1. Introduction
Mitochondria were initially observed in the 1850s and later named in 1898, drawing from the Greek words ‘mitos-’ meaning ‘thread’ and ‘-chondros’ meaning ‘granule’, inspired by their microscopic appearance [
1]. These unique intracellular organelles are maternally inherited and possess their own genome, distinct to nuclear DNA (nDNA); they encode 13 proteins that are essential components of the subunits of the oxidative phosphorylation (OXPHOS) machinery [
2]. The remaining mitochondrial proteins are encoded by nDNA and imported from the cytoplasm. Unlike the paired chromosomes of the human nuclear genome, mitochondrial DNA (mtDNA) is present in multiple copies in each cell, with cellular copy numbers ranging between 100 and 10,000, and replicates independently of the cell cycle [
3].
Mitochondria play key roles in energy production, generation of reactive oxygen species (ROS), metabolic signaling and apoptosis. These varied functions not only grant mitochondria exquisite sensitivity to cellular stressors but also the ability to adapt to changes in their environment. Tumors leverage this versatility to support cancer cell initiation, proliferation, survival and metastasis in the often harsh climates of hypoxia, acidosis, nutrient depletion and anti-cancer therapies [
4]. MtDNA is more susceptible to DNA damage than nDNA, with a background mutational rate 10–17 times higher, as a result of inefficient DNA repair mechanisms, proximity to ROS production and the lack of protective histones [
5,
6]. Coupled with high coding gene density and multiple genomic copies per cell, mtDNA provides a larger functional mutational target than nDNA.
Somatic mtDNA mutations are frequently seen in the cancer genome, including ovarian cancer, with approximately 50% of all tumors harboring at least one somatic aberration, most of which are heteroplasmic and at levels < 60% [
7]. The mutational signatures observed are similar across tumor types, with a majority of C:G > T:A substitutions, followed by T:A > C:G substitutions [
7]. A large whole-genome sequencing (WGS) study of 1916 patients across 24 cancer types found an overrepresentation of mtDNA variants in tumor genomes as compared to normal tissue [
8]. In high-grade serous ovarian cancer (HGSOC) cohorts, higher rates of truncating and missense mutations have been identified in mtDNA compared to nuclear genes (with the exception of TP53, which is almost ubiquitously mutated in HGSOC) [
9]. These deleterious effects of mtDNA mutations predict poorer patient prognosis [
9]. Studies in ovarian cancer cells have also shown altered OXPHOS, increased mitochondrial biogenesis, a process where cells increase their mitochondrial mass, and changes in mitochondrial morphology [
10,
11].
MtDNA mutations are clearly present in cancer genomes, and it is conceivable how aberrations to mitochondrial function can modulate tumorigenesis. A deeper understanding of the underlying biology will enrich proposed efforts to target mtDNA mutations therapeutically in oncology. This review will provide a summary of the functional impact of mtDNA mutations, their role in ovarian cancer oncogenesis and the clinical implications of mtDNA mutations on diagnosis, chemoresistance and future therapeutic strategies.
2. Mitochondrial DNA: Structure and Genetics
The human mitochondrial genome is a small, double-stranded and closed DNA molecule consisting of 16,569 base pairs encoding 37 genes: 13 protein-coding, 22 tRNAs and 2 rRNAs. The 13 hydrophobic proteins insert into the inner mitochondrial membrane and form core subunits of respiratory chain complexes I, III, IV and ATP synthase [
2]. MtDNA is composed of a guanine-rich ‘heavy strand’ and a cytosine-rich ‘light strand’ due to a disproportionate distribution of nucleotides across both strands [
2]. The compact nature of mtDNA, due to the economical organization of genes, close together and often overlapping each other, results in little genetic redundancy. Nevertheless, there exists an approximately 1 kb long non-coding region (NCR) of mtDNA which regulates transcription and translation [
3]. The displacement loop (D-loop) nests within the NCR and is a triple-stranded section of DNA made up of the heavy strand, light strand and a segment of partially replicated DNA known as 7S DNA. Replication of mtDNA is initiated within the D-loop [
12].
Heteroplasmy is a phenomenon unique to mtDNA in non-plant eukaryotes. Human cells generally contain fifty to hundreds of mitochondria, each containing 5 to 10 mtDNA copies, hence amounting to a relatively large and varied total copy number of mtDNA within and between cells. Typically, only a proportion of mtDNA copies within a single cell are affected when mutations arise, generating a heterogenous mix of mutant and wildtype DNA described as heteroplasmy [
13]. Homoplasmy is only present if every single copy of mtDNA is genetically identical within a cell. The level of heteroplasmy fluctuates with cell division due to random segregation of mutant and wildtype mtDNA molecules to the daughter cells, a process known as vegetative segregation. Even in non-dividing cells, mtDNA undergoes constant turnover, and individual molecules are selected at random for replication via a process known as relaxed replication. Both mechanisms can occur in concert to influence the mutational level of a cell through random genetic drift [
14].
In the case of large-scale mtDNA deletions, selective clonal expansion of mutant mtDNA has been proposed to occur via replicative advantage of smaller mtDNA molecules with deletion mutations, as compared to larger wildtype counterparts [
15,
16,
17], as well as negative feedback mechanisms that encourage an increase in mtDNA replication to compensate for deletion mutations causing reduced protein production [
17]. The mechanism of clonal expansion of mtDNA molecules with point mutations is still unknown, but it has been shown computationally to fit with a pattern of random genetic drift [
18,
19].
The rate of mtDNA mutation and random genetic drift is likely to be significantly higher in rapidly dividing cancer cells than in their normal counterparts, resulting in a broad spectrum of mtDNA mutations in tumors. This could provide an opportunity to select mtDNA variants which favor cancer cell growth and survival. Cancer cells may have an increased tolerance for mtDNA variants due to their predilection for metabolizing glucose and lactate for energy production through glycolysis, known as the Warburg effect, instead of via the mitochondria-dependent OXPHOS [
8,
20]. However, near-homoplasmic or protein-altering mtDNA mutations can confer a negative selection pressure in some tumor types [
21].
Epigenetics in mitochondria, coined ‘mitoepigenetics’, comprising mtDNA methylation or hydroxylmethylation, mitochondrial nucleoid modifications, mtRNA modifications and mtDNA-derived or nDNA-derived non-coding RNA modulations during mtDNA-encoded gene translation, have also shown a role in cancer development [
22]. For example, decreased methylation of the D-loop region is seen in colorectal cancer cells as compared to benign tissues [
23]. There also appears to be an inverse relationship between methylation levels of the D-loop region and mtDNA copy number and cancer cell proliferation in colorectal cancer, osteosarcoma and glioblastoma [
23,
24,
25]. However, conflicting studies have also suggested that low levels of mtDNA methylation in colorectal adenoma do not affect mitochondrial gene transcription [
26]. Further studies with a more precise methodology are needed to identify methylation sites on mtDNA to further understand the significance of mitoepigenetics in downstream mtDNA gene expression and its impact on tumor growth.
3. Ovarian Cancer and MtDNA
Ovarian cancer is the third most common gynecological cancer, with an incidence of 6.6% worldwide [
27]. It is also the deadliest gynecological cancer, with a 5-year survival rate of only 17% in advanced ovarian cancer patients. This high mortality is largely due to patients predominantly presenting at advanced stages. Hereditary genetic mutation syndromes, such as BRCA1 and BRCA2 mutations and Lynch syndrome, are known strong risk factors for ovarian cancer [
28].
More than 90% of ovarian cancers arise from epithelial cells, of which there are five main histological subtypes; high-grade serous, low-grade serous, endometrioid, clear cell and mucinous carcinomas. These subtypes have distinct genetic profiles and underlying biology, resulting in markedly different treatment responses and overall patient outcomes [
29]. Unlike other cancers, which primarily metastasize via the hematogenous route, ovarian cancer cells metastasize either via local invasion of neighboring organs or are exfoliated and transported through peritoneal fluid, superficially seeding the peritoneal cavity [
30]. Widespread intraperitoneal disease is often associated with recurrent ascites and bowel obstruction, especially if cytoreductive surgery is not performed or systemic drug treatment is ineffective. A small study of HGSOC patients with 22 matched plasma-to-tissue samples and 5 matched ascitic fluid-to-tissue samples showed that tumor-derived mtDNA mutations were more easily identified in ascitic fluid than plasma samples. This could reflect the cancer’s preferred mode of invasion and suggests a possible higher value in testing ascitic fluid instead of blood for mtDNA mutations [
31].
