Serial Combination of Toxic and Ischemic Renal Damages Causes Subsequent Chronic, Irreversible, and Progressive Renal Disease in Rats
Abstract
1. Introduction
2. Results
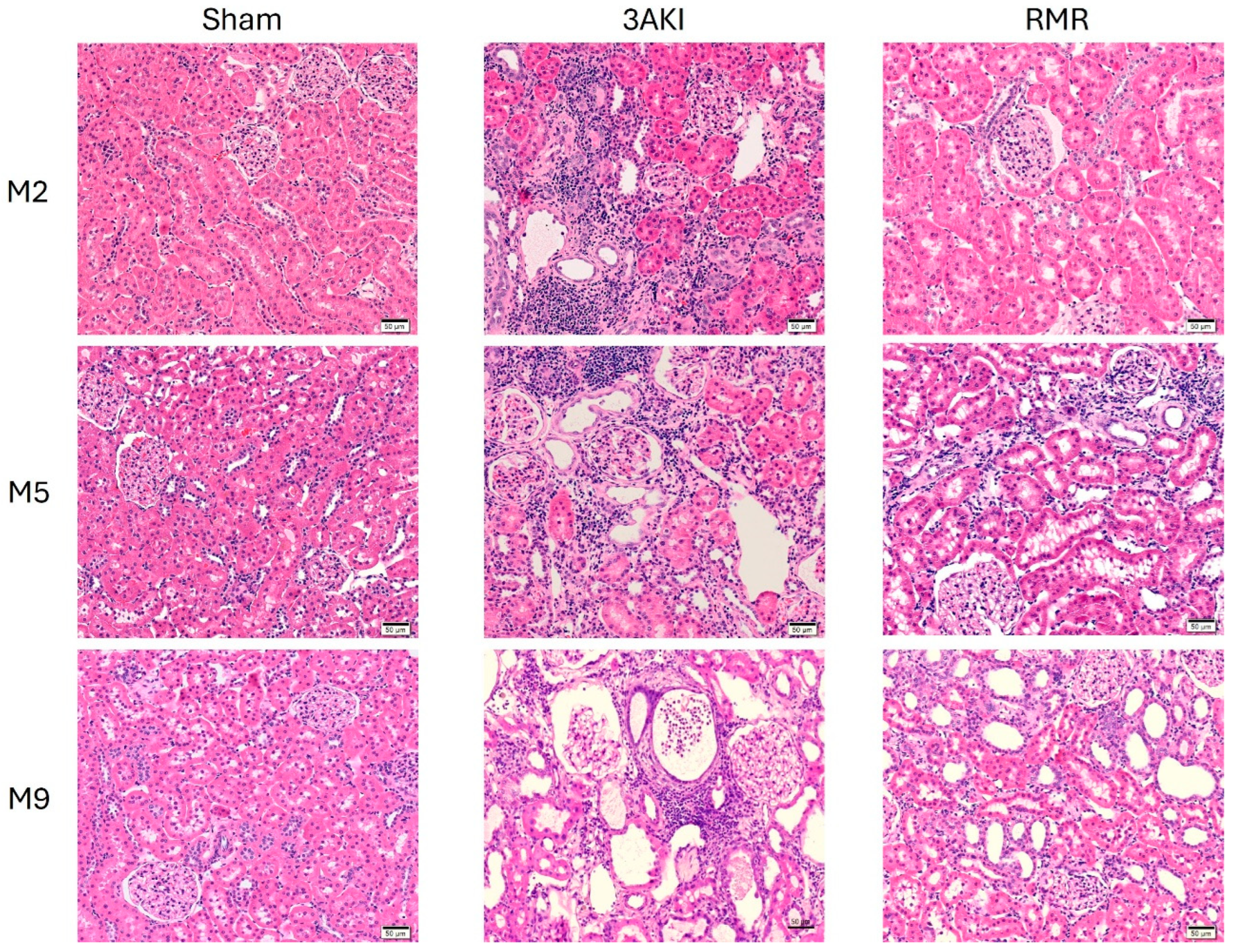
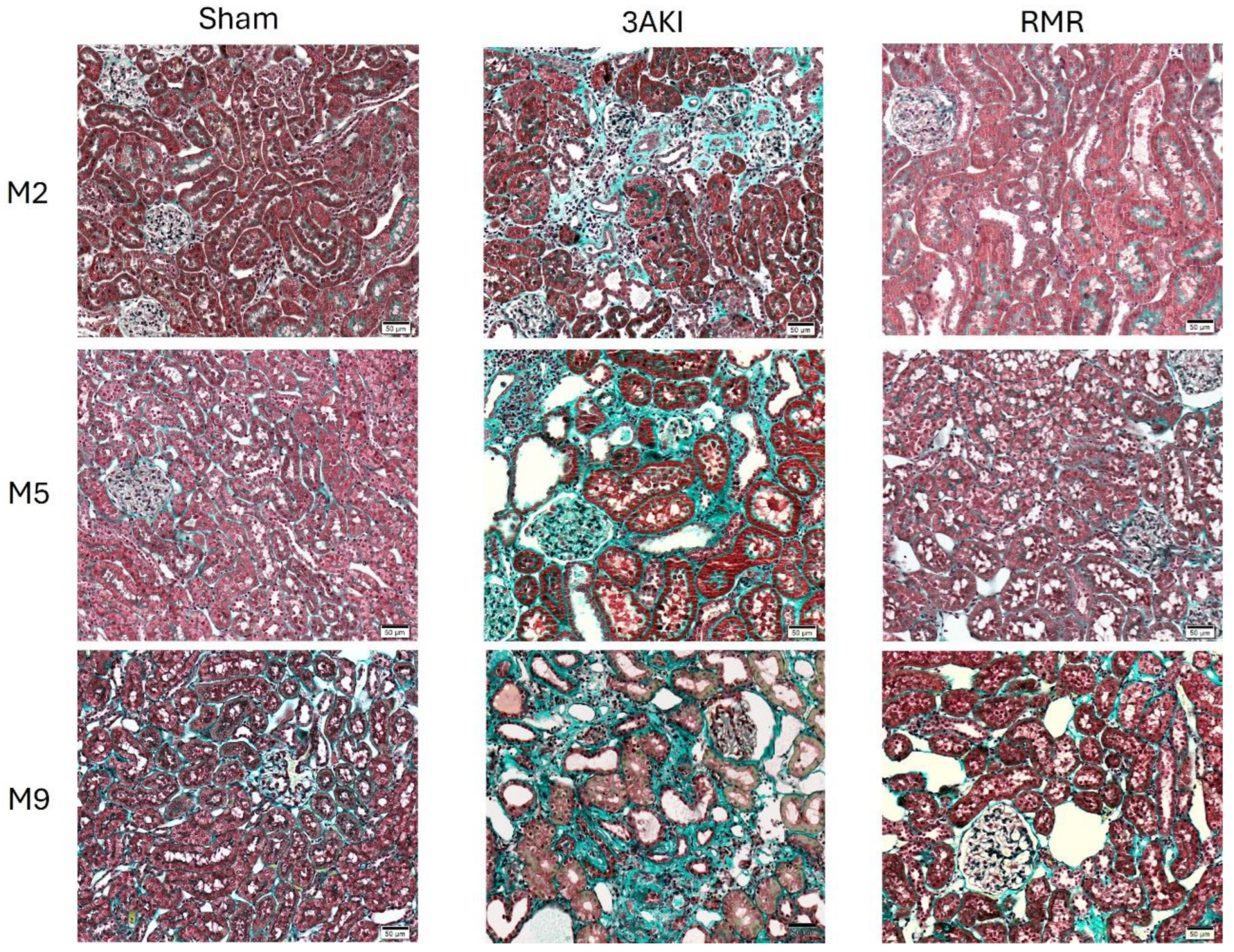
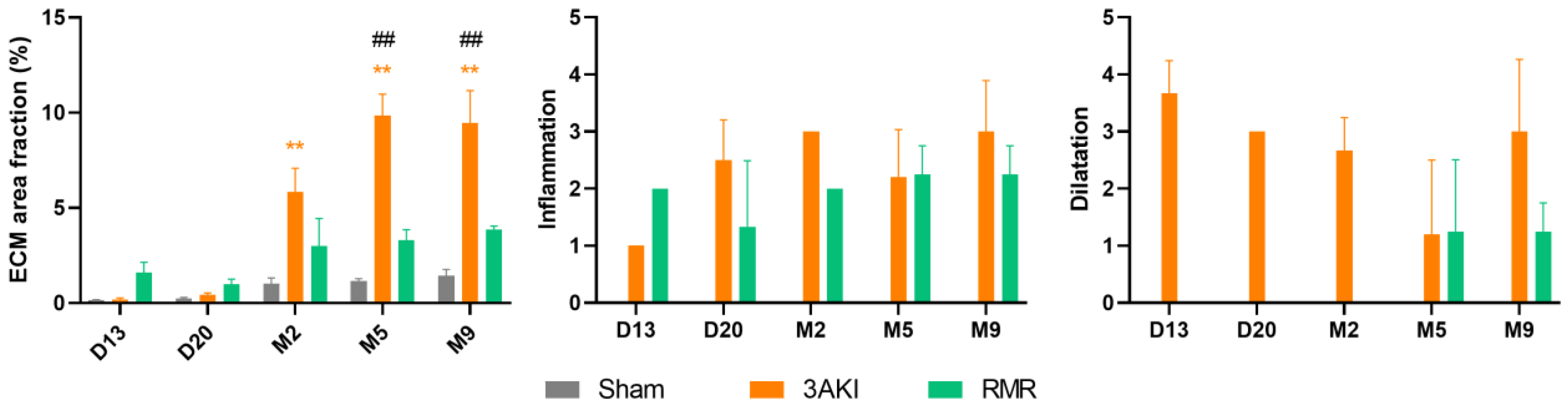
2.1. Renal Structure Progressively Deteriorates over Time Following Triple AKI and RMR
2.2. Albuminuria and Transferrinuria Progressively Increase Following Triple AKI and RMR
2.3. Glomerular Filtration Remains Almost Stably Reduced in the Long-Term Following Triple AKI and RMR
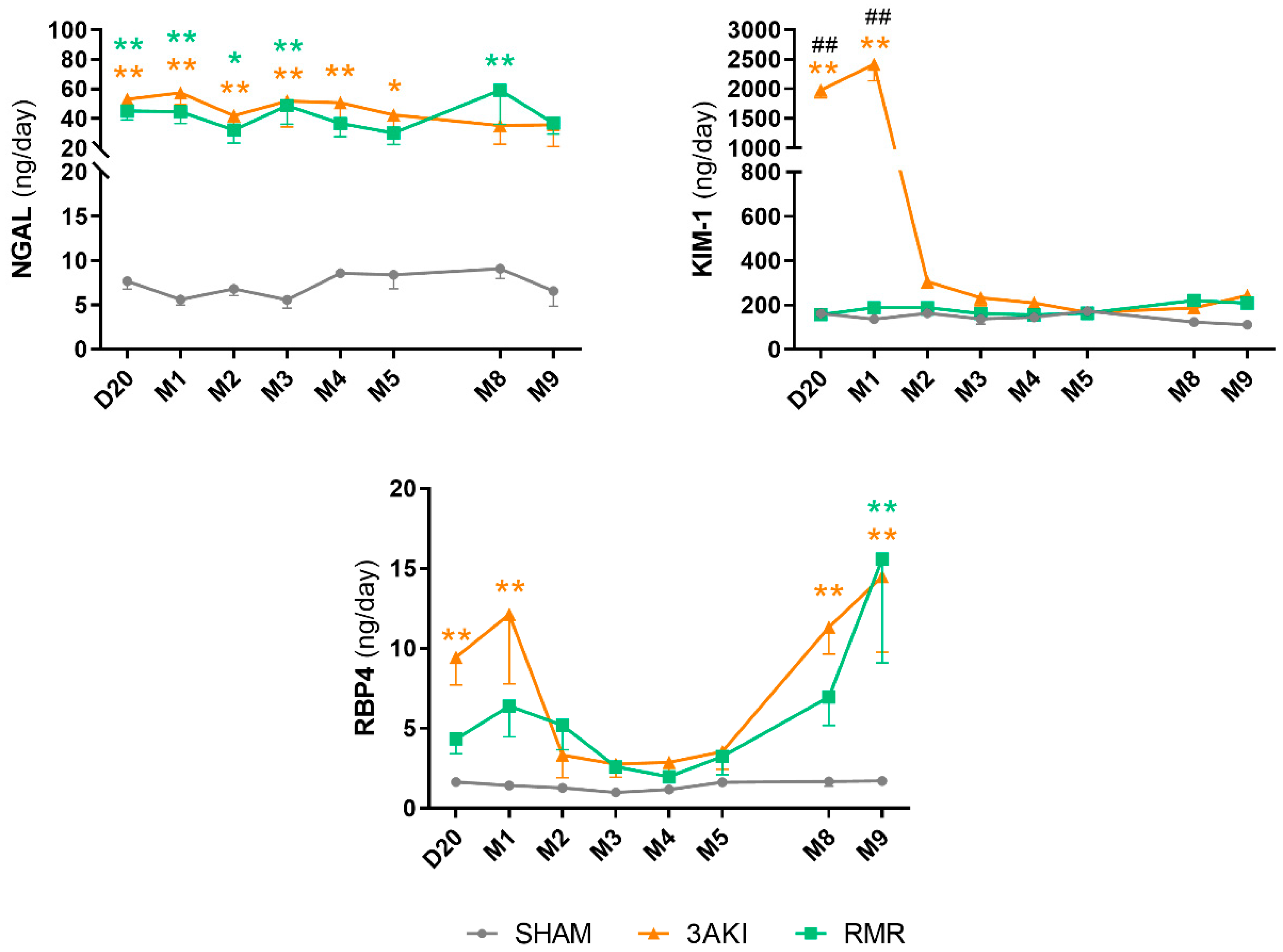
2.4. Urinary Injury Biomarkers Show Particular Trends Following Triple AKI and RMR
3. Discussion
4. Materials and Methods
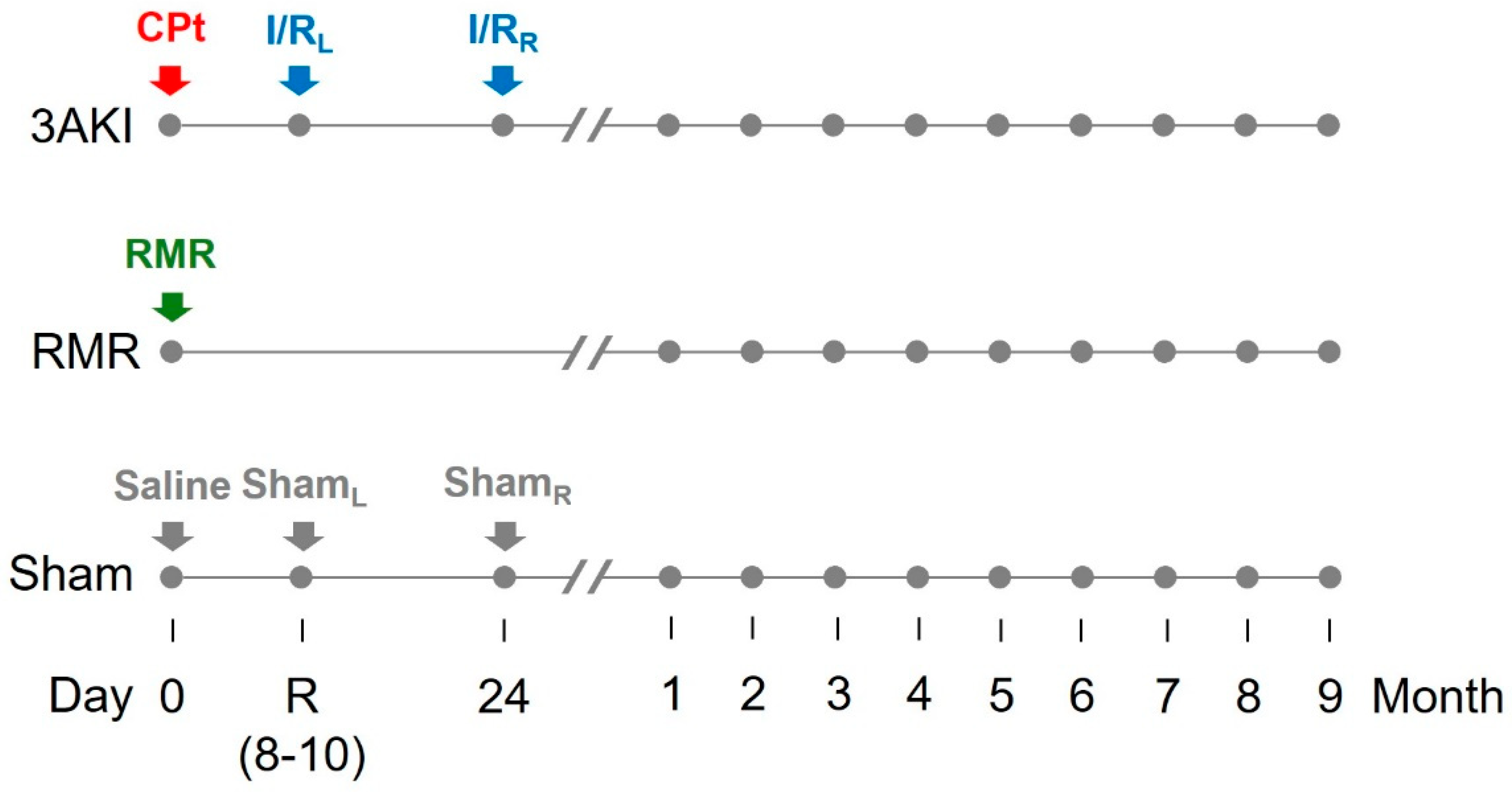
4.1. Animal Model and Experimental Protocol
- Triple AKI group (3AKI): rats received a single i.p. dose of cisplatin (5 mg/kg) on day 0 (basal), and were subsequently subjected to a 60-min ischemia by placing a clamp in the renal artery of the left kidney on day R (i.e., recovery from first AKI as by plasma creatinine concentration (pCr) normalization, typically 7–10 days after the insult), and to a 60-min ischemia by placing a clamp in the renal artery of the right kidney on the day 24. Renal ischemia/reperfusion was carried out basically as described [60]. Briefly, rats were anesthetized with a mixture of 80 mg/kg ketamine and 20 mg/kg xylazine. Kidneys were accessed through a medial laparotomy and the renal artery gently exposed, cleaned from surrounding fat, and clamped for 60 min, after which the clamp was removed to allow reperfusion. Incisions were then sutured, and animals were allowed to recover under 30 μg/kg buprenorphine analgesia (every 12 h for two days).
- Renal mass reduction (RMR) group: rats were subjected to renal mass reduction at day 0 (basal). Renal mass reduction was achieved by right nephrectomy and polectomy of the left kidney, as described [61]. Briefly, rats were anesthetized with a mixture of 80 mg/kg ketamine and 20 mg/kg xylazine. Kidneys were then accessed through a medial laparotomy. The right kidney pedicle was ligated, and the kidney was dissected. The poles of the left kidney were severed with a scalpel and absorbable hemostatic tissue (Espongostan® Film, Takeda, Madrid, Spain) was applied to prevent bleeding. Animals were sutured and allowed to recover under 30 μg/kg buprenorphine analgesia (as above).
- Control group (sham): rats were administered physiological saline on day 0 (basal) and were subjected to sham-operations on day R and 24.
4.2. Renal Histology
