Impact of Octenyl Succinylation and Bee Products on Maize Starch Films and Apple Storage Quality
Abstract
1. Introduction
2. Results and Discussion
2.1. Quantitative Determination of the Effectiveness of the Starch Esterification Process
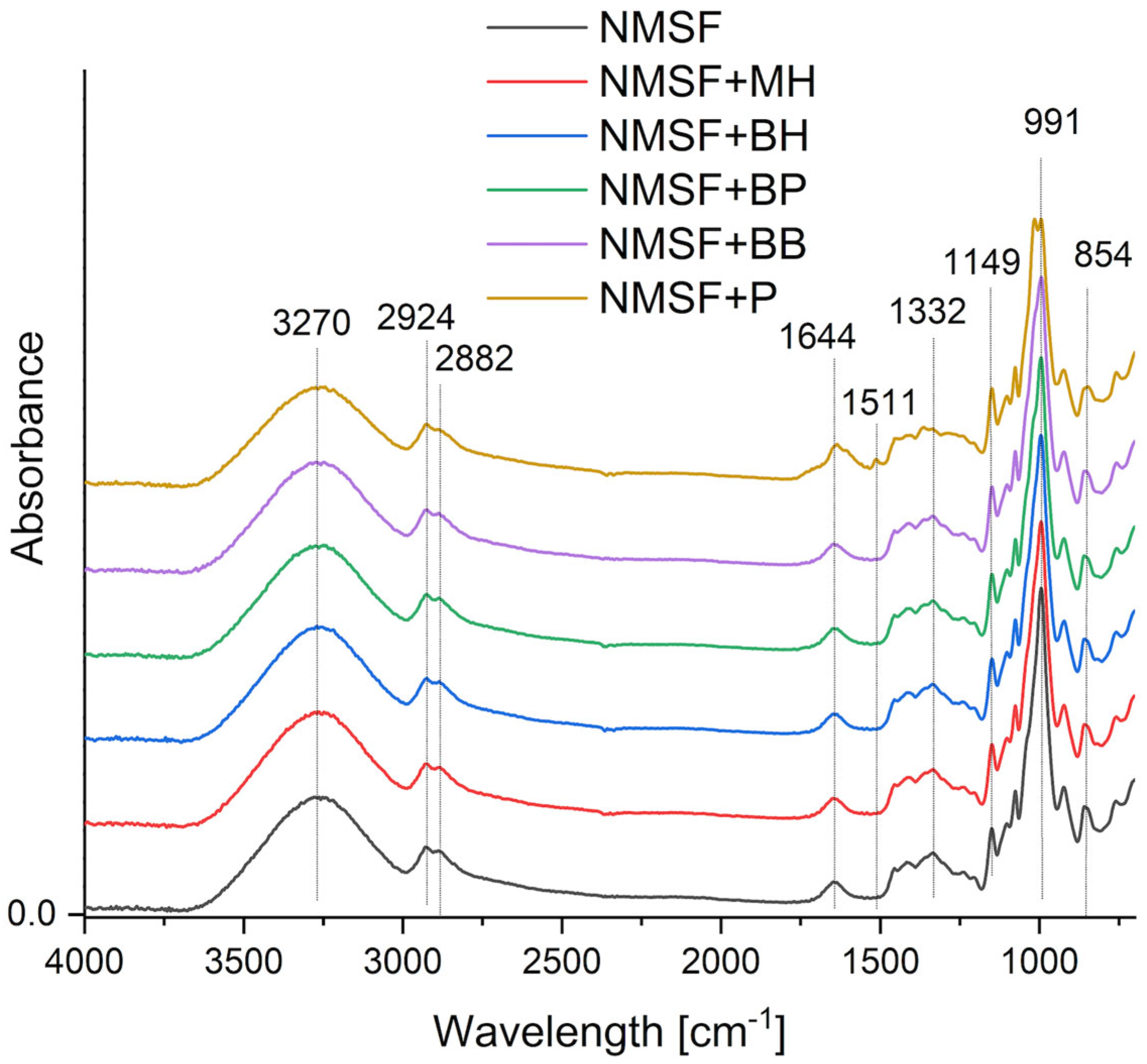
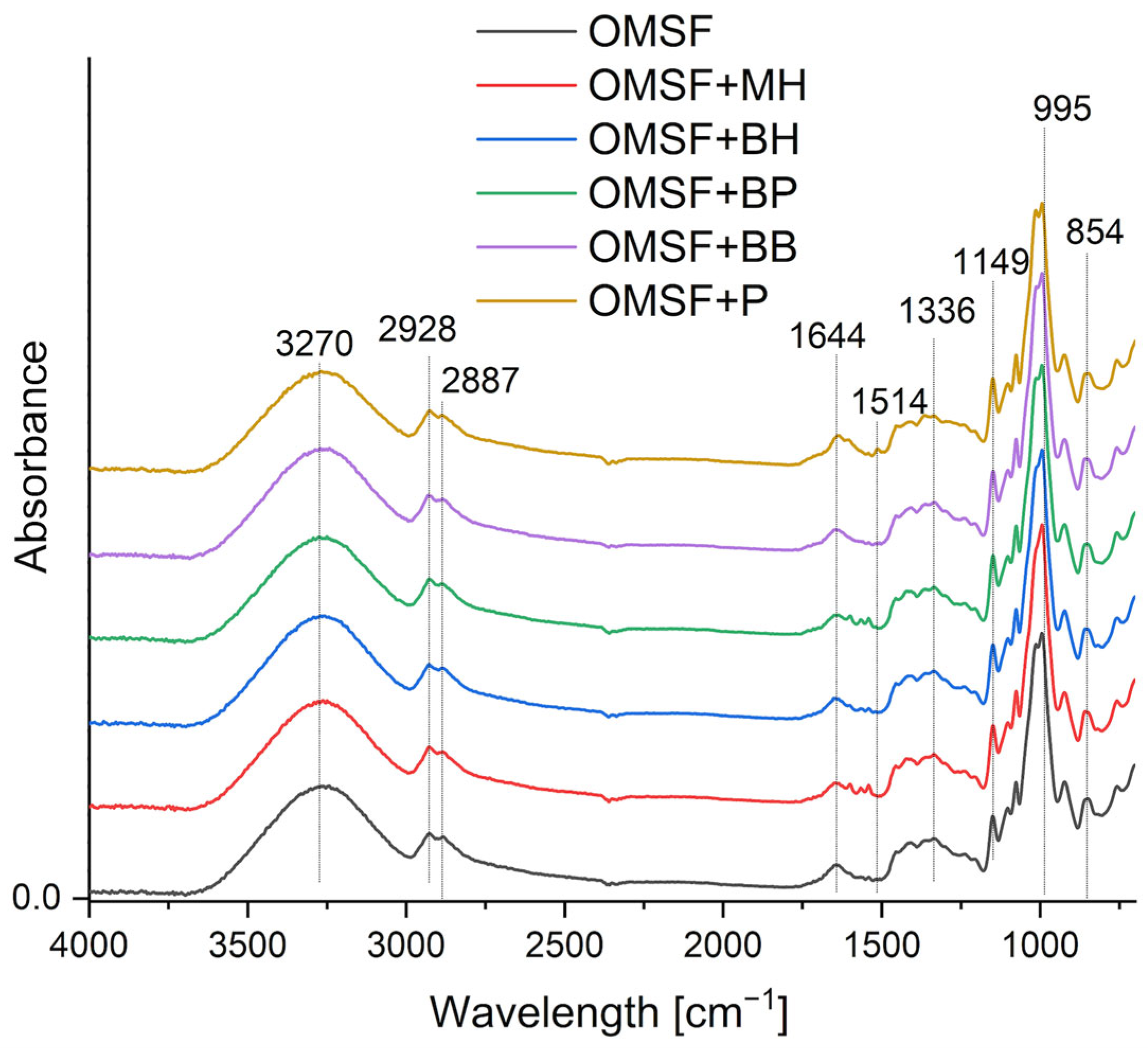
2.2. Fourier Transform Infrared Spectroscopy with Attenuated Total Reflectance (FTIR-ATR)
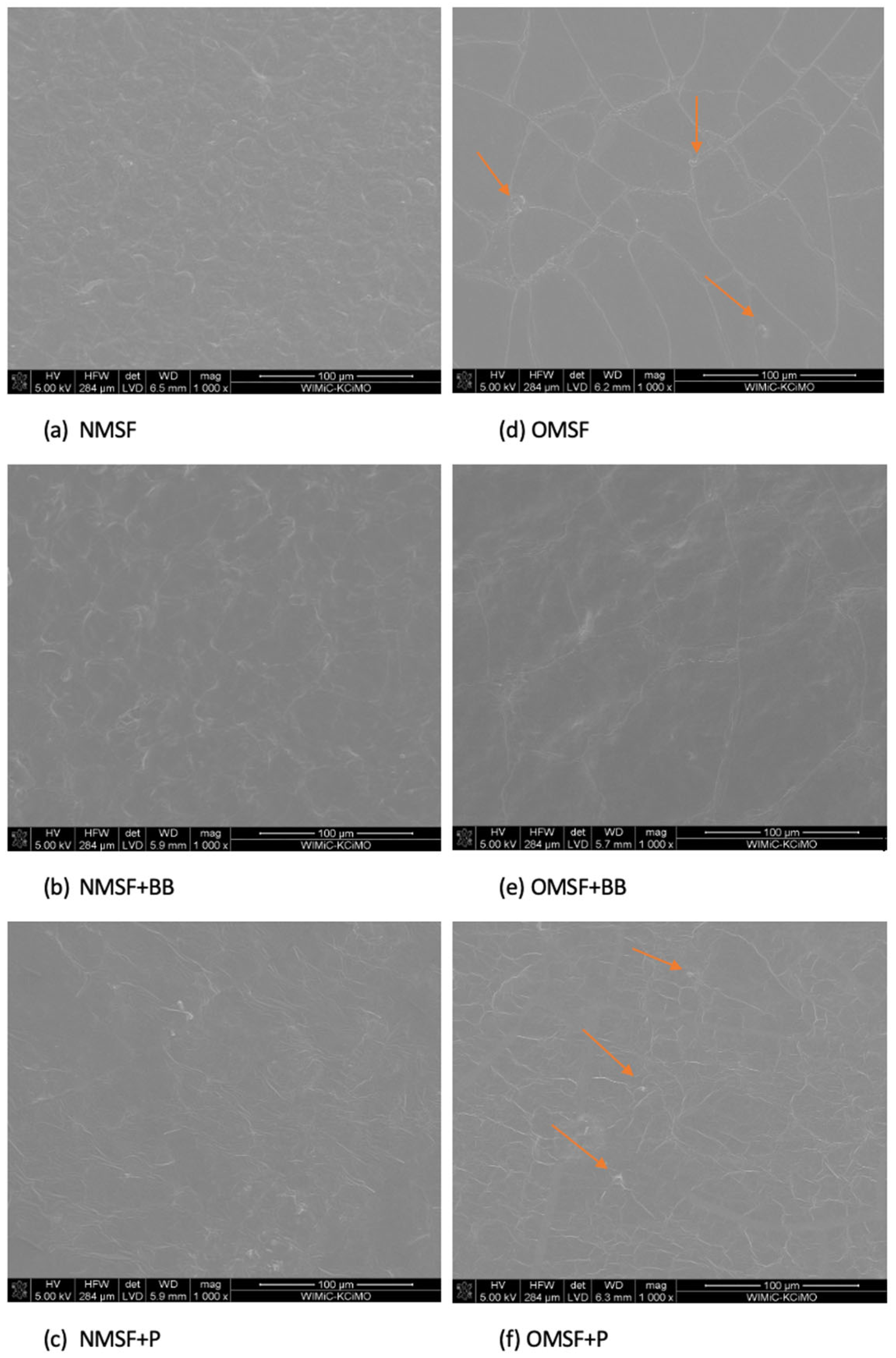
2.3. Scanning Electron Microscopy (SEM)
2.4. Visual Characteristic and Optical Properties
2.5. Thickness Measurement
2.6. Moisture Content, Solubility in Water and Swelling Ratio
2.7. Water Vapor Permeability (WVP)
2.8. Mechanical Properties
2.9. Thermal Properties
2.10. Total Phenolic Content and Antioxidant Activities Measured Using ABTS•+ (2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid)) and DPPH• (2,2-diphenyl-1-picrylhydrazyl) Assays
2.11. Antimicrobial Properties
2.12. Apple Storage
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Preparation of Octenyl-Succinylated Starches and Quantitative Determination of the Effectiveness of Esterification Process
3.2.2. The Extraction Procedure of Phenolic Compounds
3.2.3. Films Preparation
3.2.4. Characterization of Starch Films
3.2.5. Apple Storage
- Visual appearance—the visual appearance of fresh and stored apple slices was assessed.
- Color measurements—the L*, a*, and b* parameters were determined as described in Section 3.2.4. Data were collected from six different locations on the apple surface, and mean values were calculated. The browning index (BI) was calculated using the following equations [5]:
- 3.