HGSOC, the most common epithelial subtype, is characterized by significant nuclear genomic instability on a background of frequent homologous recombination deficiency (HRD), whole-genome duplication (WGD) or both [
9]. Targeted therapies that have been developed are focused solely on nDNA mutations and their downstream effects. A clearer understanding of mtDNA alterations within ovarian cancer may open new doors for targeted treatments.
3.1. Mitochondrial DNA Alterations in Ovarian Cancer
There are several published studies which have sequenced the mitochondrial genome, either in whole or in part, in ovarian cancer (
Table 1).
3.1.1. Non-Coding Regions
A small study of ten matched ovarian carcinoma and control pairs identified five unique somatic mtDNA mutations within the NCR in each of the five samples, all of which were homoplasmic. Notably, two of these samples harbored homoplasmic mutations within the D-loop [
32]. The D-loop, which lies in the NCR, is felt to be a mutational ‘hot-spot’ from an evolutionary perspective due to presence of three hypervariable regions: HV1 (nucleotides 16,024–16,383), HV2 (nucleotides 57–372) and HV3 (438–574) [
42,
43]. Analysis of matched tumor and non-tumor samples from 49 ovarian cancer patients revealed that somatic and germline mtDNA single-nucleotide variants (SNVs) predominantly occurred within hypervariable regions, and their presence was associated with poorer prognosis compared to mutations in coding regions (CRs) [
44]. This result was also echoed in a separate large dataset of more than 350 ovarian cancer patients, with negative selection seen against tRNA loop areas [
40]. Analysis of 395 sequenced tumor samples from 35 HGSOC patients showed a significantly higher mutational frequency in the mtDNA D-loop as opposed to the mtDNA CR [
31]. A further study reported subtype-specific mtDNA mutations within the NCR that could allow distinction between endometrioid and serous ovarian tumors [
35].
Accumulation of D-loop mutations may have an impact on mtDNA copy number and expression, as the D-loop forms part of the control region which governs mtDNA replication and transcription [
44]. The presence of D-loop mutations in ovarian cancer samples is associated with significantly higher mtDNA copy numbers than samples without D-loop mutations [
31]. Correspondingly, mtDNA copy number is significantly elevated in ovarian carcinomas (range: 444–16,772 copies) compared with normal ovarian tissue [
34]. Furthermore, ovarian cancer exhibited the most abundant mtDNA copy number in a large WGS project, generated by the International Cancer Genome Consortium (ICGC) and The Cancer Genome Atlas (TCGA), analyzing 2658 cancers across 38 tumor types [
7]. A preliminary study of 38 primary epithelial ovarian cancers and 4 borderline ovarian tumors showed that mtDNA copy number was unrelated to disease stage but significantly higher in borderline, Grade 1, and Grade 2 tumors compared with Grade 3 tumors. Interpretation of subtype-specific differences was limited by the use of an outdated Type I/Type II classification, which does not adequately reflect the distinct cellular origins, genetics, and molecular features of each histological subtype [
34].
Mutations at the 12S and 16S rRNA gene have also been identified in ovarian cancer [
32,
45]. Interestingly, no ovarian tumors were found to harbor somatic mutations at a highly polymorphic homopolymeric C stretch (D310) located within the D-loop, albeit seen in most other tumor groups [
45].
3.1.2. Coding Regions
In 2012, a study looking at paired tumor and non-tumor samples in 5 different cancers identified both inherited and somatic mtDNA mutations within CRs of 28 ovarian serous cystadenocarcinomas. Among the 236 inherited and 14 somatic CR variants, non-synonymous variants were markedly more frequent in somatic than inherited mutations (93% vs. 31%). Heteroplasmy levels of somatic CR mutations ranged from 33.5% to 95.1% [
36].
More recently, deeply sequenced HGSOC datasets have shown that predicted deleterious mitochondrial gene mutations affecting Complex I and IV function are most frequently implicated in HGSOC and occur at a higher rate than mutations in most nuclear tumor suppressor genes. The MT-ND5 gene, encoding NADH dehydrogenase 5—a subunit of Complex I—is the most commonly mutated mtDNA gene in HGSOC and across most other cancer types [
7,
9]. The aforementioned ICGC/TCGA WGS dataset also demonstrated that, among 113 ovarian cancer samples, mtDNA coding region mutations occurred most frequently in the MT-ND5, MT-ND4 and MT-CO1 genes [
7] (
Figure 1). Survival data has also shown that patients with HGSOC and predicted deleterious mtDNA somatic mutations have an inferior prognosis, with the extent of adverse outcomes directly correlating with increasing heteroplasmy levels, suggesting a gene–dosage effect [
9]. However, exact tumor heteroplasmy levels were not reported in this study, limiting assessment of the upper heteroplasmy threshold to which this association remains true and of whether mutations nearing homoplasmy could instead impede cancer cell growth and potentially improve prognosis. Interestingly, synonymous mtDNA mutations and mutations within mitochondrial RNA genes were not associated with worse patient outcomes, emphasizing the damaging effects of compromised mitochondrial respiratory chain function.
MtDNA alterations occur more frequently within HGSOC tumors demonstrating WGD and less frequently in tumors demonstrating HRD [
9]. Recent single-cell studies have revealed that elevated mtDNA copy numbers in HGSOC is strongly linked to WGD events in an attempt to maintain a balanced mtDNA-to-nDNA ratio [
46]. The accumulation of mitochondrial DNA mutations and copy numbers hence appears to be differentially influenced by nuclear genomic instability, highlighting the complex interdependence between nuclear and mitochondrial genome dynamics.
A separate mtDNA sequencing study revealed an evolutionary pattern of greater mitochondrial genomic instability in epithelial ovarian cancer as compared to benign ovary tissue. This accumulation of somatic mtDNA mutations was characterized by a markedly lower proportion of pathogenic and likely pathogenic variants in genes encoding Complex V, compared with Complexes I, III, and IV [
40]. The apparent negative selection against Complex V mutations underscores its critical role in ATP synthesis, contributing to cellular viability. Conversely, potential positive selection was observed in mutations within the MT-CYB gene of Complex III [
40].
3.2. MtDNA Mutations in Primary vs. Metastatic Ovarian Tumors
Comparison of the mtDNA mutational pattern in paired primary and metastatic tumor samples of 35 HGSOC patients showed a significantly higher mutational density within the D-loop and higher mutation heteroplasmy levels in metastatic than primary tumors [
31]. This suggests possible evolutionary selection of certain mtDNA mutations as tumors develop metastatic potential. No differences in mtDNA copy number or mutation density of CRs were found across paired samples [
31]. Analysis of 17 bilateral ovarian tumor cases revealed 4 patients with distinct D-loop variants between paired tumors, suggesting independent clonal origins. Metastatic deposits, arising in these bilateral ovarian cancer cases, do however retain mtDNA variants identical to at least one of the primary tumors [
33].
These mtDNA mutational signatures have been a useful tool to illustrate genetic divergence between primary and metastatic ovarian tumors. A linear metastasis model with low intratumoral genetic divergence and a parallel metastasis model with high intratumoral genetic divergence have been described in ovarian tumors. A small number of tumors demonstrated a mixed linear and parallel metastasis model. This translated to a poorer CA125 response in patients with parallel as compared to linear metastasis patterns and potential treatment resistance [
31].
