Probiotic Modulation in Aging: Strain-Specific Geroprotective Effects in Caenorhabditis elegans
Abstract
1. Introduction
2. Results
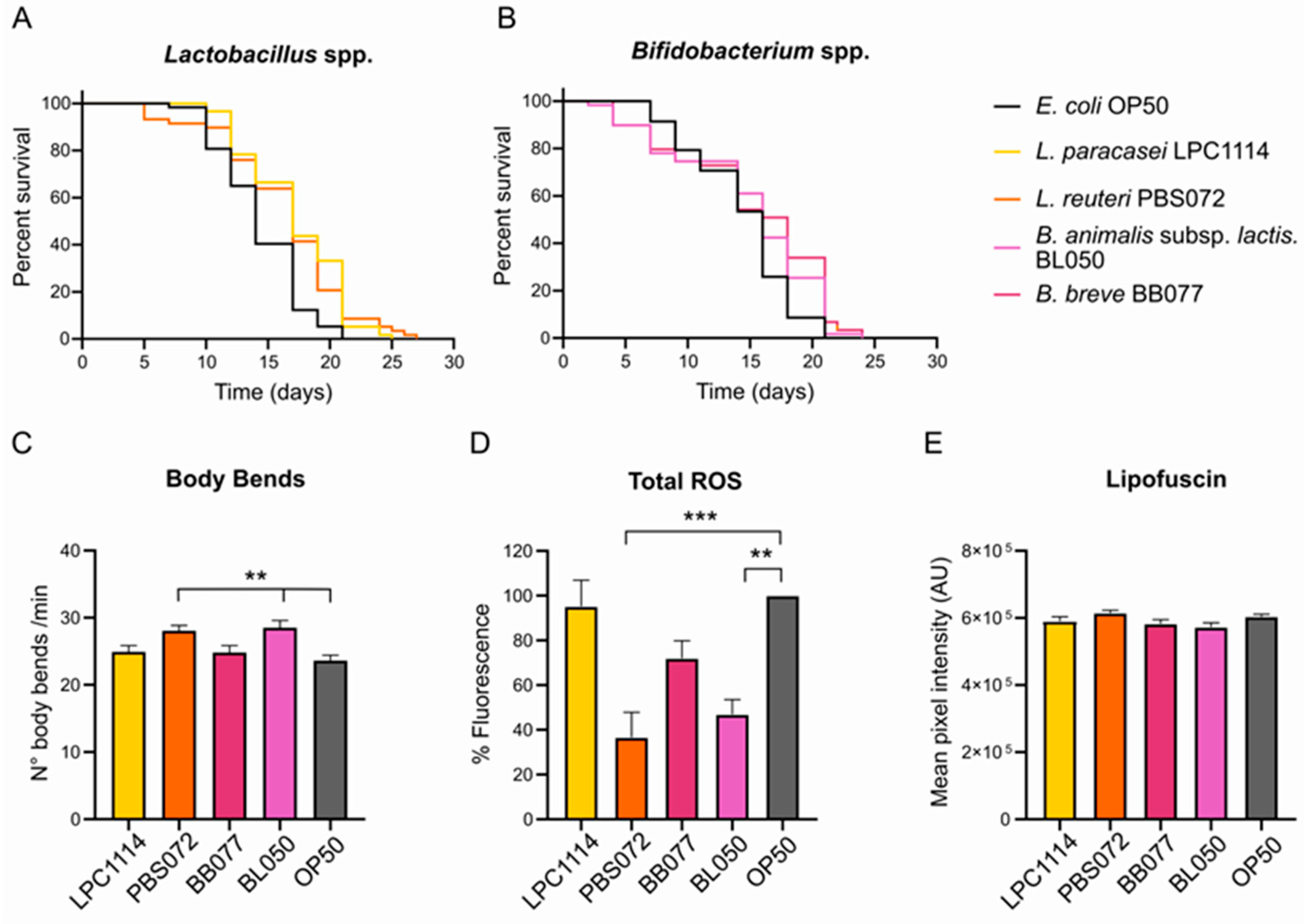
2.1. The Administration of Lactobacillus and Bifidobacterium spp. Ameliorates Phenotypic Aging Parameters in a Strain-Specific Manner
2.2. Probiotic Diets Do Not Affect Neuromuscular Trasmission but Enhance Cognitive Functions in Aged C. elegans
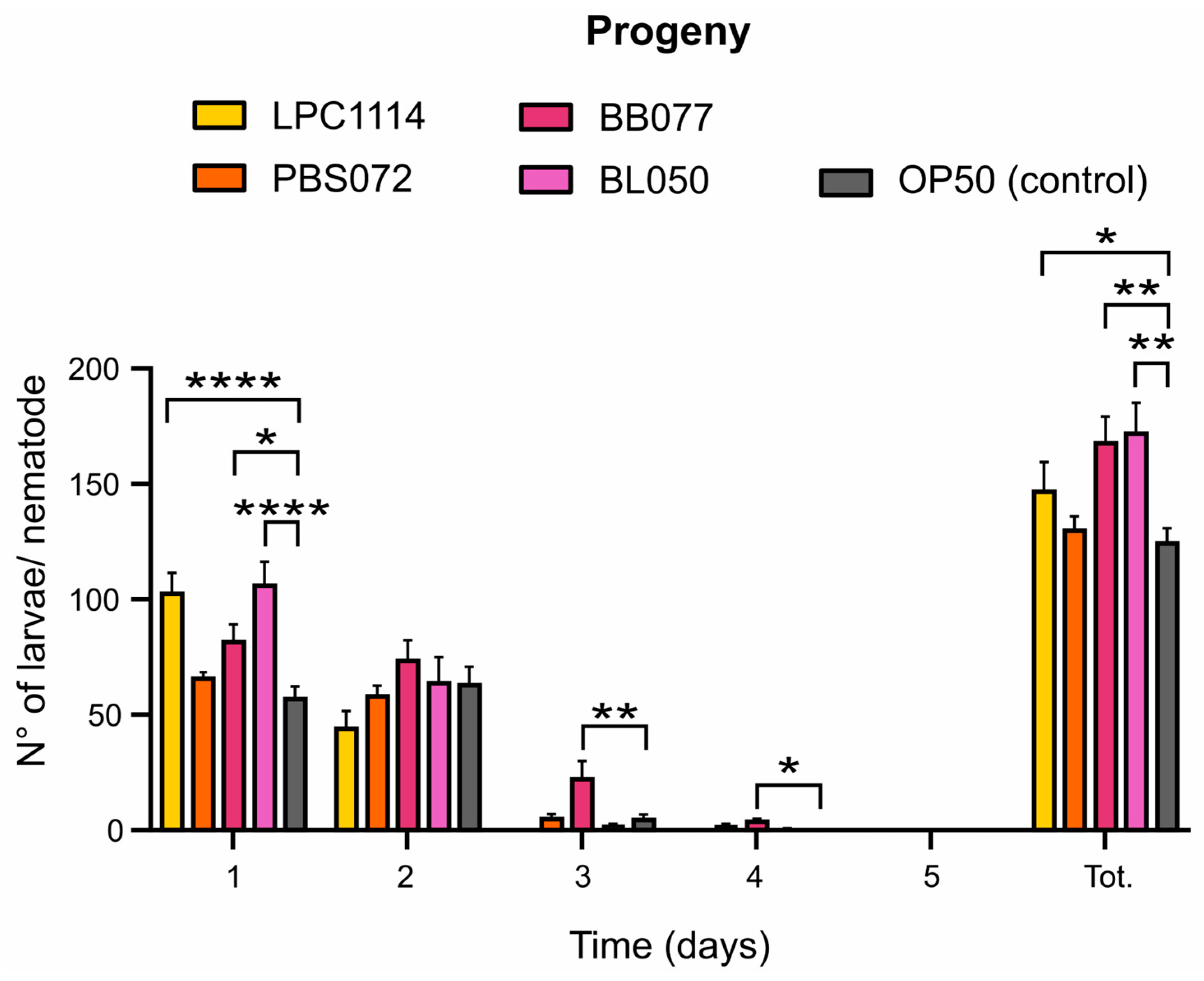
2.3. Probiotic Diets Differently Increase Progeny Production
2.4. Identification of Genes Involved in Lifespan Extension
3. Discussion
4. Materials and Methods
4.1. Caenorhabditis elegans Strains and Maintenance
4.2. Bacterial Strains and Culture Preparations
4.3. Lifespan Assay
4.4. Colony-Forming Unit (CFU) Count
4.5. Body Bends Assay
4.6. ROS Mesurements
4.7. Lipofuscin Accumulation
4.8. Aldicarb Assay
4.9. Cognitive Tests
4.10. Fertility
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Harman, D. The Aging Process. Proc. Natl. Acad. Sci. USA 1981, 78, 7124–7128. [Google Scholar] [CrossRef] [PubMed]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative Stress, Aging, and Diseases. CIA 2018, 13, 757–772. [Google Scholar] [CrossRef]
- Ferrucci, L.; Corsi, A.; Lauretani, F.; Bandinelli, S.; Bartali, B.; Taub, D.D.; Guralnik, J.M.; Longo, D.L. The Origins of Age-Related Proinflammatory State. Blood 2005, 105, 2294–2299. [Google Scholar] [CrossRef]
- Bixby, R.L. Impacts of Aging on the Federal Budget and Economy: A Cross-Cutting Challenge. Public Policy Aging Rep. 2020, 30, 46–51. [Google Scholar] [CrossRef]
- WHO Results Report 2024–2025. Available online: https://www.who.int/about/accountability/results/who-results-report-2024-2025 (accessed on 15 July 2025).
