Naturally Derived SENP1 Inhibitors with Anticancer Activity
Abstract
1. Introduction
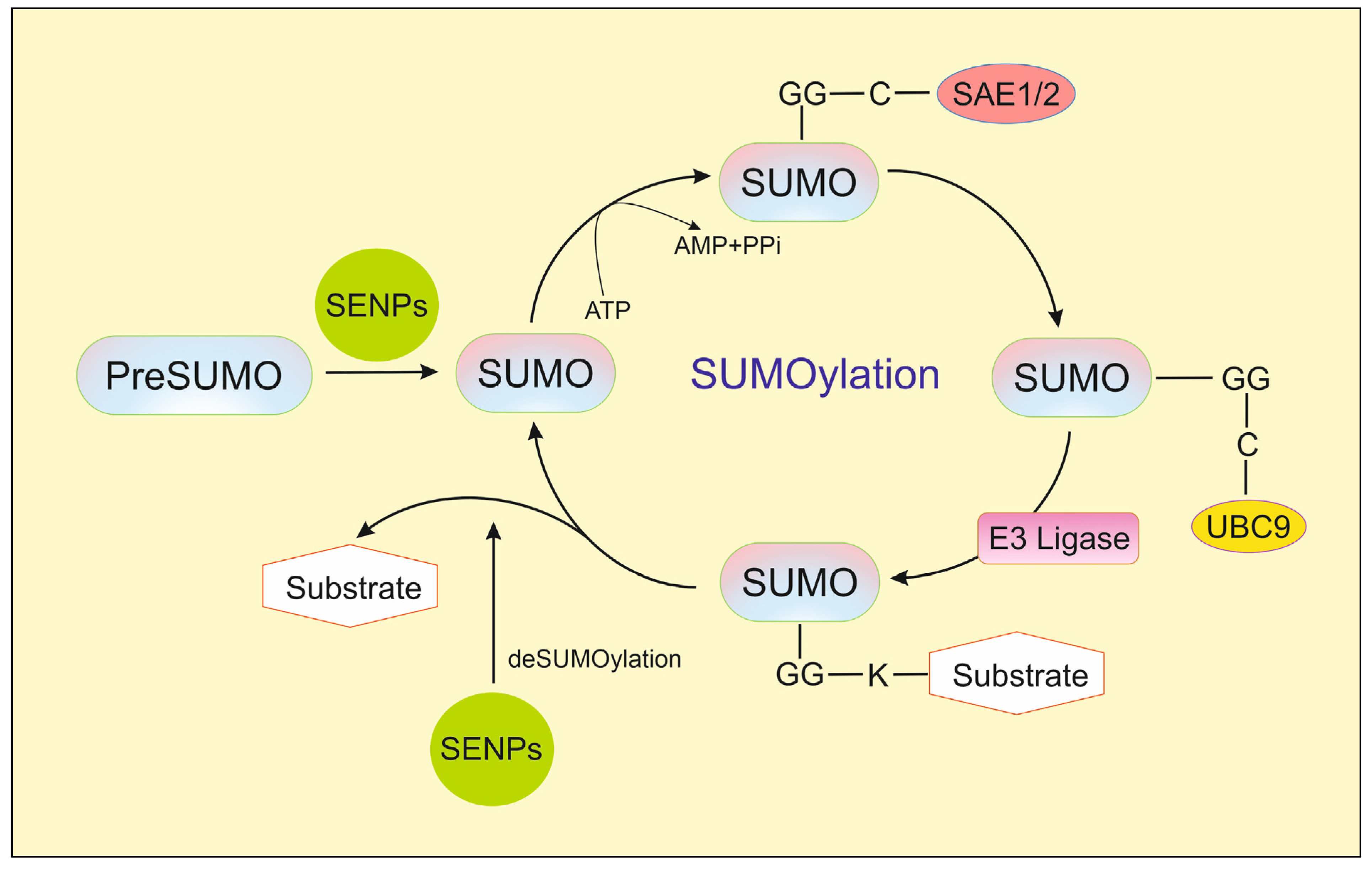
2. SENP1
3. Natural Inhibitors of SENP1
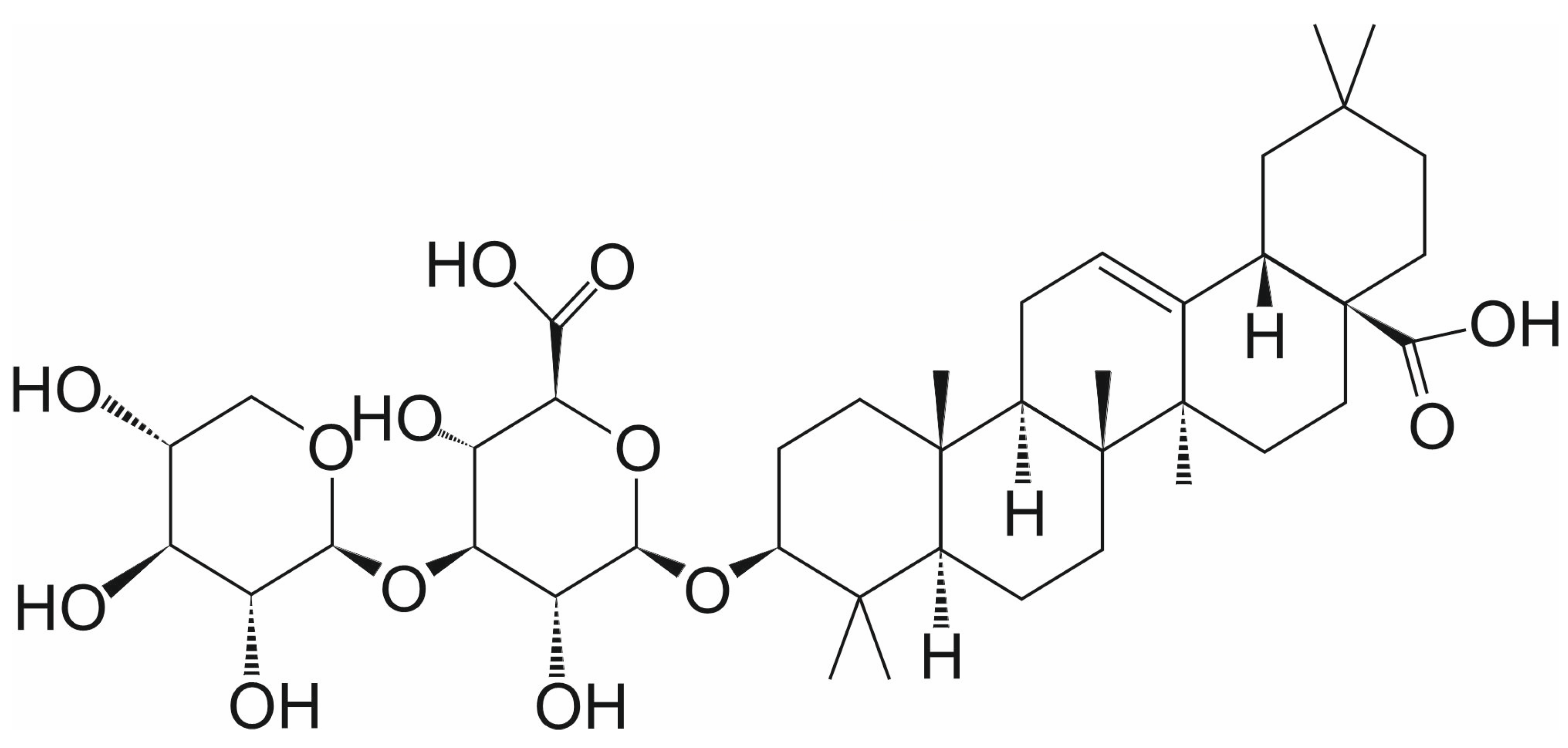
3.1. Momordin Ic
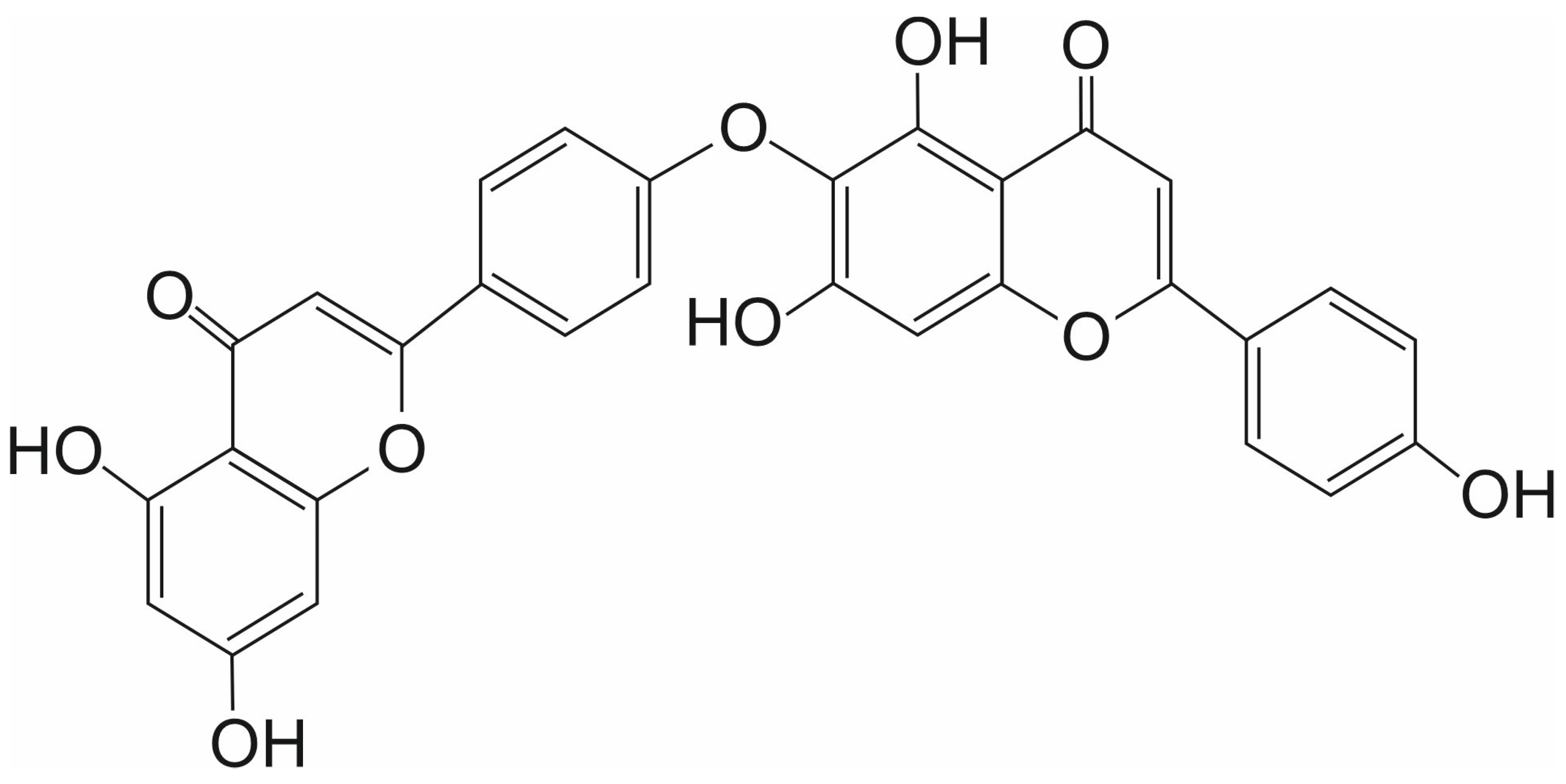
3.2. Hinokiflavone
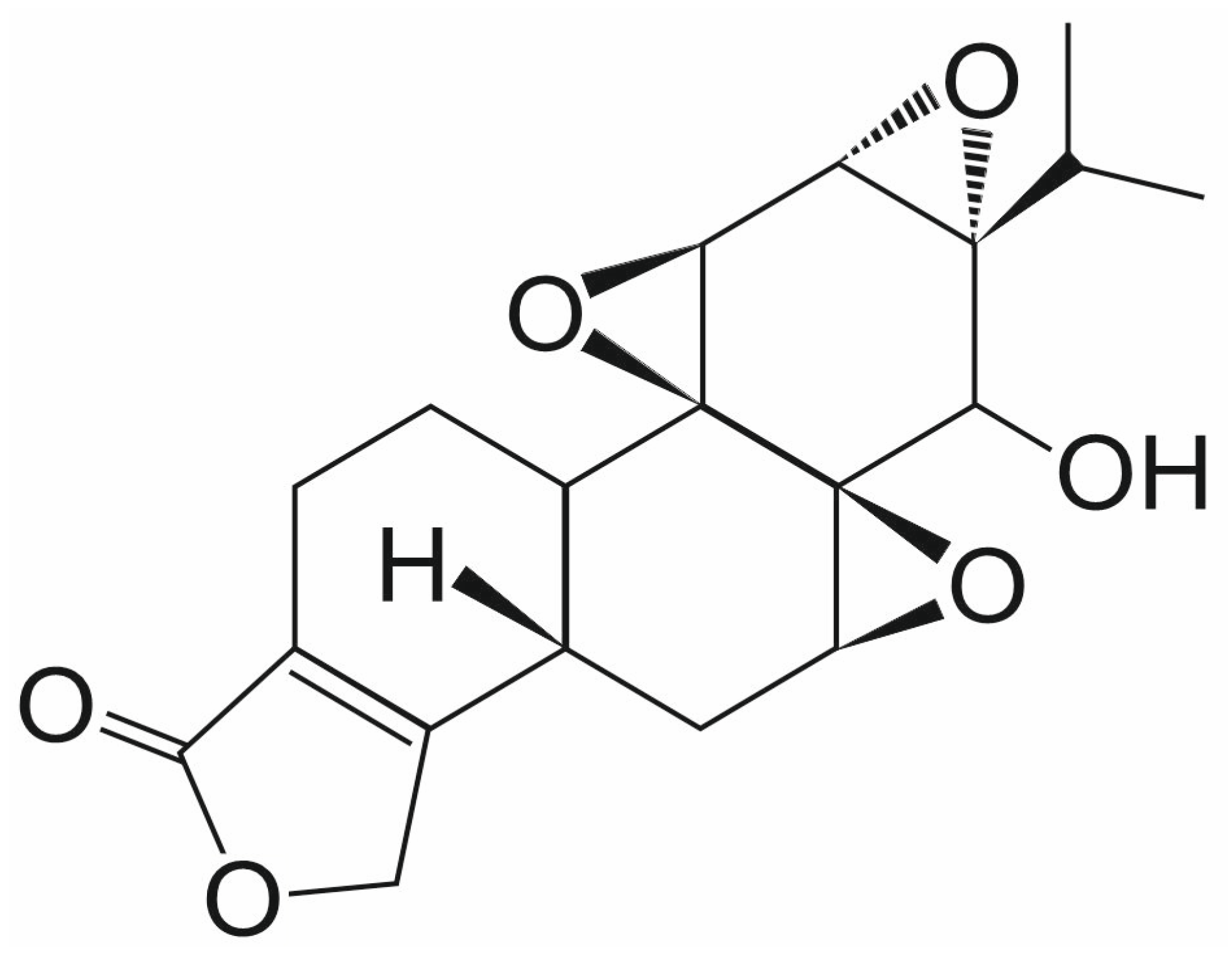
3.3. Triptolide
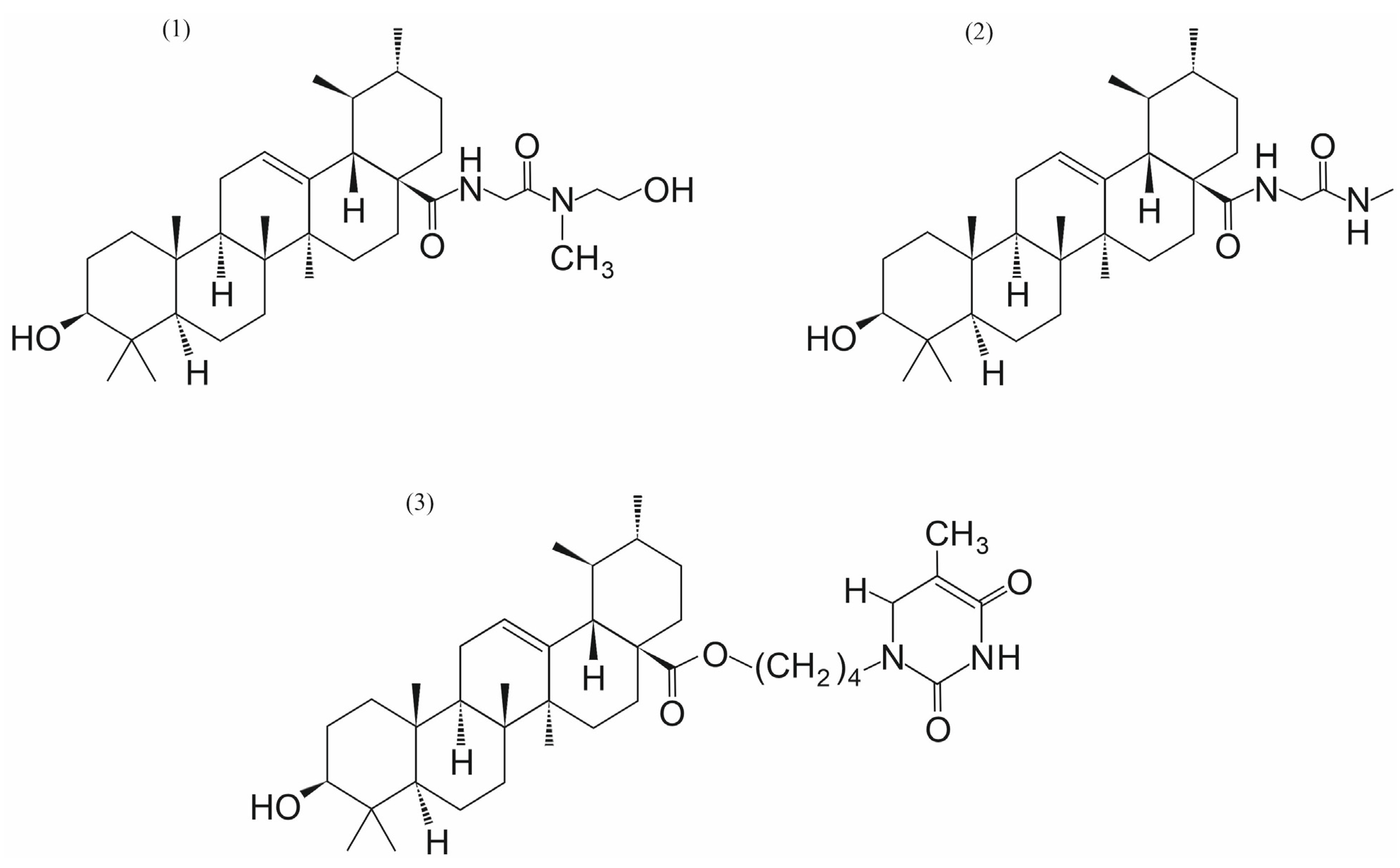
3.4. Ursolic Acid and Its Analogs
3.5. Streptonigrin
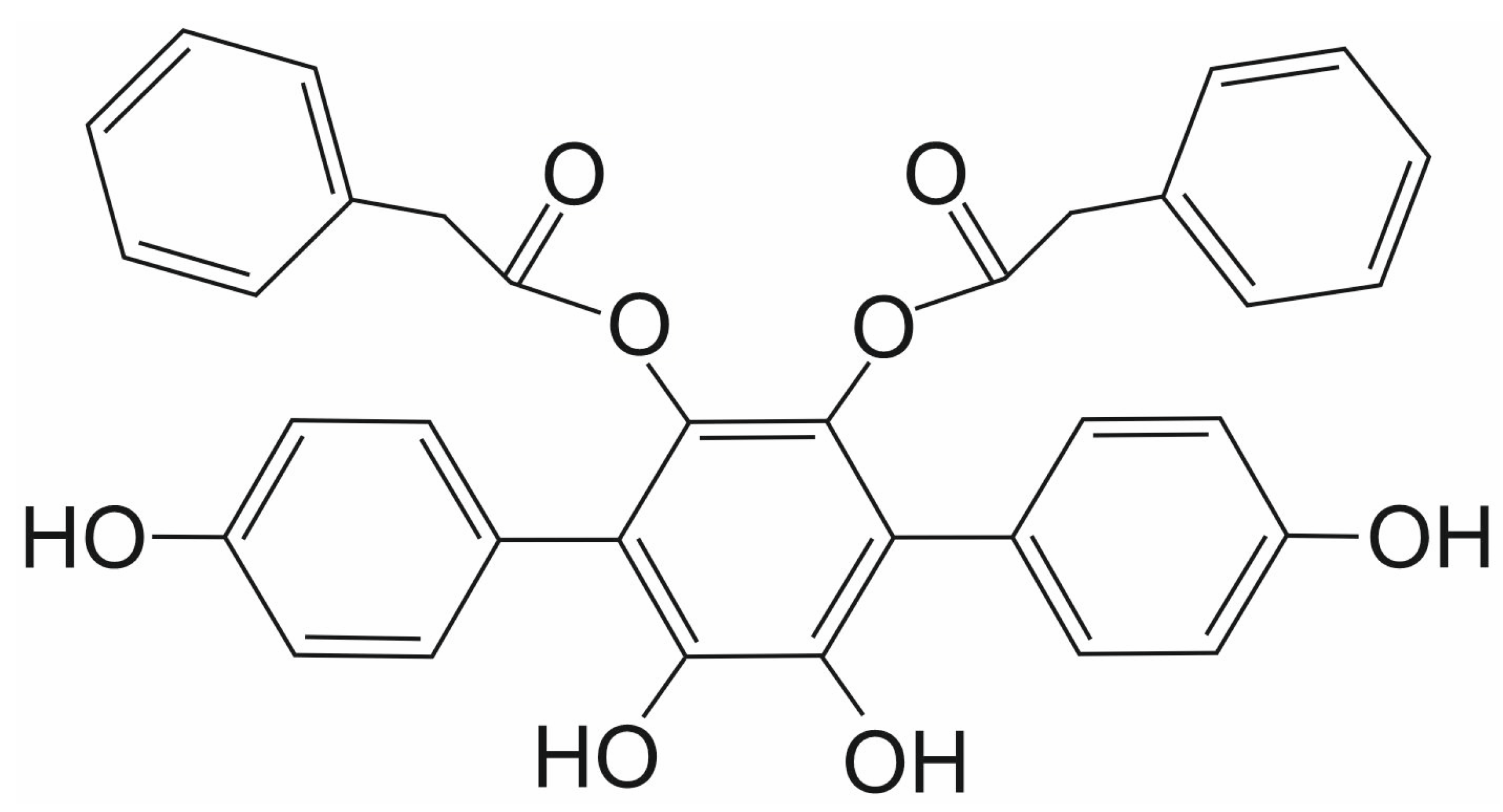
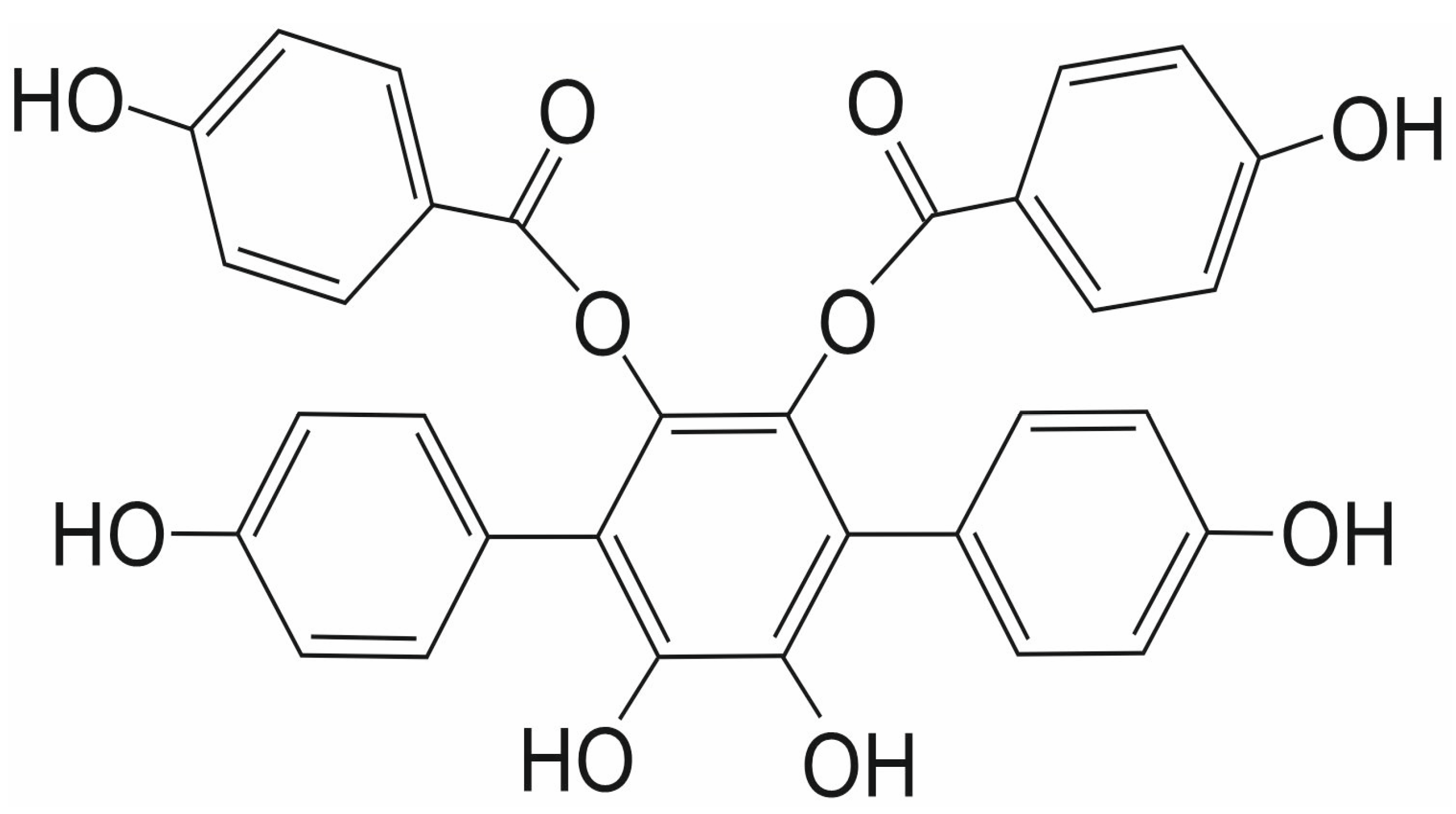
3.6. Vialinin A and Thelephantin G
3.7. Other Natural SENP1 Inhibitors
4. Conclusions and Prospects for Further Research
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADAM10 | metalloprotease-10 |
| AKT | protein kinase B (PKB) |
| AML | acute myeloid leukemia |
| AR | androgen receptor |
| CDK | cyclin-dependent kinase |
| CESTA | cellular thermal shift assay |
| CI | combination index |
| COX-2 | cyclooxygenase 2 |
| DARTS | drug affinity responsive target stability |
| DCTP1 | dCTP pyro-phosphatase 1 |
| DNA-PKcs | DNA-dependent protein kinase catalytic subunit |
| HDAC1 | histone deacetylase 1 |
| HIF1α | hypoxia-inducible factor 1-alpha |
| HNK | Hinokiflavone |
| HO-1 | heme oxygenase 1 |
| HSP70 | 70 kDa heat shock protein |
| IC50 | half maximal inhibitory concentration |
| iNOS | inducible nitric oxide synthase |
| JAK2 | Janus kinase 2 |
| MAPK | mitogen-activated protein kinase |
| Mc | momordin Ic |
| MCL1 | Myeloid Cell Leukemia 1 protein |
| MCL1-L | long isoform of Myeloid Cell Leukemia 1 protein |
| MCL1-S | short isoform of Myeloid Cell Leukemia 1 protein |
| MDM2 | mouse double minute 2 homolog |
| MMPs | matrix metalloproteinases |
| MUTYH | DNA glycosylase |
| NF-κB | nuclear factor kappa-light-chain-enhancer of activated B cells |
| OGG1 | 8-oxoguanine glycosylase |
| p300 | histone acetyltransferase |
| PC-2 | polycystin-2 |
| PIAS proteins | protein inhibitors of activated signal transducer and activator of transcription |
| PI3K | phosphoinositide 3-kinase |
| PPARγ | peroxisome proliferator-activated receptor gamma |
| Prx-I | peroxiredoxin-I |
| rfSENP1 | recombinant full-length human SENP1 |
| ROS | reactive oxygen species |
| RUNX2 | Runt-related transcription factor 2 |
| SAE1 and SAE2 | SUMO-activating enzymes 1 and 2 |
| SENP | sentrin-specific protease |
| SER | radiosensitization enhancement ratio |
| SN | streptonigrin |
| SRC-1 | steroid receptor coactivator-1 |
| SRC-2/GRIP1 | steroid receptor coactivator-2/glucocorticoid receptor-interacting protein 1 |
| SUMO | small ubiquitin-like modifier |
| TAB1 | TAK1 binding protein |
| TAK1 | transforming growth factor-β-activated kinase 1 |
| TCM | TPL solid lipid nanoparticles |
| Tf-Tp@Lip | transferrin-modified liposomes with TPL |
| TG | thelephantin G |
| TPL | triptolide |
| UA | ursolic acid |
| UBC9 | SUMO conjugating enzyme E2 |
| VA | vialinin A |
