The Carotid Body as Part of a Unified Sympathoadrenal System of Neural Crest Derivatives: Insights from Two Centuries of Research
Abstract
1. Introduction
2. A Brief History of Carotid Body Research
2.1. The First Descriptions of the Carotid Body
2.2. The Carotid Body as a Chemoreceptor Organ: A Key Morphofunctional Contradiction
3. Morphology of the Carotid Body
3.1. Anatomy of the Carotid Body
3.1.1. Shape, Size and Location
3.1.2. Blood Supply
3.1.3. Innervation
3.2. Histological Organization
3.2.1. Light Microscopy
3.2.2. Immunohistochemistry
3.2.3. Electron Microscopy
Type I Cells
Type II Cells
Ganglionic Neurons
Intralobular Nerve Endings
Stroma and Vessels
Nerve Endings in the Stroma and Vessels
4. Morphofunctional Theories of Carotid Body Function
- Catecholamines contained in type I cell granules, regarded as neurotransmitters [7].
- The ability of type I cells to alter their membrane potential in response to changes in the partial pressures of oxygen and carbon dioxide [101].
- The release of catecholamines by type I cells in response to hypoxia [102].
- Enlargement of the carotid body in high-altitude dwellers and patients with cardiovascular and pulmonary disease, due to proliferation of type I cells [54].
- Restoration of hypoxia-induced impulses after reinnervation of the organ by a nerve normally lacking chemoreceptors [103].
5. The Role of the Carotid Body in Disease Development and Pathological Conditions
5.1. Sudden Infant Death Syndrome (SIDS)
5.2. Bronchial Asthma
5.3. Arterial Hypertension
6. A New Theory of the Unified Sympathoadrenal System
6.1. Ontogenesis of the Human Carotid Body
6.2. The Carotid Body as Part of the Unified Sympathoadrenal System
6.3. A Proposed Model of Carotid Body Function
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TH | Tyrosine hydroxylase |
| βIII | βIII-tubulin |
| GABA | gamma-aminobutyric acid |
| PGP9.5 | ubiquitin carboxy-terminal hydrolase L1 |
| TASK-1 | potassium two pore domain channel subfamily K |
| ATP | Adenosine triphosphate |
| ER | endoplasmic reticulum |
| SIDS | Sudden Infant Death Syndrome |
References
- Eames, B.F.; Medeiros, D.M.; Adameyko, I. (Eds.) Evolving Neural Crest Cells; CRC Press: Boca Raton, FL, USA, 2020. [Google Scholar] [CrossRef]
- Bronner, M.E.; LeDouarin, N.M. Development and Evolution of the Neural Crest: An Overview. Dev. Biol. 2012, 366, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Mayor, R.; Theveneau, E. The Neural Crest. Development 2013, 140, 2247–2251. [Google Scholar] [CrossRef]
- Ono-Minagi, H.; Nohno, T.; Serizawa, T.; Usami, Y.; Sakai, T.; Okano, H.; Ohuchi, H. The Germinal Origin of Salivary and Lacrimal Glands and the Contributions of Neural Crest Cell-Derived Epithelium to Tissue Regeneration. Int. J. Mol. Sci. 2023, 24, 13692. [Google Scholar] [CrossRef]
- Ortega-Sáenz, P.; López-Barneo, J. Physiology of the Carotid Body: From Molecules to Disease. Annu. Rev. Physiol. 2020, 82, 127–149. [Google Scholar] [CrossRef]
- Lazarov, N.E.; Atanasova, D.Y. Morphofunctional and Neurochemical Aspects of the Mammalian Carotid Body; Advances in Anatomy, Embryology and Cell Biology; Springer: Cham, Switzerland, 2023; Volume 237. [Google Scholar] [CrossRef]
- Zak, F.G.; Lawson, W. The Paraganglionic Chemoreceptor System: Physiology, Pathology and Clinical Medicine; Springer: New York, NY, USA, 1982. [Google Scholar] [CrossRef]
- Biscoe, T.J. Carotid Body: Structure and Function. Physiol. Rev. 1971, 51, 437–495. [Google Scholar] [CrossRef] [PubMed]
- Pallot, D.J. The Mammalian Carotid Body. Adv. Anat. Embryol. Cell Biol. 1987, 102, 1–91. [Google Scholar] [CrossRef] [PubMed]
- Fidone, S.J.; Gonzalez, C. Initiation and Control of Chemoreceptor Activity in the Carotid Body. In Handbook of Physiology. Section 3: The Respiratory System. Volume II: Control of Breathing, Part 2; Cherniack, N.S., Widdicombe, J.G., Eds.; American Physiological Society: Bethesda, MD, USA, 1986; pp. 247–312. [Google Scholar] [CrossRef]
- Porzionato, A.; Macchi, V.; Parenti, A.; Matturri, L.; De Caro, R. Peripheral Chemoreceptors: Postnatal Development and Cytochemical Findings in Sudden Infant Death Syndrome. Histol. Histopathol. 2008, 23, 351–365. [Google Scholar] [CrossRef]
- Verna, A. Ultrastructure of the Carotid Body in the Mammals. Int. Rev. Cytol. 1979, 60, 271–330. [Google Scholar] [CrossRef]
- Böck, P. Das Glomus Caroticum der Maus; Springer: Berlin/Heidelberg, Germany, 1973; (In German). [Google Scholar] [CrossRef]
- Heath, D.; Smith, P. Diseases of the Human Carotid Body; Springer: London, UK, 1992. [Google Scholar] [CrossRef]
- Taube, H.W.L. Dissertationem Inauguralem de Vera Nervi Intercostalis Origine; Abram Vandenhoeck: Gottingae, Germany, 1743. (In Latin) [Google Scholar]
- von Haller, A. Elementa Physiologiae Corporis Humani. Tomus Quartus. Cerebrum. Nervi. Musculi; Apud Vincentium Ursinum: Neapoli, Italy, 1776. (In Latin) [Google Scholar]
- Luschka, H. Ueber die drüsenartige Natur des sogenannten Ganglion intercaroticum. Arch. Anat. Physiol. 1862, 4, 405–414. (In German) [Google Scholar]
- Arnold, J. Ueber die Structur des Ganglion intercaroticum. Arch. Pathol. Anat. Physiol. Klin. Med. 1865, 33, 190–209. (In German) [Google Scholar] [CrossRef]
- Carmichael, S.W. The History of the Adrenal Medulla. Rev. Neurosci. 1989, 2, 83–100. [Google Scholar] [CrossRef]
- Kohn, A. Die Paraganglien. Arch. Mikrosk. Anat. 1903, 62, 263–365. (In German) [Google Scholar] [CrossRef]
- Stilling, H. Die chromophilen Zellen und Körperchen des Sympathicus: Eine Berichtigung. Anat. Anz. 1898, 15, 229–233. (In German) [Google Scholar]
- Hering, H.E. Die Karotissinusreflexe auf Herz und Gefässe; Theodor Steinkopff: Dresden/Leipzig, Germany, 1927. (In German) [Google Scholar]
- De Castro, F. Sur la structure et l’innervation de la glande intercarotidienne (glomus caroticum) de l’homme et des mammifères, et sur un nouveau système d’innervation autonome du nerf glossopharyngien. Trav. Lab. Rech. Biol. Univ. Madrid 1926, 24, 365–432. (In Spanish) [Google Scholar]
- De Castro, F. Sur la structure et l’innervation du sinus carotidien de l’homme et des mammifères. Nouveaux faits sur l’innervation et la fonction du glomus caroticum. Trav. Lab. Rech. Biol. Univ. Madrid 1928, 25, 331–380. (In Spanish) [Google Scholar]
- De Castro, F. Über die Struktur und Innervation des Glomus caroticum beim Menschen und bei den Säugetieren—Anatomisch-Experimentelle Untersuchungen. Z. Anat. Entwicklungsgesch. 1929, 89, 250–265. (In German) [Google Scholar] [CrossRef]
- Bouckaert, J.J.; Heymans, C. Carotid Sinus Reflexes. Influence of Central Blood-Pressure and Blood Supply on Respiratory and Vaso-Motor Centres. J. Physiol. 1933, 79, 49–66. [Google Scholar] [CrossRef] [PubMed]
- Heymans, C. Role of the Cardioaortic and Carotid-Sinus Nerves in the Reflex Control of the Respiratory Center. N. Engl. J. Med. 1938, 219, 157–159. [Google Scholar] [CrossRef]
- Heymans, C.; Bouckaert, J.J. Sinus Caroticus and Respiratory Reflexes: I. Cerebral Blood Flow and Respiration. Adrenaline Apnœa. J. Physiol. 1930, 69, 254–266. [Google Scholar] [CrossRef]
- Nobel Prize Outreach. The Nobel Prize in Physiology or Medicine 1938. NobelPrize.org. 2025. Available online: https://www.nobelprize.org/prizes/medicine/1938/summary/ (accessed on 3 September 2025).
