Reduced Activity of Soluble Fibroblast Activation Protein (sFAP) Represents a Biomarker of Aggressive Disease in Lymphoid Malignancies
Abstract
1. Introduction
2. Results
2.1. Patient Characteristics
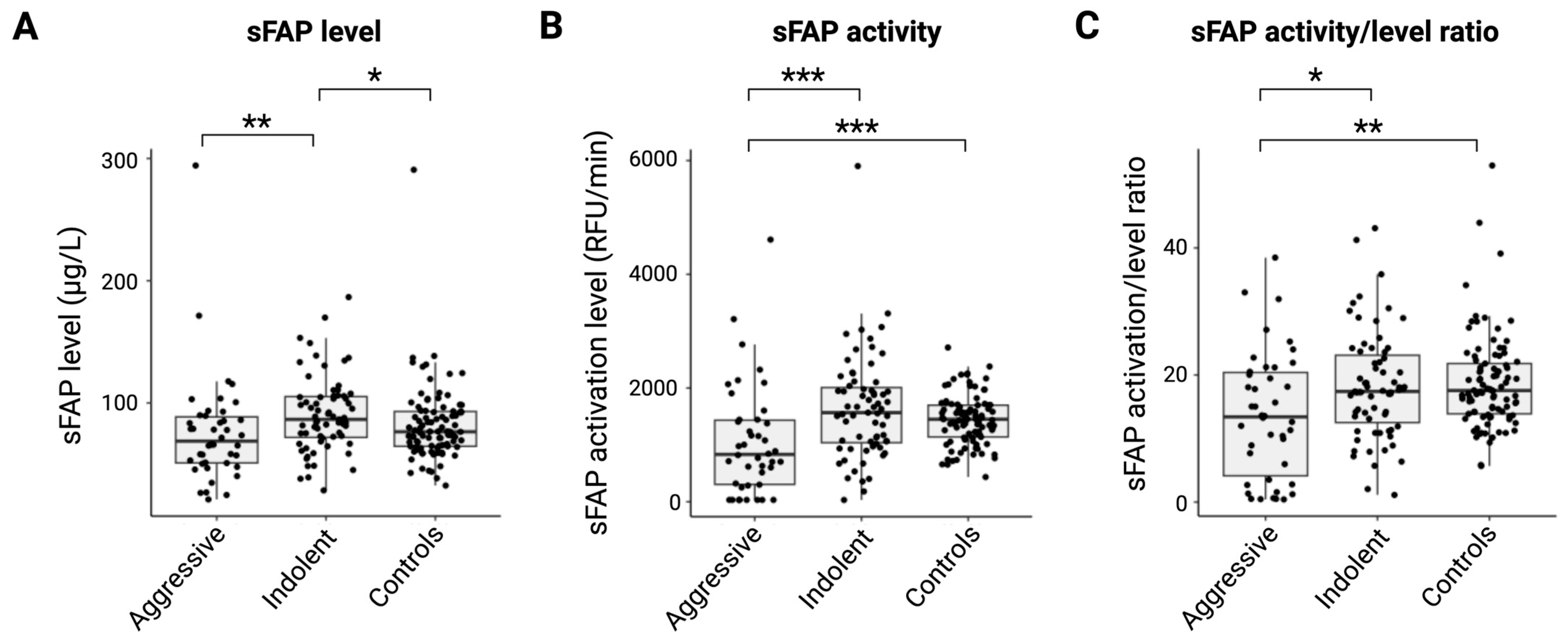
2.2. sFAP Level and Activity Are Reduced in Aggressive Lymphoid Malignancies
2.3. Ratio of sFAP Activity/sFAP Level Indicates That sFAP Is a Marker of Aggressive Disease
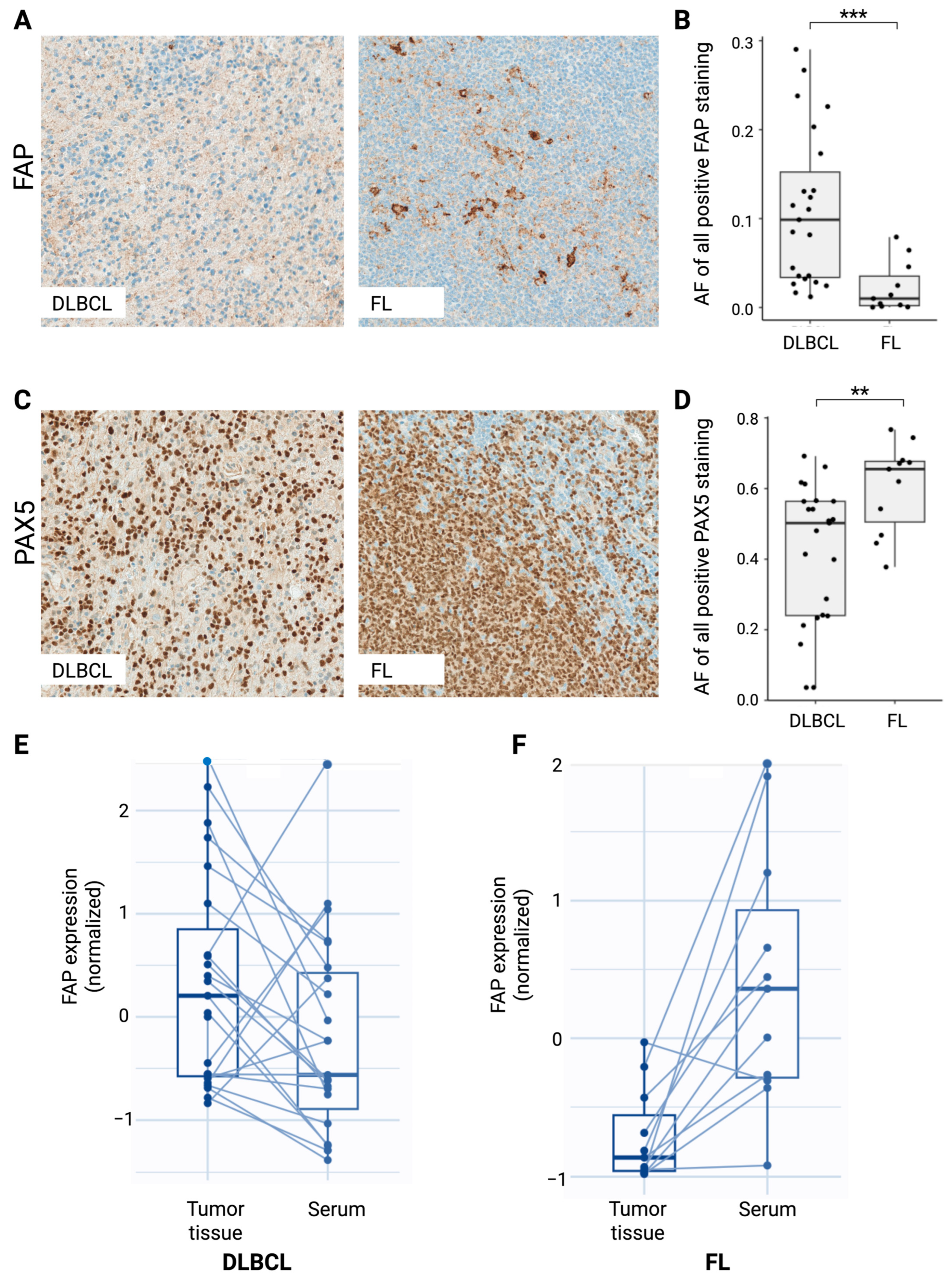
2.4. FAP Expression in DLBCL and FL Tumor Tissues Indicates Opposite Regulation of Serum sFAP Expression
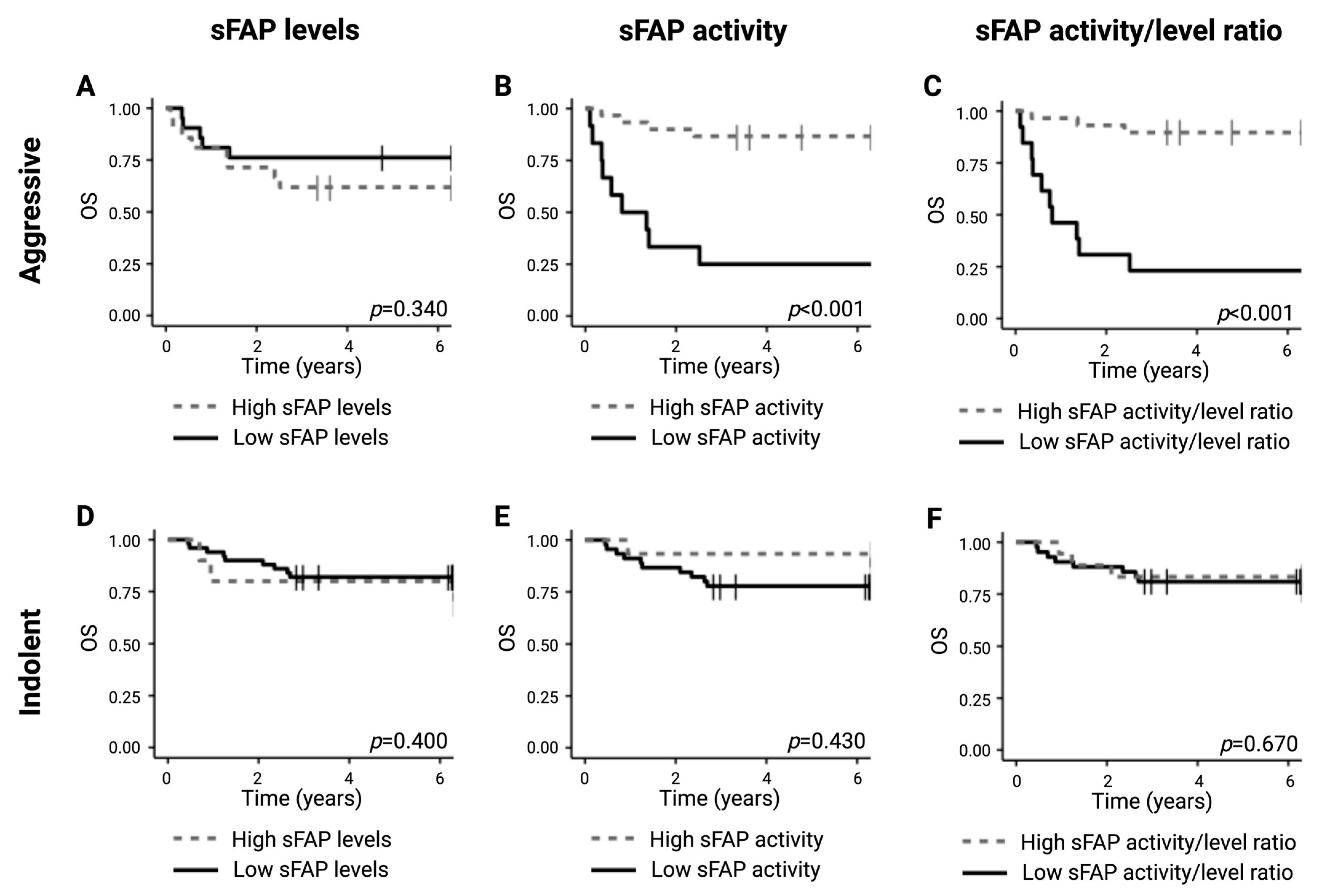
2.5. Lower sFAP Activity Is Correlated with Inferior Survival Outcome for Aggressive Lymphomas
3. Discussion
4. Materials and Methods
4.1. Patient Samples
4.2. Circulating sFAP Levels and sFAP Activity
4.3. Immunohistochemistry
4.4. Digital Image Analysis
4.5. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Decker, A.; Vliegen, G.; Van Rompaey, D.; Peeraer, A.; Bracke, A.; Verckist, L.; Jansen, K.; Geiss-Friedlander, R.; Augustyns, K.; De Winter, H.; et al. Novel Small Molecule-Derived, Highly Selective Substrates for Fibroblast Activation Protein (FAP). ACS Med. Chem. Lett. 2019, 10, 1173–1179. [Google Scholar] [CrossRef]
- Fitzgerald, A.A.; Weiner, L.M. The role of fibroblast activation protein in health and malignancy. Cancer Metastasis Rev. 2020, 39, 783–803. [Google Scholar] [CrossRef]
- Xin, L.; Gao, J.; Zheng, Z.; Chen, Y.; Lv, S.; Zhao, Z.; Yu, C.; Yang, X.; Zhang, R. Fibroblast Activation Protein-α as a Target in the Bench-to-Bedside Diagnosis and Treatment of Tumors: A Narrative Review. Front. Oncol. 2021, 11. [Google Scholar] [CrossRef]
- Liu, F.; Qi, L.; Liu, B.; Liu, J.; Zhang, H.; Che, D.; Cao, J.; Shen, J.; Geng, J.; Bi, Y.; et al. Fibroblast Activation Protein Overexpression and Clinical Implications in Solid Tumors: A Meta-Analysis. PLoS ONE 2015, 10, e0116683. [Google Scholar] [CrossRef]
