Enhanced Antibacterial and Anti-Inflammatory Activities of the Combination of Cannabis sativa and Propolis Extracts: An In Vitro Study
Abstract
1. Introduction
2. Results
2.1. HPTLC-Based Preliminary Chemical Profiling of C. sativa and Propolis Extract
2.2. CS and PE Combination Exhibits an Additive Effect on Antibacterial Activity
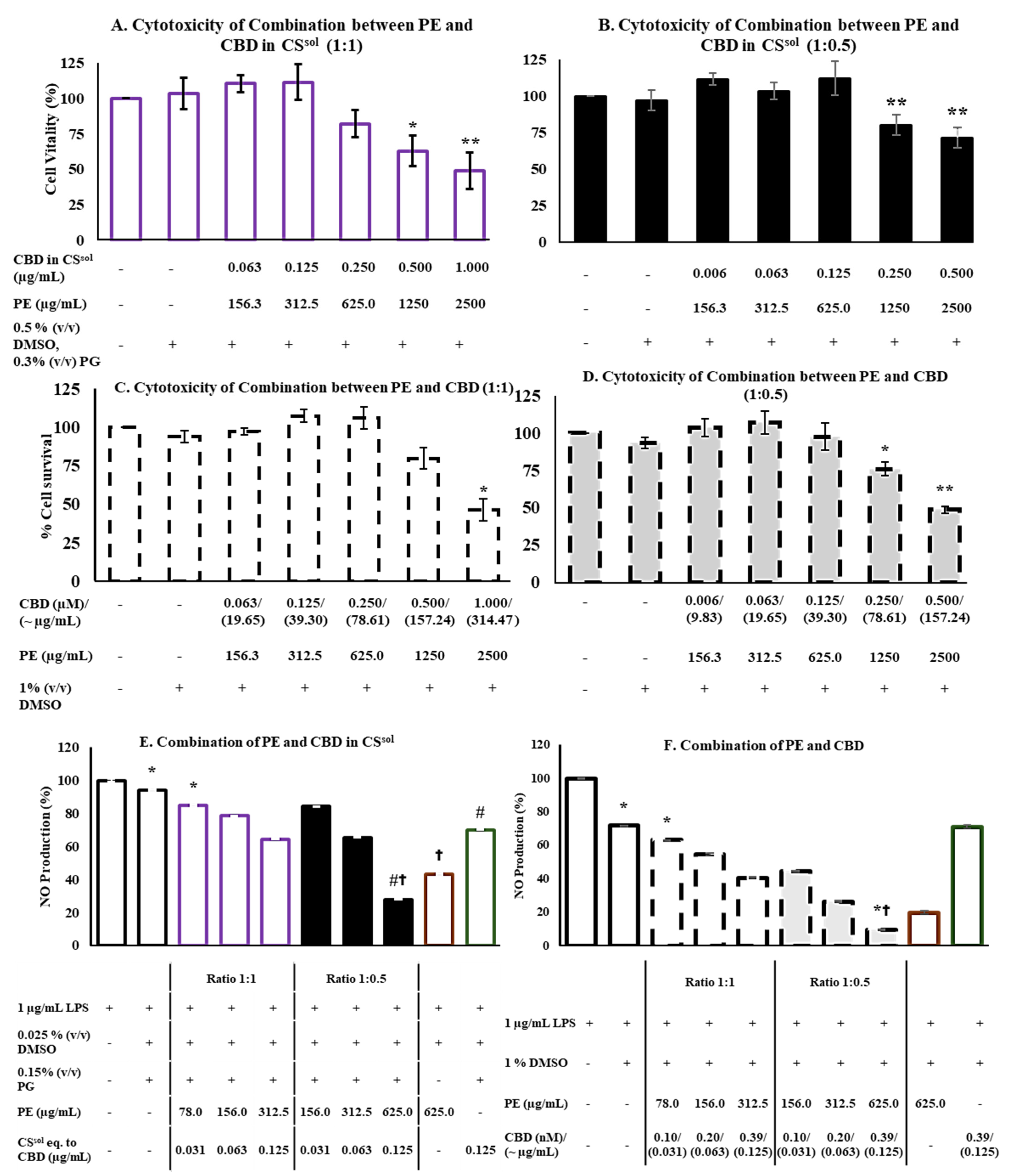
2.3. The Optimization of Combination Between CS and PE
2.4. Cytotoxicity
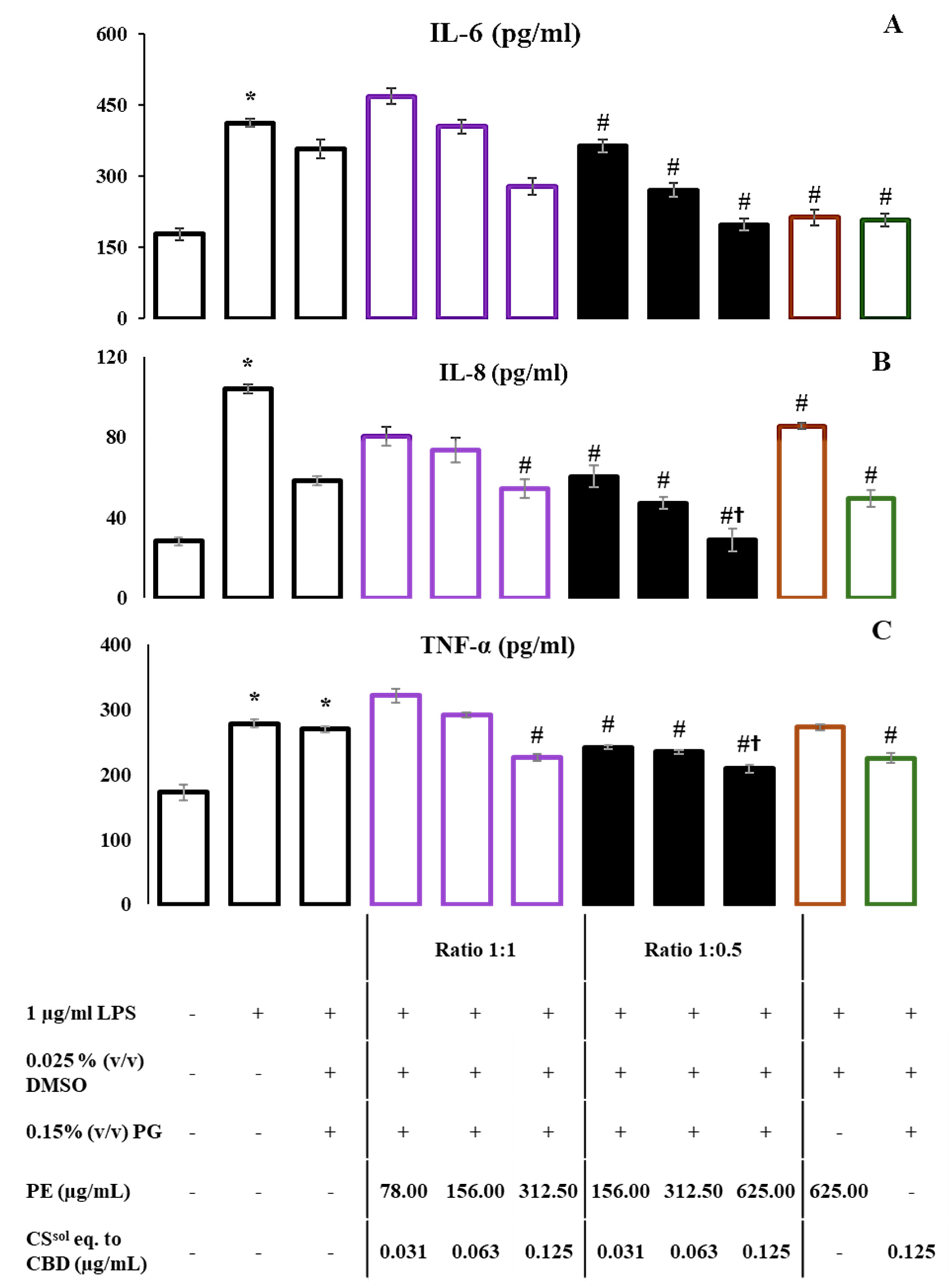
2.5. CS and PE Combination Significantly Reduced NO Production and Suppressed the Release of Proinflammatory Cytokines
3. Discussion
4. Materials and Methods
4.1. Materials and Sample Preparation
4.2. CS Sample Preparation
4.3. Propolis Sample Preparation
4.4. High-Performance Thin-Layer Chromatography Analysis
4.5. Antibacterial Activity
4.5.1. Microorganisms, Culture Media, and Maintenance Protocols
4.5.2. Agar Disk Diffusion Method
4.5.3. Minimum Inhibitory Concentration and Minimum Bactericidal Concentration
4.5.4. Fractional Inhibitory Concentration and Index Using the Broth Microdilution Checkerboard Method
4.5.5. Thin-Layer Chromatography-Bioautography
4.5.6. Anti-Inflammatory Activity
4.5.7. NO Inhibitory Assay and ELISA
4.5.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cappelletty, D. Microbiology of bacterial respiratory infections. Pediatr. Infect. Dis. J. 1998, 17, S55–S61. [Google Scholar] [CrossRef] [PubMed]
- Ghlichloo, I.; Gerriets, V. Nonsteroidal Anti-Inflammatory Drugs (NSAIDs); StatPearls Publishing: Treasure Island, FL, USA, 2019. [Google Scholar]
- Department of Thai Traditional and Alternative Medicine, Ministry of Public Health of Thailand. Thai Herbal Medicine in Thailand National List of Essential Medicines Replacing Modern Medicine; Ministry of Public Health of Thailand: Nonthaburi, Thailand, 2024; pp. 1–12. [Google Scholar]
- Herbal Product Division. National List of Essential Medicines for Herbs 2023 Manual; Department of Food and Drug Administration (FDA), Ministry of Public Health: Nonthaburi, Thailand, 2023; pp. 1–132. [Google Scholar]
- Maneekul, U.; Samranjit, K. Textbook of General Thai Traditional Medicine, Branch of Thai Pharmacy, 2nd ed.; Division of Healing Arts Department of Health Service Support, Thai Phum Publishing: Nonthaburi, Thailand, 1998. [Google Scholar]
- Odieka, A.E.; Obuzor, G.U.; Oyedeji, O.O.; Gondwe, M.; Hosu, Y.S.; Oyedeji, A.O. The medicinal natural products of Cannabis sativa Linn.: A review. Molecules 2022, 27, 1689. [Google Scholar] [CrossRef]
- Notcutt, W.; Price, M.; Miller, R.; Newport, S.; Phillips, C.; Simmons, S.; Sansom, C. Initial experiences with medicinal extracts of cannabis for chronic pain: Results from 34 ‘N of 1’studies. Anaesthesia 2004, 59, 440–452. [Google Scholar] [CrossRef] [PubMed]