- Scott, A.J.; Ellison, M.; Sinclair, D.A. The Economic Value of Targeting Aging. Nat. Aging 2021, 1, 616–623. [Google Scholar] [CrossRef]
- Choudhary, P.; Kathuria, D.; Suri, S.; Bahndral, A.; Kanthi Naveen, A. Probiotics- Its Functions and Influence on the Ageing Process: A Comprehensive Review. Food Biosci. 2023, 52, 102389. [Google Scholar] [CrossRef]
- Bahour, N.; Cortez, B.; Pan, H.; Shah, H.; Doria, A.; Aguayo-Mazzucato, C. Diabetes Mellitus Correlates with Increased Biological Age as Indicated by Clinical Biomarkers. GeroScience 2022, 44, 415–427. [Google Scholar] [CrossRef]
- Miller, B.C.; Mathai, M.; Yadav, H.; Jain, S. Geroprotective Potential of Microbiome Modulators in the Caenorhabditis elegans Model. GeroScience 2024, 46, 129–151. [Google Scholar] [CrossRef]
- Stolbov, L.; Rudik, A.; Lagunin, A.; Druzhilovskiy, D.; Filimonov, D.; Poroikov, V. In Silico Assessment of Potential Geroprotectors: From Separate Endpoints to Complex Pharmacotherapeutic Effects. Int. J. Mol. Sci. 2025, 26, 8858. [Google Scholar] [CrossRef] [PubMed]
- Rayson, A.; Boudiffa, M.; Naveed, M.; Griffin, J.; Dall’Ara, E.; Bellantuono, I. Geroprotectors and Skeletal Health: Beyond the Headlines. Front. Cell Dev. Biol. 2022, 10, 682045. [Google Scholar] [CrossRef] [PubMed]
- Moskalev, A.; Chernyagina, E.; Kudryavtseva, A.; Shaposhnikov, M. Geroprotectors: A Unified Concept and Screening Approaches. Aging Dis. 2017, 8, 354–363. [Google Scholar] [CrossRef]
- Ling, Z.; Liu, X.; Cheng, Y.; Yan, X.; Wu, S. Gut Microbiota and Aging. Crit. Rev. Food Sci. Nutr. 2022, 62, 3509–3534. [Google Scholar] [CrossRef]
- Kim, S.; Jazwinski, S.M. The Gut Microbiota and Healthy Aging: A Mini-Review. Gerontology 2018, 64, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Biagi, E.; Nylund, L.; Candela, M.; Ostan, R.; Bucci, L.; Pini, E.; Nikkïla, J.; Monti, D.; Satokari, R.; Franceschi, C.; et al. Through Ageing, and Beyond: Gut Microbiota and Inflammatory Status in Seniors and Centenarians. PLoS ONE 2010, 5, e10667, Correction in PLoS ONE 2010, 8, 5. [Google Scholar] [CrossRef]
- Nicoletti, C. Age-Associated Changes of the Intestinal Epithelial Barrier: Local and Systemic Implications. Expert Rev. Gastroenterol. Hepatol. 2015, 9, 1467–1469. [Google Scholar] [CrossRef] [PubMed]
- Leung, K.; Thuret, S. Gut Microbiota: A Modulator of Brain Plasticity and Cognitive Function in Ageing. Healthcare 2015, 3, 898–916. [Google Scholar] [CrossRef]
- Iyer, S.R.; Shah, S.B.; Lovering, R.M. The Neuromuscular Junction: Roles in Aging and Neuromuscular Disease. Int. J. Mol. Sci. 2021, 22, 8058. [Google Scholar] [CrossRef]
- Faith, J.J.; Guruge, J.L.; Charbonneau, M.; Subramanian, S.; Seedorf, H.; Goodman, A.L.; Clemente, J.C.; Knight, R.; Heath, A.C.; Leibel, R.L.; et al. The Long-Term Stability of the Human Gut Microbiota. Science 2013, 341, 1237439. [Google Scholar] [CrossRef]
- Joint FAO/WHO Expert Consultation, on. 2001. Available online: https://openknowledge.fao.org/server/api/core/bitstreams/382476b3-4d54-4175-803f-2f26f3526256/content (accessed on 17 November 2025).
- Guarner, F.; Schaafsma, G.J. Probiotics. Int. J. Food Microbiol. 1998, 39, 237–238. [Google Scholar] [CrossRef] [PubMed]
- The Prolongation of Life: Optimistic Studies—Metchnikoff, Elie—Ebook in Inglese—EPUB2 Con Adobe DRM|IBS. Available online: https://www.ibs.it/prolongation-of-life-optimistic-studies-ebook-inglese-elie-metchnikoff/e/4057664619976?srsltid=AfmBOoraVlcYg_gt2lXHFOkSb0BwpN6q4gMbxoFVAihbMxfc1aJwyIQT (accessed on 15 September 2025).
