A muscle-specific calpain, CAPN3, forms a homotrimer. Biochim. Biophys. Acta Proteins Proteom. 2020, 1868, 140411. [Google Scholar] [CrossRef]
- Ye, Q.; Henrickson, A.; Demeler, B.; Serrão, V.H.B.; Davies, P.L. Human calpain-3 and its structural plasticity: Dissociation of a homohexamer into dimers on binding titin. bioRxiv 2024. [Google Scholar] [CrossRef]
- Zhong, H.; Zheng, Y.; Zhao, Z.; Lin, P.; Xi, J.; Zhu, W.; Lin, J.; Lu, J.; Yu, M.; Zhang, W.; et al. Molecular landscape of CAPN3 mutations in limb-girdle muscular dystrophy type R1: From a Chinese multicentre analysis to a worldwide perspective. J. Med. Genet. 2021, 58, 729–736. [Google Scholar] [CrossRef]
- Anderson, L.V.; Davison, K.; Moss, J.A.; Richard, I.; Fardeau, M.; Tomé, F.M.; Hübner, C.; Lasa, A.; Colomer, J.; Beckmann, J.S. Characterization of monoclonal antibodies to calpain 3 and protein expression in muscle from patients with limb-girdle muscular dystrophy type 2A. Am. J. Pathol. 1998, 153, 1169–1179. [Google Scholar] [CrossRef]
- Fanin, M.; Nascimbeni, A.C.; Angelini, C. Screening of calpain-3 autolytic activity in LGMD muscle: A functional map of CAPN3 gene mutations. J. Med. Genet. 2007, 44, 38–43. [Google Scholar] [CrossRef]
- Groen, E.J.; Charlton, R.; Barresi, R.; Anderson, L.V.; Eagle, M.; Hudson, J.; Koref, M.S.; Straub, V.; Bushby, K.M. Analysis of the UK diagnostic strategy for limb girdle muscular dystrophy 2A. Brain 2007, 130 Pt 12, 3237–3249. [Google Scholar] [CrossRef]
- Vissing, J.; Barresi, R.; Witting, N.; Van Ghelue, M.; Gammelgaard, L.; Bindoff, L.A.; Straub, V.; Lochmüller, H.; Hudson, J.; Wahl, C.M.; et al. A heterozygous 21-bp deletion in CAPN3 causes dominantly inherited limb girdle muscular dystrophy. Brain 2016, 139 Pt 8, 2154–2163. [Google Scholar] [CrossRef]
- Martinez-Thompson, J.M.; Niu, Z.; Tracy, J.A.; Moore, S.A.; Swenson, A.; Wieben, E.D.; Milone, M. Autosomal dominant calpainopathy due to heterozygous CAPN3 C.643_663del21. Muscle Nerve 2018, 57, 679–683. [Google Scholar]
- Nallamilli, B.R.R.; Chakravorty, S.; Kesari, A.; Tanner, A.; Ankala, A.; Schneider, T.; da Silva, C.; Beadling, R.; Alexander, J.J.; Askree, S.H.; et al. Genetic landscape and novel disease mechanisms from a large LGMD cohort of 4656 patients. Ann. Clin. Transl. Neurol. 2018, 5, 1574–1587. [Google Scholar] [CrossRef]
- Cerino, M.; Campana-Salort, E.; Salvi, A.; Cintas, P.; Renard, D.; Juntas Morales, R.; Tard, C.; Leturcq, F.; Stojkovic, T.; Bonello-Palot, N.; et al. Novel CAPN3 variant associated with an autosomal dominant calpainopathy. Neuropathol. Appl. Neurobiol. 2020, 46, 564–578. [Google Scholar] [CrossRef]
- Vissing, J.; Dahlqvist, J.R.; Roudaut, C.; Poupiot, J.; Richard, I.; Duno, M.; Krag, T. A single c.1715G>C calpain 3 gene variant causes dominant calpainopathy with loss of calpain 3 expression and activity. Hum. Mutat. 2020, 41, 1507–1513. [Google Scholar] [CrossRef]
- González-Mera, L.; Ravenscroft, G.; Cabrera-Serrano, M.; Ermolova, N.; Domínguez-González, C.; Arteche-López, A.; Soltanzadeh, P.; Evesson, F.; Navas, C.; Mavillard, F.; et al. Heterozygous CAPN3 missense variants causing autosomal-dominant calpainopathy in seven unrelated families. Neuropathol. Appl. Neurobiol. 2021, 47, 283–296. [Google Scholar] [CrossRef]