- Kalluri, R. The biology and function of fibroblasts in cancer. Nat. Rev. Cancer 2016, 16, 582–598. [Google Scholar] [CrossRef] [PubMed]
- Basalova, N.; Alexandrushkina, N.; Grigorieva, O.; Kulebyakina, M.; Efimenko, A. Fibroblast Activation Protein Alpha (FAPα) in Fibrosis: Beyond a Perspective Marker for Activated Stromal Cells? Biomolecules 2023, 13, 1718. [Google Scholar] [CrossRef] [PubMed]
- Christiansen, V.J.; Jackson, K.W.; Lee, K.N.; McKee, P.A. Effect of fibroblast activation protein and α2-antiplasmin cleaving enzyme on collagen Types I, III, and IV. Arch. Biochem. Biophys. 2007, 457, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Solano-Iturri, J.D.; Beitia, M.; Errarte, P.; Calvete-Candenas, J.; Etxezarraga, M.C.; Loizate, A.; Echevarria, E.; Badiola, I.; Larrinaga, G. Altered expression of fibroblast activation protein-α (FAP) in colorectal adenoma-carcinoma sequence and in lymph node and liver metastases. Aging 2020, 12, 10337–10358. [Google Scholar] [CrossRef]
- Lv, B.; Xie, F.; Zhao, P.; Ma, X.; Jiang, W.; Yu, J.; Zhang, X.; Jia, J. Promotion of Cellular Growth and Motility Is Independent of Enzymatic Activity of Fibroblast Activation Protein-α. Cancer Genom. Proteom. 2016, 13, 201–208. [Google Scholar]
- LeBleu, V.S.; Kalluri, R. A peek into cancer-associated fibroblasts: Origins, functions and translational impact. Dis. Model. Mech. 2018, 11. [Google Scholar] [CrossRef]
- Najafi, M.; Mortezaee, K.; Majidpoor, J. Stromal reprogramming: A target for tumor therapy. Life Sci. 2019, 239, 117049. [Google Scholar] [CrossRef]
- Hamson, E.J.; Keane, F.M.; Tholen, S.; Schilling, O.; Gorrell, M.D. Understanding fibroblast activation protein (FAP): Substrates, activities, expression and targeting for cancer therapy. Proteom.—Clin. Appl. 2014, 8, 454–463. [Google Scholar] [CrossRef]