- Biagi, E.; Franceschi, C.; Rampelli, S.; Severgnini, M.; Ostan, R.; Turroni, S.; Consolandi, C.; Quercia, S.; Scurti, M.; Monti, D.; et al. Gut Microbiota and Extreme Longevity. Curr. Biol. 2016, 26, 1480–1485. [Google Scholar] [CrossRef]
- Du, Y.; Gao, Y.; Zeng, B.; Fan, X.; Yang, D.; Yang, M. Effects of Anti-Aging Interventions on Intestinal Microbiota. Gut Microbes 2021, 13, 1994835. [Google Scholar] [CrossRef] [PubMed]
- Sandionigi, A.; De Giani, A.; Tursi, F.; Michelotti, A.; Cestone, E.; Giardina, S.; Zampolli, J.; Di Gennaro, P. Effectiveness of Multistrain Probiotic Formulation on Common Infectious Disease Symptoms and Gut Microbiota Modulation in Flu-Vaccinated Healthy Elderly Subjects. Biomed. Res. Int. 2022, 2022, 3860896. [Google Scholar] [CrossRef] [PubMed]
- Prasad, J.; Gill, H.; Smart, J.; Gopal, P.K. Selection and Characterisation of Lactobacillus and Bifidobacterium Strains for Use as Probiotics. Int. Dairy J. 1998, 8, 993–1002. [Google Scholar] [CrossRef]
- Ramos, C.L.; Thorsen, L.; Schwan, R.F.; Jespersen, L. Strain-Specific Probiotics Properties of Lactobacillus fermentum, Lactobacillus plantarum and Lactobacillus brevis Isolates from Brazilian Food Products. Food Microbiol. 2013, 36, 22–29. [Google Scholar] [CrossRef]
- Sharma, M.; Wasan, A.; Sharma, R.K. Recent Developments in Probiotics: An Emphasis on Bifidobacterium. Food Biosci. 2021, 41, 100993. [Google Scholar] [CrossRef]
- Balaguer, F.; Barrena, M.; Enrique, M.; Maicas, M.; Álvarez, B.; Tortajada, M.; Chenoll, E.; Ramón, D.; Martorell, P. Bifidobacterium Animalis Subsp. Lactis BPL1TM and Its Lipoteichoic Acid Modulate Longevity and Improve Age/Stress-Related Behaviors in Caenorhabditis elegans. Antioxidants 2023, 12, 2107. [Google Scholar] [CrossRef]
- Kumar, A.; Joishy, T.; Das, S.; Kalita, M.C.; Mukherjee, A.K.; Khan, M.R. A Potential Probiotic Lactobacillus Plantarum JBC5 Improves Longevity and Healthy Aging by Modulating Antioxidative, Innate Immunity and Serotonin-Signaling Pathways in Caenorhabditis elegans. Antioxidants 2022, 11, 268. [Google Scholar] [CrossRef]
- Kishimoto, S.; Nono, M.; Makizaki, Y.; Tanaka, Y.; Ohno, H.; Nishida, E.; Uno, M. Lactobacillus paracasei subsp. paracasei 2004 Improves Health and Lifespan in Caenorhabditis elegans. Sci. Rep. 2024, 14, 10453. [Google Scholar] [CrossRef]
- Hu, T.; Chen, R.; Qian, Y.; Ye, K.; Long, X.; Park, K.-Y.; Zhao, X. Antioxidant Effect of Lactobacillus fermentum HFY02-Fermented Soy Milk on D-Galactose-Induced Aging Mouse Model. Food Sci. Hum. Wellness 2022, 11, 1362–1372. [Google Scholar] [CrossRef]
- Kim, H.; Shin, J.; Kim, S.; Kim, S.; Cho, B.; Park, S.; Park, G.; Shin, H.; Park, M.S.; Kim, J. Bifidobacterium bifidum BGN4 and Bifidobacterium longum BORI Promotes Neuronal Rejuvenation in Aged Mice. Biochem. Biophys. Res. Commun. 2022, 603, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Baek, J.-S.; Shin, Y.-J.; Ma, X.; Park, H.-S.; Hwang, Y.-H.; Kim, D.-H. Bifidobacterium bifidum and Lactobacillus paracasei Alleviate Sarcopenia and Cognitive Impairment in Aged Mice by Regulating Gut Microbiota-Mediated AKT, NF-κB, and FOXO3a Signaling Pathways. Immun. Ageing 2023, 20, 56. [Google Scholar] [CrossRef]
- Lee, H.-Y.; Lee, J.-H.; Kim, S.H.; Jo, S.-Y.; Min, K.-J. Probiotic Limosilactobacillus reuteri (Lactobacillus reuteri) Extends the Lifespan of Drosophila Melanogaster Through Insulin/IGF-1 Signaling. Aging Dis. 2023, 14, 1407–1424. [Google Scholar] [CrossRef]
- Girard, L.R.; Fiedler, T.J.; Harris, T.W.; Carvalho, F.; Antoshechkin, I.; Han, M.; Sternberg, P.W.; Stein, L.D.; Chalfie, M. WormBook: The Online Review of Caenorhabditis elegans Biology. Nucleic Acids Res. 2007, 35, D472–D475. [Google Scholar] [CrossRef]