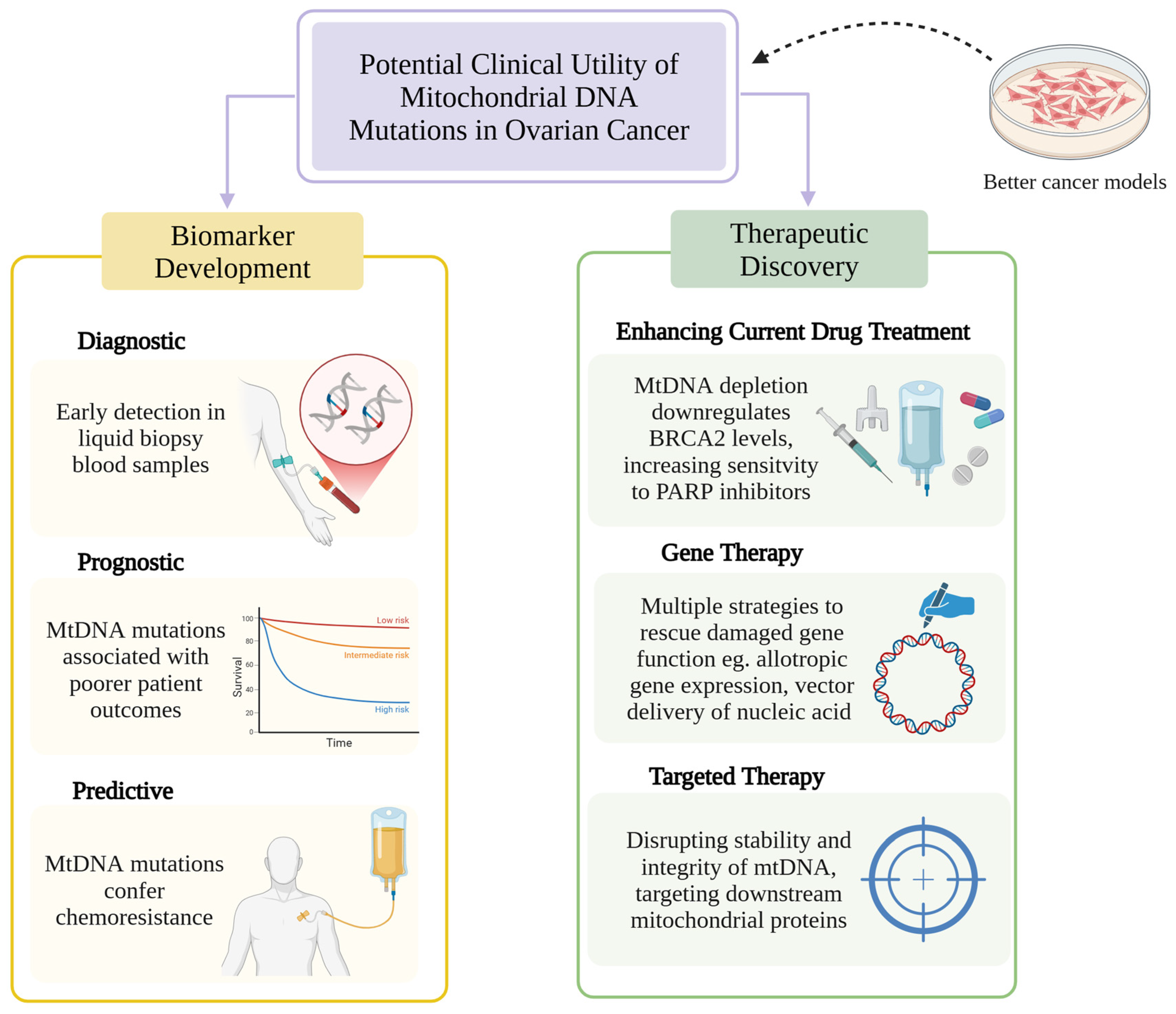
4. Clinical Utility of mtDNA Mutations in Ovarian Cancer
Understanding mitochondrial genomic variation and its significance in ovarian cancer has led to research exploring the translational application of mtDNA mutations in biomarker development and drug discovery (
Figure 2).
4.1. MtDNA as a Cancer Biomarker
Biomarker development has been a burgeoning field of research in the new era of cancer therapeutics. Biomarkers can be used for screening, diagnosis, prediction of treatment response or resistance and prognosis across different malignancies. As efforts now move from the nuclear to the mitochondrial genome, greater insights into mtDNA alterations may lead to discovery of new cancer biomarkers [
47,
48,
49]. A pilot observational retrospective study built a prediction model for HGSOC trained from mtDNA variations in 20 whole-exome-sequenced HGSOC samples and 14 controls, which was further validated with the TCGA dataset. This model identified that alterations in the cytochrome b gene, the only mitochondrial gene to encode a protein subunit of Complex III, increased the risk of HGSOC by over 30% [
41]. The consequent expression of this gene, analyzed via RNA sequencing of paired HGSOC tumor and control specimens, was significantly decreased in this population. Continued efforts are needed to explore its potential role as a biomarker for early detection of HGSOC via liquid biopsy blood samples. While evaluation of somatic nDNA mutations such as mutant TP53 allelic fraction and reverse BRCA mutations in plasma cell-free DNA have demonstrated predictive and prognostic value in HGSOC, further research is needed to evaluate the utility of tumor-specific mtDNA mutations in plasma in this context [
50]. Promisingly, mtDNA fragments have been detected in blood plasma and exosome samples in non-small-cell lung cancer, with the presence of exosome mtDNA closely associated with aggressive features of lung cancer [
51].
Studies have also shown that the presence of single-nucleotide polymorphisms (SNPs) within the D-loop, identified from peripheral blood samples, are a risk factor and a predictive marker of age of onset, as well as a prognostic marker in epithelial ovarian cancer patients [
37,
38,
39]. While not looking specifically for mtDNA mutations, increasing levels of detectable circulating cell-free mtDNA in peripheral blood are significantly associated with more aggressive epithelial ovarian cancer and poorer overall survival [
52].
4.2. MtDNA Mutations and Chemoresistance
Platinum-based doublet chemotherapy has been the established first-line treatment for advanced ovarian cancer for nearly three decades [
53]. Platinum agents, including cisplatin, carboplatin and oxaliplatin, induce intra- and inter-strand DNA crosslinking, which interferes with DNA replication and transcription, ultimately leading to cell death. This effect is seen in both nDNA and mtDNA, with some studies showing platinum agents having a greater affinity for damaging mtDNA over nDNA [
54]. To escape chemotherapy-induced cytotoxic cell death, cancer cells must adapt to evade apoptosis. MtDNA mutations have been shown to confer chemoresistance through a variety of mechanisms, including metabolic remodeling, ROS generation, mito-nuclear crosstalk and mitochondrial translocation [
55].
4.2.1. Metabolic Remodeling and ROS Generation in Cancer Cells
A small sequencing study of 16 HGSOC patients found that patients harboring heteroplasmic pathogenic somatic mtDNA mutations exhibited higher rates of platinum resistance and cancer relapse [
45]. These mutations, predicted to be deleterious or possibly deleterious by PolyPhen2 and SIFT, had heteroplasmy levels ranging between 8% and 73% and were all located within coding regions for Complex I and IV. Mitochondrial functional assays to evidence dysfunction confirmed a higher lactate-to-pyruvate ratio, indicating a shift from oxidative phosphorylation to glycolysis consistent with underlying mitochondrial dysfunction. On the contrary, platinum-sensitive patients had more synonymous somatic mtDNA mutations than their platinum-resistant counterparts [
45].
Further evidence of metabolic remodeling was observed in the study of two human ovarian cancer cell lines—the 2008 cisplatin-sensitive cell line and C13 cisplatin-resistant cell line. MtDNA depletion in the 2008 cisplatin-sensitive cells led to platinum resistance as a result of mitochondrial dysfunction, while the cisplatin-resistant C13 cells showed decreased apoptosis, decreased mitochondrial membrane potential and lower basal oxygen consumption following platinum chemotherapy as compared to the 2008 cells [
56]. Enhanced tumorigenesis with increased ROS generation and apoptotic resistance has also been associated with tumors derived from cybrids with heteroplasmic MT-ND5 and MT-ND6 mutations, both of which impact Complex I function [
57]. Ovarian cancer cells possess a complex antioxidant defense system consisting of antioxidants such as NADPH, Nrf2, GST, GPxs, glutathione, peroxiredoxin and CD44v9 to protect themselves from mounting oxidative stress. The overexpression of these antioxidants in an attempt to neutralize extra ROS is often associated with an aggressive tumor biology, chemoresistance and poor survival outcomes in ovarian cancer patients [
58].
4.2.2. Mitochondrial–Nuclear Crosstalk
The mitochondria lie at a key intersection of nuclear and mitochondrial DNA and epigenetic control, facilitating bi-directional communication [
59]. There is evidence to suggest that increased DNA methylation in gene promoter regions of post-chemotherapy ovarian cancer stem cells contributes to treatment resistance. Sensitivity to chemotherapy can be restored thereafter with the use of a DNA methyltransferase inhibitor, SGI-110 [
60]. Epigenetic modifications are regulated in a dynamic manner by enzymes, environmental factors and cellular signaling pathways, with mtDNA mutations potentially contributing to this process. Cybrid models with a single mtDNA tRNALeu (UUR) m.3243G mutation showed distinct nuclear epigenetic changes at different levels of heteroplasmy. At high levels of heteroplasmy, a decrease in acetyl-CoA levels caused reduced histone H4 acetylation. At mid-levels of heteroplasmy, raised alpha-ketoglutarate levels was inversely correlated with histone H3 methylation [
61]. This could suggest a mitochondrial–nuclear crosstalk basis for the development of chemoresistance through epigenetic modification of nDNA.
