Root Cementum Molecular Structure and Its Role in Maintaining Oral Health—Systematic Review
Abstract
1. Introduction
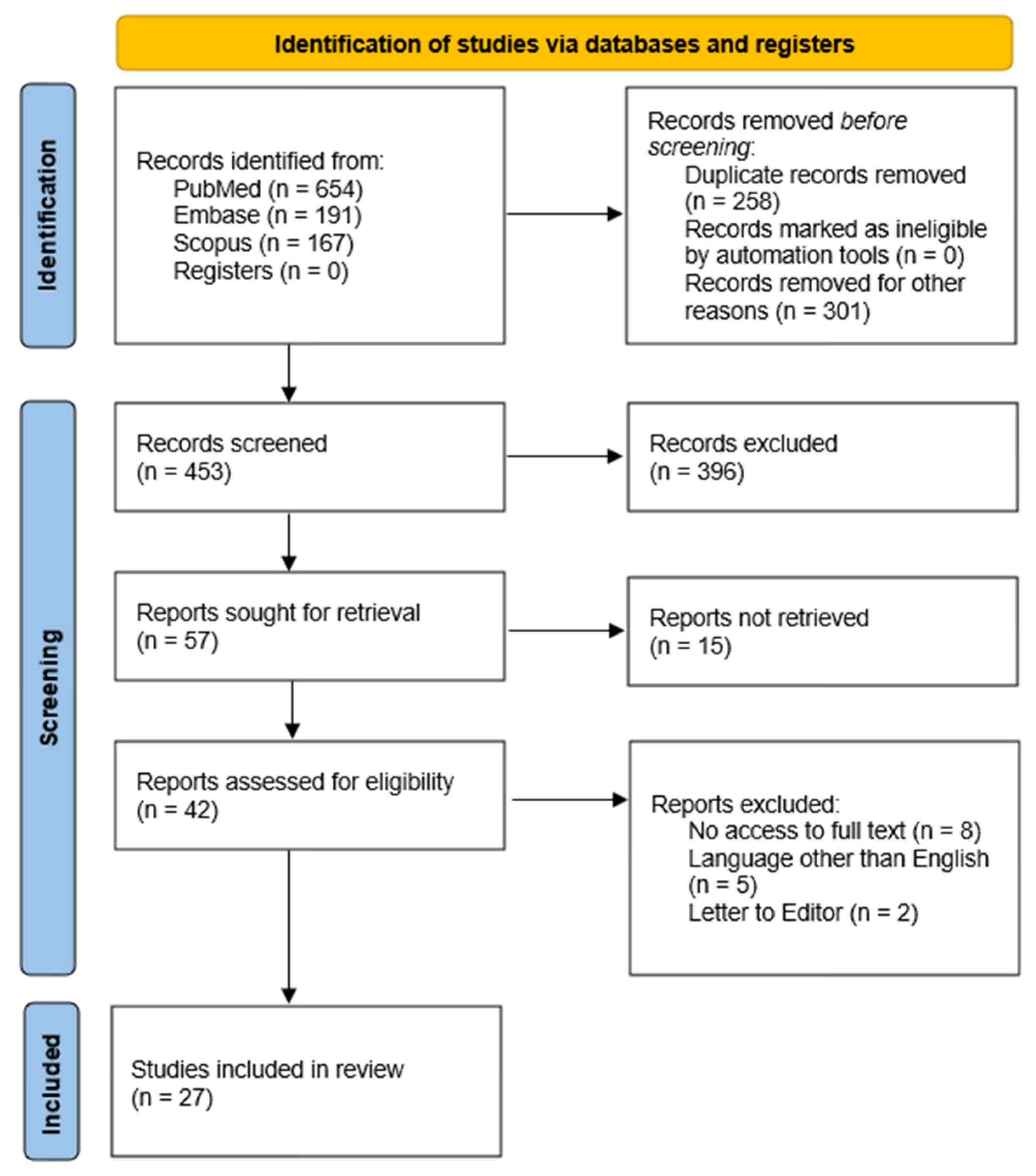
2. Materials and Methods
2.1. Data Source and Search Strategy
2.2. Focused Questions
2.3. Inclusion and Exclusion Criteria
3. Results
3.1. Periodontitis and Periodontal Regeneration
3.2. Orthodontic Tooth Movement
3.3. Root Caries
3.4. Selected Stimulants
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BSP | Bone sialoprotein |
| OPN | Osteopontin |
| PDL | Periodontal ligament |
| CAP | Cementum attachment protein |
| CEMP | Cementum protein |
| AEFC | Acellular extrinsic fiber cementum |
| CIFC | Cellular intrinsic fiber cementum |
| CMSC | Cellular mixed stratified cementum |
| AAC | Acellular afibrillar cementum |
| CEJ | Cemento-enamel junction |
| OCN | Osteocalcin |
| GTR | Guided tissue regeneration |
| RC | Root caries |
| PPD | Periodontal probing depth |
| OPG | Osteoprotegerin |
| PLGA | Polylactic-co-glycolic acid |
| nBGC | Nanobioactive glass ceramic |
| EPS | Exopolysaccharide |
| ALP | Alkaline phosphatase |
| RUNX2 | Runt-related transcription factor 2 |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| FGF | Fibroblast growth factor |
| FGFR | Fibroblast growth factor receptor |
| GLUT | Glucose transporter |
| ACP | Amorphous calcium phosphate |
| β-TCP | β-tricalcium phosphate |
| ACL | Alternative collagen lamellae |
| OTM | Orthodontic tooth movement |
| TRAP+ | Tartrate-resistant acid phosphatase-positive |
| RANKL | Receptor activator of nuclear factor-κB ligand |
| RANK | Receptor activator of nuclear factor-κB |
| SOST | Sclerostin |
| MMPs | Matrix metalloproteinases |
| VHN | Vicker’s hardness numbers |
| IL-1β | Interleukin 1β |
| TNF-α | Tumor necrosis factor α |
| PGE2 | Prostaglandin E2 |
| SIBLING | Small-Integrin-Binding Ligand, N-linked Glycoprotein |
References
- Bosshardt, D.D.; Selvig, K.A. Dental cementum: The dynamic tissue covering of the root. Periodontol. 2000 1997, 13, 41–75. [Google Scholar] [CrossRef]
- Metwally, S.; Stachewicz, U. Teeth resorption at cement–enamel junction (CEJ)—Microscopy analysis. Micron 2020, 137, 102913. [Google Scholar] [CrossRef] [PubMed]
- Christner, P.; Robinson, P.; Clark, C.C. A preliminary characterization of human cementum collagen. Calcif. Tissue Res. 1977, 23, 147–150. [Google Scholar] [CrossRef]
- Yamamoto, T.; Hasegawa, T.; Yamamoto, T.; Hongo, H.; Amizuka, N. Histology of human cementum: Its structure, function, and development. Jpn. Dent. Sci. Rev. 2016, 52, 63–74. [Google Scholar] [CrossRef]
- Nagasaki, K.; Chavez, M.B.; Nagasaki, A.; Taylor, J.M.; Tan, M.H.; Ma, M.; Ralston, E.; Thew, M.E.; Kim, D.-G.; Somerman, M.J.; et al. The Bone Sialoprotein RGD Domain Modulates and Maintains Periodontal Development. J. Dent. Res. 2022, 101, 1238–1247. [Google Scholar] [CrossRef]
- Chun, Y.H.P.; Foster, B.L.; Liang, T.; Kawasaki, K. Functions of secretory calcium-binding phosphoproteins in dental mineralization. J. Bone Miner. Res. 2025, 40, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Ababneh, K.T.; Hall, R.C.; Embery, G. Immunolocalization of glycosaminoglycans in ageing, healthy and periodontally diseased human cementum. Arch. Oral Biol. 1998, 43, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Ababneh, K.T.; Hall, R.C.; Embery, G. The proteoglycans of human cementum: Immunohistochemical localization in healthy, periodontally involved and ageing teeth. J. Periodontal Res. 1999, 34, 87–96. [Google Scholar] [CrossRef]
- Foster, B.L.; Popowics, T.E.; Fong, H.K.; Somerman, M.J. Advances in Defining Regulators of Cementum Development and Periodontal Regeneration. In Current Topics in Developmental Biology; Elsevier: Amsterdam, The Netherlands, 2007; Volume 78, pp. 47–126. [Google Scholar]
- Sarna-Boś, K.; Skic, K.; Boguta, P.; Adamczuk, A.; Vodanovic, M.; Chałas, R. Elemental mapping of human teeth enamel, dentine and cementum in view of their microstructure. Micron 2023, 172, 103485. [Google Scholar] [CrossRef]
