Supramolecular Chirogenesis in Porphyrin-Based Systems: Chirality Transfer from Anionic Chiral Surfactants to Cationic, Achiral Porphyrins
Abstract
1. Introduction
2. Results
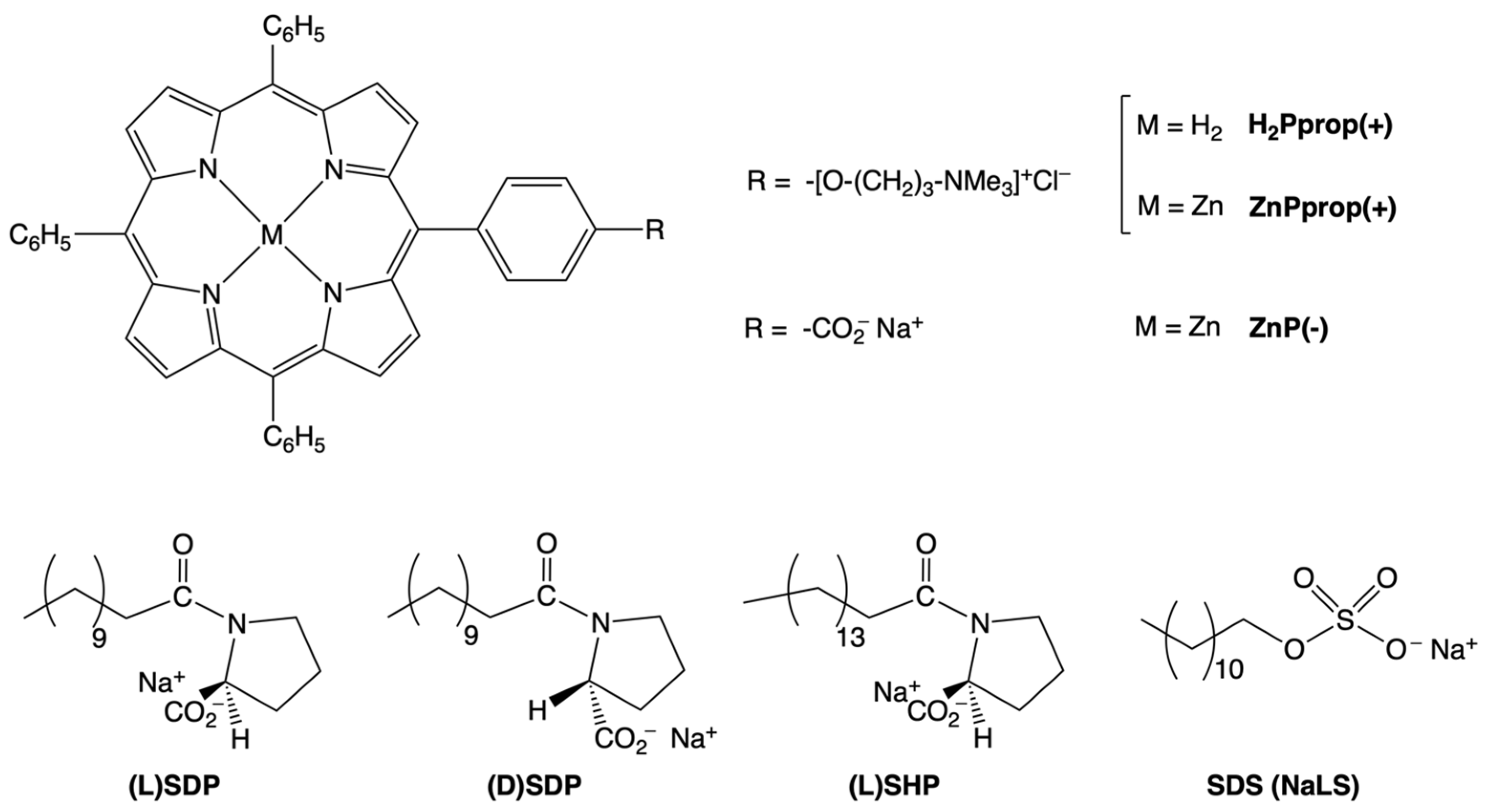
2.1. Chirality Transfer from SDP and SHP Chiral Surfactants
2.1.1. The Effect of the Chiral Configuration of the Surfactant Polar Head: Studies on (L)/(D)SDP
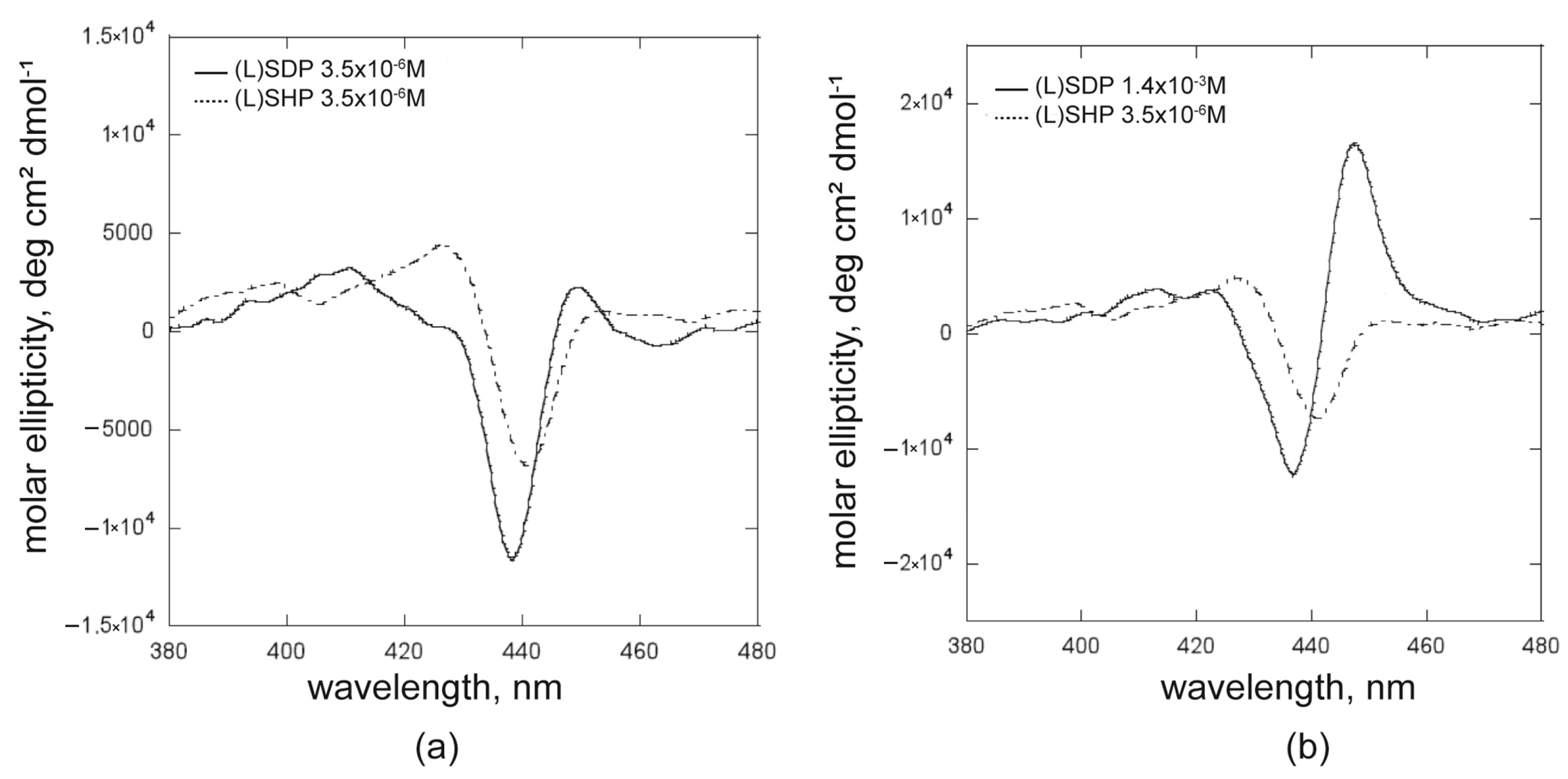
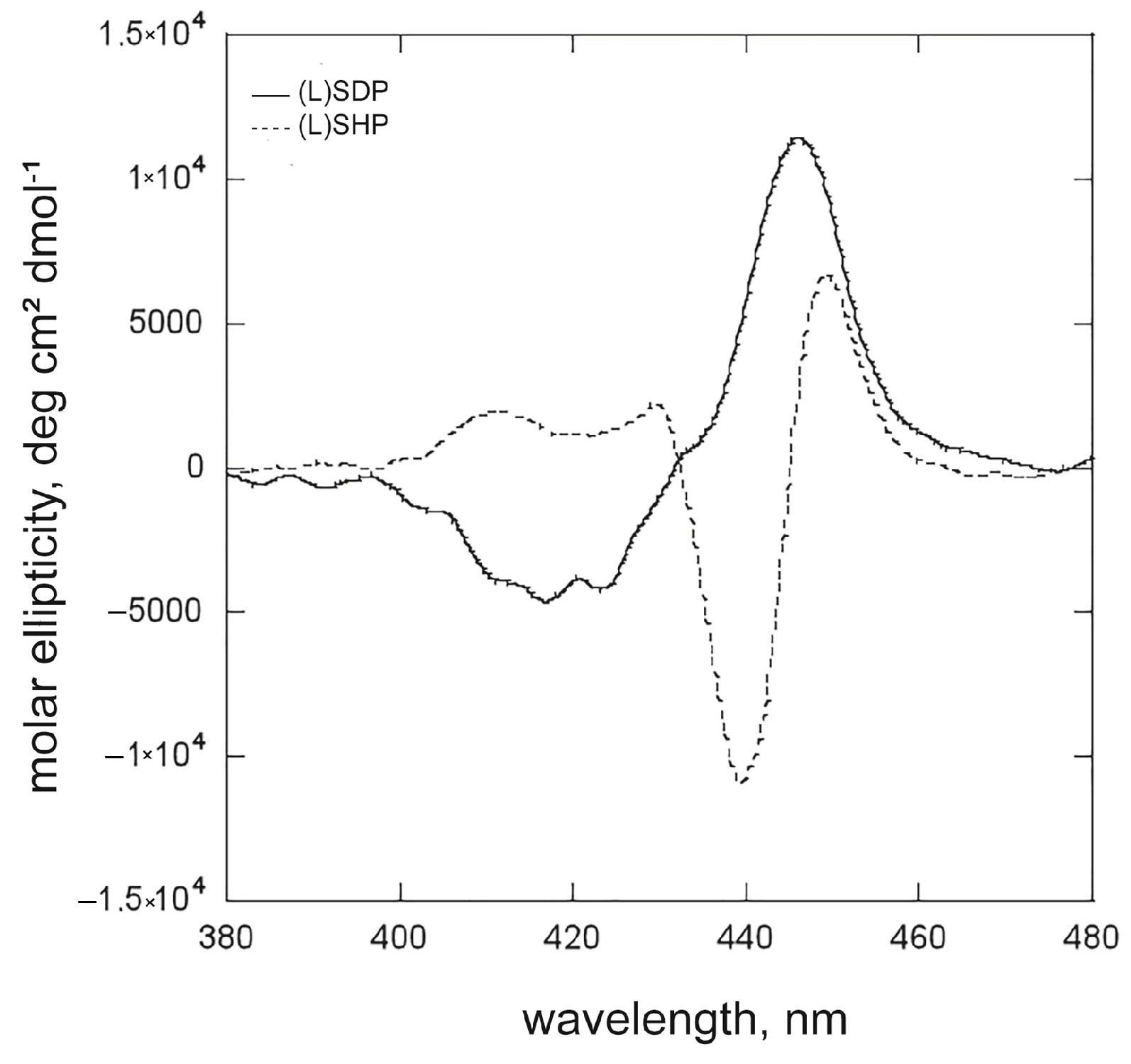
2.1.2. The Effect of the Surfactant Alkyl Chain Length: (L)SDP Versus (L)SHP
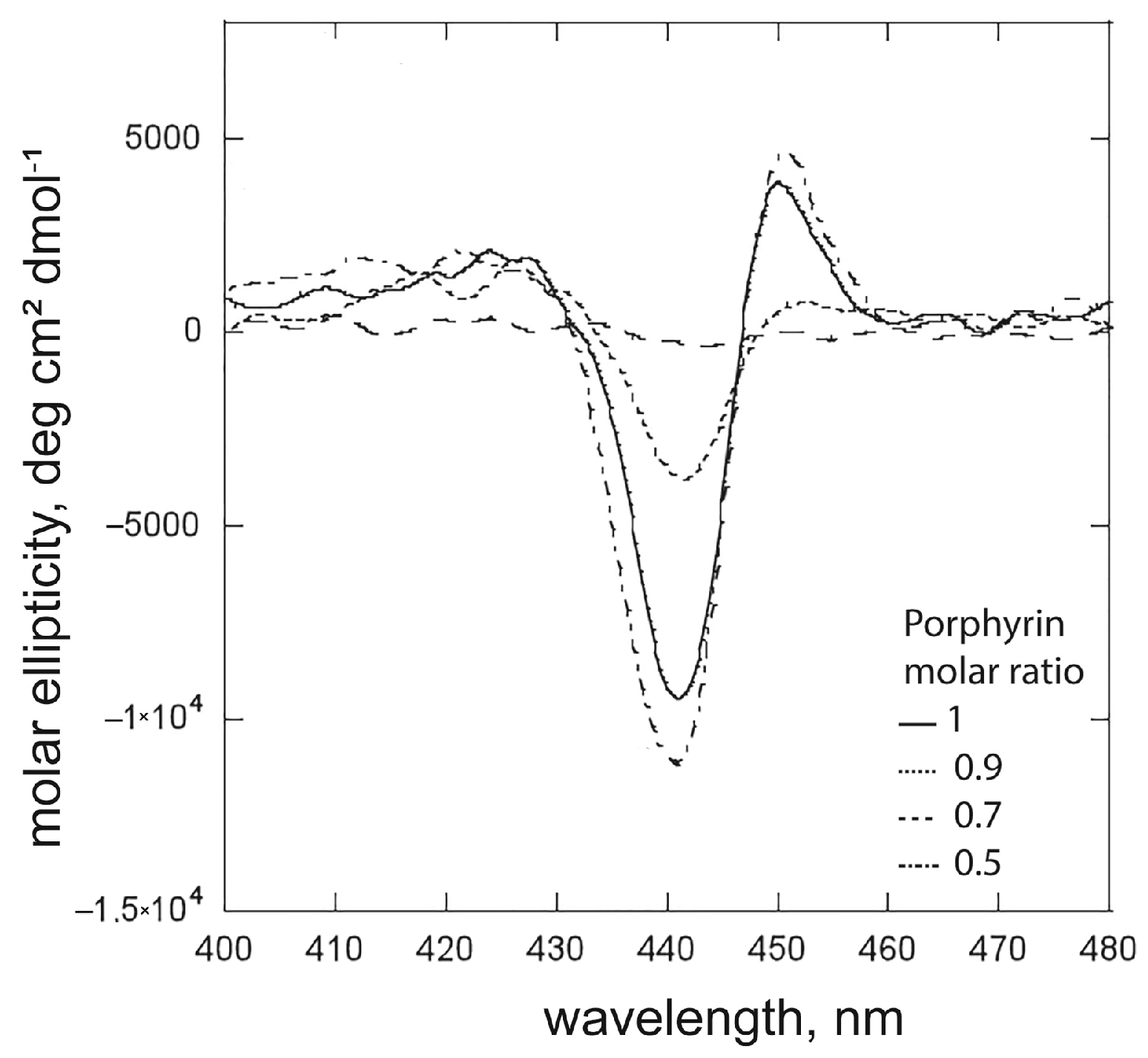
2.1.3. The Effect of the Surfactant Concentration on Porphyrin Aggregation: The Case of (L)SHP
2.2. Fluorescence and Resonance Light Scattering Studies for the Surfactant–Porphyrin Systems
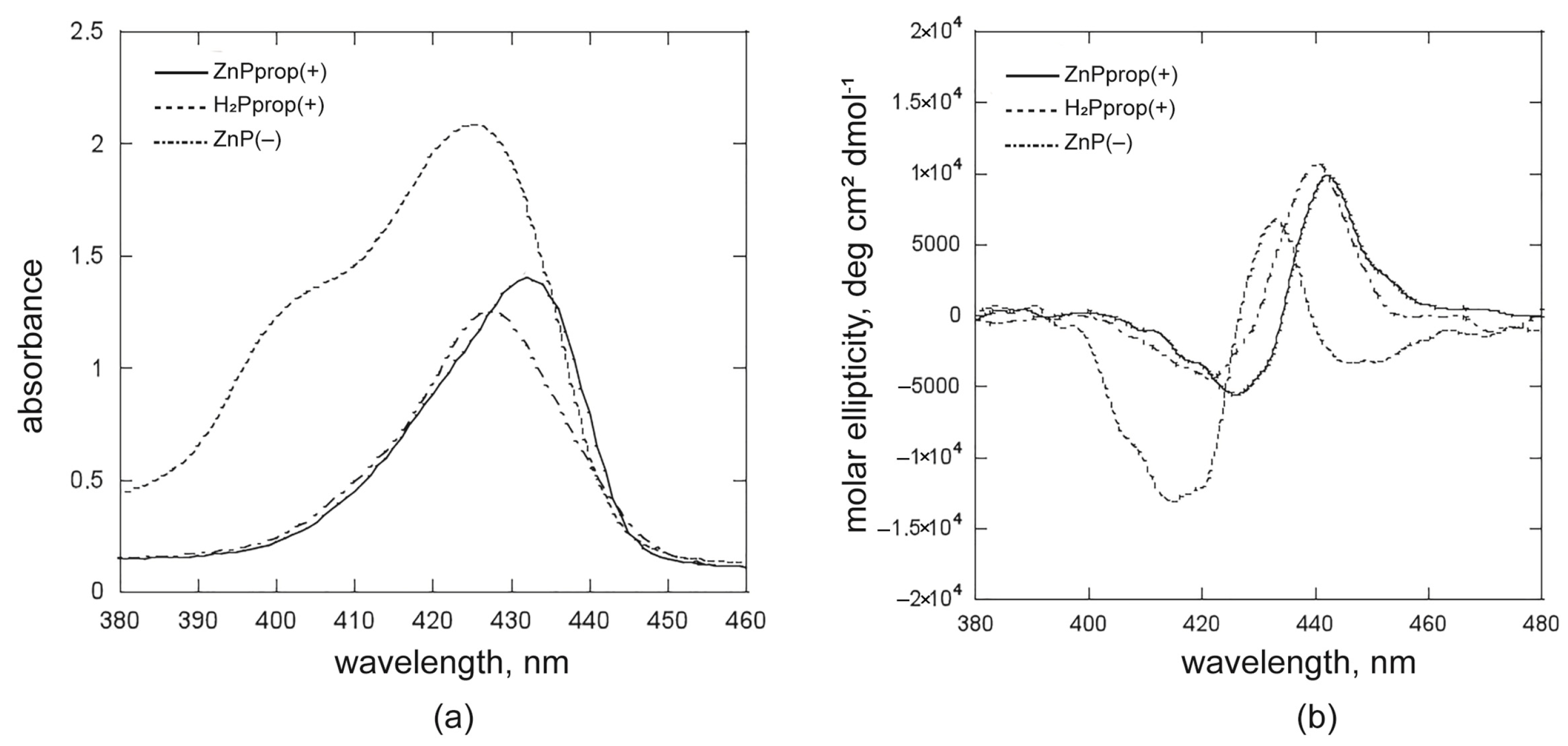
2.3. Evaluation of the Interactions Involved in the Supramolecular Chirogenesis. The Case of H2Pprop(+) and ZnP(−) Derivatives
2.3.1. Studies on H2Pprop(+) and ZnP(−), with [(L)SHP] < cmc
2.3.2. Studies on H2Pprop(+) and ZnP(−), with [(L)SHP] > cmc
3. Materials and Methods
3.1. Synthesis
3.2. Determination of the Molar Extinction Coefficient of ZnPprop(+) in Micelles
3.3. Spectroscopic Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| cmc | Critical Micellar Concentration |
| SDP | Sodium Dodecyl Sulphate |
| SHP | Sodium Hexadecyl Sulphate |
| H2Pprop(+) | [5-{4-(3-trimethylammonium)propyloxyphenyl}-10,15,20-triphenylporphyrin]chloride |
| ZnPprop(+) | Zn(II) [5-{4-(3-trimethylammonium)propyloxyphenyl}-10,15,20-triphenylporphyrin]chloride |
| ZnP(−) | Zn(II) [5-{4-(carboxyphenyl}-10,15,20-triphenylporphyrinato |
| UV-Vis | Ultraviolet–Visible |
| CD | Circular Dichroism |
| RLS | Resonance Light Scattering |
| EtOH | Ethanol |
References
- Johnson, L.N. Asymmetry at the molecular level in biology. Eur. Rev. 2005, 13, 77–95. [Google Scholar] [CrossRef]
- Garg, A.; Rendina, D.; Bendale, H.; Akiyama, T.; Ojima, I. Recent advances in catalytic asymmetric synthesis. Front. Chem. 2024, 12, 1398397. [Google Scholar] [CrossRef]
- Chen, Y. Circularly polarised luminescence based on small organic fluorophores. Mater. Today Chem. 2022, 23, 100651. [Google Scholar] [CrossRef]
