Exploring the Effects of Palm Tocotrienol-Rich Fraction in Diabetic Peripheral Neuropathy Rat’s Model: An Untargeted Metabolomic Profiling and Correlation Study
Abstract
1. Introduction
2. Results
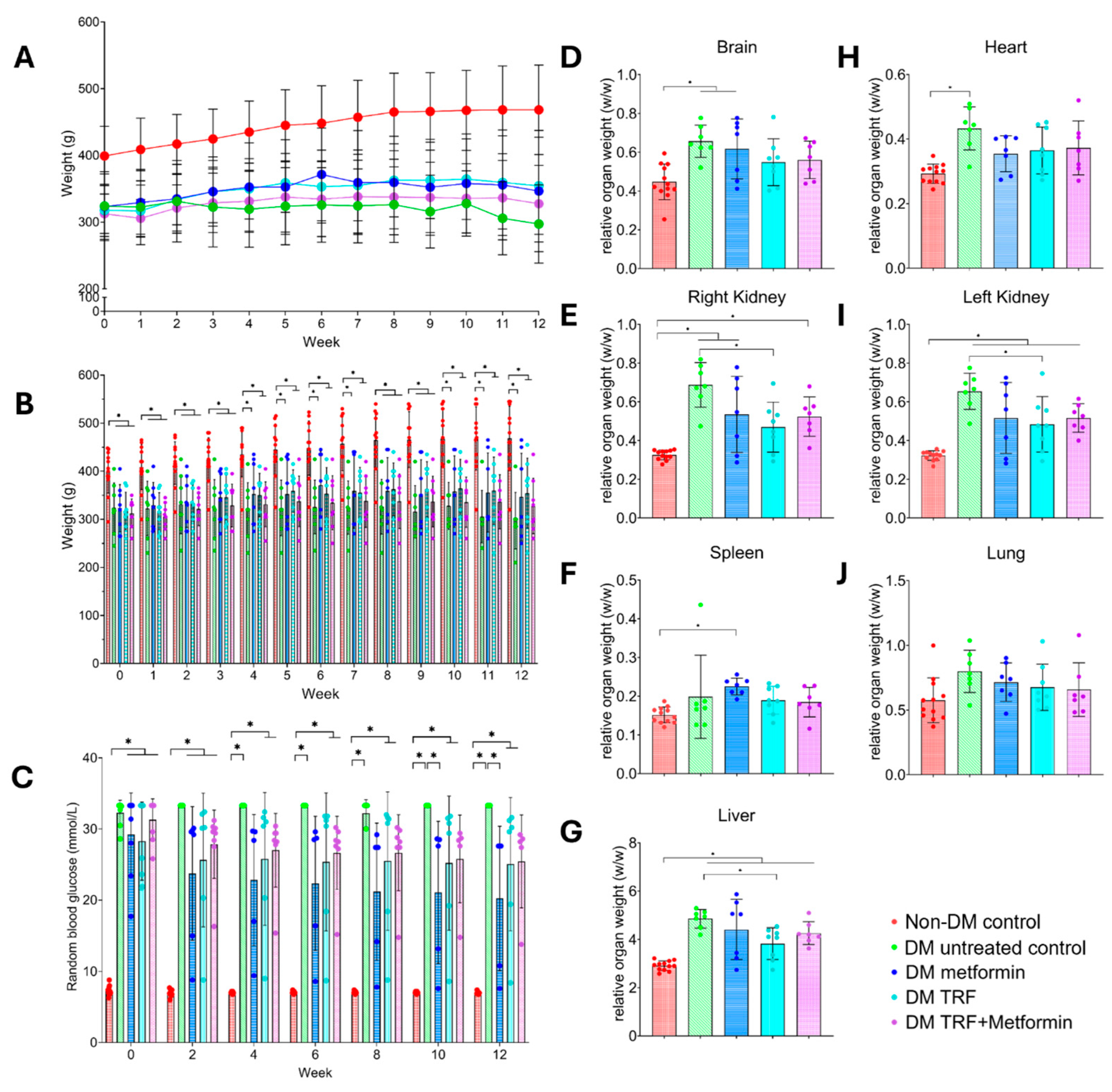
2.1. Effect of Palm TRF on Body Weight, Blood Glucose Level, and Relative Organ Weight of Diabetic Rats with DPN
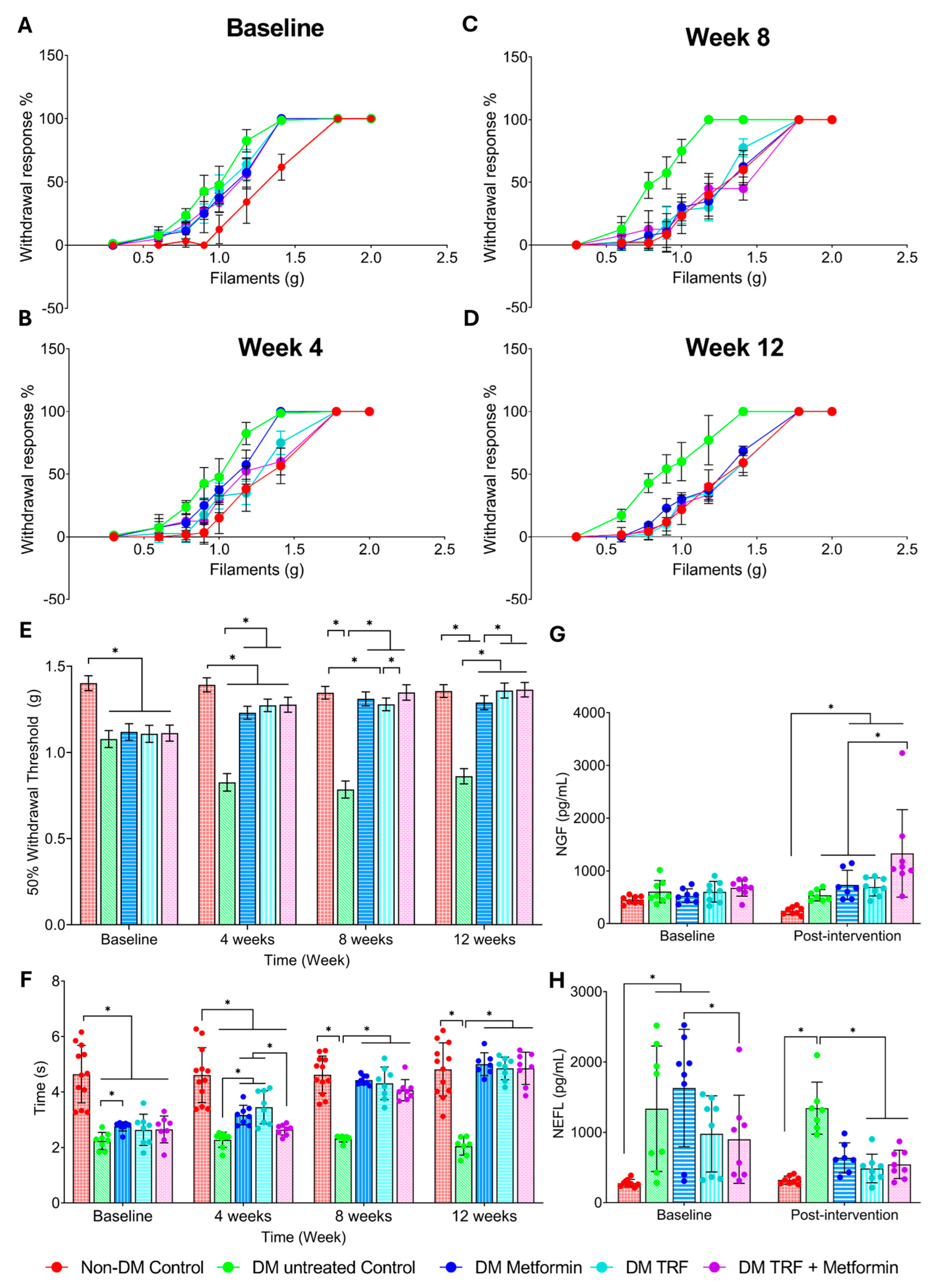
2.2. Effect of Palm TRF on Mechanical Allodynia and Thermal Hyperalgesia on Diabetic Rats with DPN
2.2.1. Mechanical Allodynia
2.2.2. Thermal Hyperalgesia
2.3. Effect of Palm TRF on Serum Nerve Growth Factor (NGF) and Neurofilament Light Chain (NEFL) Levels in Diabetic Rats with DPN
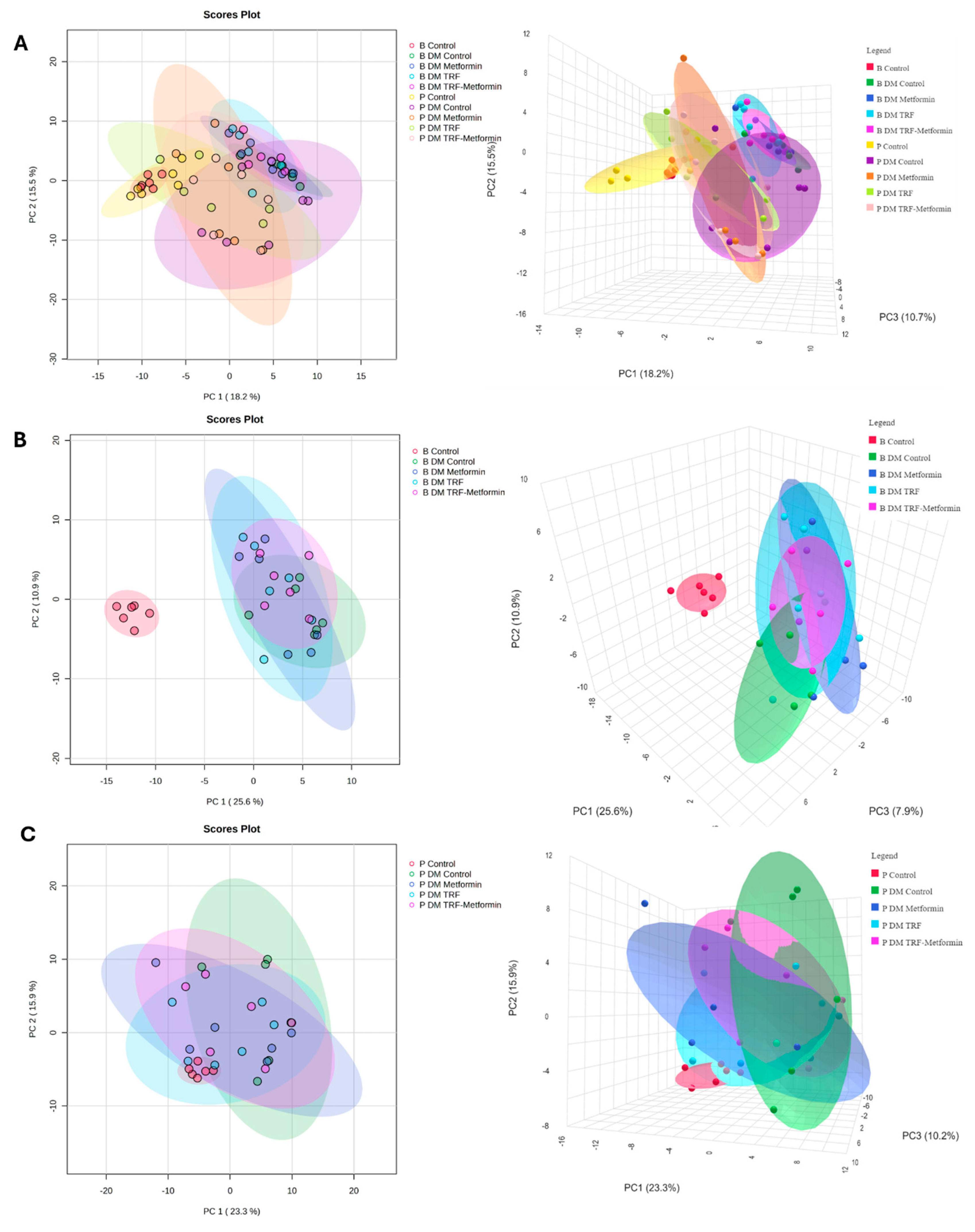
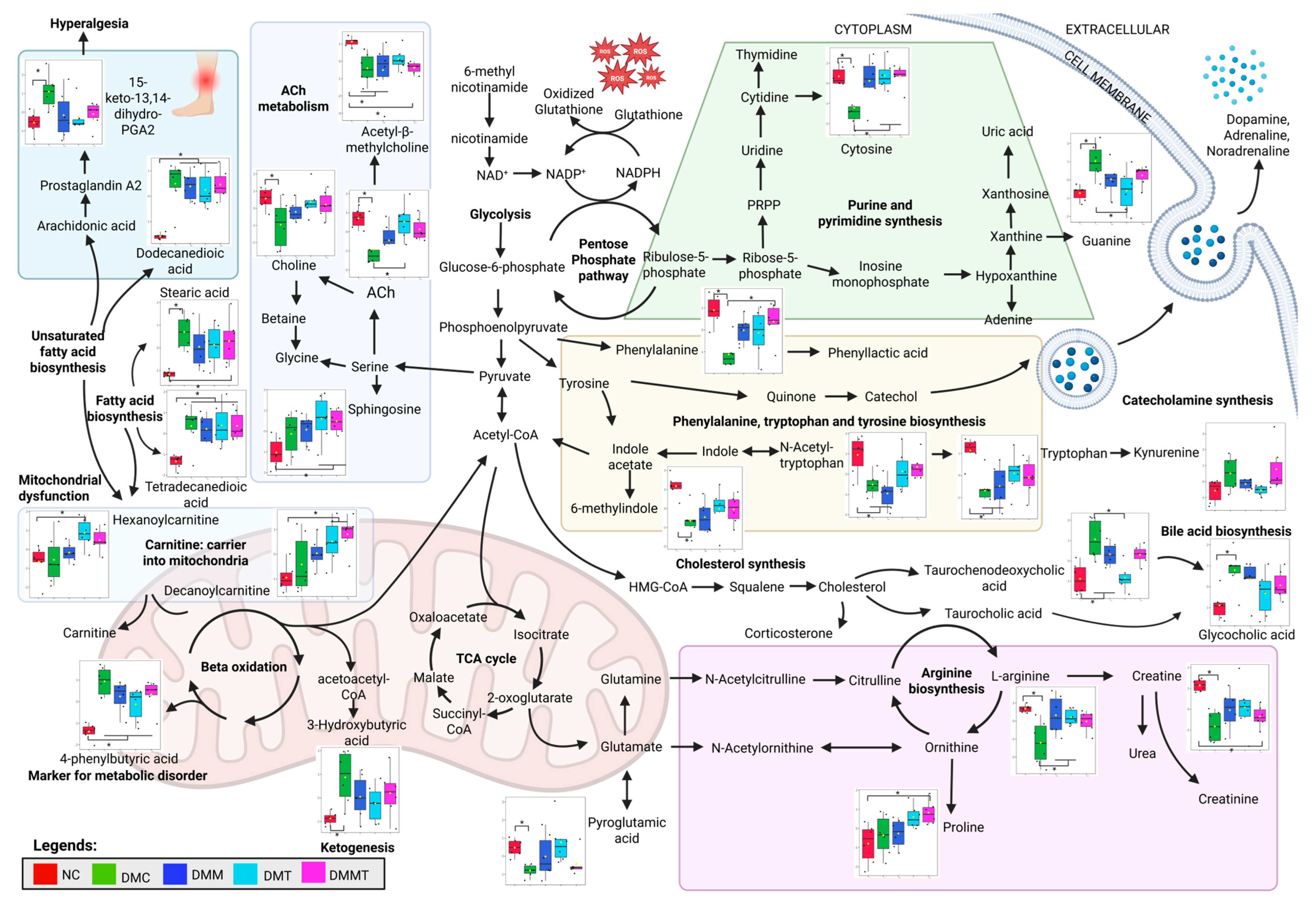
2.4. Effect of Palm TRF on the Plasma Metabolomic Profile of Diabetic Rats with DPN
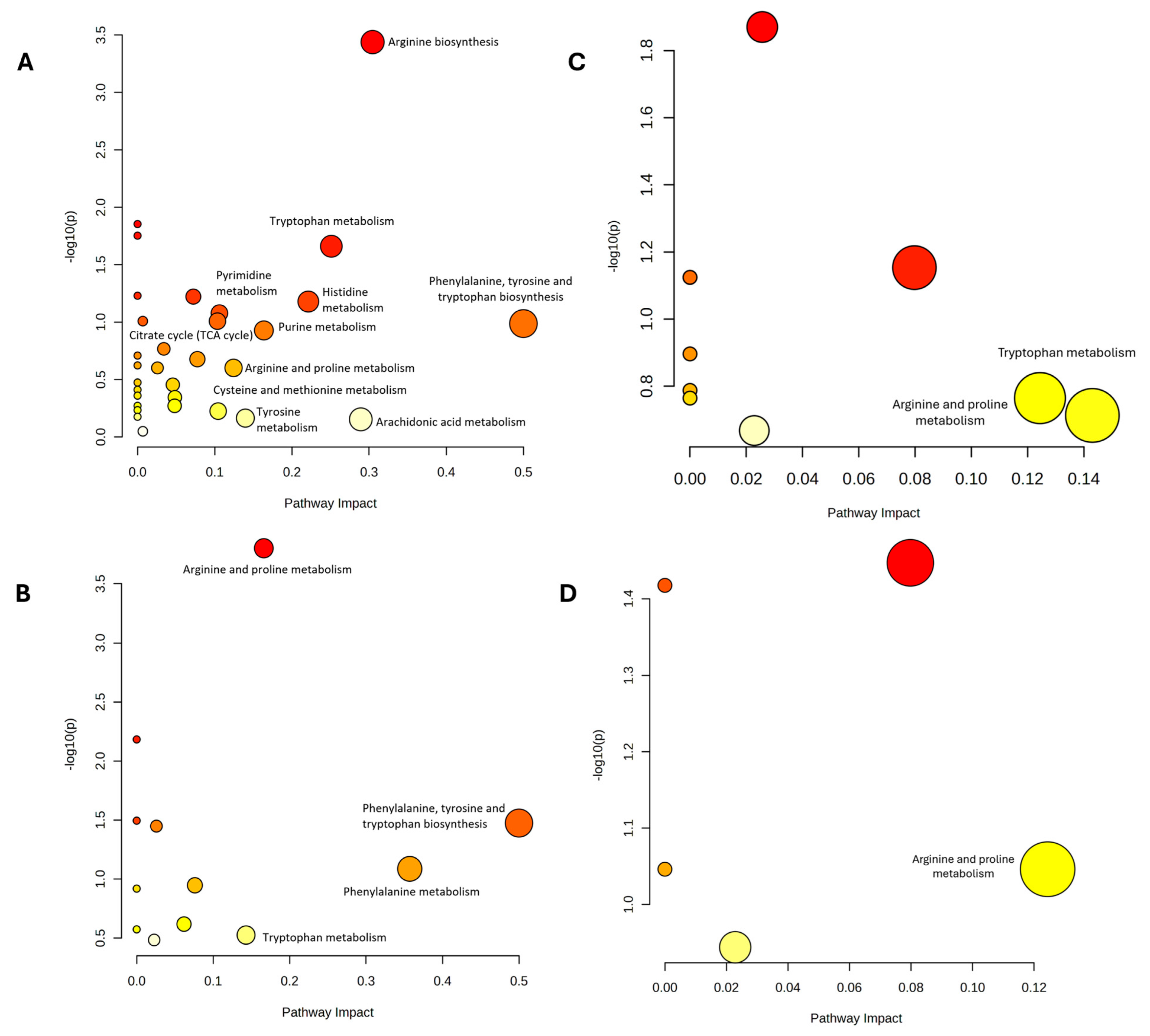
2.5. Pathway Analysis of the DEMs Following Palm TRF Intervention in Diabetic Rats with DPN
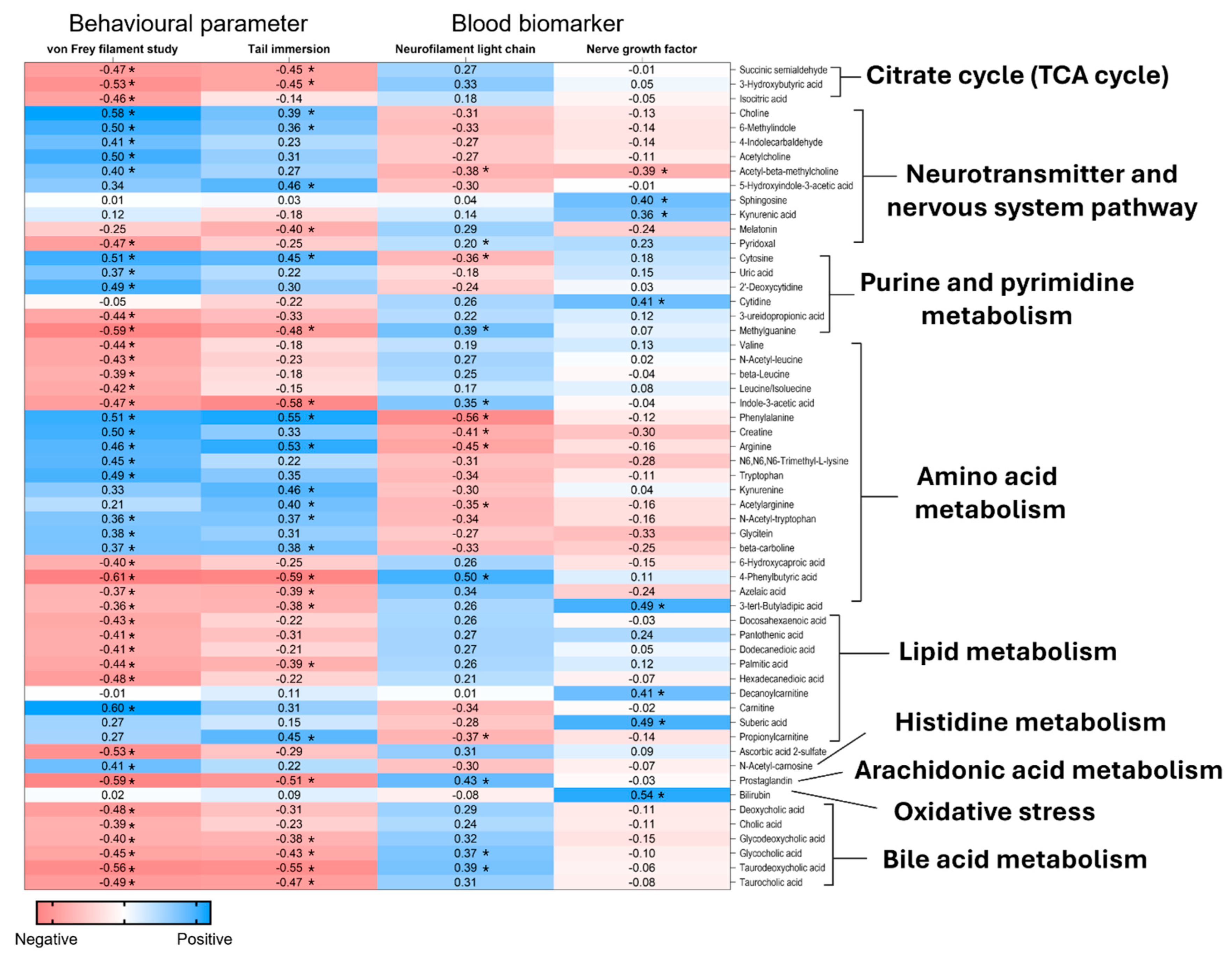
2.6. Correlation Analysis Between the Plasma Metabolites and the DPN’s Behavioural Parameters and Serum Neuronal Biomarkers
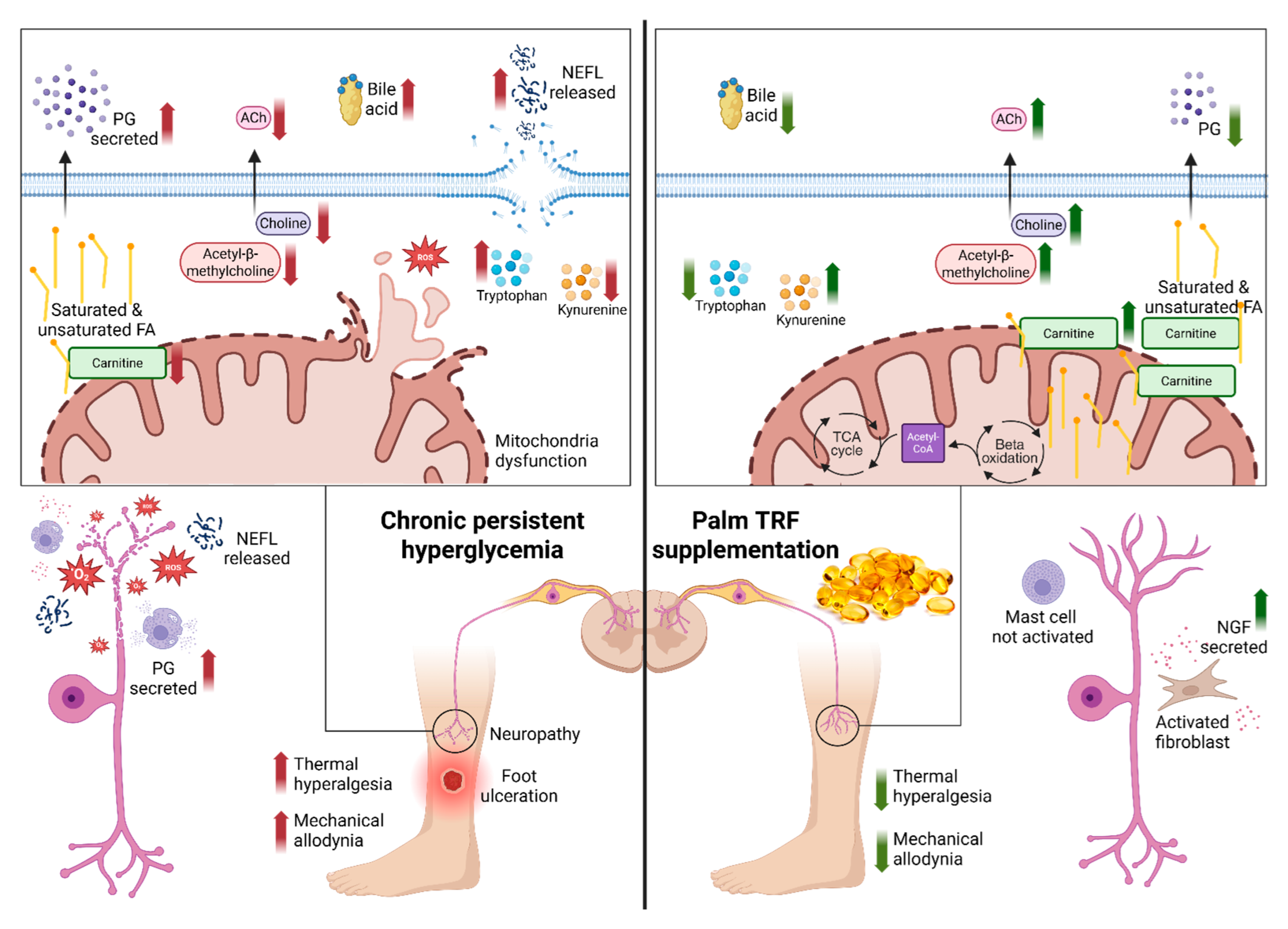
3. Discussion
3.1. Behavioural Assessment
3.2. Serum Neuropathy Biomarkers
3.3. Untargeted Metabolomic Profiling
4. Materials and Methods
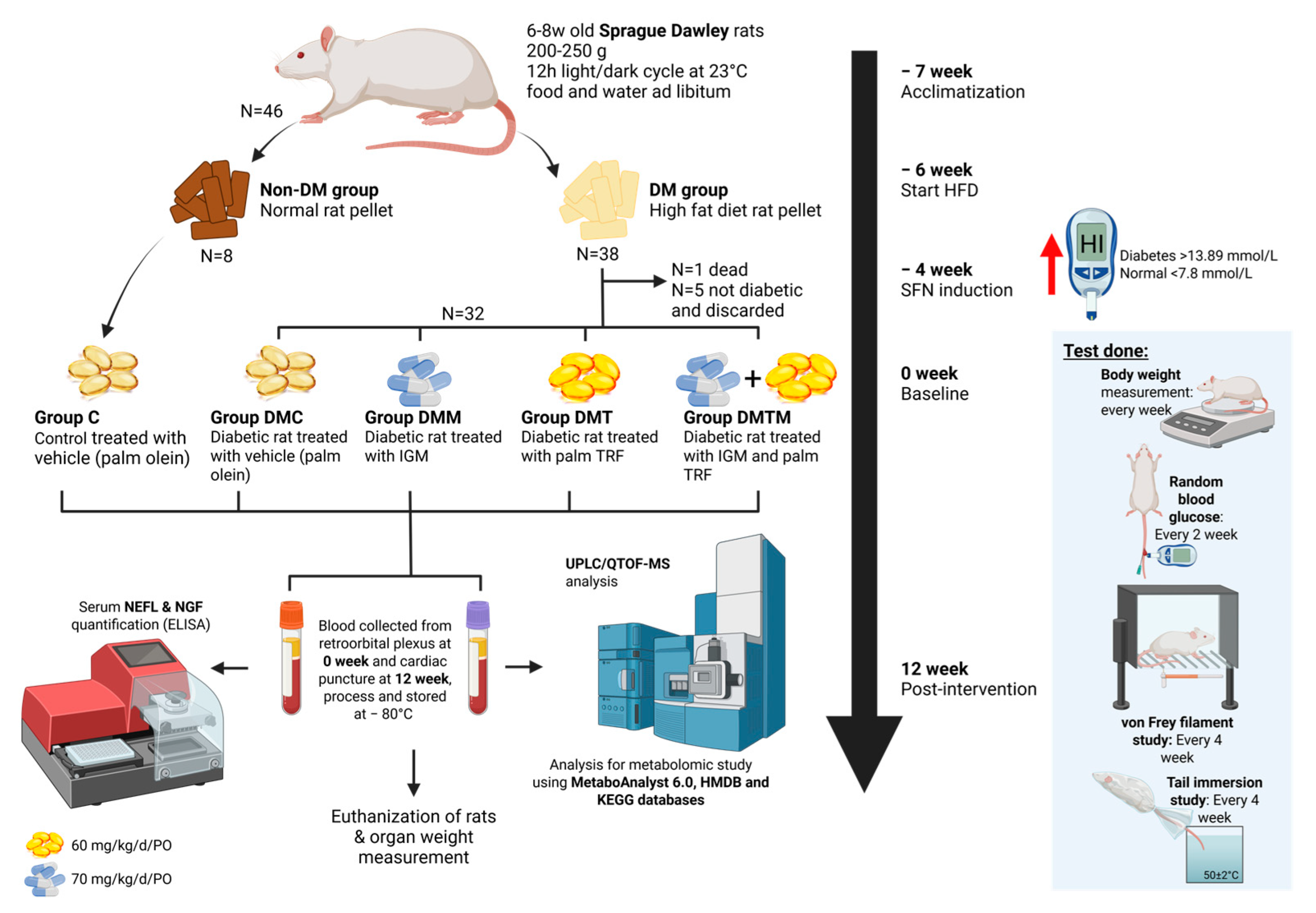
4.1. Animal Model and Treatment with Palm Tocotrienol-Rich Fraction
4.2. Neuropathic Behaviour Study
4.2.1. Von Frey Filament Study
4.2.2. Tail Immersion Study
4.3. Blood Collection
4.4. Euthanization of Animal and Organ Collection
4.5. Serum Biomarkers Assessment
4.6. Plasma Sample Preparation for Metabolite Extraction
4.7. Metabolomic Data Acquisition by UHPLC-MS/MS
4.8. Metabolomic Data Processing and Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACh | Acetylcholine |
| ACN | Acetonitrile |
| AGEs | Advanced Glycation End Products |
| ANOVA | Analysis of Variance |
| CDP-choline | Cytidine Diphosphate Choline |
| CSF | Cerebrospinal Fluid |
| DEM(s) | Differentially Expressed Metabolite(s) |
| DM | Diabetes Mellitus |
| DMC | Diabetes Mellitus Untreated Control |
| DMM | Diabetes Mellitus + Metformin |
| DMT | Diabetes Mellitus + Tocotrienol-Rich Fraction |
| DMMT | Diabetes Mellitus + Metformin + Tocotrienol-Rich Fraction |
| DPN | Diabetic Peripheral Neuropathy |
| EDTA | Ethylenediaminetetraacetic Acid |
| ELISA | Enzyme-Linked Immunosorbent Assay |
| ER | Endoplasmic Reticulum |
| FA | Fatty Acids |
| HFD | High-Fat Diet |
| HODE | Hydroxyoctadecadienoic Acid |
| IENFD | Intraepidermal Nerve Fibre Density |
| IP | Intraperitoneal |
| IVC | Individually Ventilated Cage |
| KEGG | Kyoto Encyclopaedia of Genes and Genomes |
| KTX | Ketamine–Xylazine–Tiletamine–Zolazepam |
| LARU | Laboratory Animal Resource Unit |
| LC-MS/MS | Liquid Chromatography Tandem Mass Spectrometry |
| MASLD | Metabolic Dysfunction-Associated Steatotic Liver Disease |
| MF(s) | Metabolite Feature(s) |
| MS | Mass Spectrometry |
| m/z | Mass-to-Charge Ratio |
| MW | Molecular Weight |
| NAD+ | Nicotinamide Adenine Dinucleotide |
| NEFL | Neurofilament Light Chain |
| NGF | Nerve Growth Factor |
| PCA | Principal Component Analysis |
| PGE2 | Prostaglandin E2 |
| PGI2 | Prostaglandin I2 |
| PG | Prostaglandin |
| PPARγ | Peroxisome Proliferator-Activated Receptor Gamma |
| QC | Quality Control |
| ROS | Reactive Oxygen Species |
| RT | Retention Time |
| SD | Sprague Dawley |
| SFN | Small Fibre Neuropathy |
| STZ | Streptozotocin |
| TCA | Tricarboxylic Acid |
| TCDCA | Taurochenodeoxycholic Acid |
| TGR5 | Takeda G-Protein-Coupled Receptor 5 |
| T2DM | Type 2 Diabetes Mellitus |
| TRF | Tocotrienol-Rich Fraction |