- Lee, K.N.; Jackson, K.W.; Christiansen, V.J.; Lee, C.S.; Chun, J.-G.; McKee, P.A. Antiplasmin-cleaving enzyme is a soluble form of fibroblast activation protein. Blood 2006, 107, 1397–1404. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, A.K.; Hage, C.; Jessen, N.; Mellbin, L.; Bjerre, M. Sitagliptin reduces FAP-activity and increases intact FGF21 levels in patients with newly detected glucose abnormalities. Mol. Cell. Endocrinol. 2022, 556, 111738. [Google Scholar] [CrossRef] [PubMed]
- Alaggio, R.; Amador, C.; Anagnostopoulos, I.; Attygalle, A.D.; Araujo, I.B.d.O.; Berti, E.; Bhagat, G.; Borges, A.M.; Boyer, D.; Calaminici, M.; et al. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Lymphoid Neoplasms. Leukemia 2022, 36, 1720–1748, Correction in Leukemia 2023, 37, 1944–1951. [Google Scholar] [CrossRef]
- Ge, Y.; Zhan, F.; Barlogie, B.; Epstein, J.; Shaughnessy, J.S., Jr.; Yaccoby, S. Fibroblast activation protein (FAP) is upregulated in myelomatous bone and supports myeloma cell survival. Br. J. Haematol. 2006, 133, 83–92. [Google Scholar] [CrossRef]
- Zi, F.-M.; He, J.-S.; Li, Y.; Wu, C.; Wu, W.-J.; Yang, Y.; Wang, L.-J.; He, D.-H.; Yang, L.; Zhao, Y.; et al. Fibroblast activation protein protects bortezomib-induced apoptosis in multiple myeloma cells through β-catenin signaling pathway. Cancer Biol. Ther. 2014, 15, 1413–1422. [Google Scholar] [CrossRef]
- Mei, S.; Zhang, Y.; Yu, L.; Chen, G.; Zi, F. Expression and role of fibroblast activation protein α in acute myeloid leukemia. Oncol. Rep. 2020, 45, 641–651. [Google Scholar] [CrossRef]
- Swerdlow, S.H.; Campo, E.; Pileri, S.A.; Harris, N.L.; Stein, H.; Siebert, R.; Advani, R.; Ghielmini, M.; Salles, G.A.; Zelenetz, A.D.; et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 2016, 127, 2375–2390. [Google Scholar] [CrossRef]
