Correlation Between Intrafollicular IL-10, Progesterone, and Bovine Oocyte Developmental Competence
Abstract
1. Introduction
2. Results
2.1. Oocyte and Follicle Characteristics and Their Impact on Developmental Competence
2.2. Relationships Between Biomarker Concentrations, Follicle Traits, and Embryo Development
2.3. Predictive Value of IL-10 for Oocyte Developmental Competence
3. Discussion
4. Materials and Methods
4.1. Ethical Statement
4.2. Animal Stimulation and Follicular Fluid/Oocyte Collection
4.3. In Vitro Embryo Production (IVEP)
4.4. Sample Processing and Biomarker Analysis
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Outcome | Model | Predictor | Coefficient (β) | 95% Confidence Interval (CI) | p-Value |
|---|---|---|---|---|---|
| D3 Success | Model 1 | IL-10 [pg/mL] | 0.0021 | 0.000–0.004 | 0.041 |
| (Cleavage) | (IL-10 only) | Intercept | −0.711 | −1.649–0.228 | 0.138 |
| Model 2 | Oocyte Grade 2 (vs. 1) | −0.79 | −2.148–0.568 | 0.254 | |
| (Grade only) | Oocyte Grade 3 (vs. 1) | −0.028 | −1.404–1.348 | 0.968 | |
| Oocyte Grade 4 (vs. 1) | −0.762 | −2.079–0.554 | 0.257 | ||
| Model 3 | IL-10 [pg/mL] | 0.0022 | 0.000–0.004 | 0.046 | |
| (Combined) | Oocyte Grade 2 (vs. 1) | −0.863 | −2.261–0.536 | 0.227 | |
| Oocyte Grade 3 (vs. 1) | −0.481 | −1.968–1.007 | 0.526 | ||
| Oocyte Grade 4 (vs. 1) | −0.889 | −2.254–0.476 | 0.202 | ||
| D7 Success | Model 1 | IL-10 [pg/mL] | 0.0022 | 0.000–0.004 | 0.034 |
| (Blastocyst) | (IL-10 only) | Intercept | −1.575 | −2.616–(−0.533) | 0.003 |
| Model 2 | Oocyte Grade 2 (vs. 1) | −1.204 | −2.650–0.242 | 0.103 | |
| (Grade only) | Oocyte Grade 3 (vs. 1) | −0.357 | −1.693–0.980 | 0.601 | |
| Oocyte Grade 4 (vs. 1) | −1.715 | −3.247–(−0.183) | 0.028 | ||
| Model 3 | IL-10 [pg/mL] | 0.0025 | 0.000–0.005 | 0.03 | |
| (Combined) | Oocyte Grade 2 (vs. 1) | −1.324 | −2.831–0.182 | 0.085 | |
| Oocyte Grade 3 (vs. 1) | −0.936 | −2.445–0.573 | 0.224 | ||
| Oocyte Grade 4 (vs. 1) | −1.954 | −3.575–(−0.333) | 0.018 |
| Outcome | Model | Predictors | Log-Likelihood | Pseudo R2 | AIC | LLR p-Value |