- Narongvit, T.N. The effectiveness of cannabis used in Thai Traditional way on the palliative care patients in Thailand. J. Assoc. Res. 2021, 26, 219–232. [Google Scholar]
- Mlost, J.; Bryk, M.; Starowicz, K. Cannabidiol for pain treatment: Focus on pharmacology and mechanism of action. Int. J. Mol. Sci. 2020, 21, 8870. [Google Scholar] [CrossRef]
- Pitakbut, T.; Nguyen, G.N.; Kayser, O. Activity of THC, CBD, and CBN on human ACE2 and SARS-COV1/2 main protease to understand antiviral defense mechanism. Planta Med. 2022, 88, 1047–1059. [Google Scholar] [CrossRef] [PubMed]
- Muhammad Zen, N.A.; Kobtrakul, K.; Khositanon, P.; Sanookpan, K.; Buranasudja, V.; Vimolmangkang, S. Vegetable Oil-Based Cannabis: Its Cannabinoid Profiling and Photoprotective Effect on UVA-Irradiated Human Skin Keratinocytes. Thai J. Pharm. Sci. 2023, 46, 720–733. [Google Scholar] [CrossRef]
- Duangnin, N.; Klangjorhor, J.; Tipparat, P.; Pinmanee, S.; Phitak, T.; Pothacharoen, P.; Kongtawelert, P. Anti-inflammatory effect of methanol extracts of hemp leaf in IL-1β-induced synovitis. Trop. J. Pharm. Res. 2017, 16, 1553–1563. [Google Scholar] [CrossRef]
- Niyangoda, D.; Aung, M.L.; Qader, M.; Tesfaye, W.; Bushell, M.; Chiong, F.; Tsai, D.; Ahmad, D.; Samarawickrema, I.; Sinnollareddy, M.; et al. Cannabinoids as Antibacterial Agents: A Systematic and Critical Review of In Vitro Efficacy Against Streptococcus and Staphylococcus. Antibiotics 2024, 13, 1023. [Google Scholar] [CrossRef]
- Rajan, T.S.; Giacoppo, S.; Iori, R.; De Nicola, G.R.; Grassi, G.; Pollastro, F.; Bramanti, P.; Mazzon, E. Anti-inflammatory and antioxidant effects of a combination of cannabidiol and moringin in LPS-stimulated macrophages. Fitoterapia 2016, 112, 104–115. [Google Scholar] [CrossRef]
- Ayad, A.S.; Benchaabane, S.; Daas, T.; Smagghe, G.; Loucif-Ayad, W. Propolis Stands out as a Multifaceted Natural Product: Meta-Analysis on Its Sources, Bioactivities, Applications, and Future Perspectives. Life 2025, 15, 764. [Google Scholar] [CrossRef] [PubMed]
- Silva-Carvalho, R.; Baltazar, F.; Almeida-Aguiar, C. Propolis: A Complex Natural Product with a Plethora of Biological Activities That Can Be Explored for Drug Development. Evid. Based Complement. Altern. Med. 2015, 2015, 206439. [Google Scholar] [CrossRef]
- Kamiya, T.; Nishihara, H.; Hara, H.; Adachi, T. Ethanol extract of Brazilian red propolis induces apoptosis in human breast cancer MCF-7 cells through endoplasmic reticulum stress. J. Agric. Food Chem. 2012, 60, 11065–11070. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Yang, H.; Zhang, X.; Yu, L. Identification and quantification of phytochemical composition and anti-inflammatory and radical scavenging properties of methanolic extracts of chinese propolis. J. Agric. Food Chem. 2012, 60, 12403–12410. [Google Scholar] [CrossRef]