- Schroeder, H.E. Biological Problems of Regenerative Cementogenesis: Synthesis and Attachment of Collagenous Matrices on Growing and Established Root Surfaces. In International Review of Cytology; Elsevier: Amsterdam, The Netherlands, 1992; Volume 142, pp. 1–59. [Google Scholar]
- Schroeder, H.E. Human cellular mixed stratified cementum: A tissue with alternating layers of acellular extrinsic- and cellular intrinsic fiber cementum. Schweiz. Monatsschrift Zahnmed. 1993, 103, 550–560. [Google Scholar]
- Hopewell-Smith, A. Concerning Human Cementum. J. Dent. Res. 1920, 2, 59–76. [Google Scholar] [CrossRef]
- Harrison, J.W.; Roda, R.S. Intermediate cementum. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontology 1995, 79, 624–633. [Google Scholar] [CrossRef]
- Harokopakis-Hajishengallis, E. Physiologic root resorption in primary teeth: Molecular and histological events. J. Oral Sci. 2007, 49, 1–12. [Google Scholar] [CrossRef]
- Giovani, P.A.; Martins, L.; Salmon, C.R.; Mofatto, L.S.; Leme, A.F.P.; Puppin-Rontani, R.M.; Kolli, T.N.; Foster, B.L.; Nociti, F.H., Jr.; Kantovitz, K.R. Comparative proteomic analysis of dental cementum from deciduous and permanent teeth. J. Periodontal Res. 2021, 56, 173–185. [Google Scholar] [CrossRef]
- Arzate, H.; Zeichner-David, M.; Mercado-Celis, G. Cementum proteins: Role in cementogenesis, biomineralization, periodontium formation and regeneration. Periodontol. 2000 2015, 67, 211–233. [Google Scholar] [CrossRef]
- Abdalla, H.B.; Marchioro, R.R.; Galvão, K.E.A.; Teixeira, L.N.; Kantovitz, K.R.; Millás, A.L.G.M.; Nociti, F.H., Jr. Polycaprolactone scaffolds as a biomaterial for cementoblast delivery: An in vitro study. J. Periodontal Res. 2022, 57, 1014–1023. [Google Scholar] [CrossRef] [PubMed]
- Morgenthal, A.; Zaslansky, P.; Fleck, C. Cementum thickening leads to lower whole tooth mobility and reduced root stresses: An in silico study on aging effects during mastication. J. Struct. Biol. 2021, 213, 107726. [Google Scholar] [CrossRef]
- Hassell, T.M. Tissues and cells of the periodontium. Periodontol. 2000 1993, 3, 9–38. [Google Scholar] [CrossRef] [PubMed]
- Michanowicz, A.E.; Michanowicz, J.P.; Abou-Rass, M. Cementogenic Repair of Root Fractures. J. Am. Dent. Assoc. 1971, 82, 569–579. [Google Scholar] [CrossRef] [PubMed]
- Andreasen, J.O.; Hjorting-Hansen, E. Intraalveolar root fractures: Radiographic and histologic study of 50 cases. J. Oral Surg. Am. Dent. Assoc. 1965 1967, 25, 414–426. [Google Scholar]
- Lee, A.H.C.; Neelakantan, P.; Dummer, P.M.H.; Zhang, C. Cemental tear: Literature review, proposed classification and recommendations for treatment. Int. Endod. J. 2021, 54, 2044–2073. [Google Scholar] [CrossRef]
- Pedercini, A.; Weitz, D.; Heyse, J.D., Jr.; Pedercini, C.; Kormas, I.; Koutlas, I.G.; Johnson, D.K.; McClanahan, S.B. Cemental tear: An overlooked finding associated with rapid periodontal destruction. A case series. Aust. Dent. J. 2021, 66, S82–S87. [Google Scholar] [CrossRef] [PubMed]
- Mathew, R.; Estrella, V.; Fan, J. Diagnosis and treatment of cemental tear: A case series. Gen. Dent. 2025, 73, 61–69. [Google Scholar] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Aizenbud, I.; Wilensky, A.; Almoznino, G. Periodontal Disease and Its Association with Metabolic Syndrome—A Comprehensive Review. Int. J. Mol. Sci. 2023, 24, 13011. [Google Scholar] [CrossRef]
- Caton, J.G.; Armitage, G.; Berglundh, T.; Chapple, I.L.C.; Jepsen, S.; Kornman, K.S.; Mealey, B.L.; Papapanou, P.N.; Sanz, M.; Tonetti, M.S. A new classification scheme for periodontal and peri-implant diseases and conditions—Introduction and key changes from the 1999 classification. J. Periodontol. 2018, 89, S1–S8. [Google Scholar] [CrossRef]
- Villoria, G.E.M.; Fischer, R.G.; Tinoco, E.M.B.; Meyle, J.; Loos, B.G. Periodontal disease: A systemic condition. Periodontol. 2000 2024, 96, 7–19. [Google Scholar] [CrossRef]