|---|---|---|---|---|---|---|
| D3 Success | Model 1 | IL-10 only | −45.384 | 0.047 | 94.77 | 0.033 |
| Model 2 | Grade only | −46.419 | 0.026 | 100.84 | 0.484 | |
| Model 3 | Combined | −44.297 | 0.07 | 98.59 | 0.153 | |
| D7 Success | Model 1 | IL-10 only | −42.21 | 0.053 | 88.42 | 0.029 |
| Model 2 | Grade only | −41.216 | 0.075 | 90.43 | 0.081 | |
| Model 3 | Combined | −38.685 | 0.132 | 87.37 | 0.019 |
References
- Ferre, L.B.; Kjelland, M.E.; Strobech, L.B.; Hyttel, P.; Mermillod, P.; Ross, P.J. Review: Recent advances in bovine in vitro embryo production: Reproductive biotechnology history and methods. Animal 2020, 14, 991–1004. [Google Scholar] [CrossRef]
- Pytel, A.T.; Zyzynska-Galenska, K.; Gajewski, Z.; Papis, K. Factors defining developmental competence of bovine oocytes collected for in vitro embryo productiondagger. Biol. Reprod. 2024, 111, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Luciano, A.M.; Sirard, M.A. Successful in vitro maturation of oocytes: A matter of follicular differentiation. Biol. Reprod. 2018, 98, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Lodde, V.; Modina, S.; Galbusera, C.; Franciosi, F.; Luciano, A.M. Large-scale chromatin remodeling in germinal vesicle bovine oocytes: Interplay with gap junction functionality and developmental competence. Mol. Reprod. Dev. 2007, 74, 740–749. [Google Scholar] [CrossRef] [PubMed]
- Sirard, M.A. Folliculogenesis and acquisition of oocyte competence in cows. Anim. Reprod. 2019, 16, 449–454. [Google Scholar] [CrossRef]
- Nagy, B.; Szekeres-Bartho, J.; Kovacs, G.L.; Sulyok, E.; Farkas, B.; Varnagy, A.; Vertes, V.; Kovacs, K.; Bodis, J. Key to Life: Physiological Role and Clinical Implications of Progesterone. Int. J. Mol. Sci. 2021, 22, 11039. [Google Scholar] [CrossRef]
- Costa, C.B.; Fair, T.; Seneda, M.M. Review: Environment of the ovulatory follicle: Modifications and use of biotechnologies to enhance oocyte competence and increase fertility in cattle. Animal 2023, 17 (Suppl. 1), 100866. [Google Scholar] [CrossRef]