- Comroe, J.H. The Location and Function of the Chemoreceptors of the Aorta. Am. J. Physiol. 1939, 127, 176–191. [Google Scholar] [CrossRef]
- Watt, J.G.; Dumke, P.R.; Comroe, J.H. Effects of Inhalation of 100 Per Cent and 14 Per Cent Oxygen upon Respiration of Unanesthetized Dogs before and after Chemoreceptor Denervation. Am. J. Physiol. 1943, 138, 610–617. [Google Scholar] [CrossRef]
- Gernandt, B. A Study of the Respiratory Reflexes Elicited from the Aortic and Carotid Bodies. Acta Physiol. Scand. 1946, 11 (Suppl. S35), 1–81. [Google Scholar]
- Daly, M.D.B.; Schweitzer, A. Effects of Sino-Aortic Nerve Stimulation on the Bronchi. Acta Physiol. Scand. 1951, 22, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Nadel, J.A.; Widdicombe, J.G. Effect of Changes in Blood Gas Tensions and Carotid Sinus Pressure on Tracheal Volume and Total Lung Resistance to Airflow. J. Physiol. 1962, 163, 13–33. [Google Scholar] [CrossRef]
- Nakayama, K. Surgical Removal of the Carotid Body for Bronchial Asthma. Dis. Chest 1961, 40, 595–604. [Google Scholar] [CrossRef] [PubMed]
- Winter, B. Carotid Body Resection: Controversy—Confusion—Conflict. Ann. Thorac. Surg. 1973, 16, 648–659. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.C.; Morrill, C.G.; Chai, H. Impaired Response to Hypoxia after Bilateral Carotid Body Resection for Treatment of Bronchial Asthma. Chest 1978, 73, 667–669. [Google Scholar] [CrossRef]
- Watzka, M. Über die Entwicklung des Paraganglion caroticum der Säugetiere. Z. Anat. Entwicklungsgesch 1937, 108, 61–73. (In German) [Google Scholar] [CrossRef]
- Gomez, L.P. The Anatomy and Pathology of the Carotid Gland. Am. J. Med. Sci. 1908, 136, 98–106. [Google Scholar]
- De Kock, L.L. Histology of the Carotid Body. Nature 1951, 167, 611–612. [Google Scholar] [CrossRef]
- De Kock, L.L. The Intra-Glomerular Tissues of the Carotid Body. Acta Anat. 1954, 21, 101–116. [Google Scholar] [CrossRef]
- Gould, R.P. Fine Innervation of the Carotid Body of the Rhesus Monkey. Nature 1960, 185, 183–184. [Google Scholar] [CrossRef]
- Grigor’eva, T.A. The Innervation of Blood Vessels; Pergamon Press: New York, NY, USA, 1962. [Google Scholar]
- Smitten, N.A. Sympatho-Adrenal System in Phylogenesis and Ontogenesis; Nauka: Moscow, Russia, 1972. (In Russian) [Google Scholar]
- Karnauchow, P.N. The Carotid Body: A Pathologist’s View. Can. Med. Assoc. J. 1965, 92, 1298–1301. [Google Scholar] [PubMed]
- Milsom, W.K.; Burleson, M.L. Peripheral Arterial Chemoreceptors and the Evolution of the Carotid Body. Respir. Physiol. Neurobiol. 2007, 157, 4–11. [Google Scholar] [CrossRef]
- Jonz, M.G.; Buck, L.T.; Perry, S.F.; Schwerte, T.; Zaccone, G. Sensing and Surviving Hypoxia in Vertebrates. Ann. N. Y. Acad. Sci. 2016, 1365, 43–58. [Google Scholar] [CrossRef] [PubMed]
- Hockman, D.; Burns, A.J.; Schlosser, G.; Gates, K.P.; Jevans, B.; Mongera, A.; Fisher, S.; Unlu, G.; Knapik, E.W.; Kaufman, C.K.; et al. Evolution of the Hypoxia-Sensitive Cells Involved in Amniote Respiratory Reflexes. eLife 2017, 6, e21231. [Google Scholar] [CrossRef] [PubMed]
- Otlyga, D.; Tsvetkova, E.; Junemann, O.; Saveliev, S. Immunohistochemical Characteristics of the Human Carotid Body in the Antenatal and Postnatal Periods of Development. Int. J. Mol. Sci. 2021, 22, 8222. [Google Scholar] [CrossRef]
- Heath, D. The Human Carotid Body. Thorax 1983, 38, 561–564. [Google Scholar] [CrossRef]
- Khan, Q.; Heath, D.; Smith, P. Anatomical Variations in Human Carotid Bodies. J. Clin. Pathol. 1988, 41, 1196–1199. [Google Scholar] [CrossRef]
- Smith, P.; Jago, R.; Heath, D. Anatomical Variation and Quantitative Histology of the Normal and Enlarged Carotid Body. J. Pathol. 1982, 137, 287–304. [Google Scholar] [CrossRef]
- Boyd, J.D. Absence of the Right Common Carotid Artery. J. Anat. 1934, 68, 551–557. [Google Scholar]
- Heath, D.; Edwards, C.; Harris, P. Post-Mortem Size and Structure of the Human Carotid Body. Thorax 1970, 25, 129–140. [Google Scholar] [CrossRef]
- Heath, D.; Edwards, C. The Glomic Arteries. Cardiovasc. Res. 1971, 5, 303–312. [Google Scholar] [CrossRef]
- Jago, R.; Heath, D.; Smith, P. Structure of the Glomic Arteries. J. Pathol. 1982, 138, 205–218. [Google Scholar] [CrossRef] [PubMed]
- Heath, D.; Jago, R.; Smith, P. The Vasculature of the Carotid Body. Cardiovasc. Res. 1983, 17, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Habeck, J.O.; Honig, A.; Huckstorf, C.; Pfeiffer, C. Arteriovenous Anastomoses at the Carotid Bodies of Rats. Anat. Anz. 1984, 156, 209–215. [Google Scholar] [PubMed]
- Schäfer, D.; Seidl, E.; Acker, H.; Keller, H.P.; Lübbers, D.W. Arteriovenous Anastomoses in the Cat Carotid Body. Z. Zellforsch. Mikrosk. Anat. 1973, 142, 515–524. [Google Scholar] [CrossRef]
