Characterization of Terpenoids in Aromatic Plants Using Raman Spectroscopy and Gas Chromatography–Mass Spectrometry (GC–MS)
Abstract
1. Introduction
2. Results and Discussion
2.1. Analysis of Raman Spectral Data
2.2. Multivariate Analysis of Raman Spectra
2.3. Gas Chromatography–Mass Spectrometry (GC–MS) Results Analysis
2.4. Multivariate Analysis of GC-MS Data
2.5. Limitations and Future Perspectives
3. Materials and Methods
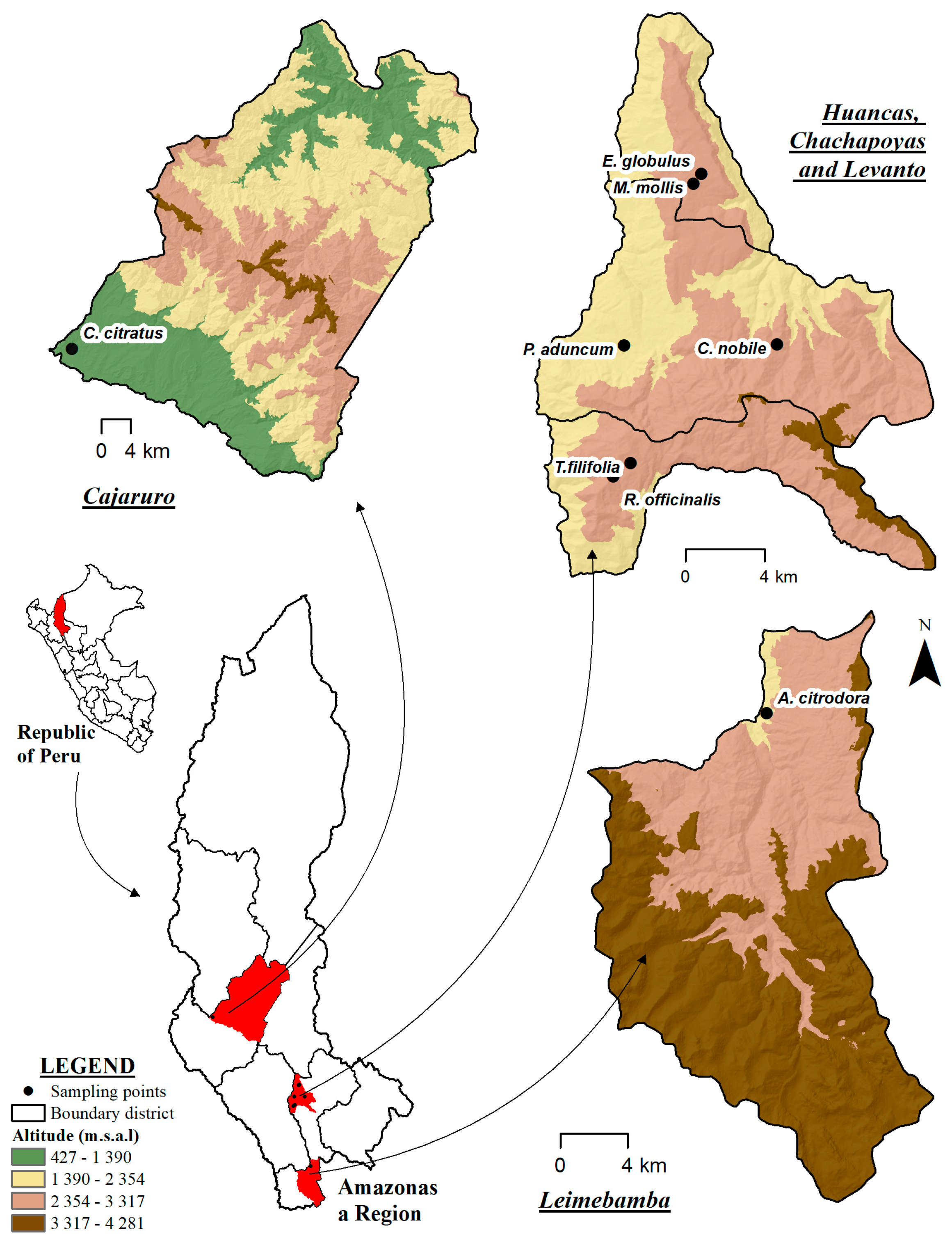
3.1. Plant Material and Sampling
3.2. Essential Oil Extraction (EO)
3.3. Raman Spectral Data Acquisition
3.4. Chemical Characterization by Gas Chromatography–Mass Spectrometry (GC–MS)
3.5. Analysis of Raman and GC–MS Spectral Data
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cravero Ponso, C.; Juncos, N.S.; Di Francisco, G.; Olmedo, H.R. Antioxidant efficiency of Minthostachys mollis (Benth) Griseb. essential oil with low pulegone/MENT relation in combination with BHT. Food Biosci. 2025, 68, 106787. [Google Scholar] [CrossRef]
- Wawoczny, A.; Gillner, D. The Most Potent Natural Pharmaceuticals, Cosmetics, and Food Ingredients Isolated from Plants with Deep Eutectic Solvents. J. Agric. Food Chem. 2023, 71, 10877–10900. [Google Scholar] [CrossRef] [PubMed]
- Bolouri, P.; Salami, R.; Kouhi, S.; Kordi, M.; Asgari Lajayer, B.; Hadian, J.; Astatkie, T. Applications of Essential Oils and Plant Extracts in Different Industries. Molecules 2022, 27, 8999. [Google Scholar] [CrossRef]
- Damtie, D. A Review Article on the Antimicrobial and Antioxidant Essential Oils of Aromatic Plants in Ethiopia. J. Herb. Med. 2024, 48, 100948. [Google Scholar] [CrossRef]
- Aimad, A.; Sanae, R.; Anas, F.; Abdelfattah, E.M.; Bourhia, M.; Mohammad Salamatullah, A.; Alzahrani, A.; Alyahya, H.K.; Albadr, N.A.; Abdelkrim, A.; et al. Chemical Characterization and Antioxidant, Antimicrobial, and Insecticidal Properties of Essential Oil from Mentha pulegium L. Evid. Based Complement. Alt. Med. 2021, 2021, 1108133. [Google Scholar] [CrossRef]
- Nabi, M.H.B.; Ahmed, M.M.; Mia, M.S.; Islam, S.; Zzaman, W. Essential Oils: Advances in Extraction Techniques, Chemical Composition, Bioactivities, and Emerging Applications. Food Chem. Adv. 2025, 8, 101048. [Google Scholar] [CrossRef]
- Lawson, S.K.; Satyal, P.; Setzer, W.N. The Volatile Phytochemistry of Seven Native American Aromatic Medicinal Plants. Plants 2021, 10, 1061. [Google Scholar] [CrossRef] [PubMed]
- Mirković, S.; Martinović, M.; Tadić, V.M.; Nešić, I.; Jovanović, A.S.; Žugić, A. Antimicrobial and Antioxidant Activity of Essential Oils from Selected Pinus Species from Bosnia and Herzegovina. Antibiotics 2025, 14, 677. [Google Scholar] [CrossRef]
- Mukaila, Y.O.; Pfukwa, T.M.; Fawole, O.A. Essential Oils from South African Indigenous Plants: Extraction Techniques, Phytochemistry, Biological Activities and Applications. S. Afr. J. Bot. 2025, 180, 774–794. [Google Scholar] [CrossRef]
- Al-Maharik, N.; Salama, Y.; Al-Hajj, N.; Jaradat, N.; Jobran, N.T.; Warad, I.; Hamdan, L.; Alrob, M.A.; Sawafta, A.; Hidmi, A. Chemical Composition, Anticancer, Antimicrobial Activity of Aloysia citriodora Palau Essential Oils from Four Different Locations in Palestine. BMC Complement. Med. Ther. 2024, 24, 94. [Google Scholar] [CrossRef]
- Elangovan, S.; Mudgil, P. Antibacterial Properties of Eucalyptus Globulus Essential Oil against MRSA: A Systematic Review. Antibiotics 2023, 12, 474. [Google Scholar] [CrossRef]
- Monzote, L.; Scull, R.; Cos, P.; Setzer, W.N. Essential Oil from Piper aduncum: Chemical Analysis, Antimicrobial Assessment, and Literature Review. Medicines 2017, 4, 49. [Google Scholar] [CrossRef]
- Nieto, G.; Ros, G.; Castillo, J. Antioxidant and Antimicrobial Properties of Rosemary (Rosmarinus officinalis L.): A Review. Medicines 2018, 5, 98. [Google Scholar] [CrossRef]
- Olivas-Méndez, P.; Chávez-Martínez, A.; Santellano-Estrada, E.; Guerrero Asorey, L.; Sánchez-Vega, R.; Rentería-Monterrubio, A.L.; Chávez-Flores, D.; Tirado-Gallegos, J.M.; Méndez-Zamora, G. Antioxidant and Antimicrobial Activity of Rosemary (Rosmarinus officinalis) and Garlic (Allium sativum) Essential Oils and Chipotle Pepper Oleoresin (Capsicum annum) on Beef Hamburgers. Foods 2022, 11, 2018. [Google Scholar] [CrossRef]
- Valková, V.; Ďúranová, H.; Galovičová, L.; Borotová, P.; Vukovic, N.L.; Vukic, M.; Kačániová, M. Cymbopogon citratus Essential Oil: Its Application as an Antimicrobial Agent in Food Preservation. Agronomy 2022, 12, 155. [Google Scholar] [CrossRef]
