Perinatal Ethanol Exposure Induces Astrogliosis and Decreases GRP55/PEA-Mediated Neuroprotection in Hippocampal Astrocytes of the 3×Tg Alzheimer’s Animal Model
Abstract
1. Introduction
2. Results
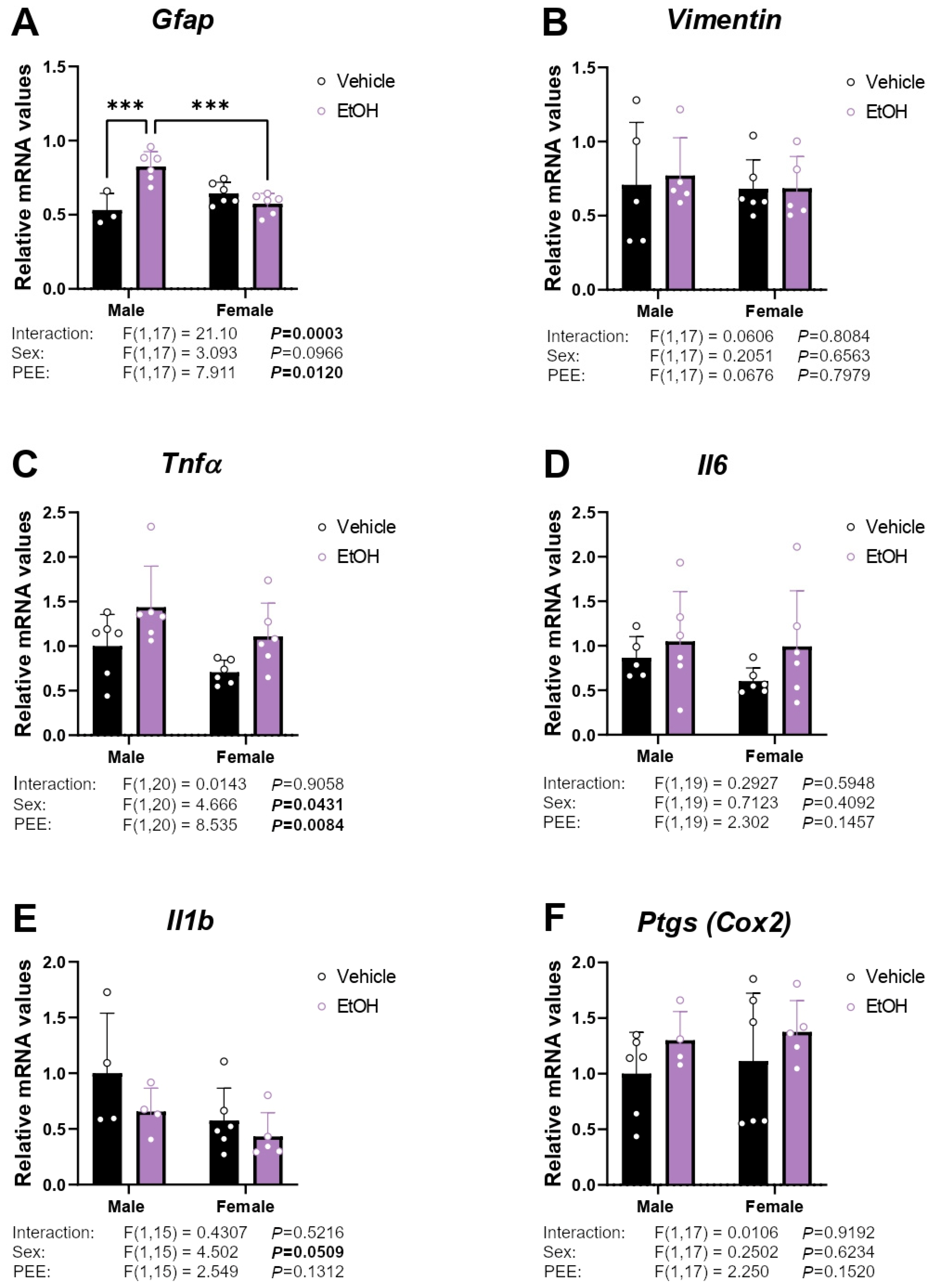
2.1. mRNA Expression of Inflammatory/Astrogliosis Factors
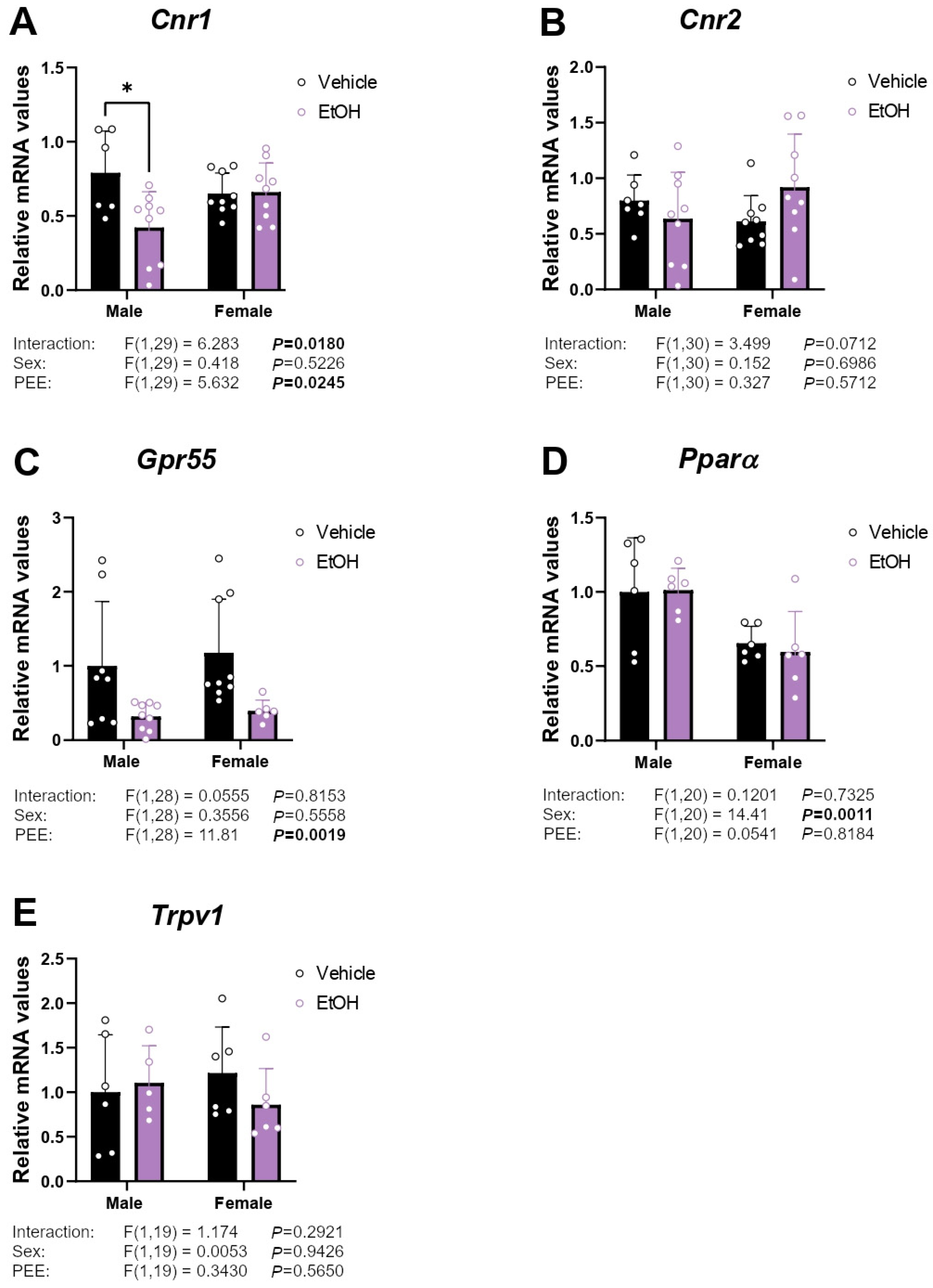
2.2. mRNA Expression of Cannabinoid-Related Receptors
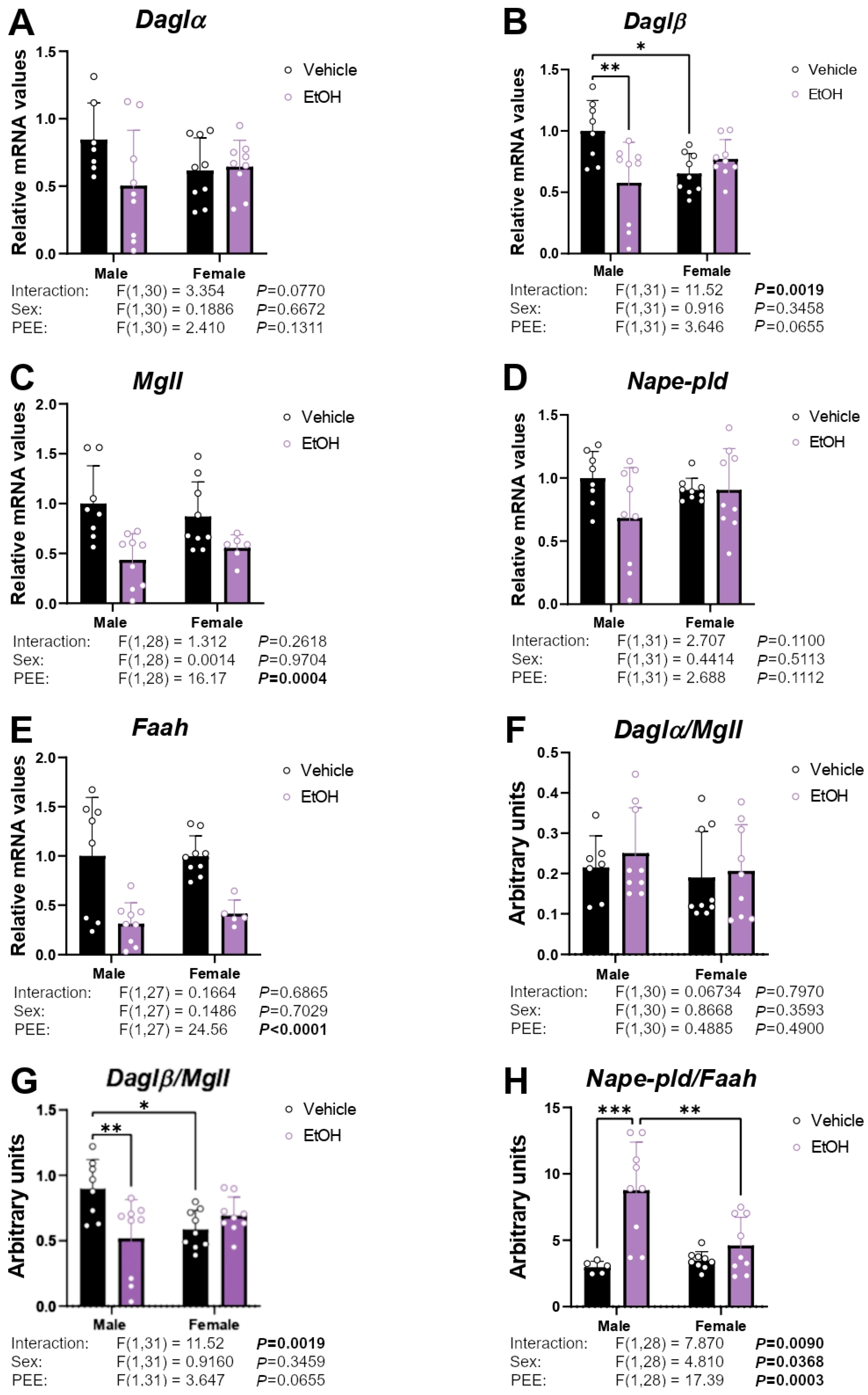
2.3. mRNA Expression of Enzymes Responsible for Endocannabinoid Synthesis and Degradation
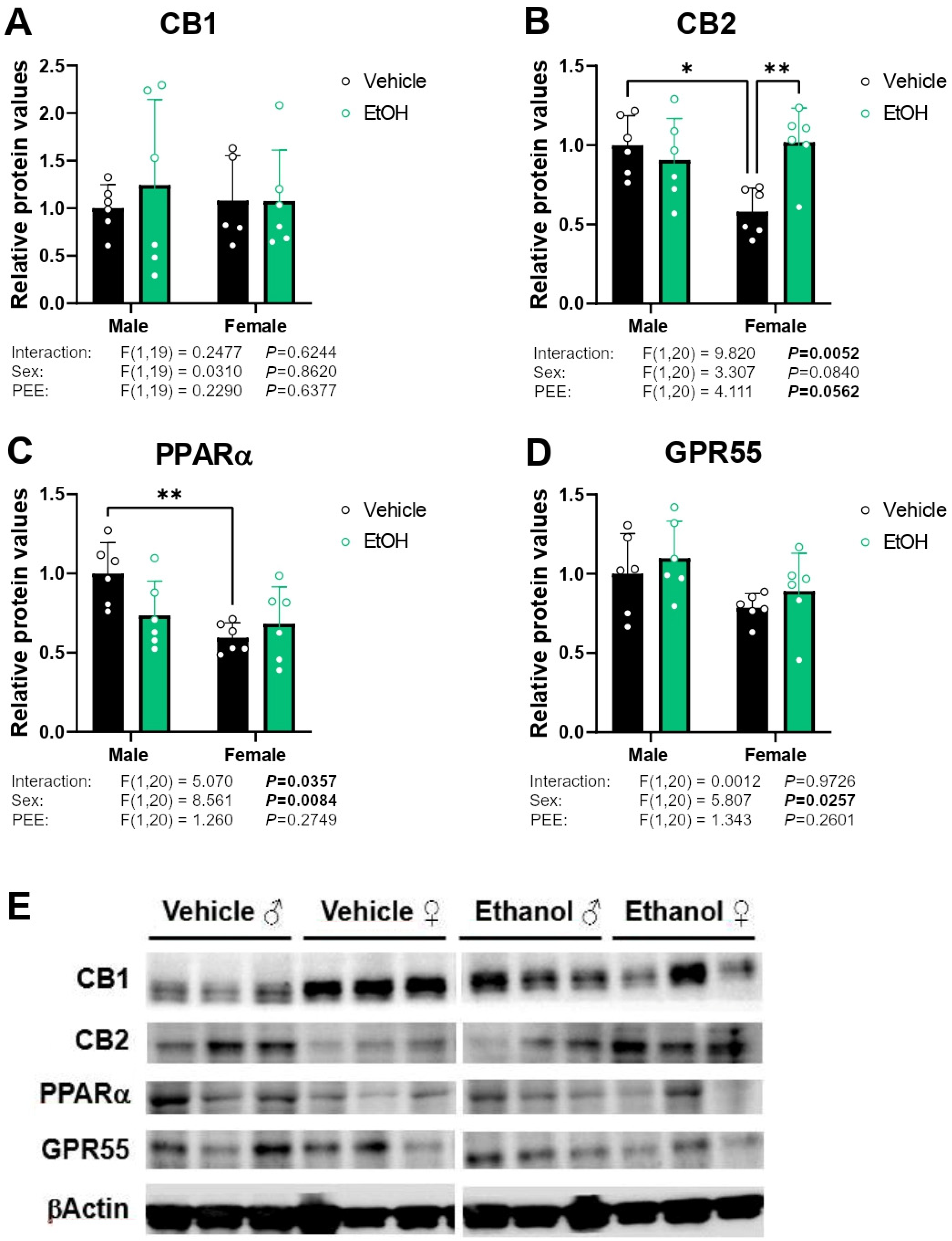
2.4. Protein Expression of Cannabinoid-Related Receptors
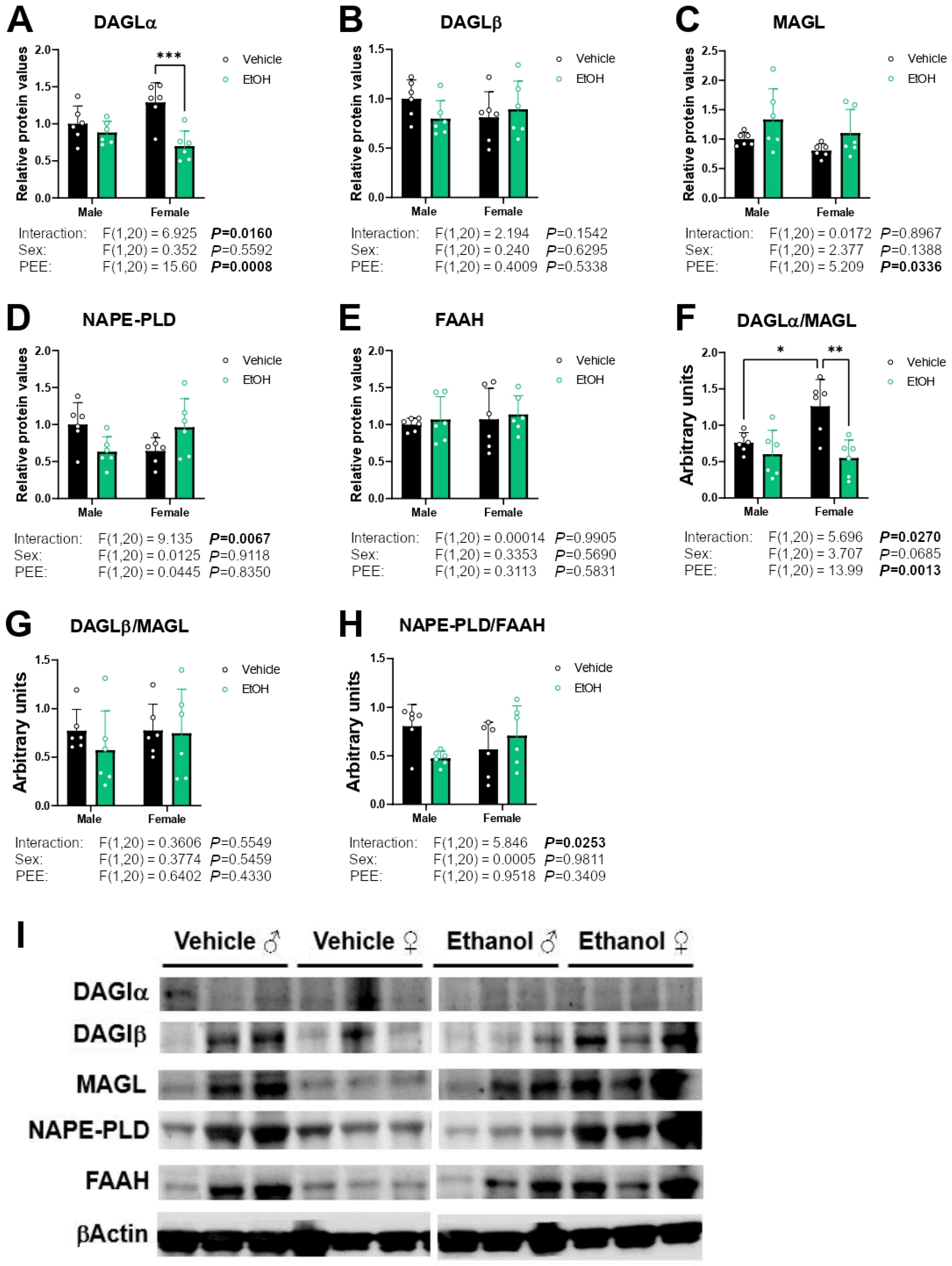
2.5. Protein Expression of Enzymes Responsible for Endocannabinoid Synthesis and Degradation
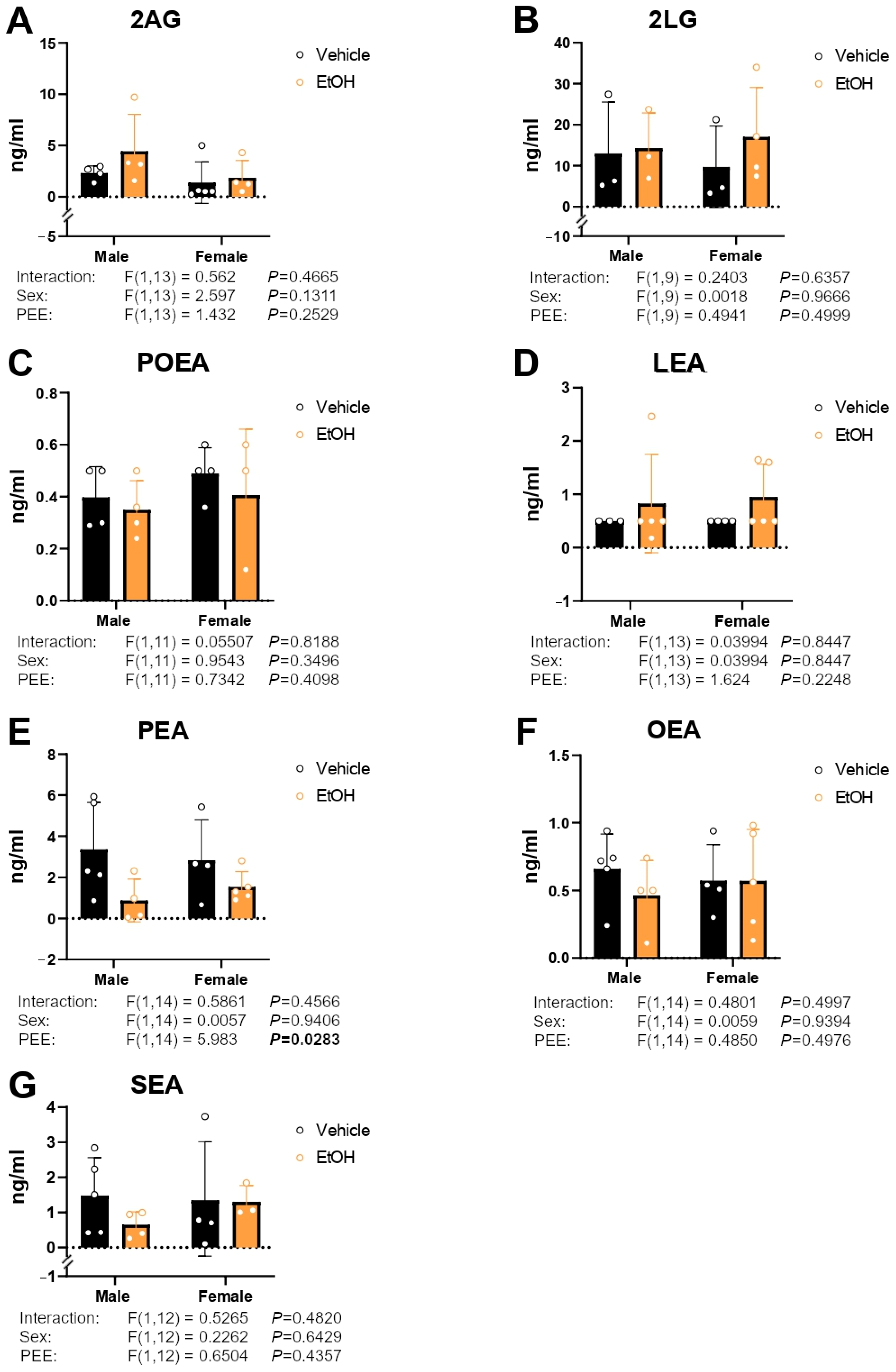
2.6. Culture Medium Endocannabinoid and Paracannabinoid Levels
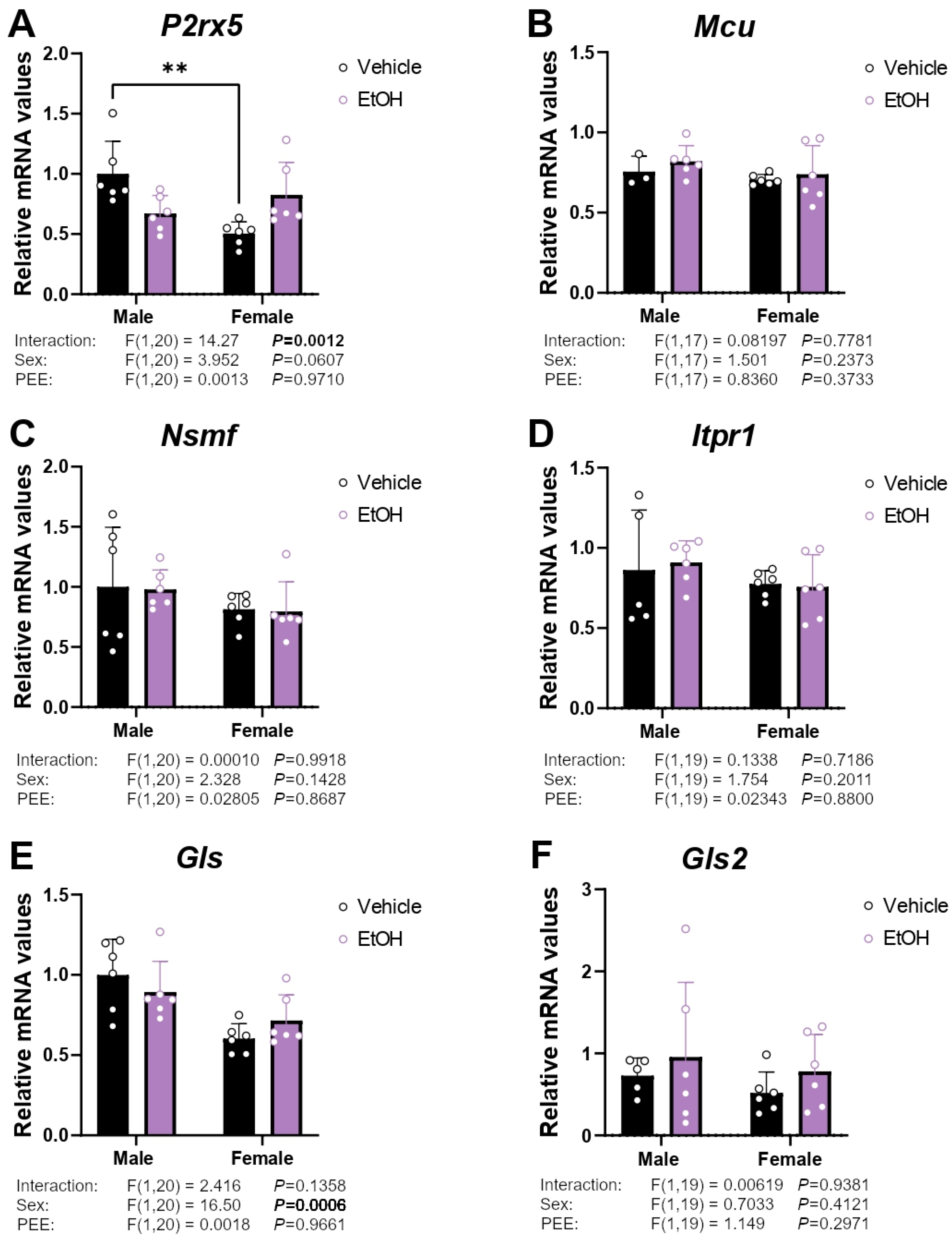
2.7. mRNA Expression of Ca2+ Signaling-Related Genes
3. Discussion
4. Materials and Methods
4.1. Ethics Statement
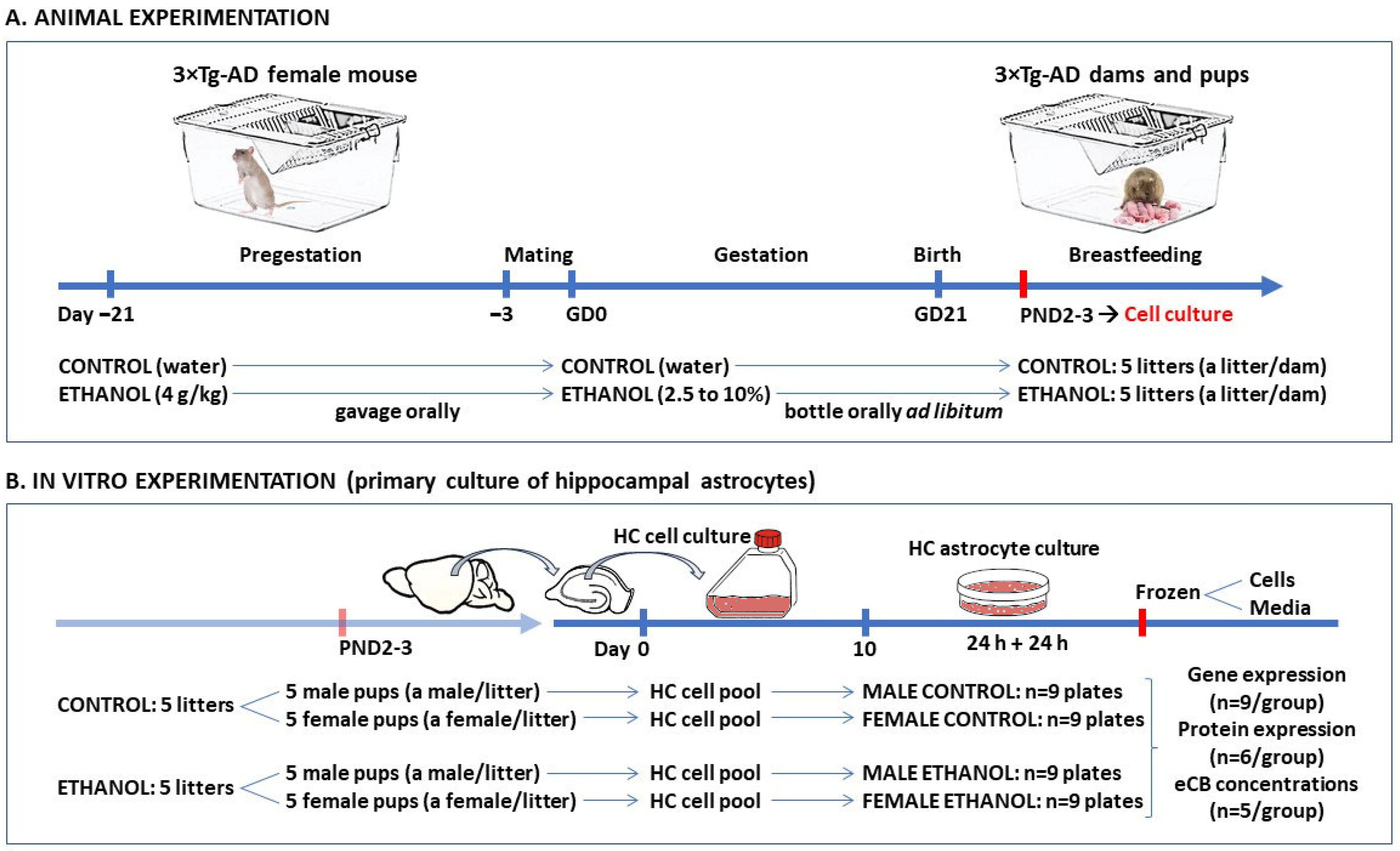
4.2. Animal Model
4.3. Experimental Procedures on Animals
4.4. Primary Cultures of Hippocampal Astrocytes
4.5. RNA Isolation and Real-Time Quantitative PCR Analysis
4.6. Western Blot Analysis
4.7. Endocannabinoid and Paracannabinoid Quantification
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bhatia, S.; Yan, Y.; Ly, M.; Wells, P.G. Sex-and OGG1-Dependent Reversal of in Utero Ethanol-Initiated Changes in Postnatal Behaviour by Neonatal Treatment with the Histone Deacetylase Inhibitor Trichostatin A (TSA) in Oxoguanine Glycosylase 1 (Ogg1) Knockout Mice. Toxicol. Lett. 2021, 356, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.; Bodenstein, D.; Cheng, A.P.; Wells, P.G. Altered Epigenetic Marks and Gene Expression in Fetal Brain, and Postnatal Behavioural Disorders, Following Prenatal Exposure of Ogg1 Knockout Mice to Saline or Ethanol. Cells 2023, 12, 2308. [Google Scholar] [CrossRef] [PubMed]
- Montagud-Romero, S.; Cantacorps, L.; Fernández-Gómez, F.J.; Núñez, C.; Miñarro, J.; Rodríguez-Arias, M.; Milanés, M.V.; Valverde, O. Unraveling the Molecular Mechanisms Involved in Alcohol Intake and Withdrawal in Adolescent Mice Exposed to Alcohol during Early Life Stages. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2021, 104, 110025. [Google Scholar] [CrossRef] [PubMed]