- Serafini-Fracassini, A.; Volpin, D. Some Features of the Vascularization of the Carotid Body in the Dog. Acta Anat. 1966, 63, 571–579. [Google Scholar] [CrossRef]
- Seidl, E. On the Morphology of the Vascular System of the Carotid Body of Cat and Rabbit and Its Relation to the Glomus Type I Cells. In The Peripheral Arterial Chemoreceptors; Purves, M.J., Ed.; Cambridge University Press: Cambridge, UK, 1975; pp. 293–299. [Google Scholar]
- McDonald, D.M.; Larue, D.T. The Ultrastructure and Connections of Blood Vessels Supplying the Rat Carotid Body and Carotid Sinus. J. Neurocytol. 1983, 12, 117–153. [Google Scholar] [CrossRef]
- De Castro, F. Towards the sensory nature of the carotid body: Hering, De Castro and Heymans. Front. Neuroanat. 2009, 3, 786. [Google Scholar] [CrossRef]
- Kumar, P.; Prabhakar, N.R. Peripheral Chemoreceptors: Function and Plasticity of the Carotid Body. Compr. Physiol. 2012, 2, 141–219. [Google Scholar] [CrossRef]
- Gerard, M.W.; Billingsley, P.R. The Innervation of the Carotid Body. Anat. Rec. 1923, 25, 391–400. [Google Scholar] [CrossRef]
- Toorop, R.J.; Scheltinga, M.R.; Moll, F.L.; Bleys, R.L. Anatomy of the Carotid Sinus Nerve and Surgical Implications in Carotid Sinus Syndrome. J. Vasc. Surg. 2009, 50, 177–182. [Google Scholar] [CrossRef]
- Boyd, J.D. Observations on the Human Carotid Sinus and Its Nerve Supply. Anat. Anz. 1937, 84, 386–399. [Google Scholar]
- Sheehan, D.; Mulholland, J.H.; Shafiroff, B. Surgical Anatomy of the Carotid Sinus Nerve. Anat. Rec. 1941, 80, 431–442. [Google Scholar] [CrossRef]
- Svitzer, E. Nogle Undersøgelser om Ganglion intercaroticum, med en Tavle. Bibliothek Læger. 1863, 7, 1–22. (In Danish) [Google Scholar]
- Hervonen, A.; Korkala, O. Fine Structure of the Carotid Body of the Midterm Human Fetus. Z. Anat. Entwicklungsgesch. 1972, 138, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Heath, D.; Lowe, P.; Smith, P. Mast Cells in the Human Carotid Body. J. Clin. Pathol. 1987, 40, 9–12. [Google Scholar] [CrossRef]
- McDonald, D.M.; Mitchell, R.A. The Innervation of Glomus Cells, Ganglion Cells and Blood Vessels in the Rat Carotid Body: A Quantitative Ultrastructural Analysis. J. Neurocytol. 1975, 4, 177–230. [Google Scholar] [CrossRef]
- Pávai, Z.; Töro, K.; Keller, E.; Jung, J. Morphometric Investigation of Carotid Body in Sudden Infant Death Syndrome. Rom. J. Morphol. Embryol. 2005, 46, 93–97. [Google Scholar]
- Pallot, D.J.; Seker, M.; Abramovici, A. Post-Mortem Changes in the Normal Rat Carotid Body: Possible Implications for Human Histopathology. Virchows Arch. A Pathol. Anat. Histopathol. 1992, 420, 31–35. [Google Scholar] [CrossRef]
- Seker, M.; Pallot, D.J.; Habeck, J.-O.; Abramovici, A. Postmortem Changes in the Human Carotid Body. In Arterial Chemoreceptors; O’Regan, R.G., Nolan, P., McQueen, D.S., Paterson, D.J., Eds.; Springer: Boston, MA, USA, 1994; Volume 360, pp. 349–351. [Google Scholar] [CrossRef]
- Otlyga, D.A.; Junemann, O.A.; Dzhalilova, D.S.; Tsvetkova, E.G.; Saveliev, S.V. Immunohistochemical Study of Dark and Progenitor Carotid Body Cells: Artefacts or Real Subtypes? Bull. Exp. Biol. Med. 2020, 168, 807–811. [Google Scholar] [CrossRef]
- Fagerlund, M.J.; Kåhlin, J.; Ebberyd, A.; Schulte, G.; Mkrtchian, S.; Eriksson, L.I. The Human Carotid Body: Expression of Oxygen Sensing and Signaling Genes of Relevance for Anesthesia. Anesthesiology 2010, 113, 1270–1279. [Google Scholar] [CrossRef] [PubMed]
- Izal-Azcárate, A.; Belzunegui, S.; Sebastián, W.S.; Garrido-Gil, P.; Vázquez-Claverie, M.; López, B.; Marcilla, I.; Luquin, M.R. Immunohistochemical Characterization of the Rat Carotid Body. Respir. Physiol. Neurobiol. 2008, 161, 95–99. [Google Scholar] [CrossRef]
- Kåhlin, J.; Eriksson, L.I.; Ebberyd, A.; Fagerlund, M.J. Presence of Nicotinic, Purinergic and Dopaminergic Receptors and the TASK-1 K+-Channel in the Mouse Carotid Body. Respir. Physiol. Neurobiol. 2010, 172, 122–128. [Google Scholar] [CrossRef]
- Otlyga, D.A.; Junemann, O.A.; Tsvetkova, E.G.; Gorokhov, K.R.; Saveliev, S.V. Immunohistochemical Features of the Human Carotid Body. Clin. Exp. Morphol. 2020, 9, 61–67. (In Russian) [Google Scholar] [CrossRef]
- Kent, C.; Rowe, H.L. The Immunolocalisation of Ubiquitin Carboxyl-Terminal Hydrolase (PGP9.5) in Developing Paraneurons in the Rat. Dev. Brain Res. 1992, 68, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Habeck, J.O.; Pallot, D.J.; Kummer, W. Serotonin Immunoreactivity in the Carotid Body of Adult Humans. Histol. Histopathol. 1994, 9, 227–232. [Google Scholar]
- Kummer, W.; Habeck, J.O. Chemoreceptor A-Fibres in the Human Carotid Body Contain Tyrosine Hydroxylase and Neurofilament Immunoreactivity. Neuroscience 1992, 47, 713–725. [Google Scholar] [CrossRef] [PubMed]