- Hajishengallis, G. Interconnection of periodontal disease and comorbidities: Evidence, mechanisms, and implications. Periodontol. 2000 2022, 89, 9–18. [Google Scholar] [CrossRef]
- Genco, R.J.; Sanz, M. Clinical and public health implications of periodontal and systemic diseases: An overview. Periodontol. 2000 2020, 83, 7–13. [Google Scholar] [CrossRef]
- Herrera, D.; Sanz, M.; Shapira, L.; Brotons, C.; Chapple, I.; Frese, T. Periodontal diseases and cardiovascular diseases, diabetes, and respiratory diseases: Summary of the consensus report by the European Federation of Periodontology and WONCA Europe. Eur. J. Gen. Pract. 2024, 30, 2320120. [Google Scholar] [CrossRef]
- Stöhr, J.; Barbaresko, J.; Neuenschwander, M.; Schlesinger, S. Bidirectional association between periodontal disease and diabetes mellitus: A systematic review and meta-analysis of cohort studies. Sci. Rep. 2021, 11, 13686. [Google Scholar] [CrossRef]
- Vinel, A.; Al Halabi, A.; Roumi, S.; Le Neindre, H.; Millavet, P.; Simon, M.; Cuny, C.; Barthet, J.S.; Barthet, P.; Laurencin-Dalicieux, S. Non-surgical Periodontal Treatment: SRP and Innovative Therapeutic Approaches. In Advances in Experimental Medicine and Biology; Springer International Publishing: Berlin/Heidelberg, Germany, 2022; pp. 303–327. [Google Scholar]
- Kwon, T.; Lamster, I.B.; Levin, L. Current Concepts in the Management of Periodontitis. Int. Dent. J. 2021, 71, 462–476. [Google Scholar] [CrossRef] [PubMed]
- Sälzer, S.; Graetz, C.; Dörfer, C.E.; Slot, D.E.; Van Der Weijden, F.A. Contemporary practices for mechanical oral hygiene to prevent periodontal disease. Periodontol. 2000 2020, 84, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Sundaram, S.; Ramaseshan, R.; Dash, S.; Rao, S.R. Evaluation of the nanostructure of cervical third cementum in health and chronic periodontitis: An in vitro study. J. Indian Soc. Periodontol. 2014, 18, 560–566. [Google Scholar] [CrossRef] [PubMed]
- Antunovic, F.; Tolosa, F.; Klein, C.; Ocaranza, R. Polycaprolactone-based scaffolds for guided tissue regeneration in periodontal therapy: A systematic review. J. Appl. Biomater. Funct. Mater. 2023, 21, 22808000231211416. [Google Scholar] [CrossRef]
- Yen, C.; Tu, Y.; Chen, T.; Lu, H. Comparison of treatment effects of guided tissue regeneration on infrabony lesions between animal and human studies: A systematic review and meta-analysis. J. Periodontal Res. 2014, 49, 415–424. [Google Scholar] [CrossRef]
- Kao, R.T.; Nares, S.; Reynolds, M.A. Periodontal Regeneration—Intrabony Defects: A Systematic Review From the AAP Regeneration Workshop. J. Periodontol. 2015, 86, S77–S104. [Google Scholar] [CrossRef]
- Liu, J.; Dai, Q.; Weir, M.D. Biocompatible Nanocomposite Enhanced Osteogenic and Cementogenic Differentiation of Periodontal Ligament Stem Cells In Vitro for Periodontal Regeneration. Materials 2020, 13, 4951. [Google Scholar] [CrossRef]
- Koch, F.; Meyer, N.; Valdec, S.; Jung, R.E.; Mathes, S.H. Development and application of a 3D periodontal in vitro model for the evaluation of fibrillar biomaterials. BMC Oral Health 2020, 20, 148. [Google Scholar] [CrossRef]
- El-Sayed, B.; Davies, R.P.W.; El-Zehery, R.R.; Ibrahim, F.M.; Grawish, M.E.; Kirkha, J.; El-Gendy, R. An in-vivo Intraoral Defect Model for Assessing the Use of P11-4 Self-Assembling Peptide in Periodontal Regeneration. Front. Bioeng. Biotechnol. 2020, 8, 559494. [Google Scholar] [CrossRef]
- Sowmya, S.; Mony, U.; Jayachandran, P.; Reshma, S.; Kumar, R.A.; Arzate, H.; Nair, S.V.; Jayakumar, R. Tri-Layered Nanocomposite Hydrogel Scaffold for the Concurrent Regeneration of Cementum, Periodontal Ligament, and Alveolar Bone. Adv. Healthc. Mater. 2017, 6, 1601251. [Google Scholar] [CrossRef] [PubMed]
- Kibar, H.; Arslan, Y.E.; Ceylan, A.; Karaca, B.; Haliscelik, O.; Kiran, F. Weissella cibaria EIR/P2-derived exopolysaccharide: A novel alternative to conventional biomaterials targeting periodontal regeneration. Int. J. Biol. Macromol. 2020, 165, 2900–2908. [Google Scholar] [CrossRef]