- Basavarajappa, B.S. Epigenetics in Fetal Alcohol Spectrum Disorder. Prog. Mol. Biol. Transl. Sci. 2023, 197, 211–239. [Google Scholar]
- Li, Y.; Zhang, L.N.; Chong, L.; Liu, Y.; Xi, F.Y.; Zhang, H.; Duan, X.L. Prenatal Ethanol Exposure Impairs the Formation of Radial Glial Fibers and Promotes the Transformation of GFAPδ-Positive Radial Glial Cells into Astrocytes. Mol. Med. Rep. 2021, 23, 274. [Google Scholar] [CrossRef]
- Saito, M.; Chakraborty, G.; Hui, M.; Masiello, K.; Saito, M. Ethanol-Induced Neurodegeneration and Glial Activation in the Developing Brain. Brain Sci. 2016, 6, 31. [Google Scholar] [CrossRef]
- Siqueira, M.; Araujo, A.P.B.; Gomes, F.C.A.; Stipursky, J. Ethanol Gestational Exposure Impairs Vascular Development and Endothelial Potential to Control BBB-Associated Astrocyte Function in the Developing Cerebral Cortex. Mol. Neurobiol. 2021, 58, 1755–1768. [Google Scholar] [CrossRef]
- Eraso-Pichot, A.; Pouvreau, S.; Olivera-Pinto, A.; Gomez-Sotres, P.; Skupio, U.; Marsicano, G. Endocannabinoid Signaling in Astrocytes. Glia 2023, 71, 44–59. [Google Scholar] [CrossRef]
- Shu-Jung Hu, S.; Mackie, K. Distribution of the Endocannabinoid System in the Central Nervous System. Handb. Exp. Pharmacol. 2015, 231, 59–93. [Google Scholar]
- Cristino, L.; Bisogno, T.; Di Marzo, V. Cannabinoids and the Expanded Endocannabinoid System in Neurological Disorders. Nat. Rev. Neurol. 2020, 16, 9–29. [Google Scholar] [CrossRef]
- Simankowicz, P.; Stępniewska, J. The Role of Endocannabinoids in Physiological Processes and Disease Pathology: A Comprehensive Review. J. Clin. Med. 2025, 14, 2851. [Google Scholar] [CrossRef] [PubMed]
- Bonilla-Del Rίo, I.; Puente, N.; Peñasco, S.; Rico, I.; Gutiérrez-Rodrίguez, A.; Elezgarai, I.; Ramos, A.; Reguero, L.; Gerrikagoitia, I.; Christie, B.R.; et al. Adolescent Ethanol Intake Alters Cannabinoid Type-1 Receptor Localization in Astrocytes of the Adult Mouse Hippocampus. Addict. Biol. 2019, 24, 182–192. [Google Scholar] [CrossRef] [PubMed]
- Aguirre-Rueda, D.; Guerra-Ojeda, S.; Aldasoro, M.; Iradi, A.; Obrador, E.; Mauricio, M.D.; Vila, J.M.; Marchio, P.; Valles, S.L. WIN 55,212-2, Agonist of Cannabinoid Receptors, Prevents Amyloid β1-42 Effects on Astrocytes in Primary Culture. PLoS ONE 2015, 10, e0122843. [Google Scholar] [CrossRef] [PubMed]