- Lazarov, N.E.; Reindl, S.; Fischer, F.; Gratzl, M. Histaminergic and Dopaminergic Traits in the Human Carotid Body. Respir. Physiol. Neurobiol. 2009, 165, 131–136. [Google Scholar] [CrossRef]
- Kondo, H.; Iwanaga, T.; Nakajima, T. Immunocytochemical Study on the Localization of Neuron-Specific Enolase and S-100 Protein in the Carotid Body of Rats. Cell Tissue Res. 1982, 227, 291–295. [Google Scholar] [CrossRef]
- Alba, E.; García-Mesa, Y.; Cobo, R.; Cuendias, P.; Martín-Cruces, J.; Suazo, I.; Martínez-Barbero, G.; Vega, J.A.; García-Suárez, O.; Cobo, T. Immunohistochemical Detection of PIEZO Ion Channels in the Human Carotid Sinus and Carotid Body. Biomolecules 2025, 15, 386. [Google Scholar] [CrossRef] [PubMed]
- Kummer, W.; Habeck, J.O. Substance P- and Calcitonin Gene-Related Peptide-like Immunoreactivities in the Human Carotid Body Studied at Light and Electron Microscopical Level. Brain Res. 1991, 554, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Grimley, P.M.; Glenner, G.G. Ultrastructure of the Human Carotid Body: A Perspective on the Mode of Chemoreception. Circulation 1968, 37, 648–665. [Google Scholar] [CrossRef]
- Ross, L.L. Electron Microscopic Observations of the Carotid Body of the Cat. J. Biophys. Biochem. Cytol. 1959, 6, 253–262. [Google Scholar] [CrossRef]
- Lever, J.D.; Lewis, P.R.; Boyd, J.D. Observations on the Fine Structure and Histochemistry of the Carotid Body in the Cat and Rabbit. J. Anat. 1959, 93 Pt 4, 478–490. [Google Scholar] [PubMed]
- Biscoe, T.J.; Stehbens, W.E. Ultrastructure of the Carotid Body. J. Cell Biol. 1966, 30, 563–578. [Google Scholar] [CrossRef]
- Morgan, M.; Pack, R.J.; Howe, A. Nerve Endings in Rat Carotid Body. Cell Tissue Res. 1975, 157, 255–272. [Google Scholar] [CrossRef]
- Abbott, C.P.; De Burgh Daly, M.; Howe, A. Early Ultrastructural Changes in the Carotid Body after Degenerative Section of the Carotid Sinus Nerve in the Cat. Cells Tissues Organs 1972, 83, 161–185. [Google Scholar] [CrossRef]
- Kobayashi, S. Fine Structure of the Carotid Body of the Dog. Arch. Histol. Jpn. 1968, 30, 95–120. [Google Scholar] [CrossRef]
- Kondo, H. Innervation of the Carotid Body of the Adult Rat—A Serial Ultrathin Section Analysis. Cell Tissue Res. 1976, 173, 1–15. [Google Scholar] [CrossRef]
- Nishi, K.; Stensaas, L.J. The Ultrastructure and Source of Nerve Endings in the Carotid Body. Cell Tissue Res. 1974, 154, 303–319. [Google Scholar] [CrossRef] [PubMed]
- King, A.S.; King, D.Z.; Hodges, R.D.; Henry, J. Synaptic Morphology of the Carotid Body of the Domestic Fowl. Cell Tissue Res. 1975, 162, 459–473. [Google Scholar] [CrossRef]
- Biscoe, T.J.; Stehbens, W.E. Ultrastructure of the Denervated Carotid Body. Q. J. Exp. Physiol. Cogn. Med. Sci. 1967, 52, 31–36. [Google Scholar] [CrossRef]
- Biscoe, T.J.; Silver, A. The Distribution of Cholinesterases in the Cat Carotid Body. J. Physiol. 1966, 183, 501–512. [Google Scholar] [CrossRef]
- Gonzalez, C.; Conde, S.V.; Gallego-Martín, T.; Olea, E.; Gonzalez-Obeso, E.; Ramirez, M.; Yubero, S.; Agapito, M.T.; Gomez-Niño, A.; Obeso, A.; et al. Fernando de Castro and the Discovery of the Arterial Chemoreceptors. Front. Neuroanat. 2014, 8, 25. [Google Scholar] [CrossRef]
- López-Barneo, J.; Ortega-Sáenz, P.; González-Rodríguez, P.; Fernández-Agüera, M.C.; Macías, D.; Pardal, R.; Gao, L. Oxygen-Sensing by Arterial Chemoreceptors: Mechanisms and Medical Translation. Mol. Aspects Med. 2016, 47–48, 90–108. [Google Scholar] [CrossRef]
- Prabhakar, N.R. Neurotransmitters in the Carotid Body. In Arterial Chemoreceptors: Cell to System; O’Regan, R.G., Nolan, P., McQueen, D.S., Paterson, D.J., Eds.; Advances in Experimental Medicine and Biology; Springer: New York, NY, USA, 1994; Volume 360, pp. 57–69. [Google Scholar] [CrossRef]
- Zapata, P.; Hess, A.; Eyzaguirre, C. Reinnervation of Carotid Body and Sinus with Superior Laryngeal Nerve Fibers. J. Neurophysiol. 1969, 32, 215–228. [Google Scholar] [CrossRef] [PubMed]
- Biscoe, T.J.; Lall, A.; Sampson, S.R. Electron Microscopic and Electrophysiological Studies on the Carotid Body Following Intracranial Section of the Glossopharyngeal Nerve. J. Physiol. 1970, 208, 133–152. [Google Scholar] [CrossRef]
- Pallot, D.J.; Biscoe, T.J. Studies of Normal and Wobbler Mutant Carotid Bodies. In Chemoreception in the Carotid Body; Acker, H., Fidone, S., Pallot, D., Eyzaguirre, C., Lübbers, D.W., Torrance, R.W., Eds.; Springer: Berlin/Heidelberg, Germany, 1977; pp. 51–54. [Google Scholar] [CrossRef]
- Eyzaguirre, C.; Baron, M.; Gallego, R. Effects of Temperature and Stimulating Agents on Carotid Body Cells. In Chemoreception in the Carotid Body; Acker, H., Fidone, S., Pallot, D., Eyzaguirre, C., Lübbers, D.W., Torrance, R.W., Eds.; Springer: Berlin/Heidelberg, Germany, 1977; pp. 71–78. [Google Scholar] [CrossRef]