- Mutafcilar Velioğlu, E.E.; Buket Bozkurt, S.; Götz, W.; Hakki, S.S. Comparison of different graft materials on the proliferation, mineralization and mineralized tissue-related gene expressions of cementoblasts. J. Mater. Sci. Mater. Med. 2025, 36, 30. [Google Scholar] [CrossRef]
- Mancini, L.; Fratini, A.; Marchetti, E. Periodontal Regeneration. Encyclopedia 2021, 1, 87–98. [Google Scholar] [CrossRef]
- Narita, L.E.; Mester, A.; Onisor, F.; Bran, S.; Onicas, M.I.; Voina-Tonea, A. The Outcomes of Enamel Matrix Derivative on Periodontal Regeneration under Diabetic Conditions. Medicina 2021, 57, 1071. [Google Scholar] [CrossRef]
- Miron, R.J.; Shirakata, Y.; Ahmad, P.; Romandini, M.; Estrin, N.E.; Farshidfar, N.; Bosshardt, D.D.; Sculean, A. 30 years of enamel matrix derivative: Mimicking tooth development as a clinical concept. Periodontol. 2000 2025. [Google Scholar] [CrossRef] [PubMed]
- Mohammadipour, H.S.; Forouzanfar, F.; Forouzanfar, A. The Role of Type 2 Fibroblast Growth Factor in Periodontal Therapy. Curr. Drug Targets 2021, 22, 310–317. [Google Scholar] [CrossRef]
- Khehra, A.; Shiba, T.; Chen, C.Y.; Kim, D.M. Latest update on the use of recombinant growth factors for periodontal regeneration: Existing evidence and clinical applications. Ther. Adv. Chronic Dis. 2024, 15, 20406223241302707. [Google Scholar] [CrossRef]
- Atarbashi-Moghadam, F.; Atarbashi-Moghadam, S.; Sarrafan Sadeghi, T.; Taghipour, N.; Azadi, A. Histologic evidence of the effect of fibroblast growth factor 2 on periodontal regeneration: A scoping review of animal studies. J. Adv. Periodontol. Implant Dent. 2025, 17, 90–102. [Google Scholar] [CrossRef]
- Rikimaru, S.; Nakao-Kuroishi, K.; Kometani-Gunjigake, K.; Mizuhara, M.; Nakatomi, C.; Toyono, T.; Ono, K.; Kawamoto, T. Fibroblast growth factor 2 stimulates differentiation of mechanically-stressed human periodontal ligament fibroblasts into cementoblasts. Am. J. Orthod. Dentofac. Orthop. 2025, 168, 477–488. [Google Scholar] [CrossRef] [PubMed]
- Saito, M.M.; Onuma, K.; Yamakoshi, Y. Cementum is key to periodontal tissue regeneration: A review on apatite microstructures for creation of novel cementum-based dental implants. Genesis 2023, 61, e23514. [Google Scholar] [CrossRef]
- Yang, T.; Li, Y.; Hong, Y.; Chi, L.; Liu, C.; Lan, Y.; Wang, Q.; Yu, Y.; Xu, Q.; Teng, W. The Construction of Biomimetic Cementum Through a Combination of Bioskiving and Fluorine-Containing Biomineralization. Front. Bioeng. Biotechnol. 2020, 8, 341. [Google Scholar] [CrossRef]
- Park, J.C.; Um, Y.J.; Jung, U.W.; Kim, C.S.; Choi, S.H.; Kim, C.K. Histological characteristics of newly formed cementum in surgically created one-wall intrabony defects in a canine model. J. Periodontal Implant Sci. 2010, 40, 3. [Google Scholar] [CrossRef]
- Deng, R.; Xie, Y.; Chan, U.; Xu, T.; Huang, Y. Biomaterials and biotechnology for periodontal tissue regeneration: Recent advances and perspectives. J. Dent. Res. Dent. Clin. Dent. Prospect. 2022, 16, 1–10. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, S.; Zhu, B.; Xu, Q.; Ding, Y.; Jin, Y. Composite cell sheet for periodontal regeneration: Crosstalk between different types of MSCs in cell sheet facilitates complex periodontal-like tissue regeneration. Stem Cell Res. Ther. 2016, 7, 168. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Li, J.; Hu, Y.; Sun, J.; Guo, W.; Li, H.; Chen, J.; Huo, F.; Tian, W.; Li, S. Treated dentin matrix particles combined with dental follicle cell sheet stimulate periodontal regeneration. Dent. Mater. 2019, 35, 1238–1253. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Chen, J.; Zhao, L.; Deng, J.; Li, Q. A simvastatin-releasing scaffold with periodontal ligament stem cell sheets for periodontal regeneration. J. Appl. Biomater. Funct. Mater. 2020, 18, 2280800019900094. [Google Scholar] [CrossRef] [PubMed]