- Mao, B.; Yang, J.; Zhao, X.; Jia, X.; Shi, X.; Zhao, L.; Banerjee, S.; Zhang, L.; Ma, X. Identification and functional characterization of a novel heterozygous splice-site mutation in the calpain 3 gene causes rare autosomal dominant limb-girdle muscular dystrophy. Exp. Ther. Med. 2024, 27, 97. [Google Scholar] [CrossRef]
- Krag, T.; Nasho, E.; Brady, L.; Verebi, C.; Leturcq, F.; Malfatti, E.; Duno, M.; Tarnopolsky, M.; Vissing, J. Variants in CAPN3 Causing Autosomal Dominant Limb-Girdle Muscular Dystrophy Combined With Calpain-3 Deficiency. Hum. Mutat. 2025, 2025, 9301465. [Google Scholar] [CrossRef]
- Massucco, S.; Fossa, P.; Fiorillo, C.; Faedo, E.; Gemelli, C.; Barresi, R.; Ripolone, M.; Patrone, S.; Gaudio, A.; Mandich, P.; et al. Case report: A single novel calpain 3 gene variant associated with mild myopathy. Front. Genet. 2024, 15, 1437859. [Google Scholar] [CrossRef]
- Ono, Y.; Ojima, K.; Shinkai-Ouchi, F.; Hata, S.; Sorimachi, H. An eccentric calpain, CAPN3/p94/calpain-3. Biochimie 2016, 122, 169–187. [Google Scholar] [CrossRef]
- Sáenz, A.; Leturcq, F.; Cobo, A.M.; Poza, J.J.; Ferrer, X.; Otaegui, D.; Camaño, P.; Urtasun, M.; Vílchez, J.; Gutiérrez-Rivas, E.; et al. LGMD2A: Genotype-phenotype correlations based on a large mutational survey on the calpain 3 gene. Brain 2005, 128, 732–742. [Google Scholar] [CrossRef]
- Fanin, M.; Fulizio, L.; Nascimbeni, A.C.; Spinazzi, M.; Piluso, G.; Ventriglia, V.M.; Ruzza, G.; Siciliano, G.; Trevisan, C.; Politano, L.; et al. Molecular diagnosis in LGMD2A: Mutation analysis or protein testing? Hum. Mutat. 2004, 24, 52–62. [Google Scholar] [CrossRef]
- Todorova, A.; Georgieva, B.; Tournev, I.; Todorov, T.; Bogdanova, N.; Mitev, V.; Mueller, C.R.; Kremensky, I.; Horst, J. A large deletion and novel point mutations in the calpain 3 gene (CAPN3) in Bulgarian LGMD2A patients. Neurogenetics 2007, 8, 225–229. [Google Scholar] [CrossRef]
- Guglieri, M.; Magri, F.; D’Angelo, M.G.; Prelle, A.; Morandi, L.; Rodolico, C.; Cagliani, R.; Mora, M.; Fortunato, F.; Bordoni, A.; et al. Clinical, molecular, and protein correlations in a large sample of genetically diagnosed Italian limb girdle muscular dystrophy patients. Hum. Mutat. 2008, 29, 258–266. [Google Scholar] [CrossRef]
- Perez, F.; Vital, A.; Martin-Negrier, M.L.; Ferrer, X.; Sole, G. Diagnostic procedure of limb girdle muscular dystrophies 2A or calpainopathies: French cohort from a neuromuscular center (Bordeaux). Rev. Neurol. 2010, 166, 502–508. [Google Scholar] [CrossRef]
- Rubegni, A.; Malandrini, A.; Dosi, C.; Astrea, G.; Baldacci, J.; Battisti, C.; Bertocci, G.; Donati, M.A.; Dotti, M.T.; Federico, A.; et al. Next-generation sequencing approach to hyperCKemia: A 2-year cohort study. Neurol. Genet. 2019, 5, e352. [Google Scholar] [CrossRef]
- Bevilacqua, J.A.; Guecaimburu Ehuletche, M.D.R.; Perna, A.; Dubrovsky, A.; Franca, M.C.; Vargas, S.; Hegde, M.; Claeys, K.G.; Straub, V.; Daba, N.; et al. The Latin American experience with a next generation sequencing genetic panel for recessive limb-girdle muscular weakness and Pompe disease. Orphanet J. Rare Dis. 2020, 15, 11. [Google Scholar] [CrossRef]
- Gonzalez-Quereda, L.; Rodriguez, M.J.; Diaz-Manera, J.; Alonso-Perez, J.; Gallardo, E.; Nascimento, A.; Ortez, C.; Benito, D.N.-D.; Olive, M.; Gonzalez-Mera, L.; et al. Targeted Next-Generation Sequencing in a Large Cohort of Genetically Undiagnosed Patients with Neuromuscular Disorders in Spain. Genes 2020, 11, 539. [Google Scholar] [CrossRef]