- Acker, H.; Pietruschka, F. Meaning of the Type I Cell for the Chemoreceptive Process—An Electrophysiological Study on Cultured Type I Cells of the Carotid Body. In Chemoreception in the Carotid Body; Acker, H., Fidone, S., Pallot, D., Eyzaguirre, C., Lübbers, D.W., Torrance, R.W., Eds.; Springer: Berlin/Heidelberg, Germany, 1977; pp. 92–96. [Google Scholar] [CrossRef]
- Hellström, S. Effects of Hypoxia on Carotid Body Type I Cells and Their Catecholamines: A Biochemical and Morphologic Study. In Chemoreception in the Carotid Body; Acker, H., Fidone, S., Pallot, D., Eyzaguirre, C., Lübbers, D.W., Torrance, R.W., Eds.; Springer: Berlin/Heidelberg, Germany, 1977; pp. 122–129. [Google Scholar] [CrossRef]
- Hanbauer, I. Molecular Biology of Chemoreceptor Function: Induction of Tyrosine Hydroxylase in the Rat Carotid Body Elicited by Hypoxia. In Chemoreception in the Carotid Body; Acker, H., Fidone, S., Pallot, D., Eyzaguirre, C., Lübbers, D.W., Torrance, R.W., Eds.; Springer: Berlin/Heidelberg, Germany, 1977; pp. 114–121. [Google Scholar] [CrossRef]
- Nishi, K. A Pharmacologic Study on a Possible Inhibitory Role of Dopamine in the Cat Carotid Body Chemoreceptor. In Chemoreception in the Carotid Body; Acker, H., Fidone, S., Pallot, D., Eyzaguirre, C., Lübbers, D.W., Torrance, R.W., Eds.; Springer: Berlin/Heidelberg, Germany, 1977; pp. 145–151. [Google Scholar] [CrossRef]
- Zapata, P. Effects of Dopamine on Carotid Chemo- and Baroreceptors in Vitro. J. Physiol. 1975, 244, 235–251. [Google Scholar] [CrossRef] [PubMed]
- Zapata, P.; Llados, F. Blockade of Carotid Body Chemosensory Inhibition. In Chemoreception in the Carotid Body; Acker, H., Fidone, S., Pallot, D., Eyzaguirre, C., Lübbers, D.W., Torrance, R.W., Eds.; Springer: Berlin/Heidelberg, Germany, 1977; pp. 152–159. [Google Scholar] [CrossRef]
- Christie, R.V. The Function of the Carotid Gland. I. The Action of Extracts of a Carotid Gland Tumor in Man. Endocrinology 1933, 17, 421–432. [Google Scholar] [CrossRef]
- Fidone, S.; Weintraub, S.; Stavinoha, W.; Stirling, C.; Jones, L. Endogenous Acetylcholine Levels in Cat Carotid Body and the Autoradiographic Localization of a High-Affinity Component of Choline Uptake. In Chemoreception in the Carotid Body; Acker, H., Fidone, S., Pallot, D., Eyzaguirre, C., Lübbers, D.W., Torrance, R.W., Eds.; Springer: Berlin/Heidelberg, Germany, 1977; pp. 106–113. [Google Scholar] [CrossRef]
- Eyzaguirre, C.; Koyano, H. Effects of Some Pharmacological Agents on Chemoreceptor Discharges. J. Physiol. 1965, 178, 410–437. [Google Scholar] [CrossRef] [PubMed]
- Eyzaguirre, C.; Koyano, H. Effects of Hypoxia, Hypercapnia, and pH on the Chemoreceptor Activity of the Carotid Body in Vitro. J. Physiol. 1965, 178, 385–409. [Google Scholar] [CrossRef]
- Schweitzer, A.; Wright, S. Action of Prostigmine and Acetylcholine on Respiration. Q. J. Exp. Physiol. 1938, 28, 33–47. [Google Scholar] [CrossRef]
- Anichkov, S.V.; Belen’kii, M.L. Pharmacology of the Carotid Body Chemoreceptors; Medgiz: Leningrad, USSR, 1962. (In Russian) [Google Scholar]
- Douglas, W.W. The Effect of a Ganglion-Blocking Drug, Hexamethonium, on the Response of the Cat’s Carotid Body to Various Stimuli. J. Physiol. 1952, 118, 373–383. [Google Scholar] [CrossRef]
- Eyzaguirre, C.; Zapata, P. Pharmacology of pH Effects on Carotid Body Chemoreceptors in Vitro. J. Physiol. 1968, 195, 557–588. [Google Scholar] [CrossRef]
- Eyzaguirre, C.; Zapata, P. The Release of Acetylcholine from Carotid Body Tissues: Further Study on the Effects of Acetylcholine and Cholinergic Blocking Agents on the Chemosensory Discharge. J. Physiol. 1968, 195, 589–607. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.K.; Prabhakar, N.R.; Kumar, G.K. Acetylcholine Release from the Carotid Body by Hypoxia: Evidence for the Involvement of Autoinhibitory Receptors. J. Appl. Physiol. 2004, 96, 376–383. [Google Scholar] [CrossRef]
- Kåhlin, J.; Mkrtchian, S.; Ebberyd, A.; Hammarstedt-Nordenvall, L.; Nordlander, B.; Yoshitake, T.; Kehr, J.; Prabhakar, N.; Poellinger, L.; Fagerlund, M.J.; et al. The Human Carotid Body Releases Acetylcholine, ATP and Cytokines during Hypoxia. Exp. Physiol. 2014, 99, 1089–1098. [Google Scholar] [CrossRef]
- Fitzgerald, R.S.; Shirahata, M.; Chang, I.; Kostuk, E. The Impact of Hypoxia and Low Glucose on the Release of Acetylcholine and ATP from the Incubated Cat Carotid Body. Brain Res. 2009, 1270, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Zapata, P. Is ATP a Suitable Co-Transmitter in Carotid Body Arterial Chemoreceptors? Respir. Physiol. Neurobiol. 2007, 157, 106–115. [Google Scholar] [CrossRef]