| UHPLC-MS/MS | Ultra-High-Performance Liquid Chromatography Tandem Mass Spectrometry |
| UKMAEC | Universiti Kebangsaan Malaysia Animal Ethics Committee |
| UPLC/QTOF-MS | Ultra-Performance Liquid Chromatography–Quadrupole Time-of-Flight Mass Spectrometry |
References
- Khan, M.A.B.; Hashim, M.J.; King, J.K.; Govender, R.D.; Mustafa, H.; Al Kaabi, J. Epidemiology of Type 2 Diabetes—Global Burden of Disease and Forecasted Trends. J. Epidemiol. Glob. Health 2019, 10, 107–111. [Google Scholar] [CrossRef]
- Standl, E.; Khunti, K.; Hansen, T.B.; Schnell, O. The Global Epidemics of Diabetes in the 21st Century: Current Situation and Perspectives. Eur. J. Prev. Cardiol. 2019, 26, 14–17. [Google Scholar] [CrossRef] [PubMed]
- Hicks, C.W.; Selvin, E. Epidemiology of Peripheral Neuropathy and Lower Extremity Disease in Diabetes. Curr. Diabetes Rep. 2019, 19, 86. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Wang, Y.; Zhang, X.; Zhu, S.; He, H. Prevalence of Peripheral Neuropathy in Patients with Diabetes: A Systematic Review and Meta-Analysis. Prim. Care Diabetes 2020, 14, 435–444. [Google Scholar] [CrossRef]
- de Souza, L.R.; Debiasi, D.; Ceretta, L.B.; Simões, P.W.; Tuon, L. Meta-Analysis and Meta-Regression of the Prevalence of Diabetic Peripheral Neuropathy among Patients with Type 2 Diabetes Mellitus. Int. Arch. Med. 2016, 9, 65. [Google Scholar] [CrossRef]
- Zhu, J.; Hu, Z.; Luo, Y.; Liu, Y.; Luo, W.; Du, X.; Luo, Z.; Hu, J.; Peng, S. Diabetic Peripheral Neuropathy: Pathogenetic Mechanisms and Treatment. Front. Endocrinol. 2024, 14, 1265372. [Google Scholar] [CrossRef] [PubMed]
- Todorovic, S.M. Is Diabetic Nerve Pain Caused by Dysregulated Ion Channels in Sensory Neurons? Diabetes 2015, 64, 3987–3989. [Google Scholar] [CrossRef]
- Iqbal, Z.; Azmi, S.; Yadav, R.; Ferdousi, M.; Kumar, M.; Cuthbertson, D.J.; Lim, J.; Malik, R.A.; Alam, U. Diabetic Peripheral Neuropathy: Epidemiology, Diagnosis, and Pharmacotherapy. Clin. Ther. 2018, 40, 828–849. [Google Scholar] [CrossRef]
- Anastasi, J.K.; Devine, D.K.; Capili, B. Distal Sensory Peripheral Neuropathy: An Undervalued Determinant of Wellbeing. Health Educ. Public Health 2021, 4, 450–454. [Google Scholar]
- Aso, Y. Updates in Diabetic Neuropathy: A Call for New Diagnostic and Treatment Approaches. J. Diabetes Investig. 2021, 13, 432–434. [Google Scholar] [CrossRef]
- Tanashyan, M.M.; Antonova, K.V.; Spryshkov, N.E.; Panina, A.A.; Lagoda, O.V.; Shchukina, E.P. Diabetic Polyneuropathy: From Pathogenesis to Pathogenetic Therapy. Eff. Pharmacother. 2024, 20, 101–108. [Google Scholar] [CrossRef]
- Kang, S.M. Pathogenesis and Treatment of Diabetic Peripheral Neuropathy. J. Korean Diabetes 2022, 23, 222–229. [Google Scholar] [CrossRef]
- Mizukami, H.; Osonoi, S. Collateral Glucose-Utlizing Pathwaya in Diabetic Polyneuropathy. Int. J. Mol. Sci. 2020, 22, 94. [Google Scholar] [CrossRef]
- Kawanami, D.; Matoba, K.; Utsunomiya, K. Signaling Pathways in Diabetic Nephropathy. Histol. Histopathol. 2016, 31, 1059–1067. [Google Scholar]
- Maiuolo, J.; Gliozzi, M.; Musolino, V.; Carresi, C.; Nucera, S.; Macrì, R.; Scicchitano, M.; Bosco, F.; Scarano, F.; Ruga, S.; et al. The Role of Endothelial Dysfunction in Peripheral Blood Nerve Barrier: Molecular Mechanisms and Pathophysiological Implications. Int. J. Mol. Sci. 2019, 20, 3022. [Google Scholar] [CrossRef]
- Ismail, M.; Alsalahi, A.; Imam, M.U.; Ooi, D.J.; Khaza’ai, H.; Aljaberi, M.A.; Shamsudin, M.N.; Idrus, Z. Safety and Neuroprotective Efficacy of Palm Oil and Tocotrienol-Rich Fraction from Palm Oil: A Systematic Review. Nutrients 2020, 12, 521. [Google Scholar] [CrossRef]
- Ramanathan, N.; Tan, E.; Loh, L.J.; Soh, B.S.; Yap, W.N. Tocotrienol Is a Cardioprotective Agent against Ageing-Associated Cardiovascular Disease and Its Associated Morbidities. Nutr. Metab. 2018, 15, 6. [Google Scholar] [CrossRef]