- Töpf, A.; Johnson, K.; Bates, A.; Phillips, L.; Chao, K.R.; England, E.M.; Laricchia, K.M.; Mullen, T.; Valkanas, E.; Xu, L.; et al. Sequential targeted exome sequencing of 1001 patients affected by unexplained limb-girdle weakness. Genet. Med. 2020, 22, 1478–1488. [Google Scholar]
- Macias, A.; Fichna, J.P.; Topolewska, M.; Rȩdowicz, M.J.; Kaminska, A.M.; Kostera-Pruszczyk, A. Targeted Next-Generation Sequencing Reveals Mutations in Non-coding Regions and Potential Regulatory Sequences of Calpain-3 Gene in Polish Limb-Girdle Muscular Dystrophy Patients. Front. Neurosci. 2021, 15, 692482. [Google Scholar] [CrossRef]
- Ozyilmaz, B.; Kirbiyik, O.; Ozdemir, T.R.; Ozer, O.K.; Kutbay, Y.B.; Erdogan, K.M.; Guvenc, M.S.; Arıkan, Ş.; Turk, T.S.; Kale, M.Y.; et al. Experiences in the molecular genetic and histopathological evaluation of calpainopathies. Neurogenetics 2022, 23, 103–114. [Google Scholar] [CrossRef]
- Çavdarlı, B.; Köken, Ö.; Satılmış, S.B.A.; Bilen, Ş.; Ardıçlı, D.; Ceylan, A.C.; Gündüz, C.N.S.; Topaloğlu, H. High diagnostic yield of targeted next-generation sequencing panel as a first-tier molecular test for the patients with myopathy or muscular dystrophy. Ann. Hum. Genet. 2023, 87, 104–114. [Google Scholar] [CrossRef]
- Hanna, R.A.; Campbell, R.L.; Davies, P.L. Calcium-bound structure of calpain and its mechanism of inhibition by calpastatin. Nature 2008, 456, 409–412. [Google Scholar] [CrossRef]
- Fanin, M.; Nascimbeni, A.C.; Aurino, S.; Tasca, E.; Pegoraro, E.; Nigro, V.; Angelini, C. Frequency of LGMD gene mutations in Italian patients with distinct clinical phenotypes. Neurology 2009, 72, 1432–1435. [Google Scholar] [CrossRef]
- Aguti, S.; Gallus, G.N.; Bianchi, S.; Salvatore, S.; Rubegni, A.; Berti, G.; Formichi, P.; De Stefano, N.; Malandrini, A.; Lopergolo, D. Novel Biomarkers for Limb Girdle Muscular Dystrophy (LGMD). Cells 2024, 13, 329. [Google Scholar] [CrossRef]
- Piluso, G.; Politano, L.; Aurino, S.; Fanin, M.; Ricci, E.; Ventriglia, V.M.; Belsito, A.; Totaro, A.; Saccone, V.; Topaloglu, H.; et al. Extensive scanning of the calpain-3 gene broadens the spectrum of LGMD2A phenotypes. J. Med. Genet. 2005, 42, 686–693. [Google Scholar] [CrossRef]
- Fanin, M.; Nascimbeni, A.C.; Fulizio, L.; Trevisan, C.P.; Meznaric-Petrusa, M.; Angelini, C. Loss of calpain-3 autocatalytic activity in LGMD2A patients with normal protein expression. Am. J. Pathol. 2003, 163, 1929–1936. [Google Scholar] [CrossRef]
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E.; et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015, 17, 405–424. [Google Scholar] [CrossRef]
- Bruno, C.; Cassandrini, D.; Martinuzzi, A.; Toscano, A.; Moggio, M.; Morandi, L.; Servidei, S.; Mongini, T.; Angelini, C.; Musumeci, O.; et al. McArdle disease: The mutation spectrum of PYGM in a large Italian cohort. Hum. Mutat. 2006, 27, 718. [Google Scholar] [CrossRef]
- Anderson, L.V.; Davison, K. Multiplex Western blotting system for the analysis of muscular dystrophy proteins. Am. J. Pathol. 1999, 154, 1017–1022. [Google Scholar] [CrossRef]
- Ermolova, N.; Kramerova, I.; Spencer, M.J. Autolytic activation of calpain 3 proteinase is facilitated by calmodulin protein. J. Biol. Chem. 2015, 290, 996–1004. [Google Scholar] [CrossRef]