- El-Kased, R.F.; El-Kersh, D.M. GC–MS Profiling of Naturally Extracted Essential Oils: Antimicrobial and Beverage Preservative Actions. Life 2022, 12, 1587. [Google Scholar] [CrossRef] [PubMed]
- Quispe-Sanchez, L.; Mestanza, M.; Oliva-Cruz, M.; Rimarachín, N.; Caetano, A.C.; Chuquizuta, T.; Goñas, M.; Gill, E.R.A.; Chavez, S.G. Oxidative Stability and Physicochemical Changes of Dark Chocolates with Essential Oils Addition. Heliyon 2023, 9, e18139. [Google Scholar] [CrossRef]
- Huang, X.; Li, H.; Ruan, Y.; Li, Z.; Yang, H.; Xie, G.; Yang, Y.; Du, Q.; Ji, K.; Yang, M. An Integrated Approach Utilizing Raman Spectroscopy and Chemometrics for Authentication and Detection of Adulteration of Agarwood Essential Oils. Front. Chem. 2022, 10, 1036082. [Google Scholar] [CrossRef] [PubMed]
- Elshibani, F.A.; Farouk, A.; Naili, E.E.; Mahmoud, E.; Othman, A.; Mohammed, H.A.; Elkazza, N.; Sharkasi, M.A.; Alzunaidy, N.A. GC-MS and HPLC Chemical Profile, Antioxidant, Anti-Acetylcholinesterase, and Anti-Diabetic Activities of Libyan Salvia Lanigera Herb Extract and Essential Oil. Sci. Rep. 2025, 15, 31853. [Google Scholar] [CrossRef] [PubMed]
- Gamal El-Din, M.I.; Youssef, F.S.; Altyar, A.E.; Ashour, M.L. GC/MS Analyses of the Essential Oils Obtained from Different Jatropha Species, Their Discrimination Using Chemometric Analysis and Assessment of Their Antibacterial and Anti-Biofilm Activities. Plants 2022, 11, 1268. [Google Scholar] [CrossRef]
- Sander, A.; Bival Štefan, M.; Radetić, A.; Petračić, A.; Kučić Grgić, D.; Cvetnić, M.; Parlov Vuković, J. Advanced Spectroscopic Characterization, Antioxidant and Antibacterial Activity Evaluation, and Trace Metal Analyses of Essential Oils from Star Anise, Nutmeg, Clove, Oregano, Bay Leaves, and Lemon Peel. Appl. Sci. 2024, 14, 11094. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, M.; Lee, J.; Khan, I.A. Characterization, differentiation, and adulteration detection of peppermint essential oil: An NMR approach. J. Pharm. Biomed. Anal. 2025, 263, 116941. [Google Scholar] [CrossRef]
- Ait benlabchir, A.; Fikri-Benbrahim, K.; Moutawalli, A.; Alanazi, M.M.; Halmoune, A.; Benkhouili, F.Z.; Oubihi, A.; Kabra, A.; Hanoune, E.; Assila, H.; et al. GC–MS Characterization and Bioactivity Study of Eucalyptus globulus Labill. (Myrtaceae) Essential Oils and Their Fractions: Antibacterial and Antioxidant Properties and Molecular Docking Modeling. Pharmaceuticals 2024, 17, 1552. [Google Scholar] [CrossRef] [PubMed]
- El-Kersh, D.M.; Mostafa, N.M.; Fayez, S.; Al-Warhi, T.; Abourehab, M.A.S.; Eldehna, W.M.; Salem, M.A. GC-MS Metabolites Profiling of Anethole-Rich Oils by Different Extraction Techniques: Antioxidant, Cytotoxicity and in-Silico Enzymes Inhibitory Insights. J. Enzyme Inhib. Med. Chem. 2022, 37, 1974–1986. [Google Scholar] [CrossRef]
- Fenghour, H.; Bouabida, H. GC/MS Analysis, Antimicrobial, Fungicidal Activities and Toxicity of Rosmarinus Officinalis Essential Oil Plant from Algeria. Pol. J. Environ. Stud. 2025, 34, 2665–2672. [Google Scholar] [CrossRef] [PubMed]
- Ieri, F.; Cecchi, L.; Giannini, E.; Clemente, C.; Romani, A. GC-MS and HS-SPME-GC×GC-TOFMS Determination of the Volatile Composition of Essential Oils and Hydrosols (By-Products) from Four Eucalyptus Species Cultivated in Tuscany. Molecules 2019, 24, 226. [Google Scholar] [CrossRef] [PubMed]
- Qasem, A. GC-MS Analysis, Antimicrobial Activity, and Genotoxicity of Pimpinella Anisum Essential Oil: In Vitro, ADMET and Molecular Docking Investigations. Phyton 2025, 94, 809–824. [Google Scholar] [CrossRef]
- Huang, W.; Wang, Y.; Tian, W.; Cui, X.; Tu, P.; Li, J.; Shi, S.; Liu, X. Biosynthesis Investigations of Terpenoid, Alkaloid, and Flavonoid Antimicrobial Agents Derived from Medicinal Plants. Antibiotics 2022, 11, 1380. [Google Scholar] [CrossRef]
- Oliveira, M.F.L.D.; Pinheiro, J.F.; Freitas, A.S.; Mendonça, C.D.J.S.; Silva, G.S.D.; Sousa, E.R.D. Exploratory Analysis of Adulterant Detection in Essential Oils Using Infrared Spectroscopy and Chemometric Tools. J. Essent. Oil Res. 2025, 37, 357–367. [Google Scholar] [CrossRef]
- Rashid, H.M.; Mahmod, A.I.; Afifi, F.U.; Talib, W.H. Antioxidant and Antiproliferation Activities of Lemon Verbena (Aloysia citrodora): An In Vitro and In Vivo Study. Plants 2022, 11, 785. [Google Scholar] [CrossRef]