- Berry, A.J.; Zubko, O.; Reeves, S.J.; Howard, R.J. Endocannabinoid System Alterations in Alzheimer’s Disease: A Systematic Review of Human Studies. Brain Res. 2020, 1749, 147135. [Google Scholar] [CrossRef]
- Ajenikoko, M.K.; Ajagbe, A.O.; Onigbinde, O.A.; Okesina, A.A.; Tijani, A.A. Review of Alzheimer’s Disease Drugs and Their Relationship with Neuron-Glia Interaction. IBRO Neurosci. Rep. 2023, 14, 64–76. [Google Scholar] [CrossRef]
- Pacheco-Sánchez, B.; Tovar, R.; Ben Rabaa, M.; Sánchez-Salido, L.; Vargas, A.; Suárez, J.; Rodríguez de Fonseca, F.; Rivera, P. Sex-Dependent Altered Expression of Cannabinoid Signaling in Hippocampal Astrocytes of the Triple Transgenic Mouse Model of Alzheimer’s Disease: Implications for Controlling Astroglial Activity. Int. J. Mol. Sci. 2023, 24, 12598. [Google Scholar] [CrossRef]
- Loeffler, D.A. Modifiable, Non-Modifiable, and Clinical Factors Associated with Progression of Alzheimer’s Disease. J. Alzheimer’s Dis. 2021, 80, 1–27. [Google Scholar] [CrossRef]
- Chandrashekar, D.V.; Steinberg, R.A.; Han, D.; Sumbria, R.K. Alcohol as a Modifiable Risk Factor for Alzheimer’s Disease—Evidence from Experimental Studies. Int. J. Mol. Sci. 2023, 24, 9492. [Google Scholar] [CrossRef]
- Heymann, D.; Stern, Y.; Cosentino, S.; Tatarina-Nulman, O.; Dorrejo, J.N.; Gu, Y. The Association Between Alcohol Use and the Progression of Alzheimer’s Disease. Curr. Alzheimer Res. 2016, 13, 1356–1362. [Google Scholar] [CrossRef]
- Aso, E.; Ferrer, I. CB2 Cannabinoid Receptor As Potential Target against Alzheimer’s Disease. Front. Neurosci. 2016, 10, 243. [Google Scholar] [CrossRef]
- Gerlikhman, L.; Das, U.; Sarkar, D.K. Prenatal and Adolescent Alcohol Exposure, Neuroinflammation, and Alzheimer’s Disease: A Network Meta Analysis Approach. NeuroImmune Pharmacol. Ther. 2023, 2, 353–363. [Google Scholar] [CrossRef]
- Collier, J.M.; Metwally, S.; Mcfarland, M.; Krishna, S.; Fiesler, V.; Stauffer, M.; Begum, G.; Kofler, J.; Sun, D. Upregulation of Na/H Exchanger in Astrogliosis and Early Alzheimer’s Disease Pathogenesis. Aging Dis. 2025, 16, 3546. [Google Scholar]
- Metna-Laurent, M.; Marsicano, G. Rising Stars: Modulation of Brain Functions by Astroglial Type-1 Cannabinoid Receptors. Glia 2015, 63, 353–364. [Google Scholar] [CrossRef]
