The Mitigating Effect and Mechanism of Polydeoxyribonucleotide Against Zoledronic Acid-Induced Growth Suppression of Human Gingival Fibroblasts
Abstract
1. Introduction
2. Results
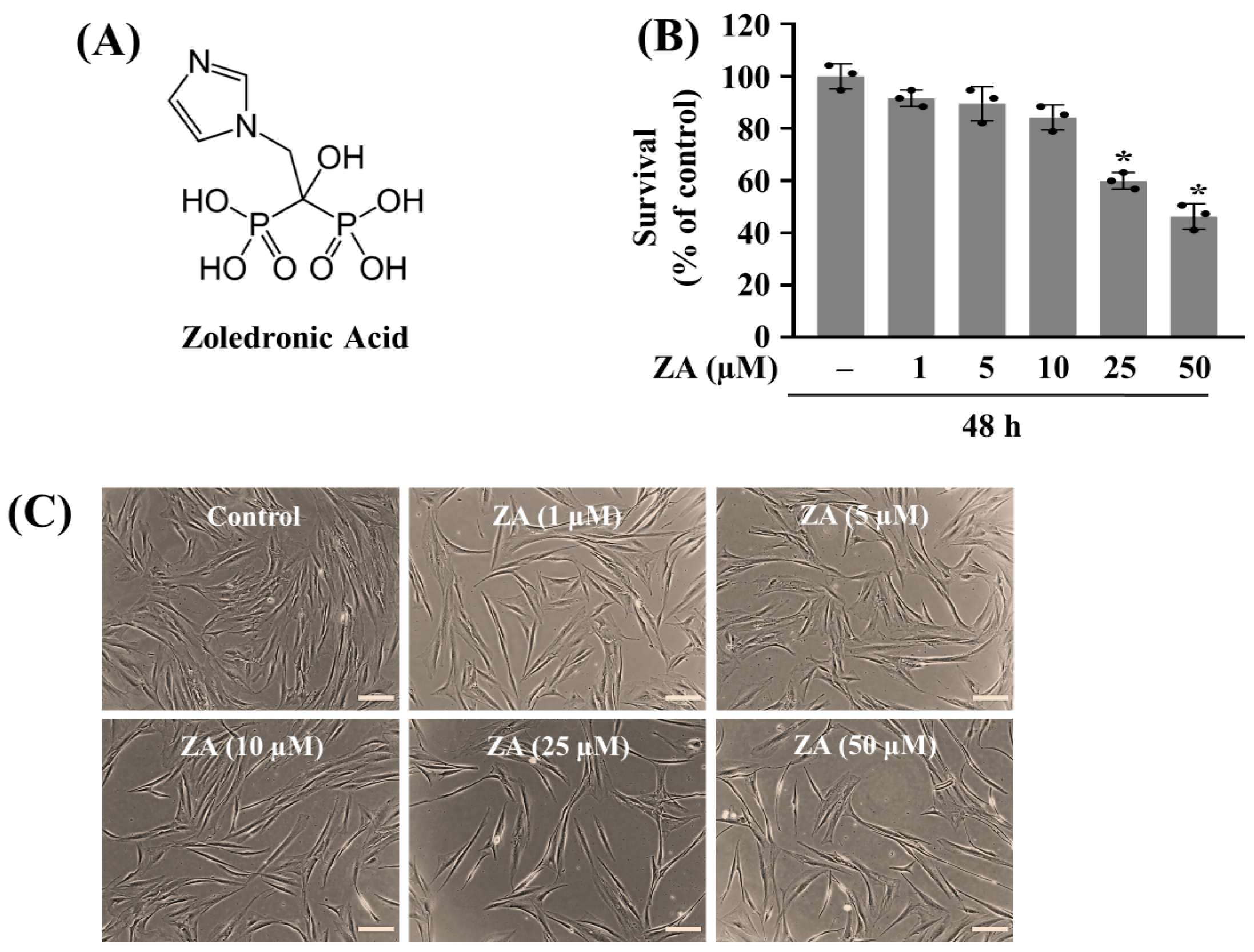
2.1. Treatment with ZA at 50 µM Markedly Reduces the Growth of HGF-1 Cells
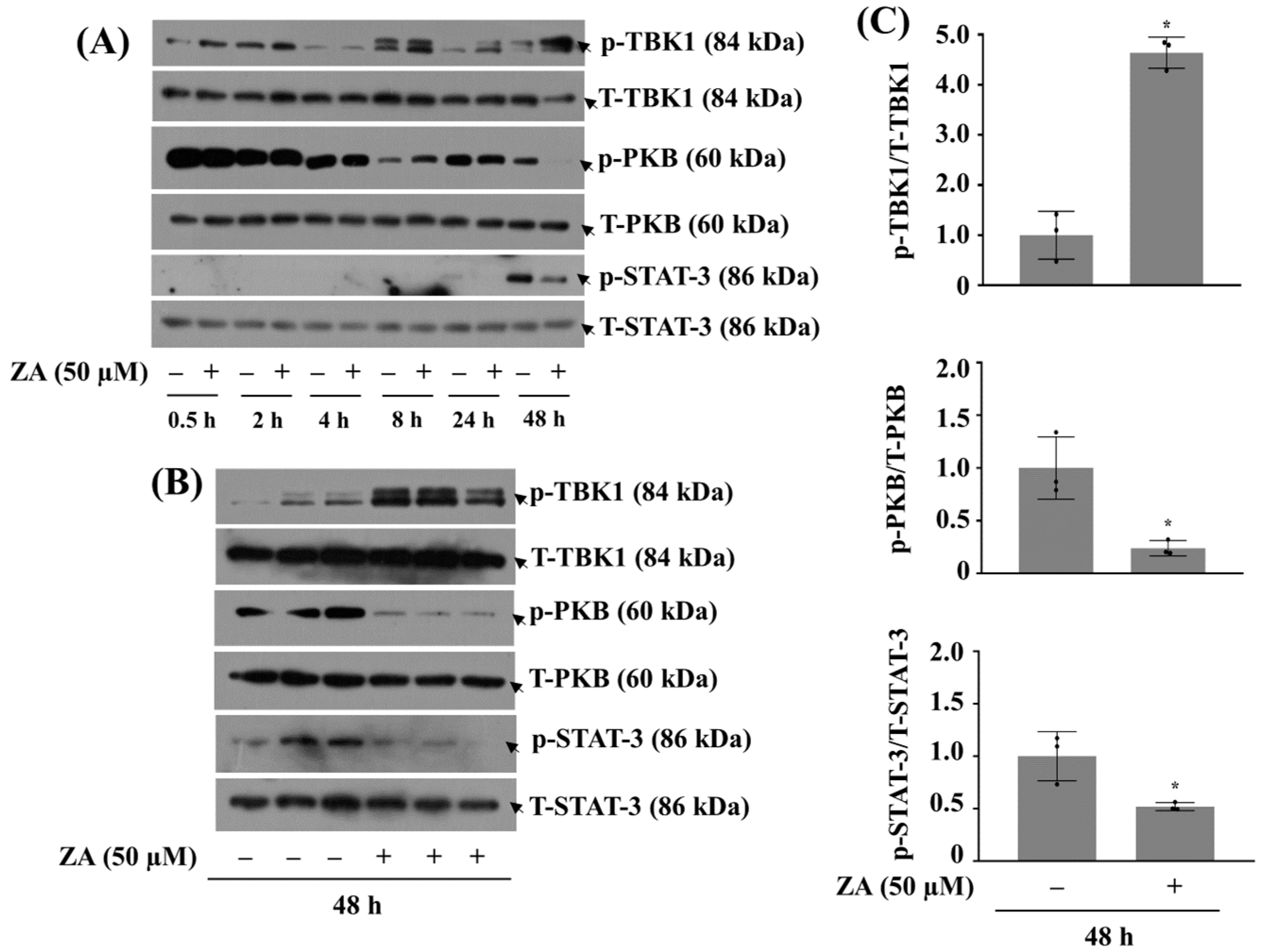
2.2. Treatment with ZA at 50 µM Significantly Decreases Phosphorylation of PKB and STAT-3 While Increasing That of TBK1 in HGF-1 Cells
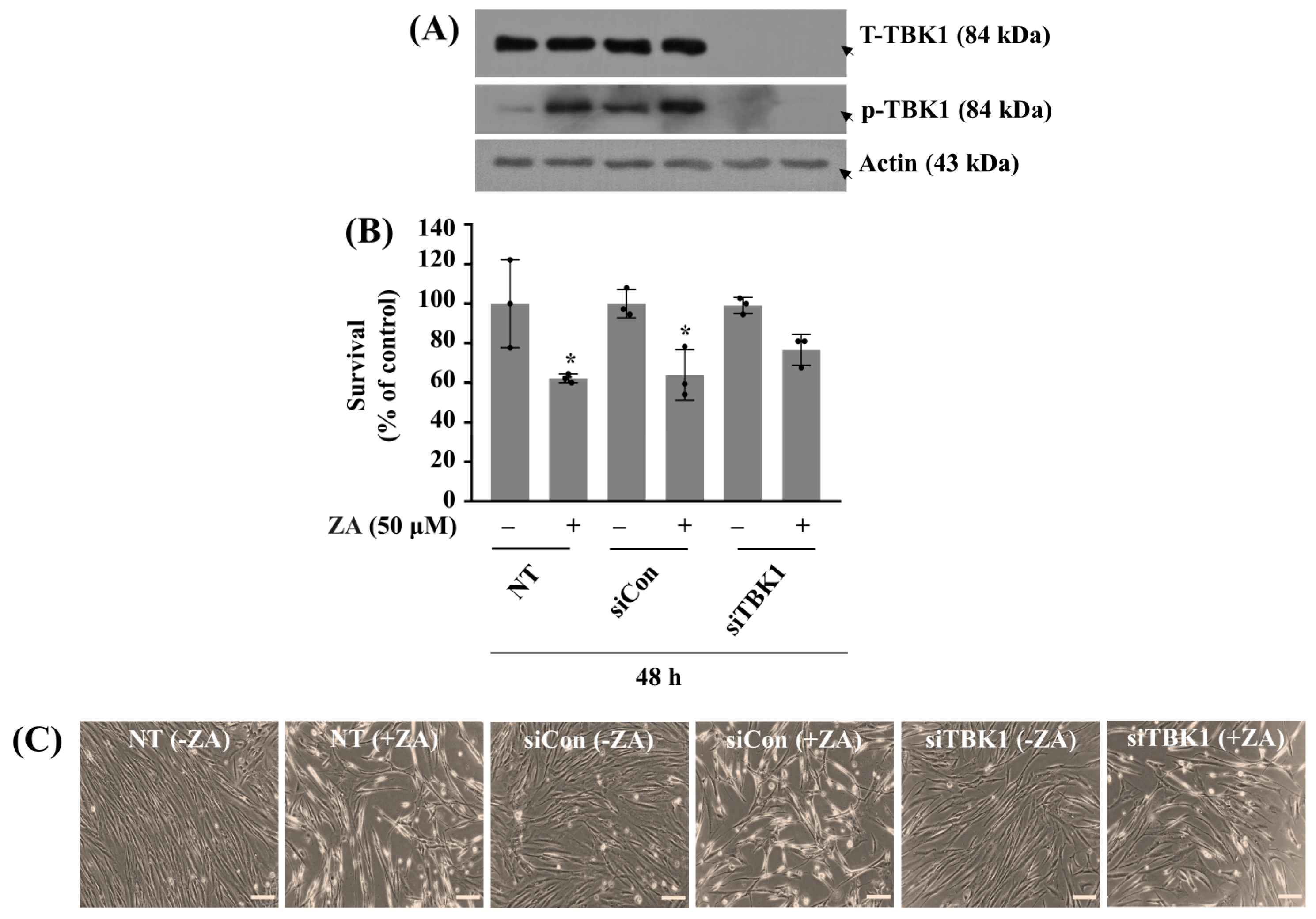
2.3. Silencing of TBK1 Partially Blocks ZA-Induced HGF-1 Cytotoxicity
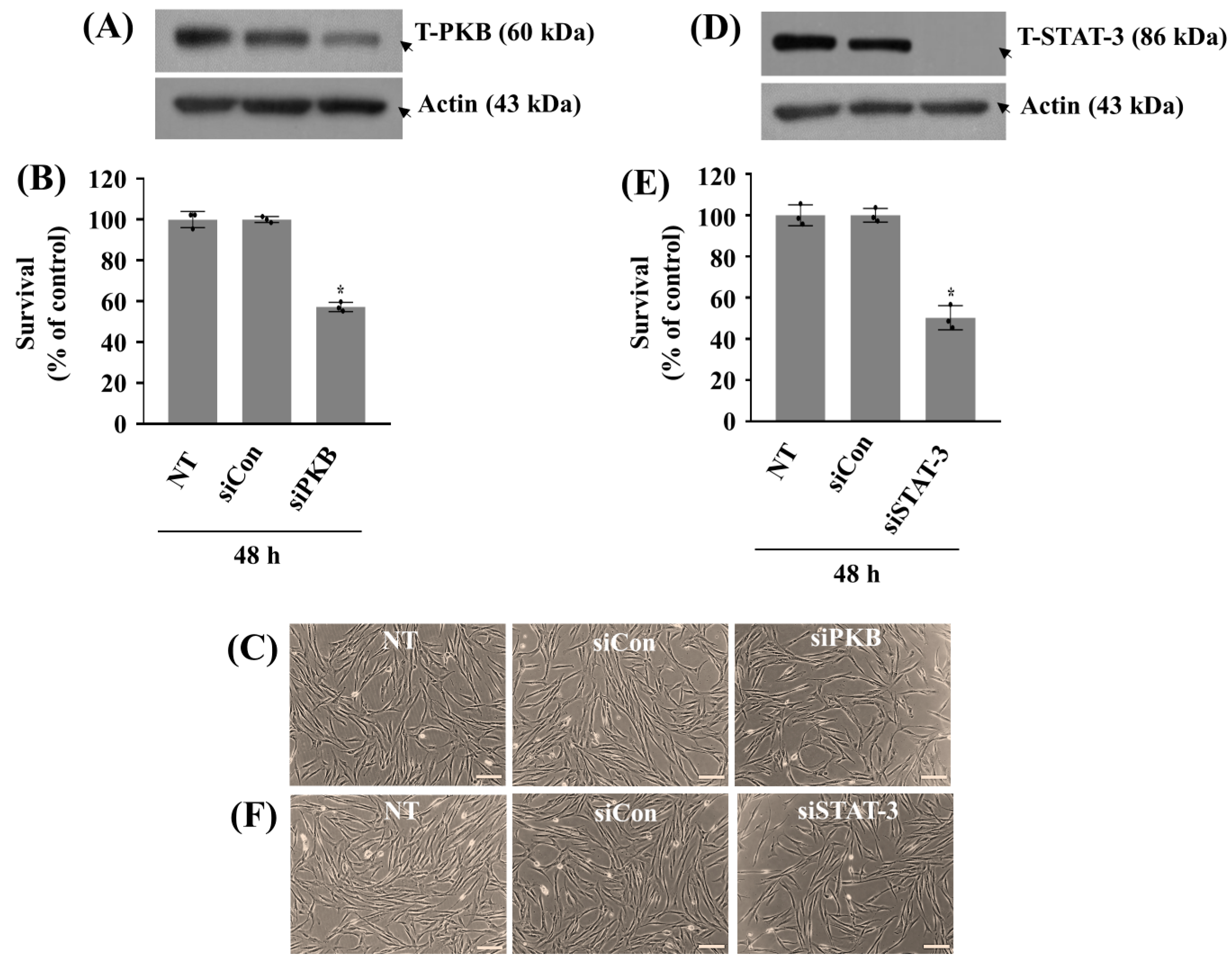
2.4. Knockdown of PKB or STAT-3 Further Reduces the Growth of HGF-1 Cells in the Absence of ZA
2.5. Co-Treatment with PDRN at 100 µg/mL Effectively Blocks ZA-Induced Growth Inhibition and Leads to Alterations of the Increased TBK1 or Decreased PKB Phosphorylation in HGF-1 Cells
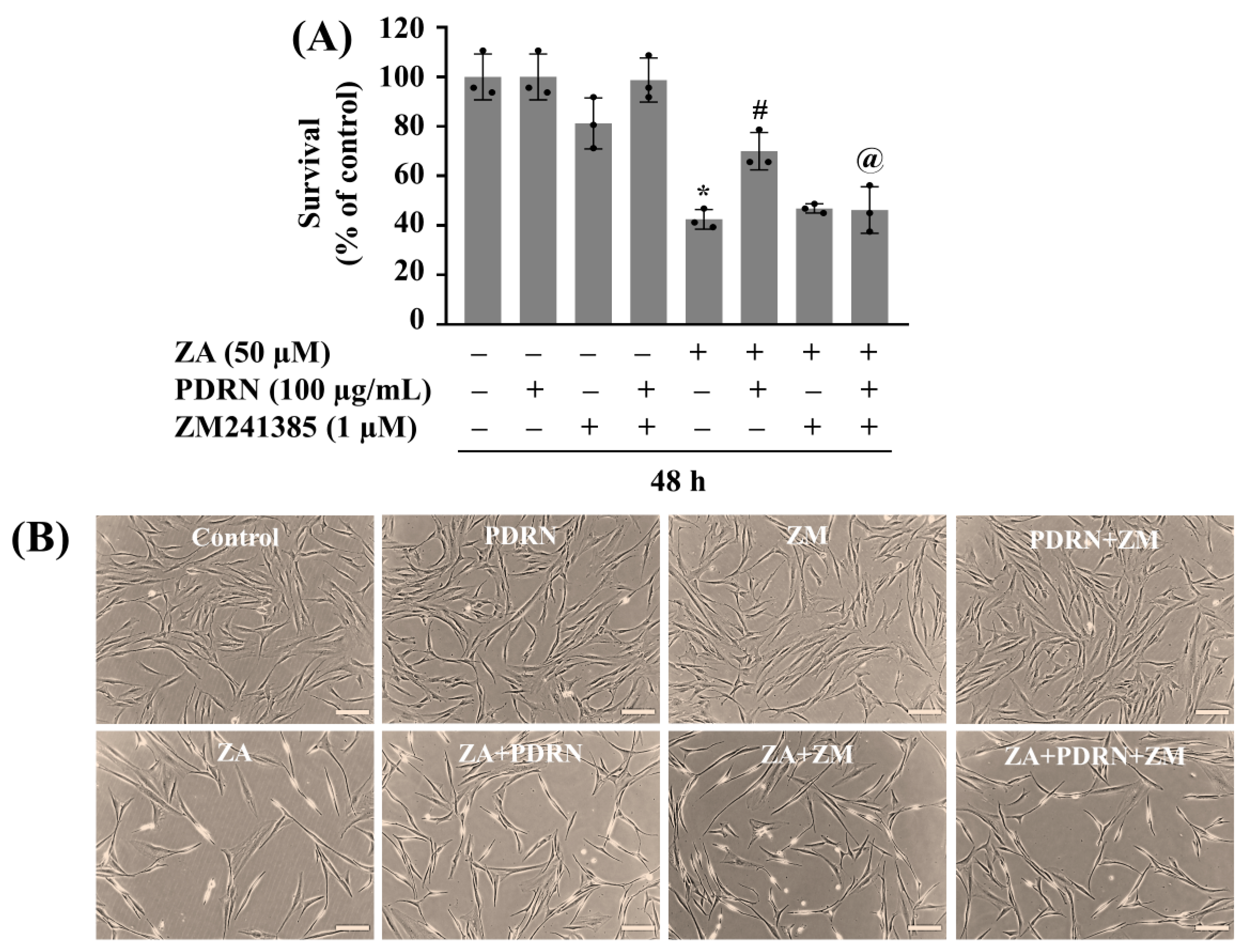
2.6. PDRN’s Protective Action Against ZA-Induced Growth Suppression of HGF-1 Cells Is at Least Partly Mediated by A2AR
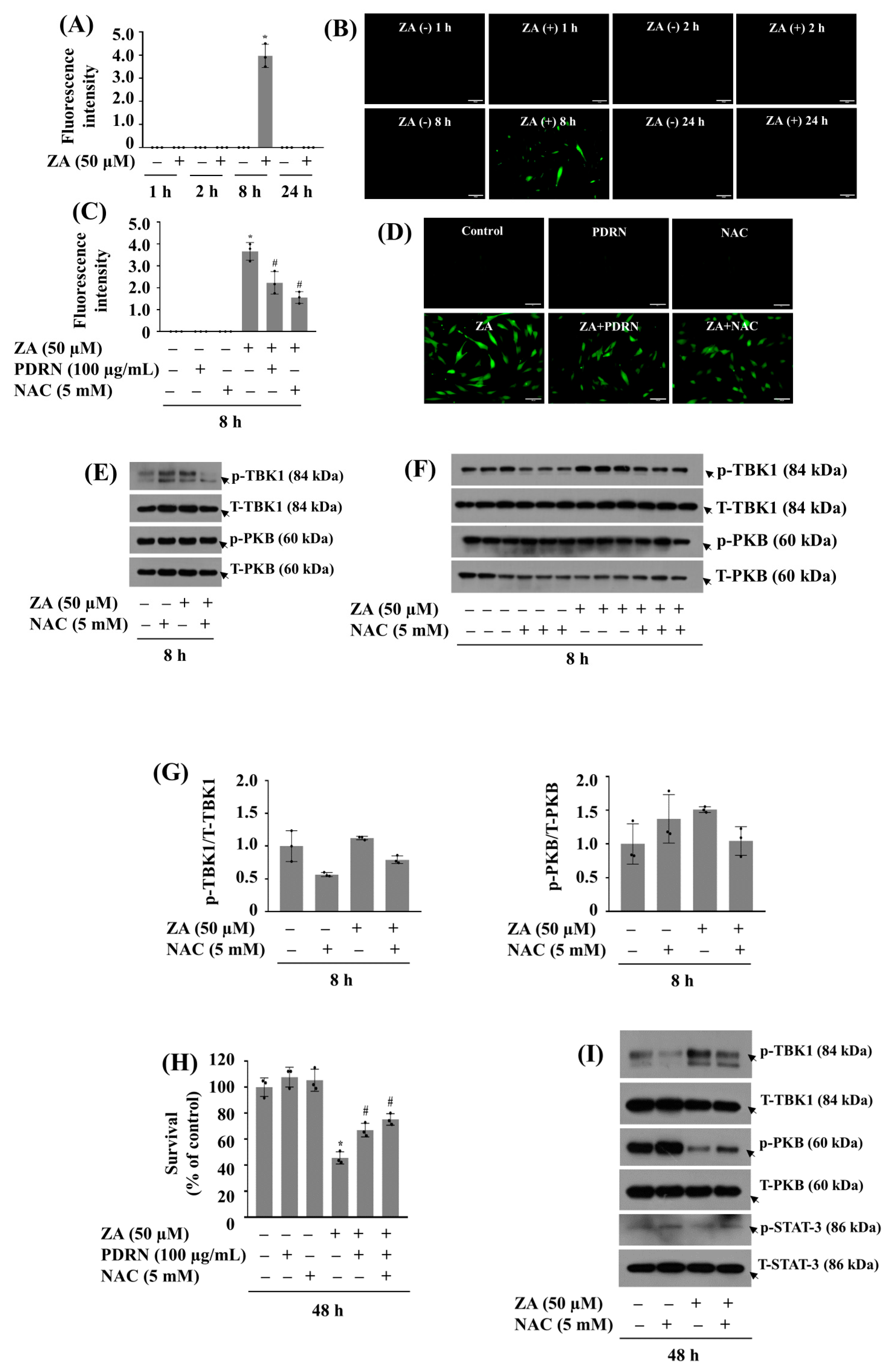
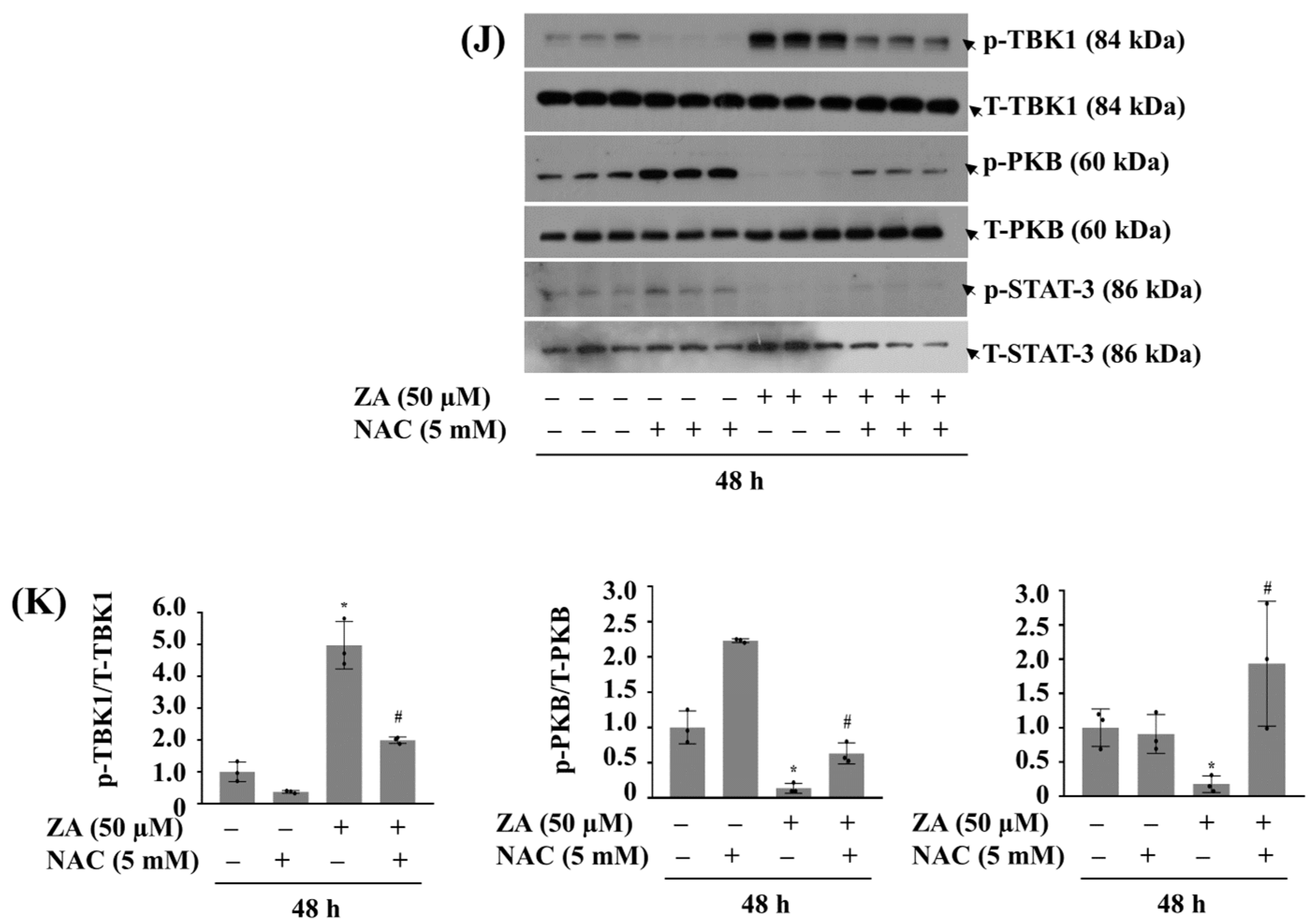
2.7. PDRN Effectively Reduces ZA-Induced ROS Production and Mitigates Growth Suppression of HGF-1 Cells
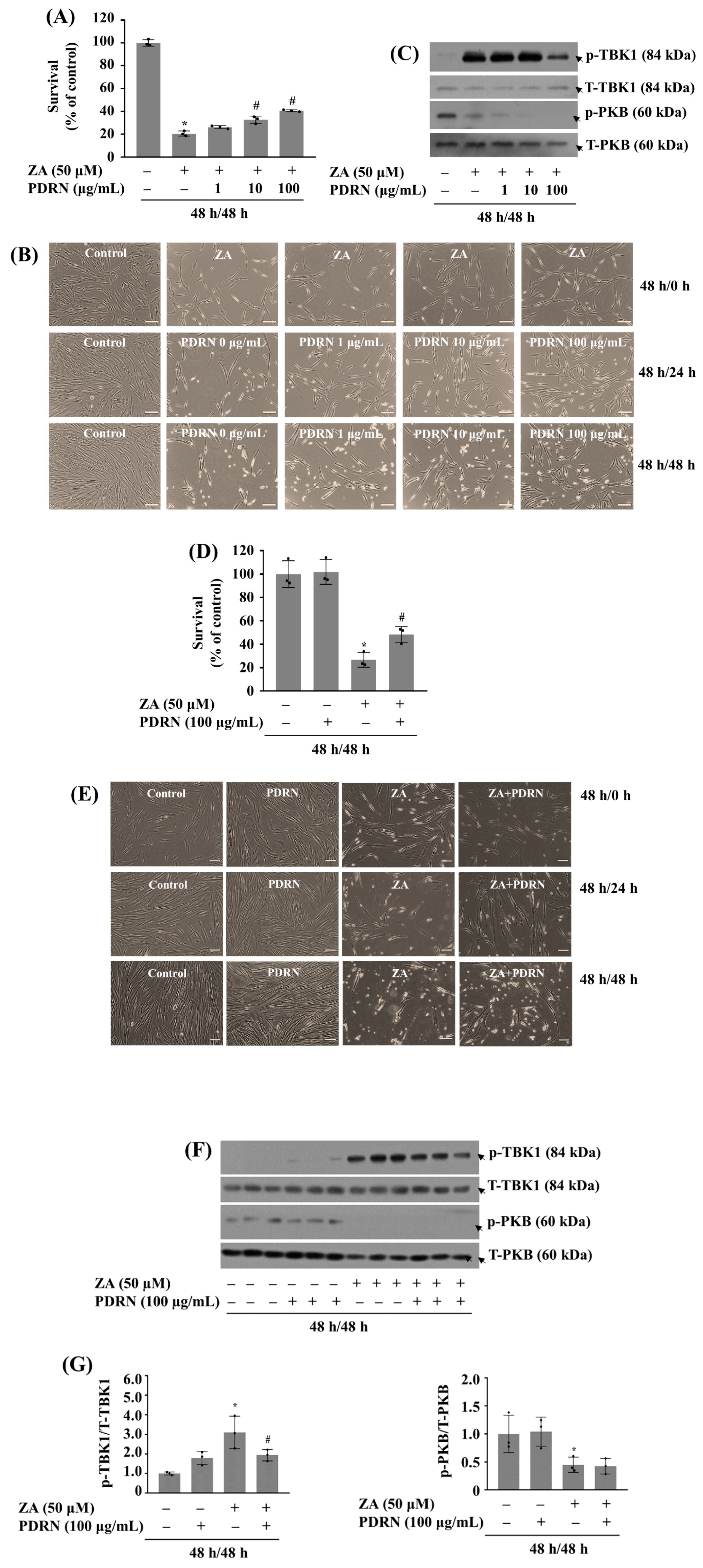
2.8. 48 H Post-Treatment of PDRN Mitigates the Growth Inhibition of HGF-1 Cells and TBK1 Phosphorylation Induced by 48 H (Pre)Treatment with ZA
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Cell Culture
5.2. Materials
5.3. Cell Count Analysis
5.4. Preparation of Whole-Cell Lysates