- Abdulrahman Alrabiah, N.; Evans, A.C.O.; Fahey, A.G.; Cantwell, N.; Lonergan, P.; McCormack, J.; Browne, J.A.; Fair, T. Immunological aspects of ovarian follicle ovulation and corpus luteum formation in cattle. Reproduction 2021, 162, 209–225. [Google Scholar] [CrossRef]
- Kobayashi, T.; Oda, T.; Yoshimura, Y.; Takehara, Y.; Natori, M.; Nozawa, S. Androstenedione and progesterone concentrations in preovulatory follicular fluid correlate with successful fertilization and cleavage of human oocytes in vitro. Fertil. Steril. 1991, 56, 301–305. [Google Scholar] [CrossRef]
- Mendoza, C.; Cremades, N.; Ruiz-Requena, E.; Martinez, F.; Ortega, E.; Bernabeu, S.; Tesarik, J. Relationship between fertilization results after intracytoplasmic sperm injection, and intrafollicular steroid, pituitary hormone and cytokine concentrations. Hum. Reprod. 1999, 14, 628–635. [Google Scholar] [CrossRef]
- Lamb, J.D.; Zamah, A.M.; Shen, S.; McCulloch, C.; Cedars, M.I.; Rosen, M.P. Follicular fluid steroid hormone levels are associated with fertilization outcome after intracytoplasmic sperm injection. Fertil. Steril. 2010, 94, 952–957. [Google Scholar] [CrossRef]
- Nagy, B.; Poto, L.; Farkas, N.; Koppan, M.; Varnagy, A.; Kovacs, K.; Papp, S.; Bohonyi, N.; Bodis, J. Follicular fluid progesterone concentration is associated with fertilization outcome after IVF: A systematic review and meta-analysis. Reprod. Biomed. Online 2019, 38, 871–882. [Google Scholar] [CrossRef]
- Zhao, H.; He, X.; Zhang, X.; Shi, J.; Zhou, R.; Mai, R.; Su, Q.; Cai, G.; Huang, S.; Xu, Z.; et al. Progesterone and Androstenedione Are Important Follicular Fluid Factors Regulating Porcine Oocyte Maturation Quality. Animals 2023, 13, 1811. [Google Scholar] [CrossRef] [PubMed]
- Ben-Rafael, Z.; Meloni, F.; Strauss, J.F., 3rd; Blasco, L.; Mastroianni, L., Jr.; Flickinger, G.L. Relationships between polypronuclear fertilization and follicular fluid hormones in gonadotropin-treated women. Fertil. Steril. 1987, 47, 284–288. [Google Scholar] [CrossRef] [PubMed]