- Pasqualucci, L. Molecular pathogenesis of germinal center-derived B cell lymphomas. Immunol. Rev. 2019, 288, 240–261. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Xing, S.; Xu, B.; Liu, W.; Zhang, G. Evaluation of the circulating level of fibroblast activation protein α for diagnosis of esophageal squamous cell carcinoma. Oncotarget 2017, 8, 30050–30062. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, S.; Lai, Y.; Wang, G.; Wei, M.; Jin, X.; Ding, J.; Zhang, Y.; Shi, Y.; Wang, F.; et al. Fibroblast Activation Protein and Glycolysis in Lymphoma Diagnosis: Comparison of 68Ga-FAPI PET/CT and 18F-FDG PET/CT. J. Nucl. Med. 2023, 64, 1399–1405. [Google Scholar] [CrossRef]
- Jin, X.; Wei, M.; Wang, S.; Wang, G.; Lai, Y.; Shi, Y.; Zhang, Y.; Yang, Z.; Wang, X. Detecting Fibroblast Activation Proteins in Lymphoma Using 68Ga-FAPI PET/CT. J. Nucl. Med. 2021, 63, 212–217. [Google Scholar] [CrossRef]
- Quartuccio, N.; Nicolosi, S.; Pulizzi, S.; D’Oppido, D.; Ialuna, S. The role of FAPI PET/CT in patients with lymphoma: A systematic review. Front Nucl Med. 2025, 5, 1589903. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Guglielmo, P.; Alongi, P.; Baratto, L.; Abenavoli, E.; Buschiazzo, A.; Celesti, G.; Conte, M.; Filice, R.; Gorica, J.; Jonghi-Lavarini, L.; et al. Head-to-Head Comparison of FDG and Radiolabeled FAPI PET: A Systematic Review of the Literature. Life 2023, 13, 1821. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, Y.; Tang, H.; Cai, J.; Zhang, T.; Guo, J.; Feng, D.; Wang, Z. Ovarian cancer-associated fibroblasts contribute to epithelial ovarian carcinoma metastasis by promoting angiogenesis, lymphangiogenesis and tumor cell invasion. Cancer Lett. 2011, 303, 47–55. [Google Scholar] [CrossRef]
