Preliminary Assessment of the Possible Environmental Risks of Photopolymerizing Resin Particles Produced by Finishing Stereolithography 3D-Printed Objects, Employing Toxicity Test on Tropical House Crickets (Gryllodes sigillatus)
Abstract
1. Introduction
2. Results
2.1. Characteristics of Photopolymerizing Resin Particles
2.2. Mortality and Observations Made During the Toxicity Test
2.3. Biochemical Assay Results
3. Discussion
4. Materials and Methods
4.1. Ethical Statement
4.2. Acquisition of Photopolymerizing Resin Particles
4.3. Characterization of Particles
4.4. Selection of the Tested Xenobiotic Concentrations
4.5. Agarose Gel Preparation
4.6. Scheme of the Experiment
4.7. Morphometric Measurements
4.8. Mortality Calculations
4.9. Enzyme Activity Assays
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zastrow, M. 3D Printing Gets Bigger, Faster and Stronger. Nature 2020, 578, 20–23. [Google Scholar] [CrossRef]
- Jandyal, A.; Chaturvedi, I.; Wazir, I.; Raina, A.; Ul Haq, M.I. 3D Printing—A Review of Processes, Materials and Applications in Industry 4.0. Sustain. Oper. Comput. 2022, 3, 33–42. [Google Scholar] [CrossRef]
- Wulff, J.; Schweikl, H.; Rosentritt, M. Cytotoxicity of Printed Resin-Based Splint Materials. J. Dent. 2022, 120, 104097. [Google Scholar] [CrossRef]
- Min, K.; Li, Y.; Wang, D.; Chen, B.; Ma, M.; Hu, L.; Liu, Q.; Jiang, G. 3D Printing-Induced Fine Particle and Volatile Organic Compound Emission: An Emerging Health Risk. Environ. Sci. Technol. Lett. 2021, 8, 616–625. [Google Scholar] [CrossRef]
- Rodríguez-Hernández, A.G.; Chiodoni, A.; Bocchini, S.; Vazquez-Duhalt, R. 3D Printer Waste, a New Source of Nanoplastic Pollutants. Environ. Pollut. 2020, 267, 115609. [Google Scholar] [CrossRef] [PubMed]
- Dobrzyńska, E.; Kondej, D.; Kowalska, J.; Szewczyńska, M. State of the Art in Additive Manufacturing and Its Possible Chemical and Particle Hazards—Review. Indoor Air 2021, 31, 1733–1758. [Google Scholar] [CrossRef] [PubMed]
- Maturi, M.; Locatelli, E.; Sanz de Leon, A.; Comes Franchini, M.; Molina, S.I. Sustainable Approaches in Vat Photopolymerization: Advancements, Limitations, and Future Opportunities. Green Chem. 2025, 27, 8710–8754. [Google Scholar] [CrossRef]
- Zhang, Q.; Davis, A.Y.; Black, M.S. Emissions and Chemical Exposure Potentials from Stereolithography Vat Polymerization 3D Printing and Post-Processing Units. ACS Chem. Health Saf. 2022, 29, 184–191. [Google Scholar] [CrossRef]