- Inui, S.; Hosoya, T.; Shimamura, Y.; Masuda, S.; Ogawa, T.; Kobayashi, H.; Shirafuji, K.; Moli, R.T.; Kozone, I.; Shin-ya, K.; et al. Solophenols b–d and solomonin: New prenylated polyphenols isolated from propolis collected from the Solomon islands and their antibacterial activity. J. Agric. Food Chem. 2012, 60, 11765–11770. [Google Scholar] [CrossRef]
- Al-Waili, N.; Al-Ghamdi, A.; Ansari, M.J.; Al-Attal, Y.; Salom, K. Synergistic effects of honey and propolis toward drug multi-resistant Staphylococcus aureus, Escherichia coli and Candida albicans isolates in single and polymicrobial cultures. Int. J. Med. Sci. 2012, 9, 793–800. [Google Scholar] [CrossRef]
- Özkök, A.; Karlıdağ, S.; Keskin, M.; Bayram, S.; Keskin, Ş.; Karabulut, E.; Çiçek, F.; Yılmaz, İ. Palynological, chemical, antimicrobial, and enzyme inhibition properties of Cannabis sativa L. propolis. Eur. Food Res. Technol. 2023, 249, 2175–2187. [Google Scholar] [CrossRef]
- Shivatare, R.S.; Nagore, D.H.; Nipanikar, S.U. HPTLC’an important tool in standardization of herbal medical product: A review. J. Sci. Innov. Res. 2013, 2, 1086–1096. [Google Scholar]
- Department of Medical Sciences, Ministry of Public Health. Cannabis Extract (TP Monograph). In Thai Pharmacopoeia II SUPPLEMENT 2020; The Thai Pharmacopoeia Committee; The Agricultural Co-operative Federation of Thailand, Ltd.: Bangkok, Thailand, 2020; Volume 1, Part 1; pp. 17–19. [Google Scholar]
- Milojković Opsenica, D.; Ristivojević, P.; Trifković, J.; Vovk, I.; Lušić, D.; Tešić, Ž. TLC fingerprinting and pattern recognition methods in the assessment of authenticity of poplar-type propolis. J. Chromatogr. Sci. 2016, 54, 1077–1083. [Google Scholar] [CrossRef]
- United Nations Office on Drugs and Crime. Recommended Methods for the Identification and Analysis of Cannabis and Cannabis Products: Manual for Use by National Drug Testing Laboratories; United Nations Publications: Vienna, Austria, 2009.
- The International Association for the Advancement of High Performance Thin Layer Chromatography (HPTLC Association). Cannabis (Cannabis sativa)—Normal Phase Separation; The International Association for the Advancement of HPTLC: Zurich, Switzerland, 2017; pp. 1–2. [Google Scholar]
- EUCAST. Terminology relating to methods for the determination of susceptibility of bacteria to antimicrobial agents. Clin. Microbiol. Infect. 2000, 6, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Department of Medical Sciences, Ministry of Public Health. KANCHA, RUEAN CHO DOK PHET MIA (Cannabis sativa L.). In Thai Herbal Pharmacopoeia; The Thai Herbal Pharmacopoeia Committee; The Agricultural Co-operative Federation of Thailand, Ltd.: Bangkok, Thailand, 2021; Volume 1, pp. 153–163. [Google Scholar]