- Water activity—the water activity (aw) of fresh and stored apple slices was measured using Swift Lab water activity meter (Swift Lab, Lachen, Switzerland).
- 4.
- Weight loss—weight loss during storage was determined by comparing the initial and final weights of the samples.
3.3. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Farhan, A.; Fazial, F.F.; Azfaralariff, A.; Costa, M.J.; Cerqueira, M.A. Production of Polysaccharide and Protein Edible Films: Challenges and Strategies to Scale-Up. Int. J. Biol. Macromol. 2025, 307, 141909. [Google Scholar] [CrossRef]
- Available online: http://Data.Europa.Eu/Eli/Dir/1994/62/2018-07-04 (accessed on 4 July 2018).
- Łupina, K.; Kowalczyk, D.; Lis, M.; Basiura-Cembala, M. Antioxidant Polysaccharide/Gelatin Blend Films Loaded with Curcumin—A Comparative Study. Int. J. Biol. Macromol. 2023, 236, 123945. [Google Scholar] [CrossRef]
- Chen, N.; Gao, H.X.; He, Q.; Zeng, W.C. Potential Application of Phenolic Compounds with Different Structural Complexity in Maize Starch-Based Film. Food Struct. 2023, 36, 100318. [Google Scholar] [CrossRef]
- Pająk, P.; Socha, R.; Łakoma, P.; Fortuna, T. Antioxidant Properties of Apple Slices Stored in Starch-Based Films. Int. J. Food Prop. 2017, 20, 1117–1128. [Google Scholar] [CrossRef]
- Punia, S.; Sandhu, K.S.; Dhull, S.B.; Kaur, M. Dynamic, Shear and Pasting Behaviour of Native and Octenyl Succinic Anhydride (OSA) Modified Wheat Starch and Their Utilization in Preparation of Edible Films. Int. J. Biol. Macromol. 2019, 133, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Leon-Bejarano, M.; Durmus, Y.; Ovando-Martínez, M.; Simsek, S. Physical, Barrier, Mechanical, and Biodegradability Properties of Modified Starch Films with Nut by-Products Extracts. Foods 2020, 9, 226. [Google Scholar] [CrossRef]
- Chen, N.; Gao, H.X.; He, Q.; Yu, Z.L.; Zeng, W.C. Interaction and Action Mechanism of Starch with Different Phenolic Compounds. Int. J. Food Sci. Nutr. 2020, 71, 726–737. [Google Scholar] [CrossRef]
- Socha, R.; Juszczak, L.; Pietrzyk, S.; Gałkowska, D.; Fortuna, T.; Witczak, T. Phenolic Profile and Antioxidant Properties of Polish Honeys. Int. J. Food Sci. Technol. 2011, 46, 528–534. [Google Scholar] [CrossRef]
- Socha, R.; Gałkowska, D.; Bugaj, M.; Juszczak, L. Phenolic Composition and Antioxidant Activity of Propolis from Various Regions of Poland. Nat. Prod. Res. 2015, 29, 416–422. [Google Scholar] [CrossRef]
- Pająk, P.; Gałkowska, D.; Juszczak, L.; Khachatryan, G. Octenyl Succinylated Potato Starch-Based Film Reinforced by Honey-Bee Products: Structural and Functional Properties. Food Packag. Shelf Life 2022, 34, 100995. [Google Scholar] [CrossRef]
- Pająk, P.; Socha, R.; Królikowska, K.; Grzyb, J.; Hetmańczyk, J.; Zachariasz, P. Characterization of Octenyl Succinylated Potato-Starch Based Films Enriched with Extracts from Various Honey-Bee Products. Int. J. Biol. Macromol. 2025, 285, 138293. [Google Scholar] [CrossRef] [PubMed]
- Jaramillo, C.M.; González Seligra, P.; Goyanes, S.; Bernal, C.; Famá, L. Biofilms Based on Cassava Starch Containing Extract of Yerba Mate as Antioxidant and Plasticizer. Starch/Staerke 2015, 67, 780–789. [Google Scholar] [CrossRef]
- Falcão, L.d.S.; Oliveira, I.d.L.; Gurgel, R.S.; de Souza, A.T.F.; Mendonça, L.d.S.; Usuda, É.O.; do Amaral, T.S.; Veggi, P.C.; Campelo, P.H.; de Vasconcellos, M.C.; et al. Development of Cassava Starch-Based Films Incorporated with Phenolic Compounds Produced by an Amazonian Fungus. Int. J. Biol. Macromol. 2024, 258, 128882. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F. Starch Based Films and Coatings for Food Packaging: Interactions with Phenolic Compounds. Food Res. Int. 2025, 204, 115758. [Google Scholar] [CrossRef]
- Yang, Q.; Zhao, Y.; Hu, X.; Bao, Q.; Zhao, Q.; Zhang, F.; Guo, K.; Li, S.; Li, T. Effect of Starch Nanoparticles and Tea Polyphenol Inclusion on Physicochemical and Mechanical Properties of Starch-Based Films. Starch/Staerke 2024, 76, 2300140. [Google Scholar] [CrossRef]
- Trehan, S.; Singh, N.; Kaur, A. Diversity and Relationship among Grain, Flour and Starch Characteristics of Indian Himalayan Colored Corn Accessions. J. Food Sci. Technol. 2020, 57, 3801–3813. [Google Scholar] [CrossRef]
- Gómez-Aldapa, C.A.; Velazquez, G.; Gutierrez, M.C.; Rangel-Vargas, E.; Castro-Rosas, J.; Aguirre-Loredo, R.Y. Effect of Polyvinyl Alcohol on the Physicochemical Properties of Biodegradable Starch Films. Mater. Chem. Phys. 2020, 239, 122027. [Google Scholar] [CrossRef]
- Guo, Z.; Gou, Q.; Yang, L.; Yu, Q.l.; Han, L. Dielectric Barrier Discharge Plasma: A Green Method to Change Structure of Potato Starch and Improve Physicochemical Properties of Potato Starch Films. Food Chem. 2022, 370, 130992. [Google Scholar] [CrossRef]
- Pająk, P.; Przetaczek-Rożnowska, I.; Juszczak, L. Development and Physicochemical, Thermal and Mechanical Properties of Edible Films Based on Pumpkin, Lentil and Quinoa Starches. Int. J. Biol. Macromol. 2019, 138, 441–449. [Google Scholar] [CrossRef]
- Sivapragasam, N.; Maqsood, S.; Rupasinghe, H.P.V. Berry Bioactive Compounds Immobilized in Starch Matrix for Active and Intelligent Packaging: A Review. Future Foods 2024, 10, 100397. [Google Scholar] [CrossRef]
- Guerreiro, A.C.; Gago, C.M.L.; Faleiro, M.L.; Miguel, M.G.C.; Antunes, M.D.C. The Effect of Alginate-Based Edible Coatings Enriched with Essential Oils Constituents on Arbutus Unedo L. Fresh Fruit Storage. Postharvest Biol. Technol. 2015, 100, 226–233. [Google Scholar] [CrossRef]
- Chen, N.; Gao, H.X.; He, Q.; Zeng, W.C. Potato Starch-Based Film Incorporated with Tea Polyphenols and Its Application in Fruit Packaging. Polymers 2023, 15, 588. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Silva, M.; Bello-Perez, L.A.; Agama-Acevedo, E.; Alvarez-Ramirez, J. Effect of Amylose Content in Morphological, Functional and Emulsification Properties of OSA Modified Corn Starch. Food Hydrocoll. 2019, 97, 105212. [Google Scholar] [CrossRef]
- Available online: https://www.legislation.gov.uk/eur/2012/231 (accessed on 18 November 2025).
