Synovial Fluid and Serum MicroRNA Signatures in Equine Osteoarthritis
Abstract
1. Introduction
2. Results
2.1. Stage 1: Exploration Stage
2.1.1. Sample and Group Characterization in the Sequencing Cohort
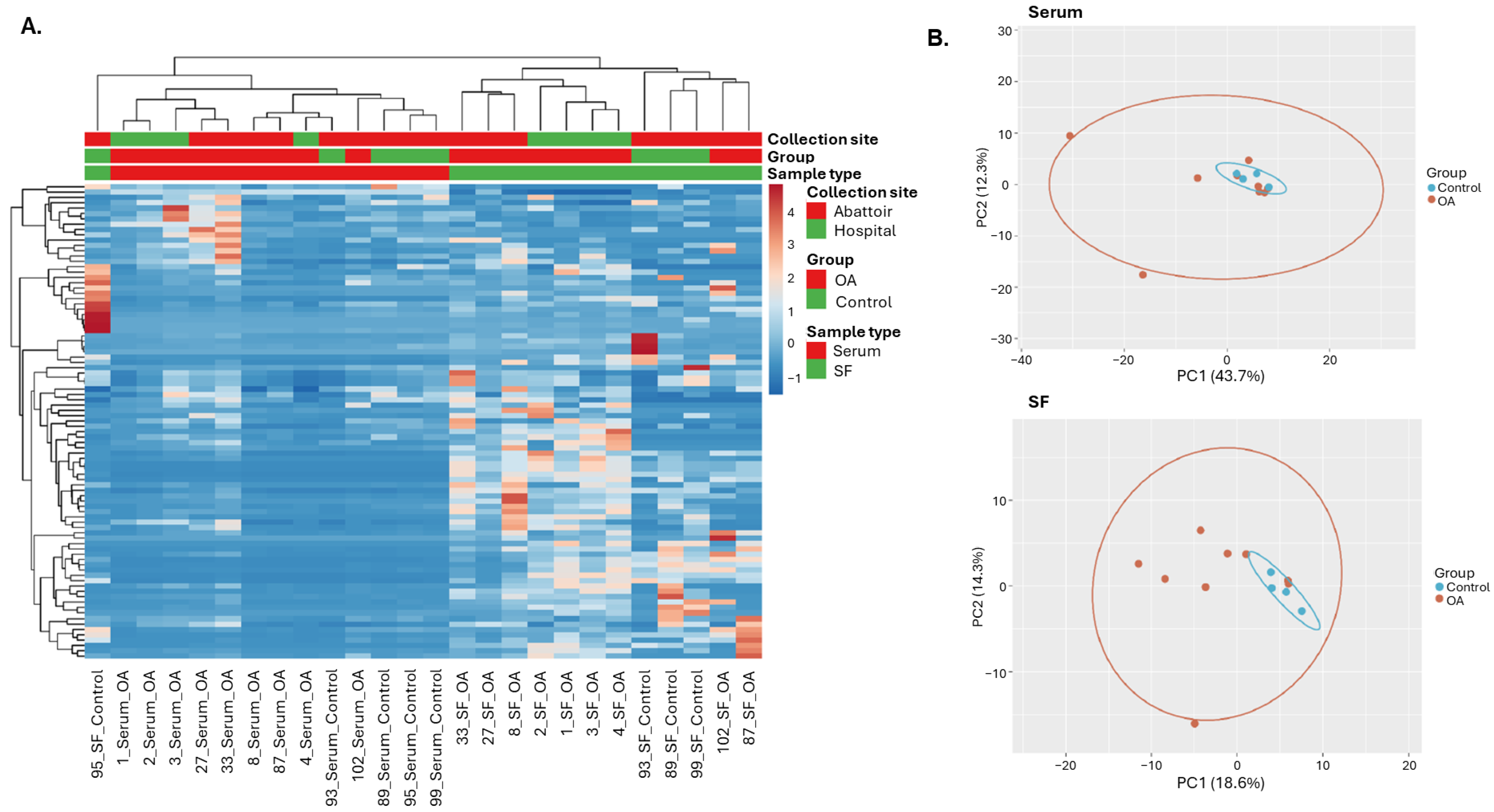
2.1.2. Sequencing Data Overview
2.1.3. Differential Expression Analysis
2.1.4. Target Prediction and Pathway Analysis
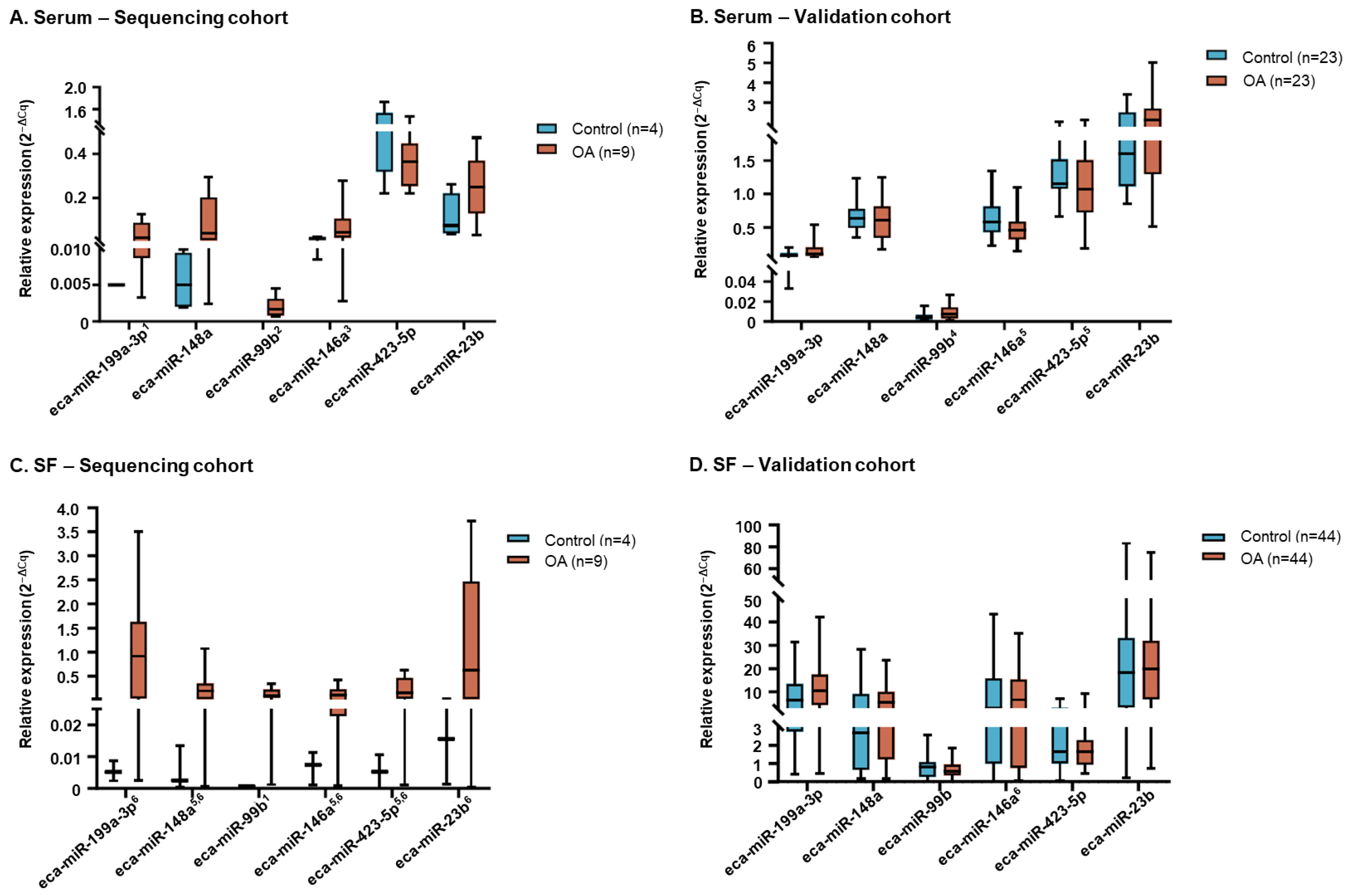
2.1.5. Candidate Biomarker miRNAs
2.2. Stage 2: Validation Stage
2.2.1. Sample and Group Characterization in the Validation Cohort
2.2.2. miRNA Expression Analysis
2.2.3. Influence of Clinical Variables in miRNA Expression
3. Discussion
4. Methods
4.1. Sample Collection
4.1.1. Sequencing Cohort
4.1.2. Validation Cohort
4.1.3. Ethical Considerations
4.2. Group Allocation
4.2.1. Sequencing Cohort
4.2.2. Validation Cohort
4.3. Demographics
4.4. Small RNA Sequencing
4.4.1. RNA Extraction
4.4.2. Library Preparation and Sequencing
4.4.3. Small RNA Sequencing Data Processing and Differential Expression Analysis
4.5. Pathway Analysis and Target Prediction
4.6. Selection of Differentially Expressed miRNAs for Validation
4.7. Sample Size Calculation
4.8. RT-qPCR
4.9. Analysis of Clinical Variables
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BCS | Body condition score |
| cDNA | Complementary DNA |
| CPM | Counts per million |
| Cq | Quantification cycle |
| FC | Fold change |
| FDR | False discovery rate |
| IL | Interleukin |
| IPA | Ingenuity Pathway Analysis |
| lncRNA | Long non-coding RNA |
| Max | Maximum |
| Min | Minimum |
| MMP | Metalloproteinase |
| mRNA | Messenger RNA |
| miRNA | microRNA |
| OA | Osteoarthritis |
| OARSI | Osteoarthritis Research Society International |
| PC | Principal component |
| PCA | Principal component analysis |
| piRNA | Piwi-interfering RNA |
| QC | Quality control |
| qPCR | Quantitative polymerase chain reaction |
| RPM | Reads per million |
| rRNA | Ribosomal RNA |
| RT-qPCR | Reverse transcription quantitative polymerase chain reaction |
| scRNA | Small conditional RNA |
| SD | Standard deviation |
| SF | Synovial fluid |
| siRNA | Small interfering RNA |
| snRNA | Small nuclear RNA |
| snoRNA | Small nucleolar RNA |
| tRNA | Transfer RNA |
References
- Morris, K.V.; Mattick, J.S. The Rise of Regulatory RNA. Nat. Rev. Genet. 2014, 15, 423–437. [Google Scholar] [CrossRef]
- Hombach, S.; Kretz, M. Non-Coding RNAs: Classification, Biology and Functioning. In Non-Coding RNAs in Colorectal Cancer; Slaby, O., Calin, G.A., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 3–17. ISBN 978-3-319-42059-2. [Google Scholar]
- Saliminejad, K.; Khorram Khorshid, H.R.; Soleymani Fard, S.; Ghaffari, S.H. An Overview of MicroRNAs: Biology, Functions, Therapeutics, and Analysis Methods. J. Cell Physiol. 2019, 234, 5451–5465. [Google Scholar] [CrossRef]
- Orang, A.V.; Safaralizadeh, R.; Kazemzadeh-Bavili, M. Mechanisms of MiRNA-Mediated Gene Regulation from Common Downregulation to MRNA-Specific Upregulation. Int. J. Genom. 2014, 2014, 970607. [Google Scholar] [CrossRef]
- Blondal, T.; Jensby Nielsen, S.; Baker, A.; Andreasen, D.; Mouritzen, P.; Wrang Teilum, M.; Dahlsveen, I.K. Assessing Sample and MiRNA Profile Quality in Serum and Plasma or Other Biofluids. Methods 2013, 59, S1–S6. [Google Scholar] [CrossRef] [PubMed]
- Ireland, J.L.; Clegg, P.D.; McGowan, C.M.; McKane, S.A.; Chandler, K.J.; Pinchbeck, G.L. Disease Prevalence in Geriatric Horses in the United Kingdom: Veterinary Clinical Assessment of 200 Cases. Equine Vet. J. 2012, 44, 101–106. [Google Scholar] [CrossRef] [PubMed]