4.3. Renal Function
4.4. Urinary Biomarkers
4.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Glossary
| AKI | acute kidney injury |
| ClCr | creatinine clearance |
| CKD | chronic kidney disease |
| KIM-1 | kidney injury molecule 1 |
| GFB | glomerular filtration barrier |
| GFR | glomerular filtration rate |
| HE | hematoxylin and eosin |
| KDIGO | Kidney Disease Improving Global Clinical Practice Guidelines |
| MT | Masson’s Trichrome |
| NGAL | neutrophil gelatinase-associated lipocalin |
| pCr | plasma creatinine concentration |
| RBP4 | retinol binding protein 4 |
| RMR | renal mass reduction |
| RRT | renal replacement therapies |
| SEM | standard error of the mean |
| SOX9 | SRY-Box transcription factor 9 |
| TGF | transforming growth factor |
| uCr | urinary creatinine concentration |
| UF | urinary flow |
References
- Bikbov, B.; Purcell, C.A.; Levey, A.S.; Smith, M.; Abdoli, A.; Abebe, M.; Adebayo, O.M.; Afarideh, M.; Agarwal, S.K.; Agudelo-Botero, M.; et al. Global, Regional, and National Burden of Chronic Kidney Disease, 1990–2017: A Systematic Analysis for the Global Burden of Disease Study 2017. Lancet 2020, 395, 709–733. [Google Scholar] [CrossRef]
- Ortiz, A.; Sanchez-Niño, M.D.; Crespo-Barrio, M.; De-Sequera-Ortiz, P.; Fernández-Giráldez, E.; García-Maset, R.; Macía-Heras, M.; Pérez-Fontán, M.; Rodríguez-Portillo, M.; Salgueira-Lazo, M.; et al. The Spanish Society of Nephrology (SENEFRO) Commentary to the Spain GBD 2016 Report: Keeping Chronic Kidney Disease out of Sight of Health Authorities Will Only Magnify the Problem. Nefrología 2019, 39, 29–34. [Google Scholar] [CrossRef]
- Romagnani, P.; Remuzzi, G.; Glassock, R.; Levin, A.; Jager, K.J.; Tonelli, M.; Massy, Z.; Wanner, C.; Anders, H.-J. Chronic Kidney Disease. Nat. Rev. Dis. Primers 2017, 3, 17088. [Google Scholar] [CrossRef]
- López-Novoa, J.M.; Martínez-Salgado, C.; Rodríguez-Peña, A.B.; Hernández, F.J.L. Common Pathophysiological Mechanisms of Chronic Kidney Disease: Therapeutic Perspectives. Pharmacol. Ther. 2010, 128, 61–81. [Google Scholar] [CrossRef]
- López-Novoa, J.M.; Rodríguez-Peña, A.B.; Ortiz, A.; Martínez-Salgado, C.; López Hernández, F.J. Etiopathology of Chronic Tubular, Glomerular and Renovascular Nephropathies: Clinical Implications. J. Transl. Med. 2011, 9, 13. [Google Scholar] [CrossRef]
- Levey, A.S.; Eckardt, K.-U.; Dorman, N.M.; Christiansen, S.L.; Hoorn, E.J.; Ingelfinger, J.R.; Inker, L.A.; Levin, A.; Mehrotra, R.; Palevsky, P.M.; et al. Nomenclature for Kidney Function and Disease: Report of a Kidney Disease: Improving Global Outcomes (KDIGO) Consensus Conference. Kidney Int. 2020, 97, 1117–1129. [Google Scholar] [CrossRef] [PubMed]