- Abdullah, A.H.D.; Chalimah, S.; Primadona, I.; Hanantyo, M.H.G. Physical and Chemical Properties of Corn, Cassava, and Potato Starchs. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Jakarta, Indonesia, 23–24 October 2017; Institute of Physics Publishing: Jakarta, Indonesia, 2018; Volume 160, p. 012003. [Google Scholar] [CrossRef]
- Juneja, P.; Kaur, B.; Odeku, O.A.; Singh, I. Development of Corn Starch-Neusilin UFL2 Conjugate as Tablet Superdisintegrant: Formulation and Evaluation of Fast Disintegrating Tablets. J. Drug Deliv. 2014, 2014, 27035. [Google Scholar] [CrossRef] [PubMed]
- Khachatryan, G.; Khachatryan, K.; Krystyjan, M.; Krzemińska-Fiedorowicz, L.; Lenart-Boroń, A.; Białecka, A.; Krupka, M.; Krzan, M.; Blaszyńska, K.; Hanula, M.; et al. Synthesis and Investigation of Physicochemical and Biological Properties of Films Containing Encapsulated Propolis in Hyaluronic Matrix. Polymers 2023, 15, 1271. [Google Scholar] [CrossRef] [PubMed]
- Lang, S.; Sui, C.; Wang, L. Jet Milling-Activated Direct Synthesis of Octenyl Succinic Anhydride Modified Starch: An Analysis of Structural and Application Properties. Int. J. Food Sci. Technol. 2024, 59, 5490–5501. [Google Scholar] [CrossRef]
- Prdun, S.; Svečnjak, L.; Valentić, M.; Marijanović, Z.; Jerković, I. Characterization of Bee Pollen: Physico-Chemical Properties, Headspace Composition and Ftir Spectral Profiles. Foods 2021, 10, 2103. [Google Scholar] [CrossRef]
- Pérez-Vergara, L.D.; Cifuentes, M.T.; Franco, A.P.; Pérez-Cervera, C.E.; Andrade-Pizarro, R.D. Development and Characterization of Edible Films Based on Native Cassava Starch, Beeswax, and Propolis. NFS J. 2020, 21, 39–49. [Google Scholar] [CrossRef]
- Martins, P.C.; Martins, V.G. Effect of Rice Starch Hydrolysis and Esterification Processes on the Physicochemical Properties of Biodegradable Films. Starch/Staerke 2021, 73, 2100022. [Google Scholar] [CrossRef]
- Thiré, R.M.S.M.; Simão, R.A.; Andrade, C.T. High Resolution Imaging of the Microstructure of Maize Starch Films. Carbohydr. Polym. 2003, 54, 149–158. [Google Scholar] [CrossRef]
- Garcia, M.A.; Pinotti, A.; Zaritzky, N.E. Physicochemical, Water Vapor Barrier and Mechanical Properties of Corn Starch and Chitosan Composite Films. Starch/Staerke 2006, 58, 453–463. [Google Scholar] [CrossRef]
- Menchaca-Rivera, J.A.; Gonzalez-Reyna, M.A.; Avilés-Arellano, L.M.; Fernández-Loyola, R.; Morales-Sánchez, E.; Pérez Robles, J.F. Determination of Optical Properties of a Corn Starch Biofilm. J. Appl. Polym. Sci. 2019, 136, 47111. [Google Scholar] [CrossRef]
- Luchese, C.L.; Spada, J.C.; Tessaro, I.C. Starch Content Affects Physicochemical Properties of Corn and Cassava Starch-Based Films. Ind. Crops Prod. 2017, 109, 619–626. [Google Scholar] [CrossRef]
- Gao, W.; Wu, W.; Liu, P.; Hou, H.; Li, X.; Cui, B. Preparation and Evaluation of Hydrophobic Biodegradable Films Made from Corn/Octenylsuccinated Starch Incorporated with Different Concentrations of Soybean Oil. Int. J. Biol. Macromol. 2020, 142, 376–383. [Google Scholar] [CrossRef]
- Acevedo-Fani, A.; Salvia-Trujillo, L.; Rojas-Graü, M.A.; Martín-Belloso, O. Edible Films from Essential-Oil-Loaded Nanoemulsions: Physicochemical Characterization and Antimicrobial Properties. Food Hydrocoll. 2015, 47, 168–177. [Google Scholar] [CrossRef]
- Song, X.; Zuo, G.; Chen, F. Effect of Essential Oil and Surfactant on the Physical and Antimicrobial Properties of Corn and Wheat Starch Films. Int. J. Biol. Macromol. 2018, 107, 1302–1309. [Google Scholar] [CrossRef]
- Wei, X.; Li, J.; Li, B. Multiple Steps and Critical Behaviors of the Binding of Tannic Acid to Wheat Starch: Effect of the Concentration of Wheat Starch and the Mass Ratio of Tannic Acid to Wheat Starch. Food Hydrocoll. 2019, 94, 174–182. [Google Scholar] [CrossRef]
- Li, J.; Ye, F.; Liu, J.; Zhao, G. Effects of Octenylsuccination on Physical, Mechanical and Moisture-Proof Properties of Stretchable Sweet Potato Starch Film. Food Hydrocoll. 2015, 46, 226–232. [Google Scholar] [CrossRef]
- Song, X.; Zhao, Q.; Li, Z.; Fu, D.; Dong, Z. Effects of Amylose Content on the Paste Properties and Emulsification of Octenyl Succinic Starch Esters. Starch/Staerke 2013, 65, 112–122. [Google Scholar] [CrossRef]