- van Weeren, P.R.; de Grauw, J.C. Pain in Osteoarthritis. Vet. Clin. North Am. Equine Pract. 2010, 26, 619–642. [Google Scholar] [CrossRef]
- Rocha, F.A.C.; Ali, S.A. Soluble Biomarkers in Osteoarthritis in 2022: Year in Review. Osteoarthr. Cartil. 2023, 31, 167–176. [Google Scholar] [CrossRef]
- Stanciugelu, S.I.; Homorogan, C.; Selaru, C.; Patrascu, J.M.; Patrascu, J.M.; Stoica, R.; Nitusca, D.; Marian, C. Osteoarthritis and MicroRNAs: Do They Provide Novel Insights into the Pathophysiology of This Degenerative Disorder? Life 2022, 12, 1914. [Google Scholar] [CrossRef]
- Beyer, C.; Zampetaki, A.; Lin, N.-Y.; Kleyer, A.; Perricone, C.; Iagnocco, A.; Distler, A.; Langley, S.R.; Gelse, K.; Sesselmann, S.; et al. Signature of Circulating MicroRNAs in Osteoarthritis. Ann. Rheum. Dis. 2015, 74, e18. [Google Scholar] [CrossRef]
- Munjal, A.; Bapat, S.; Hubbard, D.; Hunter, M.; Kolhe, R.; Fulzele, S. Advances in Molecular Biomarker for Early Diagnosis of Osteoarthritis. Biomol. Concepts 2019, 10, 111–119. [Google Scholar] [CrossRef]
- Ntoumou, E.; Tzetis, M.; Braoudaki, M.; Lambrou, G.; Poulou, M.; Malizos, K.; Stefanou, N.; Anastasopoulou, L.; Tsezou, A. Serum MicroRNA Array Analysis Identifies MiR-140-3p, MiR-33b-3p and MiR-671-3p as Potential Osteoarthritis Biomarkers Involved in Metabolic Processes. Clin. Epigenetics 2017, 9, 127. [Google Scholar] [CrossRef]
- Li, Y.-H.; Tavallaee, G.; Tokar, T.; Nakamura, A.; Sundararajan, K.; Weston, A.; Sharma, A.; Mahomed, N.N.; Gandhi, R.; Jurisica, I.; et al. Identification of Synovial Fluid MicroRNA Signature in Knee Osteoarthritis: Differentiating Early- and Late-Stage Knee Osteoarthritis. Osteoarthr. Cartil. 2016, 24, 1577–1586. [Google Scholar] [CrossRef]
- Castanheira, C.; Balaskas, P.; Falls, C.; Ashraf-Kharaz, Y.; Clegg, P.; Burke, K.; Fang, Y.; Dyer, P.; Welting, T.J.M.; Peffers, M.J. Equine Synovial Fluid Small Non-Coding RNA Signatures in Early Osteoarthritis. BMC Vet. Res. 2021, 17, 26. [Google Scholar] [CrossRef] [PubMed]
- Skiöldebrand, E.; Lorenzo, P.; Zunino, L.; Rucklidge, G.J.; Sandgren, B.; Carlsten, J.; Ekman, S. Concentration of Collagen, Aggrecan and Cartilage Oligomeric Matrix Protein (COMP) in Synovial Fluid from Equine Middle Carpal Joints. Equine Vet. J. 2001, 33, 394–402. [Google Scholar] [CrossRef] [PubMed]
- McIlwraith, C.W.; Frisbie, D.D.; Kawcak, C.E.; Fuller, C.J.; Hurtig, M.; Cruz, A. The OARSI Histopathology Initiative—Recommendations for Histological Assessments of Osteoarthritis in the Horse. Osteoarthr. Cartil. 2010, 18, S93–S105. [Google Scholar] [CrossRef] [PubMed]
- Glyn-Jones, S.; Palmer, A.J.R.; Agricola, R.; Price, A.J.; Vincent, T.L.; Weinans, H.; Carr, A.J. Osteoarthritis. Lancet 2015, 386, 376–387. [Google Scholar] [CrossRef]
- Steel, C.M. Equine Synovial Fluid Analysis. Vet. Clin. North Am. Equine Pract. 2008, 24, 437–454. [Google Scholar] [CrossRef]
- Baker, M.E.; Lee, S.; Clinton, M.; Hackl, M.; Castanheira, C.; Peffers, M.J.; Taylor, S.E. Investigation of MicroRNA Biomarkers in Equine Distal Interphalangeal Joint Osteoarthritis. Int. J. Mol. Sci. 2022, 23, 15526. [Google Scholar] [CrossRef]
- Castanheira, C.; James, V.; Taylor, S.; Skiöldebrand, E.; Clegg, P.D.; Peffers, M.J. Synovial Fluid and Serum Small Non-Coding RNA Signatures in Equine Osteoarthritis. Osteoarthr. Cart. 2021, 29, S162. [Google Scholar] [CrossRef]
