Beyond Thrombopoiesis: The Immune Functions of Megakaryocytes in Bacterial Infections and Sepsis
Abstract
1. Introduction
2. Megakaryocyte Biogenesis and Heterogeneity
3. Tissue-Specific Features of Megakaryocytes
3.1. Lung Megakaryocytes
3.2. Spleen Megakaryocytes
3.3. Circulating Megakaryocytes in Peripheral Blood
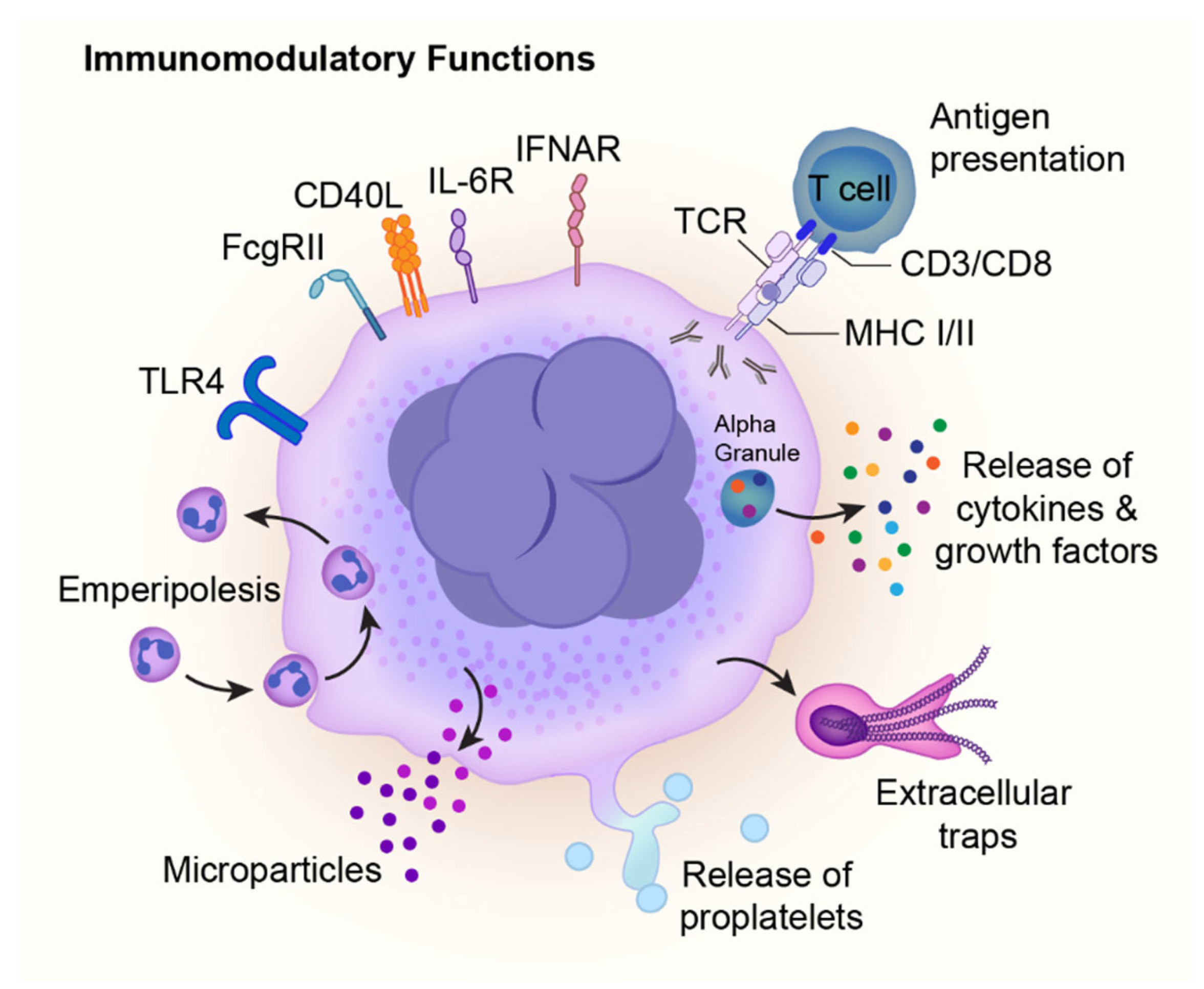
4. Immune Functions of Megakaryocytes
4.1. Megakaryocyte Immune Receptors
4.2. Megakaryocyte Immune Mediators and Responses
5. Megakaryocytes in Bacterial Infections and Sepsis
6. Therapeutic Implications
7. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Non-Standard Abbreviations and Acronyms
| AMR | Ashwell–Morell receptor |
| BM | bone marrow |
| CLEC-2 | C-type lectin-like receptor 2 |
| c-MPL | myeloproliferative leukemia protein |
| CXCL2/MIP-2 | chemokine (C-X-C motif) ligand 2/macrophage inflammatory protein-2 |
| CXCL4/PF4 | chemokine (C-X-C motif) ligand 4/platelet factor 4 |
| DAMPs | damage-associated molecular patterns |
| DIC | disseminated intravascular coagulation |
| DMS | demarcation membrane system |
| FGF-4 | fibroblast growth factor-4 |
| G-CSF | granulocyte colony-stimulating factor |
| GP | glycoprotein |
| HMGB-1 | high mobility group box 1 |
| HSCs | hematopoietic stem cells |
| HSPCs | hematopoietic stem and progenitor cells |
| IFITM3 | interferon-induced transmembrane protein 3 |
| iPSCs | inducible pluripotent stem cells |
| ITP | idiopathic thrombocytopenic purpura |
| LAMP-1 | lysosome-associated membrane protein 1 |
| LNP | lipid nanoparticles |
| LPS | lipopolysaccharide |
| MEPs | megakaryocyte-erythroid progenitors |
| MHC | major histocompatibility complex |
| MK | megakaryocyte |
| MkPs | megakaryocyte precursors |
| MPPs | multipotent progenitors |
| NLRs | NOD-like Receptors |
| PAMPs | pathogen-associated molecular patterns |
| PS | phosphatidylserine |
| RANTES | regulated on activation, normal T cell expressed, and secreted |
| ROS | reactive oxygen species |
| S1P | sphingosine-1-phosphate |
| SDF-1 | stromal cell-derived factor-1 |
| SIC | sepsis-induced coagulopathy |
| TGFβ | transforming growth factor beta |
| TLR | toll-like receptor |
| TNF-α | tumor necrosis factor alpha |
| TPO | thrombopoietin |
References
- Machlus, K.R.; Italiano, J.E., Jr. The incredible journey: From megakaryocyte development to platelet formation. J. Cell Biol. 2013, 201, 785–796. [Google Scholar] [CrossRef]
- Deutsch, V.R.; Tomer, A. Megakaryocyte development and platelet production. Br. J. Haematol. 2006, 134, 453–466. [Google Scholar] [CrossRef] [PubMed]
- Kaushansky, K. The molecular mechanisms that control thrombopoiesis. J. Clin. Investig. 2005, 115, 3339–3347. [Google Scholar] [CrossRef] [PubMed]
- Niswander, L.M.; McGrath, K.E.; Kennedy, J.C.; Palis, J. Improved quantitative analysis of primary bone marrow megakaryocytes utilizing imaging flow cytometry. Cytom. A 2014, 85, 302–312. [Google Scholar] [CrossRef]
- Wang, H.; He, J.; Xu, C.; Chen, X.; Yang, H.; Shi, S.; Liu, C.; Zeng, Y.; Wu, D.; Bai, Z.; et al. Decoding Human Megakaryocyte Development. Cell Stem Cell 2021, 28, 535–549.e8. [Google Scholar] [CrossRef]
- Stegner, D.; vanEeuwijk, J.M.M.; Angay, O.; Gorelashvili, M.G.; Semeniak, D.; Pinnecker, J.; Schmithausen, P.; Meyer, I.; Friedrich, M.; Dutting, S.; et al. Thrombopoiesis is spatially regulated by the bone marrow vasculature. Nat. Commun. 2017, 8, 127. [Google Scholar] [CrossRef]
- Ravid, K.; Lu, J.; Zimmet, J.M.; Jones, M.R. Roads to polyploidy: The megakaryocyte example. J. Cell Physiol. 2002, 190, 7–20. [Google Scholar] [CrossRef]
- Hancock, V.; Martin, J.F.; Lelchuk, R. The relationship between human megakaryocyte nuclear DNA content and gene expression. Br. J. Haematol. 1993, 85, 692–697. [Google Scholar] [CrossRef]
- Raslova, H.; Roy, L.; Vourc’h, C.; Le Couedic, J.P.; Brison, O.; Metivier, D.; Feunteun, J.; Kroemer, G.; Debili, N.; Vainchenker, W. Megakaryocyte polyploidization is associated with a functional gene amplification. Blood 2003, 101, 541–544. [Google Scholar] [CrossRef]
- Schulze, H.; Korpal, M.; Hurov, J.; Kim, S.W.; Zhang, J.; Cantley, L.C.; Graf, T.; Shivdasani, R.A. Characterization of the megakaryocyte demarcation membrane system and its role in thrombopoiesis. Blood 2006, 107, 3868–3875. [Google Scholar] [CrossRef]
- Eckly, A.; Heijnen, H.; Pertuy, F.; Geerts, W.; Proamer, F.; Rinckel, J.Y.; Leon, C.; Lanza, F.; Gachet, C. Biogenesis of the demarcation membrane system (DMS) in megakaryocytes. Blood 2014, 123, 921–930. [Google Scholar] [CrossRef]
- Patel, S.R.; Hartwig, J.H.; Italiano, J.E., Jr. The biogenesis of platelets from megakaryocyte proplatelets. J. Clin. Investig. 2005, 115, 3348–3354. [Google Scholar] [CrossRef] [PubMed]
- Malara, A.; Abbonante, V.; Di Buduo, C.A.; Tozzi, L.; Currao, M.; Balduini, A. The secret life of a megakaryocyte: Emerging roles in bone marrow homeostasis control. Cell Mol. Life Sci. 2015, 72, 1517–1536. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.V.; Lucerne, A.; Ravid, K. Immune and Inflammatory Properties of Megakaryocytes. Cells 2025, 14, 1053. [Google Scholar] [CrossRef] [PubMed]