- Husna, A.; Ashrafi, S.; Tomal, A.A.; Tuli, N.T.; Bin Rashid, A. Recent Advancements in Stereolithography (SLA) and Their Optimization of Process Parameters for Sustainable Manufacturing. Hybrid Adv. 2024, 7, 100307. [Google Scholar] [CrossRef]
- Alijagic, A.; Suljevic, D.; Engwall, M.; Särndahl, E. 3D Printing: Balancing Innovation for Sustainability with Emerging Environmental and Health Risks. iScience 2025, 28, 113185. [Google Scholar] [CrossRef]
- Sacchi, F.; Colucci, G.; Bondioli, F.; Sangermano, M.; Messori, M. Review: Bio-Based Photopolymers for Additive Manufacturing. J. Mater. Sci. 2025, 60, 11191–11220. [Google Scholar] [CrossRef]
- Ballentine, M.; Kennedy, A.; Melby, N.; Bednar, A.; Moser, R.; Moores, L.C.; Alberts, E.M.; Laber, C.H.; Crouch, R.A. Acute and Chronic Toxicity of Uncured Resin Feedstocks for Vat Photopolymerization 3D Printing to a Cladoceran (Ceriodaphnia Dubia). Bull. Environ. Contam. Toxicol. 2023, 110, 56. [Google Scholar] [CrossRef]
- Rogers, H.B.; Zhou, L.T.; Kusuhara, A.; Zaniker, E.; Shafaie, S.; Owen, B.C.; Duncan, F.E.; Woodruff, T.K. Dental Resins Used in 3D Printing Technologies Release Ovo-Toxic Leachates. Chemosphere 2021, 270, 129003. [Google Scholar] [CrossRef]
- Macdonald, N.P.; Zhu, F.; Hall, C.J.; Reboud, J.; Crosier, P.S.; Patton, E.E.; Wlodkowic, D.; Cooper, J.M. Assessment of Biocompatibility of 3D Printed Photopolymers Using Zebrafish Embryo Toxicity Assays. Lab Chip 2016, 16, 291–297. [Google Scholar] [CrossRef]
- Alt, F.; Heinemann, C.; Kruppke, B. Class I Biocompatible DLP—Printed Acrylate Impairs Adhesion and Proliferation of Human Mesenchymal Stromal Cells in Indirect Cytotoxicity Assay. Biomed Res. Int. 2023, 2023, 8305995. [Google Scholar] [CrossRef]
- Camassa, L.M.A.; Ervik, T.K.; Zegeye, F.D.; Mdala, I.; Valen, H.; Ansteinsson, V.; Zienolddiny, S. Characterization and Toxicity Evaluation of Air-Borne Particles Released by Grinding from Two Dental Resin Composites in Vitro. Dent. Mater. 2021, 37, 1121–1133, Correction in Dent. Mater. 2022, 38, 1564. [Google Scholar] [CrossRef] [PubMed]
- Stefaniak, A.B.; Bowers, L.N.; Knepp, A.K.; Luxton, T.P.; Peloquin, D.M.; Baumann, E.J.; Ham, J.E.; Wells, J.R.; Johnson, A.R.; LeBouf, R.F.; et al. Particle and Vapor Emissions from Vat Polymerization Desktop-Scale 3-Dimensional Printers. J. Occup. Environ. Hyg. 2019, 16, 519–531. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.K.; Saxena, P.; Pant, V.A.; Pant, A.B. Release and Toxicity of Dental Resin Composite. Toxicol. Int. 2012, 19, 225. [Google Scholar] [CrossRef] [PubMed]
