The Endocannabinoid System in Human Disease: Molecular Signaling, Receptor Pharmacology, and Therapeutic Innovation
Abstract
1. Introduction to the Endocannabinoid System and Its Integrative Role
1.1. Overview of the Endocannabinoid System (ECS)
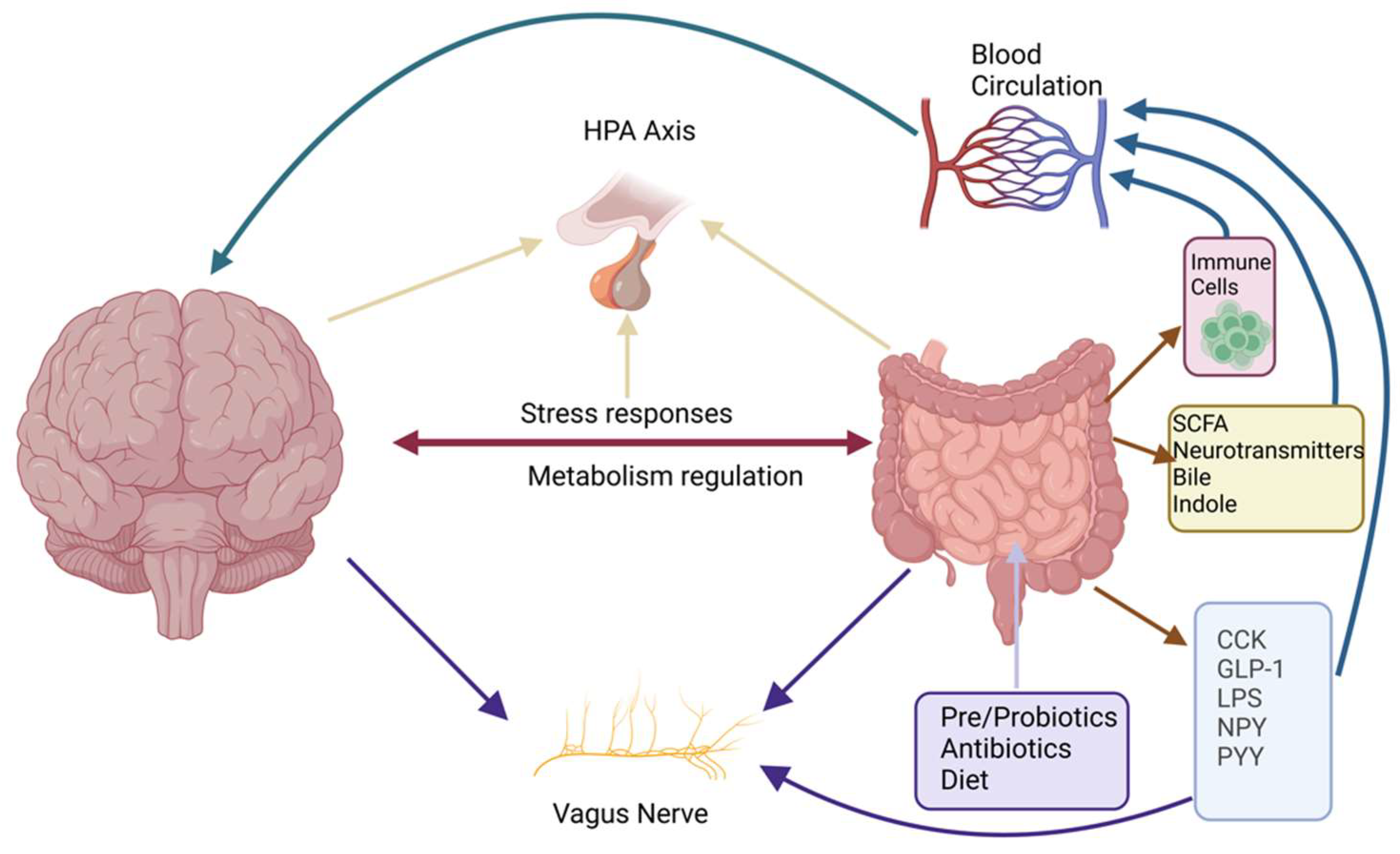
1.2. The Triad of Neuroscience, Microbiota, and Immunology
1.3. Objectives of This Review
2. Cannabinoid Receptors: Convergence Point of the Triad
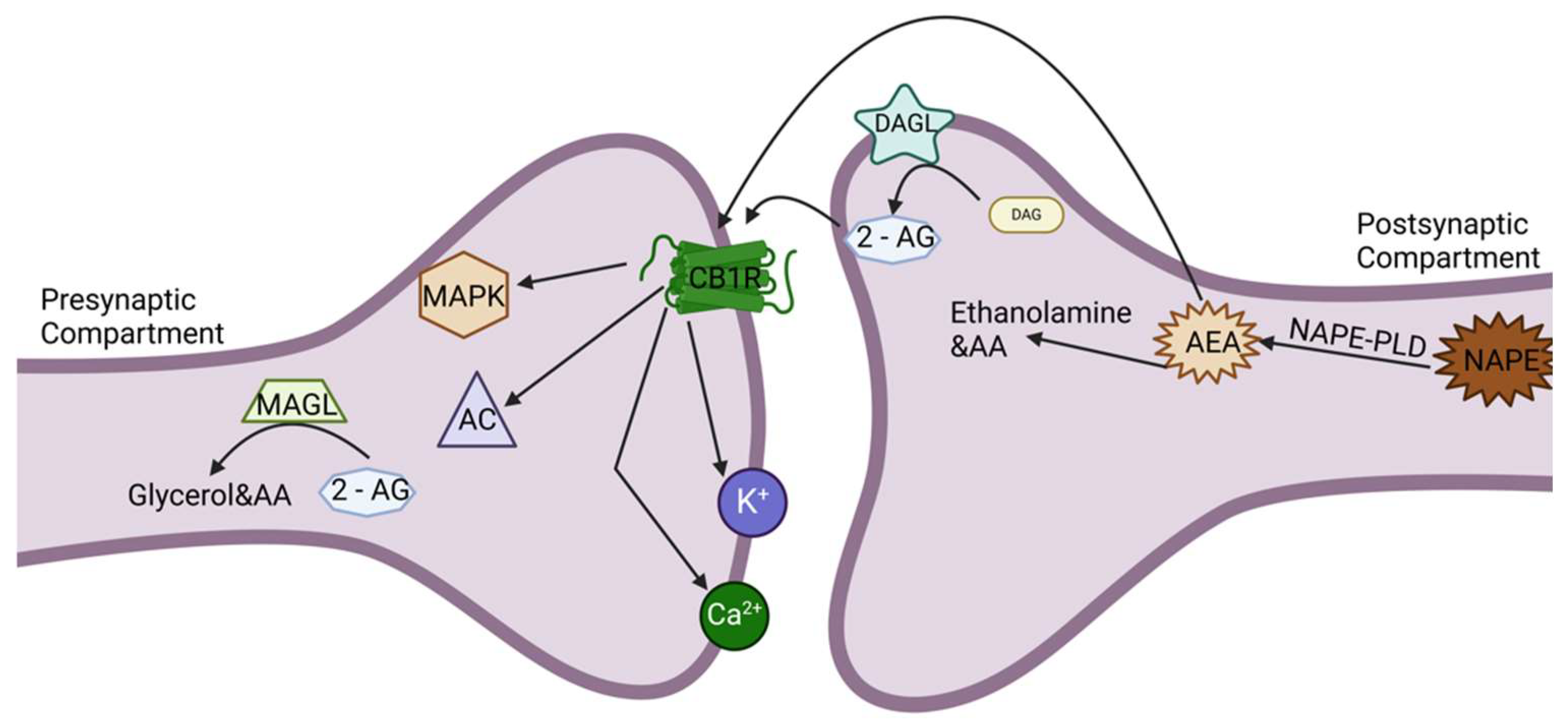
2.1. Cannabinoid Receptor Type 1 (CB1R)
2.2. Cannabinoid Receptor Type 2 (CB2R)
2.3. Putative Cannabinoid-Related Receptors
2.4. Receptor Localization in Microbiota and Gut Barrier Function
3. Endocannabinoid Signaling in the Neuroscience–Microbiota–Immunology Axis
3.1. Mechanisms of ECS Signaling
3.1.1. Retrograde Signaling in the CNS and the Gut
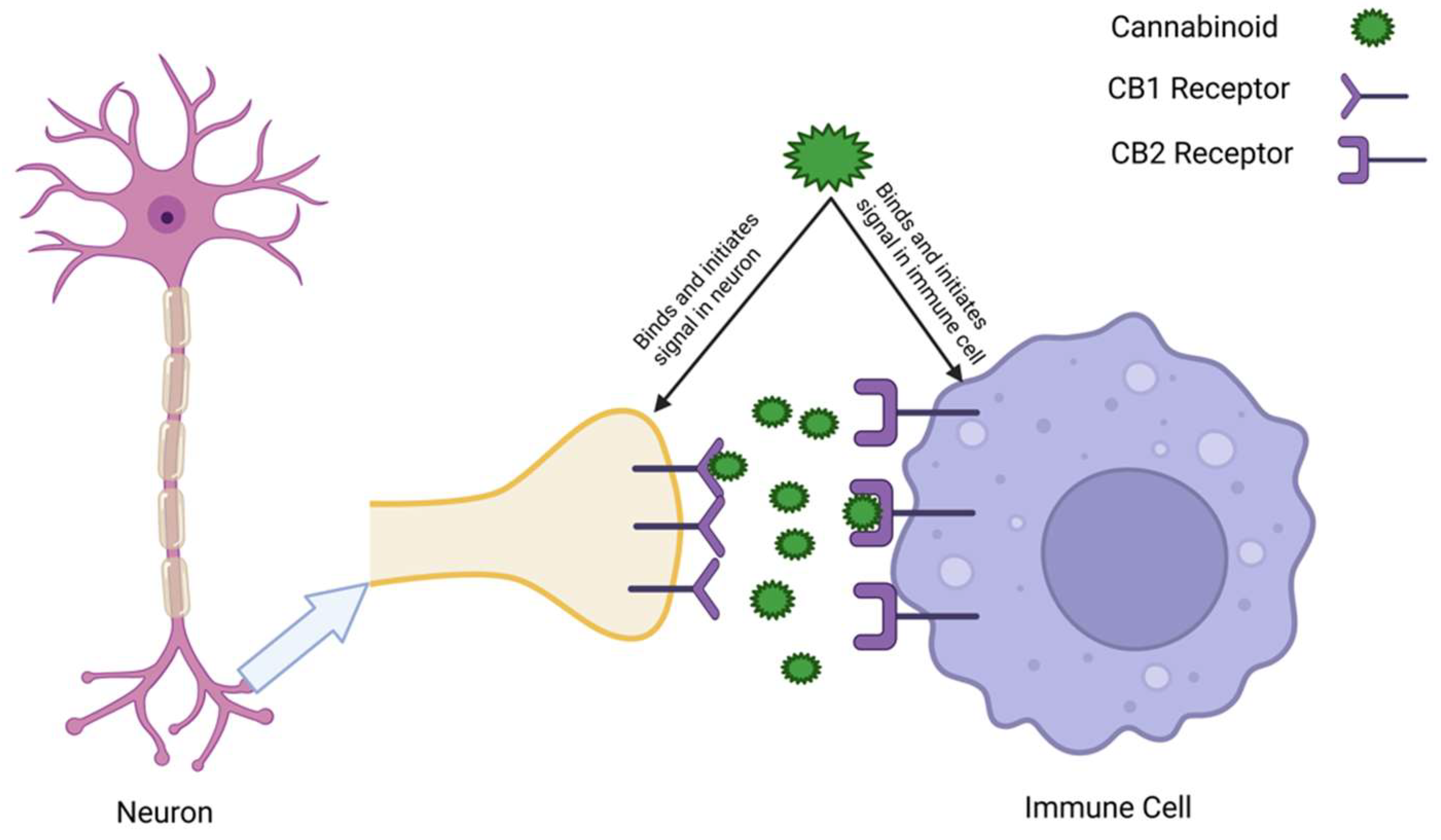
3.1.2. Autocrine and Paracrine Signaling in Immune Cells
3.1.3. ECS and the HPA Axis
3.2. Signal Transduction Pathways in ECS Signaling