- Kovesdy, C.P. Epidemiology of Chronic Kidney Disease: An Update 2022. Kidney Int. Suppl. 2022, 12, 7–11. [Google Scholar] [CrossRef]
- Erfanpoor, S.; Etemad, K.; Kazempour, S.; Hadaegh, F.; Hasani, J.; Azizi, F.; Parizadeh, D.; Khalili, D. Diabetes, Hypertension, and Incidence of Chronic Kidney Disease: Is There Any Multiplicative or Additive Interaction? Int. J. Endocrinol. Metab. 2020, 19, e101061. [Google Scholar] [CrossRef] [PubMed]
- Ostermann, M.; Zarbock, A.; Goldstein, S.; Kashani, K.; Macedo, E.; Murugan, R.; Bell, M.; Forni, L.; Guzzi, L.; Joannidis, M.; et al. Recommendations on Acute Kidney Injury Biomarkers from the Acute Disease Quality Initiative Consensus Conference: A Consensus Statement. JAMA Netw. Open 2020, 3, e2019209. [Google Scholar] [CrossRef]
- Heung, M.; Steffick, D.E.; Zivin, K.; Gillespie, B.W.; Banerjee, T.; Hsu, C.; Powe, N.R.; Pavkov, M.E.; Williams, D.E.; Saran, R.; et al. Acute Kidney Injury Recovery Pattern and Subsequent Risk of CKD: An Analysis of Veterans Health Administration Data. Am. J. Kidney Dis. 2016, 67, 742–752. [Google Scholar] [CrossRef] [PubMed]
- Ronco, C.; Bellomo, R.; Kellum, J.A. Acute Kidney Injury. Lancet 2019, 394, 1949–1964. [Google Scholar] [CrossRef]
- Bucaloiu, I.D.; Kirchner, H.L.; Norfolk, E.R.; Hartle, J.E.; Perkins, R.M. Increased Risk of Death and de Novo Chronic Kidney Disease Following Reversible Acute Kidney Injury. Kidney Int. 2012, 81, 477–485. [Google Scholar] [CrossRef]
- Fiorentino, M.; Grandaliano, G.; Gesualdo, L.; Castellano, G. Acute Kidney Injury to Chronic Kidney Disease Transition. In Contributions to Nephrology; Ding, X., Rosner, M.H., Ronco, C., Eds.; S. Karger AG: Basel, Switzerland, 2018; Volume 193, pp. 45–54. ISBN 978-3-318-06310-3. [Google Scholar]
- Hsu, R.K.; Hsu, C. The Role of Acute Kidney Injury in Chronic Kidney Disease. Semin. Nephrol. 2016, 36, 283–292. [Google Scholar] [CrossRef]
- Endo, T.; Nakamura, J.; Sato, Y.; Asada, M.; Yamada, R.; Takase, M.; Takaori, K.; Oguchi, A.; Iguchi, T.; Higashi, A.Y.; et al. Exploring the Origin and Limitations of Kidney Regeneration. J. Pathol. 2015, 236, 251–263. [Google Scholar] [CrossRef]
- Zhang, T.; Widdop, R.E.; Ricardo, S.D. Transition from Acute Kidney Injury to Chronic Kidney Disease: Mechanisms, Models, and Biomarkers. Am. J. Physiol. Ren. Physiol. 2024, 327, F788–F805. [Google Scholar] [CrossRef] [PubMed]