- Montenont, E.; Bhatlekar, S.; Jacob, S.; Kosaka, Y.; Manne, B.K.; Lee, O.; Parra-Izquierdo, I.; Tugolukova, E.; Tolley, N.D.; Rondina, M.T.; et al. CRISPR-edited megakaryocytes for rapid screening of platelet gene functions. Blood Adv. 2021, 5, 2362–2374. [Google Scholar] [CrossRef]
- Campbell, R.A.; Manne, B.K.; Banerjee, M.; Middleton, E.A.; Ajanel, A.; Schwertz, H.; Denorme, F.; Stubben, C.; Montenont, E.; Saperstein, S.; et al. IFITM3 regulates fibrinogen endocytosis and platelet reactivity in nonviral sepsis. J. Clin. Investig. 2022, 132, e153014. [Google Scholar] [CrossRef]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef]
- Rudd, K.E.; Johnson, S.C.; Agesa, K.M.; Shackelford, K.A.; Tsoi, D.; Kievlan, D.R.; Colombara, D.V.; Ikuta, K.S.; Kissoon, N.; Finfer, S.; et al. Global, regional, and national sepsis incidence and mortality, 1990-2017: Analysis for the Global Burden of Disease Study. Lancet 2020, 395, 200–211. [Google Scholar] [CrossRef]
- Iba, T.; Helms, J.; Connors, J.M.; Levy, J.H. The pathophysiology, diagnosis, and management of sepsis-associated disseminated intravascular coagulation. J. Intensive Care 2023, 11, 24. [Google Scholar] [CrossRef]
- Frydman, G.H.; Ellett, F.; Jorgensen, J.; Marand, A.L.; Zukerberg, L.; Selig, M.K.; Tessier, S.N.; Wong, K.H.K.; Olaleye, D.; Vanderburg, C.R.; et al. Megakaryocytes respond during sepsis and display innate immune cell behaviors. Front. Immunol. 2023, 14, 1083339. [Google Scholar] [CrossRef]
- Woolthuis, C.M.; Park, C.Y. Hematopoietic stem/progenitor cell commitment to the megakaryocyte lineage. Blood 2016, 127, 1242–1248. [Google Scholar] [CrossRef]
- Velten, L.; Haas, S.F.; Raffel, S.; Blaszkiewicz, S.; Islam, S.; Hennig, B.P.; Hirche, C.; Lutz, C.; Buss, E.C.; Nowak, D.; et al. Human haematopoietic stem cell lineage commitment is a continuous process. Nat. Cell Biol. 2017, 19, 271–281. [Google Scholar] [CrossRef]
- Kaushansky, K. Thrombopoietin: The primary regulator of megakaryocyte and platelet production. Thromb. Haemost. 1995, 74, 521–525. [Google Scholar] [CrossRef] [PubMed]
- Bartley, T.D.; Bogenberger, J.; Hunt, P.; Li, Y.S.; Lu, H.S.; Martin, F.; Chang, M.S.; Samal, B.; Nichol, J.L.; Swift, S.; et al. Identification and cloning of a megakaryocyte growth and development factor that is a ligand for the cytokine receptor Mpl. Cell 1994, 77, 1117–1124. [Google Scholar] [CrossRef] [PubMed]
- de Sauvage, F.J.; Hass, P.E.; Spencer, S.D.; Malloy, B.E.; Gurney, A.L.; Spencer, S.A.; Darbonne, W.C.; Henzel, W.J.; Wong, S.C.; Kuang, W.J.; et al. Stimulation of megakaryocytopoiesis and thrombopoiesis by the c-Mpl ligand. Nature 1994, 369, 533–538. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, A.; Chin, D.; Tan, D.; Abdul Majeed, A.B.B.; Nakamura-Ishizu, A.; Suda, T. Thrombopoietin maintains cell numbers of hematopoietic stem and progenitor cells with megakaryopoietic potential. Haematologica 2021, 106, 1883–1891. [Google Scholar] [CrossRef]
- Khatib-Massalha, E.; Mendez-Ferrer, S. Megakaryocyte Diversity in Ontogeny, Functions and Cell-Cell Interactions. Front. Oncol. 2022, 12, 840044. [Google Scholar] [CrossRef]
- Italiano, J.E., Jr.; Lecine, P.; Shivdasani, R.A.; Hartwig, J.H. Blood platelets are assembled principally at the ends of proplatelet processes produced by differentiated megakaryocytes. J. Cell Biol. 1999, 147, 1299–1312. [Google Scholar] [CrossRef]
- Grozovsky, R.; Begonja, A.J.; Liu, K.; Visner, G.; Hartwig, J.H.; Falet, H.; Hoffmeister, K.M. The Ashwell-Morell receptor regulates hepatic thrombopoietin production via JAK2-STAT3 signaling. Nat. Med. 2015, 21, 47–54. [Google Scholar] [CrossRef]
- Xu, M.; Li, J.; Neves, M.A.D.; Zhu, G.; Carrim, N.; Yu, R.; Gupta, S.; Marshall, J.; Rotstein, O.; Peng, J.; et al. GPIbalpha is required for platelet-mediated hepatic thrombopoietin generation. Blood 2018, 132, 622–634. [Google Scholar] [CrossRef]
- Kaser, A.; Brandacher, G.; Steurer, W.; Kaser, S.; Offner, F.A.; Zoller, H.; Theurl, I.; Widder, W.; Molnar, C.; Ludwiczek, O.; et al. Interleukin-6 stimulates thrombopoiesis through thrombopoietin: Role in inflammatory thrombocytosis. Blood 2001, 98, 2720–2725. [Google Scholar] [CrossRef]
- Machlus, K.R.; Johnson, K.E.; Kulenthirarajan, R.; Forward, J.A.; Tippy, M.D.; Soussou, T.S.; El-Husayni, S.H.; Wu, S.K.; Wang, S.; Watnick, R.S.; et al. CCL5 derived from platelets increases megakaryocyte proplatelet formation. Blood 2016, 127, 921–926. [Google Scholar] [CrossRef] [PubMed]
- Avecilla, S.T.; Hattori, K.; Heissig, B.; Tejada, R.; Liao, F.; Shido, K.; Jin, D.K.; Dias, S.; Zhang, F.; Hartman, T.E.; et al. Chemokine-mediated interaction of hematopoietic progenitors with the bone marrow vascular niche is required for thrombopoiesis. Nat. Med. 2004, 10, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Hirschi, K.K.; Nicoli, S.; Walsh, K. Hematopoiesis Lineage Tree Uprooted: Every Cell Is a Rainbow. Dev. Cell 2017, 41, 7–9. [Google Scholar] [CrossRef] [PubMed]