- Poupet, C.; Chassard, C.; Nivoliez, A.; Bornes, S. Caenorhabditis elegans, a Host to Investigate the Probiotic Properties of Beneficial Microorganisms. Front. Nutr. 2020, 7, 135. [Google Scholar] [CrossRef]
- Presti, I.; D’Orazio, G.; Labra, M.; La Ferla, B.; Mezzasalma, V.; Bizzaro, G.; Giardina, S.; Michelotti, A.; Tursi, F.; Vassallo, M.; et al. Evaluation of the Probiotic Properties of New Lactobacillus and Bifidobacterium Strains and Their In Vitro Effect. Appl. Microbiol. Biotechnol. 2015, 99, 5613–5626. [Google Scholar] [CrossRef] [PubMed]
- Mezzasalma, V.; Manfrini, E.; Ferri, E.; Boccarusso, M.; Di Gennaro, P.; Schiano, I.; Michelotti, A.; Labra, M. Orally Administered Multispecies Probiotic Formulations to Prevent Uro-Genital Infections: A Randomized Placebo-Controlled Pilot Study. Arch. Gynecol. Obstet. 2017, 295, 163–172, Erratum in Arch. Gynecol. Obstet. 2017, 295, 527. [Google Scholar] [CrossRef]
- Malfa, P.; Brambilla, L.; Giardina, S.; Masciarelli, M.; Squarzanti, D.F.; Carlomagno, F.; Meloni, M. Evaluation of Antimicrobial, Antiadhesive and Co-Aggregation Activity of a Multi-Strain Probiotic Composition Against Different Urogenital Pathogens. Int. J. Mol. Sci. 2023, 24, 1323. [Google Scholar] [CrossRef]
- Squarzanti, D.F.; Dell’Atti, F.; Scalia, A.C.; Najmi, Z.; Cochis, A.; Malfa, P. Exploring the In Vitro Antibacterial Potential of Specific Probiotic Strains Against Oral Pathogens. Microorganisms 2024, 12, 441. [Google Scholar] [CrossRef]
- Vicariotto, F.; Malfa, P.; Viciani, E.; Dell’Atti, F.; Squarzanti, D.F.; Marcante, A.; Castagnetti, A.; Ponchia, R.; Governini, L.; De Leo, V. Efficacy of Lactiplantibacillus plantarum PBS067, Bifidobacterium animalis subsp. lactis BL050, and Lacticaseibacillus rhamnosus LRH020 in the Amelioration of Vaginal Microbiota in Post-Menopausal Women: A Prospective Observational Clinical Trial. Nutrients 2024, 16, 402. [Google Scholar] [CrossRef]
- Lungaro, L.; Malfa, P.; Manza, F.; Costanzini, A.; Valentini, G.; Squarzanti, D.F.; Viciani, E.; Velichevskaya, A.; Castagnetti, A.; Barbalinardo, M.; et al. Clinical Efficacy of Probiotics for Allergic Rhinitis: Results of an Exploratory Randomized Controlled Trial. Nutrients 2024, 16, 4173. [Google Scholar] [CrossRef] [PubMed]
- Lungaro, L.; Malfa, P.; Manza, F.; Negrelli, M.; Costanzini, A.; Squarzanti, D.F.; Lo Re, M.; Cariani, A.; Ghisellini, S.; Caputo, F.; et al. Clinical Efficacy of Probiotics for Relieving Cold Symptoms in Healthy Individuals: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Nutrients 2025, 17, 1490. [Google Scholar] [CrossRef]
- Nobile, V.; Giardina, S.; Puoci, F. The Effect of a Probiotic Complex on the Gut-Brain Axis: A Translational Study. Neuropsychobiology 2021, 81, 116–126. [Google Scholar] [CrossRef]
- Nobile, V.; Puoci, F. Effect of a Multi-Strain Probiotic Supplementation to Manage Stress During the COVID-19 Pandemic: A Randomized, Double-Blind, Placebo-Controlled, Cross-Over Clinical Trial. Neuropsychobiology 2023, 82, 61–71. [Google Scholar] [CrossRef]
- Vicariotto, F.; Malfa, P.; Torricelli, M.; Lungaro, L.; Caio, G.; De Leo, V. Beneficial Effects of Limosilactobacillus reuteri PBS072 and Bifidobacterium breve BB077 on Mood Imbalance, Self-Confidence, and Breastfeeding in Women During the First Trimester Postpartum. Nutrients 2023, 15, 3513. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, B.; Lei, H.; Feng, Z.; Liu, J.; Hsu, A.-L.; Xu, X.Z.S. Functional Aging in the Nervous System Contributes to Age-Dependent Motor Activity Decline in C. elegans. Cell Metab. 2013, 18, 392–402. [Google Scholar] [CrossRef]
- Mahoney, T.R.; Luo, S.; Nonet, M.L. Analysis of Synaptic Transmission in Caenorhabditis elegans Using an Aldicarb-Sensitivity Assay. Nat. Protoc. 2006, 1, 1772–1777. [Google Scholar] [CrossRef] [PubMed]