- Yamada, M.; Kimura, T.; Nakamura, N.; Watanabe, J.; Kartikasari, N.; He, X.; Tiskratok, W.; Yoshioka, H.; Shinno, H.; Egusa, H. Titanium Nanosurface with a Biomimetic Physical Microenvironment to Induce Endogenous Regeneration of the Periodontium. ACS Appl. Mater. Interfaces 2022, 14, 27703–27719. [Google Scholar] [CrossRef]
- Safi, I.N.; Hussein, B.M.A.; Al-Shammari, A.M. Bio-hybrid dental implants prepared using stem cells with β-TCP-coated titanium and zirconia. J. Periodontal Implant Sci. 2022, 52, 242. [Google Scholar] [CrossRef]
- Bellon, B.; Pippenger, B.; Stähli, A.; Degen, M.; Parisi, L. Cementum and enamel surface mimicry influences soft tissue cell behavior. J. Periodontal Res. 2025, 60, 64–76. [Google Scholar] [CrossRef]
- Kim, H.-Y.; Im, H.-Y.; Chang, H.-K.; Jeong, H.-D.; Park, J.-H.; Kim, H.-I.; Yi, H.-S.; Kim, Y.-S. Correlation between Collagen Type I/III Ratio and Scar Formation in Patients Undergoing Immediate Reconstruction with the Round Block Technique after Breast-Conserving Surgery. Biomedicines 2023, 11, 1089. [Google Scholar] [CrossRef] [PubMed]
- Lira Dos Santos, E.J.; De Almeida, A.B.; Chavez, M.B.; Salmon, C.R.; Mofatto, L.S.; Camara-Souza, M.B.; Tan, M.H.; Kolli, T.N.; Mohamed, F.F.; Chu, E.Y.; et al. Orthodontic tooth movement alters cementocyte ultrastructure and cellular cementum proteome signature. Bone 2021, 153, 116139. [Google Scholar] [CrossRef]
- Matsuzawa, H.; Toriya, N.; Nakao, Y.; Konno-Nagasaka, M.; Arakawa, T.; Okayama, M.; Mizoguchi, I. Cementocyte cell death occurs in rat cellular cementum during orthodontic tooth movement. Angle Orthod. 2017, 87, 416–422. [Google Scholar] [CrossRef]
- Wei, T.; Xie, Y.; Wen, X.; Zhao, N.; Shen, G. Establishment of in vitro three-dimensional cementocyte differentiation scaffolds to study orthodontic root resorption. Exp. Ther. Med. 2020, 20, 3174–3184. [Google Scholar] [CrossRef] [PubMed]
- Wei, T.; Shan, Z.; Wen, X.; Zhao, N.; Shen, G. Dynamic alternations of RANKL/OPG ratio expressed by cementocytes in response to orthodontic-induced external apical root resorption in a rat model. Mol. Med. Rep. 2022, 26, 228. [Google Scholar] [CrossRef]
- AlQranei, M.S.; Balhaddad, A.A.; Melo, M.A.S. The burden of root caries: Updated perspectives and advances on management strategies. Gerodontology 2021, 38, 136–153. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, C.D.B.; Monici Silva, I.; Dame-Teixeira, N. The action of microbial collagenases in dentinal matrix degradation in root caries and potential strategies for its management: A comprehensive state-of-the-art review. J. Appl. Oral Sci. 2024, 32, e20240013. [Google Scholar] [CrossRef]
- Takahashi, N.; Nyvad, B. Ecological Hypothesis of Dentin and Root Caries. Caries Res. 2016, 50, 422–431. [Google Scholar] [CrossRef]
- Paris, S.; Banerjee, A.; Bottenberg, P.; Breschi, L.; Campus, G.; Doméjean, S.; Ekstrand, K.; Giacaman, R.A.; Haak, R.; Hannig, M.; et al. How to Intervene in the Caries Process in Older Adults: A Joint ORCA and EFCD Expert Delphi Consensus Statement. Caries Res. 2020, 54, 459–465. [Google Scholar] [CrossRef]
- Do, T.; Damé-Teixeira, N.; Naginyte, M.; Marsh, P.D. Root Surface Biofilms and Caries. Monogr. Oral Sci. 2017, 26, 26–34. [Google Scholar]
- Tjäderhane, L.; Buzalaf, M.A.R.; Carrilho, M.; Chaussain, C. Matrix Metalloproteinases and Other Matrix Proteinases in Relation to Cariology: The Era of ‘Dentin Degradomics’. Caries Res. 2015, 49, 193–208. [Google Scholar] [CrossRef]
- Barbosa, C.D.B.; Monici Silva, I.; Cena, J.A.D.; Stefani, C.M.; Dame-Teixeira, N. Presence of host and bacterial-derived collagenolytic proteases in carious dentin: A systematic review of ex vivo studies. Front. Cell. Infect. Microbiol. 2023, 13, 1278754. [Google Scholar] [CrossRef]