- Ristivojević, P.; Dimkić, I.; Trifković, J.; Berić, T.; Vovk, I.; Milojković-Opsenica, D.; Stanković, S. Antimicrobial Activity of Serbian Propolis Evaluated by Means of MIC, HPTLC, Bioautography and Chemometrics. PLoS ONE 2016, 11, e0157097. [Google Scholar] [CrossRef] [PubMed]
- Borozan, A.B.; Popescu, S.; Madosa, E.; Ciulca, A.; Moldovan, C.; Gergen, I. Comparative study on the antimicrobial activity of propolis, catechin, quercetin and gallic acid. Not. Bot. Horti Agrobot. Cluj-Napoca 2023, 51, 12826. [Google Scholar] [CrossRef]
- Department of Medical Sciences, Ministry of Public Health. KOT PHUNG PLA (Terminalia chebula Retz.). In Thai Herbal Pharmacopoeia; The Thai Herbal Pharmacopoeia Committee; The Agricultural Co-operative Federation of Thailand, Ltd.: Bangkok, Thailand, 2021; Volume 1, pp. 316–323. [Google Scholar]
- Department of Medical Sciences, Ministry of Public Health. MAKHAM POM (Phyllanthus emblica L.). In Thai Herbal Pharmacopoeia; The Thai Herbal Pharmacopoeia Committee; The Agricultural Co-operative Federation of Thailand, Ltd.: Bangkok, Thailand, 2021; Volume 1, pp. 426–433. [Google Scholar]
- Achenbach, J.; Deyerling, N.; Mello dos Santos, M.; Sultana, S.; Islam, M.K.; Locher, C. Physicochemical Properties, Antioxidant Activity, and High-Performance Thin-Layer Chromatography Profiling of Propolis Samples from Western Australia. Plants 2024, 13, 1919. [Google Scholar] [CrossRef]
- Trusheva, B.; Petkov, H.; Chimshirova, R.; Popova, M.; Dimitrova, L.; Zaharieva, M.M.; Ilieva, Y.; Vasileva, B.; Tsvetkova, I.; Najdenski, H.; et al. Insight into the influence of natural deep eutectic solvents on the extraction of phenolic compounds from poplar type propolis: Composition and in vitro biological activity. Heliyon 2024, 10, e28621. [Google Scholar] [CrossRef]
- Salehi, A.; Rezaei, A.; Damavandi, M.S.; Kharazmi, M.S.; Jafari, S.M. Almond gum-sodium caseinate complexes for loading propolis extract: Characterization, antibacterial activity, release, and in-vitro cytotoxicity. Food Chem. 2023, 405, 134801. [Google Scholar] [CrossRef]
- Jokić, S.; Jerković, I.; Pavić, V.; Aladić, K.; Molnar, M.; Kovač, M.J.; Vladimir-Knežević, S. Terpenes and Cannabinoids in Supercritical CO2 Extracts of Industrial Hemp Inflorescences: Optimization of Extraction, Antiradical and Antibacterial Activity. Pharmaceuticals 2022, 15, 1117. [Google Scholar] [CrossRef]
- Ferrante, C.; Recinella, L.; Ronci, M.; Menghini, L.; Brunetti, L.; Chiavaroli, A.; Leone, S.; Di Iorio, L.; Carradori, S.; Tirillini, B.; et al. Multiple pharmacognostic characterization on hemp commercial cultivars: Focus on inflorescence water extract activity. Food Chem. Toxicol. 2019, 125, 452–461. [Google Scholar] [CrossRef] [PubMed]