- Pizarro, B.M.; Cordeiro, A.; Reginatto, M.W.; Campos, S.P.C.; Mancebo, A.C.A.; Areas, P.C.F.; Antunes, R.A.; Souza, M.; Oliveira, K.J.; Bloise, F.F.; et al. Estradiol and Progesterone Levels are Related to Redox Status in the Follicular Fluid During in vitro Fertilization. J. Endocr. Soc. 2020, 4, bvaa064. [Google Scholar] [CrossRef] [PubMed]
- Aparicio, I.M.; Garcia-Herreros, M.; O’Shea, L.C.; Hensey, C.; Lonergan, P.; Fair, T. Expression, regulation, and function of progesterone receptors in bovine cumulus oocyte complexes during in vitro maturation. Biol. Reprod. 2011, 84, 910–921. [Google Scholar] [CrossRef]
- Stojanovic Gavrilovic, A.Z.; Cekovic, J.M.; Parandilovic, A.Z.; Nikolov, A.B.; Sazdanovic, P.S.; Velickovic, A.M.; Andjelkovic, M.V.; Sorak, M.P. IL-6 of follicular fluid and outcome of in vitro fertilization. Medicine 2022, 101, e29624. [Google Scholar] [CrossRef]
- Sabat, R.; Grutz, G.; Warszawska, K.; Kirsch, S.; Witte, E.; Wolk, K.; Geginat, J. Biology of interleukin-10. Cytokine Growth Factor Rev. 2010, 21, 331–344. [Google Scholar] [CrossRef]
- Strączyńska, P. Determination of Spatial-Molecular Parameters of Ovarian Follicles Prepared for OPU and Their Influence on Embryo Developmental Capacity in Assisted Reproductive Technology. Katedra i Oddział Kliniczny Ginekologii, Położnictwa i Ginekologii Onkologicznej. PhD Thesis, Silesian Medical University in Katowice (SUM), Katowice, Poland, 2023. [Google Scholar]
- Kumar, Y.; Prasad, S.; Khan, M.A.; Husain, S.A.; Prasad, S.; Sharma, S. Ovarian interleukin profile and pregnancy outcome in women undergoing assisted reproduction: A prospective study. Fertil. Sci. Res. 2017, 4, 93–101. [Google Scholar] [CrossRef]
- Ilhan, G.; Bacanakgil, B.H.; Vuruskan, A.K.; Eken, M.K.; Karasu, A.F.G.; Bilgic, B.E.; Kucukyurt, A.K. The effect of individual oocyte matched follicular fluid oxidant, antioxidant status, and pro- and anti-inflammatory cytokines on IVF outcomes of patients with diminished ovarian reserve. Medicine 2023, 102, e32757. [Google Scholar] [CrossRef]