- Göstemeyer, G.; Da Mata, C.; McKenna, G.; Schwendicke, F. Atraumatic vs conventional restorative treatment for root caries lesions in older patients: Meta- and trial sequential analysis. Gerodontology. 2019, 36, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Meyer-Lueckel, H.; Machiulskiene, V.; Giacaman, R.A. How to Intervene in the Root Caries Process? Systematic Review and Meta-Analyses. Caries Res. 2019, 53, 599–608. [Google Scholar] [CrossRef]
- Al Ankily, M.; Makkeyah, F.; Bakr, M.M.; Shamel, M. Evaluating the Effects of Cigarette Smoking and Heated Tobacco Products on Hard Dental Tissues: A Comparative Histological and Colorimetric Analysis. Clin. Exp. Dent. Res. 2024, 10, e941. [Google Scholar] [CrossRef] [PubMed]
- Onor, I.O.; Stirling, D.L.; Williams, S.R.; Bediako, D.; Borghol, A.; Harris, M.B.; Darensburg, T.B.; Clay, S.D.; Okpechi, S.C.; Sarpong, D.F. Clinical Effects of Cigarette Smoking: Epidemiologic Impact and Review of Pharmacotherapy Options. Int. J. Environ. Res. Public Health 2017, 14, 1147. [Google Scholar] [CrossRef]
- Zhao, C.; Xie, Y.; Zhou, X.; Zhang, Q.; Wang, N. The effect of different tobacco tar levels on DNA damage in cigarette smoking subjects. Toxicol. Res. 2020, 9, 302–307. [Google Scholar] [CrossRef]
- Karanjkar, R.R.; Preshaw, P.M.; Ellis, J.S.; Holliday, R. Effect of tobacco and nicotine in causing staining of dental hard tissues and dental materials: A systematic review and meta-analysis. Clin. Exp. Dent. Res. 2023, 9, 150–164. [Google Scholar] [CrossRef] [PubMed]
- Kurachi, T.; Chuman, S.; Suzuki, T.; Kubota, T.; Ishikawa, S. Effects of aerosols from heated tobacco products with flavors on the discoloration of bovine tooth enamel. Clin. Exp. Dent. Res. 2023, 9, 1069–1077. [Google Scholar] [CrossRef]
- Mori, Y.; Tanaka, M.; Kozai, H.; Aoyama, Y.; Shigeno, Y.; Hotta, K.; Aoike, M.; Kawamura, H.; Tsurudome, M.; Ito, M. Effects of Heat-Not-Burn Cigarette Smoking on the Secretion of Saliva and Its Innate Immune System Components. Healthcare 2022, 11, 132. [Google Scholar] [CrossRef]
- Dyasanoor, S.; Saddu, S.C. Association of Xerostomia and Assessment of Salivary Flow Using Modified Schirmer Test among Smokers and Healthy Individuals: A Preliminutesary Study. J. Clin. Diagn. Res. 2014, 8, 211–213. [Google Scholar] [CrossRef]
- Sever, E.; Božac, E.; Saltović, E.; Simonić-Kocijan, S.; Brumini, M.; Glažar, I. Impact of the Tobacco Heating System and Cigarette Smoking on the Oral Cavity: A Pilot Study. Dent. J. 2023, 11, 251. [Google Scholar] [CrossRef]
- Rajeev, G.; Lewis, A.J.; Srikant, N. A time based objective evaluation of the erosive effects of various beverages on enamel and cementum of deciduous and permanent teeth. J. Clin. Exp. Dent. 2020, 12, e1–e8. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, R.; Mesquita, C.M.; Alves, H.B.D.N.; Silva, F.G.; de Andrade Vieira, W.; de Aguiar, P.C.S.; Flores-Mir, C.; Paranhos, L.R.; de Brito-Júnior, R.B. Changes in salivary biomarkers of pain, anxiety, stress, and inflammation related to tooth movement during orthodontic treatment: A systematic review. Dent. Press J. Orthod. 2024, 29, e242436. [Google Scholar] [CrossRef]
- Flórez-Moreno, G.A.; Isaza-Guzmán, D.M.; Tobón-Arroyave, S.I. Time-related changes in salivary levels of the osteotropic factors sRANKL and OPG through orthodontic tooth movement. Am. J. Orthod. Dentofac. Orthop. 2013, 143, 92–100. [Google Scholar] [CrossRef]
- Walsh, M.C.; Choi, Y. Regulation of T cell-associated tissues and T cell activation by RANKL-RANK-OPG. J. Bone Miner. Metab. 2021, 39, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Danz, J.C.; Kantarci, A.; Bornstein, M.M.; Katsaros, C.; Stavropoulos, A. Impact of Orthodontic Forces on Plasma Levels of Markers of Bone Turnover and Inflammation in a Rat Model of Buccal Expansion. Front. Physiol. 2021, 12, 637606. [Google Scholar] [CrossRef]