- Oskui, S.M.; Diamante, G.; Liao, C.; Shi, W.; Gan, J.; Schlenk, D.; Grover, W.H. Assessing and Reducing the Toxicity of 3D-Printed Parts. Environ. Sci. Technol. Lett. 2016, 3, 1–6. [Google Scholar] [CrossRef]
- Kim, J.S.; Park, S.-K.; Lee, H.; Sniffer Worm, C. Elegans, as a Toxicity Evaluation Model Organism with Sensing and Locomotion Abilities. PLoS ONE 2023, 18, e0289493. [Google Scholar] [CrossRef]
- Zhu, F.; Skommer, J.; Friedrich, T.; Kaslin, J.; Wlodkowic, D. 3D Printed Polymers Toxicity Profiling: A Caution for Biodevice Applications. In Proceedings of the SPIE Micro + Nano Materials, Devices, and Applications, Sydney, NSW, Australia, 22 December 2015; Eggleton, B.J., Palomba, S., Eds.; SPIE: Bellingham, WA, USA; Volume 9668, p. 96680Z. [Google Scholar] [CrossRef]
- Tian, H.; Guo, H.; Zhang, H.; Zhang, T.; Tang, Y.; Ye, Z.; Zhang, J.; Yuan, G.; Zhou, Q.; Li, Y.; et al. Toxicity of Stereolithography 3D Printed Objects at the Chemical Level and Strategies to Improve Biocompatibility. Addit. Manuf. 2025, 101, 104715. [Google Scholar] [CrossRef]
- Biagio, F.P.; Tamaki, F.K.; Terra, W.R.; Ribeiro, A.F. Digestive Morphophysiology of Gryllodes Sigillatus (Orthoptera: Gryllidae). J. Insect Physiol. 2009, 55, 1125–1133. [Google Scholar] [CrossRef] [PubMed]
- Rapkin, J.; Jensen, K.; Archer, C.R.; House, C.M.; Sakaluk, S.K.; Castillo, E.D.; Hunt, J. The Geometry of Nutrient Space–Based Life-History Trade-Offs: Sex-Specific Effects of Macronutrient Intake on the Trade-Off between Encapsulation Ability and Reproductive Effort in Decorated Crickets. Am. Nat. 2018, 191, 452–474. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, M.W.; Provencher, J.F.; Allison, J.E.; Muzzatti, M.J.; MacMillan, H.A. The Digestive System of a Cricket Pulverizes Polyethylene Microplastics down to the Nanoplastic Scale. Environ. Pollut. 2024, 343, 123168. [Google Scholar] [CrossRef] [PubMed]
- Muzzatti, M.J.; McConnell, E.; Neave, S.; MacMillan, H.A.; Bertram, S.M. Fruitful Female Fecundity after Feeding Gryllodes Sigillatus (Orthoptera: Gryllidae) Royal Jelly. Can. Entomol. 2022, 154, e50. [Google Scholar] [CrossRef]
- Fudlosid, S.; Ritchie, M.W.; Muzzatti, M.J.; Allison, J.E.; Provencher, J.; MacMillan, H.A. Ingestion of Microplastic Fibres, But Not Microplastic Beads, Impacts Growth Rates in the Tropical House Cricket Gryllodes sigillatus. Front. Physiol. 2022, 13, 871149. [Google Scholar] [CrossRef]