| XPB or ERCC3 | xeroderma-pigmentosum B or ERCC excision repair 3 protein |
| XRCC1 | X-ray repair cross-complementing protein 1, DNA repair protein 1 |
References
- Han, Z.J.; Feng, Y.H.; Gu, B.H.; Li, Y.M.; Chen, H. The post-translational modification, SUMOylation, and cancer (Review). Int. J. Oncol. 2018, 52, 1081–1094. [Google Scholar] [CrossRef]
- Chen, S.; Dong, D.; Xin, W.; Zhou, H. Progress in the Discovery of Small Molecule Modulators of DeSUMOylation. Curr. Issues Mol. Biol. 2020, 35, 17–34. [Google Scholar] [CrossRef]
- Owerbach, D.; McKay, E.M.; Yeh, E.T.; Gabbay, K.H.; Bohren, K.M. A proline-90 residue unique to SUMO-4 prevents maturation and sumoylation. Biochem. Biophys. Res. Commun. 2005, 337, 517–520. [Google Scholar] [CrossRef]
- Chang, H.M.; Yeh, E.T.H. SUMO: From Bench to Bedside. Physiol. Rev. 2020, 100, 1599–1619. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Zhang, M.; Yi, B.; Chen, J.; Wen, S.; Chen, R.; Chen, T.; Li, Z. Emerging role of SENP1 in tumorigenesis and cancer therapy. Front. Pharmacol. 2024, 15, 1354323. [Google Scholar] [CrossRef]
- Bawa-Khalfe, T.; Yeh, E.T. SUMO Losing Balance: SUMO Proteases Disrupt SUMO Homeostasis to Facilitate Cancer Development and Progression. Genes Cancer 2010, 1, 748–752. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Wang, H.; Zheng, Q.; Zhang, J.; Chen, Z.; Wang, J.; Ouyang, L.; Wang, Y. Recent research and development of inhibitors targeting sentrin-specific protease 1 for the treatment of cancers. Eur. J. Med. Chem. 2022, 241, 114650. [Google Scholar] [CrossRef]
- Gao, C.; Xiao, F.; Zhang, L.; Sun, Y.; Wang, L.; Liu, X.; Sun, H.; Xie, Z.; Liang, Y.; Xu, Q.; et al. SENP1 inhibition suppresses the growth of lung cancer cells through activation of A20-mediated ferroptosis. Ann. Transl. Med. 2022, 10, 224. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, S.; Dong, Y.; Xie, Y.; Li, Q. SENP1-mediated SUMOylation of SIRT1 affects glioma development through the NF-κB pathway. Exp. Cell Res. 2023, 433, 113822. [Google Scholar] [CrossRef]
- Tao, Y.; Li, R.; Shen, C.; Li, J.; Zhang, Q.; Ma, Z.; Wang, F.; Wang, Z. SENP1 is a crucial promotor for hepatocellular carcinoma through deSUMOylation of UBE2T. Aging 2020, 12, 1563–1576. [Google Scholar] [CrossRef]
- Ao, Q.; Su, W.; Guo, S.; Cai, L.; Huang, L. SENP1 desensitizes hypoxic ovarian cancer cells to cisplatin by up-regulating HIF-1α. Sci Rep. 2015, 5, 16396. [Google Scholar] [CrossRef]
- Wang, X.; Liang, X.; Liang, H.; Wang, B. SENP1/HIF-1α feedback loop modulates hypoxia-induced cell proliferation, invasion, and EMT in human osteosarcoma cells. J. Cell Biochem. 2018, 119, 1819–1826. [Google Scholar] [CrossRef]
- Wang, L.; Wu, J.; Song, S.; Chen, H.; Hu, Y.; Xu, B.; Liu, J. Plasma Exosome-Derived Sentrin SUMO-Specific Protease 1: A Prognostic Biomarker in Patients With Osteosarcoma. Front. Oncol. 2021, 11, 625109. [Google Scholar] [CrossRef]
