Dysregulated lncRNAs in Cisplatin-Induced Nephrotoxicity and Their Association with Apoptosis and Autophagy: An Exploratory In Vitro Study
Abstract
1. Introduction
2. Results
2.1. Determination of the Half-Maximal Inhibitory Concentration (IC50)
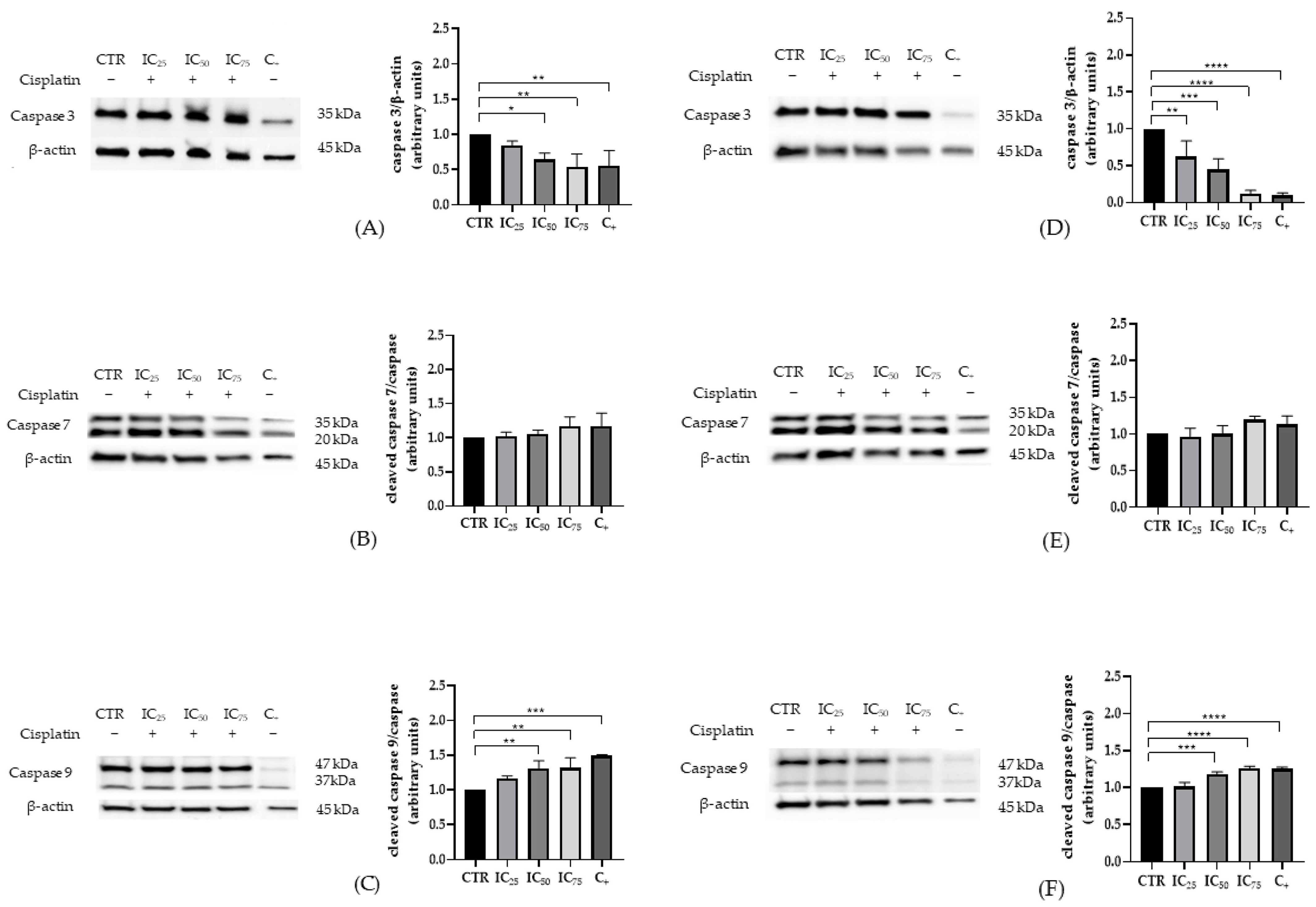
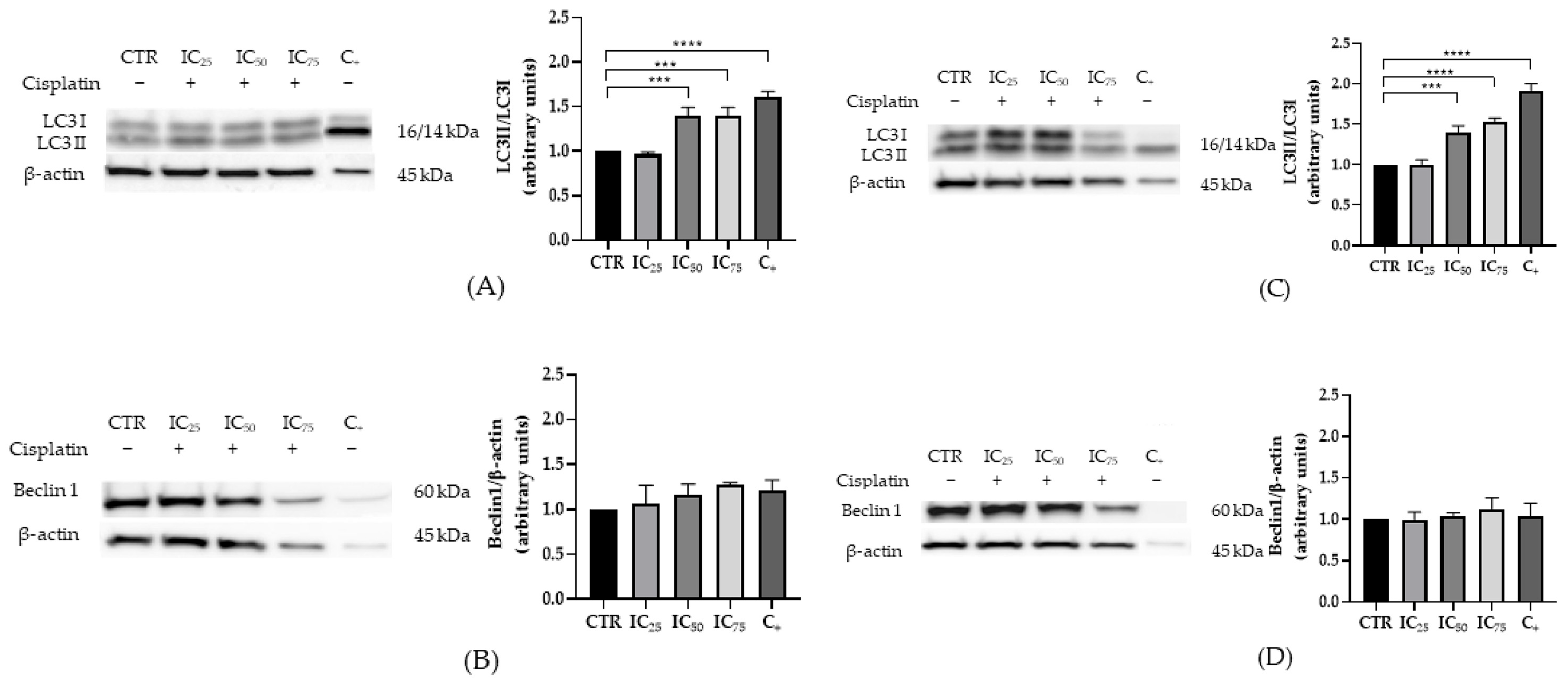
2.2. Characterization of Apoptosis and Autophagy in HEK-293 and HK-2 Cells Exposed to Cisplatin
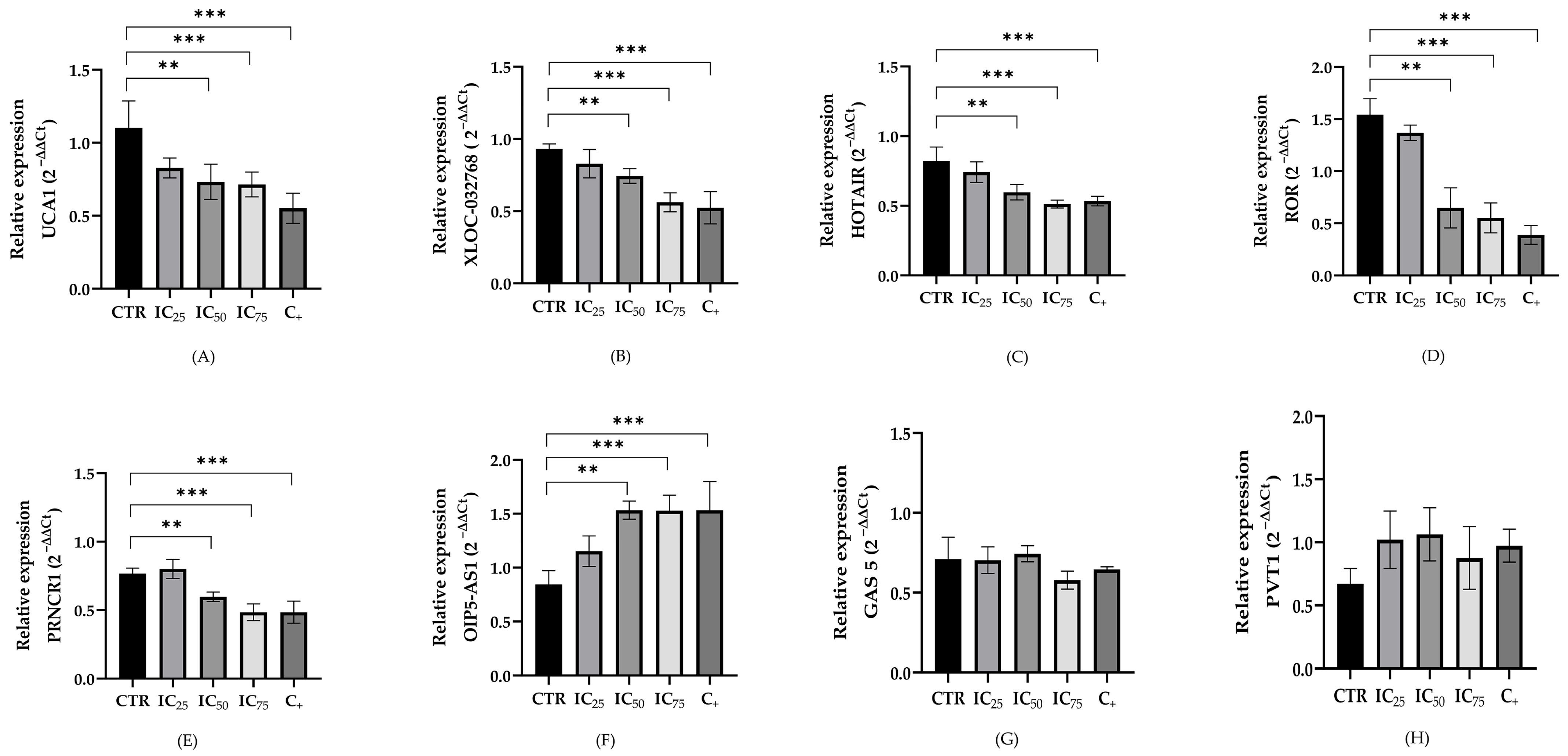
2.3. LncRNA Expression in a Cisplatin-Induced Nephrotoxicity Model
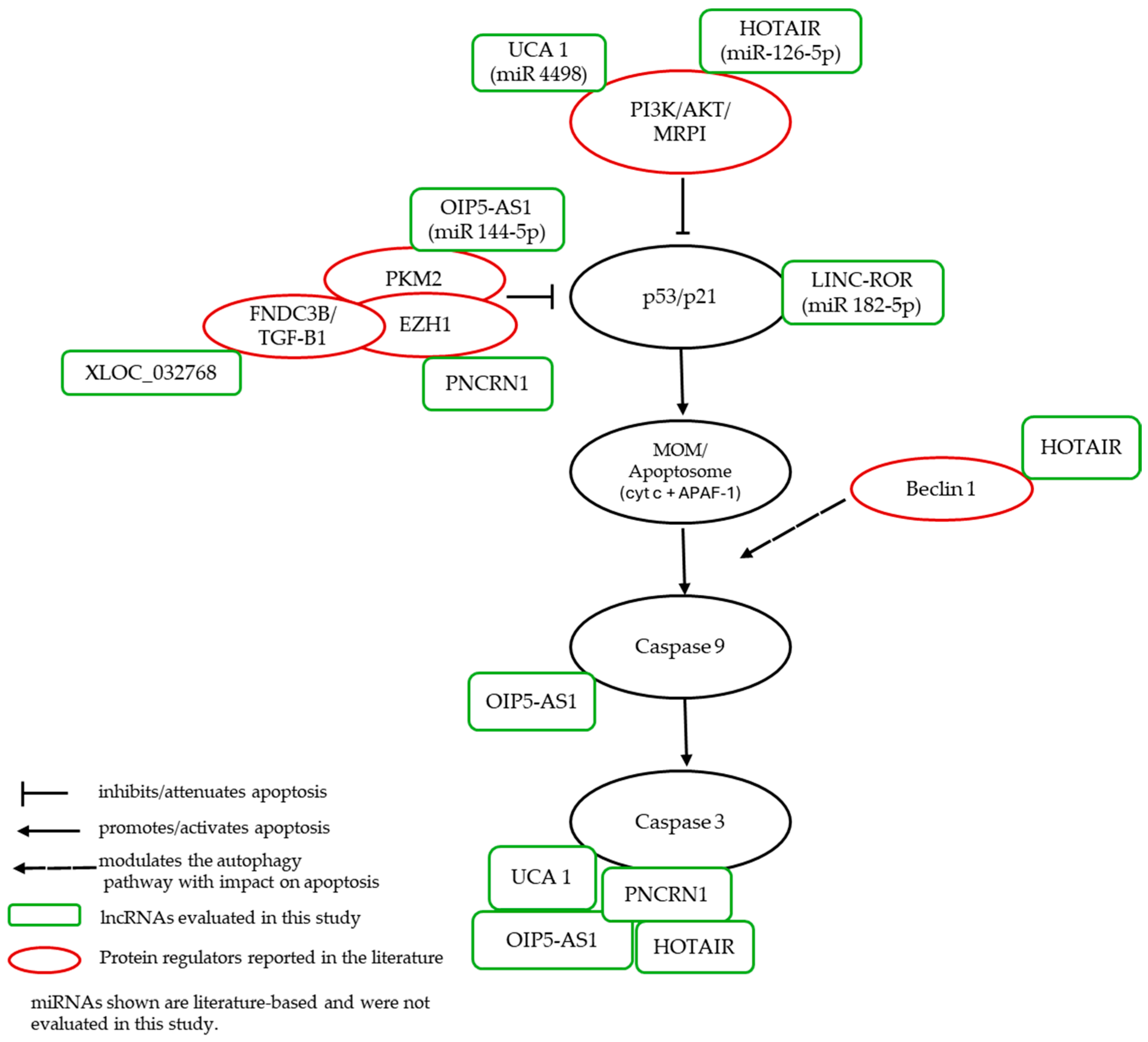
3. Discussion
3.1. Expression of Apoptotic and Autophagic Proteins in HEK-293 and HK-2 Cells Exposed to Cisplatin
3.2. LncRNAs Expression in Cisplatin-Treated Renal Cells
4. Materials and Methods
4.1. Cell Line and In Vitro Culture
4.2. Renoprotection Against Nephrotoxicity
4.3. Western Blot
4.4. Total RNA Extraction and Quantitative Real-Time PCR
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HEK-293 | Human Embryonic Kidney 293 |
| HK-2 | Human Kidney 2 |
| CDDP | cisplatin |
| CIN | Cisplatin-induced nephrotoxicity |
| lncRNAs | long non-coding RNAs |
| FBS | fetal bovine serum |
| DMSO | dimethyl sulfoxide |
| IC50 | half-maximal inhibitory concentrations |
| IC25 IC75 | concentrations required to produce 25% inhibition of cell viability concentrations required to produce 75% inhibition of cell viability |
| RNA | Ribonucleic Acid |
| cDNA | Complementary Deoxyribonucleic Acid |
| qPCR | Quantitative Polymerase Chain Reaction |
| ceRNA | competing endogenous RNA |
| PARP | Poly (ADP-ribose) Polymerase |
| mTOR | Mechanistic Target of Rapamycin |
| ULK1 | Unc-51 Like Autophagy Activating Kinase 1 |
| OCT2 | Organic Cation Transporter 2 |
References
- Siegel, R.L.; Kratzer, T.B.; Giaquinto, A.N.; Sung, H.; Jemal, A. Cancer Statistics, 2025. CA Cancer J. Clin. 2025, 75, 10–45. [Google Scholar] [CrossRef]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- INE. Anuario de Estadísticas Vitales Año 2022; INE: Santiago, Chile, 2025. [Google Scholar]
- Vermorken, J.B.; Remenar, E.; van Herpen, C.; Gorlia, T.; Mesia, R.; Degardin, M.; Stewart, J.S.; Jelic, S.; Betka, J.; Preiss, J.H.; et al. Cisplatin, Fluorouracil, and Docetaxel in Unresectable Head and Neck Cancer. N. Engl. J. Med. 2007, 357, 1695–1704. [Google Scholar] [CrossRef]
- Motzer, R.J.; Sheinfeld, J.; Mazumdar, M.; Bajorin, D.F.; Bosl, G.J.; Herr, H.; Lyn, P.; Vlamis, V. Etoposide and Cisplatin Adjuvant Therapy for Patients with Pathologic Stage II Germ Cell Tumors. J. Clin. Oncol. 1995, 13, 2700–2704. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Zhang, Y.; Xie, Y.; Xiang, J.; Zhu, Y.; Yang, J. Enhanced Tumor Suppression by Adenoviral PTEN Gene Therapy Combined with Cisplatin Chemotherapy in Small-Cell Lung Cancer. Cancer Gene Ther. 2013, 20, 251–259. [Google Scholar] [CrossRef]
- Magali, L.; Pascal, F.; Serge, A.; Mathieu, B.; Ayoube, Z.; Claire, T.; Christiane, M. Better Survival in Impaired Renal Function Patients with Metastatic Non-Small Cell Lung Cancer Treated by Cisplatin-Pemetrexed. Eur. J. Clin. Pharmacol. 2020, 76, 1573–1580. [Google Scholar] [CrossRef]
- Armstrong, D.K.; Bundy, B.; Wenzel, L.; Huang, H.Q.; Baergen, R.; Lele, S.; Copeland, L.J.; Walker, J.L.; Burger, R.A. Intraperitoneal Cisplatin and Paclitaxel in Ovarian Cancer. N. Engl. J. Med. 2006, 354, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.N.; Herzog, T.J.; Lewin, S.; Giuntoli, R.L.; Armstrong, D.K.; Rocconi, R.P.; Spannuth, W.A.; Gold, M.A. A Comparison of Cisplatin/Paclitaxel and Carboplatin/Paclitaxel in Stage IVB, Recurrent or Persistent Cervical Cancer. Gynecol. Oncol. 2007, 105, 299–303. [Google Scholar] [CrossRef]
- Coppin, C.M.; Gospodarowicz, M.K.; James, K.; Tannock, I.F.; Zee, B.; Carson, J.; Pater, J.; Sullivan, L.D. Improved Local Control of Invasive Bladder Cancer by Concurrent Cisplatin and Preoperative or Definitive Radiation. J. Clin. Oncol. 1996, 14, 2901–2907. [Google Scholar] [CrossRef]