- Sakaluk, S.K.; Burpee, D.M.; Smith, R.L. Phenotypic and Genetic Variation in the Stridulatory Organs of Male Decorated Crickets, Gryllodes Sigillatus (Orthoptera: Gryllidae). Can. J. Zool. 1992, 70, 453–457. [Google Scholar] [CrossRef]
- Bawa, M.; Songsermpong, S.; Kaewtapee, C.; Chanput, W. Effect of Diet on the Growth Performance, Feed Conversion, and Nutrient Content of the House Cricket. J. Insect Sci. 2020, 20, 10. [Google Scholar] [CrossRef]
- Vaga, M.; Berggren, Å.; Jansson, A. Growth, Survival and Development of House Crickets (Acheta Domesticus) Fed Flowering Plants. J. Insects Food Feed. 2021, 7, 151–161. [Google Scholar] [CrossRef]
- Kuo, C.; Fisher, B.L. A Literature Review of the Use of Weeds and Agricultural and Food Industry By-Products to Feed Farmed Crickets (Insecta; Orthoptera; Gryllidae). Front. Sustain. Food Syst. 2022, 5, 810421. [Google Scholar] [CrossRef]
- Seyed Alian, R.; Flasz, B.; Kędziorski, A.; Majchrzycki, Ł.; Augustyniak, M. Concentration- and Time-Dependent Dietary Exposure to Graphene Oxide and Silver Nanoparticles: Effects on Food Consumption and Assimilation, Digestive Enzyme Activities, and Body Mass in Acheta domesticus. Insects 2024, 15, 89. [Google Scholar] [CrossRef]
- Augustyniak, M.; Ajay, A.K.; Kędziorski, A.; Tarnawska, M.; Rost-Roszkowska, M.; Flasz, B.; Babczyńska, A.; Mazur, B.; Rozpędek, K.; Alian, R.S.; et al. Survival, Growth and Digestive Functions after Exposure to Nanodiamonds–Transgenerational Effects beyond Contact Time in House Cricket Strains. Chemosphere 2024, 349, 140809. [Google Scholar] [CrossRef] [PubMed]
- Augustyniak, M.; Babczyńska, A.; Dziewięcka, M.; Flasz, B.; Karpeta-Kaczmarek, J.; Kędziorski, A.; Mazur, B.; Rozpędek, K.; Seyed Alian, R.; Skowronek, M.; et al. Does Age Pay off? Effects of Three-Generational Experiments of Nanodiamond Exposure and Withdrawal in Wild and Longevity-Selected Model Animals. Chemosphere 2022, 303, 135129. [Google Scholar] [CrossRef] [PubMed]
- Komeda, Y. Discovery of Calliscelio Elegans (Hymenoptera, Scelionidae) in Japan, with a Suggestion about Its Host. Japanese J. Environ. Entomol. Zool. 2022, 33, 23–25. [Google Scholar] [CrossRef]
- Chin, W.Y.; Wilkins, V.; Sharp, A. Invasive Vegetation Encroachment Modulates Dual Threats Faced by Island-Endemic Scaly Crickets. Biol. Invasions 2024, 26, 2941–2954. [Google Scholar] [CrossRef]
- Rowe, E.; Robles López, K.Y.; Robinson, K.M.; Baudier, K.M.; Barrett, M. Farmed Cricket (Acheta Domesticus, Gryllus Assimilis, and Gryllodes Sigillatus; Orthoptera) Welfare Considerations: Recommendations for Improving Global Practice. J. Insects Food Feed. 2024, 10, 1253–1311. [Google Scholar] [CrossRef]