- Boyer, S.W.; Schroeder, A.V.; Smith-Berdan, S.; Forsberg, E.C. All hematopoietic cells develop from hematopoietic stem cells through Flk2/Flt3-positive progenitor cells. Cell Stem Cell 2011, 9, 64–73. [Google Scholar] [CrossRef]
- Noetzli, L.J.; French, S.L.; Machlus, K.R. New Insights Into the Differentiation of Megakaryocytes From Hematopoietic Progenitors. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 1288–1300. [Google Scholar] [CrossRef]
- Sanjuan-Pla, A.; Macaulay, I.C.; Jensen, C.T.; Woll, P.S.; Luis, T.C.; Mead, A.; Moore, S.; Carella, C.; Matsuoka, S.; Bouriez Jones, T.; et al. Platelet-biased stem cells reside at the apex of the haematopoietic stem-cell hierarchy. Nature 2013, 502, 232–236. [Google Scholar] [CrossRef]
- Yamamoto, R.; Morita, Y.; Ooehara, J.; Hamanaka, S.; Onodera, M.; Rudolph, K.L.; Ema, H.; Nakauchi, H. Clonal analysis unveils self-renewing lineage-restricted progenitors generated directly from hematopoietic stem cells. Cell 2013, 154, 1112–1126. [Google Scholar] [CrossRef]
- Zhu, W.; Tjin, G.; Purton, L.E. Adult megakaryopoiesis: When taking a short-cut results in a different final destination. Blood Sci. 2024, 6, e00202. [Google Scholar] [CrossRef]
- Li, J.J.; Liu, J.; Li, Y.E.; Chen, L.V.; Cheng, H.; Li, Y.; Cheng, T.; Wang, Q.F.; Zhou, B.O. Differentiation route determines the functional outputs of adult megakaryopoiesis. Immunity 2024, 57, 478–494 e476. [Google Scholar] [CrossRef]
- Rodriguez-Fraticelli, A.E.; Wolock, S.L.; Weinreb, C.S.; Panero, R.; Patel, S.H.; Jankovic, M.; Sun, J.; Calogero, R.A.; Klein, A.M.; Camargo, F.D. Clonal analysis of lineage fate in native haematopoiesis. Nature 2018, 553, 212–216. [Google Scholar] [CrossRef]
- Sun, S.; Jin, C.; Si, J.; Lei, Y.; Chen, K.; Cui, Y.; Liu, Z.; Liu, J.; Zhao, M.; Zhang, X.; et al. Single-cell analysis of ploidy and the transcriptome reveals functional and spatial divergency in murine megakaryopoiesis. Blood 2021, 138, 1211–1224. [Google Scholar] [CrossRef]
- Liu, C.; Wu, D.; Xia, M.; Li, M.; Sun, Z.; Shen, B.; Liu, Y.; Jiang, E.; Wang, H.; Su, P.; et al. Characterization of Cellular Heterogeneity and an Immune Subpopulation of Human Megakaryocytes. Adv. Sci. 2021, 8, e2100921. [Google Scholar] [CrossRef]
- Middleton, E.A.; Rowley, J.W.; Campbell, R.A.; Grissom, C.K.; Brown, S.M.; Beesley, S.J.; Schwertz, H.; Kosaka, Y.; Manne, B.K.; Krauel, K.; et al. Sepsis alters the transcriptional and translational landscape of human and murine platelets. Blood 2019, 134, 911–923. [Google Scholar] [CrossRef]
- Haas, S.; Hansson, J.; Klimmeck, D.; Loeffler, D.; Velten, L.; Uckelmann, H.; Wurzer, S.; Prendergast, A.M.; Schnell, A.; Hexel, K.; et al. Inflammation-Induced Emergency Megakaryopoiesis Driven by Hematopoietic Stem Cell-like Megakaryocyte Progenitors. Cell Stem Cell 2015, 17, 422–434. [Google Scholar] [CrossRef] [PubMed]
- Lefrancais, E.; Ortiz-Munoz, G.; Caudrillier, A.; Mallavia, B.; Liu, F.; Sayah, D.M.; Thornton, E.E.; Headley, M.B.; David, T.; Coughlin, S.R.; et al. The lung is a site of platelet biogenesis and a reservoir for haematopoietic progenitors. Nature 2017, 544, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Lefrancais, E.; Looney, M.R. Platelet Biogenesis in the Lung Circulation. Physiology 2019, 34, 392–401. [Google Scholar] [CrossRef]
- Slayton, W.B.; Georgelas, A.; Pierce, L.J.; Elenitoba-Johnson, K.S.; Perry, S.S.; Marx, M.; Spangrude, G.J. The spleen is a major site of megakaryopoiesis following transplantation of murine hematopoietic stem cells. Blood 2002, 100, 3975–3982. [Google Scholar] [CrossRef]
- Whitby, L. The significance of megakaryocytes in the peripheral circulation. Blood 1948, 3, 934–938. [Google Scholar] [CrossRef]
- Garg, N.; Gupta, R.J.; Kumar, S. Megakaryocytes in Peripheral Blood Smears. Turk. J. Haematol. 2019, 36, 212–213. [Google Scholar] [CrossRef]
- Aschoff, L. Ueber capilläre Embolie von riesenkernhaltigen Zellen. Arch. Pathol. Anat. Physiol. Klin. Med. 1893, 134, 11–25. [Google Scholar] [CrossRef]
- Pariser, D.N.; Hilt, Z.T.; Ture, S.K.; Blick-Nitko, S.K.; Looney, M.R.; Cleary, S.J.; Roman-Pagan, E.; Saunders, J., 2nd; Georas, S.N.; Veazey, J.; et al. Lung megakaryocytes are immune modulatory cells. J. Clin. Investig. 2021, 131, e137377. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.Y.; Wang, G.M.; Ke, Z.R.; Zhou, Y.; Yang, H.H.; Ma, T.L.; Guan, C.X. Megakaryocytes in pulmonary diseases. Life Sci. 2022, 301, 120602. [Google Scholar] [CrossRef] [PubMed]
- Livada, A.C.; Pariser, D.N.; Morrell, C.N. Megakaryocytes in the lung: History and future perspectives. Res. Pract. Thromb. Haemost. 2023, 7, 100053. [Google Scholar] [CrossRef] [PubMed]
- Yeung, A.K.; Villacorta-Martin, C.; Hon, S.; Rock, J.R.; Murphy, G.J. Lung megakaryocytes display distinct transcriptional and phenotypic properties. Blood Adv. 2020, 4, 6204–6217. [Google Scholar] [CrossRef]