- Lyrio, R.M.d.C.; Rocha, B.R.A.; Corrêa, A.L.R.M.; Mascarenhas, M.G.S.; Santos, F.L.; Maia, R.d.H.; Segundo, L.B.; de Almeida, P.A.A.; Moreira, C.M.O.; Sassi, R.H. Chemotherapy-Induced Acute Kidney Injury: Epidemiology, Pathophysiology, and Therapeutic Approaches. Front. Nephrol. 2024, 4, 1436896. [Google Scholar] [CrossRef] [PubMed]
- Avry, F.; Roseau, C.; Leguay, Z.; Brabant, S.; Ganea, A.; Champeaux-Orange, E.; Priou, V. Evaluation of a New Score Associated with Acute Kidney Injury in Patients Treated with Cisplatin Based EXTREME Regimen. BMC Cancer 2024, 24, 405. [Google Scholar] [CrossRef] [PubMed]
- Nourie, N.; Ghaleb, R.; Lefaucheur, C.; Louis, K. Toward Precision Medicine: Exploring the Landscape of Biomarkers in Acute Kidney Injury. Biomolecules 2024, 14, 82. [Google Scholar] [CrossRef]
- Ozkok, A.; Edelstein, C.L. Pathophysiology of Cisplatin-Induced Acute Kidney Injury. BioMed Res. Int. 2014, 2014, 967826. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.; Shen, Z.; Yin, Y. Comprehensive Review of LncRNA-Mediated Therapeutic Resistance in Non-Small Cell Lung Cancer. Cancer Cell Int. 2024, 24, 369. [Google Scholar] [CrossRef]
- Dahariya, S.; Paddibhatla, I.; Kumar, S.; Raghuwanshi, S.; Pallepati, A.; Gutti, R.K. Long Non-Coding RNA: Classification, Biogenesis and Functions in Blood Cells. Mol. Immunol. 2019, 112, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.M.; Goyal, R. LncRNA as a Therapeutic Target for Angiogenesis. Curr. Top. Med. Chem. 2017, 17, 1750–1757. [Google Scholar] [CrossRef]
- Gao, N.; Li, Y.; Li, J.; Gao, Z.; Yang, Z.; Li, Y.; Liu, H.; Fan, T. Long Non-Coding RNAs: The Regulatory Mechanisms, Research Strategies, and Future Directions in Cancers. Front. Oncol. 2020, 10, 598817. [Google Scholar] [CrossRef]
- Wang, B.; Huang, Z.; Gao, R.; Zeng, Z.; Yang, W.; Sun, Y.; Wei, W.; Wu, Z.; Yu, L.; Li, Q.; et al. Expression of Long Noncoding RNA Urothelial Cancer Associated 1 Promotes Cisplatin Resistance in Cervical Cancer. Cancer Biother. Radiopharm. 2017, 32, 101–110, Erratum in Cancer Biother. Radiopharm. 2017, 32, 147. [Google Scholar] [CrossRef]
- Liu, E.; Liu, Z.; Zhou, Y.; Mi, R.; Wang, D. Overexpression of Long Non-Coding RNA PVT1 in Ovarian Cancer Cells Promotes Cisplatin Resistance by Regulating Apoptotic Pathways. Int. J. Clin. Exp. Med. 2015, 8, 20565–20572. [Google Scholar]
- Li, L.; Gu, M.; You, B.; Shi, S.; Shan, Y.; Bao, L.; You, Y. Long Non-Coding RNA ROR Promotes Proliferation, Migration and Chemoresistance of Nasopharyngeal Carcinoma. Cancer Sci. 2016, 107, 1215–1222. [Google Scholar] [CrossRef]
- Luo, C.; Wei, L.; Qian, F.; Bo, L.; Gao, S.; Yang, G.; Mao, C. LncRNA HOTAIR Regulates Autophagy and Proliferation Mechanisms in Premature Ovarian Insufficiency through the MiR-148b-3p/ATG14 Axis. Cell Death Discov. 2024, 10, 44. [Google Scholar] [CrossRef]
- Zhou, X.; Jiang, K.; Luo, H.; Wu, C.; Yu, W.; Cheng, F. Novel LncRNA XLOC_032768 Alleviates Cisplatin-Induced Apoptosis and Inflammatory Response of Renal Tubular Epithelial Cells through TNF-α. Int. Immunopharmacol. 2020, 83, 106472. [Google Scholar] [CrossRef]
- Li, J.; Fan, X.; Wang, Q.; Gong, Y.; Guo, L. Long Noncoding RNA PRNCR1 Reduces Renal Epithelial Cell Apoptosis in Cisplatin-Induced AKI by Regulating MiR-182-5p/EZH1. Kidney Blood Press. Res. 2021, 46, 162–172. [Google Scholar] [CrossRef]
- Chang, S.; Chang, M.; Liu, G.; Xu, D.; Wang, H.; Sun, R.; Feng, M. LncRNA OIP5-AS1 Reduces Renal Epithelial Cell Apoptosis in Cisplatin-Induced AKI by Regulating the MiR-144-5p/PKM2 Axis. Biomed. J. 2022, 45, 642–653. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, S. Long Non-Coding RNA GAS5 Aggravate Renal Epithelial Cell Apoptosis in Cisplatin-Induced AKI by Regulating MiR-205-5p. arXiv 2021. [Google Scholar] [CrossRef]