- Bordoli, L.; Kiefer, F.; Arnold, K.; Benkert, P.; Battey, J.; Schwede, T. Protein structure homology modeling using SWISS-MODEL workspace. Nat. Protoc. 2009, 4, 1–13. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera--a visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef]
- Krebs, W.G.; Gerstein, M. The morph server: A standardized system for analyzing and visualizing macromolecular motions in a database framework. Nucleic Acids Res. 2000, 28, 1665–1675. [Google Scholar] [CrossRef]
| Patient | Sex | Onset (Years) | CK | First Symptoms | Neurological Evaluation | MRI/CT Scan | Biopsy | WB Calpain-3 |
|---|---|---|---|---|---|---|---|---|
| P1 | M | 46 | 4× | Asymptomatic | Unremarkable | Unremarkable | Myopathic | NP |
| P2 | F | 49 | Normal | Waddling gait | Progressive pLL > pUL weakness | NP | Myopathic | Normal |
| P3 | F | 56 | Normal | pLL > pUL | Progressive mild pLL weakness and bent spine | Fibro-fatty replacement dorsal and lumbar paraspinal, gluteal and biceps femoris | Myopathic | Reduced |
| P4 | M | 50 | 4× | Stepping and waddling gait | Progressive dLL > pLL + dUL | Unremarkable | Myopathic | Reduced |
| P5 | M | / | 2×–4× | Myalgia | Unremarkable | Unremarkable | NP | NP |
| P6 | M | 6 | Normal | Myalgia and exercise intolerance | Unremarkable | NP | NP | NP |
| P7 | M | 40 | 5× | Difficulty in walking and frequent falls | Severe pLL > dLL + pUL + axial weakness; dysphagia and respiratory involvement | Diffuse fibro-fatty replacement | Myopathic | Normal |
| P8 | M | 60 | 5× | Myalgia and fatigue | Mild pLL weakness, bilateral pes cavus (R > L) | Diffuse fibro-fatty replacement postero-lateral compartment tight and calf | Myopathic | Normal |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Este, G.; Giorgetti, A.; Cassandrini, D.; Magri, F.; Ronchi, D.; Rubegni, A.; Lopergolo, D.; Malandrini, A.; Merlini, L.; Vattemi, G.; et al. Recurrent CAPN3 p.Asp753Asn Variant Supports a Potential Dominant Calpainopathy with Variable Clinical Expressivity. Int. J. Mol. Sci. 2025, 26, 11384. https://doi.org/10.3390/ijms262311384
D’Este G, Giorgetti A, Cassandrini D, Magri F, Ronchi D, Rubegni A, Lopergolo D, Malandrini A, Merlini L, Vattemi G, et al. Recurrent CAPN3 p.Asp753Asn Variant Supports a Potential Dominant Calpainopathy with Variable Clinical Expressivity. International Journal of Molecular Sciences. 2025; 26(23):11384. https://doi.org/10.3390/ijms262311384
Chicago/Turabian StyleD’Este, Giorgia, Alejandro Giorgetti, Denise Cassandrini, Francesca Magri, Dario Ronchi, Anna Rubegni, Diego Lopergolo, Alessandro Malandrini, Luciano Merlini, Gaetano Vattemi, and et al. 2025. "Recurrent CAPN3 p.Asp753Asn Variant Supports a Potential Dominant Calpainopathy with Variable Clinical Expressivity" International Journal of Molecular Sciences 26, no. 23: 11384. https://doi.org/10.3390/ijms262311384
APA StyleD’Este, G., Giorgetti, A., Cassandrini, D., Magri, F., Ronchi, D., Rubegni, A., Lopergolo, D., Malandrini, A., Merlini, L., Vattemi, G., Tonin, P., & Barresi, R. (2025). Recurrent CAPN3 p.Asp753Asn Variant Supports a Potential Dominant Calpainopathy with Variable Clinical Expressivity. International Journal of Molecular Sciences, 26(23), 11384. https://doi.org/10.3390/ijms262311384