3.2.1. G-Protein-Coupled Receptor (GPCR) Signaling
3.2.2. ECS Integration in Lipid Signaling
3.3. ECS Dysregulation and Disease States
4. Neuroscience: The ECS at the Intersection of the Microbiota and Immunology
4.1. The ECS in Neurodevelopment and Synaptic Plasticity
4.2. The ECS in Neuroinflammation and Neurodegeneration
4.3. Psychiatric Disorders and the Gut–Brain Axis
4.4. Emerging Therapeutic Frontiers
5. Microbiota: The ECS as a Mediator of Gut–Brain–Immune Communication
5.1. Influence of Gut Microbiota on ECS Activity
5.2. ECS Regulation of Gut Barrier Function
5.3. ECS and Probiotic/Prebiotic Interventions
5.4. Novel Mechanisms in Microbiota–ECS Interactions
6. Immunology: ECS-Modulated Immune Responses in the CNS and the Gut
6.1. The ECS in Immune Cell Regulation
6.2. The ECS in Autoimmune and Inflammatory Diseases
6.3. Role in Tumor Immunology
6.4. Future Technologies: Advancing ECS Research
6.4.1. Artificial Intelligence in ECS Drug Discovery
6.4.2. Multi-Omics and AI in ECS Research
6.4.3. Biotechnology and Engineered Solutions