- Shi, S.L.; Peng, Z.F.; Yao, G.D.; Jin, H.X.; Song, W.Y.; Yang, H.Y.; Xue, R.Y.; Sun, Y.P. Expression of CD11c+HLA-DR+dendritic cells and related cytokines in the follicular fluid might be related to pathogenesis of ovarian hyperstimulation syndrome. Int. J. Clin. Exp. Pathol. 2015, 8, 15133–15137. [Google Scholar]
- Kim, T.; Kim, Y.; Lucien, F.; Zhao, Y.; Enninga, E.A.L. Decreased gremlin 1 expression in women with BMI ≥35 kg/m2 is mediated by interleukin 10 and interleukin 1beta in the follicular fluid. F S Sci. 2020, 1, 16–26. [Google Scholar] [CrossRef]
- Mocellin, S.; Panelli, M.C.; Wang, E.; Nagorsen, D.; Marincola, F.M. The dual role of IL-10. Trends Immunol. 2003, 24, 36–43. [Google Scholar] [CrossRef]
- Saraiva, M.; Vieira, P.; O’Garra, A. Biology and therapeutic potential of interleukin-10. J. Exp. Med. 2020, 217, e20190418. [Google Scholar] [CrossRef]
- Sankiewicz, A.; Zelazowska-Rutkowska, B.; Gorska, E.; Hermanowicz, A.; Gorodkiewicz, E. New Biosensor for Determination of Neuropilin-1 with Detection by Surface Plasmon Resonance Imaging. Sensors 2023, 23, 4118. [Google Scholar] [CrossRef] [PubMed]
- Zielinska, Z.; Gielazyn, J.; Dzieciol-Anikiej, Z.; Dzieciol, J.; Mrozek, P.; Reszec-Gielazyn, J.; Gorodkiewicz, E. A Study on the Levels of Selected Proangiogenic Proteins in Human Tissues and Plasma in Relation to Brain Glioma. Int. J. Mol. Sci. 2025, 26, 4802. [Google Scholar] [CrossRef] [PubMed]
- Sipka, A.; Mann, S.; Babasyan, S.; Freer, H.; Wagner, B. Development of a bead-based multiplex assay to quantify bovine interleukin-10, tumor necrosis factor-alpha, and interferon-gamma concentrations in plasma and cell culture supernatant. JDS Commun. 2022, 3, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Da Silva Rosa, P.M.; Bridi, A.; de Avila Ferronato, G.; Prado, C.M.; Bastos, N.M.; Sangalli, J.R.; Meirelles, F.V.; Perecin, F.; da Silveira, J.C. Corpus luteum presence in the bovine ovary increase intrafollicular progesterone concentration: Consequences in follicular cells gene expression and follicular fluid small extracellular vesicles miRNA contents. J. Ovarian Res. 2024, 17, 65. [Google Scholar] [CrossRef]