- Leinwand, S.G.; Chalasani, S.H. Neuropeptide Signaling Remodels Chemosensory Circuit Composition in Caenorhabditis elegans. Nat. Neurosci. 2013, 16, 1461–1467. [Google Scholar] [CrossRef]
- Leinwand, S.G.; Yang, C.J.; Bazopoulou, D.; Chronis, N.; Srinivasan, J.; Chalasani, S.H. Circuit Mechanisms Encoding Odors and Driving Aging-Associated Behavioral Declines in Caenorhabditis elegans. eLife 2015, 4, e10181. [Google Scholar] [CrossRef]
- Ferkey, D.M.; Sengupta, P.; L’Etoile, N.D. Chemosensory Signal Transduction in Caenorhabditis elegans. Genetics 2021, 217, iyab004, Erratum in Caenorhabditis elegans. Genetics 2022, 220, iyab181. [Google Scholar] [CrossRef] [PubMed]
- Torayama, I.; Ishihara, T.; Katsura, I. Caenorhabditis elegans Integrates the Signals of Butanone and Food to Enhance Chemotaxis to Butanone. J. Neurosci. 2007, 27, 741–750. [Google Scholar] [CrossRef] [PubMed]
- Scharf, A.; Pohl, F.; Egan, B.M.; Kocsisova, Z.; Kornfeld, K. Reproductive Aging in Caenorhabditis elegans: From Molecules to Ecology. Front. Cell Dev. Biol. 2021, 9, 718522. [Google Scholar] [CrossRef]
- Wood, J.G.; Rogina, B.; Lavu, S.; Howitz, K.; Helfand, S.L.; Tatar, M.; Sinclair, D. Sirtuin Activators Mimic Caloric Restriction and Delay Ageing in Metazoans. Nature 2004, 430, 686–689, Correction in Nature 2004, 430, 107. [Google Scholar] [CrossRef]
- Berdichevsky, A.; Viswanathan, M.; Horvitz, H.R.; Guarente, L.C. elegans SIR-2.1 Interacts with 14-3-3 Proteins to Activate DAF-16 and Extend Life Span. Cell 2006, 125, 1165–1177. [Google Scholar] [CrossRef]
- Lee, J.; Kwon, G.; Park, J.; Kim, J.-K.; Lim, Y.-H. Brief Communication: SIR-2.1-Dependent Lifespan Extension of Caenorhabditis elegans by Oxyresveratrol and Resveratrol. Exp. Biol. Med. 2016, 241, 1757–1763. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Feng, Y.; Zhao, J.; Chen, W.; Lu, W. Achieving Healthy Aging through Gut Microbiota-Directed Dietary Intervention: Focusing on Microbial Biomarkers and Host Mechanisms. J. Adv. Res. 2025, 68, 179–200. [Google Scholar] [CrossRef] [PubMed]
- D’Amato, A.; Di Cesare Mannelli, L.; Lucarini, E.; Man, A.L.; Le Gall, G.; Branca, J.J.V.; Ghelardini, C.; Amedei, A.; Bertelli, E.; Regoli, M.; et al. Faecal Microbiota Transplant from Aged Donor Mice Affects Spatial Learning and Memory via Modulating Hippocampal Synaptic Plasticity- and Neurotransmission-Related Proteins in Young Recipients. Microbiome 2020, 8, 140. [Google Scholar] [CrossRef]
- Li, Y.; Ning, L.; Yin, Y.; Wang, R.; Zhang, Z.; Hao, L.; Wang, B.; Zhao, X.; Yang, X.; Yin, L.; et al. Age-Related Shifts in Gut Microbiota Contribute to Cognitive Decline in Aged Rats. Aging 2020, 12, 7801–7817. [Google Scholar] [CrossRef] [PubMed]
- Donati Zeppa, S.; Agostini, D.; Ferrini, F.; Gervasi, M.; Barbieri, E.; Bartolacci, A.; Piccoli, G.; Saltarelli, R.; Sestili, P.; Stocchi, V. Interventions on Gut Microbiota for Healthy Aging. Cells 2023, 12, 34. [Google Scholar] [CrossRef]
- Yu, X.; Wu, X.; Qiu, L.; Wang, D.; Gan, M.; Chen, X.; Wei, H.; Xu, F. Analysis of the Intestinal Microbial Community Structure of Healthy and Long-Living Elderly Residents in Gaotian Village of Liuyang City. Appl. Microbiol. Biotechnol. 2015, 99, 9085–9095. [Google Scholar] [CrossRef]
- Kashtanova, D.A.; Klimenko, N.S.; Strazhesko, I.D.; Starikova, E.V.; Glushchenko, O.E.; Gudkov, D.A.; Tkacheva, O.N. A Cross-Sectional Study of the Gut Microbiota Composition in Moscow Long-Livers. Microorganisms 2020, 8, 1162. [Google Scholar] [CrossRef]
- Mytilinaiou, E.; Kitopoulou, K.; Palikaras, K. Caenorhabditis elegans as a Screening Platform for Anti-Aging Compounds. Methods Mol. Biol. 2025, 2906, 301–313. [Google Scholar] [CrossRef] [PubMed]
- Olsen, A.; Vantipalli, M.C.; Lithgow, G.J. Using Caenorhabditis elegans as a Model for Aging and Age-Related Diseases. Ann. N. Y. Acad. Sci. 2006, 1067, 120–128. [Google Scholar] [CrossRef]