- Navarro, S.; Mitjavila, M.T.; Katz, A.; Doly, J.; Vainchenker, W. Expression of interleukin 6 and its specific receptor by untreated and PMA-stimulated human erythroid and megakaryocytic cell lines. Exp. Hematol. 1991, 19, 11–17. [Google Scholar]
- Maratheftis, C.I.; Andreakos, E.; Moutsopoulos, H.M.; Voulgarelis, M. Toll-like receptor-4 is up-regulated in hematopoietic progenitor cells and contributes to increased apoptosis in myelodysplastic syndromes. Clin. Cancer Res. 2007, 13, 1154–1160. [Google Scholar] [CrossRef]
- Negrotto, S.; De Giusti, C.J.; Lapponi, M.J.; Etulain, J.; Rivadeneyra, L.; Pozner, R.G.; Gomez, R.M.; Schattner, M. Expression and functionality of type I interferon receptor in the megakaryocytic lineage. J. Thromb. Haemost. 2011, 9, 2477–2485. [Google Scholar] [CrossRef]
- Zufferey, A.; Speck, E.R.; Machlus, K.R.; Aslam, R.; Guo, L.; McVey, M.J.; Kim, M.; Kapur, R.; Boilard, E.; Italiano, J.E., Jr.; et al. Mature murine megakaryocytes present antigen-MHC class I molecules to T cells and transfer them to platelets. Blood Adv. 2017, 1, 1773–1785. [Google Scholar] [CrossRef]
- Gelon, L.; Fromont, L.; Lefrancais, E. Occurrence and role of lung megakaryocytes in infection and inflammation. Front. Immunol. 2022, 13, 1029223. [Google Scholar] [CrossRef]
- Nakamura-Ishizu, A.; Takubo, K.; Kobayashi, H.; Suzuki-Inoue, K.; Suda, T. Correction: CLEC-2 in megakaryocytes is critical for maintenance of hematopoietic stem cells in the bone marrow. J. Exp. Med. 2015, 212, 2323. [Google Scholar] [CrossRef]
- Tsukiji, N.; Inoue, O.; Morimoto, M.; Tatsumi, N.; Nagatomo, H.; Ueta, K.; Shirai, T.; Sasaki, T.; Otake, S.; Tamura, S.; et al. Platelets play an essential role in murine lung development through Clec-2/podoplanin interaction. Blood 2018, 132, 1167–1179. [Google Scholar] [CrossRef]
- Crist, S.A.; Elzey, B.D.; Ahmann, M.T.; Ratliff, T.L. Early growth response-1 (EGR-1) and nuclear factor of activated T cells (NFAT) cooperate to mediate CD40L expression in megakaryocytes and platelets. J. Biol. Chem. 2013, 288, 33985–33996. [Google Scholar] [CrossRef]
- Beckstead, J.H.; Stenberg, P.E.; McEver, R.P.; Shuman, M.A.; Bainton, D.F. Immunohistochemical localization of membrane and alpha-granule proteins in human megakaryocytes: Application to plastic-embedded bone marrow biopsy specimens. Blood 1986, 67, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Bury, L.; Malara, A.; Gresele, P.; Balduini, A. Outside-in signalling generated by a constitutively activated integrin alphaIIbbeta3 impairs proplatelet formation in human megakaryocytes. PLoS ONE 2012, 7, e34449. [Google Scholar] [CrossRef] [PubMed]
- Gurney, A.L.; Carver-Moore, K.; de Sauvage, F.J.; Moore, M.W. Thrombocytopenia in c-mpl-deficient mice. Science 1994, 265, 1445–1447. [Google Scholar] [CrossRef] [PubMed]
- Araki, D.; Hong, S.G.; Linde, N.; Fisk, B.; Redekar, N.; Salisbury-Ruf, C.; Krouse, A.; Engels, T.; Golomb, J.; Dagur, P.; et al. cMPL-based purification and depletion of human hematopoietic stem cells: Implications for pretransplant conditioning. Blood 2025, 145, 2978–2991. [Google Scholar] [CrossRef]
- Alexander, W.S.; Roberts, A.W.; Nicola, N.A.; Li, R.; Metcalf, D. Deficiencies in progenitor cells of multiple hematopoietic lineages and defective megakaryocytopoiesis in mice lacking the thrombopoietic receptor c-Mpl. Blood 1996, 87, 2162–2170. [Google Scholar] [CrossRef]
- Markovic, B.; Wu, Z.; Chesterman, C.N.; Chong, B.H. Quantitation of soluble and membrane-bound Fc gamma RIIA (CD32A) mRNA in platelets and megakaryoblastic cell line (Meg-01). Br. J. Haematol. 1995, 91, 37–42. [Google Scholar] [CrossRef]
- Bendas, G.; Schlesinger, M. The GPIb-IX complex on platelets: Insight into its novel physiological functions affecting immune surveillance, hepatic thrombopoietin generation, platelet clearance and its relevance for cancer development and metastasis. Exp. Hematol. Oncol. 2022, 11, 19. [Google Scholar] [CrossRef]
- Finkielsztein, A.; Schlinker, A.C.; Zhang, L.; Miller, W.M.; Datta, S.K. Human megakaryocyte progenitors derived from hematopoietic stem cells of normal individuals are MHC class II-expressing professional APC that enhance Th17 and Th1/Th17 responses. Immunol. Lett. 2015, 163, 84–95. [Google Scholar] [CrossRef]
- Coppin, E.; Florentin, J.; Vasamsetti, S.B.; Arunkumar, A.; Sembrat, J.; Rojas, M.; Dutta, P. Splenic hematopoietic stem cells display a pre-activated phenotype. Immunol. Cell Biol. 2018, 96, 772–784. [Google Scholar] [CrossRef]
- Burberry, A.; Zeng, M.Y.; Ding, L.; Wicks, I.; Inohara, N.; Morrison, S.J.; Nunez, G. Infection mobilizes hematopoietic stem cells through cooperative NOD-like receptor and Toll-like receptor signaling. Cell Host Microbe 2014, 15, 779–791. [Google Scholar] [CrossRef] [PubMed]
- Kraal, G.; Mebius, R. New insights into the cell biology of the marginal zone of the spleen. Int. Rev. Cytol. 2006, 250, 175–215. [Google Scholar] [CrossRef]