- Łosiewicz, B.; Bartoszek, T.; Kamaszewski, M. Health Control of Insects Used as Feed Materials and Experimental Models: State of the Art, Prospects for Future with Utilization of Biochemical Assays—A Review. J. Anim. Feed Sci. 2025, 34, 321–340. [Google Scholar] [CrossRef]
- Mashood, Z.; Rawski, M.; Kierończyk, B.; Skrzypczak, P.; Mazurkiewicz, J. Evaluation of the Application and Environmental Sustainability of Alternative Feed Materials in Sturgeon Nutrition. A Review. J. Anim. Feed Sci. 2025, 34, 3–19. [Google Scholar] [CrossRef]
- Zhang, S.; Duffield, K.R.; Foquet, B.; Ramirez, J.L.; Sadd, B.M.; Sakaluk, S.K.; Hunt, J.; Bailey, N.W. A High-Quality Reference Genome and Comparative Genomics of the Widely Farmed Banded Cricket (Gryllodes Sigillatus) Identifies Selective Breeding Targets. Ecol. Evol. 2025, 15, e71134. [Google Scholar] [CrossRef]
- Ritchie, M.W.; Cheslock, A.; Bourdages, M.P.T.; Hamilton, B.M.; Provencher, J.F.; Allison, J.E.; MacMillan, H.A. Quantifying Microplastic Ingestion, Degradation and Excretion in Insects Using Fluorescent Plastics. Conserv. Physiol. 2023, 11, coad052. [Google Scholar] [CrossRef]
- Archer, C.R.; Zajitschek, F.; Sakaluk, S.K.; Royle, N.J.; Hunt, J. Sexual Selection Affects the Evolution of Lifespan and Ageing in the Decorated Cricket Gryllodes sigillatus. Evolution 2012, 66, 3088–3100. [Google Scholar] [CrossRef]
- Ritchie, M.W.; McColville, E.R.; Mills, J.E.; Provencher, J.F.; Bertram, S.M.; MacMillan, H.A. The Disadvantage of Having a Big Mouth: The Relationship between Insect Body Size and Microplastic Ingestion. bioRxiv 2025. [Google Scholar] [CrossRef]
- Cuvillier-Hot, V.; Salin, K.; Devers, S.; Tasiemski, A.; Schaffner, P.; Boulay, R.; Billiard, S.; Lenoir, A. Impact of Ecological Doses of the Most Widespread Phthalate on a Terrestrial Species, the Ant Lasius niger. Environ. Res. 2014, 131, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Rondoni, G.; Chierici, E.; Agnelli, A.; Conti, E. Microplastics Alter Behavioural Responses of an Insect Herbivore to a Plant-Soil System. Sci. Total Environ. 2021, 787, 147716. [Google Scholar] [CrossRef]
- Le Hen, G.; Masoni, A.; Manuelli, M.; Falsini, S.; Corti, E.; Balzani, P.; Renault, D.; Papini, A.; Santini, G. Ants Avoid Food Contaminated with Micro- and Nanoplastics. Environ. Pollut. 2024, 360, 124625. [Google Scholar] [CrossRef]