- Martinenghi, L.D.; Jønsson, R.; Lund, T.; Jenssen, H. Isolation, Purification, and Antimicrobial Characterization of Cannabidiolic Acid and Cannabidiol from Cannabis sativa L. Biomolecules 2020, 10, 900. [Google Scholar] [CrossRef]
- Blaskovich, M.A.T.; Kavanagh, A.M.; Elliott, A.G.; Zhang, B.; Ramu, S.; Amado, M.; Lowe, G.J.; Hinton, A.O.; Pham, D.M.T.; Zuegg, J.; et al. The antimicrobial potential of cannabidiol. Commun. Biol. 2021, 4, 7. [Google Scholar] [CrossRef]
- Kasote, D.; Ahmad, A.; Chen, W.; Combrinck, S.; Viljoen, A. HPTLC-MS as an efficient hyphenated technique for the rapid identification of antimicrobial compounds from propolis. Phytochem. Lett. 2015, 11, 326–331. [Google Scholar] [CrossRef]
- Kongkadee, K.; Wisuitiprot, W.; Ingkaninan, K.; Waranuch, N. Anti-inflammation and gingival wound healing activities of Cannabis sativa L. subsp. sativa (hemp) extract and cannabidiol: An in vitro study. Arch. Oral Biol. 2022, 140, 105464. [Google Scholar] [CrossRef]
- Zhang, W.; Cai, Y.; Chen, X.; Ji, T.; Sun, L. Optimized extraction based on the terpenoids of Heterotrigona itama propolis and their antioxidative and anti-inflammatory activities. J. Food Biochem. 2020, 44, e13296. [Google Scholar] [CrossRef]
- Tristao Santini, A.; Otacílio Pinto, R.A.; Goldoni Lazarini, J.; Vieira de Morais, D.; Alves de Piloto Fernandes, A.M.; Franchin, M.; Lunardelli Negreiros de Carvalho, P.; Girotto Pressete, C.; Rosalen, P.L.; Matias de Alencar, S.; et al. Bioactives of Melipona rufiventris propolis: Exploring its antimicrobial, anti-inflammatory, and antioxidant activities. Chem. Biodivers. 2024, 21, e202302084. [Google Scholar] [CrossRef]
- Zulhendri, F.; Lesmana, R.; Tandean, S.; Christoper, A.; Chandrasekaran, K.; Irsyam, I.; Suwantika, A.A.; Abdulah, R.; Wathoni, N. Recent Update on the Anti-Inflammatory Activities of Propolis. Molecules 2022, 27, 8473. [Google Scholar] [CrossRef] [PubMed]
- Ristivojević, P.; Andrić, F.L.; Trifković, J.Đ.; Vovk, I.; Stanisavljević, L.Ž.; Tešić, Ž.L.; Milojković-Opsenica, D.M. Pattern recognition methods and multivariate image analysis in HPTLC fingerprinting of propolis extracts. J. Chemom. 2014, 28, 301–310. [Google Scholar] [CrossRef]
- Weinstein, M.P.; Lewis, J.S. The Clinical and Laboratory Standards Institute Subcommittee on Antimicrobial Susceptibility Testing: Background, Organization, Functions, and Processes. J. Clin. Microbiol. 2020, 58, e01864-01819. [Google Scholar] [CrossRef] [PubMed]
- EUCAST. Determination of Minimum Inhibitory Concentrations (MICs) of Antibacterial Agents by Broth Dilution. European Committee for Antimicrobial Susceptibility Testing of the European Society of Clinical Microbiology Infectious Diseases. 2003, Volume 9, pp. 467–474. Available online: https://www.clinicalmicrobiologyandinfection.org/article/S1198-743X(14)64063-5/fulltext (accessed on 3 June 2024).