- Nakashima, T.; Kobayashi, Y.; Yamasaki, S.; Kawakami, A.; Eguchi, K.; Sasaki, H.; Sakai, H. Protein Expression and Functional Difference of Membrane-Bound and Soluble Receptor Activator of NF-κB Ligand: Modulation of the Expression by Osteotropic Factors and Cytokines. Biochem. Biophys. Res. Commun. 2000, 275, 768–775. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Guo, L.; Yang, Y.; Liu, Y.; Zhang, C. Mechanical force regulates root resorption in rats through RANKL and OPG. BMC Oral Health 2022, 22, 290. [Google Scholar] [CrossRef]
- Maciel, G.B.M.; Maciel, R.M.; Danesi, C.C. Bone cells and their role in physiological remodeling. Mol. Biol. Rep. 2023, 50, 2857–2863. [Google Scholar] [CrossRef]
- Kenkre, J.; Bassett, J. The bone remodelling cycle. Ann. Clin. Biochem. 2018, 55, 308–327. [Google Scholar] [CrossRef]
- d’Apuzzo, F.; Cappabianca, S.; Ciavarella, D.; Monsurrò, A.; Silvestrini-Biavati, A.; Perillo, L. Biomarkers of Periodontal Tissue Remodeling during Orthodontic Tooth Movement in Mice and Men: Overview and Clinical Relevance. Sci. World J. 2013, 2013, 105873. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Fukasawa, S. Is Inflammation a Friend or Foe for Orthodontic Treatment?: Inflammation in Orthodontically Induced Inflammatory Root Resorption and Accelerating Tooth Movement. Int. J. Mol. Sci. 2021, 22, 2388. [Google Scholar] [CrossRef]
- Chavez, M.B.; Tan, M.H.; Kolli, T.N.; Andras, N.L.; Foster, B.L. Functional defects in cementoblasts with disrupted bone sialoprotein functional domains, in vitro. Bone 2024, 179, 116961. [Google Scholar] [CrossRef] [PubMed]
- Dab, S.; Abdelhay, N.; Figueredo, C.A.; Ganatra, S.; Gibson, M.P. Characterization of SIBLING Proteins in the Mineralized Tissues. Dent. J. 2022, 10, 144. [Google Scholar] [CrossRef] [PubMed]
- Foster, B.L.; Ao, M.; Willoughby, C.; Soenjaya, Y.; Holm, E.; Lukashova, L.; Tran, A.B.; Wimer, H.F.; Zerfas, P.M.; Nociti, F.H., Jr.; et al. Mineralization defects in cementum and craniofacial bone from loss of bone sialoprotein. Bone 2015, 78, 150–164. [Google Scholar] [CrossRef]
- Soenjaya, Y.; Foster, B.L.; Nociti, F.H., Jr.; Ao, M.; Holdsworth, D.W.; Hunter, G.K.; Somerman, M.J.; Goldberg, H.A. Mechanical Forces Exacerbate Periodontal Defects in Bsp-null Mice. J. Dent. Res. 2015, 94, 1276–1285. [Google Scholar] [CrossRef]
- Ao, M.; Chavez, M.B.; Chu, E.Y.; Hemstreet, K.C.; Yin, Y.; Yadav, M.C.; Millán, J.L.; Fisher, L.W.; Goldberg, H.A.; Somerman, M.J.; et al. Overlapping functions of bone sialoprotein and pyrophosphate regulators in directing cementogenesis. Bone 2017, 105, 134–147. [Google Scholar] [CrossRef]
- Macneil, R.L.; Berry, J.; D’errico, J.; Strayhorn, C.; Piotrowski, B.; Somerman, M.J. Role of Two Mineral-Associated Adhesion Molecules, Osteopontin and Bone Sialoprotein, during Cementogenesis. Connect. Tissue Res. 1995, 33, 1–7. [Google Scholar] [CrossRef]
- Foster, B.L.; Ao, M.; Salmon, C.R.; Chavez, M.B.; Kolli, T.N.; Tran, A.B.; Chu, E.Y.; Kantovitz, K.R.; Yadav, M.; Narisawa, S.; et al. Osteopontin regulates dentin and alveolar bone development and mineralization. Bone 2018, 107, 196–207. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H. Aging and Senescence of Dental Pulp and Hard Tissues of the Tooth. Front. Cell Dev. Biol. 2020, 8, 605996. [Google Scholar] [CrossRef]
- Ogawa, H.; McKenna, G.; Kettratad–Pruksapong, M. Prevention of Oral Functional Decline. Int. Dent. J. 2022, 72, S21–S26. [Google Scholar] [CrossRef] [PubMed]
- Da Mata, C.; McKenna, G.; Anweigi, L.; Hayes, M.; Cronin, M.; Woods, N.; O’Mahony, D.; Allen, P.F. An RCT of atraumatic restorative treatment for older adults: 5 year results. J. Dent. 2019, 83, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Hollis, J.H. Tooth loss and its association with dietary intake and diet quality in American adults. J. Dent. 2014, 42, 1428–1435. [Google Scholar] [CrossRef] [PubMed]
- Atanda, A.J.; Livinski, A.A.; London, S.D.; Boroumand, S.; Weatherspoon, D.; Iafolla, T.J.; Dye, B.A. Tooth retention, health, and quality of life in older adults: A scoping review. BMC Oral Health 2022, 22, 185. [Google Scholar] [CrossRef]