- Wang, L.C.; Lo, A.; Scholler, J.; Sun, J.; Majumdar, R.S.; Kapoor, V.; Antzis, M.; Cotner, C.E.; Johnson, L.A.; Durham, A.C.; et al. Targeting fibroblast activation protein in tumor stroma with chimeric antigen receptor T cells can inhibit tumor growth and augment host immunity without severe toxicity. Cancer Immunol. Res. 2014, 2, 154–166. [Google Scholar] [CrossRef]
- Mortensen, J.B.; Monrad, I.; Enemark, M.B.; Ludvigsen, M.; Kamper, P.; Bjerre, M.; D’Amore, F. Soluble programmed cell death protein 1 (sPD-1) and the soluble programmed cell death ligands 1 and 2 (sPD-L1 and sPD-L2) in lymphoid malignancies. Eur. J. Haematol. 2021, 107, 81–91. [Google Scholar] [CrossRef]
- Arlien-Søborg, M.C.; Grøndahl, C.; Bæk, A.; Dal, J.; Madsen, M.; Høgild, M.L.; Pedersen, S.B.; Bjerre, M.; Jørgensen, J.O.L. Fibroblast Activation Protein is a GH Target: A Prospective Study of Patients with Acromegaly Before and After Treatment. J. Clin. Endocrinol. Metab. 2019, 105, 106–115. [Google Scholar] [CrossRef]
- Enemark, M.B.; Monrad, I.; Madsen, C.; Lauridsen, K.L.; Honoré, B.; Plesner, T.L.; Hamilton-Dutoit, S.J.; D’Amore, F.; Ludvigsen, M. PD-1 Expression in Pre-Treatment Follicular Lymphoma Predicts the Risk of Subsequent High-Grade Transformation. OncoTargets Ther. 2021, ume 14, 481–489. [Google Scholar] [CrossRef]
- Enemark, M.B.; Hybel, T.E.; Madsen, C.; Lauridsen, K.L.; Honoré, B.; Plesner, T.L.; Hamilton-Dutoit, S.; D’amore, F.; Ludvigsen, M. Tumor-Tissue Expression of the Hyaluronic Acid Receptor RHAMM Predicts Histological Transformation in Follicular Lymphoma Patients. Cancers 2022, 14, 1316. [Google Scholar] [CrossRef]
- Hybel, T.E.; Vase, M.Ø.; Maksten, E.F.; Enemark, M.B.; Lauridsen, K.L.; Hamilton-Dutoit, S.; Andersen, C.; Møller, M.B.; Sørensen, S.S.; Jespersen, B.; et al. Intratumoral expression of CD38 in patients with post-transplant lymphoproliferative disorder. Acta Oncol. 2021, 60, 1637–1642. [Google Scholar] [CrossRef]