- Buranasudja, V.; Vimolmangkang, S.; Sanookpan, K.; Binalee, A.; Bao Nguyen, A.; Dong Thach, U.; Hang Do, B.; Son Dang, V.; Minh Do, K.; Minh Nguyen, H. Antioxidant, Anti-Skin-Aging, Anti-Inflammatory, and Anti-Acetylcholinesterase Activities of Rourea oligophlebia Extracts. Chem. Biodivers. 2023, 20, e202201096. [Google Scholar] [CrossRef]
- Perstwong, N.; Binalee, A.; Kobtrakul, K.; Phongsopitanun, W.; Sanookpan, K.; Areecheewakul, S.; Buranasudja, V.; Vimolmangkang, S. Qualitative analysis and exploration of anti-inflammatory and antibacterial effects of a Thai traditional medicine formula from Wat Pho beyond its use for COVID-19 treatment. BMC Complement. Med. Ther. 2025, 25, 159. [Google Scholar] [CrossRef]
- Promega Corporation. Techinical Bulletin: Griess Reagent System Instructions for Use of Product G2930; Promega Corporation: Madison, WI, USA, 2009; pp. 1–7. Available online: https://worldwide.promega.com/resources/protocols/technical bulletins/0/griess-reagent-system-protocol/ (accessed on 25 October 2024).
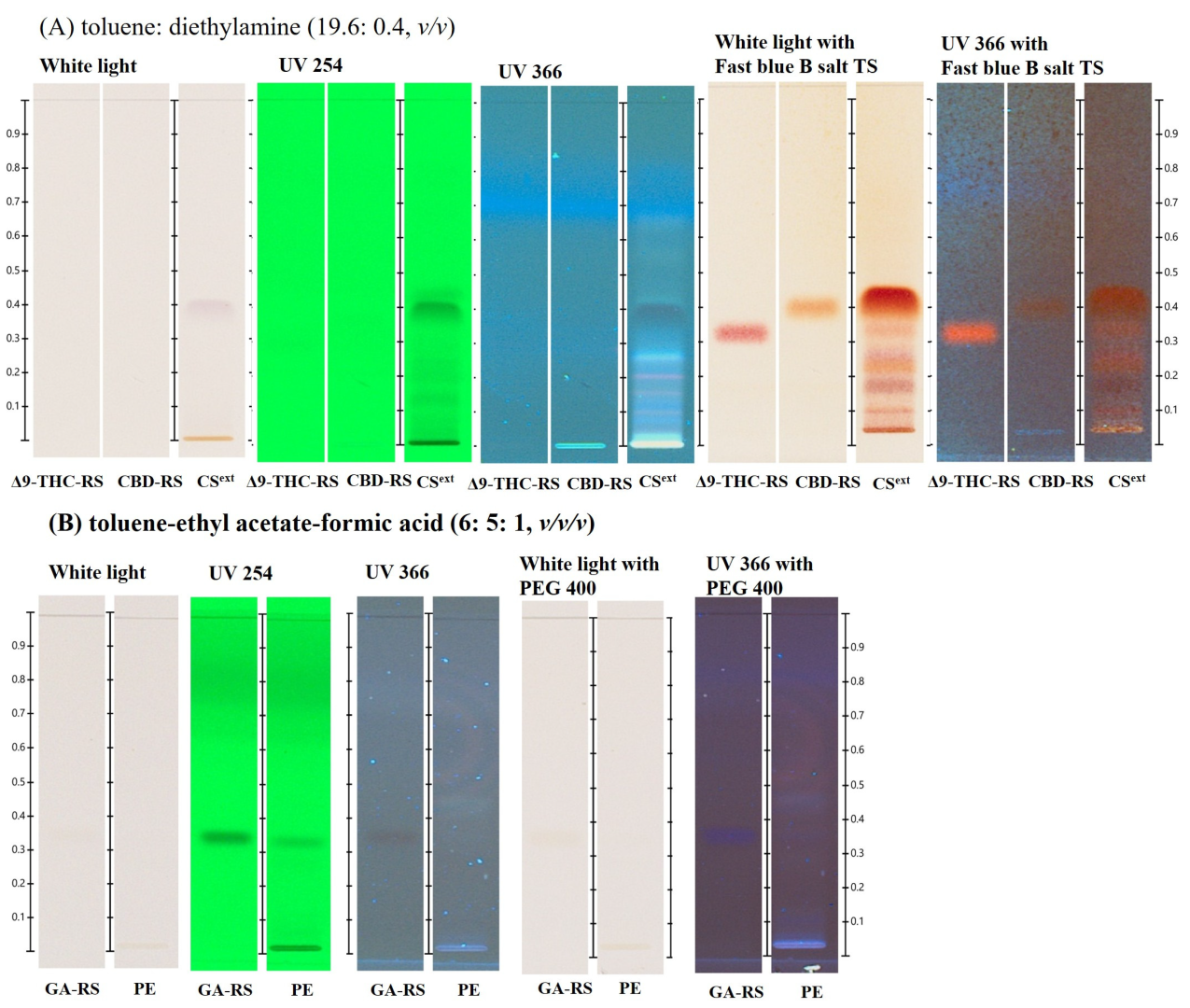
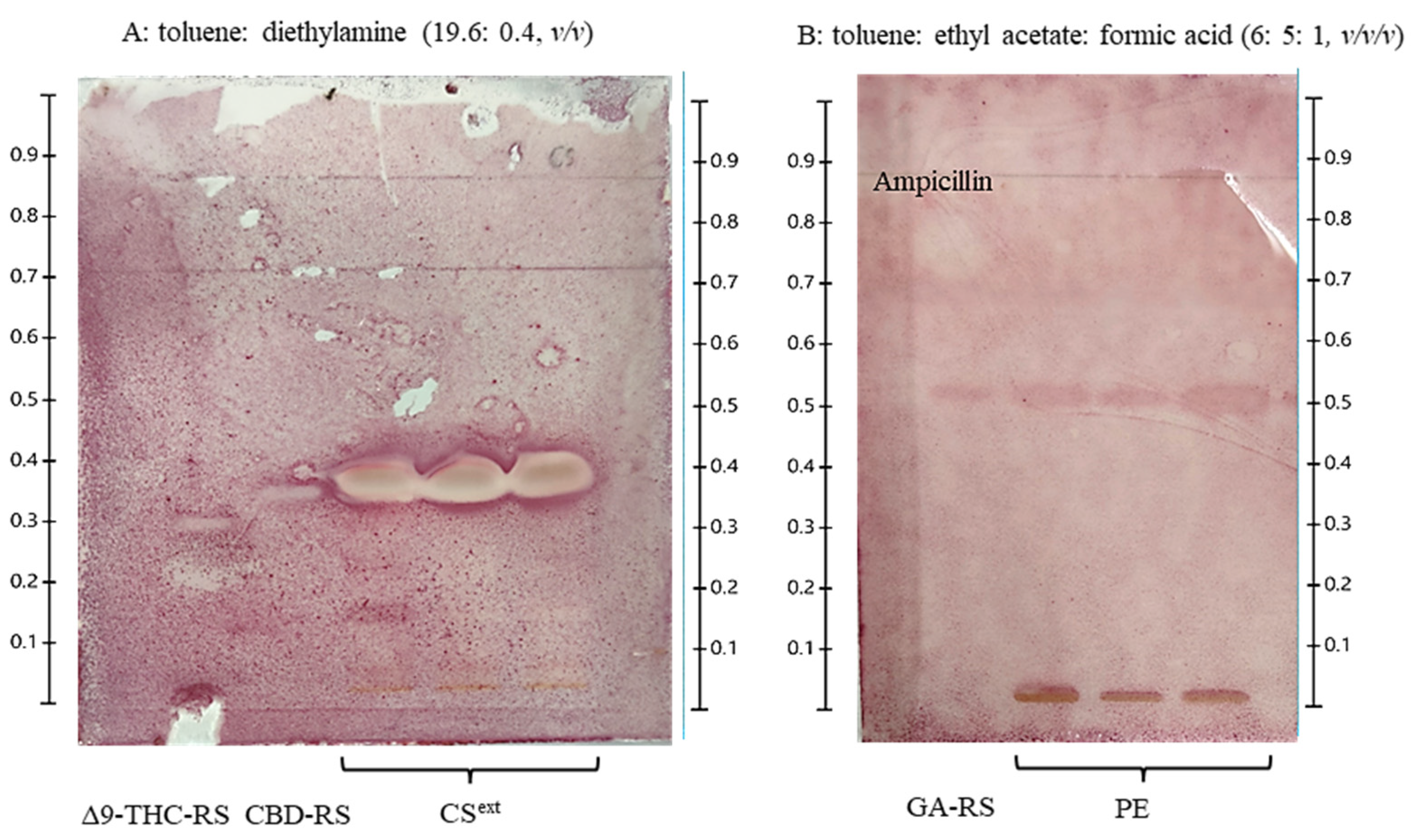

| Samples (Concentration/disc) | Zone of Inhibition (mm) (Means ± Standard Deviation) | |||
|---|---|---|---|---|
| S. pyogenes DMST 4369 | S. aureus ATCC 25923 | P. aeruginosa ATCC 9027 | K. pneumoniae ATCC 13883 | |
| 100% v/v PEsol | 12.2 ± 0.4 | 13.5 ± 1.1 | 14.7 ± 0.6 | 0.0 ± 0.0 |
| 20% v/v PEsol | 12.0 ± 0.0 | 13.0 ± 0.0 | 15.0 ± 1.7 | 0.0 ± 0.0 |
| 0.4 mg CSext (eq. to 229 µg CBD) | 17.0 ± 1.0 | 13.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| 10% v/v DMSO; negative control | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Sterile water negative control | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| 30 µg chloramphenicol; positive control | 28.0 ± 0.0 | n.d. | 16.0 ± 0.0 | 30.0 ± 0.0 |