- Majzoobi, M.; Farahnaky, A. Granular Cold-Water Swelling Starch; Properties, Preparation and Applications, a Review. Food Hydrocoll. 2021, 111, 106393. [Google Scholar] [CrossRef]
- López, O.V.; García, M.A. Starch Films from a Novel (Pachyrhizus Ahipa) and Conventional Sources: Development and Characterization. Mater. Sci. Eng. C 2012, 32, 1931–1940. [Google Scholar] [CrossRef]
- Antoniou, J.; Liu, F.; Majeed, H.; Qazi, H.J.; Zhong, F. Physicochemical and Thermomechanical Characterization of Tara Gum Edible Films: Effect of Polyols as Plasticizers. Carbohydr. Polym. 2014, 111, 359–365. [Google Scholar] [CrossRef]
- Zhu, F. Interactions between Starch and Phenolic Compound. Trends Food Sci. Technol. 2015, 43, 129–143. [Google Scholar] [CrossRef]
- Das, D.K.; Dutta, H.; Mahanta, C.L. Development of a Rice Starch-Based Coating with Antioxidant and Microbe-Barrier Properties and Study of Its Effect on Tomatoes Stored at Room Temperature. LWT 2013, 50, 272–278. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, X.; Cao, Y.; Xie, W.; Ma, X.; Yu, X. Edible Starch Sodium Octenyl Succinate Film Formation and Its Physical Properties. J. Appl. Polym. Sci. 2013, 127, 2922–2927. [Google Scholar] [CrossRef]
- Karim, A.A.; Toon, L.C.; Lee, V.P.L.; Ong, W.Y.; Fazilah, A.; Noda, T. Effects of Phosphorus Contents on the Gelatinization and Retrogradation of Potato Starch. J. Food Sci. 2007, 72, C132–C138. [Google Scholar] [CrossRef]
- Norajit, K.; Kim, K.M.; Ryu, G.H. Comparative Studies on the Characterization and Antioxidant Properties of Biodegradable Alginate Films Containing Ginseng Extract. J. Food Eng. 2010, 98, 377–384. [Google Scholar] [CrossRef]
- Daudt, R.M.; Sinrod, A.J.G.; Avena-Bustillos, R.J.; Külkamp-Guerreiro, I.C.; Marczak, L.D.F.; McHugh, T.H. Development of Edible Films Based on Brazilian Pine Seed (Araucaria angustifolia) Flour Reinforced with Husk Powder. Food Hydrocoll. 2017, 71, 60–67. [Google Scholar] [CrossRef]
- Piñeros-Hernandez, D.; Medina-Jaramillo, C.; López-Córdoba, A.; Goyanes, S. Edible Cassava Starch Films Carrying Rosemary Antioxidant Extracts for Potential Use as Active Food Packaging. Food Hydrocoll. 2017, 63, 488–495. [Google Scholar] [CrossRef]
- Medeiros Silva, V.D.; Coutinho Macedo, M.C.; Rodrigues, C.G.; Neris dos Santos, A.; de Freitas e Loyola, A.C.; Fante, C.A. Biodegradable Edible Films of Ripe Banana Peel and Starch Enriched with Extract of Eriobotrya Japonica Leaves. Food Biosci. 2020, 38, 100750. [Google Scholar] [CrossRef]
- Liu, P.; Kang, X.; Cui, B.; Gao, W.; Wu, Z.; Yu, B. Effects of Amylose Content and Enzymatic Debranching on the Properties of Maize Starch-Glycerol Monolaurate Complexes. Carbohydr. Polym. 2019, 222, 115000. [Google Scholar] [CrossRef]
- Biratu, G.; Woldemariam, H.W.; Gonfa, G. Development of Active Edible Films from Coffee Pulp Pectin, Propolis, and Honey with Improved Mechanical, Functional, Antioxidant, and Antimicrobial Properties. Carbohydr. Polym. Technol. Appl. 2024, 8, 100557. [Google Scholar] [CrossRef]
- Kurdziel, M.; Królikowska, K.; Łabanowska, M.; Pietrzyk, S.; Michalec, M. The Effect of Thermal and Irradiation Treatments on Structural and Physicochemical Properties of Octenyl Succinate Maize Starches. Food Chem. 2020, 330, 127242. [Google Scholar] [CrossRef] [PubMed]
- Bhosale, R.; Singhal, R. Effect of Octenylsuccinylation on Physicochemical and Functional Properties of Waxy Maize and Amaranth Starches. Carbohydr. Polym. 2007, 68, 447–456. [Google Scholar] [CrossRef]
- Ngo, V.T.; Kusumawardani, S.; Kunyanee, K.; Luangsakul, N. Polyphenol-Modified Starches and Their Applications in the Food Industry: Recent Updates and Future Directions. Foods 2022, 11, 3384. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Shen, M.; Xiao, W.; Li, Y.; Pan, W.; Xie, J. Regulating the Physicochemical and Structural Properties of Different Starches by Complexation with Tea Polyphenols. Food Hydrocoll. 2023, 142, 108836. [Google Scholar] [CrossRef]