- Arenas, M.; Martín, J.; Santos, J.L.; Aparicio, I.; Alonso, E. An overview of analytical methods for enantiomeric determination of chiral pollutants in environmental samples and biota. TrAC Trends Anal. Chem. 2021, 143, 116370. [Google Scholar] [CrossRef]
- Chen, W.; Li, B.; Gao, G.; Sun, T. Chiral supramolecular nanomaterials: From chirality transfer and amplification to regulation and applications. Interdiscip. Mater. 2023, 2, 689–713. [Google Scholar] [CrossRef]
- Shang, X.; Song, I.; Han, M.; Lee, J.H.; Ohtsu, H.; Choi, W.; Kim, J.C.; Ahm, J.; Kwak, S.K.; Oh, J.H. “Majority-rules” effect on supramolecular chirality and optoelectronic properties of chiral tetrachloro-perylene diimides. Adv. Opt. Mater. 2021, 9, 2001911. [Google Scholar] [CrossRef]
- Morrow, S.M.; Bissette, A.J.; Fletcher, S.P. Transmission of chirality through space and across length scales. Nat. Nanotechnol. 2017, 12, 410–419. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, D.; Kiselev, M.A.; Magazù, S.; Calandra, P. Amphiphiles self-assembly: Basic concepts and future perspectives of supramolecular approaches. Adv. Condens. Matter Phys. 2015, 2015, 1–22. [Google Scholar] [CrossRef]
- Lorenzetto, T.; Frigatti, D.; Fabris, F.; Scarso, A. Nanoconfinement effects of micellar media in asymmetric catalysis. Adv. Synth. Catal. 2022, 364, 1776–1797. [Google Scholar] [CrossRef]
- Begato, F.; Licini, G.; Zonta, C. Exploiting chirality in confined nanospaces. Angew. Chem. Int. Ed. 2023, 62, e202311153. [Google Scholar] [CrossRef]
- Fuhrhop, J.H.; Demoulin, C.; Boettcher, C.; Koning, J.; Siggel, U. Chiral micellar porphyrin fibres with 2-aminoglycosamide head groups. J. Am. Chem. Soc. 1992, 114, 4159–4165. [Google Scholar] [CrossRef]
- El-Hachemi, Z.; Mancini, G.; Ribó, J.M.; Sorrenti, A. Role of the hydrophobic effect in the transfer of chirality from molecules to complex systems: From chiral surfactants to porphyrin/surfactant aggregates. J. Am. Chem. Soc. 2008, 130, 15176–15184. [Google Scholar] [CrossRef]
- Qiu, Y.; Chen, P.; Liu, M. Evolution of various porphyrin nanostructures via an oil/aqueous medium: Controlled self-assembly, further organisation, and supramolecular chirality. J. Am. Chem. Soc. 2010, 132, 9644–9652. [Google Scholar] [CrossRef] [PubMed]