4.2.3. Mitochondrial Translocation
Horizontal mitochondrial transfer is the phenomenon where there is cell-to-cell transfer of mitochondria and its corresponding mtDNA. Methods in which this can occur include the formation of tube-like structures called tunneling nanotubes (TNTs) or extracellular vesicles (EVs) from the donor cell to the recipient cell, facilitating mitochondria transfer, cell fusion, leading to the combination of organelles between two previously separate cells, and internalization of isolated mitochondria via endocytosis [
62]. HMT allows cancer cells with defective or deleted mtDNA to overcome dysfunctional mitochondria-led respiration by transporting functional mitochondria from donor cells to cancer cells. This can restore normal respiratory function, reduce ROS levels, boost proliferation and cell migration and contribute to chemoresistance [
55]. Platinum-resistant ovarian cancer cells displayed a greater tendency for TNT formation when cultured in hypoxic conditions as compared to platinum-sensitive ovarian cancer cells or benign epithelial cells, suggesting a role for mtDNA in development of chemoresistance under stress [
63].
Mechanistically, senescent cell TNTs appear to be reliant on the mTOR signaling pathway and are partly mediated by downstream regulatory factor CDC42 [
64]. In ovarian cancer cells, the EGFR/MAPK signaling pathway appears to be a major regulator of TNTs, with MEK and ERK inhibitors both reducing TNT formation and RSK inhibition showing reduced TNT numbers [
65]. The use of Everolimus or Metformin, both mTOR inhibitors, has also shown preliminary evidence of suppression of TNT formation in chemoresistant ovarian cancer cells in vitro [
63]. Other therapeutic strategies to disrupt HMT include EV inhibitors, for which heparin has shown an inhibitory role in EV uptake in ovarian cancer cells [
66]. The process of cell fusion can be inhibited by targeting relevant signaling pathways such as NF-kB and VCAM-1/VLA-4 and proteins such as syncytin-1 and syncytin-2 [
67,
68,
69,
70].
4.2.4. The Oncojanus Effect
To add more complexity, the concept of oncojanus, where certain genes can both promote and suppress tumor growth depending on context, has been seen with mtDNA mutations in ovarian cancer [
71]. Below a certain level of heteroplasmy, mtDNA mutations which disrupt Complex I assembly are pro-tumorigenic; however, after exceeding a critical threshold level, severe dysfunction results in anti-tumorigenic effects. In a residual serous ovarian cancer case, a nearly homoplasmic novel missense mtDNA mutation in MT-ND4 was identified only in the post-chemotherapy surgical specimen which contained a previously undetected oncocytic component [
72]. Considering the oncojanus effect, this novel MT-ND4 mutation could have initially bolstered chemoresistance within the tumor up until the threshold for Complex I disruption was reached, subsequently leading to the evolution of a slowly growing, benign oncocytic tumor [
73].
4.3. Targeting mtDNA to Enhance Efficacy of Anti-Cancer Therapies
No mitochondrial-targeted anti-cancer therapies have yet been developed, but emerging evidence suggests that targeting mtDNA may enhance the efficacy of existing anti-cancer drugs. In prostate cancer cells, the presence of mtDNA depletion resulted in the downregulation of BRCA2 levels via activation of a calcium-mediated retrograde signaling pathway [
74]. This confers enhanced sensitivity to Poly (ADP-ribose) polymerase (PARP) inhibitors, which, via synthetic lethality, exploit the already defective homologous recombination DNA repair process in BRCA2-depleted cells. This promotes accumulation of single- and double-stranded DNA breaks and leads to cell death. Further studies are required to determine whether this mechanism is applicable to ovarian cancer, particularly given the higher prevalence of mtDNA alterations observed in homologous recombination proficient tumors.
5. Developing Novel Cancer Models with mtDNA Mutations
Understanding the integral role mtDNA mutations play in tumorigenesis and chemoresistance has allowed ‘mitochondrial medicine’ to emerge as a novel opportunity to develop anti-cancer therapies against mitochondrial targets. There exists a broad spectrum of mitochondrial strategies, including therapies which directly target mtDNA itself to induce genetic damage and therapies that indirectly target mtDNA by influencing pathways involved in mtDNA stability and integrity, as well as therapies which target critical downstream mitochondrial proteins within metabolic pathways [
73,
75]. Despite multiple therapeutic strategies, only one gene therapy has successfully reached Phase III clinical trials to date—an AAV2 vector which promotes allotropic ND4 gene expression in patients with Leber’s hereditary optic neuropathy, a primary mitochondrial disease (PMD) associated with point mutations in MT-ND4 [
76].
Although research into harnessing mtDNA mutations for therapeutic benefit in cancer remains in its infancy relative to PMDs, novel cancer models with mitochondrial mutations, which allow deeper biological insight and can guide future therapeutic strategies, are now being developed.
5.1. Mitochondrial Gene Editing
The impact of mtDNA on cancer biology has historically been studied using trans-mitochondrial cybrid models (donor cells providing mtDNA and recipient cells providing nuclear DNA) [
77]. While insightful, this technology does not allow for specific mtDNA base editing to study the impact of cancer-causing mutations of interest. The advent of mitochondrial gene editing technology enables precise mtDNA engineering using DddA-derived cytosine base editors (DdCBEs) [
78]. Derived from interbacterial cytidine deaminase toxins, DdCBEs induce C-G to T-A conversions in mtDNA. They consist of non-toxic split-DddA halves that activate only when combined with Transcriptor Activator-Like Effector (TALE) DNA-binding proteins at the target site. Isogenic mtDNA models can thus be generated for in vitro and in vivo analysis. Recent advances have led to the development of high-fidelity DdCBEs, which minimize off-target activities of conventional DdCBEs, caused by unwanted TALE-DNA interactions or spontaneous assembly of split DddA halves, thereby enhancing their precision and therapeutic promise [
79].
5.2. Novel Cancer Models
While mtDNA manipulation has been successfully demonstrated in mice, zebrafish, human cells, human stem-cell-derived organoids and even chloroplasts, reports of mtDNA editing in cancer cells remain scarce [
80,
81,
82,
83,
84,
85]. An impactful study demonstrated the first application of DdCBE technology in cancer research by generating murine melanoma models with engineered truncating MT-ND5 mutations at heteroplasmy levels of 40%, 60% or 80% [
86]. This study demonstrated heteroplasmy-dependent cellular redox imbalance favoring a Warburg-like shift towards aerobic glycolysis in mutant cells. Additionally, mtDNA mutants exhibited an altered tumor microenvironment with enhanced anti-tumor immune responses, with higher mutant heteroplasmy levels correlating with increased sensitivity to immune checkpoint inhibitors. These findings in mice models were mirrored in clinical data, with melanoma patients harboring mtDNA mutations at heteroplasmy > 50%, showing a 2.5-fold increase in response to Nivolumab, an anti-PD1 monoclonal antibody, compared with mtDNA wildtype patients [
86]. To date, no published studies have reported the generation of isogenic human cancer cell lines, nor any work specifically in ovarian cancer.
6. Discussion
Ovarian cancers are characterized by frequent somatic mtDNA variants, particularly within CRs of mitochondrial respiratory chain complexes I and IV, as well as HV mutational ‘hotspot’ regions within the D-loop of NCRs. Further investigation of HV region variants is needed to elucidate the selective mutational bias toward these regions. Complementary RNA-sequencing analyses will be essential to validate their predicted impact on mitochondrial transcription and biogenesis, as accumulation of D-loop mutations correlates with elevated mtDNA copy numbers in ovarian cancer.
Definitive evidence for mtDNA mutations as direct driver events in tumorigenesis remains limited; however, such alterations likely provide cancer cells with a proliferative advantage over normal tissue. Metabolic reprogramming and elevated ROS production resulting from heteroplasmic mtDNA mutations may promote tumorigenesis and contribute to chemoresistance, underscoring mitochondrial dysfunction as a potential determinant of ovarian cancer progression. Moreover, mtDNA mutational patterns can reveal genetic divergence between primary and metastatic ovarian tumors, where greater divergence has been linked to poorer CA125 responses, supporting an oncogenic and possibly increased metastatic potential with accumulating mtDNA mutations. These mutations may also influence epigenetic regulation of nuclear genes, contributing to treatment resistance in post-chemotherapy ovarian cancer stem cells.