- Zhou, M.; Bian, Z.; Liu, B.; Zhang, Y.; Cao, Y.; Cui, K.; Sun, S.; Li, J.; Zhang, J.; Wang, X.; et al. Long noncoding RNA MCM3AP-AS1 enhances cell proliferation and metastasis in colorectal cancer by regulating miR-193a-5p/SENP1. Cancer Med. 2021, 10, 2470–2481. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Jiang, X. The SUMO-specific protease family regulates cancer cell radiosensitivity. Biomed. Pharmacother. 2019, 109, 66–70. [Google Scholar] [CrossRef]
- Zhang, Y.; Wei, H.; Zhou, Y.; Li, Z.; Gou, W.; Meng, Y.; Zheng, W.; Li, J.; Li, Y.; Zhu, W. Identification of potent SENP1 inhibitors that inactivate SENP1/JAK2/STAT signaling pathway and overcome platinum drug resistance in ovarian cancer. Clin. Transl. Med. 2021, 11, e649. [Google Scholar] [CrossRef]
- Wei, H.; Guo, J.; Sun, X.; Gou, W.; Ning, H.; Shang, H.; Liu, Q.; Hou, W.; Li, Y. Discovery of Natural Ursane-type SENP1 Inhibitors and the Platinum Resistance Reversal Activity Against Human Ovarian Cancer Cells: A Structure-Activity Relationship Study. J. Nat. Prod. 2022, 85, 1248–1255. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Guo, J.; Sun, X.; Gou, W.; Ning, H.; Fang, Z.; Liu, Q.; Hou, W.; Li, Y. Discovery and radiosensitization research of ursolic acid derivatives as SENP1 inhibitors. Eur. J. Med. Chem. 2022, 227, 113918. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Xia, Z.; Wang, X.; Zhao, X.; Sheng, Z.; Ye, Y.; He, G.; Zhou, L.; Zhu, H.; Xu, N.; et al. Small-Molecule Inhibitors Targeting Protein SUMOylation as Novel Anticancer Compounds. Mol. Pharmacol. 2018, 94, 885–894. [Google Scholar] [CrossRef]
- Tokarz, P.; Woźniak, K. SENP Proteases as Potential Targets for Cancer Therapy. Cancers 2021, 13, 2059. [Google Scholar] [CrossRef]
- Li, H.; Chen, L.; Li, Y.; Hou, W. SUMO-specific protease 1 inhibitors-A literature and patent overview. Expert. Opin. Ther. Pat. 2022, 32, 1207–1216. [Google Scholar] [CrossRef]
- Zou, W.; Tang, Z.; Long, Y.; Xiao, Z.; Ouyang, B.; Liu, M. Kochiae Fructus, the Fruit of Common Potherb Kochia scoparia (L.) Schrad: A Review on Phytochemistry, Pharmacology, Toxicology, Quality Control, and Pharmacokinetics. Evid.-Based Complement. Alternat. Med. 2021, 2021, 5382684. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Lei, H.; Zhang, J.; Chen, X.; Tang, C.; Wang, W.; Xu, H.; Xiao, W.; Gu, W.; Wu, Y. Momordin Ic, a new natural SENP1 inhibitor, inhibits prostate cancer cell proliferation. Oncotarget 2016, 7, 58995–59005. [Google Scholar] [CrossRef]
- Wang, J.; Yuan, L.; Xiao, H.; Xiao, C.; Wang, Y.; Liu, X. Momordin Ic induces HepG2 cell apoptosis through MAPK and PI3K/Akt-mediated mitochondrial pathways. Apoptosis 2013, 18, 751–765. [Google Scholar] [CrossRef]