- Abd-Elhafeez, E.M.A.; Ramadan, B.R.; Abou-El-Hawa, S.H.M.; Rashwan, M.R.A. Chemical Composition and Antimicrobial Activity of Anise and Fennel Essential Oils. Assiut J. Agric. Sci. 2023, 54, 127–140. [Google Scholar] [CrossRef]
- Siatis, N.G.; Kimbaris, A.C.; Pappas, C.S.; Tarantilis, P.A.; Daferera, D.J.; Polissiou, M.G. Rapid Method for Simultaneous Quantitative Determination of Four Major Essential Oil Components from Oregano (Oreganum Sp.) and Thyme (Thymus Sp.) Using FT-Raman Spectroscopy. J. Agric. Food Chem. 2005, 53, 202–206. [Google Scholar] [CrossRef]
- Baranska, M.; Schulz, H.; Reitzenstein, S.; Uhlemann, U.; Strehle, M.A.; Krüger, H.; Quilitzsch, R.; Foley, W.; Popp, J. Vibrational Spectroscopic Studies to Acquire a Quality Control Method of Eucalyptus Essential Oils. Biopolymers 2005, 78, 237–248. [Google Scholar] [CrossRef]
- Jentzsch, P.V.; Ramos, L.A.; Ciobotă, V. Handheld Raman Spectroscopy for the Distinction of Essential Oils Used in the Cosmetics Industry. Cosmetics 2015, 2, 162–176. [Google Scholar] [CrossRef]
- Murphy, B.J.; Wilson, T.M.; Ziebarth, E.A.; Bowerbank, C.R.; Carlson, R.E. Authentication of Fennel, Star Anise, and Anise Essential Oils by Gas Chromatography (GC/MS) and Stable Isotope Ratio (GC/IRMS) Analyses. Plants 2024, 13, 214. [Google Scholar] [CrossRef] [PubMed]
- Henry, M.S. Chemical Composition and Bioactivity of Essential Oils from Lippia Alba and Aloysia Citrodora. Rev. Latinoam. Quím. 2024, 51, 13–15. [Google Scholar] [CrossRef]
- Sprea, R.M.; Fernandes, L.H.M.; Pires, T.C.S.P.; Calhelha, R.C.; Rodrigues, P.J.; Amaral, J.S. Volatile Compounds and Biological Activity of the Essential Oil of Aloysia aitrodora Paláu: Comparison of Hydrodistillation and Microwave-Assisted Hydrodistillation. Molecules 2023, 28, 4528. [Google Scholar] [CrossRef]
- Shala, A.Y.; Gururani, M.A. Phytochemical Properties and Diverse Beneficial Roles of Eucalyptus globulus Labill.: A Review. Horticulturae 2021, 7, 450. [Google Scholar] [CrossRef]
- Agnish, S.; Sharma, A.D.; Kaur, I. Nanoemulsions (O/W) Containing Cymbopogon pendulus Essential Oil: Development, Characterization, Stability Study, and Evaluation of in Vitro Anti-Bacterial, Anti-Inflammatory, Anti-Diabetic Activities. BioNanoScience 2022, 12, 540–554. [Google Scholar] [CrossRef]
- Noorbakhsh, F.; Ghasemi, M.M.; Maghbool, M.; Sorouri, M.; Firooziyan, S.; Osanloo, M. Preparation, Characterization, and Antibacterial Evaluation of Nanoemulsions and Chitosan Nanoparticles Containing Lemongrass Essential Oil and Citral against Staphylococcus aureus and Pseudomonas aeruginosa. BioNanoScience 2025, 15, 210. [Google Scholar] [CrossRef]
- Khasanah, L.U.; Ariviani, S.; Purwanto, E.; Praseptiangga, D. Chemical composition and citral content of essential oil of lemongrass (Cymbopogon citratus (DC.) Stapf) leaf waste prepared with various production methods. J. Agric. Food Res. 2025, 19, 101570. [Google Scholar] [CrossRef]
- Silva, T.L.M.D.; Rosa, G.I.D.; Santos, M.A.L.D.; Graf, S.L.; Maia, B.H.L.D.N.S.; Beltrame, F.L.; Ferrari, P.C. Lemongrass Essential Oil (Cymbopogon citratus (DC) Stapf.) Seasonal Evaluation and Microencapsulation by Spray-Drying. Braz. Arch. Biol. Technol. 2023, 66, e23230016. [Google Scholar] [CrossRef]
- Rösch, P.; Popp, J.; Kiefer, W. Raman and Surface Enhanced Raman Spectroscopic Investigation on Lamiaceae Plants. J. Mol. Struct. 1999, 480–481, 121–124. [Google Scholar] [CrossRef]
- Schulz, H.; Baranska, M.; Belz, H.-H.; Rösch, P.; Strehle, M.A.; Popp, J. Chemotaxonomic Characterisation of Essential Oil Plants by Vibrational Spectroscopy Measurements. Vib. Spectrosc. 2004, 35, 81–86. [Google Scholar] [CrossRef]
- Vargas Jentzsch, P.; Ciobotă, V. Raman Spectroscopy as an Analytical Tool for Analysis of Vegetable and Essential Oils. Flavour Fragr. J. 2014, 29, 287–295. [Google Scholar] [CrossRef]
- Santos, F.A.D.; Iulianelli, G.C.V.; Tavares, M.I.B. The Use of Cellulose Nanofillers in Obtaining Polymer Nanocomposites: Properties, Processing, and Applications. Mat. Sci. Appl. 2016, 7, 257–294. [Google Scholar] [CrossRef]
- Kampasakali, E.; Nakas, A.; Mertzanidis, D.; Kokkini, S.; Assimopoulou, A.N.; Christofilos, D. μ-Raman Determination of Essential Oils’ Constituents from Distillates and Leaf Glands of Origanum Plants. Molecules 2023, 28, 1221. [Google Scholar] [CrossRef]
- Vargas Jentzsch, P.; Sandoval Pauker, C.; Zárate Pozo, P.; Sinche Serra, M.; Jácome Camacho, G.; Rueda-Ayala, V.; Garrido, P.; Ramos Guerrero, L.; Ciobotă, V. Raman Spectroscopy in the Detection of Adulterated Essential Oils: The Case of Nonvolatile Adulterants. J. Raman Spectrosc. 2021, 52, 1055–1063. [Google Scholar] [CrossRef]