Despite growing interest in mtDNA variants in cancer, the literature on mtDNA variants specific to ovarian cancer remains limited, with most published studies involving modest cohorts. While genomic sequencing efforts have catalogued mtDNA variants in ovarian cancer, few have extended that to mechanistic or functional validation and its impact on oncogenesis. Research has largely centered on HGSOC, owing to its predominance among ovarian cancer cases. However, the distinct molecular and biological characteristics of each epithelial ovarian cancer histological subtype makes broad generalizations of mtDNA variants and its functional implications inappropriate.
Although mitochondrial gene therapy is advancing in PMDs—with one drug having reached clinical trials—no comparable mtDNA-directed therapies have been explored in cancer. Therapeutic progress is hindered by our limited and still-evolving understanding of the role mtDNA plays in cancer biology. While lessons may be drawn from drug discovery in PMDs, the complex interplay of numerous genomic alterations across both nuclear and mitochondrial DNA seen in cancer may make identification of a single therapeutic mitochondrial gene target challenging. Moreover, given the ubiquity and fundamental importance of mitochondria in nearly all human cells, off-target toxicities represent a major challenge for the development of mtDNA-targeted therapies. Encouraging progress has been made with the development of isogenic murine melanoma models carrying mtDNA mutations, but further work is required to extend this approach to other tumor types and human cancer cell lines.
7. Future Directions
Future research efforts should focus on the phenotypic characterization of mtDNA mutations for both NCRs and CRs in ovarian cancer with the use of novel cancer cell models developed with mitochondrial gene editing technology. This will allow for a deeper mechanistic understanding of the role of mtDNA variants in oncogenesis and help identify viable targets for new drug development. Additional research is warranted to explore the impact of mitochondrial–nuclear cross talk on cancer development, as well as the mitochondrial genetics underpinning tumor aggressiveness and treatment resistance. There is also an unmet need for mtDNA sequencing studies focusing on rarer ovarian cancer subtypes, such as low-grade serous and endometrioid ovarian cancer, in order to distinguish their mitochondrial mutational landscapes from that of HGSOC and examine their downstream impact.
Mitochondrial oncogenetics in ovarian cancer is still in its incipient stages. However, with precision oncology at the forefront of therapeutic discovery and patient management, we look to mtDNA mutations possibly emerging as the newest kid on the block, opening avenues for new targets and new treatments.
Author Contributions
Conceptualization, S.S.P.L. and C.G.; writing—original draft, S.S.P.L.; writing—review and editing, C.G., L.G., R.S. and C.A.S.; supervision, C.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
S.S.P.L. is supported by a TRACC Clinical Research Training Fellowship PhD programme that is funded by Cancer Research UK. L.G. is supported by Cancer Research UK—(DRCPFA-Nov22/100001) and the Medical Research Council (MC_PC_21046 and MC_PC23036). C.A.S. is supported by MRC core funding to the MRC Human Genetics Unit, University of Edinburgh, and MRC Programme funding, MC_UU_00035/1.
Conflicts of Interest
C.G. receives research funding from AstraZeneca, MSD, Novartis, GSK, BerGen Bio, Medannex, Roche, Verastem and Artios and personal fees from AstraZeneca, MSD, GSK, Clovis, Verastem, Takeda, Eisai, Cor2Ed and Peer Voice. The remaining authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AAV2 | Adeno-associated Virus 2 |
| BRCA | Breast Cancer Gene |
| CR | Coding Region |
| DdCBEs | DddA-derived Cytosine Base Editors |
| D-loop | Displacement Loop |
| ERK | Extracellular Signal-Regulated Kinase |
| EV | Extracellular Vesicle |
| GPxs | Glutathione Peroxidase |
| GST | Glutathione S-transferase |
| HGSOC | High-Grade Serous Ovarian Cancer |
| HMT | Horizontal Mitochondrial Translocation |
| HRD | Homologous Recombination Deficiency |
| HV | Hypervariable Region |
| ICGC | International Cancer Genome Consortium |
| MEK | Mitogen Activated Protein Kinase |
| mtDNA | Mitochondrial DNA |
| mTOR | Mammalian Target of Rapamycin |
| NADH | Nicotinamide Adenine Dinucleotide plus Hydrogen |
| NADPH | Nicotinamide Adenine Dinucleotide Phosphate plus Hydrogen |
| NCR | Non-Coding Region |
| NF-kB | Nuclear Factor Kappa-Light-Chain-Enhancer of Activated B Cells |
| Nrf2 | Nuclear Factor Erythroid 2-Related Factor 2 |
| nDNA | Nuclear DNA |
| OXPHOS | Oxidative Phosphorylation |
| PARP | Poly (ADP-ribose) Polymerase |
| PCR | Polymerase Chain Reaction |
| PMD | Primary Mitochondrial Disease |
| ROS | Reactive Oxygen Species |
| RSK | Ribosomal S6 Kinase |
| SIFT | Sorting Intolerant From Tolerant |
| SNP | Single Nucleotide Polymorphisms |
| SNV | Single Nucleotide Variant |
| TALE | Transcription Activator-like Effector |
| TCGA | The Cancer Genome Atlas |
| TNT | Tunnelling Nanotube |
| VCAM-1 | Vascular Adhesion Molecule-1 |
| VLA-4 | Very Late Activation Antigen-4 |
| WES | Whole-Exome Sequencing |
| WGD | Whole-Genome Duplication |
| WGS | Whole-Genome Sequencing |
References
- Benda, C. Mitochondria. In Archiv für Anatomie und Physiologie, Physiologische Abteilung; Vereinigung Wissenschaftl. Verl.: Berlin, Germany, 1898; Volume 73, p. 397. [Google Scholar]
- Anderson, S.; Bankier, A.T.; Barrell, B.G.; de Bruijn, M.H.L.; Coulson, A.R.; Drouin, J.; Eperon, I.C.; Nierlich, D.P.; Roe, B.A.; Sanger, F.; et al. Sequence and Organization of the Human Mitochondrial Genome. Nature 1981, 290, 457–465. [Google Scholar] [CrossRef]
- Sharma, P.; Sampath, H. Mitochondrial DNA Integrity: Role in Health and Disease. Cells 2019, 8, 100. [Google Scholar] [CrossRef]
- Vyas, S.; Zaganjor, E.; Haigis, M.C. Mitochondria and Cancer. Cell 2016, 166, 555–566. [Google Scholar] [CrossRef]
- Shukla, P.; Singh, K.K. The Mitochondrial Landscape of Ovarian Cancer: Emerging Insights. Carcinogenesis 2021, 42, 663–671. [Google Scholar] [CrossRef]
- Parsons, T.J.; Muniec, D.S.; Sullivan, K.; Woodyatt, N.; Alliston-Greiner, R.; Wilson, M.R.; Berry, D.L.; Holland, K.A.; Weedn, V.W.; Gill, P.; et al. A High Observed Substitution Rate in the Human Mitochondrial DNA Control Region. Nat. Genet. 1997, 15, 363–368. [Google Scholar] [CrossRef]