- Luciano, A.M.; Lodde, V.; Franciosi, F.; Ceciliani, F.; Peluso, J.J. Progesterone receptor membrane component 1 expression and putative function in bovine oocyte maturation, fertilization, and early embryonic development. Reproduction 2010, 140, 663–672. [Google Scholar] [CrossRef]
- Dieci, C.; Lodde, V.; Labreque, R.; Dufort, I.; Tessaro, I.; Sirard, M.A.; Luciano, A.M. Differences in cumulus cell gene expression indicate the benefit of a pre-maturation step to improve in-vitro bovine embryo production. Mol. Hum. Reprod. 2016, 22, 882–897. [Google Scholar] [CrossRef]
- Lodde, V.; Modina, S.; Maddox-Hyttel, P.; Franciosi, F.; Lauria, A.; Luciano, A.M. Oocyte morphology and transcriptional silencing in relation to chromatin remodeling during the final phases of bovine oocyte growth. Mol. Reprod. Dev. 2008, 75, 915–924. [Google Scholar] [CrossRef] [PubMed]
- Raes, A.; Babin, D.; Pascottini, O.B.; Opsomer, G.; Van Soom, A.; Smits, K. Artificial intelligence outperforms humans in morphology-based oocyte selection in cattle. Sci. Rep. 2025, 15, 21829. [Google Scholar] [CrossRef] [PubMed]
- Kussano, N.R.; Franco, M.M.; Dode, M.A.N. Biochemical profiling of the follicular environment to predict oocyte competence in cattle. PLoS ONE 2024, 19, e0298316. [Google Scholar] [CrossRef] [PubMed]
- Ireland, J.; Smith, G.; Scheetz, D.; Jimenez-Krassel, F.; Folger, J.; Ireland, J.; Mossa, F.; Lonergan, P.; Evans, A. Does size matter in females? An overview of the impact of the high variation in the ovarian reserve on ovarian function and fertility, utility of anti-Müllerian hormone as a diagnostic marker for fertility and causes of variation in the ovarian reserve in cattle. Reprod. Fertil. Dev. 2010, 23, 1–14. [Google Scholar]
- Mossa, F.; Ireland, J.J. Physiology and endocrinology symposium: Anti-Müllerian hormone: A biomarker for the ovarian reserve, ovarian function, and fertility in dairy cows. J. Anim. Sci. 2019, 97, 1446–1455. [Google Scholar] [CrossRef]