- Monti, D.; Cantonetti, V.; Venanzi, M.; Ceccacci, F.; Bombelli, C.; Mancini, G. Interaction of a chirally functionalised porphyrin derivative with chiral micellar aggregates. Chem. Commun. 2004, 972–973. [Google Scholar] [CrossRef]
- Maiti, N.C.; Mazumdar, S.; Periasamy, N. J- and H-aggregates of porphyrins with surfactants: Fluorescence, stopped flow and electron microscopy studies. J. Porphyr. Phthalocyanines 1998, 2, 369–376. [Google Scholar] [CrossRef]
- Maiti, N.C.; Mazumdar, S.; Periasamy, N. J- and H-aggregates of porphyrin−surfactant complexes: Time-resolved fluorescence and other spectroscopic studies. J. Phys. Chem. B 1998, 102, 1528–1538. [Google Scholar] [CrossRef]
- Gandini, S.C.M.; Yushmanov, V.E.; Borissevitch, I.E.; Tabak, M. Interaction of the tetra (4-sulfonatophenyl) porphyrin with ionic surfactants: Aggregation and location in micelles. Langmuir 1999, 15, 6233–6243. [Google Scholar] [CrossRef]
- Bellacchio, E.; Lauceri, R.; Gurrieri, S.; Monsù Scolaro, L.; Romeo, A.; Purrello, R. Template-imprinted chiral porphyrin aggregates. J. Am. Chem. Soc. 1998, 120, 12353–12354. [Google Scholar] [CrossRef]
- Purrello, R. Supramolecular chemistry: Lasting chiral memory. Nat. Mater. 2003, 2, 216–217. [Google Scholar] [CrossRef]
- Monti, D.; Venanzi, M.; Mancini, G.; Di Natale, C.; Paolesse, R. Supramolecular chirality control by solvent changes. Solvodichroic effect on chiral porphyrin aggregation. Chem. Commun. 2005, 2471–2743. [Google Scholar] [CrossRef]
- Monti, D.; Venanzi, M.; Stefanelli, M.; Sorrenti, A.; Mancini, G.; Di Natale, C.; Paolesse, R. Chiral amplification of chiral porphyrin derivatives by templated heteroaggregation. J. Am. Chem. Soc. 2007, 129, 6688–6689. [Google Scholar] [CrossRef]
- Stefanelli, M.; Magna, G.; Di Natale, C.; Paolesse, R.; Monti, D. Stereospecific self-assembly processes of porphyrin-proline conjugates: From the effect of structural features and bulk solvent properties to the application in stereoselective sensor systems. Int. J. Mol. Sci. 2022, 23, 15587. [Google Scholar] [CrossRef]
- Krzyszowska, P.; Pacholska-Dudziak, E. Porphyrins are nature’s workhorse. Nat. Chem. 2025, 17, 156. [Google Scholar] [CrossRef]