- Nigon, V.M.; Félix, M.-A. History of Research on C. elegans and Other Free-Living Nematodes as Model Organisms. WormBook 2017, 2017, 1–84. [Google Scholar] [CrossRef]
- Jeayeng, S.; Thongsroy, J.; Chuaijit, S. Caenorhabditis elegans as a Model to Study Aging and Photoaging. Biomolecules 2024, 14, 1235. [Google Scholar] [CrossRef]
- Oswal, N.; Martin, O.M.F.; Stroustrup, S.; Bruckner, M.A.M.; Stroustrup, N. A Hierarchical Process Model Links Behavioral Aging and Lifespan in C. elegans. PLOS Comput. Biol. 2022, 18, e1010415. [Google Scholar] [CrossRef]
- Newell Stamper, B.L.; Cypser, J.R.; Kechris, K.; Kitzenberg, D.A.; Tedesco, P.M.; Johnson, T.E. Movement Decline Across Lifespan of Caenorhabditis elegans Mutants in the Insulin/Insulin-like Signaling Pathway. Aging Cell 2018, 17, e12704. [Google Scholar] [CrossRef] [PubMed]
- Suryawinata, N.; Yokosawa, R.; Hui, K.; Tan, C.; Lai, A.L.; Sone, R.; Mori, I.; Noma, K. Dietary E. coli Promotes Age-Dependent Chemotaxis Decline in C. elegans. Sci. Rep. 2024, 14, 5529. Available online: https://www.nature.com/articles/s41598-024-52272-4 (accessed on 31 July 2025). [CrossRef]
- Bargmann, C.I.; Hartwieg, E.; Horvitz, H.R. Odorant-Selective Genes and Neurons Mediate Olfaction in C. elegans. Cell 1993, 74, 515–527. [Google Scholar] [CrossRef]
- L’Etoile, N.D.; Bargmann, C.I. Olfaction and Odor Discrimination Are Mediated by the C. elegans Guanylyl Cyclase ODR-1. Neuron 2000, 25, 575–586. [Google Scholar] [CrossRef]
- Aprison, E.Z.; Dzitoyeva, S.; Ruvinsky, I. The Serotonin Circuit That Coordinates Germline Proliferation and Egg Laying with Other Reproductive Functions in Caenorhabditis elegans. Proc. R. Soc. B Biol. Sci. 2022, 289, 20220913. [Google Scholar] [CrossRef] [PubMed]
- Blackwell, T.K.; Steinbaugh, M.J.; Hourihan, J.M.; Ewald, C.Y.; Isik, M. SKN-1/Nrf, Stress Responses, and Aging in Caenorhabditis elegans. Free Radic. Biol. Med. 2015, 88, 290–301. [Google Scholar] [CrossRef]
- Alcedo, J.; Kenyon, C. Regulation of C. elegans Longevity by Specific Gustatory and Olfactory Neurons. Neuron 2004, 41, 45–55. [Google Scholar] [CrossRef]
- Libina, N.; Berman, J.R.; Kenyon, C. Tissue-Specific Activities of C. elegans DAF-16 in the Regulation of Lifespan. Cell 2003, 115, 489–502. [Google Scholar] [CrossRef]
- Roy, C.; Molin, L.; Alcolei, A.; Solyga, M.; Bonneau, B.; Vachon, C.; Bessereau, J.-L.; Solari, F. DAF-2/Insulin IGF-1 Receptor Regulates Motility During Aging by Integrating Opposite Signaling from Muscle and Neuronal Tissues. Aging Cell 2022, 21, e13660. [Google Scholar] [CrossRef]
- Papp, D.; Csermely, P.; Sőti, C. A Role for SKN-1/Nrf in Pathogen Resistance and Immunosenescence in Caenorhabditis elegans. PLOS Pathog. 2012, 8, e1002673. [Google Scholar] [CrossRef]
- Roselli, M.; Schifano, E.; Guantario, B.; Zinno, P.; Uccelletti, D.; Devirgiliis, C. Caenorhabditis elegans and Probiotics Interactions from a Prolongevity Perspective. Int. J. Mol. Sci. 2019, 20, 5020. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, R.P.; Abate, J.P.; Dilks, K.; Landis, J.; Ashraf, J.; Murphy, C.T.; Blackwell, T.K. Condition-Adapted Stress and Longevity Gene Regulation by Caenorhabditis elegans SKN-1/Nrf. Aging Cell 2009, 8, 524–541. [Google Scholar] [CrossRef]
- Dou, T.; Chen, J.; Wang, R.; Pu, X.; Wu, H.; Zhao, Y. Complementary Protective Effects of Autophagy and Oxidative Response against Graphene Oxide Toxicity in Caenorhabditis elegans. Ecotoxicol. Environ. Saf. 2022, 248, 114289. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lu, Y.-Y.; Huang, L.; Shi, L.; Zheng, Z.-Y.; Chen, J.-N.; Qu, Y.; Xiao, H.-T.; Luo, H.-R.; Wu, G.-S. Para-Hydroxybenzyl Alcohol Delays the Progression of Neurodegenerative Diseases in Models of Caenorhabditis elegans Through Activating Multiple Cellular Protective Pathways. Oxidative Med. Cell. Longev. 2022, 2022, 8986287. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, K.; Zhang, Y.; Ge, S.; Zhang, S. Defense Against Oxidative Stress in Caenorhabditis elegans by Dark Tea. Front. Vet. Sci. 2024, 10, 1342747. [Google Scholar] [CrossRef]