- Bonventre, J.V. Maladaptive Proximal Tubule Repair: Cell Cycle Arrest. Nephron Clin. Pract. 2014, 127, 61–64. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, C. From AKI to CKD: Maladaptive Repair and the Underlying Mechanisms. IJMS 2022, 23, 10880. [Google Scholar] [CrossRef] [PubMed]
- Ferenbach, D.A.; Bonventre, J.V. Mechanisms of Maladaptive Repair after AKI Leading to Accelerated Kidney Ageing and CKD. Nat. Rev. Nephrol. 2015, 11, 264–276. [Google Scholar] [CrossRef]
- Fuentes-Calvo, I.; Cuesta, C.; Sancho-Martínez, S.M.; Hidalgo-Thomas, O.A.; Paniagua-Sancho, M.; López-Hernández, F.J.; Martínez-Salgado, C. Biomarkers of Persistent Renal Vulnerability after Acute Kidney Injury Recovery. Sci. Rep. 2021, 11, 21183. [Google Scholar] [CrossRef] [PubMed]
- Cuesta, C.; Fuentes-Calvo, I.; Sancho-Martinez, S.M.; Valentijn, F.A.; Düwel, A.; Hidalgo-Thomas, O.A.; Agüeros-Blanco, C.; Benito-Hernández, A.; Ramos-Barron, M.A.; Gómez-Alamillo, C.; et al. Urinary KIM-1 Correlates with the Subclinical Sequelae of Tubular Damage Persisting after the Apparent Functional Recovery from Intrinsic Acute Kidney Injury. Biomedicines 2022, 10, 1106. [Google Scholar] [CrossRef]
- Grgic, I.; Campanholle, G.; Bijol, V.; Wang, C.; Sabbisetti, V.S.; Ichimura, T.; Humphreys, B.D.; Bonventre, J.V. Targeted Proximal Tubule Injury Triggers Interstitial Fibrosis and Glomerulosclerosis. Kidney Int. 2012, 82, 172–183. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Tao, Q.; Yin, S.; Fu, G.; Wang, C.; Xiang, F.; Hu, H.; Zhang, S.; Wang, Z.; Li, D. A Single Administration of FGF2 after Renal Ischemia–Reperfusion Injury Alleviates Post-Injury Interstitial Fibrosis. Nephrol. Dial. Transplant. 2023, 38, 2537–2549. [Google Scholar] [CrossRef] [PubMed]
- Basile, D.P.; Donohoe, D.; Roethe, K.; Osborn, J.L. Renal Ischemic Injury Results in Permanent Damage to Peritubular Capillaries and Influences Long-Term Function. Am. J. Physiol. Ren. Physiol. 2001, 281, F887–F899. [Google Scholar] [CrossRef]
- Sharp, C.N.; Doll, M.A.; Dupre, T.V.; Shah, P.P.; Subathra, M.; Siow, D.; Arteel, G.E.; Megyesi, J.; Beverly, L.J.; Siskind, L.J. Repeated Administration of Low-Dose Cisplatin in Mice Induces Fibrosis. Am. J. Physiol.-Ren. Physiol. 2016, 310, F560–F568. [Google Scholar] [CrossRef]
- Lee, K.; Jang, H.R.; Jeon, J.; Yang, K.E.; Lee, J.E.; Kwon, G.Y.; Kim, D.J.; Kim, Y.-G.; Huh, W. Repair Phase Modeling of Ischemic Acute Kidney Injury: Recovery vs. Transition to Chronic Kidney Disease. Am. J. Transl. Res. 2022, 14, 554–571. [Google Scholar]