- Cavaleiro, J.A.S.; Tomé, A.C.; Neves, M.G.P.M.S. Meso-Tetraarylporphyrin derivatives: New synthetic methodologies. In Handbook of Porphyrin Science; Kadish, K.M., Smith, K.M., Guilard, R., Eds.; World Scientific Publishing Co.: Hackensack, NJ, USA; London, UK; Singapore; Beijing, China; Shanghai, China; Hong Kong, China; Taipei, Taiwan; Chennai, India, 2010; Volume 2, Chapter 9; pp. 193–294. [Google Scholar]
- Stillman, M.J. Theoretical aspects of the optical spectroscopy of porphyrinoids. In Handbook of Porphyrin Science; Kadish, K.M., Smith, K.M., Guilard, R., Eds.; World Scientific Publishing Co.: Hackensack, NJ, USA; London, UK; Singapore; Beijing, China; Shanghai, China; Hong Kong, China; Taipei, Taiwan; Chennai, India, 2011; Volume 14, Chapter 65; pp. 461–524. [Google Scholar]
- Brothers, P.J.; Senge, M.O. Fundamentals of porphyrin chemistry: A 21st century approach. In Coordination Chemistry; Brothers, P.J., Senge, M.O., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2022; Volume 1, Chapter 4; pp. 141–240. [Google Scholar]
- Hoeben, F.J.M.; Jonkheijm, P.; Meijer, E.W.; Schenning, A.P.H.J. About supramolecular assemblies of π-conjugated systems. Chem. Rev. 2005, 105, 1491–1546. [Google Scholar] [CrossRef]
- Liang, B.; Zhao, J.; Wang, J.; Li, Y.; Han, B.; Li, J.; Ding, X.; Xie, Z.; Wang, H.; Zhou, S. Nonlinear optical properties of porphyrin-based covalent organic frameworks determined by steric-orientation of conjugation. J. Mater. Chem. C 2023, 11, 3354–3359. [Google Scholar] [CrossRef]
- Imahori, H.; Kurotobi, K.; Walter, M.G.; Rudine, A.B.; Wamser, C.C. Porphyrin- and Phthalocyanine-Based Solar Cells; Kadish, K.M., Smith, K.M., Guilard, R., Eds.; World Scientific Publishing Co.: Hackensack, NJ, USA; London, UK; Singapore; Beijing, China; Shanghai, China; Hong Kong, China; Taipei, Taiwan; Chennai, India, 2012; Volume 18, Chapter 80; pp. 58–123. [Google Scholar]
- Tanaka, T.; Osuka, A. Conjugated porphyrin arrays: Synthesis, properties and applications for functional materials. Chem. Soc. Rev. 2015, 44, 943–969. [Google Scholar] [CrossRef]