- Lao, T.D.; Le, T.A.H. Data Integration Reveals the Potential Biomarkers of Circulating MicroRNAs in Osteoarthritis. Diagnostics 2021, 11, 412. [Google Scholar] [CrossRef]
- Le, L.T.T.; Swingler, T.E.; Clark, I.M. Review: The Role of MicroRNAs in Osteoarthritis and Chondrogenesis. Arthritis Rheum. 2013, 65, 1963–1974. [Google Scholar] [CrossRef] [PubMed]
- Pertusa, C.; Tarín, J.J.; Cano, A.; García-Pérez, M.Á.; Mifsut, D. Serum MicroRNAs in Osteoporotic Fracture and Osteoarthritis: A Genetic and Functional Study. Sci. Rep. 2021, 11, 19372. [Google Scholar] [CrossRef] [PubMed]
- Sondag, G.R.; Haqqi, T.M. The Role of MicroRNAs and Their Targets in Osteoarthritis. Curr. Rheumatol. Rep. 2016, 18, 56. [Google Scholar] [CrossRef] [PubMed]
- Tavallaee, G.; Rockel, J.S.; Lively, S.; Kapoor, M. MicroRNAs in Synovial Pathology Associated with Osteoarthritis. Front. Med. 2020, 7, 376. [Google Scholar] [CrossRef]
- Yu, X.-M.; Meng, H.-Y.; Yuan, X.-L.; Wang, Y.; Guo, Q.-Y.; Peng, J.; Wang, A.-Y.; Lu, S.-B. MicroRNAs’ Involvement in Osteoarthritis and the Prospects for Treatments. Evid. Based Complement. Altern. Med. 2015, 2015, 236179. [Google Scholar] [CrossRef]
- Zhang, W.; Cheng, P.; Hu, W.; Yin, W.; Guo, F.; Chen, A.; Huang, H. Downregulated MicroRNA-340-5p Promotes Proliferation and Inhibits Apoptosis of Chondrocytes in Osteoarthritis Mice through Inhibiting the Extracellular Signal-Regulated Kinase Signaling Pathway by Negatively Targeting the FMOD Gene. J. Cell Physiol. 2019, 234, 927–939. [Google Scholar] [CrossRef]
- Walters, M.; Skovgaard, K.; Heegaard, P.M.H.; Fang, Y.; Kharaz, Y.A.; Bundgaard, L.; Skovgaard, L.T.; Jensen, H.E.; Andersen, P.H.; Peffers, M.J.; et al. Identification and Characterisation of Temporal Abundance of MicroRNAs in Synovial Fluid from an Experimental Equine Model of Osteoarthritis. Equine Vet. J. 2025, 57, 1138–1150. [Google Scholar] [CrossRef]
- Rasheed, Z.; Rasheed, N.; Al-Shobaili, H.A. Epigallocatechin-3-O-Gallate up-Regulates MicroRNA-199a-3p Expression by down-Regulating the Expression of Cyclooxygenase-2 in Stimulated Human Osteoarthritis Chondrocytes. J. Cell Mol. Med. 2016, 20, 2241–2248. [Google Scholar] [CrossRef]
- Ukai, T.; Sato, M.; Akutsu, H.; Umezawa, A.; Mochida, J. MicroRNA-199a-3p, MicroRNA-193b, and MicroRNA-320c Are Correlated to Aging and Regulate Human Cartilage Metabolism. J. Orthop. Res. 2012, 30, 1915–1922. [Google Scholar] [CrossRef]
- Vonk, L.A.; Kragten, A.H.M.; Dhert, W.J.A.; Saris, D.B.F.; Creemers, L.B. Overexpression of Hsa-MiR-148a Promotes Cartilage Production and Inhibits Cartilage Degradation by Osteoarthritic Chondrocytes. Osteoarthr. Cartil. 2014, 22, 145–153. [Google Scholar] [CrossRef]
- Lu, Y.; Liu, L.; Pan, J.; Luo, B.; Zeng, H.; Shao, Y.; Zhang, H.; Guan, H.; Guo, D.; Zeng, C.; et al. MFG-E8 Regulated by MiR-99b-5p Protects against Osteoarthritis by Targeting Chondrocyte Senescence and Macrophage Reprogramming via the NF-ΚB Pathway. Cell Death Dis. 2021, 12, 533. [Google Scholar] [CrossRef] [PubMed]
- de la Rica, L.; García-Gómez, A.; Comet, N.R.; Rodríguez-Ubreva, J.; Ciudad, L.; Vento-Tormo, R.; Company, C.; Álvarez-Errico, D.; García, M.; Gómez-Vaquero, C.; et al. NF-ΚB-Direct Activation of MicroRNAs with Repressive Effects on Monocyte-Specific Genes Is Critical for Osteoclast Differentiation. Genome Biol. 2015, 16, 2. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.-N.; Lu, S.; Fu, C.-M. MiR-146a Expression Profiles in Osteoarthritis in Different Tissue Sources: A Meta-Analysis of Observational Studies. J. Orthop. Surg. Res. 2022, 17, 148. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.W.; Watkins, G.; Le Good, N.; Roberts, S.; Murphy, C.L.; Brockbank, S.M.V.; Needham, M.R.C.; Read, S.J.; Newham, P. The Identification of Differentially Expressed MicroRNA in Osteoarthritic Tissue That Modulate the Production of TNF-α and MMP13. Osteoarthr. Cartil. 2009, 17, 464–472. [Google Scholar] [CrossRef]