- Zheng, Y.; Tian, J.; Kong, X.; Yang, W.; Yin, X.; Xu, E.; Chen, S.; Liu, D.; Ye, X. Physicochemical and Digestibility Characterisation of Maize Starch–Caffeic Acid Complexes. LWT 2020, 121, 108857. [Google Scholar] [CrossRef]
- Dewi Subramaniam, S.; Hajar Abd Rahim, S.; Abdul Halim, L.; Basrawi, F.; Aini Mohd Azman, N. Study on Bee Bread Extracts as Active Ingredients in SGC-Active Film for Food Packaging Application. Mater. Today Proc. 2023, 86, 1–8. [Google Scholar] [CrossRef]
- Salimi, A.; Khodaiyan, F.; Askari, G.; Amraei, A. Effect of Propolis Extract-Loaded Films Made of Apple Pomace Pectin and Grass Pea Protein on the Shelf Life Extension of Black Mulberry. Food Hydrocoll. 2025, 158, 110549. [Google Scholar] [CrossRef]
- Velásquez, P.; Montenegro, G.; Valenzuela, L.M.; Giordano, A.; Cabrera-Barjas, G.; Martin-Belloso, O. K-Carrageenan Edible Films for Beef: Honey and Bee Pollen Phenolic Compounds Improve Their Antioxidant Capacity. Food Hydrocoll. 2022, 124, 107250. [Google Scholar] [CrossRef]
- Bahraminejad, S.; Mousavi, M.; Askari, G.; Gharaghani, M.; Pourramzan, H. Octenylsuccinated Alginate as a Delivery Agent for Encapsulation of Bergamot Essential Oil: Preparation, Functional Properties and Release Behavior. Int. J. Biol. Macromol. 2024, 282, 136616. [Google Scholar] [CrossRef]
- Pełka, K.; Otłowska, O.; Worobo, R.W.; Szweda, P. Bee Bread Exhibits Higher Antimicrobial Potential Compared to Bee Pollen. Antibiotics 2021, 10, 125. [Google Scholar] [CrossRef]
- Lu, L.C.; Chen, Y.W.; Chou, C.C. Antibacterial Activity of Propolis against Staphylococcus Aureus. Int. J. Food Microbiol. 2005, 102, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Bodini, R.B.; Sobral, P.J.A.; Favaro-Trindade, C.S.; Carvalho, R.A. Properties of Gelatin-Based Films with Added Ethanol-Propolis Extract. LWT 2013, 51, 104–110. [Google Scholar] [CrossRef]
- Grecka, K.; Kuś, P.M.; Okińczyc, P.; Worobo, R.W.; Walkusz, J.; Szweda, P. The Anti-Staphylococcal Potential of Ethanolic Polish Propolis Extracts. Molecules 2019, 24, 1732. [Google Scholar] [CrossRef] [PubMed]
- Sani, A.A.; Pereira, A.F.M.; Furlanetto, A.; de Sousa, D.S.M.; Zapata, T.B.; Rall, V.L.M.; Júnior, A.F. Inhibitory Activities of Propolis, Nisin, Melittin and Essential Oil Compounds on Paenibacillus Alvei and Bacillus Subtilis. J. Venom. Anim. Toxins Incl. Trop. Dis. 2022, 28, 20220025. [Google Scholar] [CrossRef]
- Pełka, K.; Worobo, R.W.; Walkusz, J.; Szweda, P. Bee Pollen and Bee Bread as a Source of Bacteria Producing Antimicrobials. Antibiotics 2021, 10, 713. [Google Scholar] [CrossRef]
- Pastor, C.; Sanchez-Gonzalez, L.; Marcilla, A.; Chiralt, A.; Cháfer, M.; Gonzalez-Martinez, C. Quality and Safety of Table Grapes Coated with Hydroxypropylmethylcellulose Edible Coatings Containing Propolis Extract. Postharvest Biol. Technol. 2011, 60, 64–70. [Google Scholar] [CrossRef]
- Singh, A.; Gu, Y.; Castellarin, S.D.; Kitts, D.D.; Pratap-Singh, A. Development and Characterization of the Edible Packaging Films Incorporated with Blueberry Pomace. Foods 2020, 9, 1599. [Google Scholar] [CrossRef]
- Hui, R.; Qi-he, C.; Ming-liang, F.; Qiong, X.; Guo-qing, H. Preparation and Properties of Octenyl Succinic Anhydride Modified Potato Starch. Food Chem. 2009, 114, 81–86. [Google Scholar] [CrossRef]
- Królikowska, K.; Fortuna, T.; Pietrzyk, S.; Khachatryan, K.; Pająk, P. Effect of Metal Ions on Physicochemical and Rheological Properties of Octenyl Succinate Starches. LWT 2017, 86, 447–455. [Google Scholar] [CrossRef]
- Available online: https://Store.Astm.Org/E0096-00.html (accessed on 2 September 2019).
- Jamróz, E.; Juszczak, L.; Kucharek, M. Investigation of the Physical Properties, Antioxidant and Antimicrobial Activity of Ternary Potato Starch-Furcellaran-Gelatin Films Incorporated with Lavender Essential Oil. Int. J. Biol. Macromol. 2018, 114, 1094–1101. [Google Scholar] [CrossRef]


| Film Samples 2 | Film Appearance | L* 1 | a* | b* | ΔE* | Whiteness Index (WI) | Yellowness Index (YI) |
|---|---|---|---|---|---|---|---|
| NMSF (control) |  | 86.6 ± 1.3 bc | −0.39 ± 0.01 a | 2.63 ± 0.11 c | – | 86.3 | 4.3 |
| NMSF+MH |  | 86.5 ± 1.0 bc | −0.45 ± 0.05 a | 2.47 ± 0.06 c | 0.85 ± 0.28 c | 86.3 | 4.1 |