- Li, X.-G.; Li, J.; Chen, J.F.; Rao, L.; Zheng, L.; Yu, F.; Tang, Y.; Zheng, J.; Ma, J. Porphyrin-based covalent organic frameworks from design, synthesis to biological applications. Biomater. Sci. 2024, 12, 2766–2785. [Google Scholar] [CrossRef] [PubMed]
- Fukuzumi, S. Artificial photosynthetic systems for production of hydrogen. Curr. Opin. Chem. Biol. 2015, 25, 18–26. [Google Scholar] [CrossRef]
- Das, R.; Kumar Verma, P.; Nagaraja, C.M. Design of porphyrin-based frameworks for artificial photosynthesis and environmental remediation: Recent progress and future prospects. Coord. Chem. Rev. 2024, 514, 215944. [Google Scholar] [CrossRef]
- Feiters, M.C.; Rowan, A.E.; Nolte, R.J.M. From simple to supramolecular cytochrome P450 mimics. Chem. Soc. Rev. 2000, 29, 375–384. [Google Scholar] [CrossRef]
- Chambers, R.K.; Weaver, J.D.; Kim, J.; Hoar, J.L.; Krska, S.W.; White, M.C. A preparative small-molecule mimic of liver CYP450 enzymes in the aliphatic C–H oxidation of carbocyclic N-heterocycles. Proc. Natl. Acad. Sci. USA 2023, 120, e2300315120. [Google Scholar] [CrossRef] [PubMed]
- Kou, J.; Dou, D.; Yang, L. Porphyrin photosensitisers in photodynamic therapy and its applications. Oncotarget 2017, 8, 81591–81603. [Google Scholar] [CrossRef] [PubMed]
- Akbar, A.; Khan, S.; Chatterjee, T.; Ghosh, M. Unleashing the power of porphyrin photosensitizers: Illuminating breakthroughs in photodynamic therapy. Photochem. Photobiol. B Biol. 2023, 248, 112796. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, P.; Dong, H.; Zhen, Y.; Liu, M.; Hu, W. Porphyrin supramolecular 1D structures via surfactant-assisted self-assembly. Adv. Mater. 2015, 27, 5379–5387. [Google Scholar] [CrossRef] [PubMed]
- Severoni, E.; Maniappan, S.; Liz-Marzán, L.M.; Kumar, J.; García de Abajo, F.J.; Galantini, L. Plasmon-enhanced optical chirality through hotspot formation in surfactant-directed self-assembly of gold nanorods. ACS Nano 2020, 14, 16712–16722. [Google Scholar] [CrossRef]
- D’Annibale, V.; Ariodante, L.; Marconi, C.; Piccirillo, L.; Jönsson, P.; D’Annibale, A.; Monti, D.; Scipioni, A.; Schillén, K.; Galantini, L.; et al. Tuning structure and morphology of lipidic cubosomes by encapsulation of novel porphyrin-derivatives. Colloids Surf. B Biointerfaces 2025, 252, 114646. [Google Scholar] [CrossRef]