5.5. Western Blot Analysis
5.6. siRNA Transfection
5.7. Measurement of Intracellular ROS
5.8. Apoptosis Detection by FITC Annexin V with 7-AAD and Flow Cytometry
5.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Reyes, C.; Hitz, M.; Prieto-Alhambra, D.; Abrahamsen, B. Risks and benefits of bisphosphonate therapies. J. Cell. Biochem. 2016, 117, 20–28. [Google Scholar] [CrossRef]
- Black, D.M.; Rosen, C.J. Postmenopausal osteoporosis. N. Engl. J. Med. 2016, 374, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Ruggiero, S.L.; Dodson, T.B.; Fantasia, J.; Goodday, R.; Aghaloo, T.; Mehrotra, B.; O’Ryan, F. American Association of Oral and Maxillofacial Surgeons Position Paper on Medication-Related Osteonecrosis of the Jaw—2014 Update. J. Oral Maxillofac. Surg. 2014, 72, 1938–1956, Erratum in J. Oral Maxillofac. Surg. 2015, 73, 1440; J. Oral Maxillofac. Surg. 2015, 73, 1879. [Google Scholar] [CrossRef] [PubMed]
- Hemmi, T.; Yusa, K.; Ishikawa, S.; Takano, H.; Fukuda, M.; Iino, M. Synergistic effect of zoledronate and compressive force suppresses proliferation and differentiation of human gingival fibroblasts. Br. J. Oral Maxillofac. Surg. 2024, 62, 63–70. [Google Scholar] [CrossRef]
- Zafar, S. Effects of Bisphosphonate on Cells Extracted from Oral Tissues; University of Otago: Dunedin, New Zealand, 2014. [Google Scholar]
- Ro, S.T.; Chae, Y.K.; Lee, K.E.; Kim, M.S.; Nam, O.H.; Lee, H.; Choi, S.C. Effect of Polydeoxyribonucleotide on Human Periodontal Ligament Cells as a Storage Medium for Avulsed Tooth. J. Korean Acad. Pediatr. Dent. 2023, 50, 347–359. [Google Scholar] [CrossRef]