- Sadikan, M.Z.; Nasir, N.A.A.; Bakar, N.S.; Iezhitsa, I.; Agarwal, R. Tocotrienol-Rich Fraction Reduces Retinal Inflammation and Angiogenesis in Rats with Streptozotocin-Induced Diabetes. BMC Complement. Med. Ther. 2023, 23, 179. [Google Scholar] [CrossRef]
- Wu, S.; Liu, P.; Ng, L. Tocotrienol-Rich Fraction of Palm Oil Exhibits Anti-Inflammatory Property by Suppressing the Expression of Inflammatory Mediators in Human Monocytic Cells. Mol. Nutr. Food Res. 2008, 52, 921–929, Correction in Mol. Nutr. Food Res. 2009, 53, 309. [Google Scholar] [CrossRef] [PubMed]
- Rusli, N.; Ng, C.F.; Makpol, S.; Wong, Y.P.; Isa, I.L.M.; Remli, R. Antioxidant Effect in Diabetic Peripheral Neuropathy in Rat Model: A Systematic Review. Antioxidants 2024, 13, 1041. [Google Scholar] [CrossRef] [PubMed]
- Gany, S.L.S.; Chin, K.-Y.; Tan, J.K.; Aminuddin, A.; Makpol, S. Preventative and Therapeutic Potential of Tocotrienols on Musculoskeletal Diseases in Ageing. Front. Pharmacol. 2023, 14, 1290721. [Google Scholar] [CrossRef]
- Nasir, N.A.A.; Sadikan, M.Z.; Agarwal, R. Modulation of Nfκb Signalling Pathway by Tocotrienol: A Systematic Review. Asia Pac. J. Clin. Nutr. 2021, 30, 537–555. [Google Scholar] [PubMed]
- Su, X.; Chen, W.; Fu, Y.; Wu, B.; Mao, F.; Zhao, Y.; Yang, Q.; Lan, D. Protective Role of Mertk in Diabetic Peripheral Neuropathy Via Inhibition of the Nf-Κb Signaling Pathway. Exp. Clin. Endocrinol. Diabetes 2024, 132, 396–406. [Google Scholar] [CrossRef] [PubMed]
- Chuar, P.F.; Ng, Y.T.; Phang, S.C.W.; Koay, Y.Y.; Ho, J.-I.; Ho, L.S.; Botross Henien, N.P.; Ahmad, B.; Abdul Kadir, K. Tocotrienol-Rich Vitamin E (Tocovid) Improved Nerve Conduction Velocity in Type 2 Diabetes Mellitus Patients in a Phase Ii Double-Blind, Randomized Controlled Clinical Trial. Nutrients 2021, 13, 3770. [Google Scholar] [CrossRef] [PubMed]
- Kottschade, L.A.; Sloan, J.A.; Mazurczak, M.A.; Johnson, D.B.; Murphy, B.P.; Rowland, K.M.; Smith, D.A.; Berg, A.R.; Stella, P.J.; Loprinzi, C.L. The Use of Vitamin E for the Prevention of Chemotherapy-Induced Peripheral Neuropathy: Results of a Randomized Phase Iii Clinical Trial. Support Care Cancer 2011, 19, 1769–1777. [Google Scholar] [CrossRef]
- Wei, J.; Wei, Y.; Huang, M.; Wang, P.; Jia, S. Is Metformin a Possible Treatment for Diabetic Neuropathy? J. Diabetes 2022, 14, 658–669. [Google Scholar] [CrossRef]
- Kim, S.H.; Park, T.S.; Jin, H.Y. Metformin Preserves Peripheral Nerve Damage with Comparable Effects to Alpha Lipoic Acid in Streptozotocin/High-Fat Diet Induced Diabetic Rats. Diabetes Metab. J. 2020, 44, 842–853. [Google Scholar] [CrossRef]
- Sahardi, N.F.N.M.; Jaafar, F.; Tan, J.K.; Nordin, M.F.M.; Makpol, S. Elucidating the Pharmacological Properties of Zingiber Officinale Roscoe (Ginger) on Muscle Ageing by Untargeted Metabolomic Profiling of Human Myoblasts. Nutrients 2023, 15, 4520. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Zhang, Q.; He, Y.; Cao, W.; Song, W.; Liang, X. Targeted Metabolomics Reveals the Aberrant Energy Status in Diabetic Peripheral Neuropathy and the Neuroprotective Mechanism of Traditional Chinese Medicine Jinmaitong. J. Pharm. Anal. 2023, 14, 225–243, Correction in J. Pharm. Anal. 2024, 14, 100980. [Google Scholar] [CrossRef]
- Rojas, D.R.; Kuner, R.; Agarwal, N. Metabolomic Signature of Type 1 Diabetes-Induced Sensory Loss and Nerve Damage in Diabetic Neuropathy. J. Mol. Med. 2018, 97, 845–854. [Google Scholar] [CrossRef]
- Atanasovska, E.; Tasic, V.; Slaninka-Miceska, M.; Alabakovska, S.; Zafirov, D.; Kostova, E.; Pavlovska, K.; Filipce, V.; Labacevski, N. Six Week Follow-up of Metabolic Effects Induced by a High-Fat Diet and Streptozotocin in a Rodent Model of Type 2 Diabetes Mellitus. Pril. (Makedon. Akad. Nauk. Umet. Odd. Med. Nauk.) 2014, 35, 169–179. [Google Scholar]