- Wang, J.; Yuan, L.; Xiao, H.; Wang, C.; Xiao, C.; Wang, Y.; Liu, X. A novel mechanism for momordin Ic-induced HepG2 apoptosis: Involvement of PI3K- and MAPK-dependent PPARγ activation. Food Funct. 2014, 5, 859–868. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Q.; Xiao, H.; Luo, X.; Liu, X. Suppressive effects of Momordin Ic on HepG2 cell migration and invasion by regulating MMP-9 and adhesion molecules: Involvement of p38 and JNK pathways. Toxicol. In Vitro 2019, 56, 75–83. [Google Scholar] [CrossRef]
- Wang, J.; Han, Y.; Wang, M.; Zhao, Q.; Chen, X.; Liu, X. Natural triterpenoid saponin Momordin Ic suppresses HepG2 cell invasion via COX-2 inhibition and PPARγ activation. Toxicol. In Vitro 2020, 65, 104784. [Google Scholar] [CrossRef]
- Xianjun, F.; Xirui, X.; Jie, T.; Huiwen, M.; Shaojun, Z.; Qiaoyun, L.; Yunxin, L.; Xuqun, S. Momordin Ic induces G0/1 phase arrest and apoptosis in colon cancer cells by suppressing SENP1/c-MYC signaling pathway. J. Pharmacol. Sci. 2021, 146, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.X.; Chen, Y.; Su, Y.; Wang, X.; Chauhan, K.M.; Liang, J.; Daniel, C.J.; Sears, R.C.; Dai, M.S. SUMO protease SENP1 deSUMOylates and stabilizes c-Myc. Proc. Natl. Acad. Sci. USA 2018, 115, 10983–10988. [Google Scholar] [CrossRef] [PubMed]
- Mi, Y.; Xiao, C.; Du, Q.; Wu, W.; Qi, G.; Liu, X. Momordin Ic couples apoptosis with autophagy in human hepatoblastoma cancer cells by reactive oxygen species (ROS)-mediated PI3K/Akt and MAPK signaling pathways. Free Radic. Biol. Med. 2016, 90, 230–242. [Google Scholar] [CrossRef]
- Luo, M.; Zeng, B.; Wang, H.; Yang, Z.; Peng, Y.; Zhang, Y.; Wang, C. Kochia scoparia Saponin Momordin Ic Modulates HaCaT Cell Proliferation and Apoptosis via the Wnt/β-Catenin Pathway. Evid.-Based Complement. Alternat. Med. 2021, 2021, 5522164. [Google Scholar] [CrossRef]
- Gayathri, K.G.; Shinde, P.L.; John, S.; Sivakumar, K.C.; Mishra, R. Understanding the Combined Effects of High Glucose Induced Hyper-Osmotic Stress and Oxygen Tension in the Progression of Tumourigenesis: From Mechanism to Anti-Cancer Therapeutics. Cells 2023, 12, 825. [Google Scholar] [CrossRef]
- Li, J.; Wu, R.; Yung, M.M.H.; Sun, J.; Li, Z.; Yang, H.; Zhang, Y.; Liu, S.S.; Cheung, A.N.Y.; Ngan, H.Y.S.; et al. SENP1-mediated deSUMOylation of JAK2 regulates its kinase activity and platinum drug resistance. Cell Death Dis. 2021, 12, 341. [Google Scholar] [CrossRef]
- Zhang, Y.; Shen, Y.; Wei, W.; Wang, W.; Jiang, D.; Ren, Y.; Peng, Z.; Fan, Q.; Cheng, J.; Ma, J. Dysregulation of SIRT3 SUMOylation Confers AML Chemoresistance via Controlling HES1-Dependent Fatty Acid Oxidation. Int. J. Mol. Sci. 2022, 23, 8282. [Google Scholar] [CrossRef] [PubMed]
- Goossens, J.F.; Goossens, L.; Bailly, C. Hinokiflavone and Related C-O-C-Type Biflavonoids as Anti-cancer Compounds: Properties and Mechanism of Action. Nat. Prod. Bioprospect. 2021, 11, 365–377. [Google Scholar] [CrossRef]
- Kalva, S.; Azhagiya Singam, E.R.; Rajapandian, V.; Saleena, L.M.; Subramanian, V. Discovery of potent inhibitor for matrix metalloproteinase-9 by pharmacophore based modeling and dynamics simulation studies. J. Mol. Graph. Model. 2014, 49, 25–37. [Google Scholar] [CrossRef]