- Oliveira da Cruz, J.F.; Robin, L.M.; Drago, F.; Marsicano, G.; Metna-Laurent, M. Astroglial Type-1 Cannabinoid Receptor (CB1): A New Player in the Tripartite Synapse. Neuroscience 2016, 323, 35–42. [Google Scholar] [CrossRef]
- Lovinger, D.M.; Alvarez, V.A. Alcohol and Basal Ganglia Circuitry: Animal Models. Neuropharmacology 2017, 122, 46–55. [Google Scholar] [CrossRef]
- Liu, Y.; Xing, H.; Zhang, Y.; Song, Y. The Endocannabinoid System in Alzheimer’s Disease: A Network Meta-Analysis. J. Neurosci. Res. 2024, 102, e25380. [Google Scholar] [CrossRef]
- Solas, M.; Francis, P.T.; Franco, R.; Ramirez, M.J. CB2 Receptor and Amyloid Pathology in Frontal Cortex of Alzheimer’s Disease Patients. Neurobiol. Aging 2013, 34, 805–808. [Google Scholar] [CrossRef] [PubMed]
- Medina-Vera, D.; Rosell-Valle, C.; López-Gambero, A.J.; Navarro, J.A.; Zambrana-Infantes, E.N.; Rivera, P.; Santín, L.J.; Suarez, J.; de Fonseca, F.R. Imbalance of Endocannabinoid/Lysophosphatidylinositol Receptors Marks the Severity of Alzheimer’s Disease in a Preclinical Model: A Therapeutic Opportunity. Biology 2020, 9, 377. [Google Scholar] [CrossRef] [PubMed]
- Medina-Vera, D.; Zhao, H.; Bereczki, E.; Rosell-Valle, C.; Shimozawa, M.; Chen, G.; de Fonseca, F.R.; Nilsson, P.; Tambaro, S. The Expression of the Endocannabinoid Receptors CB2 and GPR55 Is Highly Increased during the Progression of Alzheimer’s Disease in AppNL-G-F Knock-In Mice. Biology 2023, 12, 805. [Google Scholar] [CrossRef] [PubMed]
- Xiang, X.T.; Wang, X.; Wu, Y.M.; Hu, J.; Li, Y.Y.; Jin, S.Y.; Wu, X. Activation of GPR55 Attenuates Cognitive Impairment, Oxidative Stress, Neuroinflammation, and Synaptic Dysfunction in a Streptozotocin-Induced Alzheimer’s Mouse Model. Pharmacol. Biochem. Behav. 2022, 214, 173340. [Google Scholar] [CrossRef]
- Xiang, X.T.; Wang, X.; Jin, S.Y.; Hu, J.; Wu, Y.M.; Li, Y.Y.; Wu, X. Activation of GPR55 Attenuates Cognitive Impairment and Neurotoxicity in a Mouse Model of Alzheimer’s Disease Induced by Aβ1–42 through Inhibiting RhoA/ROCK2 Pathway. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2022, 112, 110423. [Google Scholar] [CrossRef] [PubMed]
- Colizzi, M.; Bortoletto, R.; Costa, R.; Zoccante, L. Palmitoylethanolamide and Its Biobehavioral Correlates in Autism Spectrum Disorder: A Systematic Review of Human and Animal Evidence. Nutrients 2021, 13, 1346. [Google Scholar] [CrossRef] [PubMed]