- Apovian, C.M.; Okemah, J.; O’neil, P.M. Body Weight Considerations in the Management of Type 2 Diabetes. Adv. Ther. 2019, 36, 44–58. [Google Scholar] [CrossRef]
- Jaliah, B.; Bin-Jaliah, I.; El-Attar, S.; Khaleel, E.F.; El-Sayed, L.A.; Haidara, M.A. Remedial Effects of Vitamin E and L-Arginine on Peripheral Neuropathy in Streptozotocin-Induced Diabetic Rats. Am. J. Pharmacol. Toxicol. 2013, 9, 13–23. [Google Scholar] [CrossRef]
- Dhaliwal, J.; Dhaliwal, N.; Akhtar, A.; Kuhad, A.; Chopra, K. Beneficial Effects of Ferulic Acid Alone and in Combination with Insulin in Streptozotocin Induced Diabetic Neuropathy in Sprague Dawley Rats. Life Sci. 2020, 255, 117856. [Google Scholar] [CrossRef]
- Lee, K.A.; Lee, N.Y.; Park, T.S.; Jin, H.Y. Comparison of Peripheral Nerve Protection between Insulin-Based Glucose Control and Alpha Lipoic Acid (Ala) in the Streptozotocin (Stz)-Induced Diabetic Rat. Endocrine 2018, 61, 58–67. [Google Scholar] [CrossRef]
- Goon, J.A.; Azman, N.H.E.N.; Ghani, S.M.A.; Hamid, Z.; Ngah, W.Z.W. Comparing Palm Oil Tocotrienol Rich Fraction with A-Tocopherol Supplementation on Oxidative Stress in Healthy Older Adults. Clin. Nutr. ESPEN 2017, 21, 1–12. [Google Scholar] [CrossRef]
- Hannan, M.A.; Dash, R.; Sohag, A.A.M.; Haque, M.N.; Moon, I.S. Neuroprotection against Oxidative Stress: Phytochemicals Targeting Trkb Signaling and the Nrf2-Are Antioxidant System. Front. Mol. Neurosci. 2020, 13, 116. [Google Scholar] [CrossRef] [PubMed]
- Dhanapalaratnam, R.; Issar, T.; Wang, L.L.; Tran, D.; Poynten, A.M.; Milner, K.-L.; Kwai, N.C.; Krishnan, A.V. Effect of Metformin on Peripheral Nerve Morphology in Type 2 Diabetes: A Cross-Sectional Observational Study. Diabetes 2024, 73, 1875–1882. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Arceneaux, L.; Culicchia, F.; Lukiw, W.J. Neurofilament Light (Nf-L) Chain Protein from a Highly Polymerized Structural Component of the Neuronal Cytoskeleton to a Neurodegenerative Disease Biomarker in the Periphery. J. Alzheimer’s Neurodegener. Dis. 2021, 7, 56. [Google Scholar]
- Pentz, R.; Iulita, M.F. The Ngf Metabolic Pathway: New Opportunities for Biomarker Research and Drug Target Discovery: Ngf Pathway Biomarkers and Drug Targets. Adv. Exp. Med. Biol. 2021, 1331, 31–48. [Google Scholar]
- Lee, Y.; Lee, B.H.; Yip, W.; Chou, P.; Yip, B.-S. Neurofilament Proteins as Prognostic Biomarkers in Neurological Disorders. Curr. Pharm. Des. 2019, 25, 4560–4569. [Google Scholar] [CrossRef]
- Maalmi, H.; Strom, A.; Petrera, A.; Hauck, S.M.; Strassburger, K.; Kuss, O.; Zaharia, O.-P.; Bönhof, G.J.; Rathmann, W.; Trenkamp, S.; et al. Serum Neurofilament Light Chain: A Novel Biomarker for Early Diabetic Sensorimotor Polyneuropathy. Diabetologia 2022, 66, 579–589. [Google Scholar] [CrossRef]
- Xu, D.; Wu, D.; Qin, M.; Nih, L.R.; Liu, C.; Cao, Z.; Ren, J.; Chen, X.; He, Z.; Yu, W.; et al. Efficient Delivery of Nerve Growth Factors to the Central Nervous System for Neural Regeneration. Adv. Mater. 2019, 31, e1900727. [Google Scholar] [CrossRef] [PubMed]
- Stino, A.M.; Smith, A.G. Peripheral Neuropathy in Prediabetes and the Metabolic Syndrome. J. Diabetes Investig. 2017, 8, 646–655. [Google Scholar] [CrossRef]
- Grisold, A.; Callaghan, B.C.; Feldman, E.L. Mediators of Diabetic Neuropathy: Is Hyperglycemia the Only Culprit? Curr. Opin. Endocrinol. Diabetes Obes. 2017, 24, 103–111. [Google Scholar] [CrossRef]
- Smith, A.G.; Rose, K.; Singleton, J.R. Idiopathic Neuropathy Patients Are at High Risk for Metabolic Syndrome. J. Neurol. Sci. 2008, 273, 25–28. [Google Scholar] [CrossRef]