- Barak, T.H.; Bölükbaş, E.; Bardakcı, H. Evaluation of Marketed Rosemary Essential Oils (Rosmarinus officinalis L.) in Terms of European Pharmacopoeia 10.0 Criteria. Turk J. Pharm. Sci. 2023, 20, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Micić, D.; Đurović, S.; Riabov, P.; Tomić, A.; Šovljanski, O.; Filip, S.; Tosti, T.; Dojčinović, B.; Božović, R.; Jovanović, D.; et al. Rosemary Essential Oils as a Promising Source of Bioactive Compounds: Chemical Composition, Thermal Properties, Biological Activity, and Gastronomical Perspectives. Foods 2021, 10, 2734. [Google Scholar] [CrossRef]
- Omar, J.; Sarmiento, A.; Olivares, M.; Alonso, I.; Etxebarria, N. Quantitative Analysis of Essential Oils from Rosemary in Virgin Olive Oil Using Raman Spectroscopy and Chemometrics. J. Raman Spectrosc. 2012, 43, 1151–1156. [Google Scholar] [CrossRef]
- Székely-Szentmiklósi, I.; Rédai, E.M.; Vlad, R.-A.; Szabó, Z.; Kovács, B.; Gergely, A.-L.; Albert, C.; Székely-Szentmiklósi, B.; Sipos, E. Microencapsulation of Salvia officinalis L. Essential Oil by Complex Coacervation Technology. Stud. UBB Chem. 2024, 69, 221–241. [Google Scholar] [CrossRef]
- Alvarado-García, P.; Soto-Vásquez, M.; Jara-Aguilar, D.; Gavidia-Valencia, J.; Guzmán-Rodríguez, N.; Rodrigo-Villanueva, E.; Beltrán, I. Antidepressant, Anxiolytic, and Antioxidant Properties of Piper Aduncum Essential Oil from Northern Peru. Pharmacogn. J. 2024, 16, 1252–1258. [Google Scholar] [CrossRef]
- van Baren, C.M.; Di Leo Lira, P.; Elechosa, M.A.; Molina, A.M.; Juárez, M.A.; Martínez, A.; Perelman, S.; Bandoni, A.L. New insights into the chemical biodiversity of Minthostachys mollis in Argentina. Biochem. Syst. Ecol. 2014, 57, 374–383. [Google Scholar] [CrossRef]
- de Luna, A.V.; Fagundes, T.D.S.F.; Ramos, Y.J.; de Araújo, M.H.; Muzitano, M.F.; Calixto, S.D.; Simão, T.L.B.V.; de Queiroz, G.A.; Guimarães, E.F.; Marques, A.M.; et al. UHPLC-HRMS/MS Chemical Fingerprinting of the Bioactive Partition from Cultivated Piper aduncum L. Molecules 2024, 29, 1690. [Google Scholar] [CrossRef] [PubMed]
- Strehle, K.R.; Rösch, P.; Berg, D.; Schulz, H.; Popp, J. Quality Control of Commercially Available Essential Oils by Means of Raman Spectroscopy. J. Agric. Food Chem. 2006, 54, 7020–7026. [Google Scholar] [CrossRef]
- Barbosa, A.J.C.; Bezerra, J.J.L.; de Araujo, H.L.S.; da Luz, M.A.; França, J.F.; Barbosa, M.F.D.S.; da Silva, M.V.; de Menezes Torres, M.D.C. Chemical Composition, Antibacterial Activity, and Antibiotic-Potentiating Effect Mediated by the Essential Oil of the Leaves of Croton urticifolius Lam. (Euphorbiaceae). Pharmacol. Res. Nat. Prod. 2025, 8, 100369. [Google Scholar] [CrossRef]
- Recio-Cázares, S.L.; López-Malo, A.; Ramírez-Corona, N.; Palou, E. Relationship Between the Chemical Composition and Transport Properties with the Antimicrobial Activity of Essential Oil from Leaves of Mexican Lippia (Aloysia citriodora) Extracted by Hydro-Distillation. Biointerface Res. Appl. Chem. 2023, 13, 560. [Google Scholar] [CrossRef]
- Malik, P.; Upadhyay, P. GC-MS Chemical profile, Antioxidant Activity, and Sun Protection Factor of Essential Oil of Tea Tree (Melaleuca alternifolia) and Rosemary (Rosmarinus officinalis L.). Orient. J. Chem. 2022, 38, 1266–1275. [Google Scholar] [CrossRef]
- Jedidi, S.; Aloui, F.; Selmi, H.; Jdaidi, N.; Ghribi, S.; Sebai, H. Phytochemical Profile and Antioxidant Properties of Essential Oils Isolated from the Aerial Parts of Rosmarinus officinalis L. Cultivated in Northwestern of Tunisia. Mor. J. Agric. Sci. 2022, 3, 229–233. [Google Scholar] [CrossRef]
- Mohammed, H.A.; Al-Omar, M.S.; Mohammed, S.A.A.; Aly, M.S.A.; Alsuqub, A.N.A.; Khan, R.A. Drying Induced Impact on Composition and Oil Quality of Rosemary Herb, Rosmarinus officinalis Linn. Molecules 2020, 25, 2830. [Google Scholar] [CrossRef]
- Zhang, G.; Ma, Z.; Piao, Y.; Li, N.; Bian, C.; Ren, C.; Gao, M.; Yue, W.; Guan, T. Revealing the Potential of Star Anise Essential Oil: Comparative Analysis and Optimization of Innovative Extraction Methods for Enhanced Yield, Aroma Characteristics, Chemical Composition, and Biological Activities. Food Sci. Nutr. 2024, 12, 9540–9554. [Google Scholar] [CrossRef]
- Zheljazkov, V.D.; Astatkie, T.; Zhalnov, I.; Georgieva, T.D. Method for Attaining Rosemary Essential Oil with Differential Composition from Dried or Fresh Material. J. Oleo Sci. 2015, 64, 485–496. [Google Scholar] [CrossRef]