- Blanco, E.; Pavón, F.J.; Palomino, A.; Luque-Rojas, M.J.; Serrano, A.; Rivera, P.; Bilbao, A.; Alen, F.; Vida, M.; Suárez, J.; et al. Cocaine-Induced Behavioral Sensitization Is Associated with Changes in the Expression of Endocannabinoid and Glutamatergic Signaling Systems in the Mouse Prefrontal Cortex. Int. J. Neuropsychopharmacol. 2015, 18, pyu024. [Google Scholar] [CrossRef] [PubMed]
- Bertolino, B.; Crupi, R.; Impellizzeri, D.; Bruschetta, G.; Cordaro, M.; Siracusa, R.; Esposito, E.; Cuzzocrea, S. Beneficial Effects of Co-Ultramicronized Palmitoylethanolamide/Luteolin in a Mouse Model of Autism and in a Case Report of Autism. CNS Neurosci. Ther. 2017, 23, 87–98. [Google Scholar] [CrossRef]
- Herrera, M.I.; Udovin, L.D.; Toro-Urrego, N.; Kusnier, C.F.; Luaces, J.P.; Capani, F. Palmitoylethanolamide Ameliorates Hippocampal Damage and Behavioral Dysfunction after Perinatal Asphyxia in the Immature Rat Brain. Front. Neurosci. 2018, 12, 145. [Google Scholar] [CrossRef]
- Petrosino, S.; Di Marzo, V. The Pharmacology of Palmitoylethanolamide and First Data on the Therapeutic Efficacy of Some of Its New Formulations. Br. J. Pharmacol. 2017, 174, 1349–1365. [Google Scholar] [CrossRef]
- Rankin, L.; Fowler, C.J. The Basal Pharmacology of Palmitoylethanolamide. Int. J. Mol. Sci. 2020, 21, 7942. [Google Scholar] [CrossRef]
- Beggiato, S.; Cassano, T.; Ferraro, L.; Tomasini, M.C. Astrocytic Palmitoylethanolamide Pre-Exposure Exerts Neuroprotective Effects in Astrocyte-Neuron Co-Cultures from a Triple Transgenic Mouse Model of Alzheimer’s Disease. Life Sci. 2020, 257, 118037. [Google Scholar] [CrossRef]
- Colizzi, M.; Bortoletto, R.; Colli, C.; Bonomo, E.; Pagliaro, D.; Maso, E.; Di Gennaro, G.; Balestrieri, M. Therapeutic Effect of Palmitoylethanolamide in Cognitive Decline: A Systematic Review and Preliminary Meta-Analysis of Preclinical and Clinical Evidence. Front. Psychiatry 2022, 13, 1038122. [Google Scholar] [CrossRef]
- Scuderi, C.; Valenza, M.; Stecca, C.; Esposito, G.; Carratù, M.R.; Steardo, L. Palmitoylethanolamide Exerts Neuroprotective Effects in Mixed Neuroglial Cultures and Organotypic Hippocampal Slices via Peroxisome Proliferator-Activated Receptor-α. J. Neuroinflammation 2012, 9, 49. [Google Scholar] [CrossRef]