- Sergi, D.; Naumovski, N.; Heilbronn, L.K.; Abeywardena, M.; O’callaghan, N.; Lionetti, L.; Luscombe-Marsh, N. Mitochondrial (Dys)Function and Insulin Resistance: From Pathophysiological Molecular Mechanisms to the Impact of Diet. Front. Physiol. 2019, 10, 532. [Google Scholar] [CrossRef]
- Taylor, E.M.; Jones, A.D.; Henagan, T.M. A Review of Mitochondrial-Derived Fatty Acids in Epigenetic Regulation of Obesity and Type 2 Diabetes. J. Nutr. Health Food Sci. 2014, 2, 1–4. [Google Scholar]
- Abu Bakar, M.H.; Kai, C.K.; Hassan, W.N.W.; Sarmidi, M.R.; Yaakob, H.; Huri, H.Z. Mitochondrial Dysfunction as a Central Event for Mechanisms Underlying Insulin Resistance: The Roles of Long Chain Fatty Acids. Diabetes/Metab. Res. Rev. 2015, 31, 453–475. [Google Scholar] [CrossRef]
- Schrauwen, P.; Schrauwen-Hinderling, V.; Hoeks, J.; Hesselink, M.K. Mitochondrial Dysfunction and Lipotoxicity. Biochim. Biophys Acta 2010, 1801, 266–271. [Google Scholar] [CrossRef]
- Fujikawa, M.; Ibuki, T.; Matsumura, K.; Sawa, T. Inflammatory Hyperalgesia: The Role of the Prostaglandin System in the Spinal Cord. Adv. Neuroimmune Biol. 2012, 3, 197–207. [Google Scholar] [CrossRef]
- Sugita, R.; Kuwabara, H.; Kubota, K.; Sugimoto, K.; Kiho, T.; Tengeiji, A.; Kawakami, K.; Shimada, K. Simultaneous Inhibition of Pge2 and Pgi2 Signals Is Necessary to Suppress Hyperalgesia in Rat Inflammatory Pain Models. Mediat. Inflamm. 2016, 2016, 984784. [Google Scholar] [CrossRef]
- Virmani, M.A.; Cirulli, M. The Role of L-Carnitine in Mitochondria, Prevention of Metabolic Inflexibility and Disease Initiation. Int. J. Mol. Sci. 2022, 23, 2717. [Google Scholar] [CrossRef]
- Modanloo, M.; Shokrzadeh, M. Analyzing Mitochondrial Dysfunction, Oxidative Stress, and Apoptosis: Potential Role of L-Carnitine. Iran. J. Kidney Dis. 2019, 13, 74–86. [Google Scholar] [PubMed]
- Marcovina, S.M.; Sirtori, C.; Peracino, A.; Gheorghiade, M.; Borum, P.; Remuzzi, G.; Ardehali, H. Translating the Basic Knowledge of Mitochondrial Functions to Metabolic Therapy: Role of L-Carnitine. Transl. Res. 2013, 161, 73–84. [Google Scholar] [CrossRef]
- Choi, J.W.; Ohn, J.H.; Jung, H.S.; Park, Y.J.; Jang, H.C.; Chung, S.S.; Park, K.S. Carnitine Induces Autophagy and Restores High-Fat Diet-Induced Mitochondrial Dysfunction. Metabolism 2018, 78, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Traina, G. The Neurobiology of Acetyl-L-Carnitine. Front. Biosci. (Landmark Ed.) 2016, 21, 1314–1329. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Moreau, R. Lipid-Regulating Properties of Butyric Acid and 4-Phenylbutyric Acid: Molecular Mechanisms and Therapeutic Applications. Pharmacol. Res. 2019, 144, 116–131. [Google Scholar] [CrossRef]
- Offermanns, S. Hydroxy-Carboxylic Acid Receptor Actions in Metabolism. Trends Endocrinol. Metab. 2017, 28, 227–236. [Google Scholar] [CrossRef]
- Hallan, S.; Afkarian, M.; Zelnick, L.R.; Kestenbaum, B.; Sharma, S.; Saito, R.; Darshi, M.; Barding, G.; Raftery, D.; Ju, W.; et al. Metabolomics and Gene Expression Analysis Reveal Down-Regulation of the Citric Acid (Tca) Cycle in Non-Diabetic Ckd Patients. EBioMedicine 2017, 26, 68–77. [Google Scholar] [CrossRef]
- Sandlers, Y.; Shah, R.R.; Pearce, R.W.; Dasarathy, J.; McCullough, A.J.; Dasarathy, S. Plasma Krebs Cycle Intermediates in Nonalcoholic Fatty Liver Disease. J. Clin. Med. 2020, 9, 314. [Google Scholar] [CrossRef]