| NMSF+BH |  | 87.0 ± 0.6 ab | −0.43 ± 0.04 a | 2.74 ± 0.03 c | 0.54 ± 0.45 c | 86.7 | 4.5 |
| NMSF+BP |  | 87.8 ± 0.2 a | −0.74 ± 0.02 b | 3.93 ± 0.07 b | 1.93 ± 0.22 b | 87.2 | 6.4 |
| NMSF+BB |  | 86.8 ± 0.2 b | −0.61 ± 0.02 b | 3.60 ± 0.10 b | 1.02 ± 0.07 c | 86.3 | 5.9 |
| NMSF+P |  | 85.8±0.5 c | −4.35 ± 0.25 c | 32.14 ± 1.14 a | 29.79 ± 1.13 a | 64.6 | 53.5 |
| OMSF (control) |  | 86.1 ± 0.2 D | −0.28 ± 0.05 A | 2.96 ± 0.14 B | – | 85.8 | 4.9 |
| OMSF+MH |  | 86.5 ± 0.3 BC | −0.31 ± 0.04 A | 2.71 ± 0.27 B | 0.51 ± 0.33 B | 86.2 | 4.5 |
| OMSF+BH |  | 86.4 ± 0.1 CD | −0.33 ± 0.03 A | 3.11 ± 0.25 B | 0.38 ± 0.18 B | 86.0 | 5.1 |
| OMSF+BP |  | 86.8 ± 0.3 AB | −0.61 ± 0.03 B | 3.02 ± 0.16 B | 0.79 ± 0.23 B | 86.4 | 4.9 |
| OMSF+BB |  | 87.0 ± 0.2 A | −0.59 ± 0.02 B | 3.25 ± 0.05 B | 1.00 ± 0.16 B | 86.6 | 5.3 |
| OMSF+P |  | 83.1 ± 0.5 E | −2.48 ± 1.49 C | 30.46 ± 1.49 A | 27.76 ± 1.51 A | 65.1 | 52.4 |
| LSD0.05 | – | 0.69 | 0.12 | 0.65 | 0.69 | – | – |
| Film Samples 2 | Thickness 1 [μm] | Moisture Content [%] | Solubility in Water [%] | Swelling Ratio [-] | WVP [g/m·s·Pa] × 10−10 |
|---|---|---|---|---|---|
| NMSF (control) | 80 ± 9 b | 29.1 ± 1.4 a | 20.6 ± 1.5 c | 2.61 ± 0.11 e | 1.52 ± 0.08 ab |
| NMSF+MH | 84 ± 5 b | 26.6 ± 1.3 b | 37.2 ± 2.0 a | 3.77 ± 0.26 c | 1.41 ± 0.08 b |
| NMSF+BH | 86 ± 5 b | 29.2 ± 2.0 a | 23.5 ± 0.6 b | 3.38 ± 0.21 d | 1.54 ± 0.10 ab |
| NMSF+BP | 84 ± 7 b | 28.6 ± 1.1 a | 10.9 ± 0.6 d | 2.30 ± 0.05 e | 1.40 ± 0.08 b |
| NMSF+BB | 84 ± 5 b | 29.8 ± 0.7 a | 23.7 ± 1.0 b | 4.57 ± 0.23 b | 1.40 ± 0.08 b |
| NMSF+P | 96 ± 11 a | 24.4 ± 0.8 c | 24.8 ± 0.2 b | 5.49 ± 0.18 a | 1.60 ± 0.09 a |
| OMSF (control) | 85 ± 11 CD | 31.3 ± 1.9 A | 24.4 ± 1.4 C | 5.39 ± 0.96 C | 1.41 ± 0.08 B |
| OMSF+MH | 83 ± 8 D | 25.1 ± 1.2 D | 26.9 ± 1.8 B | 5.52 ± 0.06 C | 1.57 ± 0.09 B |
| OMSF+BH | 92 ± 6 BC | 28.7 ± 0.9 BC | 20.4 ± 1.2 E | 5.33 ± 0.45 D | 1.54 ± 0.09 B |
| OMSF+BP | 86 ± 5 CD | 30.8 ± 0.3 AB | 8.23 ± 0.2 F | 5.91 ± 0.25 C | 1.43 ± 0.08 B |
| OMSF+BB | 94 ± 5 B | 30.3 ± 1.1 AB | 22.8 ± 0.8 D | 8.13 ± 0.45 B | 1.56 ± 0.09 B |
| OMSF+P | 115 ± 11 A | 27.5 ± 1.1 C | 39.1 ± 1.4 A | 9.27 ± 0.29 A | 1.91 ± 0.11 A |
| LSD0.05 | 6.9 | 1.94 | 2.02 | 0.43 | 0.151 |
| Film Samples 2 | TS 1 [MPa] | %EB [%] | To (°C) | Tp (°C) | Te (°C) | ΔH (J/g) |
|---|---|---|---|---|---|---|
| NMSF (control) | 8.3 ± 1.4 a | 37.0 ± 4.2 d | 269.7 ± 4.9 a | 271.4 ± 4.0 a | 275.7 ± 3.9 a | 102.0 ± 2.8 a |
| NMSF+MH | 6.4 ± 0.4 b | 56.8 ± 7.4 c | 248.7 ± 2.9 b | 250.0 ± 3.1 b | 255.2 ± 2.6 b | 87.4 ± 2.8 b |
| NMSF+BH | 6.9 ± 0.6 b | 55.0 ± 6.7 c | 254.4 ± 5.6 b | 256.3 ± 5.5 b | 260.4 ± 6.4 b | 86.6 ± 3.3 b |
| NMSF+BP | 6.1 ± 0.5 b | 74.6 ± 7.7 a | 247.1 ± 1.6 b | 249.2 ± 1.7 b | 254.7 ± 1.3 b | 83.0 ± 2.9 b |
| NMSF+BB | 4.2 ± 0.5 c | 66.2 ± 7.7 b | 252.0 ± 6.1 b | 254.5 ± 6.1 b | 261.2 ± 6.0 b | 88.9 ± 0.7 b |
| NMSF+P | 3.5 ± 0.3 d | 44.6 ± 6.8 d | 249.8 ± 5.7 b | 252.0 ± 6.2 b | 257.7 ± 6.9 b | 69.8 ± 6.3 c |
| OMSF (control) | 4.0 ± 0.4 B | 40.1 ± 6.7 D | 248.0 ± 4.0 A | 250.8 ± 4.1 A | 257.4 ± 3.6 A | 70.5 ± 1.0 AB |
| OMSF+MH | 3.5 ± 0.3 C | 67.7 ± 7.6 B | 232.3 ± 1.8 BC | 234.3 ± 1.7 BC | 239.6 ± 1.1 BC | 68.4 ± 1.3 BC |
| OMSF+BH | 4.5 ± 0.3 A | 70.4 ± 7.9 AB | 218.4 ± 8.8 D | 220.0 ± 8.7 CD | 224.9 ± 8.8 D | 67.5 ± 3.7 BC |
| OMSF+BP | 2.9 ± 0.3 D | 77.5 ± 7.5 A | 240.8 ± 3.0 AB | 243.1 ± 2.8 AB | 248.1 ± 3.7 AB | 75.0 ± 2.9 A |
| OMSF+BB | 2.4 ± 0.4 E | 70.4 ± 6.7 A | 216.4 ± 1.9 D | 218.0 ± 1.7 D | 223.6 ± 2.7 D | 55.3 ± 5.5 D |
| OMSF+P | 1.4 ± 0.1 F | 58.5 ± 6.5 C | 226.1 ± 12.1 CD | 228.0 ± 11.8 CD | 233.6 ± 12.2 C | 63.9 ± 2.8 C |
| LSD0.05 | 0.64 | 8.0 | 9.71 | 9.45 | 9.82 | 5.6 |
| Film Sample 2 | Antioxidant Activity | ||
|---|---|---|---|
| TPC 1 [mg GAE/g of Film] | DPPH [mg TE/g of Film] | ABTS [mg TE/g of Film] | |
| NMSF (control) | 0.02 ± 0.00 f | 0.06 ± 0.01 d | 0.62 ± 0.03 f |
| NMSF+MH | 0.09 ± 0.00 e | 0.08 ± 0.02 d | 0.79 ± 0.02 e |
| NMSF+BH | 0.19 ± 0.01 d | 0.09 ± 0.02 d | 1.39 ± 0.06 d |
| NMSF+BP | 0.54 ± 0.01 c | 0.19 ± 0.08 c | 2.90 ± 0.02 c |
| NMSF+BB | 0.62 ± 0.04 b | 0.29 ± 0.02 b | 3.17 ± 0.01 b |
| NMSF+P | 27.10 ± 0.10 a | 1.77 ± 0.10 a | 22.13 ± 0.46 a |