| 200 µg amoxicillin; positive control | 28.0 ± 0.0 | 29.4 ± 0.0 | 12.5 ± 0.0 | 17.5 ± 0.0 |
| Microorganisms | PEsol (% v/v) | CSsol Eq. to CBD (µg/mL) | ||
|---|---|---|---|---|
| MIC | MBC | MIC | MBC | |
| Gram-positive bacteria | ||||
| S. aureus ATCC 25923 | 2.5 | 5.0 | 1.6 | 3.1 |
| S. pyogenes DMST 4369 | 2.5 | 5.0 | 1.0 | 2.0 |
| Gram-negative bacteria | ||||
| P. aeruginosa ATCC 9027 | 5.0 | 10.0 | 40.0 | 80.0 |
| K. pneumoniae ATCC 13883 | 1.3 | 2.5 | 250.0 | 500.0 |
| Bacteria Strain | MIC | PEsol (% v/v) | CSsol Eq. to CBD (µg/mL) | FIC Index | Interpretation |
|---|---|---|---|---|---|
| Gram-positive bacteria | |||||
| S. aureus ATCC 25923 | MIC of single compounds | 2.50 | 1.56 | 1.50 | Indifference |
| MIC of Combination 1 | 1.25 | 1.56 | |||
| FIC | 0.50 | 1.00 | |||
| MIC of single compounds | 2.50 | 1.56 | 1.00 | Additive | |
| MIC of Combination 2 | 1.25 | 0.78 | |||
| FIC | 0.50 | 0.50 | |||
| S. pyogenes DMST 4369 | MIC of single compounds | 2.50 | 1.00 | 1.00 | Additive |
| MIC of Combination 1 | 1.25 | 0.50 | |||
| FIC | 0.50 | 0.50 | |||
| MIC of single compounds | 2.50 | 1.00 | 1.50 | Indifference | |
| MIC of Combination 2 | 2.50 | 0.50 | |||
| FIC | 1.00 | 0.50 | |||
| Gram-negative bacteria | |||||
| P. aeruginosa ATCC 9027 | MIC of single compounds | 5.00 | 40.00 | 1.50 | Indifference |
| MIC of Combination 1 | 5.00 | 20.00 | |||
| FIC | 1.00 | 0.50 | |||
| MIC of single compounds | 5.00 | 40.00 | 1.25 | Indifference | |
| MIC of Combination 2 | 5.00 | 10.00 | |||