- Ananieva, O.; Darragh, J.; Johansen, C.; Carr, J.M.; McIlrath, J.; Park, J.M.; Wingate, A.; Monk, C.E.; Toth, R.; Santos, S.G.; et al. The kinases MSK1 and MSK2 act as negative regulators of Toll-like receptor signaling. Nat. Immunol. 2008, 9, 1028–1036. [Google Scholar] [CrossRef]
- Hammer, M.; Mages, J.; Dietrich, H.; Schmitz, F.; Striebel, F.; Murray, P.J.; Wagner, H.; Lang, R. Control of dual-specificity phosphatase-1 expression in activated macrophages by IL-10. Eur. J. Immunol. 2005, 35, 2991–3001. [Google Scholar] [CrossRef]
- Villa-Diaz, L.G.; Miyano, T. Activation of p38 MAPK during porcine oocyte maturation. Biol. Reprod. 2004, 71, 691–696. [Google Scholar] [CrossRef]
- Snider, A.P.; Gomes, R.S.; Summers, A.F.; Tenley, S.C.; Abedal-Majed, M.A.; McFee, R.M.; Wood, J.R.; Davis, J.S.; Cupp, A.S. Identification of Lipids and Cytokines in Plasma and Follicular Fluid before and after Follicle-Stimulating Hormone Stimulation as Potential Markers for Follicular Maturation in Cattle. Animals 2023, 13, 3289. [Google Scholar] [CrossRef]
- Demetrio, D.; Barfield, J. Appendix 2: Photographic illustrations of bovine cumulus–oocyte complexes. In Manual of the International Embryo Technology Society; IETS: Denver, CO, USA, 2022; Volume 2. [Google Scholar]
- Pytel, A.; Tobolski, D.; Skup, P.; Gargaś, J.; Flis, S.; Gajewski, Z.; Gorodkiewicz, E.; Papis, K. A Novel Surface Plasmon Resonance Imaging (SPRi) Biosensor for the Determination of Bovine Interleukin-10: Development, Validation, and Application in Biological Fluids. Int. J. Mol. Sci. 2025, 26, 10395. [Google Scholar] [CrossRef]
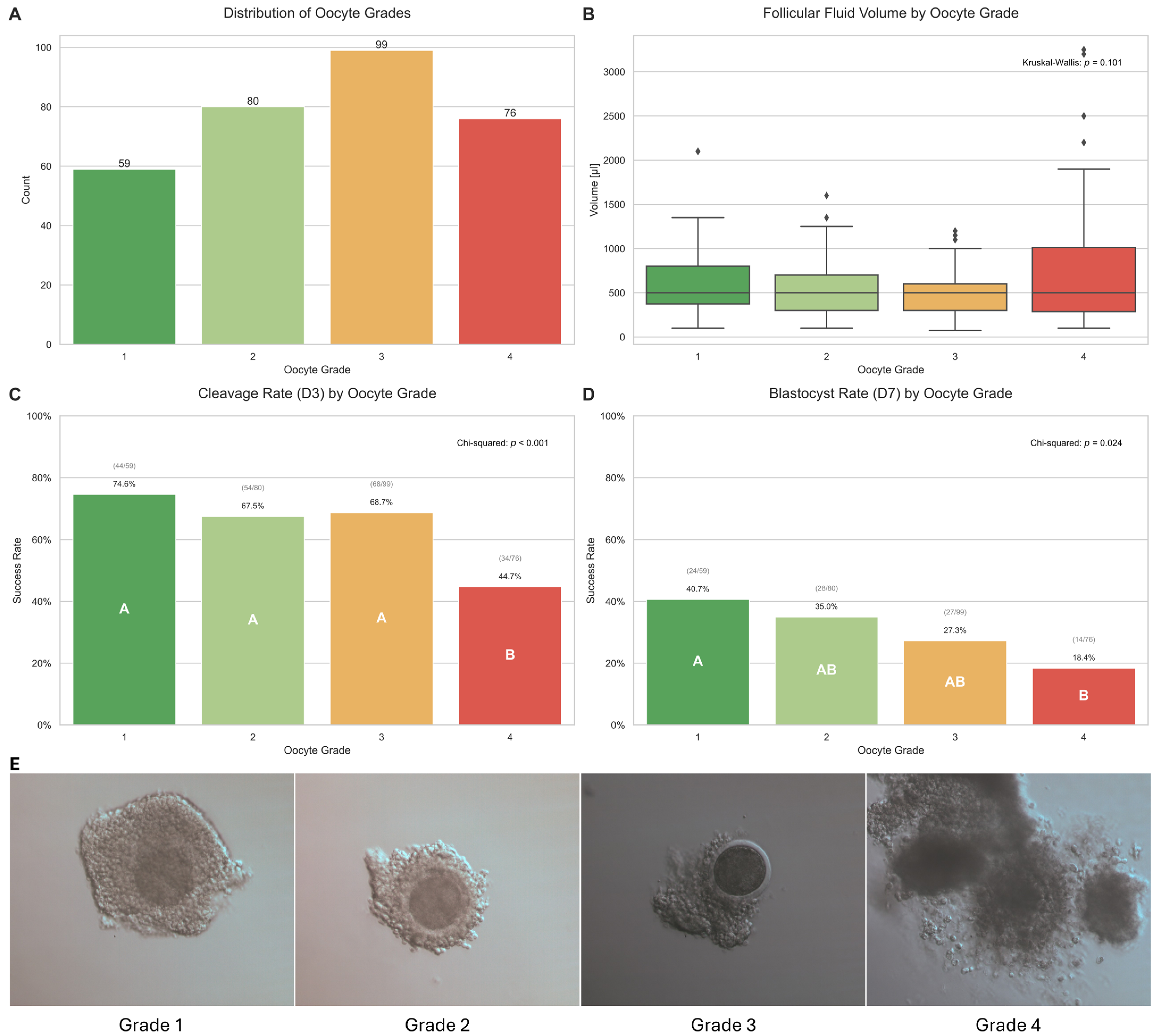
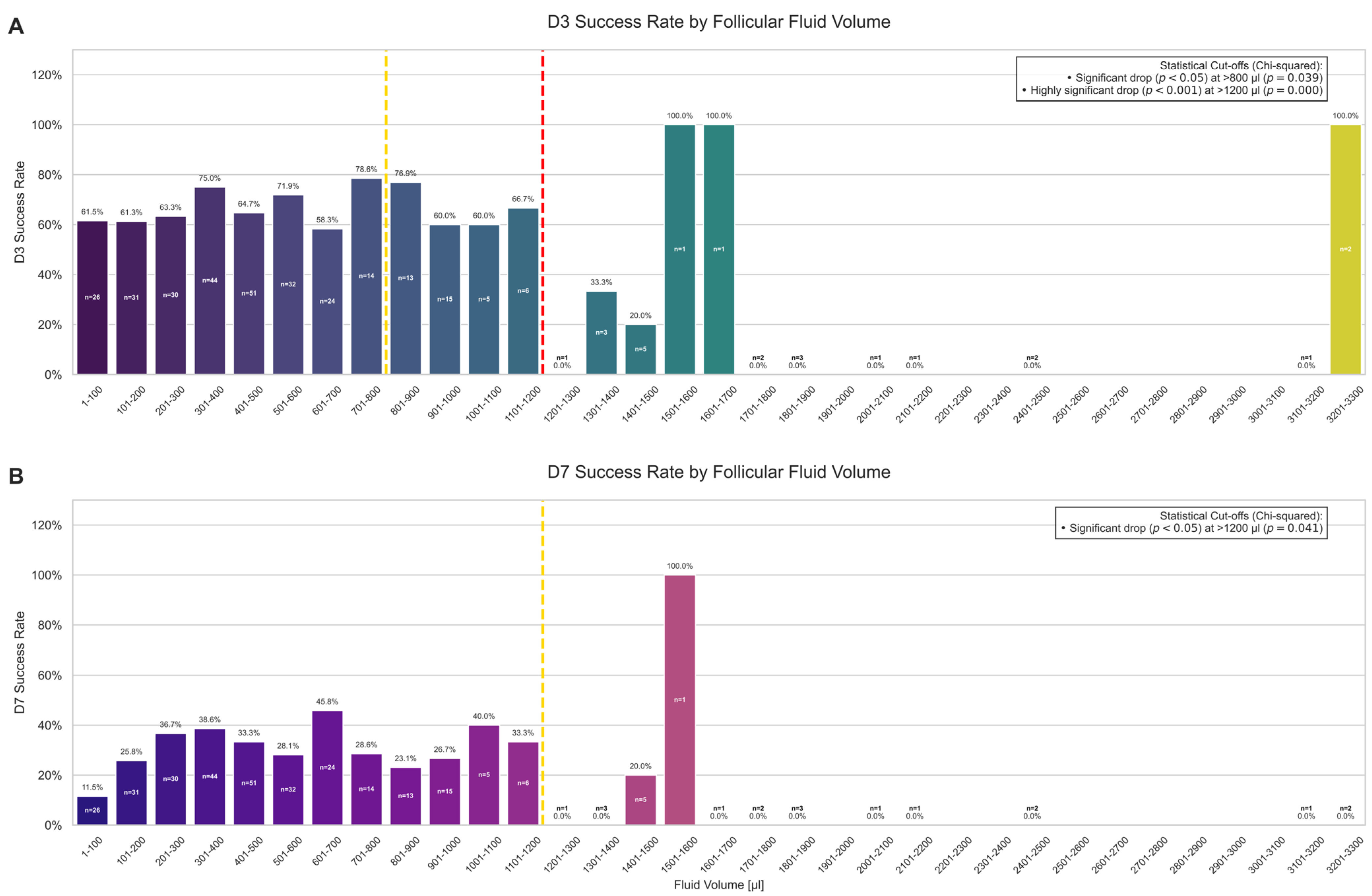
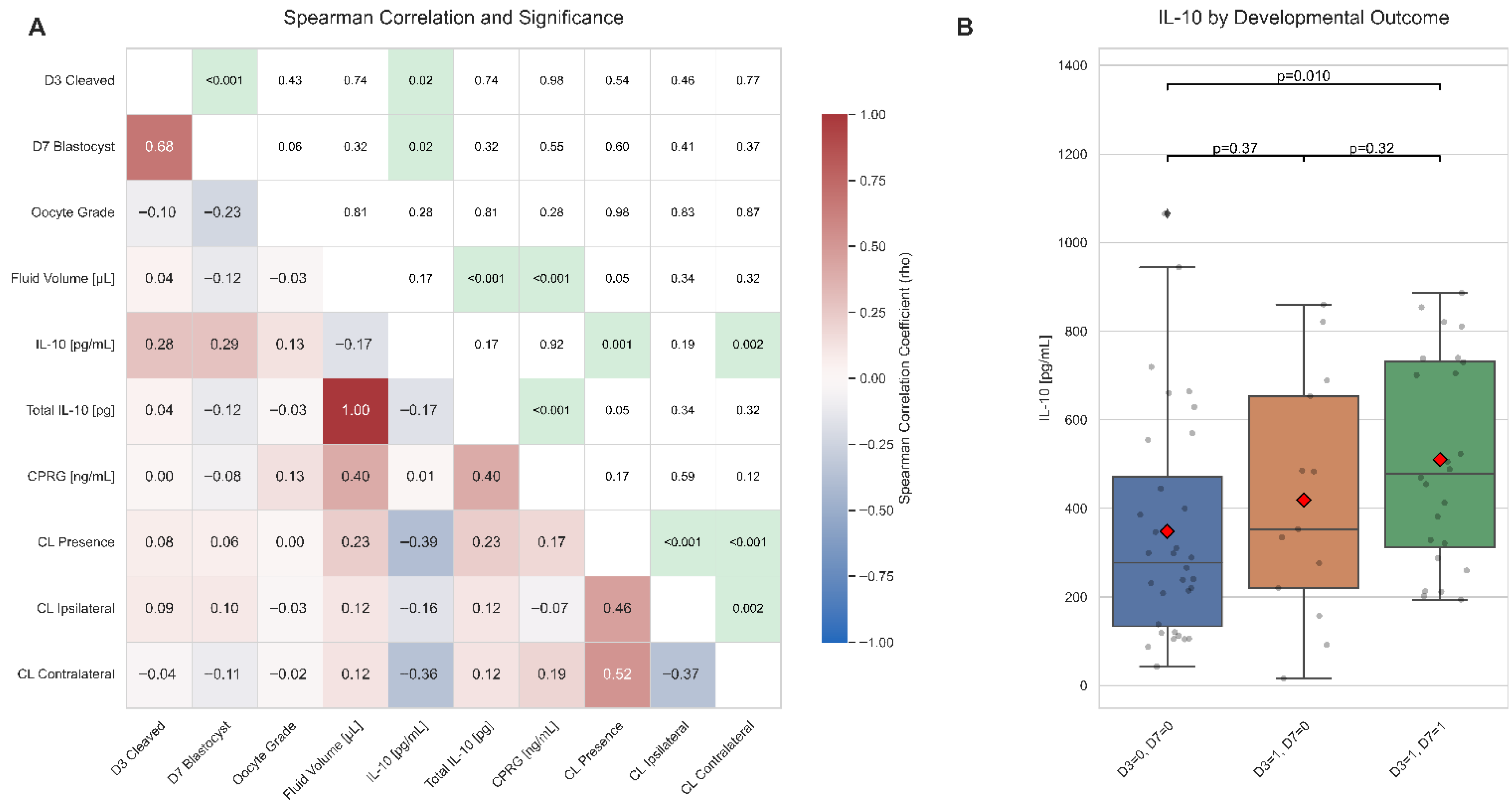
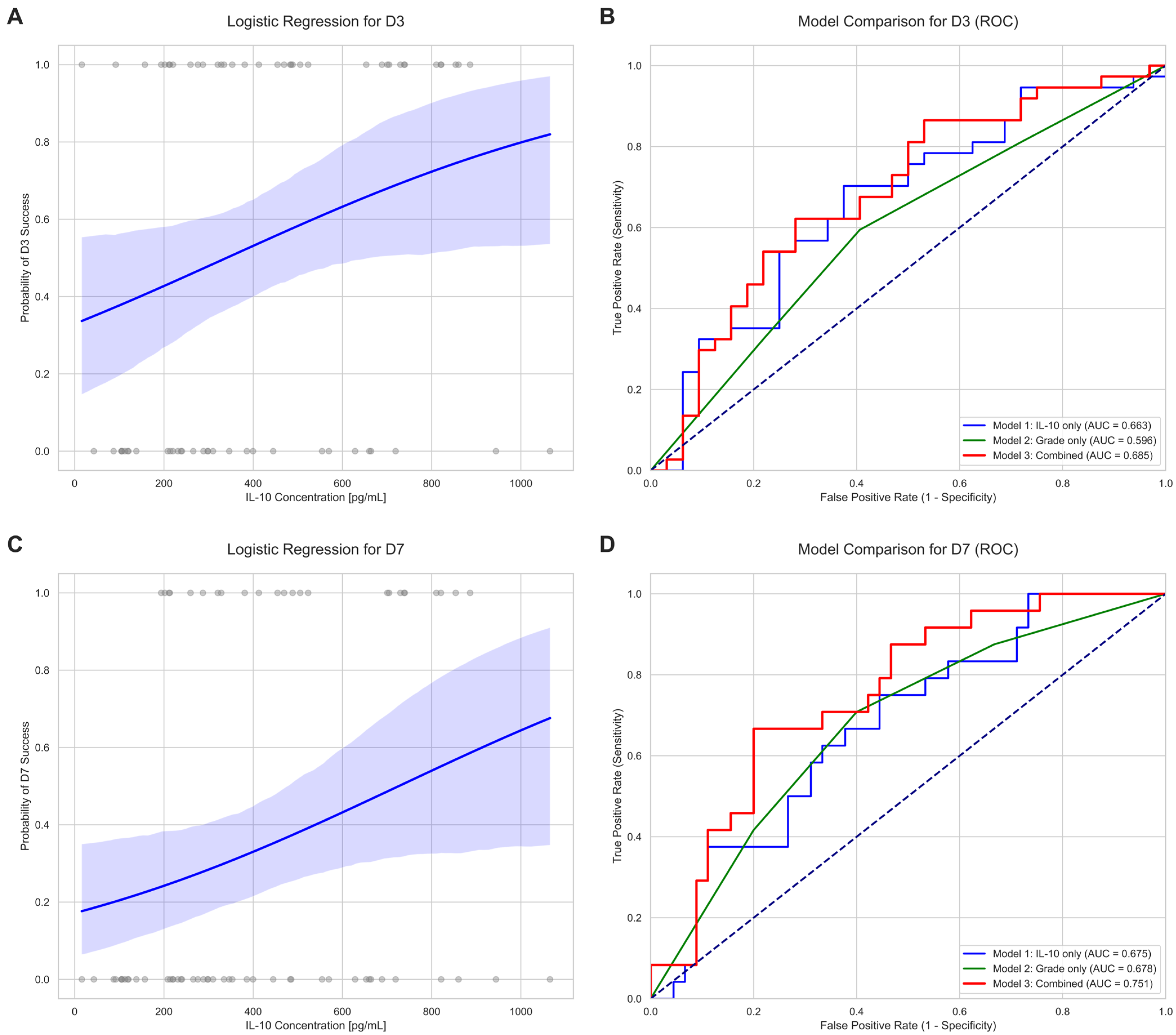
| Predictor Group | Predictor/Comparison | Outcome | Odds Ratio (95% CI) | p-Value |
|---|---|---|---|---|
| Physiological Factors * | CL Presence (Yes vs. No) | D3 | 1.40 (0.48–4.10) | 0.584 |