- Zhou, J.; Zhao, R.; Ye, T.; Yang, S.; Li, Y.; Yang, F.; Wang, G.; Xie, Y.; Li, Q. Antitumor activity in colorectal cancer induced by hinokiflavone. J. Gastroenterol. Hepatol. 2019, 34, 1571–1580. [Google Scholar] [CrossRef]
- Ilic, V.K.; Egorova, O.; Tsang, E.; Gatto, M.; Wen, Y.; Zhao, Y.; Sheng, Y. Hinokiflavone Inhibits MDM2 Activity by Targeting the MDM2-MDMX RING Domain. Biomolecules 2022, 12, 643. [Google Scholar] [CrossRef]
- Pawellek, A.; Ryder, U.; Tammsalu, T.; King, L.J.; Kreinin, H.; Ly, T.; Hay, R.T.; Hartley, R.C.; Lamond, A.I. Characterisation of the biflavonoid hinokiflavone as a pre-mRNA splicing modulator that inhibits SENP. eLife 2017, 6, e27402. [Google Scholar] [CrossRef]
- Wang, H.; Guo, M.; Wei, H.; Chen, Y. Targeting MCL-1 in cancer: Current status and perspectives. J. Hematol. Oncol. 2021, 14, 67. [Google Scholar] [CrossRef]
- Hsieh, T.H.; Kuo, H.P.; Chen, M.C.; Lin, Y.C.; Lin, B.J.; Hsu, K.C.; Chen, C.H. Hinokiflavone is a novel CK2 inhibitor promoting apoptosis and synergizing with chemotherapeutic agents in cisplatin resistant bladder cancer cells. Sci. Rep. 2025, 15, 20922. [Google Scholar] [CrossRef]
- Wei, M.; Huang, X.; Liao, L.; Tian, Y.; Zheng, X. SENP1 Decreases RNF168 Phase Separation to Promote DNA Damage Repair and Drug Resistance in Colon Cancer. Cancer Res. 2023, 83, 2908–2923. [Google Scholar] [CrossRef]
- Zhang, F.L.; Yang, S.Y.; Liao, L.; Zhang, T.M.; Zhang, Y.L.; Hu, S.Y.; Deng, L.; Huang, M.Y.; Andriani, L.; Ma, X.Y.; et al. Dynamic SUMOylation of MORC2 orchestrates chromatin remodelling and DNA repair in response to DNA damage and drives chemoresistance in breast cancer. Theranostics 2023, 13, 973–990. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; He, T.; Chai, C.; Yang, Y.; Zheng, Y.; Zhou, P.; Qiao, X.; Zhang, B.; Liu, Z.; Wang, J.; et al. Triptolide inhibits the proliferation of prostate cancer cells and down-regulates SUMO-specific protease 1 expression. PLoS ONE 2012, 7, e37693. [Google Scholar] [CrossRef]
- Wu, X.; Chen, S.; Huang, K.; Lin, G. Triptolide promotes ferroptosis by suppressing Nrf2 to overcome leukemia cell resistance to doxorubicin. Mol. Med. Rep. 2023, 27, 17. [Google Scholar] [CrossRef]
- Li, X.; Shan, Y.; Wang, S.; Wang, J.; Heng, X. Triptolide induces apoptosis of glioma cells by inhibiting NF-κB activation during oxidative stress. Sci. Rep. 2024, 14, 29740. [Google Scholar] [CrossRef]
- Gorrie, D.; Bravo, M.; Fan, L. The Yin and Yang of the Natural Product Triptolide and Its Interactions with XPB, an Essential Protein for Gene Expression and DNA Repair. Genes 2024, 15, 1287. [Google Scholar] [CrossRef] [PubMed]
- Feng, K.; Li, X.; Bai, Y.; Zhang, D.; Tian, L. Mechanisms of cancer cell death induction by triptolide: A comprehensive overview. Heliyon 2024, 10, e24335. [Google Scholar] [CrossRef]