- Loren, P.; Saavedra, N.; Saavedra, K.; Zambrano, T.; Moriel, P.; Salazar, L.A. Epigenetic Mechanisms Involved in Cisplatin-Induced Nephrotoxicity: An Update. Pharmaceuticals 2021, 14, 491. [Google Scholar] [CrossRef]
- Naimi, M.S.A.; Rasheed, H.A.; Hussien, N.R.; Al-Kuraishy, H.M.; Al-Gareeb, A.I. Nephrotoxicity: Role and Significance of Renal Biomarkers in the Early Detection of Acute Renal Injury. J. Adv. Pharm. Technol. Res. 2019, 10, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, J.M. A Review of Complex in Vitro Cell Culture Stressing the Importance of Fluid Flow and Illustrated by Organ on a Chip Liver Models. Front. Toxicol. 2023, 5, 1170193. [Google Scholar] [CrossRef] [PubMed]
- Ryan, M.J.; Johnson, G.; Kirk, J.; Fuerstenberg, S.M.; Zager, R.A.; Torok-Storb, B. HK-2: An Immortalized Proximal Tubule Epithelial Cell Line from Normal Adult Human Kidney. Kidney Int. 1994, 45, 48–57. [Google Scholar] [CrossRef]
- Faria, J.; Ahmed, S.; Gerritsen, K.G.F.; Mihaila, S.M.; Masereeuw, R. Kidney-Based in Vitro Models for Drug-Induced Toxicity Testing. Arch. Toxicol. 2019, 93, 3397–3418. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Han, J.; Li, H.; Zhang, X.; Liu, L.L.; Chen, F.; Zeng, B. Human Embryonic Kidney 293 Cells: A Vehicle for Biopharmaceutical Manufacturing, Structural Biology, and Electrophysiology. Cells Tissues Organs 2018, 205, 1–8. [Google Scholar] [CrossRef]
- Singh, M.P.; Chauhan, A.K.; Kang, S.C. Morin Hydrate Ameliorates Cisplatin-Induced ER Stress, Inflammation and Autophagy in HEK-293 Cells and Mice Kidney via PARP-1 Regulation. Int. Immunopharmacol. 2018, 56, 156–167. [Google Scholar] [CrossRef]
- Nazari, A.; Mirian, M.; Aghaei, M.; Aliomrani, M. 4-Hydroxyhalcone Effects on Cisplatin-Induced Genotoxicity Model. Toxicol. Res. 2021, 10, 11–17. [Google Scholar] [CrossRef]
- Hu, J.N.; Xu, X.Y.; Jiang, S.; Liu, Y.; Liu, Z.; Wang, Y.P.; Gong, X.J.; Li, K.K.; Ren, S.; Li, W. Protective Effect of Ginsenoside Rk1, a Major Rare Saponin from Black Ginseng, on Cisplatin-Induced Nephrotoxicity in HEK-293 Cells. Kaohsiung J. Med. Sci. 2020, 36, 732–740. [Google Scholar] [CrossRef]
- Hu, J.N.; Leng, J.; Shen, Q.; Liu, Y.; Li, X.D.; Wang, S.H.; Li, H.P.; Wang, Z.; Wang, Y.P.; Li, W. Platycodin D Suppresses Cisplatin-Induced Cytotoxicity by Suppressing ROS-Mediated Oxidative Damage, Apoptosis, and Inflammation in HEK-293 Cells. J. Biochem. Mol. Toxicol. 2021, 35, e22624. [Google Scholar] [CrossRef]
- Zhou, Y.D.; Hou, J.G.; Yang, G.; Jiang, S.; Chen, C.; Wang, Z.; Liu, Y.Y.; Ren, S.; Li, W. Icariin Ameliorates Cisplatin-Induced Cytotoxicity in Human Embryonic Kidney 293 Cells by Suppressing ROS-Mediated PI3K/Akt Pathway. Biomed. Pharmacother. 2019, 109, 2309–2317. [Google Scholar] [CrossRef]
- Song, M.; Kim, M.; Hoang, D.H.; Kovale, L.M.; Lee, J.; Kim, Y.; Lee, C.; Hong, J.; Park, S.; Choe, W.; et al. A Hydrodistillate of Gynostemma Pentaphyllum and Damulin B Prevent Cisplatin-Induced Nephrotoxicity In Vitro and In Vivo via Regulation of AMPKα1 Transcription. Nutrients 2022, 14, 4997. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Xu, Y.; Weng, Y.; Fan, X.; Bai, Y.; Zheng, X.; Lou, L.J.; Zhang, F. Astilbin Ameliorates Cisplatin-Induced Nephrotoxicity through Reducing Oxidative stress and Inflammation. Food Chem. Toxicol. 2018, 114, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Wang, H.; Weng, C.; Jiang, H.; Chen, J. Caspase 3/GSDME-Dependent Pyroptosis Contributes to Chemotherapy Drug-Induced Nephrotoxicity. Cell Death Dis. 2021, 12, 186. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Yu, P.; Wang, T.T.; Du, Y.W.; Chen, Y.; Li, Z.; He, M.L.; Feng, L.; Li, H.R.; Han, X.; et al. Polydatin Attenuates Cisplatin-Induced Acute Kidney Injury by Inhibiting Ferroptosis. Oxid. Med. Cell. Longev. 2022, 2022, 9947191. [Google Scholar] [CrossRef]