- McAllister, B.S.; Leeb-Lundberg, F.; Olson, M.S. Bradykinin inhibition of EGF-and PDGF-induced DNA synthesis in human fibroblasts. Am. J. Physiol.-Cell Physiol. 1993, 265, C477–C484. [Google Scholar] [CrossRef]
- Halasi, M.; Wang, M.; Chavan, T.S.; Gaponenko, V.; Hay, N.; Gartel, A.L. ROS inhibitor N-acetyl-L-cysteine antagonizes the activity of proteasome inhibitors. Biochem. J. 2013, 454, 201–208. [Google Scholar] [CrossRef]
- Alali, Y. Does the Local Application of Teriparatide Prevent Medication-Related Osteonecrosis of the Jaw After Tooth Extraction in Rats? University of Toronto (Canada): Toronto, ON, Canada, 2020. [Google Scholar]
- Al-Attraqchi, O.H.; Attimarad, M.; Venugopala, K.N.; Nair, A.; Al-Attraqchi, N.H. Adenosine A2A receptor as a potential drug target-current status and future perspectives. Curr. Pharm. Des. 2019, 25, 2716–2740. [Google Scholar] [CrossRef]
- Khandelwal, V.; Mitrofan, L.; Hyttinen, J.; Chaudhari, K.; Buccione, R.; Kaarniranta, K.; Ravingerová, T.; Mönkkönen, J. Oxidative stress plays an important role in zoledronic acid-induced autophagy. Physiol. Res. 2014, 63, S601. [Google Scholar] [CrossRef]
- De Colli, M.; Zara, S.; di Giacomo, V.; Patruno, A.; Gallorini, M.; Sancilio, S.; Zizzari, V.; Tetè, G.; Cataldi, A. Nitric Oxide-mediated cytotoxic effect induced by zoledronic acid treatment on Human Gingival Fibroblasts. Ital. J. Anat. Embryol. 2014, 119, 1. [Google Scholar] [CrossRef]
