Unraveling the Role of BAG3 in Hepatic Fibrosis: Genetic and Biomarker Insights in Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD)
Abstract
1. Introduction
2. Results
2.1. Clinical and Biochemical Characteristics
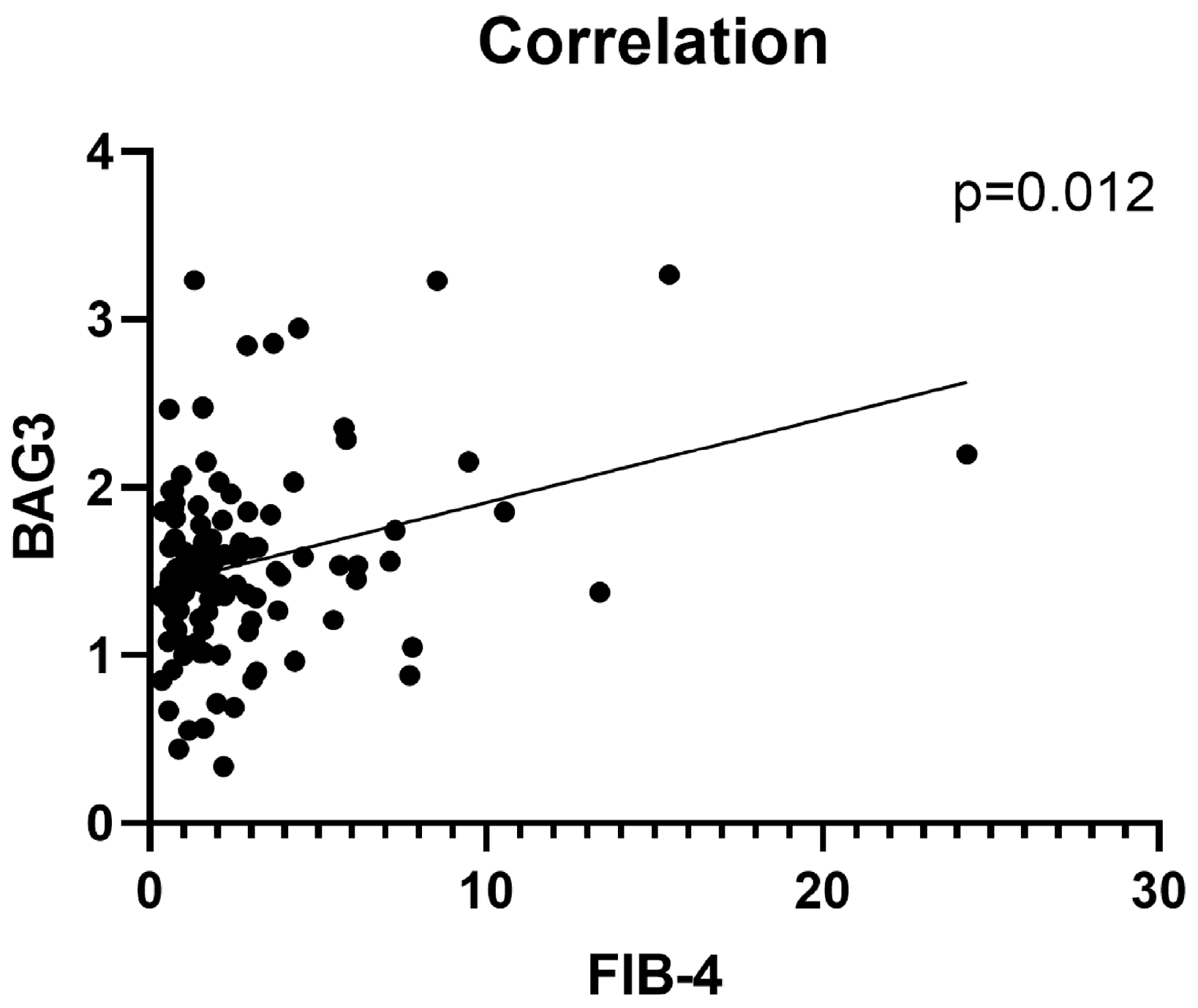
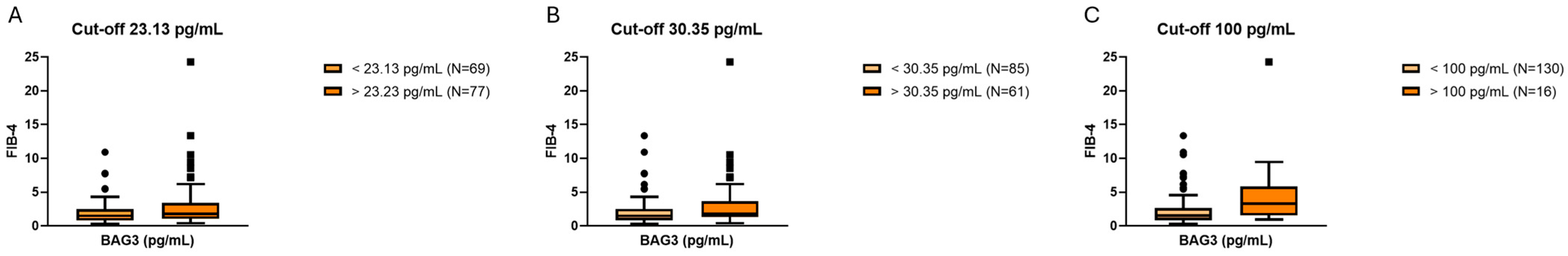
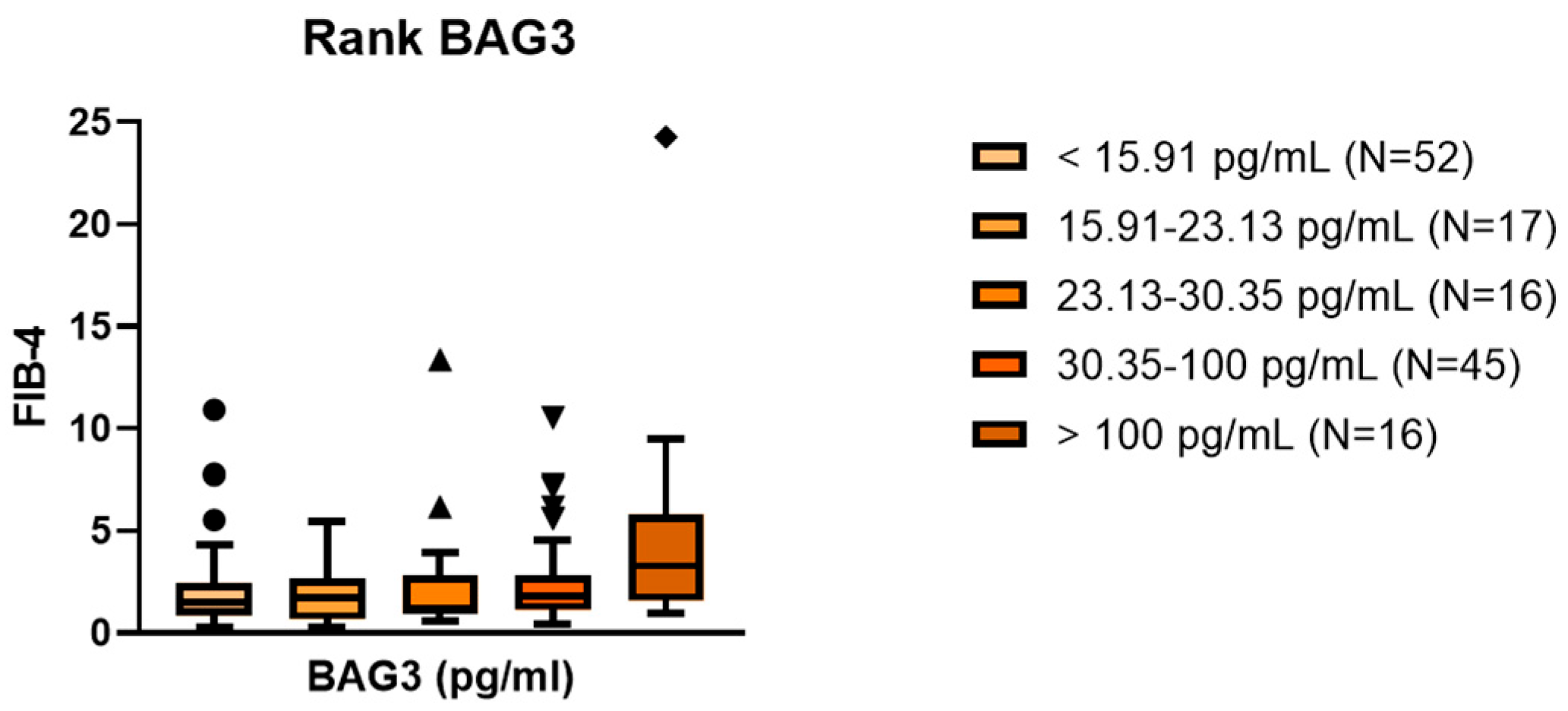
2.2. Association Between Serum BAG3 and Fibrosis
2.3. PNPLA3 and TM6F2 Genotypes Influences BAG3 Sera Levels
3. Discussion
4. Materials and Methods
4.1. Study Population
- Evidence of hepatic steatosis on imaging (ultrasound or controlled attenuation parameter [CAP]) or liver histology;
- ≥1 cardiometabolic risk factor, including overweight/obesity, dyslipidaemia, dysglycaemia, or hypertension.