- Wang, G.; Guo, H.; Ren, Y.; Chen, W.; Wang, Y.; Li, J.; Liu, H.; Xing, J.; Zhang, Y.; Li, N. Triptolide enhances carboplatin-induced apoptosis by inhibiting nucleotide excision repair (NER) activity in melanoma. Front. Pharmacol. 2023, 14, 1157433. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Zhu, Y.; Ge, S.; Liu, S.B. The roles of TPL in hematological malignancies. Hematology 2023, 28, 2231765. [Google Scholar] [CrossRef]
- Li, M.; Li, J.; Tang, Q.; Zhu, Y. Potential antitumor activity of triptolide and its derivatives: Focused on gynecological and breast cancers. Biomed. Pharmacother. 2024, 180, 117581. [Google Scholar] [CrossRef]
- Cheng, J.; Wang, D.; Wang, Z.; Yeh, E.T. SENP1 enhances androgen receptor-dependent transcription through desumoylation of histone deacetylase 1. Mol. Cell Biol. 2004, 24, 6021–6028. [Google Scholar] [CrossRef]
- Chugh, R.; Sangwan, V.; Patil, S.P.; Dudeja, V.; Dawra, R.K.; Banerjee, S.; Schumacher, R.J.; Blazar, B.R.; Georg, G.I.; Vickers, S.M.; et al. A preclinical evaluation of Minnelide as a therapeutic agent against pancreatic cancer. Sci. Transl. Med. 2012, 4, 156ra139. [Google Scholar] [CrossRef]
- Moser, R.; Annis, J.; Nikolova, O.; Whatcott, C.; Gurley, K.; Mendez, E.; Moran-Jones, K.; Dorrell, C.; Sears, R.C.; Kuo, C.; et al. Pharmacologic Targeting of TFIIH Suppresses KRAS-Mutant Pancreatic Ductal Adenocarcinoma and Synergizes with TRAIL. Cancer Res. 2022, 82, 3375–3393. [Google Scholar] [CrossRef]
- He, Q.L.; Minn, I.; Wang, Q.; Xu, P.; Head, S.A.; Datan, E.; Yu, B.; Pomper, M.G.; Liu, J.O. Targeted Delivery and Sustained Antitumor Activity of Triptolide through Glucose Conjugation. Angew. Chem. Int. Ed. Engl. 2016, 55, 12035–12039. [Google Scholar] [CrossRef]
- Datan, E.; Minn, I.; Xu, P.; He, Q.L.; Ahn, H.H.; Yu, B.; Pomper, M.G.; Liu, J.O. A Glucose-Triptolide Conjugate Selectively Targets Cancer Cells under Hypoxia. iScience 2020, 23, 101536. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Arulnangai, R.; Asia Thabassoom, H.; Vajiha Banu, H.; Thirugnanasambandham, K.; Ganesamoorthy, R. Recent developments on ursolic acid and its potential biological applications. Toxicol. Rep. 2025, 14, 101900. [Google Scholar] [CrossRef]
- Hussain, H.; Green, I.R.; Ali, I.; Khan, I.A.; Ali, Z.; Al-Sadi, A.M.; Ahmed, I. Ursolic acid derivatives for pharmaceutical use: A patent review (2012-2016). Expert Opin. Ther. Pat. 2017, 27, 1061–1072. [Google Scholar] [CrossRef]
- Yin, R.; Li, T.; Tian, J.X.; Xi, P.; Liu, R.H. Ursolic acid, a potential anticancer compound for breast cancer therapy. Crit. Rev. Food Sci. Nutr. 2018, 58, 568–574. [Google Scholar] [CrossRef] [PubMed]
- Michalak, O.; Cybulski, M.; Kubiszewski, M.; Finiuk, N.; Kozak, Y.; Milenkovic, D.; Żurawicka, S.; Roszczenko, P.; Bielawska, A.; Trzaskowski, B.; et al. Uracil derivatives/ursolic acid hybrids—Naturally derived compounds as anticancer agents. Sci. Rep. 2025, 15, 28803. [Google Scholar] [CrossRef]