- Valet, C.; Magnen, M.; Qiu, L.; Cleary, S.J.; Wang, K.M.; Ranucci, S.; Grockowiak, E.; Boudra, R.; Conrad, C.; Seo, Y.; et al. Sepsis promotes splenic production of a protective platelet pool with high CD40 ligand expression. J. Clin. Investig. 2022, 132, e153920. [Google Scholar] [CrossRef] [PubMed]
- Sorensen, O.E.; Borregaard, N. Neutrophil extracellular traps—The dark side of neutrophils. J. Clin. Investig. 2016, 126, 1612–1620. [Google Scholar] [CrossRef]
- Cunin, P.; Nigrovic, P.A. Megakaryocytes as immune cells. J. Leukoc. Biol. 2019, 105, 1111–1121. [Google Scholar] [CrossRef]
- Wang, J.; Xie, J.; Wang, D.; Han, X.; Chen, M.; Shi, G.; Jiang, L.; Zhao, M. CXCR4(high) megakaryocytes regulate host-defense immunity against bacterial pathogens. Elife 2022, 11, e78662. [Google Scholar] [CrossRef]
- Hua, T.; Yao, F.; Wang, H.; Liu, W.; Zhu, X.; Yao, Y. Megakaryocyte in sepsis: The trinity of coagulation, inflammation and immunity. Crit. Care 2024, 28, 442. [Google Scholar] [CrossRef]
- Ku, N.K.; Rashidi, H. Unusual finding of a megakaryocyte in a peripheral blood smear. Blood 2017, 130, 2573. [Google Scholar] [CrossRef]
- Zhang, L.; Orban, M.; Lorenz, M.; Barocke, V.; Braun, D.; Urtz, N.; Schulz, C.; von Bruhl, M.L.; Tirniceriu, A.; Gaertner, F.; et al. A novel role of sphingosine 1-phosphate receptor S1pr1 in mouse thrombopoiesis. J. Exp. Med. 2012, 209, 2165–2181. [Google Scholar] [CrossRef]
- Pawig, L.; Klasen, C.; Weber, C.; Bernhagen, J.; Noels, H. Diversity and Inter-Connections in the CXCR4 Chemokine Receptor/Ligand Family: Molecular Perspectives. Front. Immunol. 2015, 6, 429. [Google Scholar] [CrossRef]
- Yang, X.; Chitalia, S.V.; Matsuura, S.; Ravid, K. Integrins and their role in megakaryocyte development and function. Exp. Hematol. 2022, 106, 31–39. [Google Scholar] [CrossRef]
- Zhang, W.; Yan, C.; Liu, X.; Yang, P.; Wang, J.; Chen, Y.; Liu, W.; Li, S.; Zhang, X.; Dong, G.; et al. Global characterization of megakaryocytes in bone marrow, peripheral blood, and cord blood by single-cell RNA sequencing. Cancer Gene Ther. 2022, 29, 1636–1647. [Google Scholar] [CrossRef] [PubMed]
- Beaulieu, L.M.; Freedman, J.E. The role of inflammation in regulating platelet production and function: Toll-like receptors in platelets and megakaryocytes. Thromb. Res. 2010, 125, 205–209. [Google Scholar] [CrossRef]
- D’Atri, L.P.; Rodriguez, C.S.; Miguel, C.P.; Pozner, R.G.; Ortiz Wilczynski, J.M.; Negrotto, S.; Carrera-Silva, E.A.; Heller, P.G.; Schattner, M. Activation of toll-like receptors 2 and 4 on CD34(+) cells increases human megakaryo/thrombopoiesis induced by thrombopoietin. J. Thromb. Haemost. 2019, 17, 2196–2210. [Google Scholar] [CrossRef] [PubMed]
- Cunin, P.; Penke, L.R.; Thon, J.N.; Monach, P.A.; Jones, T.; Chang, M.H.; Chen, M.M.; Melki, I.; Lacroix, S.; Iwakura, Y.; et al. Megakaryocytes compensate for Kit insufficiency in murine arthritis. J. Clin. Investig. 2017, 127, 1714–1724. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Shen, S.; Rowley, J.W.; Tolley, N.D.; Jia, W.; Manne, B.K.; McComas, K.N.; Bolingbroke, B.; Kosaka, Y.; Krauel, K.; et al. Platelet MHC class I mediates CD8+ T-cell suppression during sepsis. Blood 2021, 138, 401–416. [Google Scholar] [CrossRef]
- Chapman, L.M.; Aggrey, A.A.; Field, D.J.; Srivastava, K.; Ture, S.; Yui, K.; Topham, D.J.; Baldwin, W.M., 3rd; Morrell, C.N. Platelets present antigen in the context of MHC class I. J. Immunol. 2012, 189, 916–923. [Google Scholar] [CrossRef]
- Kuter, D.J.; Gminski, D.M.; Rosenberg, R.D. Transforming growth factor beta inhibits megakaryocyte growth and endomitosis. Blood 1992, 79, 619–626. [Google Scholar] [CrossRef][Green Version]
- Sakamaki, S.; Hirayama, Y.; Matsunaga, T.; Kuroda, H.; Kusakabe, T.; Akiyama, T.; Konuma, Y.; Sasaki, K.; Tsuji, N.; Okamoto, T.; et al. Transforming growth factor-beta1 (TGF-beta1) induces thrombopoietin from bone marrow stromal cells, which stimulates the expression of TGF-beta receptor on megakaryocytes and, in turn, renders them susceptible to suppression by TGF-beta itself with high specificity. Blood 1999, 94, 1961–1970. [Google Scholar]
- Lambert, M.P.; Rauova, L.; Bailey, M.; Sola-Visner, M.C.; Kowalska, M.A.; Poncz, M. Platelet factor 4 is a negative autocrine in vivo regulator of megakaryopoiesis: Clinical and therapeutic implications. Blood 2007, 110, 1153–1160. [Google Scholar] [CrossRef] [PubMed]
- Lasagni, L.; Francalanci, M.; Annunziato, F.; Lazzeri, E.; Giannini, S.; Cosmi, L.; Sagrinati, C.; Mazzinghi, B.; Orlando, C.; Maggi, E.; et al. An alternatively spliced variant of CXCR3 mediates the inhibition of endothelial cell growth induced by IP-10, Mig, and I-TAC, and acts as functional receptor for platelet factor 4. J. Exp. Med. 2003, 197, 1537–1549. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Ten Dijke, P. Immunoregulation by members of the TGFbeta superfamily. Nat. Rev. Immunol. 2016, 16, 723–740. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Hu, M.; Xu, Y.; Chen, F.; Chen, S.; Chen, M.; Qi, Y.; Shen, M.; Wang, C.; Lu, Y.; et al. Megakaryocytes promote bone formation through coupling osteogenesis with angiogenesis by secreting TGF-beta1. Theranostics 2020, 10, 2229–2242. [Google Scholar] [CrossRef]