- Chen, X.; Wei, W.; Li, Y.; Huang, J.; Ci, X. Hesperetin Relieves Cisplatin-Induced Acute Kidney Injury by Mitigating Oxidative Stress, Inflammation and Apoptosis. Chem. Biol. Interact. 2019, 308, 269–278. [Google Scholar] [CrossRef]
- Yang, Q.; Qian, L.; Zhang, S. Ginsenoside Rh1 Alleviates HK-2 Apoptosis by Inhibiting ROS and the JNK/P53 Pathways. Evid. Based Complement. Altern. Med. 2020, 2020, 3401067. [Google Scholar] [CrossRef]
- Nho, J.H.; Jung, H.K.; Lee, M.J.; Jang, J.H.; Sim, M.O.; Jeong, D.E.; Cho, H.W.; Kim, J.C. Beneficial Effects of Cynaroside on Cisplatin-Induced Kidney Injury in Vitro and in Vivo. Toxicol. Res. 2018, 34, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Li, Y.; Wu, C.; Yu, W.; Cheng, F. Novel LncRNA XLOC_032768 Protects against Renal Tubular Epithelial Cells Apoptosis in Renal Ischemia–Reperfusion Injury by Regulating FNDC3B/TGF-Β1. Ren. Fail. 2020, 42, 994–1003. [Google Scholar] [CrossRef]
- Jiang, H.; Hong, Y.; Fan, G. Bismuth Reduces Cisplatin-Induced Nephrotoxicity Via Enhancing Glutathione Conjugation and Vesicular Transport. Front. Pharmacol. 2022, 13, 887876. [Google Scholar] [CrossRef] [PubMed]
- Dachuri, V.; Song, P.H.; Ku, S.K.; Song, C.H. Protective Effects of Traditional Herbal Formulas on Cisplatin-Induced Nephrotoxicity in Renal Epithelial Cells via Antioxidant and Antiapoptotic Properties. Evid. Based Complement. Altern. Med. 2020, 2020, 5807484. [Google Scholar] [CrossRef]
- Ju, S.M.; Kang, J.G.; Bae, J.S.; Pae, H.O.; Lyu, Y.S.; Jeon, B.H. The Flavonoid Apigenin Ameliorates Cisplatin-Induced Nephrotoxicity through Reduction of P53 Activation and Promotion of PI3K/Akt Pathway in Human Renal Proximal Tubular Epithelial Cells. Evid. Based Complement. Altern. Med. 2015, 2015, 186436. [Google Scholar] [CrossRef] [PubMed]
- Pabla, N.; Dong, Z. Cisplatin Nephrotoxicity: Mechanisms and Renoprotective Strategies. Kidney Int. 2008, 73, 994–1007. [Google Scholar] [CrossRef]
- Manohar, S.; Leung, N. Cisplatin Nephrotoxicity: A Review of the Literature. J. Nephrol. 2018, 31, 15–25. [Google Scholar] [CrossRef]
- Ciarimboli, G.; Ludwig, T.; Lang, D.; Pavenstädt, H.; Koepsell, H.; Piechota, H.J.; Haier, J.; Jaehde, U.; Zisowsky, J.; Schlatter, E. Cisplatin Nephrotoxicity Is Critically Mediated via the Human Organic Cation Transporter 2. Am. J. Pathol. 2005, 167, 1477–1484. [Google Scholar] [CrossRef]
- Filipski, K.K.; Mathijssen, R.H.; Mikkelsen, T.S.; Schinkel, A.H.; Sparreboom, A. Contribution of Organic Cation Transporter 2 (OCT2) to Cisplatin-Induced Nephrotoxicity. Clin. Pharmacol. Ther. 2009, 86, 396–402. [Google Scholar] [CrossRef]
- Aggarwal, A.; Dinda, A.K.; Mukhopadhyay, C.K. Effect of Cisplatin on Renal Iron Homeostasis Components: Implication in Nephropathy. ACS Omega 2022, 7, 27804–27817. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Li, Y.; Ma, Y.; Jin, Y.; Gou, X.; Yuan, Y.; Xu, F.; Wu, X. The Toxicity of Cisplatin Derives from Effects on Renal Organic Ion Transporters Expression and Serum Endogenous Substance Levels. Food Chem. Toxicol. 2024, 192, 114949. [Google Scholar] [CrossRef] [PubMed]
- Becerir, T.; Tokgün, O.; Yuksel, S. The Therapeutic Effect of Cilastatin on Drug-Induced Nephrotoxicity: A New Perspective. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 5436–5447. [Google Scholar] [CrossRef] [PubMed]
- Havasi, A.; Borkan, S.C. Apoptosis and Acute Kidney Injury. Kidney Int. 2011, 80, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Sanz, A.B.; Sanchez-Niño, M.D.; Ramos, A.M.; Ortiz, A. Regulated Cell Death Pathways in Kidney Disease. Nat. Rev. Nephrol. 2023, 19, 281–299. [Google Scholar] [CrossRef]
- Carvalho Rodrigues, M.A.; Gobe, G.; Santos, N.A.G.; Santos, A.C. Carvedilol Protects against Apoptotic Cell Death Induced by Cisplatin in Renal Tubular Epithelial Cells. J. Toxicol. Environ. Health Part A 2012, 75, 981–990. [Google Scholar] [CrossRef]