- Chang, C.-C.; Venø, M.T.; Chen, L.; Ditzel, N.; Le, D.Q.S.; Dillschneider, P.; Kassem, M.; Kjems, J. Global MicroRNA Profiling in Human Bone Marrow–Skeletal Stromal or Mesenchymal–Stem Cells Identified Candidates for Bone Regeneration. Mol. Ther. 2018, 26, 593–605. [Google Scholar] [CrossRef]
- Xu, J.-F.; Zhang, S.-J.; Zhao, C.; Qiu, B.-S.; Gu, H.-F.; Hong, J.-F.; Cao, L.; Chen, Y.; Xia, B.; Bi, Q.; et al. Altered MicroRNA Expression Profile in Synovial Fluid from Patients with Knee Osteoarthritis with Treatment of Hyaluronic Acid. Mol. Diagn. Ther. 2015, 19, 299–308. [Google Scholar] [CrossRef]
- Kopańska, M.; Szala, D.; Czech, J.; Gabło, N.; Gargasz, K.; Trzeciak, M.; Zawlik, I.; Snela, S. MiRNA Expression in the Cartilage of Patients with Osteoarthritis. J. Orthop. Surg. Res. 2017, 12, 51, Erratum in J. Orthop. Surg. Res. 2017, 12, 90.. [Google Scholar] [CrossRef]
- Guo, Y.; Min, Z.; Jiang, C.; Wang, W.; Yan, J.; Xu, P.; Xu, K.; Xu, J.; Sun, M.; Zhao, Y.; et al. Downregulation of HS6ST2 by MiR-23b-3p Enhances Matrix Degradation through P38 MAPK Pathway in Osteoarthritis. Cell Death Dis. 2018, 9, 699. [Google Scholar] [CrossRef]
- Yang, Q.; Zhou, Y.; Cai, P.; Fu, W.; Wang, J.; Wei, Q.; Li, X. Downregulation of MicroRNA-23b-3p Alleviates IL-1β-Induced Injury in Chondrogenic CHON-001 Cells. Drug Des. Devel Ther. 2019, 13, 2503–2512. [Google Scholar] [CrossRef]
- Aird, D.; Ross, M.G.; Chen, W.-S.; Danielsson, M.; Fennell, T.; Russ, C.; Jaffe, D.B.; Nusbaum, C.; Gnirke, A. Analyzing and Minimizing PCR Amplification Bias in Illumina Sequencing Libraries. Genome Biol. 2011, 12, R18. [Google Scholar] [CrossRef]
- Becker, N.; Lockwood, C.M. Pre-Analytical Variables in MiRNA Analysis. Clin. Biochem. 2013, 46, 861–868. [Google Scholar] [CrossRef]
- Cappelli, K.; Mecocci, S.; Capomaccio, S.; Beccati, F.; Palumbo, A.R.; Tognoloni, A.; Pepe, M.; Chiaradia, E. Circulating Transcriptional Profile Modulation in Response to Metabolic Unbalance Due to Long-Term Exercise in Equine Athletes: A Pilot Study. Genes 2021, 12, 1965. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.; Denham, M.M.; Spencer, S.J.; Denham, J. Exercise Regulates Shelterin Genes and MicroRNAs Implicated in Ageing in Thoroughbred Horses. Pflug. Arch. 2022, 474, 1159–1169. [Google Scholar] [CrossRef]
- de Oliveira Jr, G.P.; Porto, W.F.; Palu, C.C.; Pereira, L.M.; Reis, A.M.M.; Marçola, T.G.; Teixeira-Neto, A.R.; Franco, O.L.; Pereira, R.W. Effects of Endurance Racing on Horse Plasma Extracellular Particle MiRNA. Equine Vet. J. 2021, 53, 618–627. [Google Scholar] [CrossRef]
- Hooten, N.N.; Abdelmohsen, K.; Gorospe, M.; Ejiogu, N.; Zonderman, A.B.; Evans, M.K. MicroRNA Expression Patterns Reveal Differential Expression of Target Genes with Age. PLoS ONE 2010, 5, e10724. [Google Scholar] [CrossRef]
- Balaskas, P.; Green, J.A.; Haqqi, T.M.; Dyer, P.; Kharaz, Y.A.; Fang, Y.; Liu, X.; Welting, T.J.M.; Peffers, M.J. Small Non-Coding RNAome of Ageing Chondrocytes. Int. J. Mol. Sci. 2020, 21, 5675. [Google Scholar] [CrossRef]