- Bellumori, M.; Innocenti, M.; Congiu, F.; Cencetti, G.; Raio, A.; Menicucci, F.; Mulinacci, N.; Michelozzi, M. Within-Plant variation in Rosmarinus officinalis L. terpenes and phenols and their antimicrobial activity against the rosemary phytopathogens alternaria alternata and Pseudomonas viridiflava. Molecules 2021, 26, 3425. [Google Scholar] [CrossRef] [PubMed]
- Han, F.; Ma, G.; Yang, M.; Yan, L.; Xiong, W.; Shu, J.; Zhao, Z.; Xu, H. Chemical Composition and Antioxidant Activities of Essential Oils from Different Parts of the Oregano. J. Zhejiang Univ. Sci. B. 2017, 18, 79–84. [Google Scholar] [CrossRef]
- Valadares, A.C.F.; Alves, C.C.F.; Alves, J.M.; De Deus, I.P.B.; De Oliveira Filho, J.G.; Dos Santos, T.C.L.; Dias, H.J.; Crotti, A.E.M.; Miranda, M.L.D. Essential Oils from Piper aduncum Inflorescences and Leaves: Chemical Composition and Antifungal Activity against Sclerotinia sclerotiorum. An. Acad. Bras. Ciênc. 2018, 90, 2691–2699. [Google Scholar] [CrossRef]
- Meza, E.T.V.; Vasquez-Kool, J.; Sánchez, N.I.C.; Vieira, A.; Rodrigues, R.A.F.; Sartoratto, A.; Granados, A.D.P.F.; Tello, C.L.M.; Ruiz, A.L.T.G. Chemical Composition and Anti-Proliferative Activity of Essential Oils from Some Medicinal Plants from Cachicadán, Región La Libertad, Perú. Nat. Prod. Res. 2024, 38, 2145–2150. [Google Scholar] [CrossRef] [PubMed]
- Peralta-Canchis, L.P.; Schneid Kroning, I.; Zandoná, G.P.; Kleinubing, N.R.; Larre Oliveira, T.; Florentini, Á.M.; Valmor Rombaldi, C. Chemical composition of Minthostachys setosa (Briquet) and Piper elongatum (Vahl) essential oils, antistaphylococcal activity and effect on Staphylococcus aureus biofilm removal. Biocatal. Agric. Biotech. 2024, 58, 103170. [Google Scholar] [CrossRef]
- Ghosh, A.; Zhu, E.V.; Wang, H.; Zurek, L.; Zhu, J.J. Antibacterial Activities of Nepetalactones Against Public Health-Related Pathogens. Nat. Prod. Commun. 2021, 16, 1934578X211004875. [Google Scholar] [CrossRef]
- Voigt, V.; Franke, H.; Lachenmeier, D.W. Risk Assessment of Pulegone in Foods Based on Benchmark Dose–Response Modeling. Foods 2024, 13, 2906. [Google Scholar] [CrossRef]
- Assaggaf, H.; El Hachlafi, N.; Elbouzidi, A.; Taibi, M.; Benkhaira, N.; El Kamari, F.; Alnasseri, S.M.; Laaboudi, W.; Bouyahya, A.; Ardianto, C.; et al. Unlocking the combined action of Mentha pulegium L. essential oil and Thym honey: In vitro pharmacological activities, molecular docking, and in vivo anti-inflammatory effect. Heliyon 2024, 10, e31922. [Google Scholar] [CrossRef]
- Kokkini, S.; Hanlidou, E.; Karousou, R.; Lanaras, T. Variation of Pulegone Content in Pennyroyal (Mentha pulegium L.) Plants Growing Wild in Greece. J. Essent. Oil Res. 2002, 14, 224–227. [Google Scholar] [CrossRef]
- Luís, Â.; Domingues, F. Screening of the Potential Bioactivities of Pennyroyal (Mentha pulegium L.) Essential Oil. Antibiotics 2021, 10, 1266. [Google Scholar] [CrossRef] [PubMed]
- Mollaei, S.; Ebadi, M.; Hazrati, S.; Habibi, B.; Gholami, F.; Sourestani, M.M. Essential oil variation and antioxidant capacity of Mentha pulegium populations and their relation to ecological factors. Biochem. Syst. Ecol. 2020, 91, 104084. [Google Scholar] [CrossRef]
- Fajdek-Bieda, A.; Pawlińska, J.; Wróblewska, A.; Łuś, A. Evaluation of the Antimicrobial Activity of Geraniol and Selected Geraniol Transformation Products against Gram-Positive Bacteria. Molecules 2024, 29, 950. [Google Scholar] [CrossRef]
- Khwaza, V.; Aderibigbe, B.A. Antibacterial Activity of Selected Essential Oil Components and Their Derivatives: A Review. Antibiotics 2025, 14, 68. [Google Scholar] [CrossRef]
- Sahal, G.; Woerdenbag, H.J.; Hinrichs, W.L.J.; Visser, A.; Tepper, P.G.; Quax, W.J.; Van Der Mei, H.C.; Bilkay, I.S. Antifungal and biofilm inhibitory effect of Cymbopogon citratus (lemongrass) essential oil on biofilm forming by Candida tropicalis isolates; an in vitro study. J. Ethnopharmacol. 2020, 246, 112188. [Google Scholar] [CrossRef]
- Belhachemi, A.; Maatoug, M.; Canela-Garayoa, R. GC-MS and GC-FID analyses of the essential oil of Eucalyptus camaldulensis grown under greenhouses differentiated by the LDPE cover-films. Ind. Crops Prod. 2022, 178, 114606. [Google Scholar] [CrossRef]
- dos Santos, J.F.; Possate, M.E.D.; Almeida, M.B.D.; Melo, E.M.D.; Furtado, R.A.; Santos, L.D.S.M.; Honório, I.C.G.; de Almeida-Junior, S. Anticoagulant activity of Eucalyptus essential oils: An in vitro approach and a bioinformatics-based pharmacokinetic-pharmacodynamic analysis. J. Pharmacol. Toxicol. Methods 2025, 135, 108381. [Google Scholar] [CrossRef]