- Kang, S.; Park, H.; Ban, J.; Chung, J.; Chun, G.; Cho, J. Effects of nicotine on apoptosis in human gingival fibroblasts. Arch. Oral Biol. 2011, 56, 1091–1097. [Google Scholar] [CrossRef]
- Huang, W. Investigating the Role of Bisphosphonates on Bone Cells and the Potential of Ionic Therapy to Restore Bone Regeneration in Osteonecrosis of the Jaw; UCL (University College London): London, UK, 2024. [Google Scholar]
- Fayard, E.; Xue, G.; Parcellier, A.; Bozulic, L.; Hemmings, B.A. Protein kinase B (PKB/Akt), a key mediator of the PI3K signaling pathway. In Phosphoinositide 3-kinase in Health and Disease; Springer: Berlin/Heidelberg, Germany, 2010; Volume 1, pp. 31–56. [Google Scholar]
- Sale, E.; Sale, G. Protein kinase B: Signalling roles and therapeutic targeting. Cell. Mol. Life Sci. 2008, 65, 113–127. [Google Scholar] [CrossRef] [PubMed]
- Song, G.; Ouyang, G.; Bao, S. The activation of Akt/PKB signaling pathway and cell survival. J. Cell. Mol. Med. 2005, 9, 59–71. [Google Scholar] [CrossRef] [PubMed]
- Manning, B.D.; Toker, A. AKT/PKB signaling: Navigating the network. Cell 2017, 169, 381–405. [Google Scholar] [CrossRef] [PubMed]
- Datta, S.R.; Dudek, H.; Tao, X.; Masters, S.; Fu, H.; Gotoh, Y.; Greenberg, M.E. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell 1997, 91, 231–241. [Google Scholar] [CrossRef]
- Brazil, D.P.; Yang, Z.-Z.; Hemmings, B.A. Advances in protein kinase B signalling: AKTion on multiple fronts. Trends Biochem. Sci. 2004, 29, 233–242. [Google Scholar] [CrossRef]