- Kimura, H.; Kamiyama, K.; Imamoto, T.; Takeda, I.; Kobayashi, M.; Takahashi, N.; Kasuno, K.; Sugaya, T.; Iwano, M. Dichloroacetate Reduces Cisplatin-Induced Apoptosis by Inhibiting the JNK/14-3-3/Bax/Caspase-9 Pathway and Suppressing Caspase-8 Activation via CFLIP in Murine Tubular Cells. Sci. Rep. 2024, 14, 24307. [Google Scholar] [CrossRef]
- Kaushal, G.P.; Kaushal, V.; Hong, X.; Shah, S.V. Role and Regulation of Activation of Caspases in Cisplatin-Induced Injury to Renal Tubular Epithelial Cells. Kidney Int. 2001, 60, 1726–1736. [Google Scholar] [CrossRef]
- Jiang, M.; Wang, C.Y.; Huang, S.; Yang, T.; Dong, Z. Cisplatin-Induced Apoptosis in P53-Deficient Renal Cells via the Intrinsic Mitochondrial Pathway. Am. J. Physiol. Ren. Physiol. 2009, 296, 983–993. [Google Scholar] [CrossRef]
- Lakhani, S.A.; Masud, A.; Kuida, K.; Porter, G.A., Jr.; Booth, C.J.; Mehal, W.Z.; Inayat, I.; Flavell, R.A. Caspases 3 and 7: Key Mediators of Mitochondrial Events of Apoptosis. Science 2006, 311, 847–851. [Google Scholar] [CrossRef]
- Jiang, M.; Yi, X.; Hsu, S.; Wang, C.Y.; Dong, Z. Role of P53 in Cisplatin-Induced Tubular Cell Apoptosis: Dependence on P53 Transcriptional Activity. Am. J. Physiol. Ren. Physiol. 2004, 287, 1140–1147. [Google Scholar] [CrossRef]
- Yang, C.; Kaushal, V.; Haun, R.S.; Seth, R.; Shah, S.V.; Kaushal, G.P. Transcriptional Activation of Caspase-6 and -7 Genes by Cisplatin-Induced P53 and Its Functional Significance in Cisplatin Nephrotoxicity. Cell Death Differ. 2008, 15, 530–544. [Google Scholar] [CrossRef]
- Liu, H.; Baliga, R. Endoplasmic Reticulum Stress-Associated Caspase 12 Mediates Cisplatin-Induced LLC-PK1 Cell Apoptosis. J. Am. Soc. Nephrol. 2005, 16, 1985–1992. [Google Scholar] [CrossRef]
- Cummings, B.S.; Mchowat, J.; Schnellmann, R.G. Role of an Endoplasmic Reticulum Ca2+-Independent Phospholipase A2 in Cisplatin-Induced Renal Cell Apoptosis. J. Pharmacol. Exp. Ther. 2004, 308, 921–928. [Google Scholar] [CrossRef] [PubMed]
- Periyasamy-thandavan, S.; Jiang, M.; Wei, Q.; Smith, R.; Yin, X.; Dong, Z. Autophagy Is Cytoprotective during Cisplatin Injury of Renal Proximal Tubular Cells. Kidney Int. 2008, 74, 631–640. [Google Scholar] [CrossRef]
- Yang, C.; Kaushal, V.; Shah, S.V.; Kaushal, G.P. Autophagy Is Associated with Apoptosis in Cisplatin Injury to Renal Tubular Epithelial Cells. Am. J. Physiol.-Ren. Physiol. 2008, 294, F777–F787. [Google Scholar] [CrossRef] [PubMed]
- Kaushal, G.P.; Kaushal, V.; Herzog, C.; Yang, C. Autophagy Delays Apoptosis in Renal Tubular Epithelial Cells in Cisplatin Cytotoxicity. Autophagy 2008, 4, 710–712. [Google Scholar] [CrossRef]
- Klionsky, D.J.; Abdel-Aziz, A.K.; Abdelfatah, S.; Abdellatif, M.; Abdoli, A.; Abel, S.; Abeliovich, H.; Abildgaard, M.H.; Abudu, Y.P.; Acevedo-Arozena, A.; et al. Guidelines for the Use and Interpretation of Assays for Monitoring Autophagy (4th Edition). Autophagy 2021, 17, 1–382. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, Z.; Shu, S.; Cai, J.; Tang, C.; Dong, Z. AMPK/MTOR Signaling in Autophagy Regulation During Cisplatin-Induced Acute Kidney Injury. Front. Physiol. 2020, 11, 619730. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, C.; Cai, J.; Chen, G.; Zhang, D.; Zhang, Z.; Dong, Z. PINK1/Parkin-Mediated Mitophagy Is Activated in Cisplatin Nephrotoxicity to Protect against Kidney Injury. Cell Death Dis. 2018, 9, 1113. [Google Scholar] [CrossRef]
- Lin, Q.; Li, S.; Jiang, N.; Shao, X.; Zhang, M.; Jin, H.; Zhang, Z.; Shen, J.; Zhou, Y.; Zhou, W.; et al. PINK1-Parkin Pathway of Mitophagy Protects against Contrast-Induced Acute Kidney Injury via Decreasing Mitochondrial ROS and NLRP3 Inflammasome Activation. Redox Biol. 2019, 26, 101254. [Google Scholar] [CrossRef]
- Marquez, R.T.; Xu, L. Bcl-2:Beclin 1 Complex: Multiple, Mechanisms Regulating Autophagy/Apoptosis Toggle Switch. Am. J. Cancer Res. 2012, 2, 214–221. [Google Scholar]