- Enemark, M.B.H.; Wolter, K.; Campbell, A.J.; Andersen, M.D.; Sørensen, E.F.; Hybel, T.E.; Madsen, C.; Lauridsen, K.L.; Plesner, T.L.; Hamilton-Dutoit, S.J.; et al. Proteomics identifies apoptotic markers as predictors of histological transformation in patients with follicular lymphoma. Blood Adv. 2023, 7, 7418–7432. [Google Scholar] [CrossRef]
- Madsen, C.; Lauridsen, K.L.; Plesner, T.L.; Monrad, I.; Honoré, B.; Hamilton-Dutoit, S.; D’aMore, F.; Ludvigsen, M. High intratumoral expression of vimentin predicts histological transformation in patients with follicular lymphoma. Blood Cancer J. 2019, 9, 35. [Google Scholar] [CrossRef]
| Characteristics | All Patients (n = 120) n (%) | DLBCL (n = 30, 26%) n (%) | FL (n = 13, 12%) n (%) | HL (n = 11, 9%) n (%) | CLL/SLL (n = 38, 30%) n (%) | Other B-Cell (n = 21, 17%) n (%) | TL/L (n = 7, 6%) n (%) |
|---|---|---|---|---|---|---|---|
| Sex | |||||||
| Male | 75 (63) | 17 (57) | 8 (62) | 5 (45) | 26 (68) | 13 (62) | 6 (86) |
| Female | 45 (38) | 13 (43 | 5 (38) | 6 (55) | 12 (32) | 8 (38) | 1 (14) |
| Age at diagnosis, y | |||||||
| Mean | 66 | 68 | 65 | 34 | 70 | 63 | 62 |
| Range | 18–96 | 20–91 | 33–85 | 18–70 | 45–96 | 49–93 | 18–78 |
| Comorbidities | |||||||
| No | 27 (23) | 6 (20) | 4 (31) | 7 (64) | 6 (16) | 2 (10) | 2 (29) |
| Yes | 90 (75) | 23 (77) | 9 (69) | 4 (36) | 31 (82) | 18 (86) | 5 (71) |
| Unknown | 3 (3) | 1 (3) | 0 (0) | 0 (0) | 1 (3) | 1 (5) | 0 (0) |
| Ann Arbor stage | |||||||
| I–II | 27 (23) | 8 (27) | 5 (62) | 6 (55) | - | 7 (33) | 1 (14) |
| III–IV | 55 (46) | 22 (73) | 8 (38) | 5 (45) | - | 14 (67) | 6 (86) |
| Unknown/not relevant | 38 (32) | 0 (0) | 0 (0) | 0 (0) | - | 0 (0) | 0 (0) |
| B symptoms | |||||||
| No | 72 (60) | 14 (47) | 11 (85) | 3 (27) | 30 (79) | 12 (57) | 2 (29) |
| Yes | 48 (40) | 16 (53) | 2 (15) | 8 (73) | 8 (21) | 9 (43) | 5 (71) |
| ECOG | |||||||