- Uncomplicated MASLD (n = 121);
- Cirrhotic MASLD (n = 23);
- MASLD-related hepatocellular carcinoma (HCC) (n = 2).
4.2. Fibrosis Assessment
- Low risk: <1.30 (or <2.00 if age > 65 years).
- Intermediate risk: 1.30–2.67.
- High risk: >2.67.
4.3. Serum BAG3 Quantification
4.4. Genotyping
- PNPLA3 rs738409 (C>G, p.I148M).
- TM6SF2 rs58542926 (C>T, p.E167K).
4.5. Statistical Analysis
5. Limitations and Future Directions
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ALT | Alanine Aminotransferase |
| AST | Aspartate Aminotransferase |
| BAG3 | Bcl-2-associated Athanogene 3 |
| BMI | Body Mass Index |
| CAP | Controlled Attenuation Parameter |
| CEI | Comitato Etico Interaziendale (Ethics Committee) |
| EASD | European Association for the Study of Diabetes |
| EASL | European Association for the Study of the Liver |
| EASO | European Association for the Study of Obesity |
| ELISA | Enzyme-Linked Immunosorbent Assay |
| ER | Endoplasmic Reticulum |
| FIB-4 | Fibrosis-4 Index |
| HCC | Hepatocellular Carcinoma |
| HSC | Hepatic Stellate Cell |
| Hsp70 | Heat Shock Protein 70 |
| IRB | Institutional Review Board |
| LS | Liver Stiffness |
| MASLD | Metabolic Dysfunction-Associated Steatotic Liver Disease |
| PNPLA3 | Patatin-like Phospholipase Domain-containing Protein 3 |
| PRS | Polygenic Risk Score |
| ROS | Reactive Oxygen Species |
| SNP | Single Nucleotide Polymorphism |
| TM6SF2 | Transmembrane 6 Superfamily Member 2 |
| VLDL | Very-Low-Density Lipoprotein |
References
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef]
- Eslam, M.; Sanyal, A.J.; George, J.; Panel, I.C. MAFLD: A Consensus-Driven Proposed Nomenclature for Metabolic Associated Fatty Liver Disease. Gastroenterology 2020, 158, 1999–2014.e1991. [Google Scholar] [CrossRef]
- Dulai, P.S.; Singh, S.; Patel, J.; Soni, M.; Prokop, L.J.; Younossi, Z.; Sebastiani, G.; Ekstedt, M.; Hagstrom, H.; Nasr, P.; et al. Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: Systematic review and meta-analysis. Hepatology 2017, 65, 1557–1565. [Google Scholar] [CrossRef]
- Allen, A.M.; Lazarus, J.V.; Younossi, Z.M. Healthcare and socioeconomic costs of NAFLD: A global framework to navigate the uncertainties. J. Hepatol. 2023, 79, 209–217. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines on the management of metabolic dysfunction-associated steatotic liver disease (MASLD). J. Hepatol. 2024, 81, 492–542. [Google Scholar] [CrossRef]
- Jamialahmadi, O.; De Vincentis, A.; Tavaglione, F.; Malvestiti, F.; Li-Gao, R.; Mancina, R.M.; Alvarez, M.; Gelev, K.; Maurotti, S.; Vespasiani-Gentilucci, U.; et al. Partitioned polygenic risk scores identify distinct types of metabolic dysfunction-associated steatotic liver disease. Nat. Med. 2024, 30, 3614–3623, Erratum in Nat. Med. 2025, 31, 700. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Li, Y.; Hong, C.; Ma, P.; Zhu, H.; Cui, H.; Zou, X.; Wang, J.; Li, R.; He, J.; et al. Polygenic risk score of metabolic dysfunction-associated steatotic liver disease amplifies the health impact on severe liver disease and metabolism-related outcomes. J. Transl. Med. 2024, 22, 650. [Google Scholar] [CrossRef]