- Wei, J.; Zhang, J.; Wang, L.; Jiang, S.; Fu, L.; Buggs, J.; Liu, R. New Mouse Model of Chronic Kidney Disease Transitioned from Ischemic Acute Kidney Injury. Am. J. Physiol. Ren. Physiol. 2019, 317, F286–F295. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, X.; Wei, J.; Wang, L.; Jiang, S.; Xu, L.; Qu, L.; Yang, K.; Fu, L.; Buggs, J.; et al. A Two-Stage Bilateral Ischemia-Reperfusion Injury-Induced AKI to CKD Transition Model in Mice. Am. J. Physiol. Ren. Physiol. 2020, 319, F304–F311. [Google Scholar] [CrossRef]
- Vanmassenhove, J.; Van Biesen, W.; Vanholder, R.; Lameire, N. Subclinical AKI: Ready for Primetime in Clinical Practice? J. Nephrol. 2019, 32, 9–16. [Google Scholar] [CrossRef]
- Ratajczyk, K.; Konieczny, A.; Czekaj, A.; Piotrów, P.; Fiutowski, M.; Krakowska, K.; Kowal, P.; Witkiewicz, W.; Marek-Bukowiec, K. The Clinical Significance of Urinary Retinol-Binding Protein 4: A Review. Int. J. Environ. Res. Public Health 2022, 19, 9878. [Google Scholar] [CrossRef]
- Bolignano, D.; Donato, V.; Coppolino, G.; Campo, S.; Buemi, A.; Lacquaniti, A.; Buemi, M. Neutrophil Gelatinase–Associated Lipocalin (NGAL) as a Marker of Kidney Damage. Am. J. Kidney Dis. 2008, 52, 595–605. [Google Scholar] [CrossRef]
- Rodrigues-Díez, R.; Rayego-Mateos, S.; Orejudo, M.; Aroeira, L.S.; Selgas, R.; Ortiz, A.; Egido, J.; Ruiz-Ortega, M. TGF-Beta Blockade Increases Renal Inflammation Caused by the C-Terminal Module of the CCN2. Mediat. Inflamm. 2015, 2015, 506041. [Google Scholar] [CrossRef]
- Silver, S.A.; Harel, Z.; McArthur, E.; Nash, D.M.; Acedillo, R.; Kitchlu, A.; Garg, A.X.; Chertow, G.M.; Bell, C.M.; Wald, R. Causes of Death after a Hospitalization with AKI. J. Am. Soc. Nephrol. 2018, 29, 1001–1010. [Google Scholar] [CrossRef]
- Coca, S.G.; Singanamala, S.; Parikh, C.R. Chronic Kidney Disease after Acute Kidney Injury: A Systematic Review and Meta-Analysis. Kidney Int. 2012, 81, 442–448. [Google Scholar] [CrossRef]
- Silver, S.A.; Nadim, M.K.; O’Donoghue, D.J.; Wilson, F.P.; Kellum, J.A.; Mehta, R.L.; Ronco, C.; Kashani, K.; Rosner, M.H.; Haase, M.; et al. Community Health Care Quality Standards to Prevent Acute Kidney Injury and Its Consequences. Am. J. Med. 2020, 133, 552–560.e3. [Google Scholar] [CrossRef]
- Wonnacott, A.; Meran, S.; Amphlett, B.; Talabani, B.; Phillips, A. Epidemiology and Outcomes in Community-Acquired Versus Hospital-Acquired AKI. Clin. J. Am. Soc. Nephrol. 2014, 9, 1007. [Google Scholar] [CrossRef]
- Evans, R.D.R.; Hemmilä, U.; Craik, A.; Mtekateka, M.; Hamilton, F.; Kawale, Z.; Kirwan, C.J.; Dobbie, H.; Dreyer, G. Incidence, Aetiology and Outcome of Community-Acquired Acute Kidney Injury in Medical Admissions in Malawi. BMC Nephrol. 2017, 18, 21. [Google Scholar] [CrossRef]