- Minakuchi, S. Philosophy of Oral Hypofunction. Gerodontology 2022, 39, 1–2. [Google Scholar] [CrossRef]
| Author (Year) | Examined Factor | Results | Molecular Mechanism |
|---|---|---|---|
| Sundaram et al. (2014) [37] | Nanomechanical properties of the cervical third of the cementum in health and chronic periodontitis. |
|
|
| Liu et al. (2020) [41] | The effect of a biocompatible nanocomposite with nano-sized calcium fluoride particles on osteogenic and cementogenic induction of human PDL stem cells. |
|
|
| Koch et al. (2020) [42] | Effects of the use of self-assembling peptide P11–4 as a matrix for PDL regeneration. | P11–4 served as an efficient supporter of fibroblast activity and matrix formation in regenerative processes in PDL. |
|
| El-Sayed et al. (2020) [43] | The effects of a self- assembling peptide P11–4 on periodontal regeneration. | Enhanced regeneration of periodontal tissues when P11–4 was used to fill periodontal defects. |
|
| Sowmya et al. (2017) [44] | Regeneration of tooth supporting structures after application of tissue-specific tri-layered nanocomposite hydrogel scaffold. | The tri-layered nanocomposite hydrogel scaffold with growth factors is successful in stimulating matrix formation, mineralization in periodontal regeneration. |
|
| Kibar et al. (2020) [45] | The potential of Weissella cibaria EIR/P2 EPS for periodontal regeneration. |
|
|
| Mutafcilar et al. (2025) [46] | The effect of different graft materials, including Emdogain®, on cementoblasts’ proliferation, mineralization, and mineralized tissue-related gene expressions. |
|
|
| Rikimaru et al. (2025) [53] | The effects of fibroblast growth factor 2 (FGF2) and mechanical stress on PDL fibroblasts differentiation, focusing on cementoblast differentiation. |
|
|
| Author (Year) | Examined Factor | Results | Molecular Mechanism |
|---|---|---|---|
| Yang et al. (2019) [59] | A combination of alternative collagen lamellae (ACL) and amorphous calcium phosphate (ACP) solution to create biomimetic cementum. | Significant promotion of the adhesion, proliferation, and cementogenic differentiation of PDL cells. |
|
| Park et al. (2010) [56] | Comparison of pristine cementum and repaired cementum after surgical procedures on intrabony defect with 8 and 24-week healing period. |
|
|
| Yamada et al. (2022) [61] | Smart titanium nanosurface mimicking the surface nanotopography and micromechanical properties of the tooth root cementum in periodontal regeneration. |
|
|
| Safi et al. (2022) [62] | PDL restoration in osseointegrated implants coated with β-TCP using stem cells. | β-TCP-coated (titanium and zirconia) implants generated periodontal tissue and formed biohybrid implants. |
|
| Bellon et al. (2025) [63] | Testing whether titanium surface roughness disparity might be used to specifically guide the behavior of gingiva fibroblasts and keratinocytes, thereby improvingthe quality of soft tissue integration around abutments. |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Janik, K.; Skucha-Nowak, M. Root Cementum Molecular Structure and Its Role in Maintaining Oral Health—Systematic Review. Int. J. Mol. Sci. 2025, 26, 11178. https://doi.org/10.3390/ijms262211178
Janik K, Skucha-Nowak M. Root Cementum Molecular Structure and Its Role in Maintaining Oral Health—Systematic Review. International Journal of Molecular Sciences. 2025; 26(22):11178. https://doi.org/10.3390/ijms262211178
Chicago/Turabian StyleJanik, Katarzyna, and Małgorzata Skucha-Nowak. 2025. "Root Cementum Molecular Structure and Its Role in Maintaining Oral Health—Systematic Review" International Journal of Molecular Sciences 26, no. 22: 11178. https://doi.org/10.3390/ijms262211178
APA StyleJanik, K., & Skucha-Nowak, M. (2025). Root Cementum Molecular Structure and Its Role in Maintaining Oral Health—Systematic Review. International Journal of Molecular Sciences, 26(22), 11178. https://doi.org/10.3390/ijms262211178