- Greer, E.L.; Brunet, A. Different Dietary Restriction Regimens Extend Lifespan by Both Independent and Overlapping Genetic Pathways in C. elegans. Aging Cell 2009, 8, 113–127. [Google Scholar] [CrossRef] [PubMed]
- Palominos, M.F.; Calixto, A. Quantification of Bacteria Residing in Caenorhabditis elegans Intestine. Bio. Protoc. 2020, 10, e3605. [Google Scholar] [CrossRef] [PubMed]
- Sciandrone, B.; Kentsop, R.A.D.; Pensotti, R.; Ottolina, G.; Mascheretti, I.; Mattana, M.; Regonesi, M.E. Toxicological Analysis of the Arylnaphthalene Lignan Justicidin B Using a Caenorhabditis elegans Model. Molecules 2024, 29, 5516. [Google Scholar] [CrossRef]
- Yoon, D.S.; Lee, M.-H.; Cha, D.S. Measurement of Intracellular ROS in Caenorhabditis elegans Using 2′,7′-Dichlorodihydrofluorescein Diacetate. Bio. Protoc. 2018, 8, e2774. [Google Scholar] [CrossRef]
- Heydarian, D.; Flavel, M.; Munasinghe, M.; Jois, M.; Thomas, J. Improving Cognitive and Chemosensory Function in Caenorhabditis elegans Through Polyphenol-Rich Sugarcane Extract. Stresses 2024, 4, 816–826. [Google Scholar] [CrossRef]


| Strain | Median Lifespan (Days) 1 | Maximum Lifespan (Days) 2 | p-Value 3 |
|---|---|---|---|
| LPC1114 | 15.5 ± 1.73 | 25 ± 0.00 | 0.0217 |
| PBS072 | 15.5 ± 1.53 | 26.3 ± 2.08 | 0.0007 |
| BB077 | 15.5 ± 1.91 | 26.5 ± 2.52 | n.s. |
| BL050 | 15.2 ± 1.50 | 24.3 ± 2.52 | n.s. |
| OP50 | 14.7 ± 1.15 | 21.0 ± 0.00 | n.s. |
| Strain | CFU/mL |
|---|---|
| LPC1114 | 8.7 ± 2.9 × 101 |
| PBS072 | 11.7 ± 1.5 × 101 |
| BB077 | 5.3 ± 1.5 × 101 |
| BL050 | 22.3 ± 6.8 × 101 |
| OP50 | 16.5 ± 2.1 × 106 |
| Strain | Ci After Conditioning 1 | p-Value 2 |
|---|---|---|
| LPC1114 | 0.210 ± 0.042 | n.s. |
| PBS072 | 0.404 ± 0.088 | n.s. |
| BB077 | 0.465 ± 0.057 | 0.0266 |
| BL050 | 0.537 ± 0.045 | 0.0103 |
| OP50 | 0.166 ± 0.017 | n.s. |
| C. elegans Mutant | Strain | Median Lifespan (Days) 1 | Maximum Lifespan (Days) 2 | p-Value 3 |
|---|---|---|---|---|
| GR1307 (Δdaf16) | LPC1114 | 13.2 ± 1.75 | 17.5 ± 2.63 | n.s. |
| PBS072 | 14.0 ± 0.00 | 20.0 ± 1.50 | 0.0077 | |
| OP50 | 11.0 ± 1.73 | 18.0 ± 2.08 | ||
| EU1 (Δskn1) | LPC1114 | 12.0 ± 1.53 | 14.0 ± 2.31 | n.s. |
| PBS072 | 11.0 ± 0.58 | 18.0 ± 1.73 | n.s. | |
| OP50 | 12.5 ± 2.57 | 16.0 ± 1.73 | ||
| VC199 (Δsir-2.1) | LPC1114 | 14.0 ± 0.00 | 18.5 ± 3.56 | n.s. |
| PBS072 | 14.0 ± 2.45 | 21.5 ± 3.40 | n.s. | |
| OP50 | 14.0 ± 1.00 | 18.0 ± 3.61 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sciandrone, B.; Squarzanti, D.F.; Malfa, P.; Regonesi, M.E. Probiotic Modulation in Aging: Strain-Specific Geroprotective Effects in Caenorhabditis elegans. Int. J. Mol. Sci. 2025, 26, 11205. https://doi.org/10.3390/ijms262211205
Sciandrone B, Squarzanti DF, Malfa P, Regonesi ME. Probiotic Modulation in Aging: Strain-Specific Geroprotective Effects in Caenorhabditis elegans. International Journal of Molecular Sciences. 2025; 26(22):11205. https://doi.org/10.3390/ijms262211205
Chicago/Turabian StyleSciandrone, Barbara, Diletta Francesca Squarzanti, Patrizia Malfa, and Maria Elena Regonesi. 2025. "Probiotic Modulation in Aging: Strain-Specific Geroprotective Effects in Caenorhabditis elegans" International Journal of Molecular Sciences 26, no. 22: 11205. https://doi.org/10.3390/ijms262211205
APA StyleSciandrone, B., Squarzanti, D. F., Malfa, P., & Regonesi, M. E. (2025). Probiotic Modulation in Aging: Strain-Specific Geroprotective Effects in Caenorhabditis elegans. International Journal of Molecular Sciences, 26(22), 11205. https://doi.org/10.3390/ijms262211205