- Castanheira, C.I.G.D.; Anderson, J.R.; Fang, Y.; Milner, P.I.; Goljanek-Whysall, K.; House, L.; Clegg, P.D.; Peffers, M.J. Mouse MicroRNA Signatures in Joint Ageing and Post-Traumatic Osteoarthritis. Osteoarthr. Cartil. Open 2021, 3, 100186. [Google Scholar] [CrossRef]
- Loeser, R.F. The Role of Aging in the Development of Osteoarthritis. Trans. Am. Clin. Climatol. Assoc. 2017, 128, 44–54. [Google Scholar]
- Chugh, P.; Dittmer, D.P. Potential Pitfalls in MicroRNA Profiling. WIREs RNA 2012, 3, 601–616. [Google Scholar] [CrossRef]
- Ou, F.-S.; Michiels, S.; Shyr, Y.; Adjei, A.A.; Oberg, A.L. Biomarker Discovery and Validation: Statistical Considerations. J. Thorac. Oncol. 2021, 16, 537–545. [Google Scholar] [CrossRef]
- Kawcak, C.E.; Frisbie, D.D.; Werpy, N.M.; Park, R.D.; McIlwraith, C.W. Effects of Exercise vs Experimental Osteoarthritis on Imaging Outcomes. Osteoarthr. Cartil. 2008, 16, 1519–1525. [Google Scholar] [CrossRef]
- Diendorfer, A.; Khamina, K.; Pultar, M.; Hackl, M. MiND (MiRNA NGS Discovery Pipeline): A Small RNA-Seq Analysis Pipeline and Report Generator for MicroRNA Biomarker Discovery Studies. F1000Res 2022, 11, 233. [Google Scholar] [CrossRef]
- Ewels, P.; Magnusson, M.; Lundin, S.; Käller, M. MultiQC: Summarize Analysis Results for Multiple Tools and Samples in a Single Report. Bioinformatics 2016, 32, 3047–3048. [Google Scholar] [CrossRef] [PubMed]
- Martin, M. Cutadapt Removes Adapter Sequences from High-Throughput Sequencing Reads. EMBnet J. 2011, 17, 10. [Google Scholar] [CrossRef]
- Langmead, B.; Trapnell, C.; Pop, M.; Salzberg, S.L. Ultrafast and Memory-Efficient Alignment of Short DNA Sequences to the Human Genome. Genome Biol. 2009, 10, R25. [Google Scholar] [CrossRef]
- Friedländer, M.R.; Mackowiak, S.D.; Li, N.; Chen, W.; Rajewsky, N. MiRDeep2 Accurately Identifies Known and Hundreds of Novel MicroRNA Genes in Seven Animal Clades. Nucleic Acids Res. 2012, 40, 37–52. [Google Scholar] [CrossRef]
- Zerbino, D.R.; Achuthan, P.; Akanni, W.; Amode, M.R.; Barrell, D.; Bhai, J.; Billis, K.; Cummins, C.; Gall, A.; Girón, C.G.; et al. Ensembl 2018. Nucleic Acids Res 2018, 46, D754–D761. [Google Scholar] [CrossRef]
- Griffiths-Jones, S. The MicroRNA Registry. Nucleic Acids Res. 2004, 32, D109–D111. [Google Scholar] [CrossRef]
- The RNAcentral Consortium. RNAcentral: A Hub of Information for Non-Coding RNA Sequences. Nucleic Acids Res. 2019, 47, D221–D229. [Google Scholar] [CrossRef]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. EdgeR: A Bioconductor Package for Differential Expression Analysis of Digital Gene Expression Data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated Estimation of Fold Change and Dispersion for RNA-Seq Data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Andersen, C.L.; Jensen, J.L.; Ørntoft, T.F. Normalization of Real-Time Quantitative Reverse Transcription-PCR Data: A Model-Based Variance Estimation Approach to Identify Genes Suited for Normalization, Applied to Bladder and Colon Cancer Data Sets. Cancer Res. 2004, 64, 5245–5250. [Google Scholar] [CrossRef]
- Tonge, D.P.; Gant, T.W. Evidence Based Housekeeping Gene Selection for MicroRNA-Sequencing (MiRNA-Seq) Studies. Toxicol. Res. 2013, 2, 328–334. [Google Scholar] [CrossRef]


| Control (N = 4) | OA (N = 9) | |
|---|---|---|
| Collection site, n (%) | ||
| Abattoir 1 | 4 (100) | 5 (55.5) |
| Hospital 2 | 0 | 4 (44.4) |
| Age, years | ||
| n | 3 | 9 |
| Mean (SD) | 6.3 (7.5) | 6.6 (3.5) |
| Min; Max | 2; 15 | 2; 14 |
| Missing/not reported | 1 | 0 |
| Sex, n (%) | ||
| n | 3 | 9 |
| Female | 2 (66.7) | 2 (22.2) |
| Male | ||
| Neutered | 6 (66.7) | |