- Kanda, T.; Sasaki-Tanaka, R.; Abe, H.; Kimura, N.; Yoshida, T.; Hayashi, K.; Sakamaki, A.; Yokoo, T.; Kamimura, H.; Tsuchiya, A.; et al. Polygenic Risk Score for Metabolic Dysfunction-Associated Steatotic Liver Disease and Steatohepatitis: A Narrative Review. Int. J. Mol. Sci. 2025, 26, 5164. [Google Scholar] [CrossRef] [PubMed]
- He, S.; McPhaul, C.; Li, J.Z.; Garuti, R.; Kinch, L.; Grishin, N.V.; Cohen, J.C.; Hobbs, H.H. A sequence variation (I148M) in PNPLA3 associated with nonalcoholic fatty liver disease disrupts triglyceride hydrolysis. J. Biol. Chem. 2010, 285, 6706–6715. [Google Scholar] [CrossRef]
- Romeo, S.; Kozlitina, J.; Xing, C.; Pertsemlidis, A.; Cox, D.; Pennacchio, L.A.; Boerwinkle, E.; Cohen, J.C.; Hobbs, H.H. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat. Genet. 2008, 40, 1461–1465. [Google Scholar] [CrossRef] [PubMed]
- Sookoian, S.; Pirola, C.J. Meta-analysis of the influence of I148M variant of patatin-like phospholipase domain containing 3 gene (PNPLA3) on the susceptibility and histological severity of nonalcoholic fatty liver disease. Hepatology 2011, 53, 1883–1894. [Google Scholar] [CrossRef] [PubMed]
- Pingitore, P.; Dongiovanni, P.; Motta, B.M.; Meroni, M.; Lepore, S.M.; Mancina, R.M.; Pelusi, S.; Russo, C.; Caddeo, A.; Rossi, G.; et al. PNPLA3 overexpression results in reduction of proteins predisposing to fibrosis. Hum. Mol. Genet. 2016, 25, 5212–5222. [Google Scholar] [CrossRef]
- Kozlitina, J.; Smagris, E.; Stender, S.; Nordestgaard, B.G.; Zhou, H.H.; Tybjærg-Hansen, A.; Vogt, T.F.; Hobbs, H.H.; Cohen, J.C. Exome-wide association study identifies a TM6SF2 variant that confers susceptibility to nonalcoholic fatty liver disease. Nat. Genet. 2014, 46, 352–356. [Google Scholar] [CrossRef] [PubMed]
- Mahdessian, H.; Taxiarchis, A.; Popov, S.; Silveira, A.; Franco-Cereceda, A.; Hamsten, A.; Eriksson, P.; van’t Hooft, F. TM6SF2 is a regulator of liver fat metabolism influencing triglyceride secretion and hepatic lipid droplet content. Proc. Natl. Acad. Sci. USA 2014, 111, 8913–8918. [Google Scholar] [CrossRef]
- Liu, Y.L.; Reeves, H.L.; Burt, A.D.; Tiniakos, D.; McPherson, S.; Leathart, J.B.; Allison, M.E.; Alexander, G.J.; Piguet, A.C.; Anty, R.; et al. TM6SF2 rs58542926 influences hepatic fibrosis progression in patients with non-alcoholic fatty liver disease. Nat. Commun. 2014, 5, 4309. [Google Scholar] [CrossRef]
- Doong, H.; Vrailas, A.; Kohn, E.C. What’s in the ‘BAG’?—A functional domain analysis of the BAG-family proteins. Cancer Lett. 2002, 188, 25–32. [Google Scholar] [CrossRef]
- Rosati, A.; Graziano, V.; De Laurenzi, V.; Pascale, M.; Turco, M.C. BAG3: A multifaceted protein that regulates major cell pathways. Cell Death Dis. 2011, 2, e141. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Bogomolovas, J.; Wu, T.; Zhang, W.; Liu, C.; Veevers, J.; Stroud, M.J.; Zhang, Z.; Ma, X.; Mu, Y.; et al. Loss-of-function mutations in co-chaperone BAG3 destabilize small HSPs and cause cardiomyopathy. J. Clin. Investig. 2017, 127, 3189–3200. [Google Scholar] [CrossRef]