- Bronzuoli, M.R.; Facchinetti, R.; Steardo, L.; Romano, A.; Stecca, C.; Passarella, S.; Cassano, T.; Scuderi, C. Palmitoylethanolamide Dampens Reactive Astrogliosis and Improves Neuronal Trophic Support in a Triple Transgenic Model of Alzheimer’s Disease: In Vitro and in Vivo Evidence. Oxid. Med. Cell. Longev. 2018, 2018, 4720532. [Google Scholar] [CrossRef]
- Di Stefano, V.; Steardo, L.; D’Angelo, M.; Monaco, F.; Steardo, L. Palmitoylethanolamide: A Multifunctional Molecule for Neuroprotection, Chronic Pain, and Immune Modulation. Biomedicines 2025, 13, 1271. [Google Scholar] [CrossRef]
- Gorzkiewicz, A.; Szemraj, J. Brain Endocannabinoid Signaling Exhibits Remarkable Complexity. Brain Res. Bull. 2018, 142, 33–46. [Google Scholar] [CrossRef]
- Talarico, G.; Trebbastoni, A.; Bruno, G.; de Lena, C. Modulation of the Cannabinoid System: A New Perspective for the Treatment of the Alzheimer’s Disease. Curr. Neuropharmacol. 2018, 17, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Flannigan, K.; Poole, N.; Cook, J.; Unsworth, K. Sex-Related Differences among Individuals Assessed for Fetal Alcohol Spectrum Disorder in Canada. Alcohol Clin. Exp. Res. 2023, 47, 613–623. [Google Scholar] [CrossRef] [PubMed]
- Fuente-Martiń, E.; García-Cáceres, C.; Granado, M.; De Ceballos, M.L.; Sánchez-Garrido, M.Á.; Sarman, B.; Liu, Z.W.; Dietrich, M.O.; Tena-Sempere, M.; Argente-Arizón, P.; et al. Leptin Regulates Glutamate and Glucose Transporters in Hypothalamic Astrocytes. J. Clin. Investig. 2012, 122, 3900–3913. [Google Scholar] [CrossRef] [PubMed]
- Tovar, R.; de Ceglia, M.; Ubaldi, M.; Rodríguez-Pozo, M.; Soverchia, L.; Cifani, C.; Rojo, G.; Gavito, A.; Hernandez-Folgado, L.; Jagerovic, N.; et al. Administration of Linoleoylethanolamide Reduced Weight Gain, Dyslipidemia, and Inflammation Associated with High-Fat-Diet-Induced Obesity. Nutrients 2023, 15, 4448. [Google Scholar] [CrossRef]
- de Ceglia, M.; Micioni Di Bonaventura, M.V.; Romano, A.; Friuli, M.; Micioni Di Bonaventura, E.; Gavito, A.L.; Botticelli, L.; Gaetani, S.; de Fonseca, F.R.; Cifani, C. Anxiety Associated with Palatable Food Withdrawal Is Reversed by the Selective FAAH Inhibitor PF-3845: A Regional Analysis of the Contribution of Endocannabinoid Signaling Machinery. Int. J. Eat. Disord. 2023, 56, 1098–1113. [Google Scholar] [CrossRef]