- Umeno, A.; Sakashita, M.; Sugino, S.; Murotomi, K.; Okuzawa, T.; Morita, N.; Tomii, K.; Tsuchiya, Y.; Yamasaki, K.; Horie, M.; et al. Comprehensive Analysis of Pparγ Agonist Activities of Stereo-, Regio-, and Enantio-Isomers of Hydroxyoctadecadienoic Acids. Biosci. Rep. 2020, 40, BSR20193767. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Yang, T.; Liu, H.; Han, L.; Zhang, K.; Hu, X.; Zhang, X.; Yin, K.-J.; Gao, Y.; Bennett, M.V.; et al. Peroxisome Proliferator-Activated Receptor Γ (Pparγ): A Master Gatekeeper in Cns Injury and Repair. Prog. Neurobiol. 2018, 163–164, 27–58. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Devi, S.; Mannan, A.; Kumar, M.; Singh, T.G. PPAR-γ Signaling and Common Protective Pathways against Obesity and Alzheimer’s Disease. Curr. Signal Transduct. Ther. 2025, 20, 1–18. [Google Scholar] [CrossRef]
- Villapol, S. Roles of Peroxisome Proliferator-Activated Receptor Gamma on Brain and Peripheral Inflammation. Cell. Mol. Neurobiol. 2018, 38, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Gibb, A.J. Choline and Acetylcholine: What a Difference an Acetate Makes! J. Physiol. 2017, 595, 1021–1022. [Google Scholar] [CrossRef]
- Calcutt, N.A.; Smith, D.R.; Frizzi, K.; Sabbir, M.G.; Chowdhury, S.K.R.; Mixcoatl-Zecuatl, T.; Saleh, A.; Muttalib, N.; Van der Ploeg, R.; Ochoa, J.; et al. Selective Antagonism of Muscarinic Receptors Is Neuroprotective in Peripheral Neuropathy. J. Clin. Investig. 2017, 127, 608–622. [Google Scholar] [CrossRef]
- Fernstrom, J.D.; Fernstrom, M.H. Tyrosine, Phenylalanine, and Catecholamine Synthesis and Function in the Brain. J. Nutr. 2007, 137 (Suppl. S1), 1539S–1547S; discussion 48S. [Google Scholar] [CrossRef]
- Srinivasan, K.; Viswanad, B.; Asrat, L.; Kaul, C.; Ramarao, P. Combination of High-Fat Diet-Fed and Low-Dose Streptozotocin-Treated Rat: A Model for Type 2 Diabetes and Pharmacological Screening. Pharmacol. Res. 2005, 52, 313–320. [Google Scholar] [CrossRef]
- Zhang, M.; Lv, X.-Y.; Li, J.; Xu, Z.-G.; Chen, L. The Characterization of High-Fat Diet and Multiple Low-Dose Streptozotocin Induced Type 2 Diabetes Rat Model. Exp. Diabetes Res. 2008, 2008, 704045. [Google Scholar] [CrossRef]
- Al-Megrin, W.A.; El-Khadragy, M.F.; Hussein, M.H.; Mahgoub, S.; Abdel-Mohsen, D.M.; Taha, H.; Bakkar, A.A.A.; Moneim, A.E.A.; Amin, H.K. Green Coffea Arabica Extract Ameliorates Testicular Injury in High-Fat Diet/Streptozotocin-Induced Diabetes in Rats. J. Diabetes Res. 2020, 2020, 6762709. [Google Scholar] [CrossRef]
- Isa, I.L.M.; Abbah, S.A.; Kilcoyne, M.; Sakai, D.; Dockery, P.; Finn, D.P.; Pandit, A. Implantation of Hyaluronic Acid Hydrogel Prevents the Pain Phenotype in a Rat Model of Intervertebral Disc Injury. Sci. Adv. 2018, 4, eaaq0597, Correction in Sci. Adv. 2018, 4, eaav0295. [Google Scholar] [PubMed]
- Kiasalari, Z.; Rahmani, T.; Mahmoudi, N.; Baluchnejadmojarad, T.; Roghani, M. Diosgenin Ameliorates Development of Neuropathic Pain in Diabetic Rats: Involvement of Oxidative Stress and Inflammation. Biomed. Pharmacother. 2017, 86, 654–661. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.T.; Kim, T.; Novotny, B.; Khan, N.; Aksamit, J.; Siegel, S.; Miranpuri, G.S.; Resnick, D.K. Thermal Hyperalgesia Assessment for Rats after Spinal Cord Injury: Developing a Valid and Useful Pain Index. Spine J. 2014, 14, 984–989. [Google Scholar] [CrossRef] [PubMed]
- Arslan, B.; Zetterberg, H. Neurofilament Light Chain as Neuronal Injury Marker—What Is Needed to Facilitate Implementation in Clinical Laboratory Practice? Clin. Chem. Lab. Med. (CCLM) 2023, 61, 1140–1149. [Google Scholar] [CrossRef]
- Leinninger, G.M.; Vincent, A.M.; Feldman, E.L. The Role of Growth Factors in Diabetic Peripheral Neuropathy. J. Peripher. Nerv. Syst. 2004, 9, 26–53. [Google Scholar] [CrossRef]
- Tan, J.K.; Zakaria, S.N.A.; Gunasekaran, G.; Sani, N.F.A.; Nasaruddin, M.L.; Jaafar, F.; Abu Bakar, Z.H.; Hamzah, A.I.Z.A.; Aripin, K.N.N.; Rani, M.D.M.; et al. Metabolomics Profiling of Age-Associated Metabolites in Malay Population. Oxid. Med. Cell. Longev. 2023, 2023, 4416410. [Google Scholar] [CrossRef]