- El Kholy, S.; Al Naggar, Y. Exposure to Polystyrene Microplastic Beads Causes Sex-Specific Toxic Effects in the Model Insect Drosophila melanogaster. Sci. Rep. 2023, 13, 204. [Google Scholar] [CrossRef]
- Wei, L.-F.; Liu, X.-Y.; Feng, H.-S.; Zhang, J.-T.; Liu, X.-P. Impact of Polystyrene Micro- and Nanoplastics on the Biological Traits of the Japanese Carpenter Ant, Camponotus Japonicus Mayr (Hymenoptera: Formicidae). Insects 2025, 16, 292. [Google Scholar] [CrossRef]
- Buteler, M.; Alma, A.M.; Stadler, T.; Gingold, A.C.; Manattini, M.C.; Lozada, M. Acute Toxicity of Microplastic Fibers to Honeybees and Effects on Foraging Behavior. Sci. Total Environ. 2022, 822, 153320. [Google Scholar] [CrossRef]
- Muhammad, A.; Zhou, X.; He, J.; Zhang, N.; Shen, X.; Sun, C.; Yan, B.; Shao, Y. Toxic Effects of Acute Exposure to Polystyrene Microplastics and Nanoplastics on the Model Insect, Silkworm Bombyx mori. Environ. Pollut. 2021, 285, 117255. [Google Scholar] [CrossRef]
- Łosiewicz, B.; Szudrowicz, H. Impact of Slaughter Method on Stress in Organic Common Carp (Cyprinus Carpio). J. Anim. Feed Sci. 2024, 33, 562–570. [Google Scholar] [CrossRef]
- Inagaki, Y.; Matsumoto, Y.; Kataoka, K.; Matsuhashi, N.; Sekimizu, K. Evaluation of Drug-Induced Tissue Injury by Measuring Alanine Aminotransferase (ALT) Activity in Silkworm Hemolymph. BMC Pharmacol. Toxicol. 2012, 13, 13. [Google Scholar] [CrossRef]
- Mirhaghparast, S.K.; Zibaee, A.; Sendi, J.J.; Hoda, H.; Fazeli-Dinan, M. Immune and Metabolic Responses of Chilo Suppressalis Walker (Lepidoptera: Crambidae) Larvae to an Insect Growth Regulator, Hexaflumuron. Pestic. Biochem. Physiol. 2015, 125, 69–77. [Google Scholar] [CrossRef]
- Muhammad, A.; He, J.; Yu, T.; Sun, C.; Shi, D.; Jiang, Y.; Xianyu, Y.; Shao, Y. Dietary Exposure of Copper and Zinc Oxides Nanoparticles Affect the Fitness, Enzyme Activity, and Microbial Community of the Model Insect, Silkworm Bombyx mori. Sci. Total Environ. 2022, 813, 152608. [Google Scholar] [CrossRef] [PubMed]
- Arvind Bharani, R.S.; Karthick Raja Namasivayam, S. Biogenic Silver Nanoparticles Mediated Stress on Developmental Period and Gut Physiology of Major Lepidopteran Pest Spodoptera Litura (Fab.) (Lepidoptera: Noctuidae)—An Eco-Friendly Approach of Insect Pest Control. J. Environ. Chem. Eng. 2017, 5, 453–467. [Google Scholar] [CrossRef]