- Jeong, Y.-H.; Yoon, H.-J.; Jang, W.-D. Dendrimer porphyrin-based self-assembled nano-devices for biomedical applications. Polym. J. 2012, 44, 512–521. [Google Scholar] [CrossRef]
- Young, K.; Yamane, S.; GharehTapeh, E.A.; Kasamatsu, S.; Ihara, H.; Hasegawa, U. Manganese porphyrin-containing polymeric micelles: A novel approach for intracellular catalytic formation of per/polysulfide species from a hydrogen sulfide donor. Adv. Healthc. Mater. 2024, 13, 2302429. [Google Scholar] [CrossRef]
- Pan, Y.; Qu, H.; Chen, H.; Cheng, W.; Duan, Z.; Yang, J.; Wang, N.; Wu, J.; Wang, Y.; Wang, C.; et al. A tumor-targeting porphyrin-micelle with enhanced STING agonist delivery and synergistic photo-/immuno- therapy for cancer treatment. Acta Biomater. 2025, 193, 377–391. [Google Scholar] [CrossRef]
- Zarenezhad, E.; Marzi, M.; Abdulabbas, H.T.; Jasim, S.A.; Kouhpayeh, S.A.; Barbaresi, S.; Ahmadi, S.; Ghasemian, A. Bilosomes as nanocarriers for the drug and vaccine delivery against gastrointestinal infections: Opportunities and challenges. J. Funct. Biomater. 2023, 14, 453. [Google Scholar] [CrossRef]
- Chae, K.; Che Mohamad, N.A.R.; Kim, J.; Won, D.-I.; Lin, Z.; Kim, J.; Kim, D.H. The promise of chiral electrocatalysis for efficient and sustainable energy conversion and storage: A comprehensive review of the CISS effect and future directions. Chem. Soc. Rev. 2024, 53, 9029–9058. [Google Scholar] [CrossRef]
- Hu, Y.; Peng, J.; Liu, R.; Gao, J.; Hua, G.; Fan, X.; Wang, S. Porphyrin-based supramolecular self-assemblies: Construction, charge separation and transfer, stability, and application in photocatalysis. Molecules 2024, 29, 6063. [Google Scholar] [CrossRef] [PubMed]
- Monti, D.; Venanzi, M.; Russo, M.; Bussetti, G.; Goletti, C.; Montalti, M.; Zaccheroni, N.; Prodi, L.; Rella, R.; Manera, M.G.; et al. Spontaneous deposition of amphiphilic porphyrin films on glass. New J. Chem. 2004, 28, 1123. [Google Scholar] [CrossRef]
- Bombelli, C.; Bernardini, C.; Elemento, G.; Mancini, G.; Sorrenti, A.; Villani, C. Concentration as the Switch for Chiral Recognition in Biomembrane Models. J. Am. Chem. Soc. 2008, 130, 2732–2733. [Google Scholar] [CrossRef]