- Li, X.; Hu, S.; Cai, Y.; Liu, X.; Luo, J.; Wu, T. Revving the engine: PKB/AKT as a key regulator of cellular glucose metabolism. Front. Physiol. 2024, 14, 1320964. [Google Scholar] [CrossRef]
- Lin, Z.; Sugai, J.V.; Jin, Q.; Chandler, L.A.; Giannobile, W.V. Platelet-derived growth factor-B gene delivery sustains gingival fibroblast signal transduction. J. Periodontal Res. 2008, 43, 440–449. [Google Scholar] [CrossRef]
- Zhou, R.; Zhang, Q.; Xu, P. TBK1, a central kinase in innate immune sensing of nucleic acids and beyond. Acta Biochim. Biophys. Sin. 2020, 52, 757–767. [Google Scholar] [CrossRef]
- Zhang, Y.; Lo, K.; Wang, C.; Zhou, G.; Feng, X.; Ni, J.; Chen, X. Herpes simplex virus-induced upregulation of inflammatory cytokines in human gingival fibroblasts. Virol. J. 2024, 21, 323. [Google Scholar] [CrossRef]
- Larabi, A.; Devos, J.M.; Ng, S.-L.; Nanao, M.H.; Round, A.; Maniatis, T.; Panne, D. Crystal structure and mechanism of activation of TANK-binding kinase 1. Cell Rep. 2013, 3, 734–746. [Google Scholar] [CrossRef]
- Chen, S.; Liu, S.; Wang, J.; Wu, Q.; Wang, A.; Guan, H.; Zhang, Q.; Zhang, D.; Wang, X.; Song, H. TBK1-mediated DRP1 targeting confers nucleic acid sensing to reprogram mitochondrial dynamics and physiology. Mol. Cell 2020, 80, 810–827. [Google Scholar] [CrossRef]
- Elmanfi, S.; Yilmaz, M.; Ong, W.W.; Yeboah, K.S.; Sintim, H.O.; Gürsoy, M.; Könönen, E.; Gürsoy, U.K. Bacterial cyclic dinucleotides and the cgas–cgamp–sting pathway: A role in periodontitis? Pathogens 2021, 10, 675. [Google Scholar] [CrossRef] [PubMed]