- Sezer Tuncsoy, B.; Tuncsoy, M.; Gomes, T.; Sousa, V.; Teixeira, M.R.; Bebianno, M.J.; Ozalp, P. Effects of Copper Oxide Nanoparticles on Tissue Accumulation and Antioxidant Enzymes of Galleria mellonella L. Bull. Environ. Contam. Toxicol. 2019, 102, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Tuncsoy, B.; Mese, Y. Influence of Titanium Dioxide Nanoparticles on Bioaccumulation, Antioxidant Defense and Immune System of Galleria mellonella L. Environ. Sci. Pollut. Res. 2021, 28, 38007–38015. [Google Scholar] [CrossRef]
- Parenti, C.C.; Binelli, A.; Caccia, S.; Della Torre, C.; Magni, S.; Pirovano, G.; Casartelli, M. Ingestion and Effects of Polystyrene Nanoparticles in the Silkworm Bombyx mori. Chemosphere 2020, 257, 127203. [Google Scholar] [CrossRef]
- Sahebzadeh, N.; Haddadi, T.; Mokhtari, A. Toxicities of Synthesized Green Iron Oxide Nanoparticles on Trialeurodes Vaporariorum and Their Effects on Antioxidant Enzymes and Lipid Peroxidation. Afghanistan Res. J. Nat. Sci. 2023, 43, 149–165. [Google Scholar] [CrossRef]
- Horie, M.; Tabei, Y. Role of Oxidative Stress in Nanoparticles Toxicity. Free Radic. Res. 2021, 55, 331–342. [Google Scholar] [CrossRef]
- Benelli, G. Mode of Action of Nanoparticles against Insects. Environ. Sci. Pollut. Res. 2018, 25, 12329–12341. [Google Scholar] [CrossRef]
- Ibrahim, A.M.A.; Alfuhaid, N.A.; Thabet, M.A.; Ali, A.M. Chronic Effects Induced by Zinc Oxide Nanoparticles against Larvae of the Northern House Mosquito Culex Pipiens (Diptera: Culicidae). Int. J. Trop. Insect Sci. 2023, 43, 1937–1945. [Google Scholar] [CrossRef]
- Kamaszewski, M.; Kawalski, K.; Wiechetek, W.; Szudrowicz, H.; Martynow, J.; Adamek-Urbańska, D.; Łosiewicz, B.; Szczepański, A.; Bujarski, P.; Frankowska-Łukawska, J.; et al. The Effect of Silver Nanoparticles on the Digestive System, Gonad Morphology, and Physiology of Butterfly Splitfin (Ameca Splendens). Int. J. Mol. Sci. 2023, 24, 14598. [Google Scholar] [CrossRef]
- Helmberger, M.S.; Miesel, J.R.; Tiemann, L.K.; Grieshop, M.J. Soil Invertebrates Generate Microplastics From Polystyrene Foam Debris. J. Insect Sci. 2022, 22, 21, Correction in J. Insect. Sci. 2022, 22, 6. [Google Scholar] [CrossRef] [PubMed]
- Bankhead, P.; Loughrey, M.B.; Fernández, J.A.; Dombrowski, Y.; McArt, D.G.; Dunne, P.D.; McQuaid, S.; Gray, R.T.; Murray, L.J.; Coleman, H.G.; et al. QuPath: Open Source Software for Digital Pathology Image Analysis. Sci. Rep. 2017, 7, 16878. [Google Scholar] [CrossRef] [PubMed]
- Kong, J.D.; Ritchie, M.W.; Vadboncoeur, É.; MacMillan, H.A.; Bertram, S.M. Growth, Development, and Life History of a Mass-Reared Edible Insect, Gryllodes Sigillatus (Orthoptera: Gryllidae). J. Econ. Entomol. 2025, 118, 1093–1103. [Google Scholar] [CrossRef] [PubMed]