- Reyes, E.P.; Fernandez, R.; Larraín, C.; Zapata, P. Effects of Combined Cholinergic–Purinergic Block upon Cat Carotid Body Chemoreceptors in Vitro. Respir. Physiol. Neurobiol. 2007, 156, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Monti-Bloch, L.; Stensaas, L.J.; Eyzaguirre, C. Carotid Body Grafts Induce Chemosensitivity in Muscle Nerve Fibers of the Cat. Brain Res. 1983, 270, 77–92. [Google Scholar] [CrossRef]
- Verna, A.; Roumy, M.; Leitner, L.M. Loss of Chemoreceptive Properties of the Rabbit Carotid Body after Destruction of the Glomus Cells. Brain Res. 1975, 100, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.; Zhang, M.; Nurse, C.A. Synapse Formation and Hypoxic Signalling in Co-Cultures of Rat Petrosal Neurones and Carotid Body Type I Cells. J. Physiol. 1997, 503, 599–612. [Google Scholar] [CrossRef] [PubMed]
- Biscoe, T.J.; Duchen, M.R. Responses of Type I Cells Dissociated from the Rabbit Carotid Body to Hypoxia. J. Physiol. 1990, 428, 39–59. [Google Scholar] [CrossRef]
- López-Barneo, J.; Ortega-Sáenz, P.; Pardal, R.; Pascual, A.; Piruat, J.I.; Durán, R.; Gómez-Díaz, R. Oxygen Sensing in the Carotid Body. Ann. N. Y. Acad. Sci. 2009, 1177, 119–131. [Google Scholar] [CrossRef]
- López-Barneo, J.; Pardal, R.; Ortega-Sáenz, P. Cellular Mechanism of Oxygen Sensing. Annu. Rev. Physiol. 2001, 63, 259–287. [Google Scholar] [CrossRef]
- Weir, E.K.; López-Barneo, J.; Buckler, K.J.; Archer, S.L. Acute Oxygen-Sensing Mechanisms. N. Engl. J. Med. 2005, 353, 2042–2055. [Google Scholar] [CrossRef]
- Rakoczy, R.J.; Wyatt, C.N. Acute Oxygen Sensing by the Carotid Body: A Rattlebag of Molecular Mechanisms. J. Physiol. 2018, 596, 2969–2976. [Google Scholar] [CrossRef]
- Vjotosh, A.N. Intracellular Mechanisms of Oxygen Sensing. Biochemistry 2020, 85, 40–53. [Google Scholar] [CrossRef]
- Gao, L.; Ortega-Sáenz, P.; López-Barneo, J. Acute Oxygen Sensing—Role of Metabolic Specifications in Peripheral Chemoreceptor Cells. Respir. Physiol. Neurobiol. 2019, 265, 100–111. [Google Scholar] [CrossRef]
- Bishop, T.; Ratcliffe, P.J. Genetic Basis of Oxygen Sensing in the Carotid Body: HIF-2α and an Isoform Switch in Cytochrome c Oxidase Subunit 4. Sci. Signal. 2020, 13, eaba1302. [Google Scholar] [CrossRef]
- Conde, S.V.; Sacramento, J.F.; Guarino, M.P. Carotid Body: A Metabolic Sensor Implicated in Insulin Resistance. Physiol. Genom. 2018, 50, 208–214. [Google Scholar] [CrossRef]
- Porzionato, A.; Macchi, V.; De Caro, R.; Di Giulio, C. Inflammatory and Immunomodulatory Mechanisms in the Carotid Body. Respir. Physiol. Neurobiol. 2013, 187, 31–40. [Google Scholar] [CrossRef]
- Zapata, P.; Larraín, C.; Reyes, P.; Fernández, R. Immunosensory Signalling by Carotid Body Chemoreceptors. Respir. Physiol. Neurobiol. 2011, 178, 370–374. [Google Scholar] [CrossRef]
- Naeye, R.L.; Fisher, R.; Ryser, M.; Whalen, P. Carotid Body in the Sudden Infant Death Syndrome. Science 1976, 191, 567–569. [Google Scholar] [CrossRef]
- Cole, S.; Lindenberg, L.B.; Galioto, F.M.; Howe, P.E.; DeGraff, A.C.; Davis, J.M.; Lubka, R.; Gross, E.M. Ultrastructural Abnormalities of the Carotid Body in Sudden Infant Death Syndrome. Pediatrics 1979, 63, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Hess, A. Histofluorescent and Ultrastructural Studies on the Effects of Reserpine and Calcium on Dense-Cored Vesicles in Glomus Cells of the Rat Carotid Body. In Chemoreception in the Carotid Body; Acker, H., Fidone, S., Pallot, D., Eyzaguirre, C., Lübbers, D.W., Torrance, R.W., Eds.; Springer: Berlin/Heidelberg, Germany, 1977; pp. 201–206. [Google Scholar] [CrossRef]
- Perrin, D.G.; Cutz, E.; Becker, L.E.; Bryan, A.C. Ultrastructure of Carotid Bodies in Sudden Infant Death Syndrome. Pediatrics 1984, 73, 646–651. [Google Scholar] [CrossRef] [PubMed]
- Lack, E.E.; Perez-Atayde, A.R.; Young, J.B. Carotid Bodies in Sudden Infant Death Syndrome: A Combined Light Microscopic, Ultrastructural, and Biochemical Study. Fetal Pediatr. Pathol. 1986, 6, 335–350. [Google Scholar] [CrossRef] [PubMed]
- Sedwitz, J.L. Unilateral Carotid Body Resection for Asthma: A Report of 350 Patients. J. Natl. Med. Assoc. 1963, 55, 384–388. [Google Scholar] [PubMed]
- Honda, Y.; Watanabe, S.; Hashizume, I.; Satomura, Y.; Hata, N.; Sakakibara, Y.; Severinghaus, J.W. Hypoxic Chemosensitivity in Asthmatic Patients Two Decades after Carotid Body Resection. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1979, 46, 632–638. [Google Scholar] [CrossRef]
- Gilevich, I.; Gorikov, N.G.; Nikulin, G.M. Morphology of the Carotid Glands in Bronchial Asthma in the Light of Indications for Glomectomy. Vestn. Khirurgii im. I.I. Grekova 1967, 99, 61–64. (In Russian) [Google Scholar]
- Makarova, N.P.; Zislin, B.D.; Simonova, Z.S.; Suganova, N.M. Comparative Evaluation of the Efficacy of Conservative and Surgical Treatment of Severe Forms of Bronchial Asthma. Sov. Med. 1975, 1, 48–52. (In Russian) [Google Scholar]