- Yuan, Y.; Ju, Y.S.; Kim, Y.; Li, J.; Wang, Y.; Yoon, C.J.; Yang, Y.; Martincorena, I.; Creighton, C.J.; Weinstein, J.N.; et al. Comprehensive Molecular Characterization of Mitochondrial Genomes in Human Cancers. Nat. Genet. 2020, 52, 342–352, Erratum in Nat. Genet. 2023, 55, 893. Erratum in Nat. Genet. 2023, 55, 892; Erratum in Nat. Genet. 2023, 55, 1078. [Google Scholar] [CrossRef]
- Grandhi, S.; Bosworth, C.; Maddox, W.; Sensiba, C.; Akhavanfard, S.; Ni, Y.; LaFramboise, T. Heteroplasmic Shifts in Tumor Mitochondrial Genomes Reveal Tissue-Specific Signals of Relaxed and Positive Selection. Hum. Mol. Genet. 2017, 26, 2912–2922. [Google Scholar] [CrossRef] [PubMed]
- Ewing, A.; Meynert, A.; Silk, R.; Aitken, S.; Bendixsen, D.P.; Churchman, M.; Brown, S.L.; Hamdan, A.; Mattocks, J.; Grimes, G.R.; et al. Divergent Trajectories to Structural Diversity Impact Patient Survival in High Grade Serous Ovarian Cancer. Nat. Commun. 2025, 16, 5586. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.Y.; Ho, Q.S.; Low, J.; Choolani, M.; Wong, K.P. Respiratory Competent Mitochondria in Human Ovarian and Peritoneal Cancer. Mitochondrion 2011, 11, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Signorile, A.; De Rasmo, D.; Cormio, A.; Musicco, C.; Rossi, R.; Fortarezza, F.; Palese, L.L.; Loizzi, V.; Resta, L.; Scillitani, G.; et al. Human Ovarian Cancer Tissue Exhibits Increase of Mitochondrial Biogenesis and Cristae Remodeling. Cancers 2019, 11, 1350. [Google Scholar] [CrossRef]
- Gammage, P.A.; Frezza, C. Mitochondrial DNA: The Overlooked Oncogenome? BMC Biol. 2019, 17, 53. [Google Scholar] [CrossRef]
- He, Y.; Wu, J.; Dressman, D.C.; Iacobuzio-Donahue, C.; Markowitz, S.D.; Velculescu, V.E.; Diaz, L.A., Jr.; Kinzler, K.W.; Vogelstein, B.; Papadopoulos, N. Heteroplasmic Mitochondrial DNA Mutations in Normal and Tumor Cells. Nature 2010, 464, 610–614. [Google Scholar] [CrossRef]
- Stewart, J.B.; Chinnery, P.F. The Dynamics of Mitochondrial DNA Heteroplasmy: Implications for Human Health and Disease. Nat. Rev. Genet. 2015, 16, 530–542. [Google Scholar] [CrossRef] [PubMed]
- Diaz, F.; Bayona-Bafaluy, M.P.; Rana, M.; Mora, M.; Hao, H.; Moraes, C.T. Human Mitochondrial DNA with Large Deletions Repopulates Organelles Faster than Full-Length Genomes under Relaxed Copy Number Control. Nucleic Acids Res. 2002, 30, 4626–4633. [Google Scholar] [CrossRef]
- Clark, K.A.; Howe, D.K.; Gafner, K.; Kusuma, D.; Ping, S.; Estes, S.; Denver, D.R. Selfish Little Circles: Transmission Bias and Evolution of Large Deletion-Bearing Mitochondrial DNA in Caenorhabditis Briggsae Nematodes. PLoS ONE 2012, 7, e41433. [Google Scholar] [CrossRef]
- Lawless, C.; Greaves, L.; Reeve, A.K.; Turnbull, D.M.; Vincent, A.E. The Rise and Rise of Mitochondrial DNA Mutations. Open Biol. 2020, 10, 200061. [Google Scholar] [CrossRef] [PubMed]
- Elson, J.L.; Samuels, D.C.; Turnbull, D.M.; Chinnery, P.F. Random Intracellular Drift Explains the Clonal Expansion of Mitochondrial DNA Mutations with Age. Am. J. Hum. Genet. 2001, 68, 802–806. [Google Scholar] [CrossRef]
- Stamp, C.; Zupanic, A.; Sachdeva, A.; Stoll, E.A.; Shanley, D.P.; Mathers, J.C.; Kirkwood, T.B.L.; Heer, R.; Simons, B.D.; Turnbull, D.M.; et al. Predominant Asymmetrical Stem Cell Fate Outcome Limits the Rate of Niche Succession in Human Colonic Crypts. EBioMedicine 2018, 31, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, K.; Mandal, S.; Roy, A. Recent Advancements in Therapeutic Targeting of the Warburg Effect in Refractory Ovarian Cancer: A Promise towards Disease Remission. Biochim. Biophys. Acta BBA-Rev. Cancer 2021, 1876, 188563. [Google Scholar] [CrossRef]
- Ju, Y.S.; Alexandrov, L.B.; Gerstung, M.; Martincorena, I.; Nik-Zainal, S.; Ramakrishna, M.; Davies, H.R.; Papaemmanuil, E.; Gundem, G.; Shlien, A.; et al. Origins and Functional Consequences of Somatic Mitochondrial DNA Mutations in Human Cancer. eLife 2014, 3, e02935. [Google Scholar] [CrossRef]
- Dong, Z.; Pu, L.; Cui, H. Mitoepigenetics and Its Emerging Roles in Cancer. Front. Cell Dev. Biol. 2020, 8, 4. [Google Scholar] [CrossRef]
- Tong, H.; Zhang, L.; Gao, J.; Wen, S.; Zhou, H.; Feng, S. Methylation of Mitochondrial DNA Displacement Loop Region Regulates Mitochondrial Copy Number in Colorectal Cancer. Mol. Med. Rep. 2017, 16, 5347–5353. [Google Scholar] [CrossRef]
- Sun, X.; Zhan, L.; Chen, Y.; Wang, G.; He, L.; Wang, Q.; Zhou, F.; Yang, F.; Wu, J.; Wu, Y.; et al. Increased mtDNA Copy Number Promotes Cancer Progression by Enhancing Mitochondrial Oxidative Phosphorylation in Microsatellite-Stable Colorectal Cancer. Signal Transduct. Target. Ther. 2018, 3, 8. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Vaghjiani, V.; Jayasekara, W.S.N.; Cain, J.E.; St. John, J.C. The Degree of Mitochondrial DNA Methylation in Tumor Models of Glioblastoma and Osteosarcoma. Clin. Epigenetics 2018, 10, 157. [Google Scholar] [CrossRef]
- Morris, M.J.; Hesson, L.B.; Poulos, R.C.; Ward, R.L.; Wong, J.W.H.; Youngson, N.A. Reduced Nuclear DNA Methylation and Mitochondrial Transcript Changes in Adenomas Do Not Associate with mtDNA Methylation. Biomark. Res. 2018, 6, 37. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Jayson, G.C.; Kohn, E.C.; Kitchener, H.C.; Ledermann, J.A. Ovarian Cancer. Lancet 2014, 384, 1376–1388. [Google Scholar] [CrossRef]
- Webb, P.M.; Jordan, S.J. Global Epidemiology of Epithelial Ovarian Cancer. Nat. Rev. Clin. Oncol. 2024, 21, 389–400. [Google Scholar] [CrossRef]
- Lengyel, E. Ovarian Cancer Development and Metastasis. Am. J. Pathol. 2010, 177, 1053–1064. [Google Scholar] [CrossRef]
- Xu, Z.; Zhou, K.; Wang, Z.; Liu, Y.; Wang, X.; Gao, T.; Xie, F.; Yuan, Q.; Gu, X.; Liu, S.; et al. Metastatic Pattern of Ovarian Cancer Delineated by Tracing the Evolution of Mitochondrial DNA Mutations. Exp. Mol. Med. 2023, 55, 1388–1398. [Google Scholar] [CrossRef] [PubMed]
- Liu, V.W.S.; Shi, H.H.; Cheung, A.N.Y.; Chiu, P.M.; Leung, T.W.; Nagley, P.; Wong, L.C.; Ngan, H.Y.S. High Incidence of Somatic Mitochondrial DNA Mutations in Human Ovarian Carcinomas. Cancer Res. 2001, 61, 5998–6001. [Google Scholar]
- Trappen, P.O.V.; Cullup, T.; Troke, R.; Swann, D.; Shepherd, J.H.; Jacobs, I.J.; Gayther, S.A.; Mein, C.A. Somatic Mitochondrial DNA Mutations in Primary and Metastatic Ovarian Cancer. Gynecol. Oncol. 2007, 104, 129–133. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, V.W.S.; Xue, W.C.; Cheung, A.N.Y.; Ngan, H.Y.S. Association of Decreased Mitochondrial DNA Content with Ovarian Cancer Progression. Br. J. Cancer 2006, 95, 1087–1091. [Google Scholar] [CrossRef] [PubMed]