- Szczepański, A.; Adamek-Urbańska, D.; Kasprzak, R.; Cieśla, W.; Szudrowicz, H.; Piotrowska, I.; Gomułka, P.; Kawalski, K.; Martynow, J.; Łosiewicz, B.; et al. Effects of a 1.5-Year Dietary Inclusion of White Lupin Meal (Lupinus Albus) on Siberian Sturgeon (Acipenser Baerii) Performance. J. Anim. Feed Sci. 2025, 34, 476–490. [Google Scholar] [CrossRef]
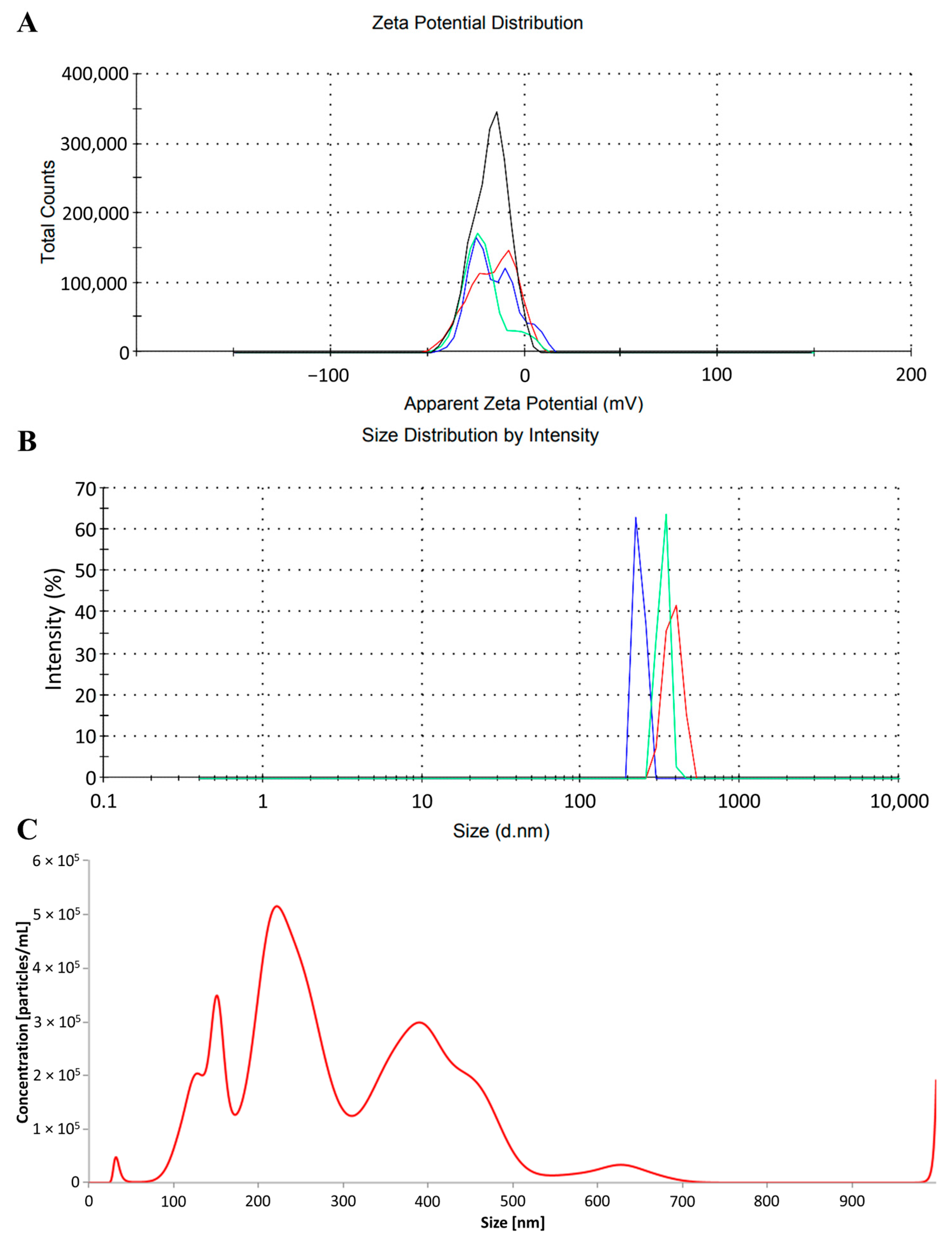
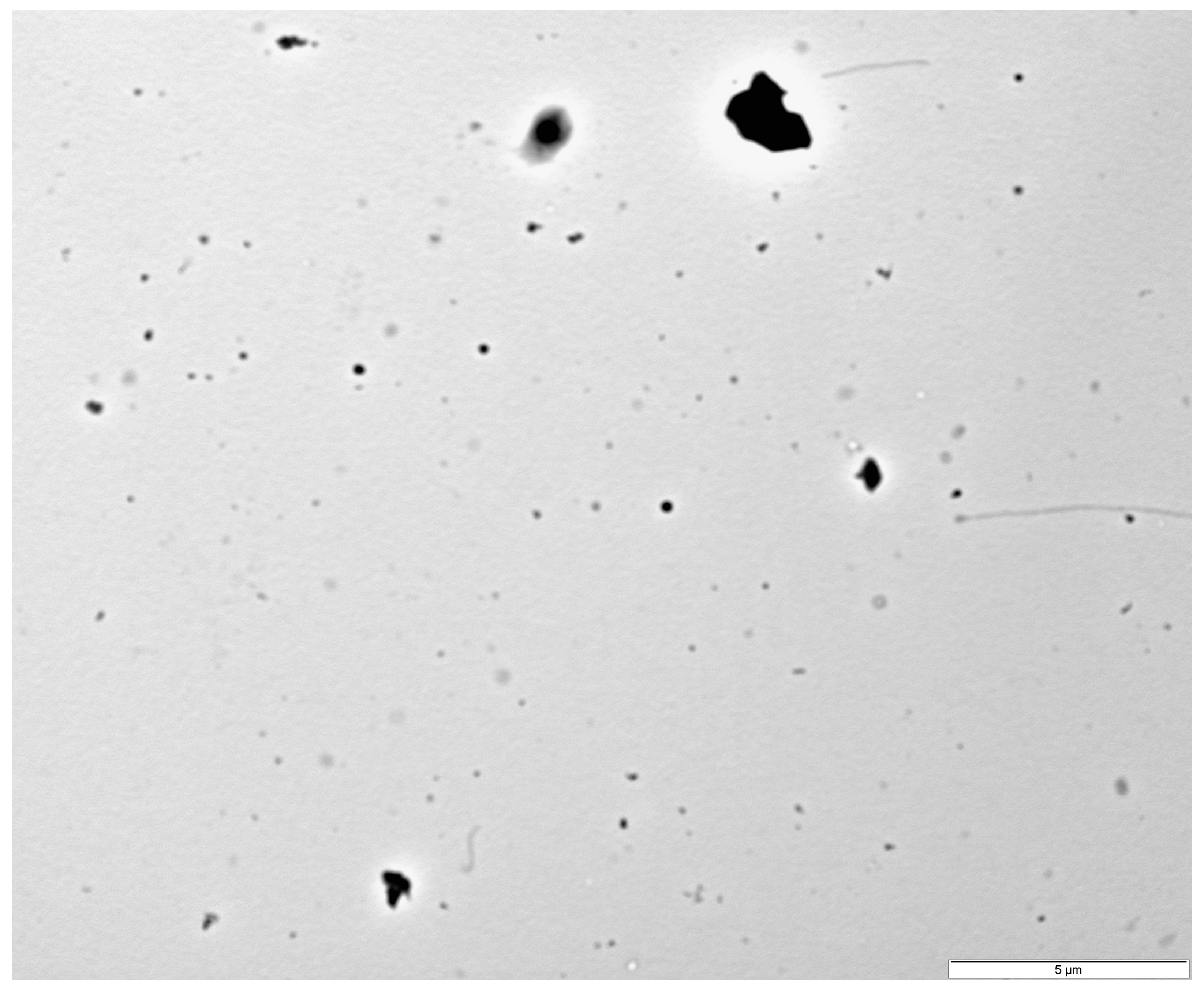

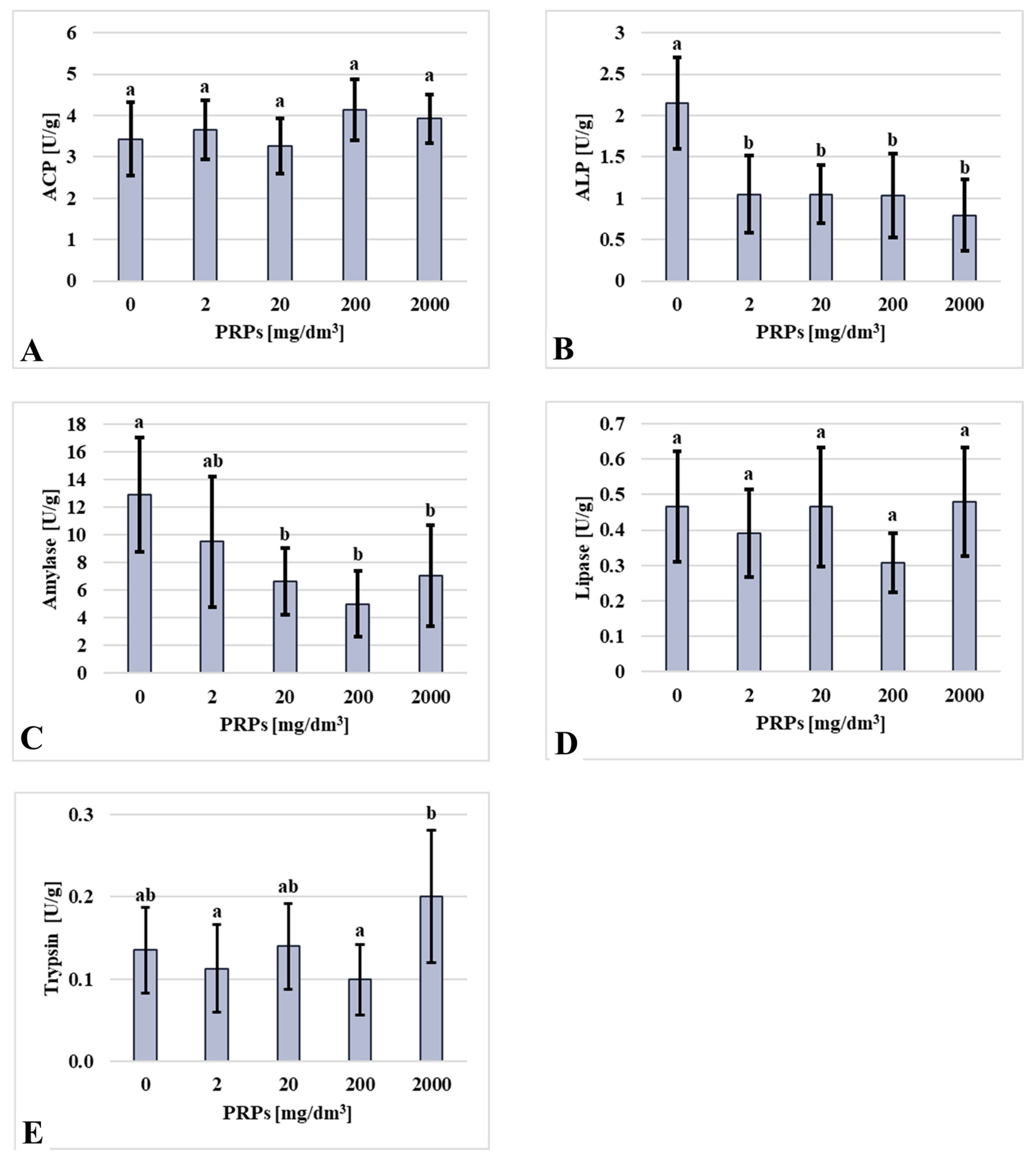
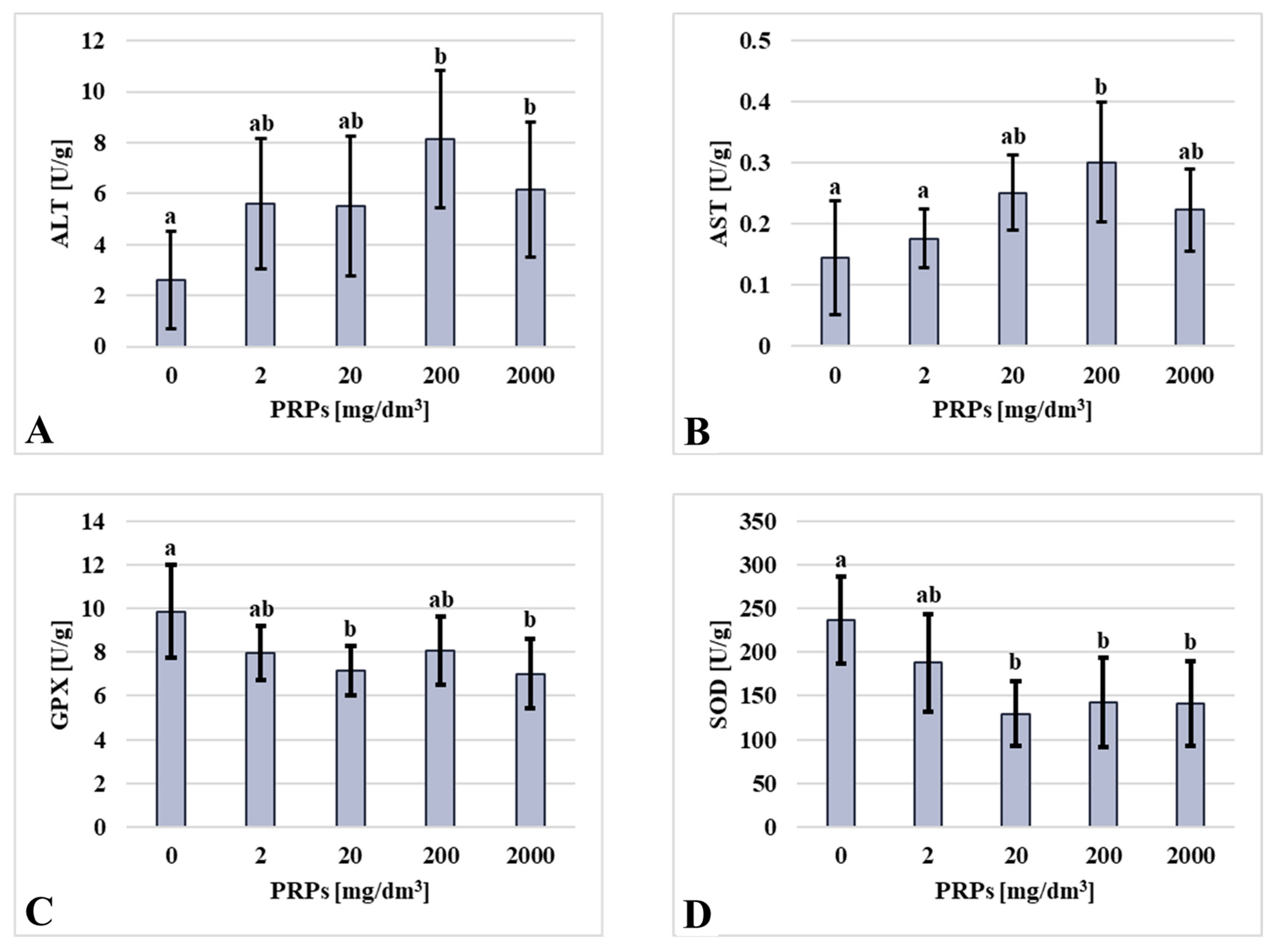
| Group [mg/dm3 of PRP] | Mortality [%] | Mortality Sex Ratio (Male/Female) [%] | Mortality Per Sex (Male/Female) [n] |
|---|---|---|---|
| 0 (control) | 26.67 ± 23.09 | 42.86:57.14 | 3:5 |
| 2 | 13.33 ± 15.28 | 100.00:0.00 | 4:0 |
| 20 | 26.67 ± 11.55 | 87.50:12.50 | 7:1 |
| 200 | 30.00 ± 10.00 | 88.89:11.11 | 8:1 |
| 2000 * | 41.38 ± 10.32 | 69.23:30.77 | 9:4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Łosiewicz, B.; Kamaszewski, M. Preliminary Assessment of the Possible Environmental Risks of Photopolymerizing Resin Particles Produced by Finishing Stereolithography 3D-Printed Objects, Employing Toxicity Test on Tropical House Crickets (Gryllodes sigillatus). Int. J. Mol. Sci. 2025, 26, 11245. https://doi.org/10.3390/ijms262311245
Łosiewicz B, Kamaszewski M. Preliminary Assessment of the Possible Environmental Risks of Photopolymerizing Resin Particles Produced by Finishing Stereolithography 3D-Printed Objects, Employing Toxicity Test on Tropical House Crickets (Gryllodes sigillatus). International Journal of Molecular Sciences. 2025; 26(23):11245. https://doi.org/10.3390/ijms262311245
Chicago/Turabian StyleŁosiewicz, Bogumił, and Maciej Kamaszewski. 2025. "Preliminary Assessment of the Possible Environmental Risks of Photopolymerizing Resin Particles Produced by Finishing Stereolithography 3D-Printed Objects, Employing Toxicity Test on Tropical House Crickets (Gryllodes sigillatus)" International Journal of Molecular Sciences 26, no. 23: 11245. https://doi.org/10.3390/ijms262311245
APA StyleŁosiewicz, B., & Kamaszewski, M. (2025). Preliminary Assessment of the Possible Environmental Risks of Photopolymerizing Resin Particles Produced by Finishing Stereolithography 3D-Printed Objects, Employing Toxicity Test on Tropical House Crickets (Gryllodes sigillatus). International Journal of Molecular Sciences, 26(23), 11245. https://doi.org/10.3390/ijms262311245