| Not neutered | 1 (33.3) | 1 (11.1) |
| Missing/not reported | 1 | 0 |
| Breed, n (%) | ||
| n | 3 | 8 |
| Arab | 1 (33.3) | 0 |
| Friesian | 0 | 1 (12.5) |
| Standardbred | 1 (33.3) | 1 (12.5) |
| Swedish Warmblood | 1 (33.3) | 4 (50.0) |
| Thoroughbred | 0 | 2 (25.0) |
| Missing/not reported | 1 | 1 |
| Occupation, n (%) | ||
| n | 1 | 3 |
| Racing | 1 (100) | 3 (100) |
| Missing | 3 | 6 |
| OA severity, n (%) 3 | ||
| n | 4 | 5 |
| Control | 4 (100) | 0 |
| Mild | 0 | 3 (60.0) |
| Moderate | 0 | 1 (20.0) |
| Severe | 0 | 1 (20.0) |
| Missing/not reported | 0 | 4 |
| Joint affected, n (%) | ||
| n | 4 | 9 |
| Carpal | 4 (100) | 6 (66.7) |
| Metacarpophalangeal | 0 | 3 (33.3) |
| miRNA | logFC 1 | p-Value | FDR | Significance |
|---|---|---|---|---|
| Serum | ||||
| eca-miR-9048 | −8.74 | <0.0001 | 0.0164 | Decreased in OA |
| eca-miR-143 | 4.09 | 0.0001 | 0.0164 | Increased in OA |
| eca-miR-25 | 1.99 | 0.0002 | 0.0164 | Increased in OA |
| eca-miR-146a | 2.67 | 0.0004 | 0.0242 | Increased in OA |
| eca-miR-1291a | −7.35 | 0.0007 | 0.0242 | Decreased in OA |
| eca-miR-8986b | −7.35 | 0.0007 | 0.0242 | Decreased in OA |
| eca-miR-1892 | −7.26 | 0.0008 | 0.0242 | Decreased in OA |
| eca-miR-8954 | −7.26 | 0.0008 | 0.0242 | Decreased in OA |
| eca-miR-330 | −6.93 | 0.0008 | 0.0242 | Decreased in OA |
| eca-miR-490-3p | −7.47 | 0.0008 | 0.0242 | Decreased in OA |
| eca-miR-191a | 1.76 | 0.0010 | 0.0255 | Increased in OA |
| eca-miR-345-5p | −6.91 | 0.0010 | 0.0255 | Decreased in OA |
| eca-miR-16 | 2.24 | 0.0014 | 0.0296 | Increased in OA |
| eca-miR-133a | 3.80 | 0.0014 | 0.0296 | Increased in OA |
| eca-miR-223 | 4.32 | 0.0022 | 0.0446 | Increased in OA |
| eca-miR-129b-3p | −5.60 | 0.0033 | 0.0580 | Decreased in OA |
| eca-miR-8951 | −5.60 | 0.0033 | 0.0580 | Decreased in OA |
| eca-miR-199a-3p | 2.15 | 0.0038 | 0.0597 | Increased in OA |
| eca-miR-199b-3p | 2.15 | 0.0038 | 0.0597 | Increased in OA |
| eca-miR-483 | −5.01 | 0.0049 | 0.0729 | Decreased in OA |
| eca-miR-142-5p | 1.69 | 0.0065 | 0.0935 | Increased in OA |
| eca-miR-15a | 3.61 | 0.0076 | 0.1032 | Increased in OA |
| eca-miR-148a | 1.63 | 0.0087 | 0.1094 | Increased in OA |
| eca-miR-423-5p | −1.06 | 0.0088 | 0.1094 | Decreased in OA |
| eca-miR-23b | −1.56 | 0.0106 | 0.1271 | Decreased in OA |
| eca-miR-93 | 1.77 | 0.0112 | 0.1287 | Increased in OA |
| eca-miR-744 | 2.64 | 0.0122 | 0.1351 | Increased in OA |
| eca-miR-130a | 3.42 | 0.0132 | 0.1410 | Increased in OA |
| eca-miR-8992 | −3.88 | 0.0143 | 0.1444 | Decreased in OA |
| eca-miR-8977 | −5.19 | 0.0144 | 0.1444 | Decreased in OA |
| eca-miR-423-3p | −1.12 | 0.0217 | 0.2064 | Decreased in OA |
| eca-miR-206 | 3.59 | 0.0221 | 0.2064 | Increased in OA |
| eca-miR-194 | 2.59 | 0.0227 | 0.2064 | Increased in OA |
| eca-miR-1 | 4.53 | 0.0235 | 0.2077 | Increased in OA |
| eca-let-7f | −1.31 | 0.0278 | 0.2358 | Decreased in OA |
| eca-miR-30e | 1.84 | 0.0283 | 0.2358 | Increased in OA |
| eca-miR-98 | −1.88 | 0.0292 | 0.2371 | Decreased in OA |
| eca-miR-340-5p | 2.30 | 0.0312 | 0.2464 | Increased in OA |
| eca-miR-140-3p | 4.07 | 0.0323 | 0.2482 | Increased in OA |
| eca-miR-23a | −1.24 | 0.0340 | 0.2547 | Decreased in OA |
| eca-miR-27b | 1.55 | 0.0470 | 0.3437 | Increased in OA |
| eca-miR-2483 | 3.24 | 0.0492 | 0.3465 | Increased in OA |
| eca-miR-7177b | 8.21 | 0.0497 | 0.3465 | Increased in OA |
| Synovial fluid | ||||
| eca-miR-324-5p | −6.66 | <0.0001 | <0.0001 | Decreased in OA |
| eca-miR-296 | −5.98 | <0.0001 | 0.0002 | Decreased in OA |