- Açil, Y.; Arndt, M.L.; Gülses, A.; Wieker, H.; Naujokat, H.; Ayna, M.; Wiltfang, J. Cytotoxic and inflammatory effects of alendronate and zolendronate on human osteoblasts, gingival fibroblasts and osteosarcoma cells. J. Cranio-Maxillofac. Surg. 2018, 46, 538–546. [Google Scholar] [CrossRef] [PubMed]
- Yazıcı, T.; Koçer, G.; Nazıroğlu, M.; Övey, İ.S.; Öz, A. Zoledronic acid, bevacizumab and dexamethasone-induced apoptosis, mitochondrial oxidative stress, and calcium signaling are decreased in human osteoblast-like cell line by selenium treatment. Biol. Trace Elem. Res. 2018, 184, 358–368. [Google Scholar] [CrossRef] [PubMed]
- Altavilla, D.; Bitto, A.; Polito, F.; Marini, H.; Minutoli, L.; Stefano, V.D.; Irrera, N.; Cattarini, G.; Squadrito, F. Polydeoxyribonucleotide (PDRN): A safe approach to induce therapeutic angiogenesis in peripheral artery occlusive disease and in diabetic foot ulcers. Cardiovasc. Hematol. Agents Med. Chem. Former. 2009, 7, 313–321. [Google Scholar] [CrossRef]
- Picciolo, G.; Mannino, F.; Irrera, N.; Altavilla, D.; Minutoli, L.; Vaccaro, M.; Arcoraci, V.; Squadrito, V.; Picciolo, G.; Squadrito, F. PDRN, a natural bioactive compound, blunts inflammation and positively reprograms healing genes in an “in vitro” model of oral mucositis. Biomed. Pharmacother. 2021, 138, 111538. [Google Scholar] [CrossRef]
- Chae, D.; Oh, S.-W.; Choi, Y.-S.; Kang, D.-J.; Park, C.-W.; Lee, J.; Seo, W.-S. First report on microbial-derived polydeoxyribonucleotide: A sustainable and enhanced alternative to salmon-based polydeoxyribonucleotide. Curr. Issues Mol. Biol. 2025, 47, 41. [Google Scholar] [CrossRef]