- Deuel, T.F.; Senior, R.M.; Chang, D.; Griffin, G.L.; Heinrikson, R.L.; Kaiser, E.T. Platelet factor 4 is chemotactic for neutrophils and monocytes. Proc. Natl. Acad. Sci. USA 1981, 78, 4584–4587. [Google Scholar] [CrossRef]
- Lagraoui, M.; Gagnon, L. Enhancement of human neutrophil survival and activation by TGF-beta 1. Cell Mol. Biol. 1997, 43, 313–318. [Google Scholar]
- Brandes, M.E.; Mai, U.E.; Ohura, K.; Wahl, S.M. Type I transforming growth factor-beta receptors on neutrophils mediate chemotaxis to transforming growth factor-beta. J. Immunol. 1991, 147, 1600–1606. [Google Scholar] [CrossRef]
- Kohler, A.; De Filippo, K.; Hasenberg, M.; van den Brandt, C.; Nye, E.; Hosking, M.P.; Lane, T.E.; Mann, L.; Ransohoff, R.M.; Hauser, A.E.; et al. G-CSF-mediated thrombopoietin release triggers neutrophil motility and mobilization from bone marrow via induction of Cxcr2 ligands. Blood 2011, 117, 4349–4357. [Google Scholar] [CrossRef]
- Blair, P.; Flaumenhaft, R. Platelet alpha-granules: Basic biology and clinical correlates. Blood Rev. 2009, 23, 177–189. [Google Scholar] [CrossRef]
- Bonci, D.; Hahne, M.; Felli, N.; Peschle, C.; De Maria, R. Potential role of APRIL as autocrine growth factor for megakaryocytopoiesis. Blood 2004, 104, 3169–3172. [Google Scholar] [CrossRef]
- Beaulieu, L.M.; Lin, E.; Mick, E.; Koupenova, M.; Weinberg, E.O.; Kramer, C.D.; Genco, C.A.; Tanriverdi, K.; Larson, M.G.; Benjamin, E.J.; et al. Interleukin 1 receptor 1 and interleukin 1beta regulate megakaryocyte maturation, platelet activation, and transcript profile during inflammation in mice and humans. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 552–564. [Google Scholar] [CrossRef]
- Jiang, S.; Levine, J.D.; Fu, Y.; Deng, B.; London, R.; Groopman, J.E.; Avraham, H. Cytokine production by primary bone marrow megakaryocytes. Blood 1994, 84, 4151–4156. [Google Scholar] [CrossRef] [PubMed]
- Stahl, C.P.; Zucker-Franklin, D.; Evatt, B.L.; Winton, E.F. Effects of human interleukin-6 on megakaryocyte development and thrombocytopoiesis in primates. Blood 1991, 78, 1467–1475. [Google Scholar] [CrossRef] [PubMed]
- Italiano, J.E., Jr.; Mairuhu, A.T.; Flaumenhaft, R. Clinical relevance of microparticles from platelets and megakaryocytes. Curr. Opin. Hematol. 2010, 17, 578–584. [Google Scholar] [CrossRef] [PubMed]
- Flaumenhaft, R.; Dilks, J.R.; Richardson, J.; Alden, E.; Patel-Hett, S.R.; Battinelli, E.; Klement, G.L.; Sola-Visner, M.; Italiano, J.E., Jr. Megakaryocyte-derived microparticles: Direct visualization and distinction from platelet-derived microparticles. Blood 2009, 113, 1112–1121. [Google Scholar] [CrossRef]
- Gitz, E.; Pollitt, A.Y.; Gitz-Francois, J.J.; Alshehri, O.; Mori, J.; Montague, S.; Nash, G.B.; Douglas, M.R.; Gardiner, E.E.; Andrews, R.K.; et al. CLEC-2 expression is maintained on activated platelets and on platelet microparticles. Blood 2014, 124, 2262–2270. [Google Scholar] [CrossRef]
- Humble, J.G.; Jayne, W.H.; Pulvertaft, R.J. Biological interaction between lymphocytes and other cells. Br. J. Haematol. 1956, 2, 283–294. [Google Scholar] [CrossRef]
- Larsen, T.E. Emperipolesis of granular leukocytes within megakaryocytes in human hemopoietic bone marrow. Am. J. Clin. Pathol. 1970, 53, 485–489. [Google Scholar] [CrossRef]
- Sahebekhitiari, H.A.; Tavassoli, M. Marrow cell uptake by megakaryocytes in routine bone marrow smears during blood loss. Scand. J. Haematol. 1976, 16, 13–17. [Google Scholar] [CrossRef]
- de Pasquale, A.; Paterlini, P.; Quaglino, D.; Quaglino, D. Emperipolesis of granulocytes within megakaryocytes. Br. J. Haematol. 1985, 60, 384–386. [Google Scholar] [CrossRef]
- Cashell, A.W.; Buss, D.H. The frequency and significance of megakaryocytic emperipolesis in myeloproliferative and reactive states. Ann. Hematol. 1992, 64, 273–276. [Google Scholar] [CrossRef]
- Thiele, J.; Schneider, G.; Hoeppner, B.; Wienhold, S.; Zankovich, R.; Fischer, R. Histomorphometry of bone marrow biopsies in chronic myeloproliferative disorders with associated thrombocytosis—Features of significance for the diagnosis of primary (essential) thrombocythaemia. Virchows Arch. A 1988, 413, 407–417. [Google Scholar] [CrossRef]
- Thiele, J.; Kuemmel, T.; Sander, C.; Fischer, R. Ultrastructure of bone marrow tissue in so-called primary (idiopathic) myelofibrosis-osteomyelosclerosis (agnogenic myeloid metaplasia). I. Abnormalities of megakaryopoiesis and thrombocytes. J. Submicrosc. Cytol. Pathol. 1991, 23, 93–107. [Google Scholar] [PubMed]