- Luís, Â.; Duarte, A.P.; Pereira, L.; Domingues, F. Chemical Profiling and Evaluation of Antioxidant and Anti-Microbial Properties of Selected Commercial Essential Oils: A Comparative Study. Medicines 2017, 4, 36. [Google Scholar] [CrossRef]
- Barbosa, L.C.A.; Filomeno, C.A.; Teixeira, R.R. Chemical Variability and Biological Activities of Eucalyptus spp. Essential Oils. Molecules 2016, 21, 1671. [Google Scholar] [CrossRef] [PubMed]
- Webb, H.; Lanfear, R.; Hamill, J.; Foley, W.J.; Külheim, C. The Yield of Essential Oils in Melaleuca alternifolia (Myrtaceae) Is Regulated through Transcript Abundance of Genes in the MEP Pathway. PLoS ONE 2013, 8, e60631. [Google Scholar] [CrossRef] [PubMed]
- Friedel, M.; Frotscher, J.; Nitsch, M.; Hofmann, M.; Bogs, J.; Stoll, M.; Dietrich, H. Light Promotes Expression of Monoterpene and Flavonol Metabolic Genes and Enhances Flavour of Winegrape Berries (Vitis vinifera L. Cv. Riesling). Aust. J. Grape Wine Res. 2016, 22, 409–421. [Google Scholar] [CrossRef]
- Seira, E.; Poulaki, S.; Hassiotis, C.; Poulios, S.; Vlachonasios, K.E. Gene Expression of Monoterpene Synthases Is Affected Rhythmically during the Day in Lavandula angustifolia Flowers. Physiologia 2023, 3, 433–441. [Google Scholar] [CrossRef]
- Dobhal, P.; Kant Purohit, V.; Chandra, S.; Rawat, S. Climate-induced changes in essential oil production and terpene composition in alpine aromatic plants. Plant Stress 2024, 12, 100445. [Google Scholar] [CrossRef]
- Zhang, X.; Niu, M.; Teixeira da Silva, J.A.; Zhang, Y.; Yuan, Y.; Jia, Y.; Xiao, Y.; Li, Y.; Fang, L.; Zeng, S.; et al. Identification and Functional Characterization of Three New Terpene Synthase Genes Involved in Chemical Defense and Abiotic Stresses in Santalum album. BMC Plant Biol. 2019, 19, 115. [Google Scholar] [CrossRef]
- Malik, T.G.; Sahu, L.K.; Gupta, M.; Mir, B.A.; Gajbhiye, T.; Dubey, R.; Clavijo McCormick, A.; Pandey, S.K. Environmental Factors Affecting Monoterpene Emissions from Terrestrial Vegetation. Plants 2023, 12, 3146. [Google Scholar] [CrossRef]
- Wu, Y.; Zhuo, Z.; Qian, Q.; Xu, D. Chemotaxonomic Variation of Volatile Components in Zanthoxylum Bungeanum Peel and Effects of Climate on Volatile Components. BMC Plant Biol. 2024, 24, 793. [Google Scholar] [CrossRef]
- Zhang, T.; Zheng, Y.; Fu, C.; Yang, H.; Liu, X.; Qiu, F.; Wang, X.; Wang, Z. Chemical Variation and Environmental Influence on Essential Oil of Cinnamomum camphora. Molecules 2023, 28, 973. [Google Scholar] [CrossRef]
- Dumitrescu, E.; Muselin, F.; Tîrziu, E.; Folescu, M.; Dumitrescu, C.S.; Orboi, D.M.; Cristina, R.T. Pimpinella anisum L. Essential Oil a Valuable Antibacterial and Antifungal Alternative. Plants 2023, 12, 2428. [Google Scholar] [CrossRef]
- Eiasu, B.K.; Dyafta, V.; Araya, H.T. Effect of Leaf Age on Essential Oil Yield and Composition in Rose-Scented Geranium. Hort. Sci. 2022, 57, 1524–1528. [Google Scholar] [CrossRef]
- Oğuz, I.K.; Kaplan, M. El efecto de la altitud y las propiedades del suelo sobre los componentes de aceites esenciales en la salvia turca (Salvia fruticosa Mill.). Bol. Latinoam. Caribe Plantas Med. Aromát. 2023, 22, 204–213. [Google Scholar] [CrossRef]
- Benković-Lačić, T.; Orehovec, I.; Mirosavljević, K.; Benković, R.; Ćavar Zeljković, S.; Štefelová, N.; Tarkowski, P.; Salopek-Sondi, B. Effect of Drying Methods on Chemical Profile of Chamomile (Matricaria chamomilla L.) Flowers. Sustainability 2023, 15, 15373. [Google Scholar] [CrossRef]
- Slavik, B.; Roehrer, S.; Loos, H.M.; Minceva, M.; Buettner, A. Isolation of Sesquiterpenoids from Matricaria chamomilla by Means of Solvent Assisted Flavor Evaporation and Centrifugal Partition Chromatography. Anal. Bioanal. Chem. 2021, 413, 4387–4396. [Google Scholar] [CrossRef]
- Rojas-Molina, J.O.; Pino, J.A.; Cevallos-Carvajal, E.R.; Zambrano-Ochoa, Z.E.; Vaca-Castro, C.E.; Molina-Borja, F.A.; Mena-Herrera, K.R. Aceite esencial de hojas de Minthostachys mollis [HBK] Griseb. del Ecuador: Extracción, composición química, capacidad antioxidante y actividad antimicrobiana. Bol. Latinoam. Caribe Plantas Med. Aromát. 2024, 23, 437–447. [Google Scholar] [CrossRef]
- Ruiz-Durán, J.; Torres, R.; Stashenko, E.E.; Ortiz, C. Antifungal and Antibiofilm Activity of Colombian Essential Oils against Different Candida Strains. Antibiotics 2023, 12, 668. [Google Scholar] [CrossRef]