| FIC | 1.00 | 0.25 | |||
| K. pneumoniae ATCC 13883 | MIC of single compounds | 1.25 | 250.00 | 4.00 | Indifference |
| MIC of Combination 1 | 2.50 | 500.00 | |||
| FIC | 2.00 | 2.00 | |||
| MIC of single compounds | 1.25 | 250.00 | 3.00 | Indifference | |
| MIC of Combination 2 | 2.50 | 250.00 | |||
| FIC | 2.00 | 1.00 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perstwong, N.; Phongsopitanun, W.; Buranasudja, V.; Vimolmangkang, S. Enhanced Antibacterial and Anti-Inflammatory Activities of the Combination of Cannabis sativa and Propolis Extracts: An In Vitro Study. Int. J. Mol. Sci. 2025, 26, 11181. https://doi.org/10.3390/ijms262211181
Perstwong N, Phongsopitanun W, Buranasudja V, Vimolmangkang S. Enhanced Antibacterial and Anti-Inflammatory Activities of the Combination of Cannabis sativa and Propolis Extracts: An In Vitro Study. International Journal of Molecular Sciences. 2025; 26(22):11181. https://doi.org/10.3390/ijms262211181
Chicago/Turabian StylePerstwong, Naruemon, Wongsakorn Phongsopitanun, Visarut Buranasudja, and Sornkanok Vimolmangkang. 2025. "Enhanced Antibacterial and Anti-Inflammatory Activities of the Combination of Cannabis sativa and Propolis Extracts: An In Vitro Study" International Journal of Molecular Sciences 26, no. 22: 11181. https://doi.org/10.3390/ijms262211181
APA StylePerstwong, N., Phongsopitanun, W., Buranasudja, V., & Vimolmangkang, S. (2025). Enhanced Antibacterial and Anti-Inflammatory Activities of the Combination of Cannabis sativa and Propolis Extracts: An In Vitro Study. International Journal of Molecular Sciences, 26(22), 11181. https://doi.org/10.3390/ijms262211181