| D7 | 1.32 (0.42–4.17) | 0.771 | ||
| Ipsilateral CL (Yes vs. No) | D3 | 1.44 (0.55–3.75) | 0.471 | |
| D7 | 1.52 (0.57–4.10) | 0.446 | ||
| Contralateral CL (Yes vs. No) | D3 | 0.87 (0.34–2.21) | 0.812 | |
| D7 | 0.64 (0.24–1.73) | 0.449 | ||
| Biochemical Marker * | IL-10 > 142.16 pg/mL | D3 | 5.74 (1.30–25.41) | 0.018 |
| IL-10 > 142.16 pg/mL | D7 | 16.33 (0.92–290.51) | 0.006 | |
| Morphological Factor * | Oocyte Grade (vs. Grade 1) | |||
| Grade 2 | D3 | 0.72 (0.34–1.50) | 0.452 | |
| D7 | 0.79 (0.40–1.57) | 0.595 | ||
| Grade 3 | D3 | 0.76 (0.37–1.55) | 0.473 | |
| D7 | 0.55 (0.28–1.08) | 0.113 | ||
| Grade 4 | D3 | 0.28 (0.14–0.59) | 0.001 | |
| D7 | 0.34 (0.16–0.73) | 0.007 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pytel, A.T.; Tobolski, D.; Skup, P.; Strączyńska, P.; Domrazek, K.; Gajewski, Z.; Gorodkiewicz, E.; Papis, K. Correlation Between Intrafollicular IL-10, Progesterone, and Bovine Oocyte Developmental Competence. Int. J. Mol. Sci. 2025, 26, 11364. https://doi.org/10.3390/ijms262311364
Pytel AT, Tobolski D, Skup P, Strączyńska P, Domrazek K, Gajewski Z, Gorodkiewicz E, Papis K. Correlation Between Intrafollicular IL-10, Progesterone, and Bovine Oocyte Developmental Competence. International Journal of Molecular Sciences. 2025; 26(23):11364. https://doi.org/10.3390/ijms262311364
Chicago/Turabian StylePytel, Aleksandra Teresa, Dawid Tobolski, Piotr Skup, Patrycja Strączyńska, Kinga Domrazek, Zdzisław Gajewski, Ewa Gorodkiewicz, and Krzysztof Papis. 2025. "Correlation Between Intrafollicular IL-10, Progesterone, and Bovine Oocyte Developmental Competence" International Journal of Molecular Sciences 26, no. 23: 11364. https://doi.org/10.3390/ijms262311364
APA StylePytel, A. T., Tobolski, D., Skup, P., Strączyńska, P., Domrazek, K., Gajewski, Z., Gorodkiewicz, E., & Papis, K. (2025). Correlation Between Intrafollicular IL-10, Progesterone, and Bovine Oocyte Developmental Competence. International Journal of Molecular Sciences, 26(23), 11364. https://doi.org/10.3390/ijms262311364