| eca-miR-615-5p | −9.25 | 0.0001 | 0.0072 | Decreased in OA |
| eca-miR-671-3p | −4.69 | 0.0004 | 0.0187 | Decreased in OA |
| eca-miR-27a | −4.42 | 0.0005 | 0.0187 | Decreased in OA |
| eca-miR-184 | −4.47 | 0.0006 | 0.0187 | Decreased in OA |
| eca-miR-1291a | −9.55 | 0.0006 | 0.0187 | Decreased in OA |
| eca-miR-148a | 2.45 | 0.0026 | 0.0646 | Increased in OA |
| eca-miR-423-5p | −1.90 | 0.0032 | 0.0646 | Decreased in OA |
| eca-miR-23b | −3.04 | 0.0032 | 0.0646 | Decreased in OA |
| eca-miR-598 | −7.76 | 0.0059 | 0.1090 | Decreased in OA |
| eca-miR-206 | −7.24 | 0.0075 | 0.1270 | Decreased in OA |
| eca-miR-199a-3p | 1.63 | 0.0122 | 0.1770 | Increased in OA |
| eca-miR-199b-3p | 1.63 | 0.0122 | 0.1770 | Increased in OA |
| eca-miR-31 | −6.76 | 0.0149 | 0.1950 | Decreased in OA |
| eca-miR-92b | −2.66 | 0.0153 | 0.1950 | Decreased in OA |
| eca-miR-99a | 4.81 | 0.0169 | 0.2020 | Increased in OA |
| eca-miR-1892 | −6.60 | 0.0360 | 0.3930 | Decreased in OA |
| eca-miR-10b | 0.89 | 0.0368 | 0.3930 | Increased in OA |
| eca-miR-27b | −2.15 | 0.0437 | 0.4350 | Decreased in OA |
| eca-miR-151-5p | 10.72 | 0.0465 | 0.4350 | Increased in OA |
| eca-miR-342-5p | 10.82 | 0.0480 | 0.4350 | Increased in OA |
| eca-miR-211 | 10.67 | 0.0495 | 0.4350 | Increased in OA |
| Serum | SF | |||
|---|---|---|---|---|
| Control (N = 23) | OA (N = 23) | Control (N = 44) | OA (N = 44) | |
| Collection site, n (%) | ||||
| Abattoir 1 | 0 | 0 | 39 (88.6) | 40 (90.9) |
| Hospital/Clinic 2 | 23 (100) | 23 (100) | 5 (11.4) | 4 (9.1) |
| A | 23 (100) | 9 (39.1) | 0 | 0 |
| B | 0 | 5 (21.7) | 5 (100) | 4 (100) |
| C | 0 | 9 (39.1) | 0 | 0 |
| Age, years | ||||
| n | 23 | 9 | 41 | 41 |
| Mean (SD) | 9.8 (4.2) | 11.1 (5.4) | 10.2 (6.8) | 15.7 (6.7) |
| Min; Max | 3; 18 | 4; 23 | 2; 20 | 3; 25 |
| Missing/not reported | 0 | 14 | 3 | 3 |
| p-value | 0.6582 1 | 0.0003 1 | ||
| Sex, n (%) | ||||
| n | 23 | 14 | 25 | 26 |
| Female | 8 (34.8) | 2 (14.3) | 17 (68.0) | 8 (30.8) |
| Neutered male | 15 (65.2) | 12 (85.7) | 8 (32.0) | 181 (69.2) |
| p-value | 0.2603 2 | 0.0227 2 | ||
| Missing/not reported | 0 | 9 | 19 | 18 |
| Body Condition Score, n (%) | ||||
| n | 23 | 9 | 0 | 0 |
| 1–3 | 0 | 0 | – | – |
| 4 | 0 | 1 (11.1) | – | – |
| 5 | 19 (82.6) | 6 (66.6) | – | – |
| 6 | 3 (13.0) | 2 (22.2) | – | – |
| 7 | 1 (4.3) | 0 | – | – |
| 8–9 | 0 | 0 | – | – |
| Missing/not reported | 0 | 14 | 44 | 44 |
| Breed, n (%) | ||||
| n | 23 3 | 23 3 | 19 4 | 16 4 |
| Appaloosa | 0 | 1 (4.3) | 0 | 0 |
| Cob 5 | 1 (4.3) | 0 | 2 (10.5) | 1 (6.3) |
| Connemara 5 | 4 (17.4) | 1 (4.3) | 0 | 0 |
| Dales 5 | 0 | 1 (4.3) | 0 | 0 |
| Dutch Warmblood | 0 | 1 (4.3) | 0 | 0 |
| Hanoverian | 0 | 2 (8.7) | 0 | 0 |
| Holsteiner | 0 | 1 (4.3) | 0 | 0 |
| Irish cob | 0 | 1 (4.3) | 0 | 0 |
| Irish Draught | 2 (8.7) | 0 | 0 | 0 |
| Irish Sport Horse 5 | 9 (39.1) | 4 (17.4) | 2 (10.5) | 3 (18.8) |
| Lusitano | 1 (4.3) | 0 | 0 | 1 (6.3) |
| Pony | 1 (4.3) | 1 (4.3) | 4 (21.4) | 0 |
| Thoroughbred 5 | 2 (8.7) | 9 (39.1) | 7 (36.8) | 9 (56.3) |
| Warmblood 5 | 1 (4.3) | 0 | 0 | 2 (12.5) |
| Welsh Pony/Cob 5 | 2 (8.7) | 1 (4.3) | 4 (21.4) | 0 |
| Missing/not reported | 0 | 0 | 25 | 25 |
| Occupation, n (%) 3 | ||||
| n | 23 | 18 | 0 | 0 |
| Not in work (‘out in the field’) | 2 (8.7) | 2 (11.1) | – | – |
| All-rounder | 5 (21.7) | 1 (5.6) | – | – |
| Dressage | 1 (4.3) | 0 | – | – |
| Eventing | 2 (8.7) | 0 | – | – |
| Hacking | 5 (21.7) | 32 (16.5) | – | – |
| Hunting | 3 (13.0) | 1 (5.6) | – | – |