| <2 | 102 (85) | 19 (63) | 13 (100) | 10 (91) | 35 (92) | 19 (90) | 6 (86) |
| ≥2 | 18 (15) | 11 (37) | 0 (0) | 1 (9) | 3 (8) | 2 (10) | 1 (14) |
| LDH elevation | |||||||
| No | 80 (67) | 18 (60) | 12 (92) | 6 (55) | 29 (76) | 12 (57) | 3 (43) |
| Yes | 40 (33) | 12 (40) | 1 (8) | 5 (45) | 9 (24) | 9 (43) | 4 (57) |
| Nodal sites | |||||||
| <2 | 89 (74) | 7 (23) | 8 (62) | 7 (64) | 34 (89) | 3 (14) | 2 (29) |
| ≥2 | 31 (26) | 23 (77) | 5 (38) | 4 (36) | 4 (11) | 18 (86) | 5 (71) |
| Bulky disease | |||||||
| No | 101 (84) | 22 (73) | 11 (85) | 9 (82) | 36 (95) | 17 (81) | 6 (86) |
| Yes | 13 (11) | 6 (20) | 2 (15) | 2 (18) | 0 (0) | 3 (14) | 0 (0) |
| Unknown | 6 (5) | 2 (7) | 0 (0) | 0 (0) | 2 (5) | 1 (5) | 1 (14) |
| BM involvement | |||||||
| No | 61 (51) | 28 (93) | 9 (69) | 9 (82) | 1 (3) | 9 (43) | 5 (71) |
| Yes | 57 (48) | 2 (7) | 3 (23) | 1 (9) | 37 (97) | 12 (57) | 2 (29) |
| Unknown | 2 (2) | 0 (0) | 1 (8) | 1 (9) | 0 (0) | 0 (0) | 0 (0) |
| IPI | |||||||
| 0–I | 19 (16) | 6 (20) | 4 (31) | 6 (55) | 2 (10) | 1 (14) | |
| II–III | 41 (34) | 11 (37) | 7 (54) | 4 (36) | 14 (67) | 4 (57) | |
| IV–V | 22 (18) | 13 (43) | 0 (0) | 1 (9) | 5 (24) | 2 (29) | |
| Unknown/not relevant | 38 (32) | 0 (0) | 2 (15) | 0 (0) | 0 (0) | 0 (0) |
| sFAP Activity | B Symptoms | ECOG | Bulky Disease | Ann Arbor Stage | IPI | BM | PAX5 | |
|---|---|---|---|---|---|---|---|---|
| (A) All patients | ||||||||
| sFAP expression | ρ = 0.59 p < 0.001 | ρ = −0.18 p = 0.046 | ρ = −0.22 p = 0.013 | ρ = −0.31 p < 0.001 | - | - | - | - |
| sFAP activity | - | ρ = −0.23 p = 0.010 | ρ = −0.36 p < 0.001 | ρ = −0.24 p = 0.009 | - | - | - | - |
| (B) B-cell lymphomas | ||||||||
| sFAP expression | ρ = 0.60 p < 0.001 | ρ = −0.24 p = 0.041 | - | ρ = −0.33 p = 0.005 | - | - | - | - |
| sFAP activity | ρ = −0.30 p = 0.009 | ρ = −0.35 p = 0.002 | ρ = −0.26 p = 0.025 | ρ = −0.28 p = 0.015 | ρ = −0.29 p = 0.012 | - | - | |