- Aikhionbare, F.O.; Mehrabi, S.; Kumaresan, K.; Zavareh, M.; Olatinwo, M.; Odunsi, K.; Partridge, E. Mitochondrial DNA Sequence Variants in Epithelial Ovarian Tumor Subtypes and Stages. J. Carcinog. 2007, 6, 1. [Google Scholar] [CrossRef]
- Larman, T.C.; DePalma, S.R.; Hadjipanayis, A.G.; The Cancer Genome Atlas Research Network; Protopopov, A.; Zhang, J.; Gabriel, S.B.; Chin, L.; Seidman, C.E.; Kucherlapati, R.; et al. Spectrum of Somatic Mitochondrial Mutations in Five Cancers. Proc. Natl. Acad. Sci. USA. 2012, 109, 14087–14091. [Google Scholar] [CrossRef]
- Kong, D.; Shi, S.; Li, Y. Single Nucleotide Polymorphisms in the D-Loop Region of Mitochondrial DNA Are Associated with Epithelial Ovarian Cancer Prognosis. Mitochondrial DNA 2015, 26, 848–850. [Google Scholar] [CrossRef]
- Kong, D.; Shi, S.; Li, Y.; Li, R.; Li, M. Single Nucleotide Polymorphisms in the Mitochondrial Displacement Loop and Age-at-Onset of Epithelial Ovarian Cancer. Mitochondrial DNA Part A 2016, 27, 1141–1143. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Shi, S.; Li, Y.; Kong, D. Identification of Sequence Nucleotide Polymorphisms in the D-Loop Region of Mitochondrial DNA as a Risk Factor for Epithelial Ovarian Cancer. Mitochondrial DNA Part A 2016, 27, 9–11. [Google Scholar] [CrossRef] [PubMed]
- Xie, F.; Guo, W.; Wang, X.; Zhou, K.; Guo, S.; Liu, Y.; Sun, T.; Li, S.; Xu, Z.; Yuan, Q.; et al. Mutational Profiling of Mitochondrial DNA Reveals an Epithelial Ovarian Cancer-specific Evolutionary Pattern Contributing to High Oxidative Metabolism. Clin. Transl. Med. 2024, 14, e1523. [Google Scholar] [CrossRef]
- Gonzalez Bosquet, J.; Wagner, V.; Polio, A.; Linder, K.E.; Bender, D.P.; Goodheart, M.J.; Schickling, B.M. Identification of Ovarian High-Grade Serous Carcinoma with Mitochondrial Gene Variation. Int. J. Mol. Sci. 2025, 26, 1347. [Google Scholar] [CrossRef]
- Stoneking, M. Hypervariable Sites in the mtDNA Control Region Are Mutational Hotspots. Am. J. Hum. Genet. 2000, 67, 1029–1032. [Google Scholar] [CrossRef]
- Gupta, R.; Kanai, M.; Durham, T.J.; Tsuo, K.; McCoy, J.G.; Kotrys, A.V.; Zhou, W.; Chinnery, P.F.; Karczewski, K.J.; Calvo, S.E.; et al. Nuclear Genetic Control of mtDNA Copy Number and Heteroplasmy in Humans. Nature 2023, 620, 839–848, Erratum in Nature 2024, 630, E10. [Google Scholar] [CrossRef]
- Ji, X.; Guo, W.; Gu, X.; Guo, S.; Zhou, K.; Su, L.; Yuan, Q.; Liu, Y.; Guo, X.; Huang, Q.; et al. Mutational Profiling of mtDNA Control Region Reveals Tumor-Specific Evolutionary Selection Involved in Mitochondrial Dysfunction. eBioMedicine 2022, 80, 104058. [Google Scholar] [CrossRef] [PubMed]
- Ni, J.; Wang, Y.; Cheng, X.; Teng, F.; Wang, C.; Han, S.; Chen, X.; Guo, W. Pathogenic Heteroplasmic Somatic Mitochondrial DNA Mutation Confers Platinum-Resistance and Recurrence of High-Grade Serous Ovarian Cancer. Cancer Manag. Res. 2020, 12, 11085–11093. [Google Scholar] [CrossRef]
- Kim, M.; Gorelick, A.N.; Vàzquez-García, I.; Williams, M.J.; Salehi, S.; Shi, H.; Weiner, A.C.; Ceglia, N.; Funnell, T.; Park, T.; et al. Single-Cell mtDNA Dynamics in Tumors Is Driven by Coregulation of Nuclear and Mitochondrial Genomes. Nat. Genet. 2024, 56, 889–899. [Google Scholar] [CrossRef] [PubMed]
- Schwarzenbach, H.; Hoon, D.S.B.; Pantel, K. Cell-Free Nucleic Acids as Biomarkers in Cancer Patients. Nat. Rev. Cancer 2011, 11, 426–437. [Google Scholar] [CrossRef] [PubMed]
- Duffy, M.J.; Crown, J. Circulating Tumor DNA as a Biomarker for Monitoring Patients with Solid Cancers: Comparison with Standard Protein Biomarkers. Clin. Chem. 2022, 68, 1381–1390. [Google Scholar] [CrossRef]
- Mohd Khair, S.Z.N.; Abd Radzak, S.M.; Mohamed Yusoff, A.A. The Uprising of Mitochondrial DNA Biomarker in Cancer. Dis. Markers 2021, 2021, 7675269. [Google Scholar] [CrossRef]
- Paracchini, L.; D’Incalci, M.; Marchini, S. Liquid Biopsy in the Clinical Management of High-Grade Serous Epithelial Ovarian Cancer—Current Use and Future Opportunities. Cancers 2021, 13, 2386. [Google Scholar] [CrossRef]
- Lou, C.; Ma, X.; Chen, Z.; Zhao, Y.; Yao, Q.; Zhou, C.; Zhao, X.; Meng, X. The mtDNA Fragments within Exosomes Might Be Novel Diagnostic Biomarkers of Non-Small Cell Lung Cancer. Pathol.-Res. Pract. 2023, 249, 154718. [Google Scholar] [CrossRef]
- Meng, X.; Schwarzenbach, H.; Yang, Y.; Müller, V.; Li, N.; Tian, D.; Shen, Y.; Gong, Z. Circulating Mitochondrial DNA Is Linked to Progression and Prognosis of Epithelial Ovarian Cancer. Transl. Oncol. 2019, 12, 1213–1220. [Google Scholar] [CrossRef] [PubMed]
- Rose, P.G. First-Line Chemotherapy for Ovarian Cancer: Inferences from Recent Studies. Oncologist 2016, 21, 1286–1290. [Google Scholar] [CrossRef]
- Yang, Z.; Schumaker, L.M.; Egorin, M.J.; Zuhowski, E.G.; Guo, Z.; Cullen, K.J. Cisplatin Preferentially Binds Mitochondrial DNA and Voltage-Dependent Anion Channel Protein in the Mitochondrial Membrane of Head and Neck Squamous Cell Carcinoma: Possible Role in Apoptosis. Clin. Cancer Res. 2006, 12, 5817–5825. [Google Scholar] [CrossRef]
- Cui, X.; Xu, J.; Jia, X. Targeting Mitochondria: A Novel Approach for Treating Platinum-Resistant Ovarian Cancer. J. Transl. Med. 2024, 22, 968. [Google Scholar] [CrossRef]
- Montopoli, M.; Bellanda, M.; Lonardoni, F.; Ragazzi, E.; Dorigo, P.; Froldi, G.; Mammi, S.; Caparrotta, L. “Metabolic Reprogramming” in Ovarian Cancer Cells Resistant to Cisplatin. Curr. Cancer Drug Targets 2011, 11, 226–235. [Google Scholar] [CrossRef]
- Smith, A.L.M.; Whitehall, J.C.; Greaves, L.C. Mitochondrial DNA Mutations in Ageing and Cancer. Mol. Oncol. 2022, 16, 3276–3294. [Google Scholar] [CrossRef]
- Kobayashi, H.; Imanaka, S.; Shigetomi, H. Revisiting Therapeutic Strategies for Ovarian Cancer by Focusing on Redox Homeostasis (Review). Oncol. Lett. 2022, 23, 80. [Google Scholar] [CrossRef]
- Wagner, A.; Kosnacova, H.; Chovanec, M.; Jurkovicova, D. Mitochondrial Genetic and Epigenetic Regulations in Cancer: Therapeutic Potential. Int. J. Mol. Sci. 2022, 23, 7897. [Google Scholar] [CrossRef]
- Wang, Y.; Cardenas, H.; Fang, F.; Condello, S.; Taverna, P.; Segar, M.; Liu, Y.; Nephew, K.P.; Matei, D. Epigenetic Targeting of Ovarian Cancer Stem Cells. Cancer Res. 2014, 74, 4922–4936. [Google Scholar] [CrossRef] [PubMed]