- Peng, H.; Lydia, M.; Liu, Z.; Wang, T.; Li, H.; Zhang, X.; Liu, G.; Ren, X. LncRNA UCA1 Regulates Immune Micro-Environment in Cisplatin-Induced AKI by MiRNA-4498/AKT3 Pathway. PLoS ONE 2025, 20, e0314654. [Google Scholar] [CrossRef]
- Yu, R.; Zhang, Y.; Lu, Z.; Li, J.; Shi, P.; Li, J. Long-Chain Non-Coding RNA UCA1 Inhibits Renal Tubular Epithelial Cell Apoptosis by Targeting MicroRNA-206 in Diabetic Nephropathy. Arch. Physiol. Biochem. 2022, 128, 231–239. [Google Scholar] [CrossRef]
- Shen, J.; Zhang, J.; Jiang, X.; Wang, H.; Pan, G. LncRNA HOX Transcript Antisense RNA Accelerated Kidney Injury Induced by Urine-Derived Sepsis through the MiR-22/High Mobility Group Box 1 Pathway. Life Sci. 2018, 210, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Qiu, Z.Z.; Yu, Z.H.; Gao, L.; He, J.M.; Zhang, Z.W.; Zheng, J. Paeonol Reverses Promoting Effect of the HOTAIR/MiR-124/Notch1 Axis on Renal Interstitial Fibrosis in a Rat Model. J. Cell Physiol. 2019, 234, 14351–14363. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Yang, T.; Zou, Y.; He, M.; Li, Q.; Chen, X.; Zhong, A. LncRNA HOX Transcript Antisense RNA Mediates Hyperglycemic-Induced Injury in the Renal Tubular Epithelial Cell via the MiR-126-5pAkt Axis. Aging Med. 2023, 6, 427–434. [Google Scholar] [CrossRef]
- Majumder, S.; Hadden, M.J.; Thieme, K.; Batchu, S.N.; Niveditha, D.; Chowdhury, S.; Yerra, V.G.; Advani, S.L.; Bowskill, B.B.; Liu, Y.; et al. Dysregulated Expression but Redundant Function of the Long Non-Coding RNA HOTAIR in Diabetic Kidney Disease. Diabetologia 2019, 62, 2129–2142. [Google Scholar] [CrossRef]
- Xue, Q.; Yang, L.; Wang, J.; Li, L.; Wang, H.; He, Y. LncRNA ROR and MiR-125b Predict the Prognosis in Heart Failure Combined Acute Renal Failure. Dis. Markers 2022, 2022, 6853939. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Zhang, W.; Tian, H.; Zhang, Q.; Men, T. LncRNA ROR Promotes the Proliferation of Renal Cancer and Is Negatively Associated with Favorable Prognosis. Mol. Med. Rep. 2017, 16, 9561–9566. [Google Scholar] [CrossRef]
- Hou, P.; Zhao, Y.; Li, Z.; Yao, R.; Ma, M.; Gao, Y.; Zhao, L.; Zhang, Y.; Huang, B.; Lu, J. LincRNA-ROR Induces Epithelial-to-Mesenchymal Transition and Contributes to Breast Cancer Tumorigenesis and Metastasis. Cell Death Dis. 2014, 5, e1287, Erratum in Cell Death Dis. 2025, 16, 340. [Google Scholar] [CrossRef]
- Deng, L.Q.; Li, S.Y.; Xie, T.; Zeng, W.Q.; Wang, Y.Y.; Shi, C.J.; Zhang, J.F. LincROR Promotes Tumor Growth of Colorectal Cancer through the MiR-145/WNT2B/WNT10A/Wnt/β-Catenin Regulatory Axis. PLoS ONE 2024, 19, e0312417. [Google Scholar] [CrossRef]
- Fu, S.; Hu, X.; Ma, Z.; Wei, Q.; Xiang, X.; Li, S.; Wen, L.; Liang, Y.; Dong, Z. P53 in Proximal Tubules Mediates Chronic Kidney Problems after Cisplatin Treatment. Cells 2022, 11, 712. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wang, B.; Ma, L.; Fu, P. An Update of Long-Noncoding RNAs in Acute Kidney Injury. Front. Physiol. 2022, 13, 849403. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Yang, Z.; Shi, L.; Zhao, L.; Liu, K.; Tang, N. The Role of LncRNAs in AKI and CKD: Molecular Mechanisms, Biomarkers, and Potential Therapeutic Targets. Genes. Dis. 2025, 12, 101509. [Google Scholar] [CrossRef]
- Fu, J.X.; Sun, G.; Wang, H.; Jiang, H. LncRNA OIP5-AS1 Induces Epithelial-to-Mesenchymal Transition and Renal Fibrosis in Diabetic Nephropathy via Binding to MiR-30c-5p. J. Biol. Regul. Homeost. Agents 2020, 34, 961–968. [Google Scholar] [CrossRef]
- Li, J.; Dong, Z.; Tang, L.; Liu, L.; Su, C.; Yu, S. LncRNA OIP5-AS1/MiR-186-5p/NLRP3 Axis Contributes to Sepsis-Induced Kidney Injury Through Enhancing NLRP3 Inflammasome Activation. J. Biochem. Mol. Toxicol. 2025, 39, e70305. [Google Scholar] [CrossRef]
- Geng, X.; Song, N.; Zhao, S.; Xu, J.; Liu, Y.; Fang, Y.; Liang, M.; Xu, X.; Ding, X. LncRNA GAS5 Promotes Apoptosis as a Competing Endogenous RNA for MiR-21 via Thrombospondin 1 in Ischemic AKI. Cell Death Discov. 2020, 6, 19. [Google Scholar] [CrossRef]