- Similie, D.; Minda, D.; Bora, L.; Kroškins, V.; Lugiņina, J.; Turks, M.; Dehelean, C.A.; Danciu, C. An Update on Pentacyclic Triterpenoids Ursolic and Oleanolic Acids and Related Derivatives as Anticancer Candidates. Antioxidants 2024, 13, 952. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, E.W.; Hymes, A.C.; Ausman, R.K.; Ferguson, D.J. An evaluation of actinomycin D and mitomycin C in patients with advanced cancer. Surgery 1961, 50, 881–885. [Google Scholar]
- Sullivan, R.D.; Miller, E.; Zurek, W.Z.; Rodriguez, F.R. Clinical Effects of Prolonged (Continuous) Infusion of Streptonigrin (Nsc-45383) in Advanced Cancer. Cancer Chemother. Rep. 1963, 33, 27–40. [Google Scholar]
- Harris, M.N.; Medrek, T.J.; Golomb, F.M.; Gumport, S.L.; Postel, A.H.; Wright, J.C. Chemotherapy with Streptonigrin in Advanced Cancer. Cancer 1965, 18, 49–57. [Google Scholar] [CrossRef]
- Sztiller-Sikorska, M.; Koprowska, K.; Majchrzak, K.; Hartman, M.; Czyz, M. Natural compounds’ activity against cancer stem-like or fast-cycling melanoma cells. PLoS ONE 2014, 9, e90783. [Google Scholar] [CrossRef]
- Madu, I.G.; Namanja, A.T.; Su, Y.; Wong, S.; Li, Y.J.; Chen, Y. Identification and characterization of a new chemotype of noncovalent SENP inhibitors. ACS Chem. Biol. 2013, 8, 1435–1441. [Google Scholar] [CrossRef]
- Ambaye, N.; Chen, C.H.; Khanna, S.; Li, Y.J.; Chen, Y. Streptonigrin Inhibits SENP1 and Reduces the Protein Level of Hypoxia-Inducible Factor 1alpha (HIF1alpha) in Cells. Biochemistry 2018, 57, 1807–1813. [Google Scholar] [CrossRef]
- Sonowal, H.; Shukla, K.; Kota, S.; Saxena, A.; Ramana, K.V. Vialinin A, an Edible Mushroom-Derived p-Terphenyl Antioxidant, Prevents VEGF-Induced Neovascularization In Vitro and In Vivo. Oxid. Med. Cell Longev. 2018, 2018, 1052102. [Google Scholar] [CrossRef]
- Xie, C.; Koshino, H.; Esumi, Y.; Takahashi, S.; Yoshikawa, K.; Abe, N. Vialinin A, a novel 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenger from an edible mushroom in China. Biosci. Biotechnol. Biochem. 2005, 69, 2326–2332. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Xu, J.; Chen, D.; Jin, L.; Chen, Z.; Tu, Y.; Huang, X.; Xue, F.; Xu, J.; Chen, M.; Wang, X.; et al. Ubiquitously specific protease 4 inhibitor-Vialinin A attenuates inflammation and fibrosis in S100-induced hepatitis mice through Rheb/mTOR signalling. J. Cell Mol. Med. 2021, 25, 1140–1150. [Google Scholar] [CrossRef] [PubMed]
- Onose, J.; Xie, C.; Ye, Y.Q.; Sugaya, K.; Takahashi, S.; Koshino, H.; Yasunaga, K.; Abe, N.; Yoshikawa, K. Vialinin A, a novel potent inhibitor of TNF-alpha production from RBL-2H3 cells. Biol. Pharm. Bull. 2008, 31, 831–833. [Google Scholar] [CrossRef][Green Version]