- Čmiková, N.; Galovičová, L.; Schwarzová, M.; Vukic, M.D.; Vukovic, N.L.; Kowalczewski, P.Ł.; Bakay, L.; Kluz, M.I.; Puchalski, C.; Kačániová, M. Chemical Composition and Biological Activities of Eucalyptus globulus Essential Oil. Plants 2023, 12, 1076. [Google Scholar] [CrossRef] [PubMed]
- Morocho, V.; Chamba, A.; Pozo, P.; Montalván, M.; Suárez, A.I. Chemical Characterization and Enantioselective Analysis of Tagetes filifolia Lag. Essential Oil and Crude Extract. Plants 2024, 13, 1921. [Google Scholar] [CrossRef]
- Cebi, N.; Arici, M.; Sagdic, O. The Famous Turkish Rose Essential Oil: Characterization and Authenticity Monitoring by FTIR, Raman and GC–MS Techniques Combined with Chemometrics. Food Chem. 2021, 354, 129495. [Google Scholar] [CrossRef]
- Khaiper, M.; Poonia, P.K.; Dhanda, S.K.; Beniwal, R.; Verma, P.; Nasir, M. Seasonal variation in chemical composition and bioactivity of Eucalyptus tereticornis leaf essential oil. Biochem. Syst. Ecol. 2025, 121, 104988. [Google Scholar] [CrossRef]
- Niu, Y.; Xu, L.; Qiao, M.; Wang, Y. The Anti-Depression Effect and Mechanism of Harmonious rosemary Essential Oil and Its Application in Microcapsules. Materials Today Bio 2025, 31, 101546. [Google Scholar] [CrossRef]
- Raffo, A.; Baiamonte, I.; De Benedetti, L.; Lupotto, E.; Marchioni, I.; Nardo, N.; Cervelli, C. Exploring volatile aroma and non-volatile bioactive compounds diversity in wild populations of rosemary (Salvia rosmarinus Schleid.). Food Chem. 2023, 404, 134532. [Google Scholar] [CrossRef] [PubMed]
- da Costa Sobral, K.G.; Neuberger, B.; Mello, F.K.; Mallmann, M.P.; Sampaio, T.B.; Oliveira, M.S. Anticonvulsant Activity of β-Caryophyllene in Association with Pregabalin in a Seizure Model in Rats. Epilepsy Res. 2022, 179, 106842. [Google Scholar] [CrossRef]
- Nunes, J.D.A.; Teixeira, L.L.; Nascimento, W.M.O.; Lopes, D.C.F.; da Silva, J.K.R.; Pinto, L.C.; Santos, P.V.L.; Figueiredo, P.L.B. Seasonal variation on chemical composition and in vitro cytotoxic and anti-inflammatory activities of Myrciaria dubia (Kunth) McVaugh essential oil from Amazon. Biochem. Syst. Ecol. 2025, 121, 105001. [Google Scholar] [CrossRef]
- Sánchez-Tito, M.A.; Cartagena-Cutipa, R.; Flores-Valencia, E.; Collantes Díaz, I. Chemical composition and antimicrobial activity of essential oil from Minthostachys mollis against oral pathogens. Rev. Cuba Estomatol. 2025, 58, e3647. [Google Scholar]
- Juncos, N.S.; Cravero, C.F.; Grosso, N.R.; Olmedo, R.H. Pulegone/MENT ratio as an indicator of antioxidant activity for the selection of industrial cultivars of peperina with high antioxidant potential. Ind. Crops Prod. 2024, 216, 118770. [Google Scholar] [CrossRef]
- Aničić, N.; Gašić, U.; Lu, F.; Ćirić, A.; Ivanov, M.; Jevtić, B.; Dimitrijević, M.; Anđelković, B.; Skorić, M.; Nestorović Živković, J.; et al. Antimicrobial and Immunomodulating Activities of Two Endemic Nepeta Species and Their Major Iridoids Isolated from Natural Sources. Pharmaceuticals 2021, 14, 414. [Google Scholar] [CrossRef]
- Batume, C.; Mulongo, I.M.; Ludlow, R.; Ssebaale, J.; Randerson, P.; Pickett, J.A.; Mukisa, I.M.; Scofield, S. Evaluating Repellence Properties of Catnip Essential Oil against the Mosquito Species Aedes Aegypti Using a Y-Tube Olfactometer. Sci. Rep. 2024, 14, 2269. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.; Gupta, S.; Singh, S.; Verma, R.S.; Srivastava, R.K.; Gupta, A.K.; Lal, R.K. Genetic Variability and Elite Line Selection for High Essential Oil and Nepetalactone Content in Catmint (Nepeta cataria L.). Am. J. Plant Sci. 2021, 12, 1135–1154. [Google Scholar] [CrossRef]
- Vasquez-Gomez, K.L.; Mori-Mestanza, D.; Caetano, A.C.; Idrogo-Vasquez, G.; Culqui-Arce, C.; Auquiñivin-Silva, E.A.; Castro-Alayo, E.M.; Cruz-Lacerna, R.; Perez-Ramos, H.A.; Balcázar-Zumaeta, C.R.; et al. Exploring Chemical Properties of Essential Oils from Citrus Peels Using Green Solvent. Heliyon 2024, 10, e40088. [Google Scholar] [CrossRef]
- Balcázar-Zumaeta, C.R.; Maicelo-Quintana, J.L.; Salón-Llanos, G.; Barrena, M.; Muñoz-Astecker, L.D.; Cayo-Colca, I.S.; Torrejón-Valqui, L.; Castro-Alayo, E.M. A Novel Technique Using Confocal Raman Spectroscopy Coupled with PLS-DA to Identify the Types of Sugar in Three Tropical Fruits. App. Sci. 2024, 14, 8476. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4.1 ed.; Allured Business Media: Carol Stream, IL, USA, 2017. [Google Scholar]