- Pastor, A.; Farré, M.; Fitó, M.; Fernandez-Aranda, F.; De La Torre, R. Analysis of ECs and Related Compounds in Plasma: Artifactual Isomerization and Ex Vivo Enzymatic Generation of 2-MGs. J. Lipid Res. 2014, 55, 966–977. [Google Scholar] [CrossRef]
| Gene Symbol | Assay ID | GenBank Accession Number | Amplicon Length (bp) |
|---|---|---|---|
| Actb | Mm02619580 | NM_007393.5 | 143 |
| Gfap | Mm01253033 | NM_001131020.1 | 75 |
| Vim (Vimentin) | Mm01333430 | NM_011701.4 | 62 |
| Tnfa | Mm00443258 | NM_001278601.1 | 81 |
| Il1b | Mm00434228 | NM_008361.3 | 90 |
| Gls | Mm01257297 | NM_001081081.2 | 114 |
| Gls2 | Mm01164862 | NM_001033264.3 | 118 |
| Cnr1 (CB1) | Mm01212171 | NM_007726.3 | 66 |
| Cnr2 (CB2) | Mm02620087 | NM_009924.4 | 171 |
| Gpr55 | Mm02621622 | NM_001033290.2 | 102 |
| Ppara | Mm00440939 | NM_001113418.1 | 74 |
| Trpv1 | Mm01246300 | NM_001001445.2 | 56 |
| Dagla | Mm00813830 | NM_198114.2 | 69 |
| Daglb | Mm00523381 | NM_144915.3 | 72 |
| Nape-pld | Mm00724596 | NM_178728.5 | 85 |
| Mgll | Mm00449274 | NM_001166249.1 | 78 |
| Faah | Mm00515684 | NM_010173.4 | 62 |
| P2rx5 | Mm00473677 | NM_033321.3 | 104 |
| Mcu | Mm01168773 | NM_001033259.4 | 71 |
| Nsmf | Mm00480341 | NM_001039386.1 | 70 |
| Itpr1 | Mm00439907 | NM_010585.5 | 58 |
| Il6 | Mm00446190 | NM_031168.1 | 78 |
| Ptgs2 | Mm00478374 | NM_011198.3 | 80 |
| Antigen | Immunogen | Manufacturing | Dilution |
|---|---|---|---|
| β-actin | A purified Arabidopsis actin protein. | Abcam (Cambridge, UK). (#ab230169) Mouse monoclonal antibody | 1:2000 |
| CB1 | Synthetic Peptide within Human CNR1 aa 450 to C-terminus. | Abcam (Cambridge, UK). (#ab23703) Rabbit polyclonal antibody | 1:200 |
| CB2 | Recombinant Fragment Protein within Rat Cnr2 aa 1–50. | Abcam (Cambridge, UK). (#ab3561) Rabbit polyclonal antibody | 1:200 |
| PPARα | Synthetic Peptide within Human PPARA aa 300–400 conjugated to KLH. | Abcam (Cambridge, UK). (#ab215270) Rabbit polyclonal antibody | 1:500 |
| GPR55 | Synthetic peptide from an internal cytoplasmic region of human GPR55 | Cayman (Ann Arbor, MI, USA). #10224 Rabbit polyclonal antibody | 1:200 |
| DAGLα | Synthetic peptide from the central region of human DAGLA (317–345 aa). | Biorbyt (Cambridge, UK) #orb1561573 Rabbit polyclonal antibody | 1:100 |