- Behl, C. Breaking BAG: The Co-Chaperone BAG3 in Health and Disease. Trends Pharmacol. Sci. 2016, 37, 672–688. [Google Scholar] [CrossRef]
- De Marco, M.; Del Papa, N.; Reppucci, F.; Iorio, V.; Basile, A.; Falco, A.; Iaccarino, R.; Brongo, S.; De Caro, F.; Capunzo, M.; et al. BAG3 induces α-SMA expression in human fibroblasts and its over-expression correlates with poorer survival in fibrotic cancer patients. J. Cell Biochem. 2022, 123, 91–101. [Google Scholar] [CrossRef]
- Rosati, A.; Basile, A.; D’Auria, R.; d’Avenia, M.; De Marco, M.; Falco, A.; Festa, M.; Guerriero, L.; Iorio, V.; Parente, R.; et al. BAG3 promotes pancreatic ductal adenocarcinoma growth by activating stromal macrophages. Nat. Commun. 2015, 6, 8695. [Google Scholar] [CrossRef]
- De Marco, M.; Gauttier, V.; Pengam, S.; Mary, C.; Ranieri, B.; Basile, A.; Festa, M.; Falco, A.; Reppucci, F.; Cammarota, A.L.; et al. Concerted BAG3 and SIRPα blockade impairs pancreatic tumor growth. Cell Death Discov. 2022, 8, 94. [Google Scholar] [CrossRef]
- Ji, J.; Yu, F.; Ji, Q.; Li, Z.; Wang, K.; Zhang, J.; Lu, J.; Chen, L.; E, Q.; Zeng, Y.; et al. Comparative proteomic analysis of rat hepatic stellate cell activation: A comprehensive view and suppressed immune response. Hepatology 2012, 56, 332–349. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.Y.; Yun, H.H.; Lim, J.H.; Lee, D.H.; Seo, S.B.; Baek, J.Y.; Lee, J.; Yoo, K.; Kim, H.; Kim, H.L.; et al. Hepatocyte-specific deletion of Bis causes senescence in the liver without deteriorating hepatic function. Biochem. Biophys. Res. Commun. 2022, 619, 42–48. [Google Scholar] [CrossRef]
- Damiani, V.; Lamolinara, A.; Cicalini, I.; Cufaro, M.C.; Del Pizzo, F.; Di Marco, F.; Del Boccio, P.; Dufrusine, B.; Hahne, M.; Lattanzio, R.; et al. Pancreatic beta-cell specific BAG3 knockout results in chronic hyperinsulinemia inducing insulin resistance. Mol. Metab. 2023, 74, 101752. [Google Scholar] [CrossRef]
- Longo, M.; Meroni, M.; Paolini, E.; Erconi, V.; Carli, F.; Fortunato, F.; Ronchi, D.; Piciotti, R.; Sabatini, S.; Macchi, C.; et al. TM6SF2/PNPLA3/MBOAT7 Loss-of-Function Genetic Variants Impact on NAFLD Development and Progression Both in Patients and in In Vitro Models. Cell Mol. Gastroenterol. Hepatol. 2022, 13, 759–788. [Google Scholar] [CrossRef]
- Puri, P.; Mirshahi, F.; Cheung, O.; Natarajan, R.; Maher, J.W.; Kellum, J.M.; Sanyal, A.J. Activation and dysregulation of the unfolded protein response in nonalcoholic fatty liver disease. Gastroenterology 2008, 134, 568–576. [Google Scholar] [CrossRef] [PubMed]
- Sanyal, A.J. Past, present and future perspectives in nonalcoholic fatty liver disease. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 377–386. [Google Scholar] [CrossRef]
- Schwabe, R.F.; Tabas, I.; Pajvani, U.B. Mechanisms of Fibrosis Development in Nonalcoholic Steatohepatitis. Gastroenterology 2020, 158, 1913–1928. [Google Scholar] [CrossRef] [PubMed]
- De Marco, M.; Armentaro, G.; Falco, A.; Minniti, A.; Cammarota, A.L.; Iannone, C.; Basile, A.; D’Ardia, A.; Zeppa, P.; Marzullo, L.; et al. Overexpression of BAG3 (Bcl2-associated athanogene 3) in serum and skin of patients with systemic sclerosis. Clin. Exp. Rheumatol. 2024, 42, 1623–1628. [Google Scholar] [CrossRef]