- Nejat, M.; Pickering, J.W.; Devarajan, P.; Bonventre, J.V.; Edelstein, C.L.; Walker, R.J.; Endre, Z.H. Some Biomarkers of Acute Kidney Injury Are Increased in Pre-Renal Acute Injury. Kidney Int. 2012, 81, 1254–1262. [Google Scholar] [CrossRef] [PubMed]
- de Geus, H.R.; Haase, M.; Jacob, L. The Cardiac Surgery–Associated Neutrophil Gelatinase–Associated Lipocalin Score for Postoperative Acute Kidney Injury: Does Subclinical Acute Kidney Injury Matter? J. Thorac. Cardiovasc. Surg. 2017, 154, 939–940. [Google Scholar] [CrossRef] [PubMed]
- Haase, M.; Kellum, J.A.; Ronco, C. Subclinical AKI—An Emerging Syndrome with Important Consequences. Nat. Rev. Nephrol. 2012, 8, 735–739. [Google Scholar] [CrossRef] [PubMed]
- Wynn, T. Cellular and Molecular Mechanisms of Fibrosis. J. Pathol. 2008, 214, 199–210. [Google Scholar] [CrossRef]
- Raza, S.; Jokl, E.; Pritchett, J.; Martin, K.; Su, K.; Simpson, K.; Birchall, L.; Mullan, A.F.; Athwal, V.S.; Doherty, D.T.; et al. SOX9 Is Required for Kidney Fibrosis and Activates NAV3 to Drive Renal Myofibroblast Function. Sci. Signal. 2021, 14, eabb4282. [Google Scholar] [CrossRef]
- Aggarwal, S.; Wang, Z.; Rincon Fernandez Pacheco, D.; Rinaldi, A.; Rajewski, A.; Callemeyn, J.; Van Loon, E.; Lamarthée, B.; Covarrubias, A.E.; Hou, J.; et al. SOX9 Switch Links Regeneration to Fibrosis at the Single-Cell Level in Mammalian Kidneys. Science 2024, 383, eadd6371. [Google Scholar] [CrossRef] [PubMed]
- Eddy, A.A. Overview of the Cellular and Molecular Basis of Kidney Fibrosis. Kidney Int. Suppl. 2014, 4, 2–8. [Google Scholar] [CrossRef]
- Hirschberg, R. Wound Healing in the Kidney: Complex Interactions in Renal Interstitial Fibrogenesis. J. Am. Soc. Nephrol. 2005, 16, 9. [Google Scholar] [CrossRef] [PubMed]
- Panizo, S.; Martínez-Arias, L.; Alonso-Montes, C.; Cannata, P.; Martín-Carro, B.; Fernández-Martín, J.L.; Naves-Díaz, M.; Carrillo-López, N.; Cannata-Andía, J.B. Fibrosis in Chronic Kidney Disease: Pathogenesis and Consequences. Int. J. Mol. Sci. 2021, 22, 408. [Google Scholar] [CrossRef]
- Talbott, H.E.; Mascharak, S.; Griffin, M.; Wan, D.C.; Longaker, M.T. Wound Healing, Fibroblast Heterogeneity, and Fibrosis. Cell Stem Cell 2022, 29, 1161–1180. [Google Scholar] [CrossRef]
- King, W.W. Renal Wound Healing: Histologic and Histochemical Sequences in the Repair of Intersegmental Nephrotomies. Investig. Urol. 1974, 11, 278–285. [Google Scholar]