- Kopinski, P.K.; Janssen, K.A.; Schaefer, P.M.; Trefely, S.; Perry, C.E.; Potluri, P.; Tintos-Hernandez, J.A.; Singh, L.N.; Karch, K.R.; Campbell, S.L.; et al. Regulation of Nuclear Epigenome by Mitochondrial DNA Heteroplasmy. Proc. Natl. Acad. Sci. USA 2019, 116, 16028–16035. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Xie, C. Unveiling the Power of Mitochondrial Transfer in Cancer Progression: A Perspective in Ovarian Cancer. J. Ovarian Res. 2024, 17, 233. [Google Scholar] [CrossRef]
- Desir, S.; Dickson, E.L.; Vogel, R.I.; Thayanithy, V.; Wong, P.; Teoh, D.; Geller, M.A.; Steer, C.J.; Subramanian, S.; Lou, E. Tunneling Nanotube Formation Is Stimulated by Hypoxia in Ovarian Cancer Cells. Oncotarget 2016, 7, 43150–43161. [Google Scholar] [CrossRef]
- Walters, H.E.; Cox, L.S. Intercellular Transfer of Mitochondria between Senescent Cells through Cytoskeleton-Supported Intercellular Bridges Requires mTOR and CDC42 Signalling. Oxid. Med. Cell. Longev. 2021, 2021, 6697861. [Google Scholar] [CrossRef]
- Cole, J.M.; Dahl, R.; Cowden Dahl, K.D. MAPK Signaling Is Required for Generation of Tunneling Nanotube-Like Structures in Ovarian Cancer Cells. Cancers 2021, 13, 274. [Google Scholar] [CrossRef] [PubMed]
- Samuel, P.; Mulcahy, L.A.; Furlong, F.; McCarthy, H.O.; Brooks, S.A.; Fabbri, M.; Pink, R.C.; Carter, D.R.F. Cisplatin Induces the Release of Extracellular Vesicles from Ovarian Cancer Cells That Can Induce Invasiveness and Drug Resistance in Bystander Cells. Philos. Trans. R. Soc. B Biol. Sci. 2018, 373, 20170065. [Google Scholar] [CrossRef] [PubMed]
- Weiler, J.; Dittmar, T. Minocycline Impairs TNF-α-Induced Cell Fusion of M13SV1-Cre Cells with MDA-MB-435-pFDR1 Cells by Suppressing NF-κB Transcriptional Activity and Its Induction of Target-Gene Expression of Fusion-Relevant Factors. Cell Commun. Signal. 2019, 17, 71. [Google Scholar] [CrossRef]
- Strick, R.; Ackermann, S.; Langbein, M.; Swiatek, J.; Schubert, S.W.; Hashemolhosseini, S.; Koscheck, T.; Fasching, P.A.; Schild, R.L.; Beckmann, M.W.; et al. Proliferation and Cell–Cell Fusion of Endometrial Carcinoma Are Induced by the Human Endogenous Retroviral Syncytin-1 and Regulated by TGF-β. J. Mol. Med. 2007, 85, 23–38. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Schekman, R. Syncytin-Mediated Open-Ended Membrane Tubular Connections Facilitate the Intercellular Transfer of Cargos Including Cas9 Protein. eLife 2023, 12, e84391. [Google Scholar] [CrossRef]
- Song, K.; Zhu, F.; Zhang, H.; Shang, Z. Tumor Necrosis Factor-α Enhanced Fusions between Oral Squamous Cell Carcinoma Cells and Endothelial Cells via VCAM-1/VLA-4 Pathway. Exp. Cell Res. 2012, 318, 1707–1715. [Google Scholar] [CrossRef]
- Gasparre, G.; Kurelac, I.; Capristo, M.; Iommarini, L.; Ghelli, A.; Ceccarelli, C.; Nicoletti, G.; Nanni, P.; De Giovanni, C.; Scotlandi, K.; et al. A Mutation Threshold Distinguishes the Antitumorigenic Effects of the Mitochondrial Gene MTND1, an Oncojanus Function. Cancer Res. 2011, 71, 6220–6229. [Google Scholar] [CrossRef]
- Guerra, F.; Perrone, A.M.; Kurelac, I.; Santini, D.; Ceccarelli, C.; Cricca, M.; Zamagni, C.; De Iaco, P.; Gasparre, G. Mitochondrial DNA Mutation in Serous Ovarian Cancer: Implications for Mitochondria-Coded Genes in Chemoresistance. J. Clin. Oncol. 2012, 30, e373–e378. [Google Scholar] [CrossRef]
- Guerra, F.; Arbini, A.A.; Moro, L. Mitochondria and Cancer Chemoresistance. Biochim. Biophys. Acta BBA-Bioenerg. 2017, 1858, 686–699. [Google Scholar] [CrossRef]
- Arbini, A.A.; Guerra, F.; Greco, M.; Marra, E.; Gandee, L.; Xiao, G.; Lotan, Y.; Gasparre, G.; Hsieh, J.-T.; Moro, L. Mitochondrial DNA Depletion Sensitizes Cancer Cells to PARP Inhibitors by Translational and Post-Translational Repression of BRCA2. Oncogenesis 2013, 2, e82. [Google Scholar] [CrossRef]
- Lin, Y.; Yang, B.; Huang, Y.; Zhang, Y.; Jiang, Y.; Ma, L.; Shen, Y.-Q. Mitochondrial DNA-Targeted Therapy: A Novel Approach to Combat Cancer. Cell Insight 2023, 2, 100113. [Google Scholar] [CrossRef]
- Di Donfrancesco, A.; Massaro, G.; Di Meo, I.; Tiranti, V.; Bottani, E.; Brunetti, D. Gene Therapy for Mitochondrial Diseases: Current Status and Future Perspective. Pharmaceutics 2022, 14, 1287. [Google Scholar] [CrossRef]
- Cavaliere, A.; Marchet, S.; Meo, I.D.; Tiranti, V. An In Vitro Approach to Study Mitochondrial Dysfunction: A Cybrid Model. J. Vis. Exp. JoVE 2022, 181, e63452. [Google Scholar] [CrossRef]
- Mok, B.Y.; de Moraes, M.H.; Zeng, J.; Bosch, D.E.; Kotrys, A.V.; Raguram, A.; Hsu, F.; Radey, M.C.; Peterson, S.B.; Mootha, V.K.; et al. A Bacterial Cytidine Deaminase Toxin Enables CRISPR-Free Mitochondrial Base Editing. Nature 2020, 583, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Lee, H.; Baek, G.; Kim, J.-S. Precision Mitochondrial DNA Editing with High-Fidelity DddA-Derived Base Editors. Nat. Biotechnol. 2023, 41, 378–386. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Lee, H.; Baek, G.; Namgung, E.; Park, J.M.; Kim, S.; Hong, S.; Kim, J.-S. Enhanced Mitochondrial DNA Editing in Mice Using Nuclear-Exported TALE-Linked Deaminases and Nucleases. Genome Biol. 2022, 23, 211. [Google Scholar] [CrossRef]
- Lee, H.; Lee, S.; Baek, G.; Kim, A.; Kang, B.-C.; Seo, H.; Kim, J.-S. Mitochondrial DNA Editing in Mice with DddA-TALE Fusion Deaminases. Nat. Commun. 2021, 12, 1190. [Google Scholar] [CrossRef] [PubMed]
- Sabharwal, A.; Kar, B.; Restrepo-Castillo, S.; Holmberg, S.R.; Mathew, N.D.; Kendall, B.L.; Cotter, R.P.; WareJoncas, Z.; Seiler, C.; Nakamaru-Ogiso, E.; et al. The FusX TALE Base Editor (FusXTBE) for Rapid Mitochondrial DNA Programming of Human Cells In Vitro and Zebrafish Disease Models In Vivo. CRISPR J. 2021, 4, 799–821. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC8742272/ (accessed on 11 November 2025). [PubMed]
- Lim, K.; Cho, S.-I.; Kim, J.-S. Nuclear and Mitochondrial DNA Editing in Human Cells with Zinc Finger Deaminases. Nat. Commun. 2022, 13, 366. [Google Scholar] [CrossRef] [PubMed]
- Tolle, I.; Tiranti, V.; Prigione, A. Modeling Mitochondrial DNA Diseases: From Base Editing to Pluripotent Stem-cell-derived Organoids. EMBO Rep. 2023, 24, e55678. [Google Scholar] [CrossRef]
- Kang, B.-C.; Bae, S.-J.; Lee, S.; Lee, J.S.; Kim, A.; Lee, H.; Baek, G.; Seo, H.; Kim, J.; Kim, J.-S. Chloroplast and Mitochondrial DNA Editing in Plants. Nat. Plants 2021, 7, 899–905. [Google Scholar] [CrossRef]
- Mahmood, M.; Liu, E.M.; Shergold, A.L.; Tolla, E.; Tait-Mulder, J.; Huerta-Uribe, A.; Shokry, E.; Young, A.L.; Lilla, S.; Kim, M.; et al. Mitochondrial DNA Mutations Drive Aerobic Glycolysis to Enhance Checkpoint Blockade Response in Melanoma. Nat. Cancer 2024, 5, 659–672. [Google Scholar] [CrossRef] [PubMed]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).