- Mao, M.; Xia, Q.; Zhan, G.; Bing, H.; Zhang, C.; Wang, J.; Tian, W.; Lian, H.; Li, X.; Chu, Q. Vialinin A alleviates oxidative stress and neuronal injuries after ischaemic stroke by accelerating Keap1 degradation through inhibiting USP4-mediated deubiquitination. Phytomedicine 2024, 124, 155304. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, Y.; Ye, Y.Q.; Okada, K.; Taniguchi, K.; Yoshida, A.; Sugaya, K.; Onose, J.; Koshino, H.; Takahashi, S.; Yajima, A.; et al. Ubiquitin-specific peptidase 5, a target molecule of vialinin A, is a key molecule of TNF-alpha production in RBL-2H3 cells. PLoS ONE 2013, 8, e80931. [Google Scholar] [CrossRef]
- Bailly, C. Anti-inflammatory and anticancer p-terphenyl derivatives from fungi of the genus Thelephora. Bioorg. Med. Chem. 2022, 70, 116935. [Google Scholar] [CrossRef]
- Wang, J.; Fang, S.; Jiang, Y.; Hua, Q. Unraveling the Mechanism of Action of Ubiquitin-Specific Protease 5 and Its Inhibitors in Tumors. Clin. Med. Insights Oncol. 2024, 18, 11795549241281932. [Google Scholar] [CrossRef]
- Yoshioka, Y.; Namiki, D.; Makiuchi, M.; Sugaya, K.; Onose, J.; Ashida, H.; Abe, N. Vialinin A and thelephantin G, potent inhibitors of tumor necrosis factor-alpha production, inhibit sentrin/SUMO-specific protease 1 enzymatic activity. Bioorg. Med. Chem. Lett. 2016, 26, 4237–4240. [Google Scholar] [CrossRef]
- Tuli, H.S.; Mistry, H.; Kaur, G.; Aggarwal, D.; Garg, V.K.; Mittal, S.; Yerer, M.B.; Sak, K.; Khan, M.A. Gallic Acid: A Dietary Polyphenol that Exhibits Anti-neoplastic Activities by Modulating Multiple Oncogenic Targets. Anticancer Agents Med. Chem. 2022, 22, 499–514. [Google Scholar] [CrossRef]
- Taghvaei, S.; Sabouni, F.; Minuchehr, Z.; Taghvaei, A. Identification of novel anti-cancer agents, applying in silico method for SENP1 protease inhibition. J. Biomol. Struct. Dyn. 2022, 40, 6228–6242. [Google Scholar] [CrossRef]
- Taghvaei, S.; Taghvaei, A.; Anvar, M.S.; Guo, C.; Sabouni, F.; Minuchehr, Z. Computational study of SENP1 in cancer by novel natural compounds and ZINC database screening. Front. Pharmacol. 2023, 14, 1144632. [Google Scholar] [CrossRef] [PubMed]
- Yan, T.; Wang, Y.; Hou, Z.; Song, P.; Wang, P.; Li, J.; Zheng, J.; Lv, D. A comprehensive overview of triptolide utilizing nanotechnology and its potential applications in prostate diseases. Front. Pharmacol. 2025, 16, 1592066. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krupa, R.; Woźniak, K. Naturally Derived SENP1 Inhibitors with Anticancer Activity. Int. J. Mol. Sci. 2025, 26, 11210. https://doi.org/10.3390/ijms262211210
Krupa R, Woźniak K. Naturally Derived SENP1 Inhibitors with Anticancer Activity. International Journal of Molecular Sciences. 2025; 26(22):11210. https://doi.org/10.3390/ijms262211210
Chicago/Turabian StyleKrupa, Renata, and Katarzyna Woźniak. 2025. "Naturally Derived SENP1 Inhibitors with Anticancer Activity" International Journal of Molecular Sciences 26, no. 22: 11210. https://doi.org/10.3390/ijms262211210
APA StyleKrupa, R., & Woźniak, K. (2025). Naturally Derived SENP1 Inhibitors with Anticancer Activity. International Journal of Molecular Sciences, 26(22), 11210. https://doi.org/10.3390/ijms262211210