- Anitua, E.; Zalduendo, M.; Troya, M.; Orive, G. PRGF exerts a cytoprotective role in zoledronic acid-treated oral cells. Clin. Oral Investig. 2016, 20, 513–521. [Google Scholar] [CrossRef]
- Rattanawonsakul, K.; Bullock, G.; Bolt, R.; Claeyssens, F.; Atkins, S.; Hearnden, V. In vitro effect of geranylgeraniol (GGOH) on bisphosphonate-induced cytotoxicity of oral mucosa cells. Front. Oral Health 2022, 3, 892615. [Google Scholar] [CrossRef]
- Galeano, M.; Pallio, G.; Irrera, N.; Mannino, F.; Bitto, A.; Altavilla, D.; Vaccaro, M.; Squadrito, G.; Arcoraci, V.; Colonna, M.R. Polydeoxyribonucleotide: A promising biological platform to accelerate impaired skin wound healing. Pharmaceuticals 2021, 14, 1103. [Google Scholar] [CrossRef]
- Goker, F.; Grecchi, E.; Grecchi, F.; Francetti, L.; Del Fabbro, M. Treatment of medication-related osteonecrosis of the jaw (MRONJ). A systematic review. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 2662–2673. [Google Scholar]
- Wan, J.T.; Sheeley, D.M.; Somerman, M.J.; Lee, J.S. Mitigating osteonecrosis of the jaw (ONJ) through preventive dental care and understanding of risk factors. Bone Res. 2020, 8, 14. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Galfo, F.; Oteri, G.; Atteritano, M.; Pallio, G.; Mannino, F.; D’Amore, A.; Pellegrino, E.; Aliquò, F. Adenosine Receptor Stimulation Improves Glucocorticoid-Induced Osteoporosis in a Rat Model. Front. Pharmacol. 2017, 8, 558, Corrigendum in: Front. Pharmacol. 2018, 9, 78. [Google Scholar]