- Dall’Oglio, M.F.; Ballarotti, L.; Passerotti, C.C.; Paluello, D.V.; Colombo, J.R.; Crippa, A.; Srougi, M. Anatrophic Nephrotomy as Nephron-Sparing Approach for Complete Removal of Intraparenchymal Renal Tumors. Int. Braz. J. Urol. 2012, 38, 356–361. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hess, E. Nephrotomy. Cal. West. Med. 1934, 41, 73–79. [Google Scholar][Green Version]
- López-Hernández, F.J.; López-Novoa, J.M. Role of TGF-β in Chronic Kidney Disease: An Integration of Tubular, Glomerular and Vascular Effects. Cell Tissue Res. 2012, 347, 141–154. [Google Scholar] [CrossRef]
- García-Sánchez, O.; López-Hernández, F.J.; López-Novoa, J.M. An Integrative View on the Role of TGF-β in the Progressive Tubular Deletion Associated with Chronic Kidney Disease. Kidney Int. 2010, 77, 950–955. [Google Scholar] [CrossRef]
- Ruiz-Ortega, M.; Rayego-Mateos, S.; Lamas, S.; Ortiz, A.; Rodrigues-Diez, R.R. Targeting the Progression of Chronic Kidney Disease. Nat. Rev. Nephrol. 2020, 16, 269–288. [Google Scholar] [CrossRef]
- Docherty, N.G.; Delles, C.; López-Hernández, F.J. Reframing Acute Kidney Injury as a Pathophysiological Continuum of Disrupted Renal Excretory Function. Acta Physiol. 2024, 240, e14181. [Google Scholar] [CrossRef]
- Sancho-Martínez, S.M.; Blanco-Gozalo, V.; Quiros, Y.; Prieto-García, L.; Montero-Gómez, M.J.; Docherty, N.G.; Martínez-Salgado, C.; Morales, A.I.; López-Novoa, J.M.; López-Hernández, F.J. Impaired Tubular Reabsorption Is the Main Mechanism Explaining Increases in Urinary NGAL Excretion Following Acute Kidney Injury in Rats. Toxicol. Sci. 2020, 175, 75–86. [Google Scholar] [CrossRef]
- Skrypnyk, N.I.; Gist, K.M.; Okamura, K.; Montford, J.R.; You, Z.; Yang, H.; Moldovan, R.; Bodoni, E.; Blaine, J.T.; Edelstein, C.L.; et al. IL-6-Mediated Hepatocyte Production Is the Primary Source of Plasma and Urine Neutrophil Gelatinase Associated Lipocalin during Acute Kidney Injury. Kidney Int. 2020, 97, 966–979. [Google Scholar] [CrossRef]
- Johnson, A.C.M.; Zager, R.A. Mechanisms Underlying Increased TIMP2 and IGFBP7 Urinary Excretion in Experimental AKI. J. Am. Soc. Nephrol. 2018, 29, 2157–2167. [Google Scholar] [CrossRef]
- Sancho-Martínez, S.M.; Herrero, M.; Fontecha-Barriuso, M.; Mercado-Hernández, J.; López-Hernández, F.J. The Urinary Level of Injury Biomarkers Is Not Univocally Reflective of the Extent of Toxic Renal Tubular Injury in Rats. Int. J. Mol. Sci. 2022, 23, 3494. [Google Scholar] [CrossRef] [PubMed]
- Sancho-Martínez, S.M.; Casanova, A.G.; Düwel, A.G.; Rivero-García, K.; García-Garrido, T.; Morales, A.I.; Martínez-Salgado, C.; López-Hernández, F.J.; Fraile, P. Identification of Pre-Renal and Intrinsic Acute Kidney Injury by Anamnestic and Biochemical Criteria: Distinct Association with Urinary Injury Biomarkers. Int. J. Mol. Sci. 2023, 24, 1826. [Google Scholar] [CrossRef] [PubMed]