- Tanaka, M.; Aze, Y.; Shinomiya, K.; Fujita, T. Morphological observations of megakaryocytic emperipolesis in the bone marrow of rats treated with lipopolysaccharide. J. Vet. Med. Sci. 1996, 58, 663–667. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Ren, J.; Liu, Q.; Li, J.; Wu, X.; Wang, W.; Wu, J.; Wang, G.; Li, J. Hypermucoviscous Klebsiella pneumoniae infections induce platelet aggregation and apoptosis and inhibit maturation of megakaryocytes. Thromb. Res. 2018, 171, 45–54. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, H.; Li, T.; Jiang, Z.; Yuan, Y.; Chen, X. Fusobacterium nucleatum Promotes Megakaryocyte Maturation in Patients with Gastric Cancer via Inducing the Production of Extracellular Vesicles Containing 14-3-3epsilon. Infect. Immun. 2023, 91, e0010223. [Google Scholar] [CrossRef]
- Gopala Krishnan, G.K.; Sethumadhavan, A.; Vellaichamy, P.; Mani, M. Pseudomonas aeruginosa infection stimulates mitogen-activated protein kinases signaling pathway in human megakaryocytes. Microbiol. Immunol. 2019, 63, 229–237. [Google Scholar] [CrossRef]
- Lin, G.L.; Chang, H.H.; Lin, W.T.; Liou, Y.S.; Lai, Y.L.; Hsieh, M.H.; Chen, P.K.; Liao, C.Y.; Tsai, C.C.; Wang, T.F.; et al. Dachshund Homolog 1: Unveiling Its Potential Role in Megakaryopoiesis and Bacillus anthracis Lethal Toxin-Induced Thrombocytopenia. Int. J. Mol. Sci. 2024, 25, 3102. [Google Scholar] [CrossRef]
- Kelly, L.S.; Darden, D.B.; Fenner, B.P.; Efron, P.A.; Mohr, A.M. The Hematopoietic Stem/Progenitor Cell Response to Hemorrhage, Injury, and Sepsis: A Review of Pathophysiology. Shock 2021, 56, 30–41. [Google Scholar] [CrossRef]
- Tang, X.; Xu, Q.; Yang, S.; Huang, X.; Wang, L.; Huang, F.; Luo, J.; Zhou, X.; Wu, A.; Mei, Q.; et al. Toll-like Receptors and Thrombopoiesis. Int. J. Mol. Sci. 2023, 24, 1010. [Google Scholar] [CrossRef]
- Handtke, S.; Thiele, T. Large and small platelets-(When) do they differ? J. Thromb. Haemost. 2020, 18, 1256–1267. [Google Scholar] [CrossRef]
- Velez-Paez, J.L.; Legua, P.; Velez-Paez, P.; Irigoyen, E.; Andrade, H.; Jara, A.; Lopez, F.; Perez-Galarza, J.; Baldeon, L. Mean platelet volume and mean platelet volume to platelet count ratio as predictors of severity and mortality in sepsis. PLoS ONE 2022, 17, e0262356. [Google Scholar] [CrossRef] [PubMed]
- Leung, G.; Middleton, E.A. The role of platelets and megakaryocytes in sepsis and ARDS. J. Physiol. 2024, 602, 6047–6063. [Google Scholar] [CrossRef] [PubMed]
- Ajanel, A.; Middleton, E.A. Alterations in the megakaryocyte transcriptome impacts platelet function in sepsis and COVID-19 infection. Thromb. Res. 2023, 231, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Thorup, C.V.; Christensen, S.; Hvas, A.M. Immature Platelets As a Predictor of Disease Severity and Mortality in Sepsis and Septic Shock: A Systematic Review. Semin. Thromb. Hemost. 2020, 46, 320–327. [Google Scholar] [CrossRef]
- Qiu, X.R.; Nair, M.G.; Jaroszewski, L.; Godzik, A. Deciphering Abnormal Platelet Subpopulations in COVID-19, Sepsis and Systemic Lupus Erythematosus through Machine Learning and Single-Cell Transcriptomics. Int. J. Mol. Sci. 2024, 25, 5941. [Google Scholar] [CrossRef]
- Yang, X.; Song, J.; Ma, H.; Hua, N.; Bai, Y.; Ju, Y.; Shen, J.W.; Zheng, W.; Jiang, S. The Crucial Roles of Platelets as Immune Mediators in Sepsis. J. Inflamm. Res. 2025, 18, 12825–12845. [Google Scholar] [CrossRef]
- Zhou, H.; Deng, M.; Liu, Y.; Yang, C.; Hoffman, R.; Zhou, J.; Loughran, P.A.; Scott, M.J.; Neal, M.D.; Billiar, T.R. Platelet HMGB1 is required for efficient bacterial clearance in intra-abdominal bacterial sepsis in mice. Blood Adv. 2018, 2, 638–648. [Google Scholar] [CrossRef]
- Kim, S.M.; Kim, S.I.; Yu, G.; Kim, J.S.; Hong, S.I.; Kim, W.Y. Hypercoagulability in Septic Shock Patients With Thrombocytopenia. J. Intensive Care Med. 2022, 37, 721–727. [Google Scholar] [CrossRef]
- Papageorgiou, C.; Jourdi, G.; Adjambri, E.; Walborn, A.; Patel, P.; Fareed, J.; Elalamy, I.; Hoppensteadt, D.; Gerotziafas, G.T. Disseminated Intravascular Coagulation: An Update on Pathogenesis, Diagnosis, and Therapeutic Strategies. Clin. Appl. Thromb. Hemost. 2018, 24, 8S–28S. [Google Scholar] [CrossRef]
- Semeraro, N.; Ammollo, C.T.; Semeraro, F.; Colucci, M. Sepsis-associated disseminated intravascular coagulation and thromboembolic disease. Mediterr. J. Hematol. Infect. Dis. 2010, 2, e2010024. [Google Scholar] [CrossRef] [PubMed]
- Cox, D. Sepsis—It is all about the platelets. Front. Immunol. 2023, 14, 1210219. [Google Scholar] [CrossRef] [PubMed]
- Couldwell, G.; Machlus, K.R. Modulation of megakaryopoiesis and platelet production during inflammation. Thromb. Res. 2019, 179, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Kao, C.Y.; Papoutsakis, E.T. Engineering human megakaryocytic microparticles for targeted delivery of nucleic acids to hematopoietic stem and progenitor cells. Sci. Adv. 2018, 4, eaau6762. [Google Scholar] [CrossRef]
- Chattapadhyaya, S.; Haldar, S.; Banerjee, S. Microvesicles promote megakaryopoiesis by regulating DNA methyltransferase and methylation of Notch1 promoter. J. Cell Physiol. 2020, 235, 2619–2630. [Google Scholar] [CrossRef]
- Escobar, C.; Kao, C.Y.; Das, S.; Papoutsakis, E.T. Human megakaryocytic microparticles induce de novo platelet biogenesis in a wild-type murine model. Blood Adv. 2020, 4, 804–814. [Google Scholar] [CrossRef]