- Castro-Alayo, E.M.; Torrejón-Valqui, L.; Cayo-Colca, I.; Cárdenas-Toro, F.P. Evaluation of the Miscibility of Novel Cocoa Butter Equivalents by Raman Mapping and Multivariate Curve Resolution–Alternating Least Squares. Foods 2021, 10, 3101. [Google Scholar] [CrossRef] [PubMed]
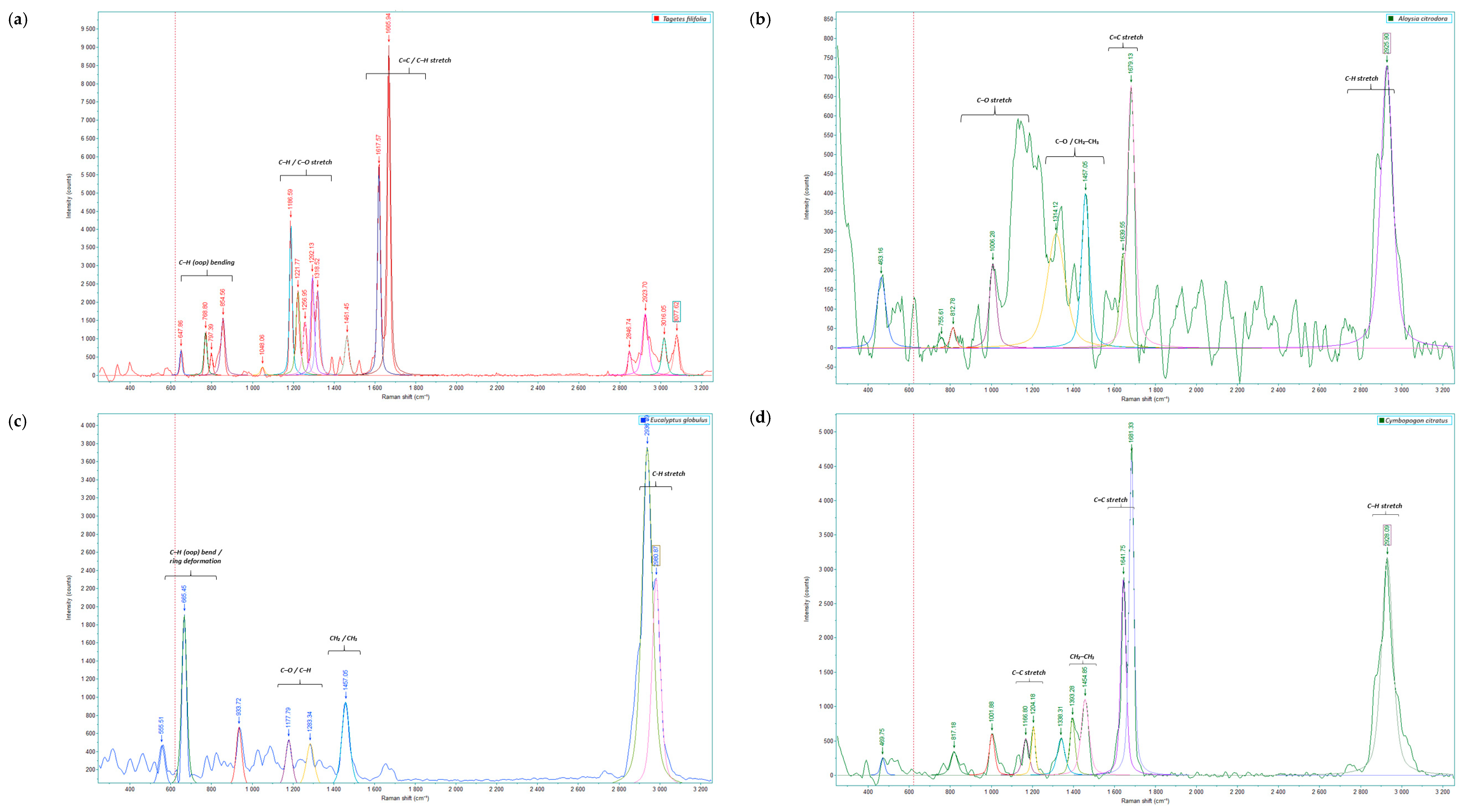
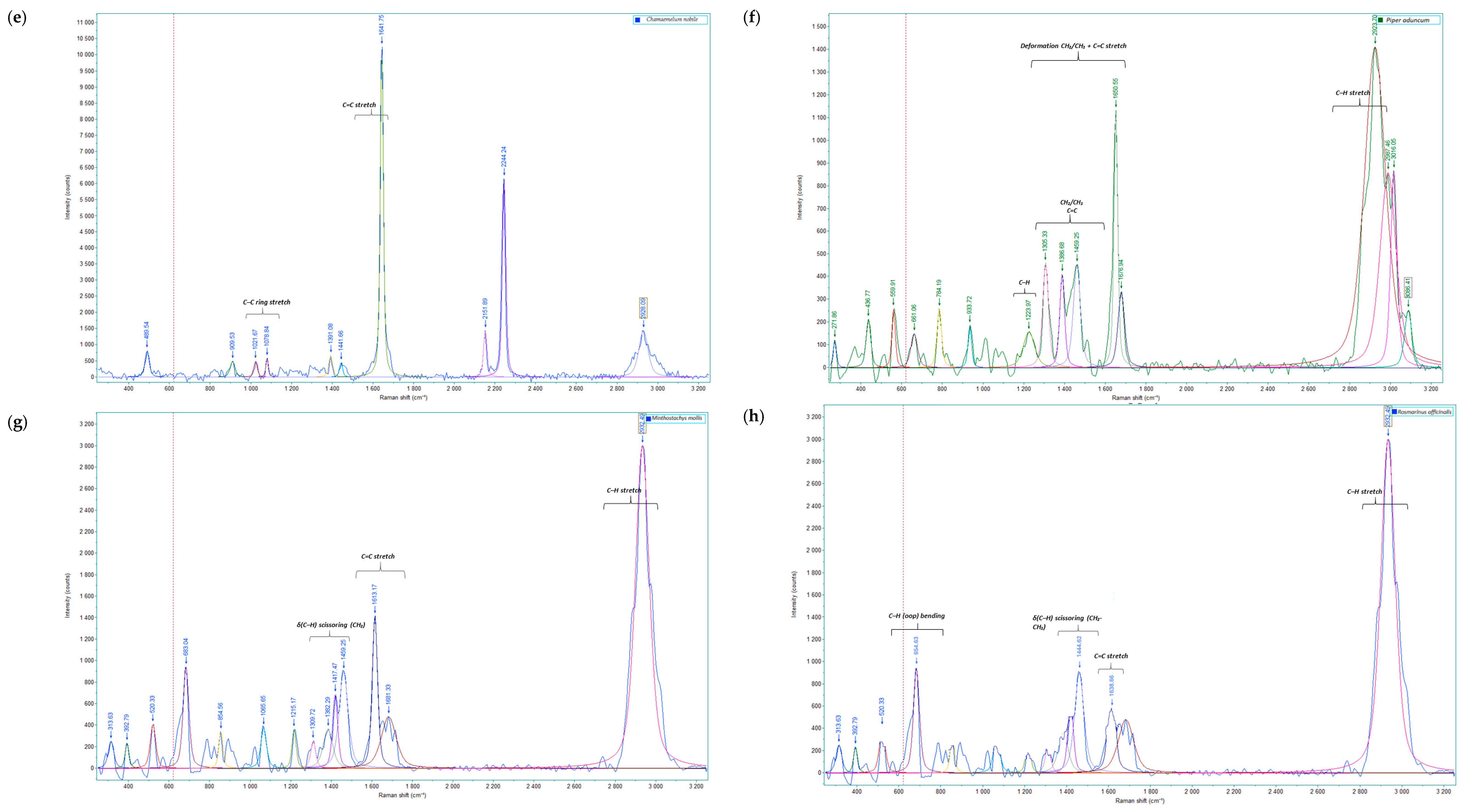

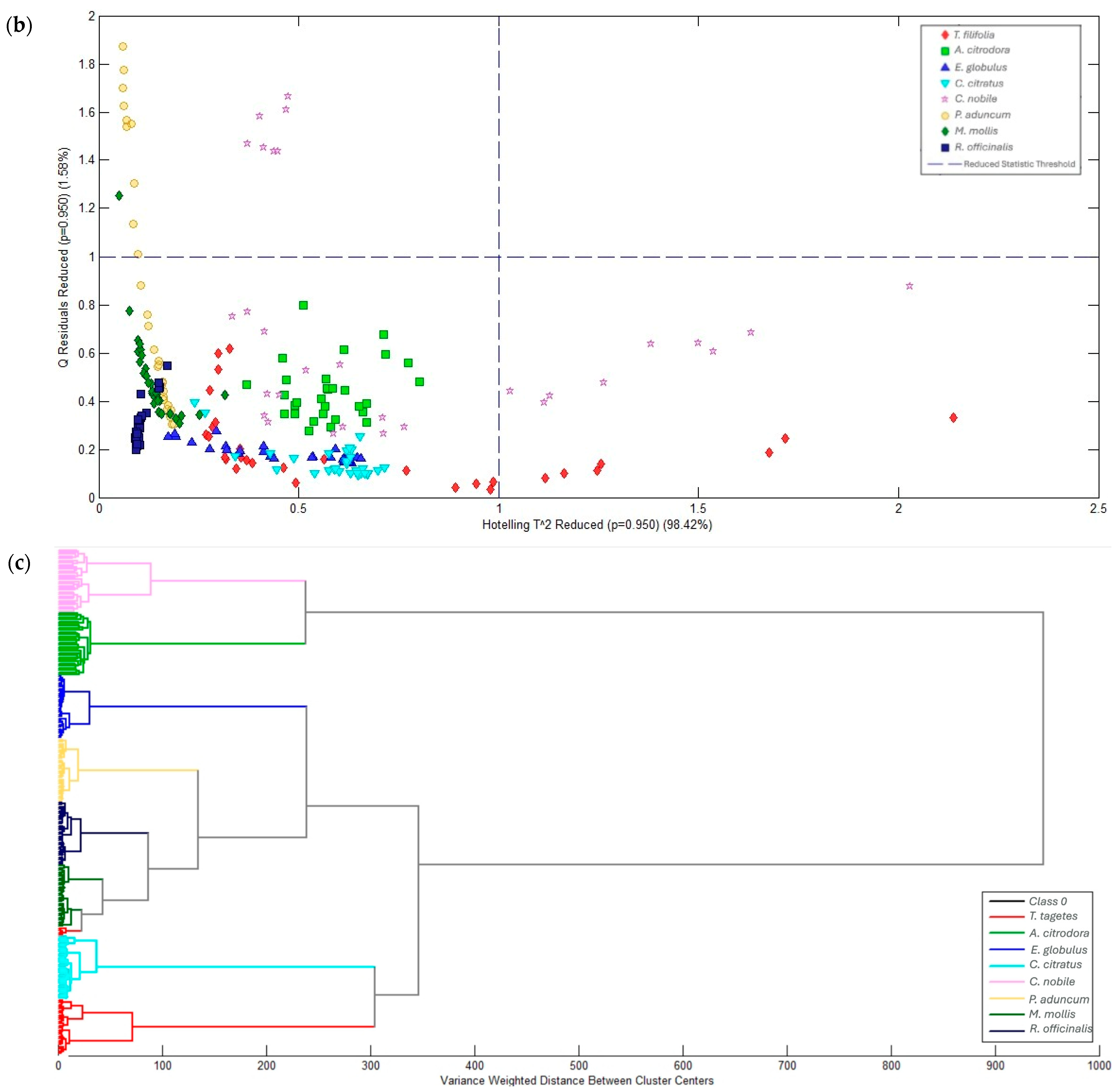
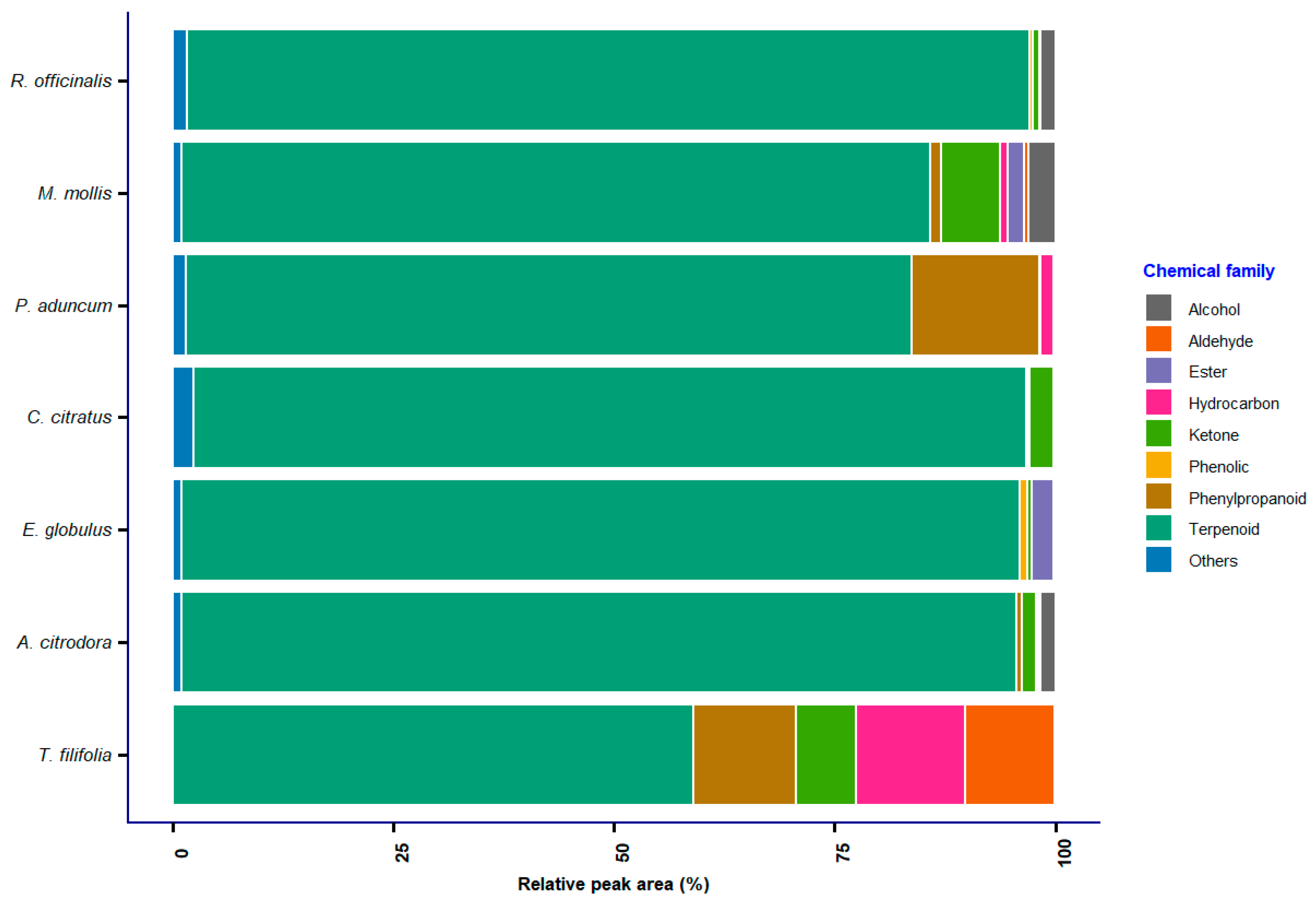
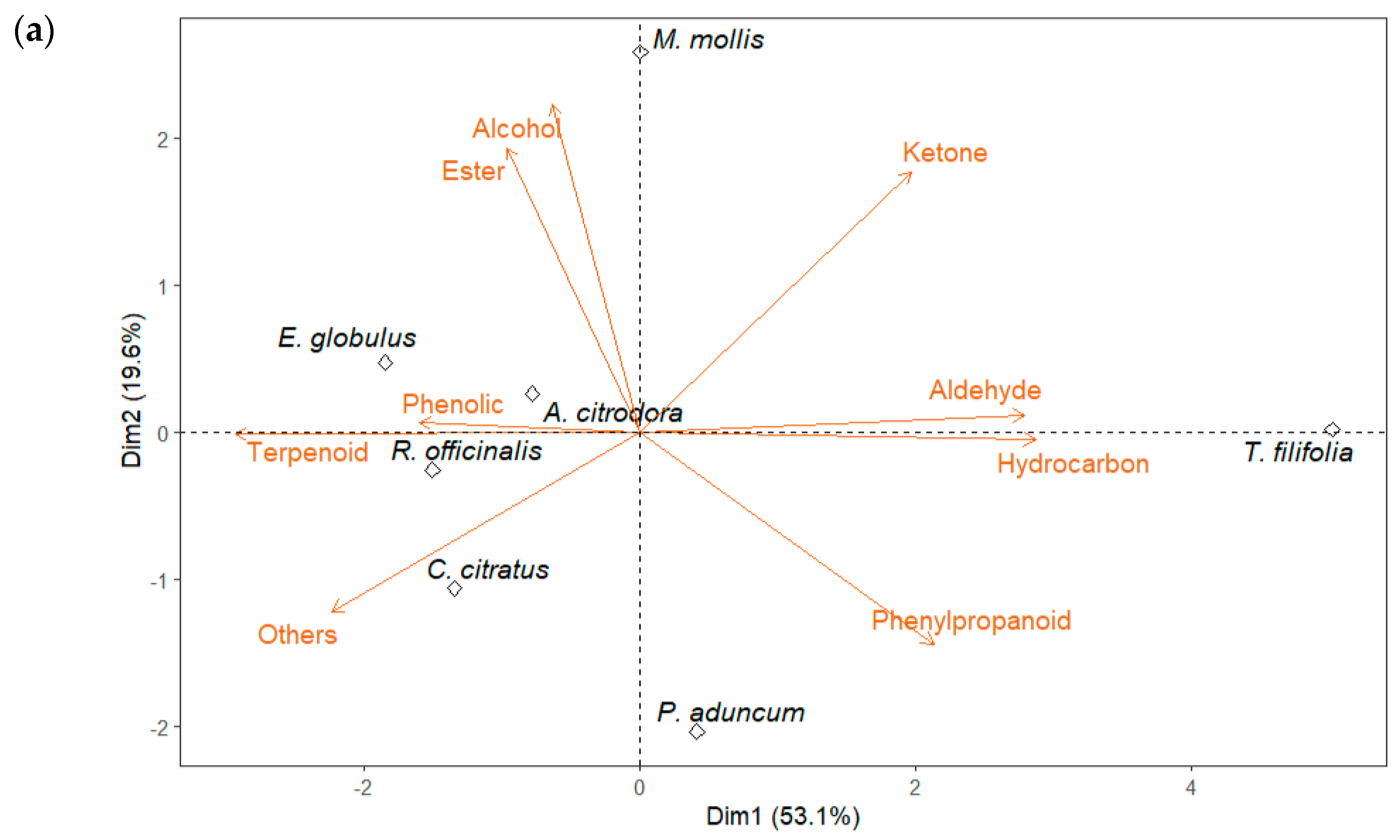
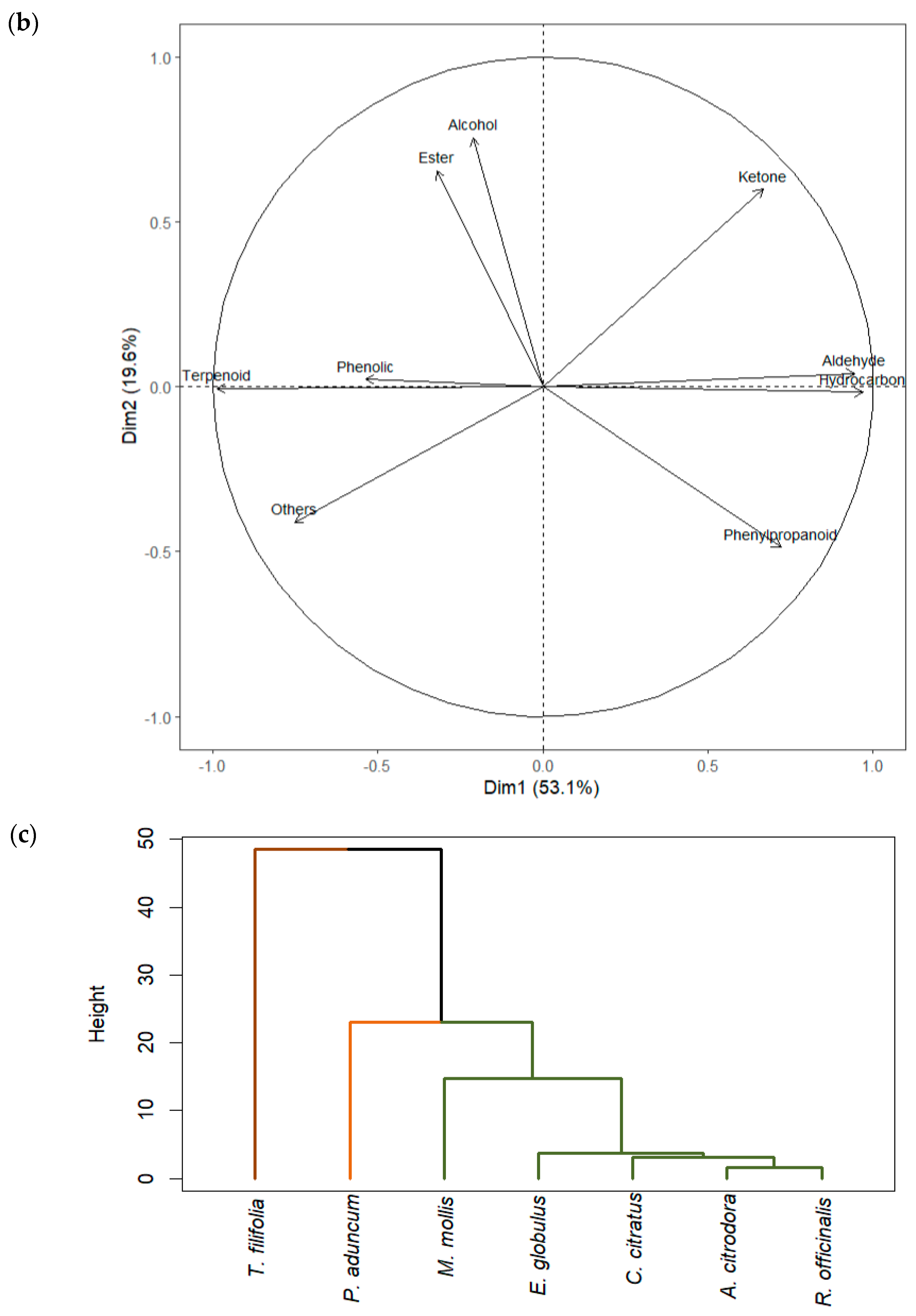
| Group | Compound | R. officinalis | M. mollis | P. aduncum | C. citratus | E. globulus | A. citrodora | T. filifolia |
|---|---|---|---|---|---|---|---|---|
| Terpenoid | cis,cis-Nepetalactone | 0.00 | 30.16 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| α-terpinene | 2.51 | 0.00 | 1.35 | 0.00 | 0.13 | 0.00 | 0.00 | |
| Citral | 0.00 | 0.00 | 0.00 | 0.00 | 0.40 | 0.00 | 2.35 | |
| Myrcene | 0.00 | 0.39 | 0.00 | 0.00 | 8.49 | 0.00 | 0.58 | |
| Sabinene | 0.49 | 0.86 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| β-caryophyllene | 18.26 | 0.00 | 11.00 | 0.22 | 0.00 | 6.00 | 0.00 | |
| Citronellol | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 10.92 | 0.00 | |
| Linalool | 2.91 | 9.63 | 0.33 | 2.33 | 0.90 | 0.59 | 1.61 | |
| Terpinen-4-ol | 0.00 | 0.00 | 2.03 | 0.00 | 3.54 | 0.15 | 0.00 | |
| Thymol | 0.00 | 2.04 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| D-limonene | 8.59 | 6.87 | 4.59 | 0.28 | 0.00 | 6.31 | 2.81 | |
| Eucalyptol | 0.00 | 0.29 | 0.00 | 0.00 | 0.00 | 1.42 | 0.35 | |
| Geraniol | 0.00 | 0.00 | 0.00 | 6.06 | 3.13 | 3.17 | 0.39 | |
| β-pinene | 0.00 | 0.00 | 3.37 | 0.00 | 8.21 | 0.00 | 0.91 |
| Common Name | Scientific Name | Abbreviation | Botanical Family | Growth Habit | Geographical Origin | Latitude–Longitude |
|---|---|---|---|---|---|---|
| Anise | Tagetes filifolia | T.filifolia | Asteraceae | Herb | Chachapoyas-Levanto | −6.30625, −77.8987 |
| Lemon verbena | Aloysia citrodora | A. citrodora | Verbenaceae | Chachapoyas-Leymebamba | −6.7023, −77.7966 | |
| Eucalyptus | Eucalyptus globulus | E. globulus | Myrtaceae | Shrub | Chachapoyas-Huancas | −6.17500, −77.7966 |
| Lemongrass | Cymbopogon citratus | C. citratus | Poaceae | Herb | Utcubamba-Bagua Grande | −5.7329, −78.4279 |
| Matico | Piper aduncum | P. aduncum | Piperaceae | Shrub | Chachapoyas-Chachapoyas | −6.2528, −77.9016 |
| Chamomile | chamaemelum nobile | C. nobile | Asteraceae | Herb | −6.2523, −77.8325 | |
| Pennyroyal | Minthostachys mollis | M. mollis | Lamiaceae | Shrub | −6.1795, −77.8702 | |
| Rosemary | Rosmarinus officinalis | R. officinalis | Lamiaceae | Herb | Chachapoyas-Levanto | −6.3121, −77.9066 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Granda-Santos, M.; Reyna-Gonzales, K.; Torrejón-Valqui, L.; Valle-Epquín, M.G.; Caetano, A.C.; Díaz-Valderrama, J.R.; Castro-Alayo, E.M.; Cayo-Colca, I.S.; Maicelo, J.L.; Balcázar-Zumaeta, C.R. Characterization of Terpenoids in Aromatic Plants Using Raman Spectroscopy and Gas Chromatography–Mass Spectrometry (GC–MS). Int. J. Mol. Sci. 2025, 26, 11254. https://doi.org/10.3390/ijms262311254
Granda-Santos M, Reyna-Gonzales K, Torrejón-Valqui L, Valle-Epquín MG, Caetano AC, Díaz-Valderrama JR, Castro-Alayo EM, Cayo-Colca IS, Maicelo JL, Balcázar-Zumaeta CR. Characterization of Terpenoids in Aromatic Plants Using Raman Spectroscopy and Gas Chromatography–Mass Spectrometry (GC–MS). International Journal of Molecular Sciences. 2025; 26(23):11254. https://doi.org/10.3390/ijms262311254
Chicago/Turabian StyleGranda-Santos, Milagros, Katherine Reyna-Gonzales, Llisela Torrejón-Valqui, Marvin G. Valle-Epquín, Aline C. Caetano, Jorge R. Díaz-Valderrama, Efraín M. Castro-Alayo, Ilse S. Cayo-Colca, Jorge L. Maicelo, and César R. Balcázar-Zumaeta. 2025. "Characterization of Terpenoids in Aromatic Plants Using Raman Spectroscopy and Gas Chromatography–Mass Spectrometry (GC–MS)" International Journal of Molecular Sciences 26, no. 23: 11254. https://doi.org/10.3390/ijms262311254
APA StyleGranda-Santos, M., Reyna-Gonzales, K., Torrejón-Valqui, L., Valle-Epquín, M. G., Caetano, A. C., Díaz-Valderrama, J. R., Castro-Alayo, E. M., Cayo-Colca, I. S., Maicelo, J. L., & Balcázar-Zumaeta, C. R. (2025). Characterization of Terpenoids in Aromatic Plants Using Raman Spectroscopy and Gas Chromatography–Mass Spectrometry (GC–MS). International Journal of Molecular Sciences, 26(23), 11254. https://doi.org/10.3390/ijms262311254