- Valdivielso, J.M.; Crespo, C.; Alonso, J.R.; Martinez-Salgado, C.; Eleno, N.; Arévalo, M.; Pérez-Barriocanal, F.; López-Novoa, J.M. Renal Ischemia in the Rat Stimulates Glomerular Nitric Oxide Synthesis. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2001, 280, R771–R779. [Google Scholar] [CrossRef] [PubMed]
- López-Hernández, F.J.; Carrón, R.; Montero, M.J.; Flores, O.; López-Novoa, J.M.; Arévalo, M.A. Antihypertensive Effect of Trandolapril and Verapamil in Rats with Induced Hypertension. J. Cardiovasc. Pharmacol. 1999, 33, 748. [Google Scholar] [CrossRef]
- Pellicer-Valero, Ó.J.; Massaro, G.A.; Casanova, A.G.; Paniagua-Sancho, M.; Fuentes-Calvo, I.; Harvat, M.; Martín-Guerrero, J.D.; Martínez-Salgado, C.; López-Hernández, F.J. Neural Network-Based Calculator for Rat Glomerular Filtration Rate. Biomedicines 2022, 10, 610. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Massaro, G.A.; Mercado-Hernández, J.; Broekhuizen, R.; Nguyen, T.Q.; Fuentes-Calvo, I.; Sancho-Martínez, S.M.; Martínez-Salgado, C.; López-Hernández, F.J. Serial Combination of Toxic and Ischemic Renal Damages Causes Subsequent Chronic, Irreversible, and Progressive Renal Disease in Rats. Int. J. Mol. Sci. 2025, 26, 9336. https://doi.org/10.3390/ijms26199336
Massaro GA, Mercado-Hernández J, Broekhuizen R, Nguyen TQ, Fuentes-Calvo I, Sancho-Martínez SM, Martínez-Salgado C, López-Hernández FJ. Serial Combination of Toxic and Ischemic Renal Damages Causes Subsequent Chronic, Irreversible, and Progressive Renal Disease in Rats. International Journal of Molecular Sciences. 2025; 26(19):9336. https://doi.org/10.3390/ijms26199336
Chicago/Turabian StyleMassaro, Giampiero A., Joana Mercado-Hernández, Roel Broekhuizen, Tri Q. Nguyen, Isabel Fuentes-Calvo, Sandra M. Sancho-Martínez, Carlos Martínez-Salgado, and Francisco J. López-Hernández. 2025. "Serial Combination of Toxic and Ischemic Renal Damages Causes Subsequent Chronic, Irreversible, and Progressive Renal Disease in Rats" International Journal of Molecular Sciences 26, no. 19: 9336. https://doi.org/10.3390/ijms26199336
APA StyleMassaro, G. A., Mercado-Hernández, J., Broekhuizen, R., Nguyen, T. Q., Fuentes-Calvo, I., Sancho-Martínez, S. M., Martínez-Salgado, C., & López-Hernández, F. J. (2025). Serial Combination of Toxic and Ischemic Renal Damages Causes Subsequent Chronic, Irreversible, and Progressive Renal Disease in Rats. International Journal of Molecular Sciences, 26(19), 9336. https://doi.org/10.3390/ijms26199336