- Park, G.Y.; Jang, B.-C. Polydeoxyribonucleotide as a Regenerative Agent in Dermatology and Wound Healing: Mechanisms, Clinical Applications, and Safety. Keimyung Med. J. 2025, 44, 9–18. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pachhapure, S.; Shin, Y.-M.; Kim, D.G.; Choi, D.-R.; Yun, J.-I.; Kim, J.-H.; Jang, B.-C. The Mitigating Effect and Mechanism of Polydeoxyribonucleotide Against Zoledronic Acid-Induced Growth Suppression of Human Gingival Fibroblasts. Int. J. Mol. Sci. 2025, 26, 11367. https://doi.org/10.3390/ijms262311367
Pachhapure S, Shin Y-M, Kim DG, Choi D-R, Yun J-I, Kim J-H, Jang B-C. The Mitigating Effect and Mechanism of Polydeoxyribonucleotide Against Zoledronic Acid-Induced Growth Suppression of Human Gingival Fibroblasts. International Journal of Molecular Sciences. 2025; 26(23):11367. https://doi.org/10.3390/ijms262311367
Chicago/Turabian StylePachhapure, Shailashree, Young-Min Shin, Duk Gyu Kim, Dong-Rak Choi, Jong-IL Yun, Jae-Hong Kim, and Byeong-Churl Jang. 2025. "The Mitigating Effect and Mechanism of Polydeoxyribonucleotide Against Zoledronic Acid-Induced Growth Suppression of Human Gingival Fibroblasts" International Journal of Molecular Sciences 26, no. 23: 11367. https://doi.org/10.3390/ijms262311367
APA StylePachhapure, S., Shin, Y.-M., Kim, D. G., Choi, D.-R., Yun, J.-I., Kim, J.-H., & Jang, B.-C. (2025). The Mitigating Effect and Mechanism of Polydeoxyribonucleotide Against Zoledronic Acid-Induced Growth Suppression of Human Gingival Fibroblasts. International Journal of Molecular Sciences, 26(23), 11367. https://doi.org/10.3390/ijms262311367



_Kim.png)