- Koroleva, N.S.; Dobrovol’skii, S.R. Results of Glomectomy in Bronchial Asthma. Klin. Khir. 1980, 10, 8–11. (In Russian) [Google Scholar]
- Karashurov, E.S.; Ostrovskii, A.G.; Mart’yanov, S.G. Indications and Contraindications for Glomectomy in Patients with Bronchial Asthma. Khirurgiia 1993, 9, 36–41. (In Russian) [Google Scholar]
- Gudovskii, L.M.; Karashurov, S.E.; Karashurov, E.S. Surgical Treatment of Bronchial Asthma. Khirurgiia 2002, 7, 14–18. (In Russian) [Google Scholar]
- Curran, W.S.; Graham, W.G. Long Term Effects of Glomectomy: Follow-Up of a Double-Blind Study. Am. Rev. Respir. Dis. 1971, 103, 566–568. [Google Scholar] [PubMed]
- Marschke, G.; Beall, G.N.; Stern, W.E.; Murray, J.F. Carotid-Body Removal in Asthma. J. Am. Med. Assoc. 1965, 191, 397. [Google Scholar] [CrossRef]
- Edwards, C.; Heath, D.; Harris, P. The Carotid Body in Emphysema and Left Ventricular Hypertrophy. J. Pathol. 1971, 104, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Paton, J.F.R.; Sobotka, P.A.; Fudim, M.; Engelman, Z.J.; Hart, E.C.J.; McBryde, F.D.; Abdala, A.P.; Marina, N.; Gourine, A.V.; Lobo, M.; et al. The Carotid Body as a Therapeutic Target for the Treatment of Sympathetically Mediated Diseases. Hypertension 2013, 61, 5–13, Erratum in Hypertension 2013, 61, e26. [Google Scholar] [CrossRef] [PubMed]
- Iturriaga, R. Carotid Body Ablation: A New Target to Address Central Autonomic Dysfunction. Curr. Hypertens. Rep. 2018, 20, 53. [Google Scholar] [CrossRef]
- Niewinski, P.; Janczak, D.; Rucinski, A.; Tubek, S.; Engelman, Z.J.; Piesiak, P.; Jazwiec, P.; Banasiak, W.; Fudim, M.; Sobotka, P.A.; et al. Carotid Body Resection for Sympathetic Modulation in Systolic Heart Failure: Results from First-in-Man Study. Eur. J. Heart Fail. 2017, 19, 391–400. [Google Scholar] [CrossRef]
- Narkiewicz, K.; Ratcliffe, L.E.K.; Hart, E.C.; Briant, L.J.B.; Chrostowska, M.; Wolf, J.; Szyndler, A.; Hering, D.; Abdala, A.P.; Manghat, N.; et al. Unilateral Carotid Body Resection in Resistant Hypertension: A Safety and Feasibility Trial. JACC Basic Transl. Sci. 2016, 1, 313–324. [Google Scholar] [CrossRef]
- Fidone, S.J.; Zapata, P.; Stensaas, L.J. Axonal Transport of Labeled Material into Sensory Nerve Endings of Cat Carotid Body. Brain Res. 1977, 124, 9–28. [Google Scholar] [CrossRef]
- Korkala, O.; Hervonen, A. Origin and Development of the Catecholamine-Storing Cells of the Human Fetal Carotid Body. Histochemie 1973, 37, 287–297. [Google Scholar] [CrossRef]
- Scraggs, M.; Smith, P.; Heath, D. Glomic Cells and Their Peptides in the Carotid Body of the Human Fetus. Fetal Pediatr. Pathol. 1992, 12, 823–834. [Google Scholar] [CrossRef] [PubMed]
- Pokorski, M.; Walski, M.; Dymecka, A.; Marczak, M. The Aging Carotid Body. J. Physiol. Pharmacol. 2004, 55 (Suppl. S3), 107–113. [Google Scholar]
- Gauda, E.B.; Bamford, O.; Gerfen, C.R. Developmental Expression of Tyrosine Hydroxylase, D2-Dopamine Receptor and Substance P Genes in the Carotid Body of the Rat. Neuroscience 1996, 75, 969–977. [Google Scholar] [CrossRef] [PubMed]
- Blanco, C.E.; Dawes, G.S.; Hanson, M.A.; McCooke, H.B. The Response to Hypoxia of Arterial Chemoreceptors in Fetal Sheep and New-Born Lambs. J. Physiol. 1984, 351, 25–37. [Google Scholar] [CrossRef]
- Carroll, J.L.; Bamford, O.S.; Fitzgerald, R.S. Postnatal Maturation of Carotid Chemoreceptor Responses to O2 and CO2 in the Cat. J. Appl. Physiol. 1993, 75, 2383–2391. [Google Scholar] [CrossRef]
- Wasicko, M.J.; Sterni, L.M.; Bamford, O.S.; Montrose, M.H.; Carroll, J.L. Resetting and Postnatal Maturation of Oxygen Chemosensitivity in Rat Carotid Chemoreceptor Cells. J. Physiol. 1999, 514, 493–503. [Google Scholar] [CrossRef] [PubMed]
- Otlyga, E.; Otlyga, D.; Junemann, O.; Krivova, Y.; Proshchina, A.; Kharlamova, A.; Gulimova, V.I.; Sonin, G.; Saveliev, S. Developmental Parallels between the Human Organs of Zuckerkandl and Adrenal Medulla. Life 2025, 15, 1214. [Google Scholar] [CrossRef] [PubMed]
- Otlyga, D.A.; Junemann, O.A.; Tsvetkova, E.G.; Kharlamova, A.S.; Besova, N.V.; Saveliev, S.V. Carotid Body, Adrenal Medulla and Zuckerkandl Organ as an Integrated Sympathoadrenal System in Human Prenatal Development. Clin. Exp. Morphol. 2020, 9, 61–69. (In Russian) [Google Scholar] [CrossRef]
- Subramanian, A.; Maker, V.K. Organs of Zuckerkandl: Their Surgical Significance and a Review of a Century of Literature. Am. J. Surg. 2006, 192, 224–234. [Google Scholar] [CrossRef]
- Hockman, D.; Adameyko, I.; Kaucka, M.; Barraud, P.; Otani, T.; Hunt, A.; Hartwig, A.C.; Sock, E.; Waithe, D.; Franck, M.C.M.; et al. Striking Parallels between Carotid Body Glomus Cell and Adrenal Chromaffin Cell Development. Dev. Biol. 2018, 444 (Suppl. S1), S308–S324. [Google Scholar] [CrossRef]