- Bombelli, C.; Borocci, S.; Cruciani, O.; Mancini, G.; Monti, D.; Segre, A.L.; Sorrenti, A.; Venanzi, M. Chiral recognition of dipeptides in bio-membrane models: The role of amphiphile hydrophobic chains. Tetrahedron Asymmetry 2008, 19, 124–130. [Google Scholar] [CrossRef]
- Simoncini, E.; Caroleo, F.; Ceccacci, F.; Mancini, G.; Stefanelli, M.; Paolesse, R.; Lettieri, R.; Venanzi, M.; Monti, D. Surfactant-induced chirality on reluctant aggregates of a chiral amphiphilic cationic (L)-proline–Zn(II)porphyrin conjugate in water. RSC Adv. 2014, 4, 55362–55366. [Google Scholar] [CrossRef]
- Marinelli, F.; Sorrenti, A.; Corvaglia, V.; Leone, V.; Mancini, G. Molecular description of the propagation of chirality from molecules to complex systems: Different mechanisms controlled by hydrophobic interactions. Chem. Eur. J. 2012, 18, 14680–14688. [Google Scholar] [CrossRef] [PubMed]
- Creatto, E.J.; Ceccacci, F.; Mancini, G.; Sabadini, E. Effect of the Hydrophobic Tail of a Chiral Surfactant on the Chirality of Aggregates and on the Formation of Wormlike Micelles. Langmuir 2018, 34, 13288–13295. [Google Scholar] [CrossRef] [PubMed]
- Ceccacci, F.; Giansanti, L.; Mancini, G.; Mauceri, A.; Scipioni, A.; Sperduto, C. Transcription of chirality from molecules to complex systems: The role of hydrophobic interactions. Supramol. Chem. 2013, 25, 741–747. [Google Scholar] [CrossRef]
- Pasternack, R.F.; Collings, P.J. Resonance light scattering: A new technique for studying chromophore aggregation. Science 1995, 269, 935–939. [Google Scholar] [CrossRef]
- Parkash, J.; Robblee, J.H.; Agnew, J.; Gibbs, E.; Collings, P.; Pasternack, R.F.; de Paula, J.C. Depolarized resonance light scattering by porphyrin and chlorophyll a aggregates. Biophys. J. 1998, 74, 2089–2099. [Google Scholar] [CrossRef] [PubMed]
- Barber, D.C.; Freitag-Beeston, R.A.; Whitten, D.G. Atropisomer-specific formation of premicellar porphyrin J-aggregates in aqueous surfactant solutions. J. Phys. Chem. 1991, 95, 4074–4086. [Google Scholar] [CrossRef]
- Stefanelli, M.; Magna, G.; Zurlo, F.; Caso, M.F.; Di Bartolomeo, E.; Antonaroli, S.; Venanzi, M.; Paolesse, R.; Di Natale, C.; Monti, D. Chiral Selectivity of Porphyrin–ZnO Nanoparticle Conjugates. ACS Appl. Mater. Interfaces 2019, 11, 12077–12087. [Google Scholar] [CrossRef] [PubMed]