| DAGLβ | KLH conjugated synthetic peptide derived from human DAGLB (51–150/672aa) | Biorbyt (Cambridge, UK) #orb182976 Rabbit polyclonal antibody | 1:200 |
| NAPE-PLD | Synthetic Peptide within Human NAPEPLD aa 150–200. | Abcam (Cambridge, UK). #ab95397 Rabbit polyclonal antibody | 1:200 |
| MAGL | Recombinant Fragment Protein within Human MGLL aa 1–50. | Abcam (Cambridge, UK). #ab24701 Rabbit polyclonal antibody | 1:200 |
| FAAH | Synthetic peptide from the C-terminal region of rat FAAH | Cayman (Ann Arbor, MI, USA). #101600 Rabbit polyclonal antibody | 1:200 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Pozo, M.; Pacheco-Sánchez, B.; Ben Rabaa, M.; de Ceglia, M.; Melgar-Locatelli, S.; Santos, I.; Rodríguez de Fonseca, F.; Suárez, J.; Rivera, P. Perinatal Ethanol Exposure Induces Astrogliosis and Decreases GRP55/PEA-Mediated Neuroprotection in Hippocampal Astrocytes of the 3×Tg Alzheimer’s Animal Model. Int. J. Mol. Sci. 2025, 26, 11154. https://doi.org/10.3390/ijms262211154
Rodríguez-Pozo M, Pacheco-Sánchez B, Ben Rabaa M, de Ceglia M, Melgar-Locatelli S, Santos I, Rodríguez de Fonseca F, Suárez J, Rivera P. Perinatal Ethanol Exposure Induces Astrogliosis and Decreases GRP55/PEA-Mediated Neuroprotection in Hippocampal Astrocytes of the 3×Tg Alzheimer’s Animal Model. International Journal of Molecular Sciences. 2025; 26(22):11154. https://doi.org/10.3390/ijms262211154
Chicago/Turabian StyleRodríguez-Pozo, Miguel, Beatriz Pacheco-Sánchez, Meriem Ben Rabaa, Marialuisa de Ceglia, Sonia Melgar-Locatelli, Ignacio Santos, Fernando Rodríguez de Fonseca, Juan Suárez, and Patricia Rivera. 2025. "Perinatal Ethanol Exposure Induces Astrogliosis and Decreases GRP55/PEA-Mediated Neuroprotection in Hippocampal Astrocytes of the 3×Tg Alzheimer’s Animal Model" International Journal of Molecular Sciences 26, no. 22: 11154. https://doi.org/10.3390/ijms262211154
APA StyleRodríguez-Pozo, M., Pacheco-Sánchez, B., Ben Rabaa, M., de Ceglia, M., Melgar-Locatelli, S., Santos, I., Rodríguez de Fonseca, F., Suárez, J., & Rivera, P. (2025). Perinatal Ethanol Exposure Induces Astrogliosis and Decreases GRP55/PEA-Mediated Neuroprotection in Hippocampal Astrocytes of the 3×Tg Alzheimer’s Animal Model. International Journal of Molecular Sciences, 26(22), 11154. https://doi.org/10.3390/ijms262211154