- Lyde, R.B.; Ahn, H.S.; Vo, K.K.; Jarocha, D.J.; Tkaczynski, J.; Treffeisen, E.; Sullivan, S.K.; Camire, R.M.; Sabatino, D.E.; French, D.L.; et al. Infused factor VIII-expressing platelets or megakaryocytes as a novel therapeutic strategy for hemophilia A. Blood Adv. 2019, 3, 1368–1378. [Google Scholar] [CrossRef]
- Borger, A.K.; Eicke, D.; Wolf, C.; Gras, C.; Aufderbeck, S.; Schulze, K.; Engels, L.; Eiz-Vesper, B.; Schambach, A.; Guzman, C.A.; et al. Generation of HLA-Universal iPSC-Derived Megakaryocytes and Platelets for Survival Under Refractoriness Conditions. Mol. Med. 2016, 22, 274–285. [Google Scholar] [CrossRef]
- Leung, J.; Primbetova, A.; Strong, C.; Hay, B.N.; Hsu, H.H.; Hagner, A.; Foster, L.J.; Devine, D.; Cullis, P.R.; Zandstra, P.W.; et al. Genetic engineering of megakaryocytes from blood progenitor cells using messenger RNA lipid nanoparticles. J. Thromb. Haemost. 2025, 23, 306–313. [Google Scholar] [CrossRef]
- Bussel, J.B.; Cheng, G.; Saleh, M.N.; Psaila, B.; Kovaleva, L.; Meddeb, B.; Kloczko, J.; Hassani, H.; Mayer, B.; Stone, N.L.; et al. Eltrombopag for the treatment of chronic idiopathic thrombocytopenic purpura. N. Engl. J. Med. 2007, 357, 2237–2247. [Google Scholar] [CrossRef]
- Hasan, M.J.; Rabbani, R.; Huq, S.M.R. The Use of High Dose Eltrombopag in the Management of Sepsis-Associated Thrombocytopenia in Critically Ill Patients. J. Crit. Care Med. 2019, 5, 123–129. [Google Scholar] [CrossRef]
- Guan, X.; Wang, L.; Wang, H.; Wang, H.; Dai, W.; Jiang, Y. Good Manufacturing Practice-Grade of Megakaryocytes Produced by a Novel Ex Vivo Culturing Platform. Clin. Transl. Sci. 2020, 13, 1115–1126. [Google Scholar] [CrossRef]
- Martinez, A.F.; Miller, W.M. Enabling Large-Scale Ex Vivo Production of Megakaryocytes from CD34(+) Cells Using Gas-Permeable Surfaces. Stem Cells Transl. Med. 2019, 8, 658–670. [Google Scholar] [CrossRef]
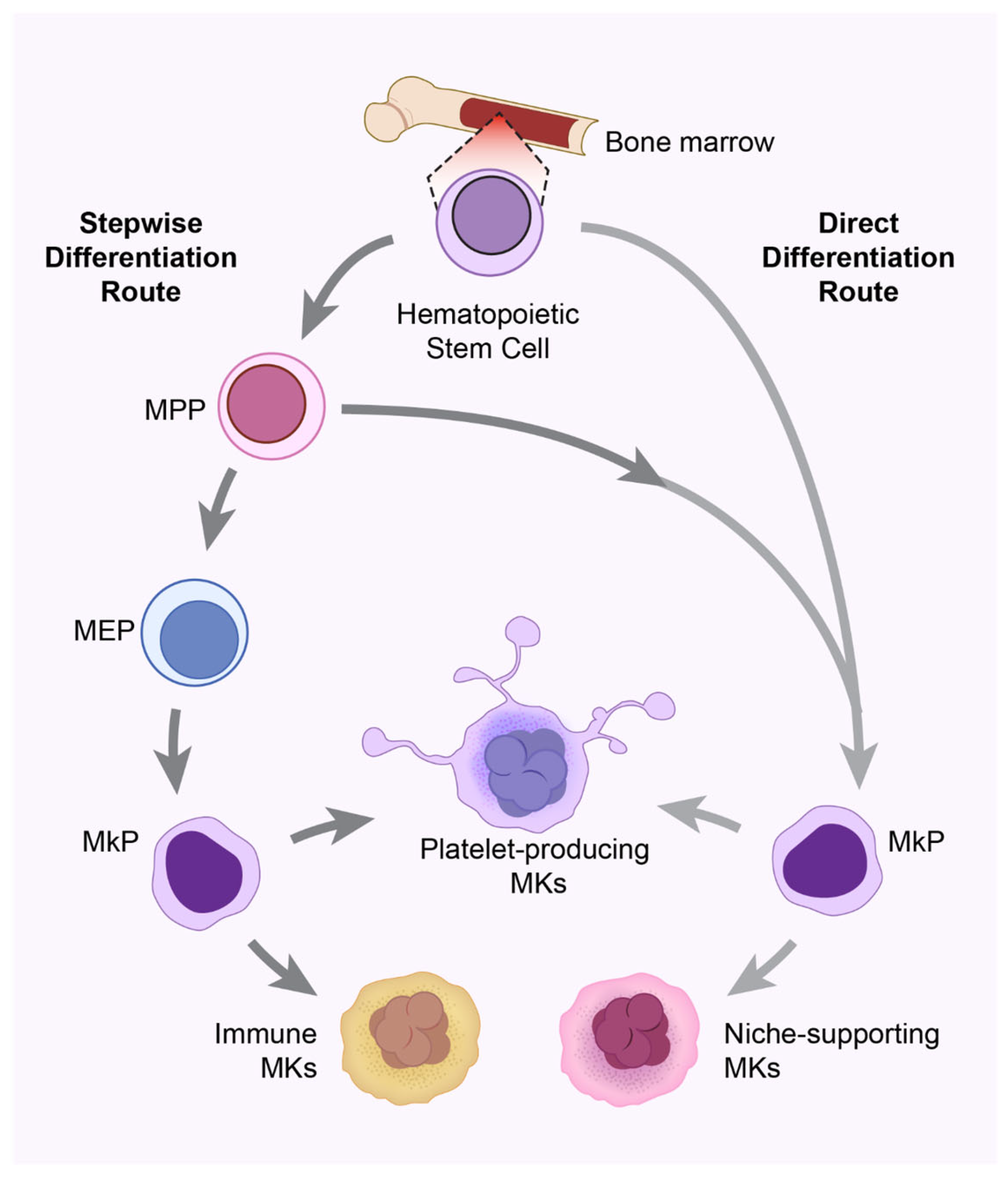
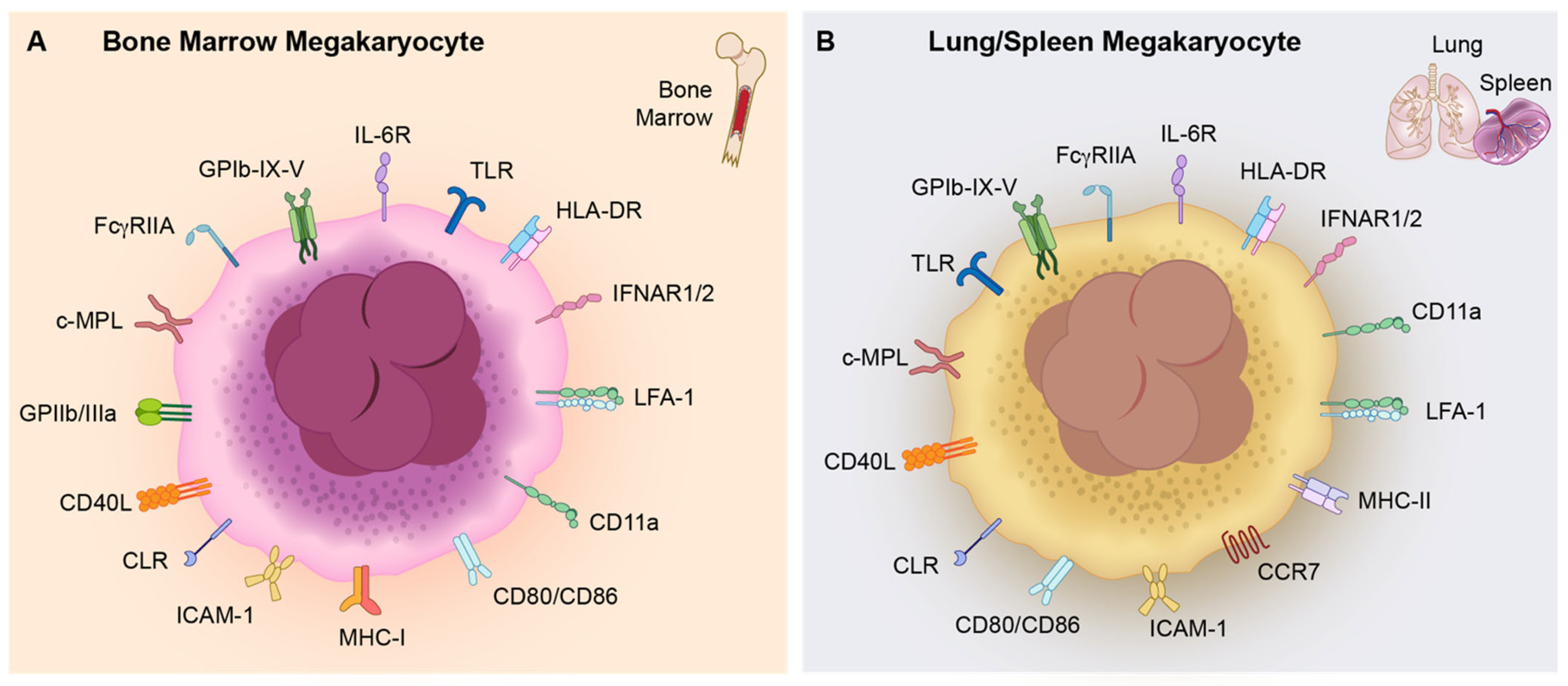
| MK Subset | Ploidy | Associated Markers | Functions |
|---|---|---|---|
| Immune MKs | 2N–8N | CD53+/LSP1+, Spi1, Cebpd, Irf5, Irf8 | Immune-related Functions (Phagocytosis, Expansion under stimuli, antigen presentation and T-cell activation), Not platelet-producing |
| HSC niche-supporting MKs | 8N–32N | MYLK4+, Igf1, Pf4, Wnt3a, Wnt4, Dkk1, Dkk2 | HSC regulation |
| Platelet-producing MKs | >32N | Tubb1, Myh9, Vwf, Gp1ba, Gp5, Gp6, P2yr1, P2yr12 | Platelet production, hemostasis |
| Polyploidization MKs | 2N–32N | Pola2, Pold2 | Cell cycle regulation |
| Ploidy Status and Surface Markers | Bone Marrow MKs | Extramedullary MKs (Lung/Spleen) | References |
|---|---|---|---|
| Ploidy status | 2–128N | 2–16N (low-ploidy cells) | [7,9,52] |
| IL-6R | ✔ | ✔ | [5,56] |
| TLR | ✔ | ✔ | [55,57] |
| HLA-DR | ✔ | ✔ | [52] |
| IFNAR1/2 | ✔ | ✔ | [58] |
| LFA-1 | ✔ | ✔ | [14,52] |
| CD11a | ✔ | ✔ | [52] |
| CD80/CD86 | ✔ | ✔ | [52,59] |
| MHC-I | ✔ | ✖ | [59,60] |
| ICAM-1 | ✔ | ✔ | [52] |
| CLR | ✔ | ✔ | [60,61,62] |
| CD40L | ✔ | ✔ | [52,63] |
| GPIIb/IIIa | ✔ | ✔ | [64,65] |
| c-MPL | ✔ | ✔ | [66,67,68] |
| FcγRIIA | ✔ | ✔ | [5,69] |
| GPIb-IX-V | ✔ | ✔ | [5,70] |
| MHC-II | ✖ | ✔ | [52,71] |
| CCR7 | * | ✔ | [52] |
| Pathogen | Mechanism | Outcomes | Classification |
|---|---|---|---|
| Klebsiella pneumoniae | Platelet activation → apoptosis → inhibited MK maturation | ↓ Platelets, ↑ bleeding | Thrombocytopenia-inducing |
| Fusobacterium nucleatum | Aberrant MK maturation → excessive thrombosis | ↑ Clotting | Thrombosis-inducing |
| Pseudomonas aeruginosa | MK cytotoxicity via p38 → reduced platelet count | ↓ Platelets | Thrombocytopenia-inducing |
| Bacillus anthracis | Downregulation of DACH1 → reduced platelet production | ↓ Platelets | Thrombocytopenia-inducing |
| Escherichia coli | Internalization via MHC receptors → antigen processing | ↑ Antigen Presentation | Activation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leardini-Tristão, M.; Banerjee, M. Beyond Thrombopoiesis: The Immune Functions of Megakaryocytes in Bacterial Infections and Sepsis. Int. J. Mol. Sci. 2025, 26, 11191. https://doi.org/10.3390/ijms262211191
Leardini-Tristão M, Banerjee M. Beyond Thrombopoiesis: The Immune Functions of Megakaryocytes in Bacterial Infections and Sepsis. International Journal of Molecular Sciences. 2025; 26(22):11191. https://doi.org/10.3390/ijms262211191
Chicago/Turabian StyleLeardini-Tristão, Marina, and Meenakshi Banerjee. 2025. "Beyond Thrombopoiesis: The Immune Functions of Megakaryocytes in Bacterial Infections and Sepsis" International Journal of Molecular Sciences 26, no. 22: 11191. https://doi.org/10.3390/ijms262211191
APA StyleLeardini-Tristão, M., & Banerjee, M. (2025). Beyond Thrombopoiesis: The Immune Functions of Megakaryocytes in Bacterial Infections and Sepsis. International Journal of Molecular Sciences, 26(22), 11191. https://doi.org/10.3390/ijms262211191