- Mancini, G.; Schiavo, C.; Cerichelli, G. Trapping of Counterions and Water on the Surface of Cationic Micelles. Langmuir 1996, 12, 3567–3573. [Google Scholar] [CrossRef]


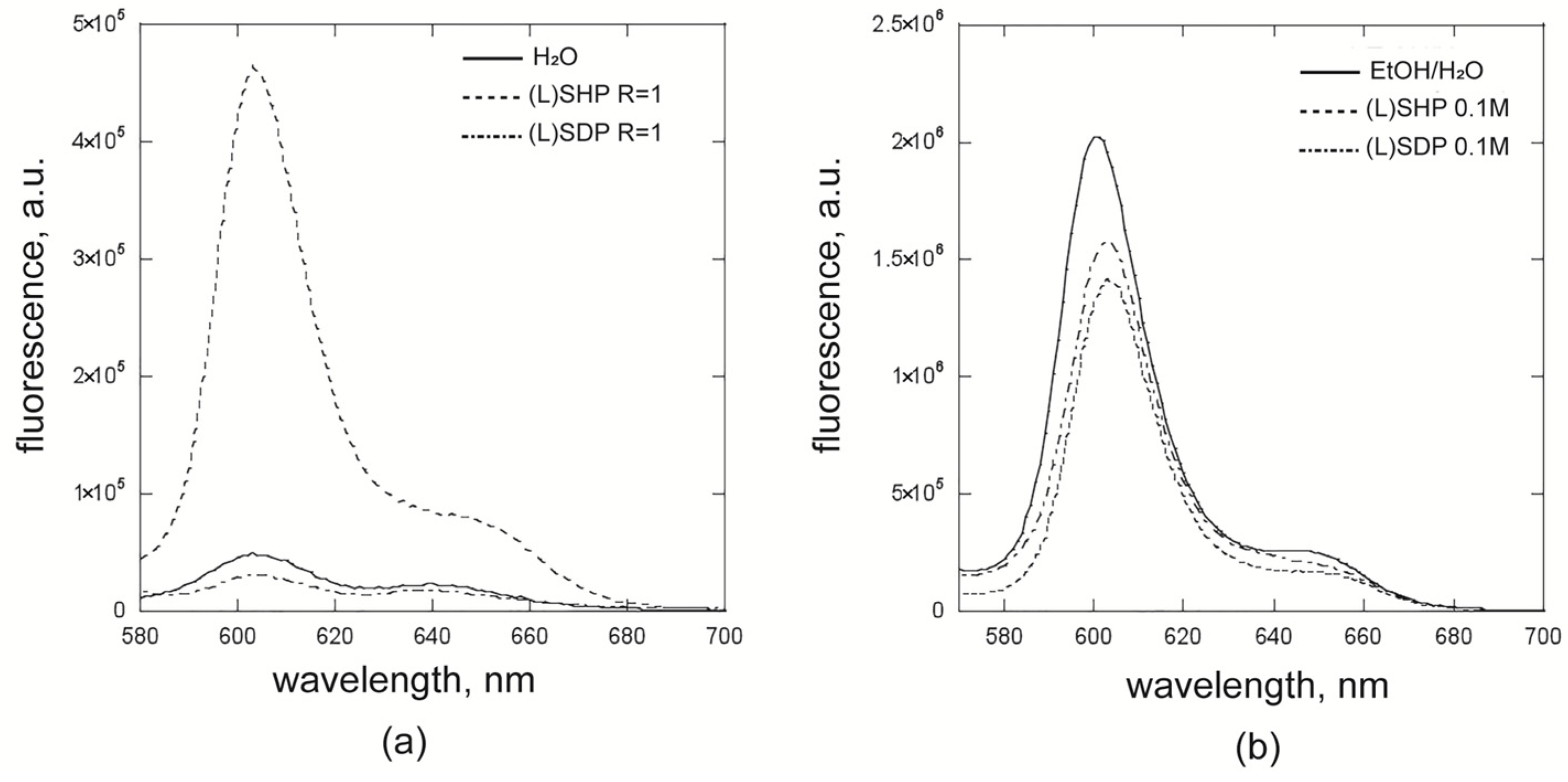
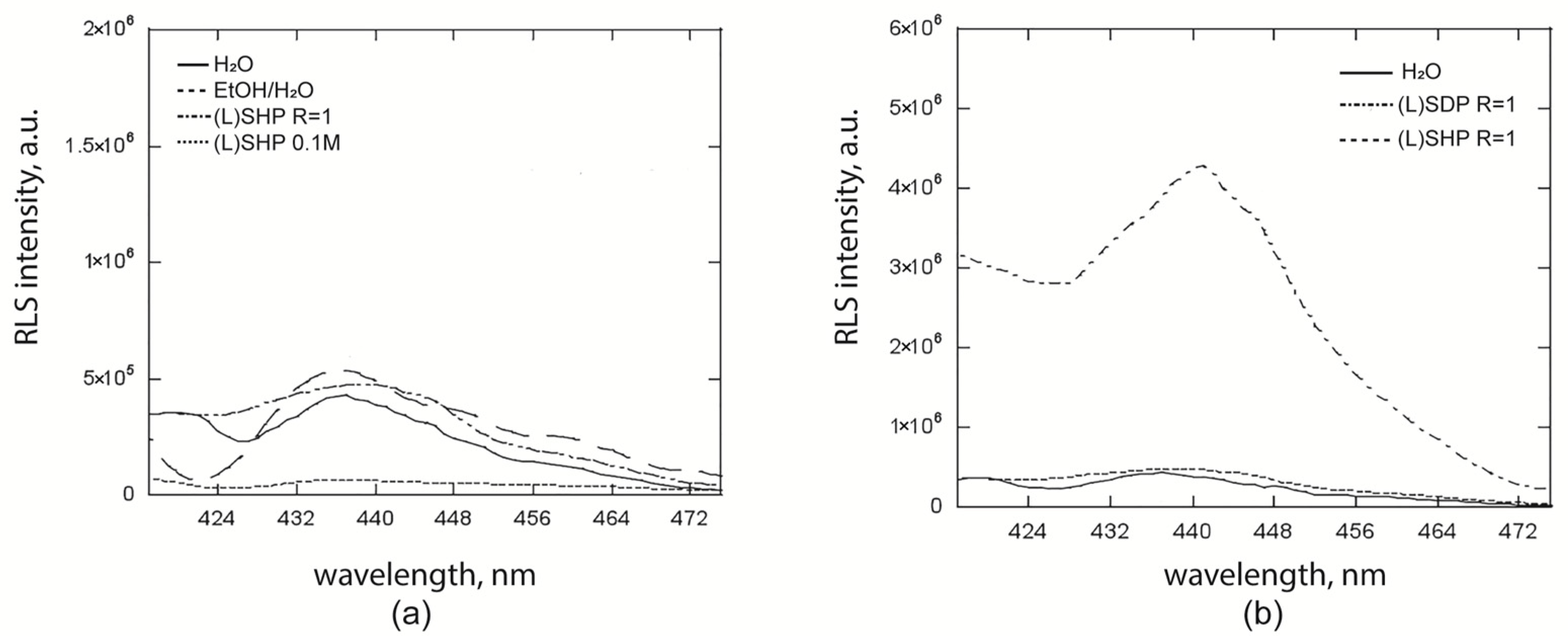
| Entry | Solution | λmax em (1), nm | λmax em (2), nm |
|---|---|---|---|
| 1 | (L)SDP 0.1 M | 603 | 645 |
| 2 | (L)SDP R = 1 | 604 | 640 |
| 3 | (L)SHP 0.1 M | 603 | 645 |
| 4 | (L)SHP R = 1 | 603 | 647 |
| 5 | (L)SHP R’ = 8 | 604 | 636 |
| 6 | (L)SHP R’ = 30 | 603 | 637 |
| 7 | H2O | 603 | 639 |
| 8 | EtOH/H2O | 601 | 645 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sbardella, P.; Stefanelli, M.; Pomarico, G.; Bombelli, C.; Ceccacci, F.; Paolesse, R.; Venanzi, M.; Monti, D. Supramolecular Chirogenesis in Porphyrin-Based Systems: Chirality Transfer from Anionic Chiral Surfactants to Cationic, Achiral Porphyrins. Int. J. Mol. Sci. 2025, 26, 11330. https://doi.org/10.3390/ijms262311330
Sbardella P, Stefanelli M, Pomarico G, Bombelli C, Ceccacci F, Paolesse R, Venanzi M, Monti D. Supramolecular Chirogenesis in Porphyrin-Based Systems: Chirality Transfer from Anionic Chiral Surfactants to Cationic, Achiral Porphyrins. International Journal of Molecular Sciences. 2025; 26(23):11330. https://doi.org/10.3390/ijms262311330
Chicago/Turabian StyleSbardella, Paola, Manuela Stefanelli, Giuseppe Pomarico, Cecilia Bombelli, Francesca Ceccacci, Roberto Paolesse, Mariano Venanzi, and Donato Monti. 2025. "Supramolecular Chirogenesis in Porphyrin-Based Systems: Chirality Transfer from Anionic Chiral Surfactants to Cationic, Achiral Porphyrins" International Journal of Molecular Sciences 26, no. 23: 11330. https://doi.org/10.3390/ijms262311330
APA StyleSbardella, P., Stefanelli, M., Pomarico, G., Bombelli, C., Ceccacci, F., Paolesse, R., Venanzi, M., & Monti, D. (2025). Supramolecular Chirogenesis in Porphyrin-Based Systems: Chirality Transfer from Anionic Chiral Surfactants to Cationic, Achiral Porphyrins. International Journal of Molecular Sciences, 26(23), 11330. https://doi.org/10.3390/ijms262311330