- Xie, C.; Wu, W.; Tang, A.; Luo, N.; Tan, Y. LncRNA GAS5/MiR-452-5p Reduces Oxidative Stress and Pyroptosis of High-Glucose-Stimulated Renal Tubular Cells. Diabetes Metab. Syndr. Obes. 2019, 12, 2609–2617. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.; Xu, B.; Xu, W.; Xia, L.; Xu, Z.; Shen, L.; Peng, W.; Huang, S. Long Noncoding RNA GAS5 Inhibits Cell Proliferation and Fibrosis in Diabetic Nephropathy by Sponging MiR-221 and Modulating SIRT1 Expression. Aging 2019, 11, 8745–8759. [Google Scholar] [CrossRef]
- Zhang, Y.-Y.; Tan, R.-Z.; Yu, Y.; Niu, Y.-Y.; Yu, C. LncRNA GAS5 Protects against TGF-β-Induced Renal Fibrosis via the Smad3/MiRNA-142-5p Axis. Am. J. Physiol.-Ren. Physiol. 2021, 321, F517–F526. [Google Scholar] [CrossRef]
- Guo, Y.; Li, G.; Gao, L.; Cheng, X.; Wang, L.; Qin, Y.; Zhang, D. Exaggerated Renal Fibrosis in LncRNA Gas5-Deficient Mice after Unilateral Ureteric Obstruction. Life Sci. 2021, 264, 118656. [Google Scholar] [CrossRef] [PubMed]
- Ling, H.; Li, Q.; Duan, Z.P.; Wang, Y.J.; Hu, B.Q.; Dai, X.G. LncRNA GAS5 Inhibits MiR-579-3p to Activate SIRT1/PGC-1a/Nrf2 Signaling Pathway to Reduce Cell Pyroptosis in Sepsis-Associated Renal Injury. Am. J. Physiol. Cell Physiol. 2021, 321, C117–C133. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Jin, F.Y.; Xia, R.; Kong, R.; Li, J.H.; Xu, T.P.; Liu, Y.W.; Zhang, E.B.; Liu, X.H.; De, W. Decreased Expression of Long Noncoding RNA GAS5 Indicates a Poor Prognosis and Promotes Cell Proliferation in Gastric Cancer. BMC Cancer 2014, 14, 319. [Google Scholar] [CrossRef] [PubMed]
- Frank, F.; Kavousi, N.; Bountali, A.; Dammer, E.B.; Mourtada-Maarabouni, M.; Ortlund, E.A. The LncRNA Growth Arrest Specific 5 Regulates Cell Survival via Distinct Structural Modules with Independent Functions. Cell Rep. 2020, 32, 107933. [Google Scholar] [CrossRef]
- Liu, D.W.; Zhang, J.H.; Liu, F.X.; Wang, X.T.; Pan, S.K.; Jiang, D.K.; Zhao, Z.H.; Liu, Z.S. Silencing of Long Noncoding RNA PVT1 Inhibits Podocyte Damage and Apoptosis in Diabetic Nephropathy by Upregulating FOXA1. Exp. Mol. Med. 2019, 51, 1–15. [Google Scholar] [CrossRef]
- Dieter, C.; Lemos, N.E.; Girardi, E.; Ramos, D.T.; Pellenz, F.M.; Canani, L.H.; Assmann, T.S.; Crispim, D. The Rs3931283/PVT1 and Rs7158663/MEG3 Polymorphisms Are Associated with Diabetic Kidney Disease and Markers of Renal Function in Patients with Type 2 Diabetes Mellitus. Mol. Biol. Rep. 2023, 50, 2159–2169. [Google Scholar] [CrossRef]
- Hanson, R.L.; Craig, D.W.; Millis, M.P.; Yeatts, K.A.; Kobes, S.; Pearson, J.V.; Lee, A.M.; Knowler, W.C.; Nelson, R.G.; Wolford, J.K. Identification of PVT1 as a Candidate Gene for End-Stage Renal Disease in Type 2 Diabetes Using a Pooling-Based Genome-Wide Single Nucleotide Polymorphism Association Study. Diabetes 2007, 56, 975–983. [Google Scholar] [CrossRef]
- Chang, Y.; Liu, C.; Wang, J.; Feng, J.; Chen, Y.; Qi, M.; Guo, Y. Downregulation of LncRNA-PVT1 Participates in the Development of Progressive Chronic Kidney Disease among Patients with Congestive Heart Failure. J. Inflamm. 2021, 18, 27. [Google Scholar] [CrossRef]
- Yang, Q.; Sun, Q.; Jin, P. Long Non-Coding RNA PVT1 Regulates LPS-Induced Acute Kidney Injury in an in Vitro Model of HK-2 Cells by Modulating the MiR-27a-3p/OXSR1 Axis. Exp. Ther. Med. 2022, 24, 552. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Lu, Y.; Wang, Y.; Wang, X.; Kang, X.; Tang, W.; Chen, L.S.E. Long Noncoding RNA Urothelial Carcinoma Associated 1 Protects Human Placental Vascular Endothelial Cells from Hypoxia-Induced Damage by Regulating the MiR-197-3p/Histone Deacetylase-2 Axis in Patients with Pregnancy-Induced Hypertension. Am. J. Transl. Res. 2022, 14, 6137–6149. [Google Scholar] [PubMed]