| Leisure | 1 (4.3) | 0 | – | – |
| Racing | 0 | 9 (50.0) | – | – |
| Schooling | 4 (17.4) | 1 (5.6) | – | – |
| Showjumping | 0 | 1 (5.6) | – | – |
| Missing/not reported | 0 | 5 | 44 | 44 |
| Current level of work (0–3), n (%) 5 | ||||
| n | 23 | 18 | 0 | 0 |
| 0 (‘out in the field’) | 2 (8.7) | 2 (11.1) | – | – |
| 1 (‘light work’) | 12 (52.2) | 4 (22.2) | – | – |
| 2 (‘medium work’) | 7 (30.4) | 2 (11.1) | – | – |
| 3 (‘intense work’) | 2 (8.7) | 10 (55.6) | – | – |
| Missing/not reported | 0 | 10 | 44 | 44 |
| p-value | 0.0214 6 | – | ||
| Shoes, n (%) | ||||
| n | 18 | 8 | 0 | 0 |
| All four feet | 13 (72.2) | 3 (37.5) | n/a | n/a |
| Front feet | 2 (11.1) | 1 (12.5) | n/a | n/a |
| Unshod | 3 (16.7) | 4 (50.0) | n/a | n/a |
| Missing/not reported | 5 | 15 | 44 | 44 |
| Joints affected, n (%) 7,8 | ||||
| Distal interphalangeal | – | 2 (9.1) 3 | 0 | 0 |
| Intervertebral | – | 2 (9.1) | 0 | 0 |
| Metacarpophalangeal | – | 7 (31.8) 3 | 44 (100) 8 | 44 (100) 8 |
| Metatarsophalangeal | – | 4 (18.1) 3 | 0 | 0 |
| Sacroiliac | – | 2 (9.1) 3 | 0 | 0 |
| Scapulohumeral | – | 1 (4.5) 3 | 0 | 0 |
| Tarsometatarsal | – | 2 (9.1) 3 | 0 | 0 |
| Front limb 9 | – | 2 (9.1) 3 | 0 | 0 |
| Joint gross/macroscopic score, n (%) 10 | ||||
| n | 0 | 0 | 44 | 44 |
| 0 | – | – | 21 (47.7) | 0 |
| 1 | – | – | 23 (52.3) | 0 |
| 2 | – | – | 0 | 10 (22.7) |
| 3 | – | – | 0 | 16 (36.4) |
| 4 | – | – | 0 | 11 (25.0) |
| 5 | – | – | 0 | 4 (9.1) |
| 6 | – | – | 0 | 2 (4.5) |
| 7 | – | – | 0 | 1 (2.3) |
| 8–9 | – | – | 0 | 0 |
| Mean (SD) | – | – | 0.5 (0.5) | 3.4 (1.2) |
| Min; Max | – | – | 0; 1 | 2; 7 |
| p-value | – | <0.0001 6 | ||
| Missing/not reported | 23 | 23 | 0 | 0 |
| Articular cartilage microscopic score, n (%) 11 | ||||
| n | 0 | 0 | 34 | 30 |
| 0 | – | – | 0 | 0 |
| 1 | – | – | 5 (14.7) | 0 |
| 2 | – | – | 8 (23.5) | 5 (16.7) |
| 3 | – | – | 7 (20.6) | 4 (13.3) |
| 4 | – | – | 9 (26.5) | 5 (16.7) |
| 5 | – | – | 2 (5.9) | 5 (16.7) |
| 6 | – | – | 1 (2.9) | 5 (16.7) |
| 7 | – | – | 1 (2.9) | 4 (13.3) |
| 8 | – | – | 1 (2.9) | 1 (3.3) |
| 9–15 | – | – | 0 | 0 |
| 16 | – | – | 0 | 1 (3.3) |
| 17–20 | – | – | 0 | 0 |
| Mean (SD) | – | – | 3.2 (1.7) | 5.0 (2.9) |
| Min; Max | – | – | 1; 8 | 2; 16 |
| p-value | – | 0.0015 6 | ||
| Missing/not reported | 23 | 23 | 10 | 14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castanheira, C.I.G.D.; Taylor, S.; Skiöldebrand, E.; Rubio-Martinez, L.M.; Hackl, M.; Clegg, P.D.; Peffers, M.J. Synovial Fluid and Serum MicroRNA Signatures in Equine Osteoarthritis. Int. J. Mol. Sci. 2025, 26, 11190. https://doi.org/10.3390/ijms262211190
Castanheira CIGD, Taylor S, Skiöldebrand E, Rubio-Martinez LM, Hackl M, Clegg PD, Peffers MJ. Synovial Fluid and Serum MicroRNA Signatures in Equine Osteoarthritis. International Journal of Molecular Sciences. 2025; 26(22):11190. https://doi.org/10.3390/ijms262211190
Chicago/Turabian StyleCastanheira, Catarina I. G. D., Sarah Taylor, Eva Skiöldebrand, Luis M. Rubio-Martinez, Matthias Hackl, Peter D. Clegg, and Mandy J. Peffers. 2025. "Synovial Fluid and Serum MicroRNA Signatures in Equine Osteoarthritis" International Journal of Molecular Sciences 26, no. 22: 11190. https://doi.org/10.3390/ijms262211190
APA StyleCastanheira, C. I. G. D., Taylor, S., Skiöldebrand, E., Rubio-Martinez, L. M., Hackl, M., Clegg, P. D., & Peffers, M. J. (2025). Synovial Fluid and Serum MicroRNA Signatures in Equine Osteoarthritis. International Journal of Molecular Sciences, 26(22), 11190. https://doi.org/10.3390/ijms262211190