| (C) DLBCL and FL | ||||||||
| FAP | - | - | - | - | - | - | ρ = −0.38 p = 0.028 | ρ = −0.38 p = 0.022 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hemmingsen, J.K.; Enemark, M.H.; Pedersen, A.K.N.; Sørensen, E.F.; Lauridsen, K.L.; Løhde, J.B.; d’Amore, F.; Hamilton-Dutoit, S.J.; Bjerre, M.; Ludvigsen, M. Reduced Activity of Soluble Fibroblast Activation Protein (sFAP) Represents a Biomarker of Aggressive Disease in Lymphoid Malignancies. Int. J. Mol. Sci. 2025, 26, 11248. https://doi.org/10.3390/ijms262311248
Hemmingsen JK, Enemark MH, Pedersen AKN, Sørensen EF, Lauridsen KL, Løhde JB, d’Amore F, Hamilton-Dutoit SJ, Bjerre M, Ludvigsen M. Reduced Activity of Soluble Fibroblast Activation Protein (sFAP) Represents a Biomarker of Aggressive Disease in Lymphoid Malignancies. International Journal of Molecular Sciences. 2025; 26(23):11248. https://doi.org/10.3390/ijms262311248
Chicago/Turabian StyleHemmingsen, Jonas Klejs, Marie Hairing Enemark, Anne Kathrine Nissen Pedersen, Emma Frasez Sørensen, Kristina Lystlund Lauridsen, Julie Bondgaard Løhde, Francesco d’Amore, Stephen Jacques Hamilton-Dutoit, Mette Bjerre, and Maja Ludvigsen. 2025. "Reduced Activity of Soluble Fibroblast Activation Protein (sFAP) Represents a Biomarker of Aggressive Disease in Lymphoid Malignancies" International Journal of Molecular Sciences 26, no. 23: 11248. https://doi.org/10.3390/ijms262311248
APA StyleHemmingsen, J. K., Enemark, M. H., Pedersen, A. K. N., Sørensen, E. F., Lauridsen, K. L., Løhde, J. B., d’Amore, F., Hamilton-Dutoit, S. J., Bjerre, M., & Ludvigsen, M. (2025). Reduced Activity of Soluble Fibroblast Activation Protein (sFAP) Represents a Biomarker of Aggressive Disease in Lymphoid Malignancies. International Journal of Molecular Sciences, 26(23), 11248. https://doi.org/10.3390/ijms262311248