- Sumner, L.W.; Amberg, A.; Barrett, D.; Beale, M.H.; Beger, R.; Daykin, C.A.; Fan, T.W.-M.; Fiehn, O.; Goodacre, R.; Griffin, J.L.; et al. Proposed Minimum Reporting Standards for Chemical Analysis. Metabolomics 2007, 3, 211–221. [Google Scholar] [CrossRef]
| Metabolite | MW | RT | HMDB ID | Mode | Fold Change | ||||
|---|---|---|---|---|---|---|---|---|---|
| DMC vs. C | DMM vs. DMC | DMT vs. DMC | DMMT vs. DMC | DMMT vs. DMT | |||||
| 13,14-Dihydro-15-keto Prostaglandin A2 | 334.21 | 11.64 | HMDB0001244 | n | 3.29 | ||||
| 3-Hydroxybutyric acid | 104.05 | 1.65 | HMDB0000011 | n | 16.98 | ||||
| 4-Hydroxy-6-methyl-2-pyrone | 126.03 | 4.02 | HMDB0341406 | n | 8.35 | ||||
| 4-Oxoproline | 129.04 | 1.18 | HMDB0304793 | n | 1.68 | ||||
| 4-Phenylbutyric acid | 164.08 | 6.95 | HMDB0000543 | p | 73.06 | ||||
| 6-Methylquinoline | 143.07 | 3.78 | HMDB0033115 | p | −1.82 | ||||
| Acetyl-beta-methylcholine | 159.13 | 0.73 | HMDB0015654 | p | −2.47 | ||||
| Acetylcholine | 145.11 | 0.54 | HMDB0000895 | p | −2.14 | 2.09 | |||
| Arginine | 174.11 | 0.48 | HMDB0003416/HMDB0000517 | p | −2.35 | 2.21 | 1.85 | ||
| Choline | 103.10 | 0.48 | HMDB0000097 | p | −1.32 | ||||
| Creatine | 131.07 | 0.54 | HMDB0000064 | p | −3.73 | ||||
| Cytosine | 111.04 | 0.89 | HMDB0000630 | p | −1.56 | 1.60 | 1.56 | ||
| Dodecanedioic acid | 230.15 | 8.77 | HMDB0000623 | n | 22.87 | ||||
| Glycocholic acid | 465.31 | 7.61 | HMDB0000138 | n | 42.94 | ||||
| 7-Methylguanine/1-Methylguanine | 165.06 | 1.15 | HMDB0000897/HMDB0003282 | p | 1.48 | −1.40 | |||
| Isomer: Tauroursodeoxycholic acid/Taurochenodeoxycholic acid/Taurodeoxycholic acid | 499.30 | 9.76 | HMDB0000874/HMDB0000951/HMDB0000896 | n | 38.48 | −38.83 | |||
| Metformin | 129.10 | 0.60 | HMDB0001921 | p | 6.10 | 13.87 | 39.05 | ||
| N-Acetyl-tryptophan | 246.10 | 5.93 | HMDB0255052 | n | −2.06 | ||||
| Phenylalanine | 165.08 | 3.15 | HMDB0000159 | n | −1.40 | 1.29 | |||
| Stearic acid | 284.27 | 11.79 | HMDB0000827 | n | 14.91 | ||||
| Tetradecanedioic acid | 258.19 | 10.20 | HMDB0000872 | n | 5.12 | ||||
| Tryptophan | 204.09 | 3.783 | HMDB0000929/HMDB0013609/HMDB0030396 | p | −1.85 | ||||
| (A) Comparison Baseline and Post-Intervention for All Group | |||
| Pathway Name | Match Status | p Value | Impact Value |
| Phenylalanine, tyrosine and tryptophan biosynthesis | 1/4 | 0.103 | 0.50 # |
| Arginine biosynthesis | 4/14 | 0.001 * | 0.30 # |
| Arachidonic acid metabolism | 1/44 | 0.702 | 0.29 # |
| Tryptophan metabolism | 4/41 | 0.022 * | 0.25 # |
| Histidine metabolism | 2/16 | 0.066 | 0.22 # |
| Purine metabolism | 4/71 | 0.118 | 0.16 # |
| Tyrosine metabolism | 1/42 | 0.685 | 0.14 # |
| Arginine and proline metabolism | 2/36 | 0.250 | 0.12 # |
| Pyrimidine metabolism | 3/39 | 0.084 | 0.11 # |
| Cysteine and methionine metabolism | 1/33 | 0.595 | 0.10 # |
| Citrate cycle (TCA cycle) | 2/20 | 0.098 | 0.10 # |
| Sphingolipid metabolism | 2/32 | 0.210 | 0.08 |
| Glycine, serine and threonine metabolism | 3/34 | 0.060 | 0.07 |
| Pentose phosphate pathway | 1/22 | 0.452 | 0.05 |
| Alanine, aspartate and glutamate metabolism | 1/28 | 0.535 | 0.05 |
| Primary bile acid biosynthesis | 2/46 | 0.351 | 0.05 |
| Glutathione metabolism | 2/28 | 0.171 | 0.03 |
| Glycerophospholipid metabolism | 2/36 | 0.250 | 0.03 |
| Steroid hormone biosynthesis | 1/80 | 0.892 | 0.01 |
| Pantothenate and CoA biosynthesis | 2/20 | 0.098 | 0.01 |
| Biosynthesis of unsaturated fatty acids | 2/8 | 0.014 | 0.00 |
| Valine, leucine and isoleucine biosynthesis | 4/36 | 0.018 | 0.00 |
| Butanoate metabolism | 2/15 | 0.059 | 0.00 |
| Taurine and hypotaurine metabolism | 1/8 | 0.195 | 0.00 |
| Phenylalanine metabolism | 1/10 | 0.238 | 0.00 |