| OMSF (control) | 0.08 ± 0.01 E | 0.07 ± 0.03 E | 0.54 ± 0.05 F |
| OMSF+MH | 0.14 ± 0.01 D | 0.12 ± 0.04 D | 0.75 ± 0.01 E |
| OMSF+BH | 0.17 ± 0.00 D | 0.12 ± 0.01 D | 2.32 ± 0.02 D |
| OMSF+BP | 1.23 ± 0.00 C | 0.58 ± 0.01 C | 5.96 ± 0.03 C |
| OMSF+BB | 1.32 ± 0.05 B | 0.63 ± 0.02 B | 6.52 ± 0.04 B |
| OMSF+P | 28.28 ± 0.35 A | 2.20 ± 0.15 A | 24.59 ± 1.21 A |
| LSD0.05 | 0.76 | 0.10 | 0.63 |
| Sample Name 2 | Inhibition Zone [mm] | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| St1 1 | St2 | St3 | St4 | St5 | St6 | St7 | St8 | St9 | Bs | Sal | Ec | |
| NMSF | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| NMSF+MH | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| NMSF+BH | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| NMSF+BP | 9.5 ± 1.0 | 9.5 ± 1.0 | 0 | 0 | 10.0 ± 1.2 | 0 | 0 | 0 | 10.0 ± 1.2 | 0 | 0 | 0 |
| NMSF+BB | 0 | 13.5 ± 2.5 | 0 | 0 | 12.5 ± 1.9 | 0 | 0 | 0 | 0 | 25.0 ± 0.0 | 0 | 11.0 ± 0.0 |
| NMSF+P | 0 | 11.0 ± 1.6 | 15.5 ± 1.0 | 13.0 ± 1.6 | 0 | 13.5 ± 1.0 | 0 | 21.0 ± 0.0 | 13.5 ± 1.9 | 11.0 ± 0.0 | 15.0 ± 0.0 | 17.0 ± 5.4 |
| OMSF | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| OMSF+MH | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| OMSF+BH | 0 | 0 | 0 | 0 | 7.3 ± 4.9 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| OMSF+BP | 10.5 ± 1.9 | 12.0 ± 2.6 | 0 | 0 | 11.5 ± 1.0 | 0 | 0 | 4.5 ± 5.2 | 0 | 0 | 0 | 0 |
| OMSF+BB | 0 | 11.0 ± 1.6 | 0 | 0 | 8.3 ± 5.7 | 0 | 0 | 11.0 ± 1.6 | 0 | 11.0 ± 0.0 | 0 | 25.0 ± 0.0 |
| OMSF+P | 0 | 9.5 ± 1.0 | 13.0 ± 0.0 | 21.5 ± 2.5 | 0 | 10.5 ± 1.9 | 0 | 23.0 ± 3.7 | 16.0 ± 2.6 | 15.0 ± 2.8 | 25.0 ± 0.0 | 18.8 ± 6.1 |
| Name of Sample 2 | Lightness 1 L* [-] | Browning Index BI [-] | Water Activity (aw) [-] | Weight Loss [%] |
|---|---|---|---|---|
| fresh apple | 79.9 ± 1.1 a | 46.0 ± 3.6 d | 0.854 ± 0.001 a | - |
| After 7 days of storage | ||||
| apple in NMSF apple in NMSF+MH apple in NMSF+BH apple in NMSF+BP apple in NMSF+BB apple in NMSF+P | 42.2 ± 4.6 de 40.9 ± 3.8 def 37.4 ± 1.5 f 43.1 ± 1.7 de 40.8±4.1 def 47.8±1.5 c | 112.3 ± 6.5 a 108.6 ± 8.8 a 109.3 ± 6.3 a 115.2 ± 4.7 a 119.4 ± 5.4 a 120.0± 12.5 a | 0.732± 0.008 bc 0.758 ± 0.011 b 0.715 ± 0.008b cd 0.736 ± 0.001 bc 0.732 ± 1.13 bc 0.697 ± 0.040 cd | 59.48 ± 5.37 ab 55.48 ± 4.09 c 60.14 ± 1.55 abc 56.13 ± 2.99 c 56.45 ± 4.96 bc 58.74 ± 1.14 abc |
| apple in OMSF apple in OMSF+MH apple in OMSF+BH apple in OMSF+BP apple in OMSF+BB apple in OMSF+P | 44.9 ± 3.7 cd 43.5 ± 2.0 cde 40.6 ± 3.6 def 40.8 ± 4.1 def 44.7 ± 3.5 cd 42.1 ± 3.5 de | 105.9 ± 5.9 b 107.8 ± 11.7 b 103.3 ± 11.3 b 105.7 ± 10.1 b 122.6 ± 8.0 a 120.0 ± 11.9 a | 0.719 ± 0.004b cd 0.695 ± 0.009 c 0.683 ± 0.013 d 0.683 ± 0.003 d 0.697 ± 0.026 cd 0.634 ± 0.055 e | 60.59 ± 1.91 ab 59.67 ± 2.18 abc 59.19 ± 1.25 abc 61.88 ± 2.27 a 61.02 ± 1.75 ab 63.04 ± 0.44 a |
| apple in LDPEF | 52.3 ± 4.6 b | 76.0 ± 7.3 c | 0.850 ± 0.0115 a | 2.37 ± 0.35 a |
| LSD0.05 | 4.34 | 11.66 | 0.045 | 4.69 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pająk, P.; Królikowska, K.; Juszczak, L.; Khachatryan, G.; Grzyb, J. Impact of Octenyl Succinylation and Bee Products on Maize Starch Films and Apple Storage Quality. Int. J. Mol. Sci. 2025, 26, 11270. https://doi.org/10.3390/ijms262311270
Pająk P, Królikowska K, Juszczak L, Khachatryan G, Grzyb J. Impact of Octenyl Succinylation and Bee Products on Maize Starch Films and Apple Storage Quality. International Journal of Molecular Sciences. 2025; 26(23):11270. https://doi.org/10.3390/ijms262311270
Chicago/Turabian StylePająk, Paulina, Karolina Królikowska, Lesław Juszczak, Gohar Khachatryan, and Jacek Grzyb. 2025. "Impact of Octenyl Succinylation and Bee Products on Maize Starch Films and Apple Storage Quality" International Journal of Molecular Sciences 26, no. 23: 11270. https://doi.org/10.3390/ijms262311270
APA StylePająk, P., Królikowska, K., Juszczak, L., Khachatryan, G., & Grzyb, J. (2025). Impact of Octenyl Succinylation and Bee Products on Maize Starch Films and Apple Storage Quality. International Journal of Molecular Sciences, 26(23), 11270. https://doi.org/10.3390/ijms262311270