- Barry, M.A.; Frank, M.E. Response of the Gustatory System to Peripheral Nerve Injury. Exp. Neurol. 1992, 115, 60–64. [Google Scholar] [CrossRef]
- Fujimoto, S.; Murray, R.G. Fine Structure of Degeneration and Regeneration in Denervated Rabbit Vallate Taste Buds. Anat. Rec. 1970, 168, 393–413. [Google Scholar] [CrossRef]
- May, R.M. The Relation of Nerves to Degenerating and Regenerating Taste Buds. J. Exp. Zool. 1925, 42, 371–410. [Google Scholar] [CrossRef]
- Yaglov, V.V.; Mikhailyuk, I.A.; Yaglova, N.V. Biology of the Diffuse Endocrine Epithelial System; Symfoniya forte: Ivano-Frankivsk, Ukraine, 2013. (In Russian) [Google Scholar]
- Levitsky, K.L.; López-Barneo, J. Developmental Change of T-Type Ca2+ Channel Expression and Its Role in Rat Chromaffin Cell Responsiveness to Acute Hypoxia. J. Physiol. 2009, 587, 1917–1929. [Google Scholar] [CrossRef]
- Seidler, F.J.; Slotkin, T.A. Ontogeny of Adrenomedullary Responses to Hypoxia and Hypoglycemia: Role of Splanchnic Innervation. Brain Res. Bull. 1986, 16, 11–14. [Google Scholar] [CrossRef] [PubMed]
- Gosney, J.R. Adrenal Corticomedullary Hyperplasia in Hypobaric Hypoxia. J. Pathol. 1985, 146, 59–64. [Google Scholar] [CrossRef]
- McDonald, D.M.; Mitchell, R.A. The Neural Pathway Involved in “Efferent Inhibition” of Chemoreceptors in the Cat Carotid Body. J. Comp. Neurol. 1981, 201, 457–476. [Google Scholar] [CrossRef]
- Zavarzin, A.A. Selected Works, Vol. III: Essays on the Evolutionary Histology of the Nervous System; Academy of Sciences of the USSR: Moscow/Leningrad, USSR, 1950. (In Russian) [Google Scholar]
- Portbury, A.L.; Chandra, R.; Groelle, M.; McMillian, M.K.; Elias, A.; Herlong, J.R.; Rios, M.; Roffler-Tarlov, S.; Chikaraishi, D.M. Catecholamines Act via a β-Adrenergic Receptor to Maintain Fetal Heart Rate and Survival. Am. J. Physiol. Heart Circ. Physiol. 2003, 284, H2069–H2077. [Google Scholar] [CrossRef]
- Phillippe, M. Fetal Catecholamines. Am. J. Obstet. Gynecol. 1983, 146, 840–855. [Google Scholar] [CrossRef]
- Hoermann, H.; van Faassen, M.; Roeper, M.; Hagenbeck, C.; Herebian, D.; Muller Kobold, A.C.; Dukart, J.; Kema, I.P.; Mayatepek, E.; Meissner, T.; et al. Association of Fetal Catecholamines with Neonatal Hypoglycemia. J. Am. Med. Assoc. Pediatr. 2024, 178, 577–585. [Google Scholar] [CrossRef] [PubMed]
- Maruhashi, J.; Wright, E.B. Effect of Oxygen Lack on the Single Isolated Mammalian (Rat) Nerve Fiber. J. Neurophysiol. 1967, 30, 434–452. [Google Scholar] [CrossRef] [PubMed]
- Zapata, P.; Stensaas, L.J.; Eyzaguirre, C. Recovery of Chemosensory Function of Regenerating Carotid Nerve Fibers. In Chemoreception in the Carotid Body; Acker, H., Fidone, S., Pallot, D., Eyzaguirre, C., Lübbers, D.W., Torrance, R.W., Eds.; Springer: Berlin/Heidelberg, Germany, 1977; pp. 44–50. [Google Scholar] [CrossRef]
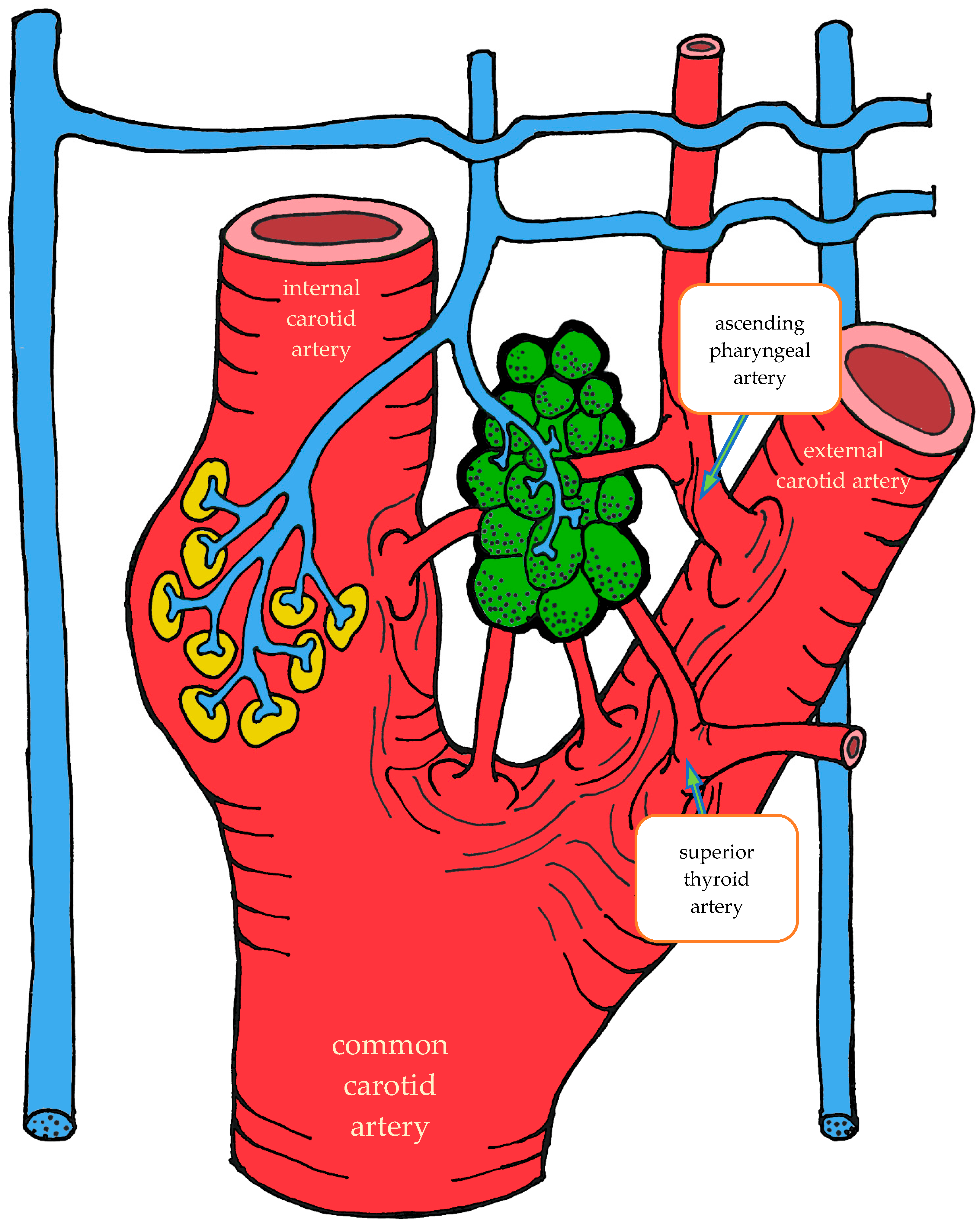

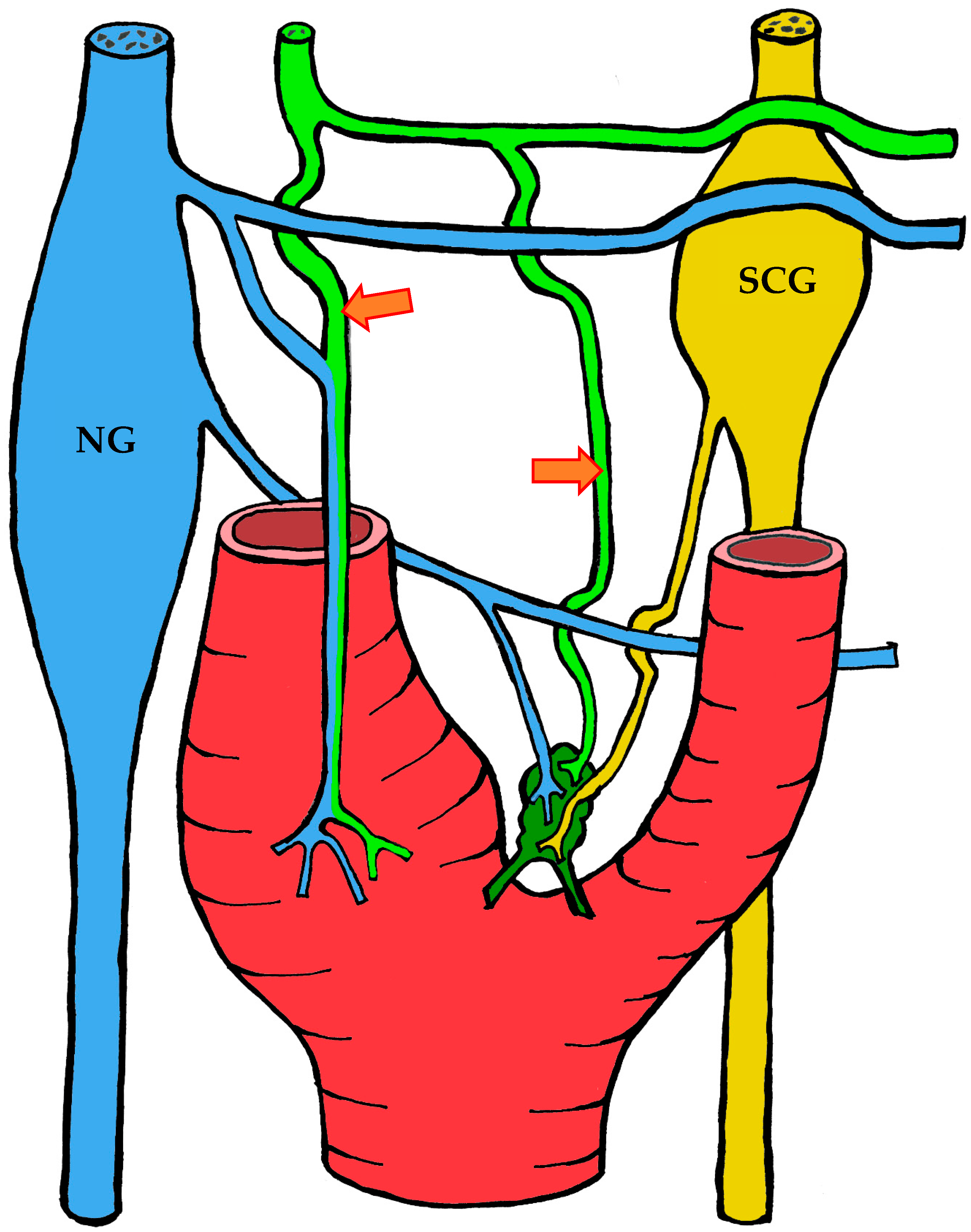
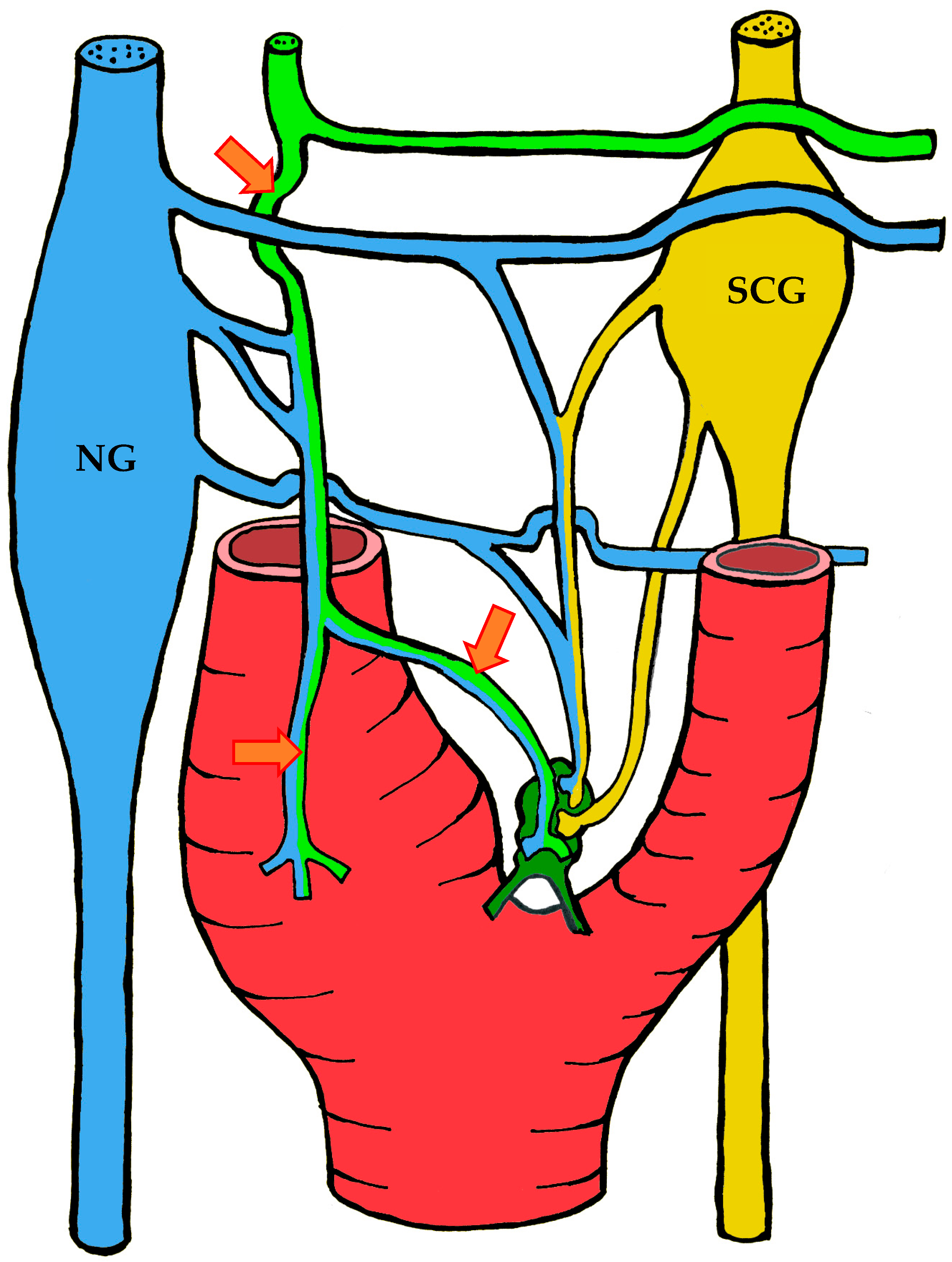


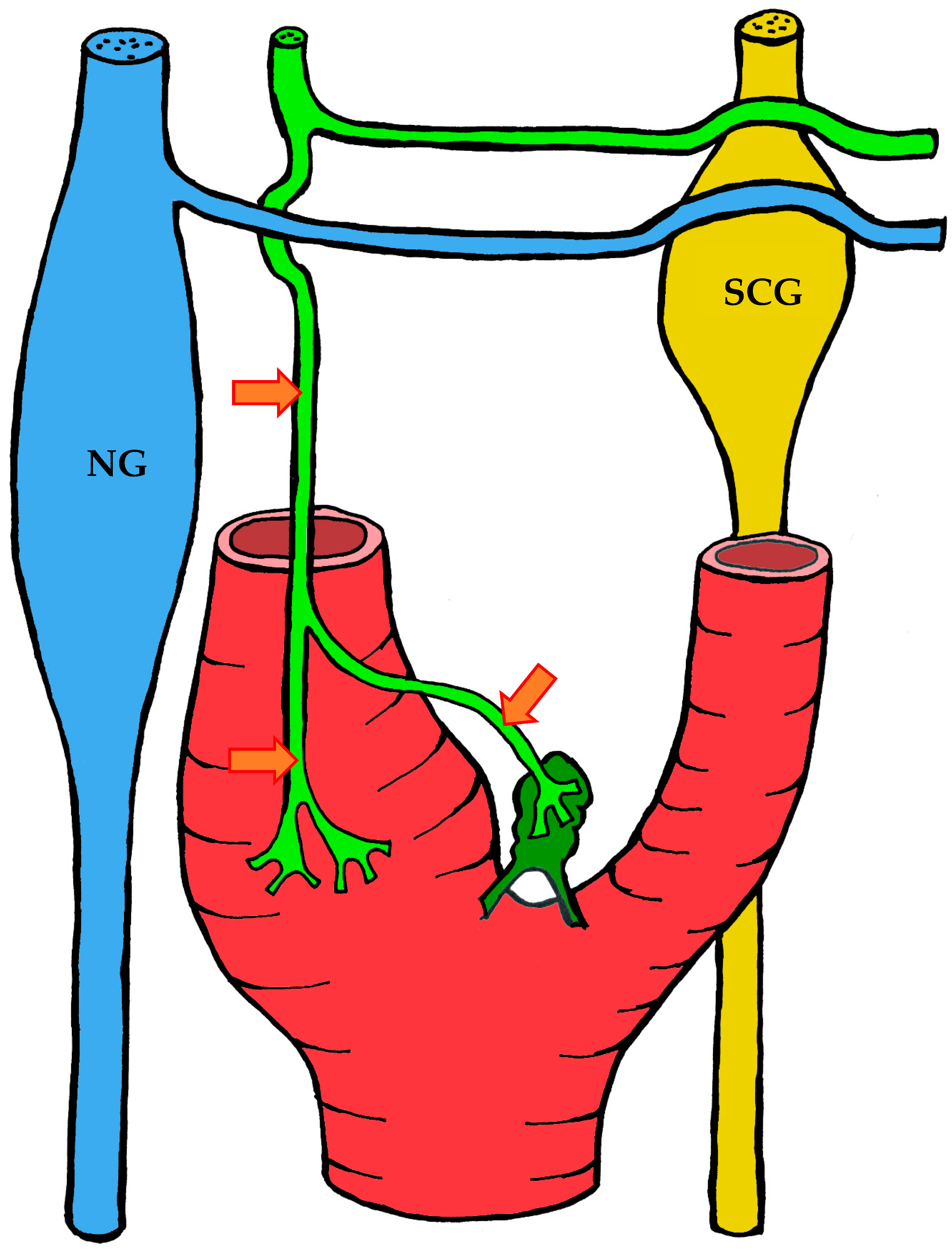
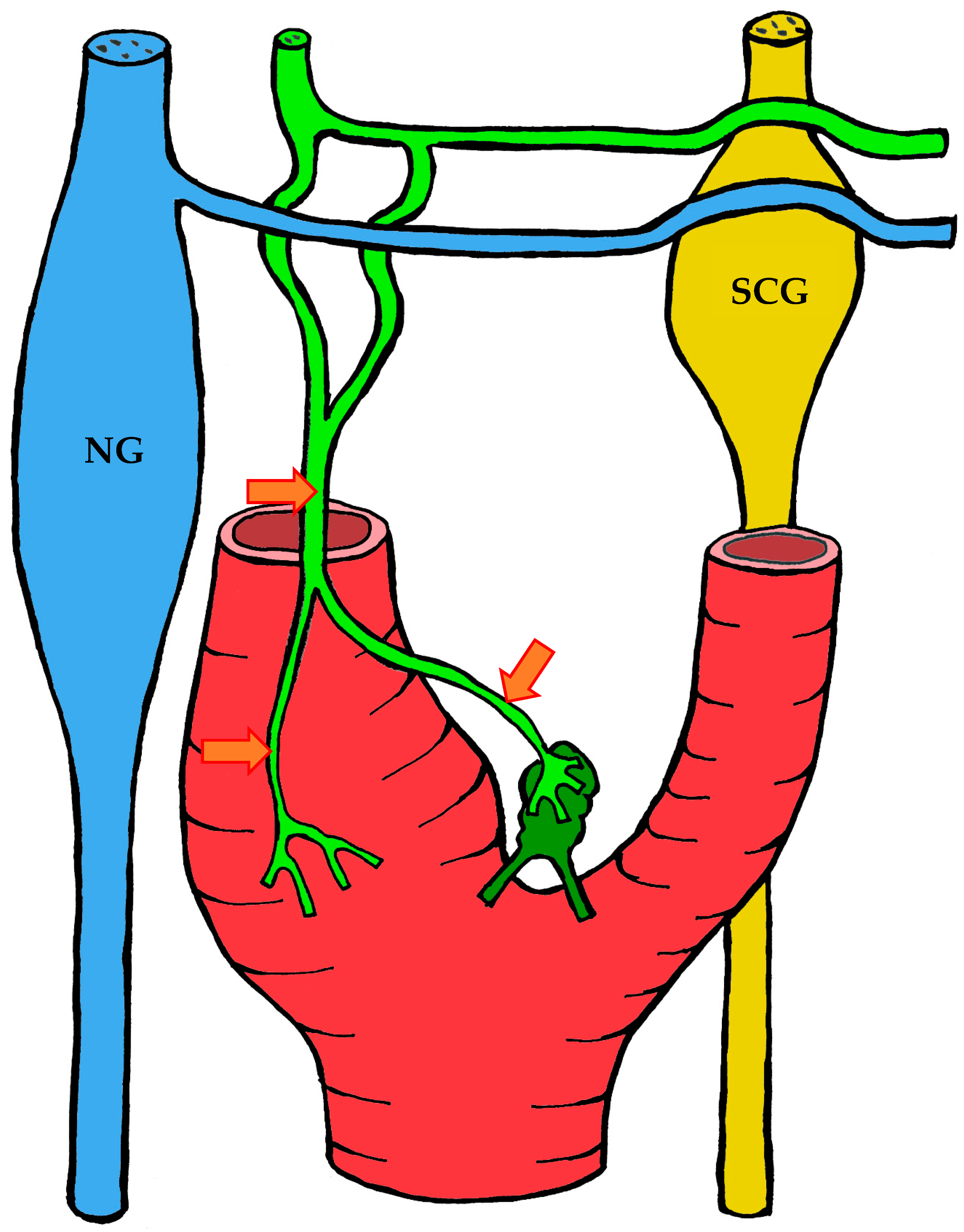
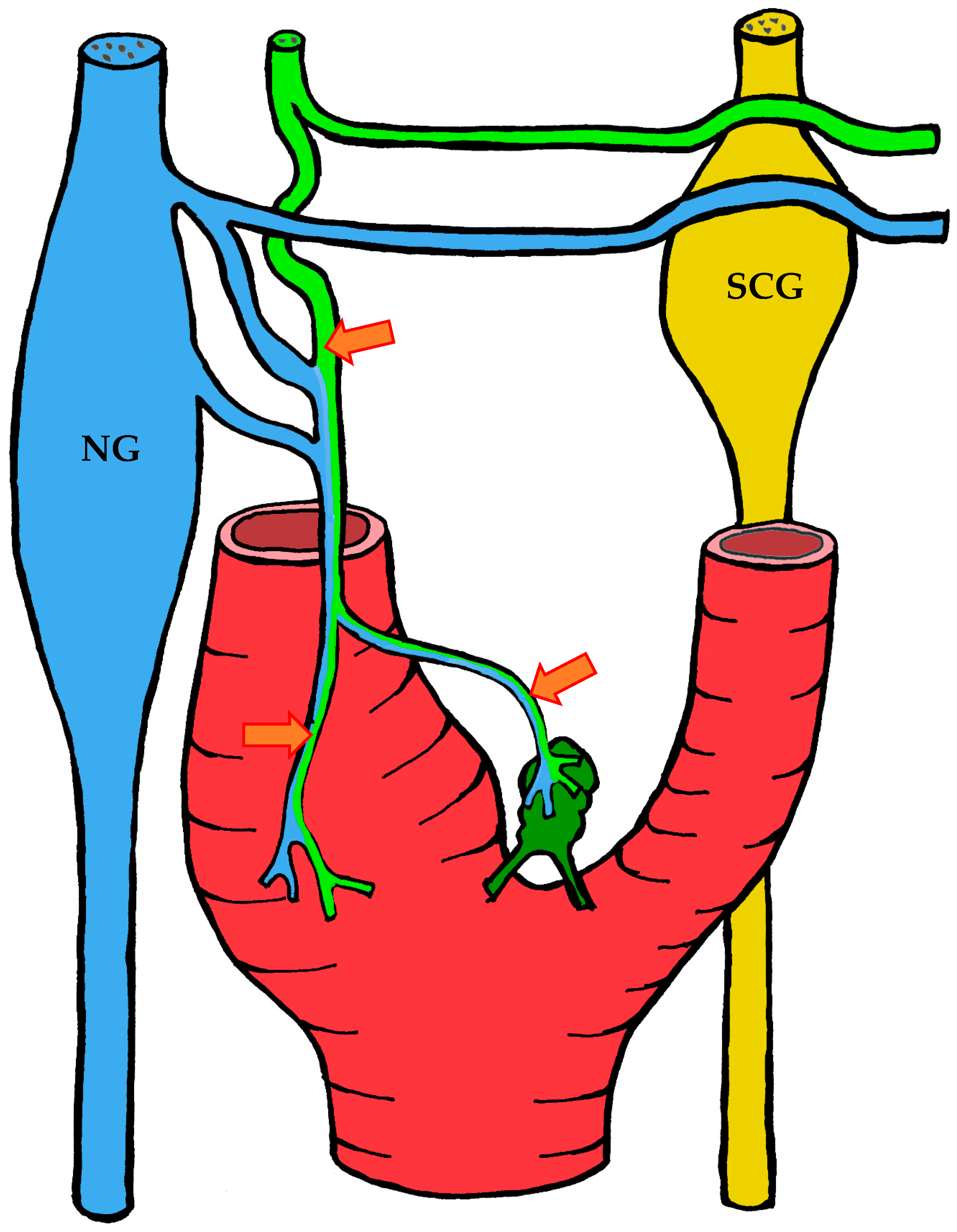
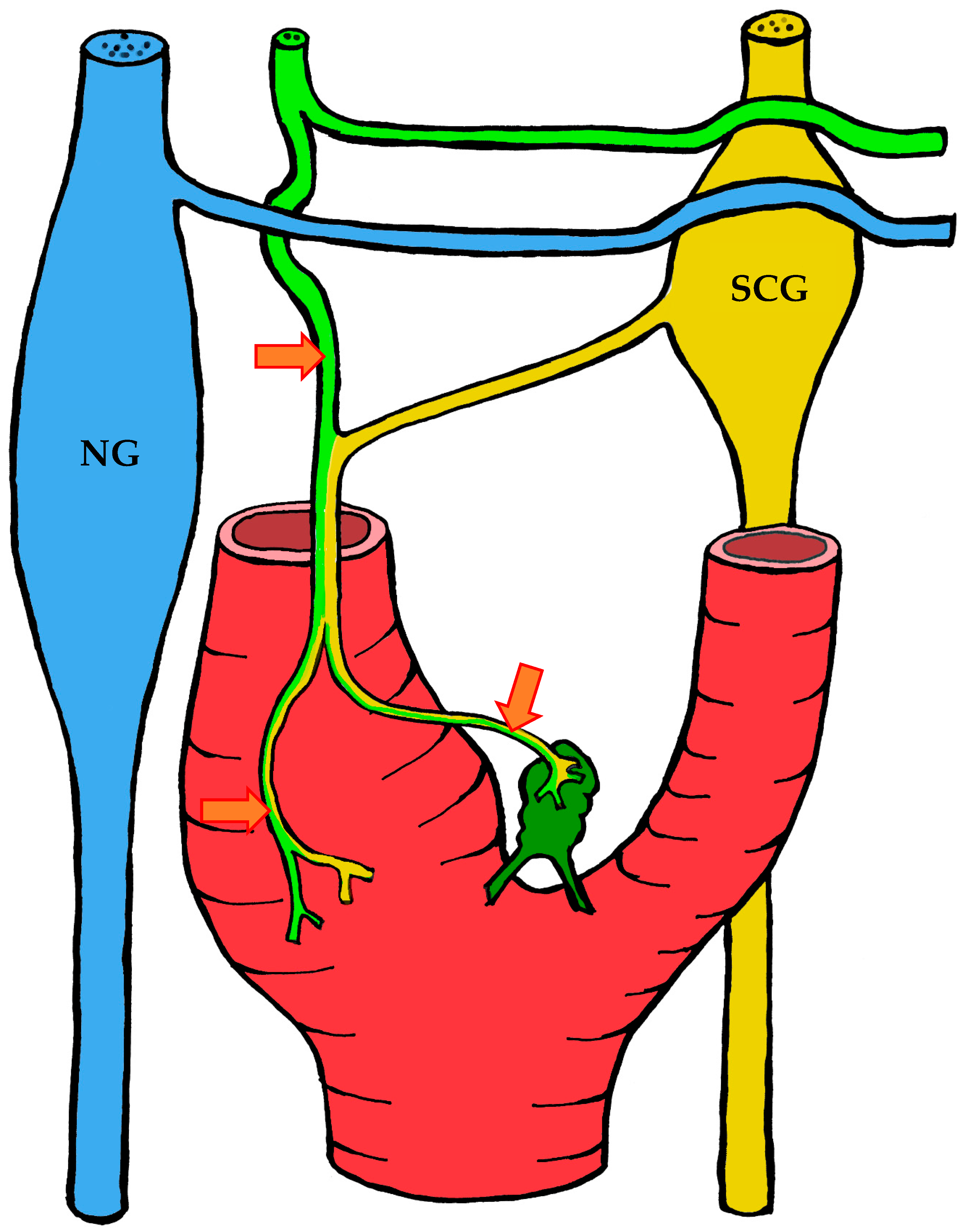


| Location | Right | Left | Both Sides |
|---|---|---|---|
| Carotid bifurcation | 87.2 | 87.2 | 87.2 |
| External carotid artery | 5.6 | 6.4 | 6.0 |
| Internal carotid artery | 5.2 | 3.6 | 4.4 |
| Ascending pharyngeal artery | 1.6 | 2.4 | 2.0 |
| Common carotid artery | 0.4 | 0.4 | 0.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Otlyga, D.; Otlyga, E.; Junemann, O.; Krivova, Y.; Saveliev, S. The Carotid Body as Part of a Unified Sympathoadrenal System of Neural Crest Derivatives: Insights from Two Centuries of Research. Int. J. Mol. Sci. 2025, 26, 11129. https://doi.org/10.3390/ijms262211129
Otlyga D, Otlyga E, Junemann O, Krivova Y, Saveliev S. The Carotid Body as Part of a Unified Sympathoadrenal System of Neural Crest Derivatives: Insights from Two Centuries of Research. International Journal of Molecular Sciences. 2025; 26(22):11129. https://doi.org/10.3390/ijms262211129
Chicago/Turabian StyleOtlyga, Dmitry, Ekaterina Otlyga, Olga Junemann, Yuliya Krivova, and Sergey Saveliev. 2025. "The Carotid Body as Part of a Unified Sympathoadrenal System of Neural Crest Derivatives: Insights from Two Centuries of Research" International Journal of Molecular Sciences 26, no. 22: 11129. https://doi.org/10.3390/ijms262211129
APA StyleOtlyga, D., Otlyga, E., Junemann, O., Krivova, Y., & Saveliev, S. (2025). The Carotid Body as Part of a Unified Sympathoadrenal System of Neural Crest Derivatives: Insights from Two Centuries of Research. International Journal of Molecular Sciences, 26(22), 11129. https://doi.org/10.3390/ijms262211129