- Venkatesan, N.; Doskey, L.C.; Malhi, H. The Role of Endoplasmic Reticulum in Lipotoxicity during Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD) Pathogenesis. Am. J. Pathol. 2023, 193, 1887–1899. [Google Scholar] [CrossRef] [PubMed]
- Sterling, R.K.; Lissen, E.; Clumeck, N.; Sola, R.; Correa, M.C.; Montaner, J.; Sulkowski, M.S.; Torriani, F.J.; Dieterich, D.T.; Thomas, D.L.; et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 2006, 43, 1317–1325. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines on non-invasive tests for evaluation of liver disease severity and prognosis—2021 update. J. Hepatol. 2021, 75, 659–689. [Google Scholar] [CrossRef]
- Boursier, J.; Hagström, H.; Ekstedt, M.; Moreau, C.; Bonacci, M.; Cure, S.; Ampuero, J.; Nasr, P.; Tallab, L.; Canivet, C.M.; et al. Non-invasive tests accurately stratify patients with NAFLD based on their risk of liver-related events. J. Hepatol. 2022, 76, 1013–1020. [Google Scholar] [CrossRef] [PubMed]

| Variable | Total (n = 146) | MASLD (n = 121) | Cirrhosis + HCC (n = 25) | p-Value |
|---|---|---|---|---|
| Age (years) | 62.9 ± 14.0 | 61.8 ± 14.8 | 67.9 ± 8.4 | 0.020 |
| Male sex | 82 (56.2%) | 64 (52.9%) | 18 (72.0%) | — |
| BMI (kg/m2) | 32.3 ± 6.8 | 32.0 ± 6.8 | 34.2 ± 6.5 | — |
| AST (U/L) | 35.5 ± 23.5 | 35.6 ± 21.1 | 44.7 ± 31.6 | 0.040 |
| ALT (U/L) | 40.8 ± 33.0 | 42.9 ± 35.0 | 30.3 ± 18.1 | — |
| LS (kPa) | 9.36 ± 9.3 | 7.24 ± 4.02 | 19.2 ± 17.5 | 2.98 × 10−8 |
| FIB-4 | 2.51 ± 2.89 | 1.74 ± 1.35 | 6.25 ± 4.89 | 2.11 × 10−10 |
| BAG3 (pg/mL) | 75.9 ± 226.5 | 61.8 ± 220.9 | 143.8 ± 245.1 | 0.011 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Motta, B.M.; Rosati, A.; Festa, M.; Basile, A.; Sarcina, T.; Turco, M.C.; Persico, M. Unraveling the Role of BAG3 in Hepatic Fibrosis: Genetic and Biomarker Insights in Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD). Int. J. Mol. Sci. 2025, 26, 11286. https://doi.org/10.3390/ijms262311286
Motta BM, Rosati A, Festa M, Basile A, Sarcina T, Turco MC, Persico M. Unraveling the Role of BAG3 in Hepatic Fibrosis: Genetic and Biomarker Insights in Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD). International Journal of Molecular Sciences. 2025; 26(23):11286. https://doi.org/10.3390/ijms262311286
Chicago/Turabian StyleMotta, Benedetta Maria, Alessandra Rosati, Mariano Festa, Anna Basile, Tommaso Sarcina, Maria Caterina Turco, and Marcello Persico. 2025. "Unraveling the Role of BAG3 in Hepatic Fibrosis: Genetic and Biomarker Insights in Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD)" International Journal of Molecular Sciences 26, no. 23: 11286. https://doi.org/10.3390/ijms262311286
APA StyleMotta, B. M., Rosati, A., Festa, M., Basile, A., Sarcina, T., Turco, M. C., & Persico, M. (2025). Unraveling the Role of BAG3 in Hepatic Fibrosis: Genetic and Biomarker Insights in Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD). International Journal of Molecular Sciences, 26(23), 11286. https://doi.org/10.3390/ijms262311286