| D-Amino acid metabolism | 1/15 | 0.336 | 0.00 |
| Ubiquinone and other terpenoid–quinone biosynthesis | 1/18 | 0.388 | 0.00 |
| beta-Alanine metabolism | 1/21 | 0.436 | 0.00 |
| Lipoic acid metabolism | 1/28 | 0.535 | 0.00 |
| Glyoxylate and dicarboxylate metabolism | 1/32 | 0.584 | 0.00 |
| Valine, leucine and isoleucine degradation | 1/40 | 0.667 | 0.00 |
| (B) Comparison All Groups at 12 Weeks | |||
| Pathway Name | Match Status | p Value | Impact Value |
| Phenylalanine, tyrosine and tryptophan biosynthesis | 1/4 | 0.034 * | 0.50 # |
| Phenylalanine metabolism | 1/10 | 0.082 | 0.36 # |
| Arginine and proline metabolism | 4/36 | 0.001 * | 0.17 # |
| Tryptophan metabolism | 1/41 | 0.298 | 0.14 # |
| Arginine biosynthesis | 1/14 | 0.113 | 0.08 |
| Sphingolipid metabolism | 1/32 | 0.241 | 0.06 |
| Glycerophospholipid metabolism | 2/36 | 0.036 * | 0.03 |
| Primary bile acid biosynthesis | 1/46 | 0.328 | 0.02 |
| D-Amino acid metabolism | 2/15 | 0.007 * | 0.00 |
| Glycine, serine and threonine metabolism | 2/34 | 0.032 * | 0.00 |
| Butanoate metabolism | 1/15 | 0.121 | 0.00 |
| Biosynthesis of unsaturated fatty acids | 1/36 | 0.267 | 0.00 |
| (C) Comparison Between DMC and Non-DM Control | |||
| Pathway Name | Match Status | p Value | Impact Value |
| Tryptophan metabolism | 1/41 | 0.019 * | 0.14 # |
| Arginine and proline metabolism | 1/36 | 0.027 * | 0.12 # |
| Arginine biosynthesis | 1/14 | 0.070 | 0.08 |
| Glycerophospholipid metabolism | 2/36 | 0.013 * | 0.03 |
| Primary bile acid biosynthesis | 1/46 | 0.215 | 0.02 |
| D-Amino acid metabolism | 1/15 | 0.075 | 0.00 |
| Butanoate metabolism | 1/15 | 0.075 | 0.00 |
| One carbon pool by folate | 1/26 | 0.127 | 0.00 |
| Glycine, serine and threonine metabolism | 1/34 | 0.163 | 0.00 |
| Biosynthesis of unsaturated fatty acids | 1/36 | 0.172 | 0.00 |
| (D) Comparison Between DMT and DMC | |||
| Pathway Name | Match Status | p Value | Impact Value |
| Arginine and proline metabolism | 1/36 | 0.050 * | 0.12 # |
| Arginine biosynthesis | 1/14 | 0.036 * | 0.08 |
| Primary bile acid biosynthesis | 1/46 | 0.114 | 0.02 |
| D-Amino acid metabolism | 1/15 | 0.038 * | 0.00 |
| Glycerophospholipid metabolism | 1/36 | 0.090 | 0.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rusli, N.; Tan, J.K.; Makpol, S.; Mohd Isa, I.L.; Hakimi, N.H.; Ab Rani, N.; Remli, R. Exploring the Effects of Palm Tocotrienol-Rich Fraction in Diabetic Peripheral Neuropathy Rat’s Model: An Untargeted Metabolomic Profiling and Correlation Study. Int. J. Mol. Sci. 2025, 26, 11247. https://doi.org/10.3390/ijms262311247
Rusli N, Tan JK, Makpol S, Mohd Isa IL, Hakimi NH, Ab Rani N, Remli R. Exploring the Effects of Palm Tocotrienol-Rich Fraction in Diabetic Peripheral Neuropathy Rat’s Model: An Untargeted Metabolomic Profiling and Correlation Study. International Journal of Molecular Sciences. 2025; 26(23):11247. https://doi.org/10.3390/ijms262311247
Chicago/Turabian StyleRusli, Noradliyanti, Jen Kit Tan, Suzana Makpol, Isma Liza Mohd Isa, Nur Haleeda Hakimi, Nazirah Ab Rani, and Rabani Remli. 2025. "Exploring the Effects of Palm Tocotrienol-Rich Fraction in Diabetic Peripheral Neuropathy Rat’s Model: An Untargeted Metabolomic Profiling and Correlation Study" International Journal of Molecular Sciences 26, no. 23: 11247. https://doi.org/10.3390/ijms262311247
APA StyleRusli, N., Tan, J. K., Makpol, S., Mohd Isa, I. L., Hakimi, N. H., Ab Rani, N., & Remli, R. (2025). Exploring the Effects of Palm Tocotrienol-Rich Fraction in Diabetic Peripheral Neuropathy Rat’s Model: An Untargeted Metabolomic Profiling and Correlation Study. International Journal of Molecular Sciences, 26(23), 11247. https://doi.org/10.3390/ijms262311247


_Kim.png)