- Zhang, S.; Zhang, G.; Liu, J. Long Noncoding RNA PVT1 Promotes Cervical Cancer Progression through Epigenetically Silencing MiR-200b. Apmis 2016, 124, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Shi, Y.; Chen, L.; Jiang, Y.; Mao, C.; Yan, B.; Liu, S.; Shan, B.; Tao, Y.; Wang, X. The Ratio of FoxA1 to FoxA2 in Lung Adenocarcinoma Is Regulated by LncRNA HOTAIR and Chromatin Remodeling Factor LSH. Sci. Rep. 2015, 5, 17826. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhu, Z.; Watabe, K.; Zhang, X.; Bai, C.; Xu, M.; Wu, F.; Mo, Y.Y. Negative Regulation of LncRNA GAS5 by MiR-21. Cell Death Differ. 2013, 20, 1558–1568. [Google Scholar] [CrossRef]
- Deng, J.; Deng, H.; Liu, C.; Liang, Y.; Wang, S. Long Non-Coding RNA OIP5-AS1 Functions as an Oncogene in Lung Adenocarcinoma through Targeting MiR-448/Bcl-2. Biomed. Pharmacother. 2018, 98, 102–110. [Google Scholar] [CrossRef]
- Paez, I.; Prado, Y.; Ubilla, C.G.; Zambrano, T.; Salazar, L.A. Atorvastatin Increases the Expression of Long Non-Coding RNAs ARSR and CHROME in Hypercholesterolemic Patients: A Pilot Study. Pharmaceuticals 2020, 13, 382. [Google Scholar] [CrossRef]
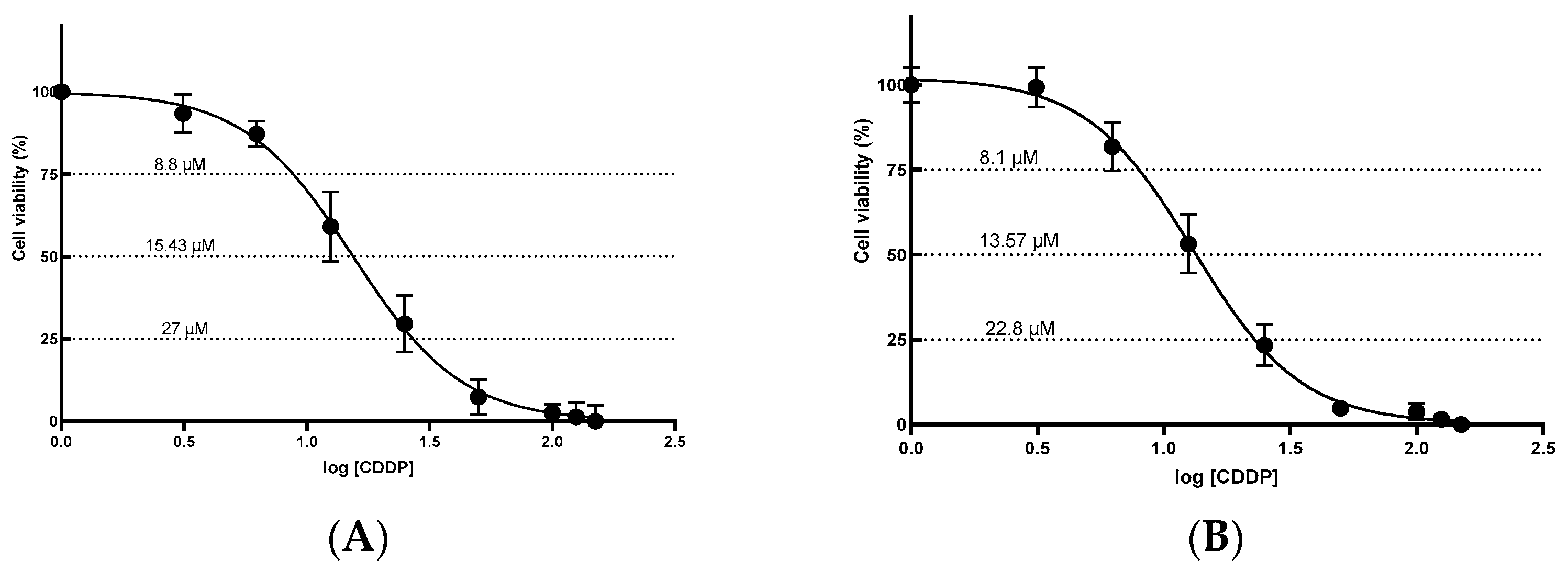
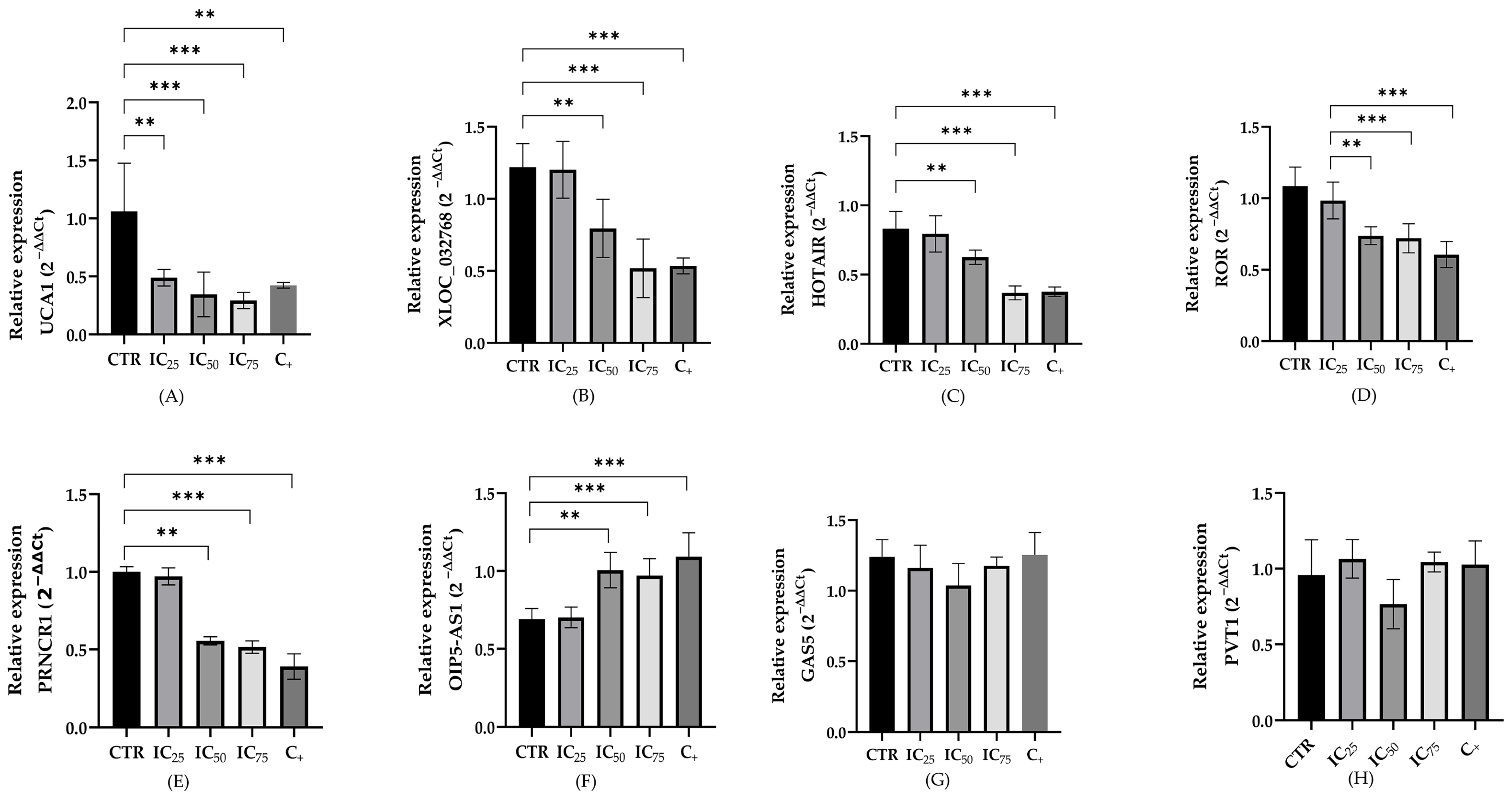
| Cell Line | IC25 | IC50 | IC75 | R2 |
|---|---|---|---|---|
| HEK-293 | 8.8 ± 1.08 | 15.43 ± 1.08 | 27.0 ± 1.03 | 0.9804 |
| HK-2 | 8.1 ± 0.86 | 13.57 ± 0.69 | 22.8 ± 0.70 | 0.9876 |
| lncRNA | IC25 | IC50 | IC75 | DMSO 10% |
|---|---|---|---|---|
| UCA1 | - | ↓ | ↓ | ↓ |
| XLOC_032768 | - | ↓ | ↓ | ↓ |
| HOTAIR | - | ↓ | ↓ | ↓ |
| LINC-ROR | - | ↓ | ↓ | ↓ |
| PRNCR1 | - | ↓ | ↓ | ↓ |
| OIP5-AS1 | - | ↑ | ↑ | ↑ |
| GAS5 | - | - | - | - |
| PVT1 | - | - | - | - |
| LncRNA | Forward Primer (5′-3′) | Reverse Primer (5′-3′) | Reference |
|---|---|---|---|
| LINC-ROR | CTCCAGCTATGCAGACCACTC | GTGACGCCTGACCTGTTGAC | [103] |
| PVT1 | ATAGATCCTGCCCTGTTTGC | CATTTCCTGCTGCCGTTTTC | [104] |
| UCA1 | CTCTCCATTGGGTTCACCATTC | GCGGCAGGTCTTAAGAGATGAG | [19] |
| HOTAIR | GCAGTGGAATGGAACGGATT | CGTGGCATTTCTGGTCTTGTA | [105] |
| PRNCR1 | CCAGATTCCAAGGGCTGATA | GATGTTTGGAGGCATCTGGT | [24] |
| GAS5 | CTTCTGGGCTCAAGTGATCCT | TTGTGCCATGAGACTCCATCAG | [106] |
| OIP5-AS1 | TGCGAAGATGGCGGAGTAAG | TAGTTCCTCTCCTCTGGCCG | [107] |
| XLOC_032768 | CATTGCCGACAGCACAACATAC | GCCTATTTAGCAGCCCACCTC | [45] |
| U6 | CTCGCTTCGGCAGCACATATAC | GGAACGCTTCACGAATTTGC | [108] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lugones, Y.; Loren, P.; Matus, C.E.; Rodriguez, N.M.; Leal-Rojas, P.; San Martín, R.; Saavedra, K.; Saavedra, N.; Moriel, P.; Salazar, L.A. Dysregulated lncRNAs in Cisplatin-Induced Nephrotoxicity and Their Association with Apoptosis and Autophagy: An Exploratory In Vitro Study. Int. J. Mol. Sci. 2025, 26, 11201. https://doi.org/10.3390/ijms262211201
Lugones Y, Loren P, Matus CE, Rodriguez NM, Leal-Rojas P, San Martín R, Saavedra K, Saavedra N, Moriel P, Salazar LA. Dysregulated lncRNAs in Cisplatin-Induced Nephrotoxicity and Their Association with Apoptosis and Autophagy: An Exploratory In Vitro Study. International Journal of Molecular Sciences. 2025; 26(22):11201. https://doi.org/10.3390/ijms262211201
Chicago/Turabian StyleLugones, Yuliannis, Pía Loren, Carola E. Matus, Nelia M. Rodriguez, Pamela Leal-Rojas, Rody San Martín, Kathleen Saavedra, Nicolás Saavedra, Patricia Moriel, and Luis A. Salazar. 2025. "Dysregulated lncRNAs in Cisplatin-Induced Nephrotoxicity and Their Association with Apoptosis and Autophagy: An Exploratory In Vitro Study" International Journal of Molecular Sciences 26, no. 22: 11201. https://doi.org/10.3390/ijms262211201
APA StyleLugones, Y., Loren, P., Matus, C. E., Rodriguez, N. M., Leal-Rojas, P., San Martín, R., Saavedra, K., Saavedra, N., Moriel, P., & Salazar, L. A. (2025). Dysregulated lncRNAs in Cisplatin-Induced Nephrotoxicity and Their Association with Apoptosis and Autophagy: An Exploratory In Vitro Study. International Journal of Molecular Sciences, 26(22), 11201. https://doi.org/10.3390/ijms262211201