7. Genetics and Epigenetics: Shaping ECS Function in the Triad
8. Translational Applications of ECS-Targeted Therapies
9. The ECS as a Central Integrator in Health and Disease
10. Conclusions: The ECS as a Central Regulator of Systemic Homeostasis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviation
| 2-AG | 2-Arachidonoylglycerol |
| AA | Arachidonic Acid |
| AC | Adenylyl Cyclase |
| AD | Alzheimer’s Disease |
| AEA | Anandamide |
| AI | Artificial Intelligence |
| AI-Enhanced | Artificial-Intelligence-Enhanced |
| ALS | Amyotrophic Lateral Sclerosis |
| BBB | Blood–Brain Barrier |
| BDNF | Brain-Derived Neurotrophic Factor |
| CB1 | Cannabinoid Receptor Type 1 |
| CB2 | Cannabinoid Receptor Type 2 |
| CBD | Cannabidiol |
| CCK | Cholecystokinin |
| CNR1/CNR2 | Cannabinoid Receptor Genes 1 and 2 |
| CNS | Central Nervous System |
| CRH | Corticotropin-Releasing Hormone |
| CRISPR/Cas9 | Clustered Regularly Interspaced Short Palindromic Repeats/CRISPR-Associated Protein 9 |
| CRISPR-Cas9 RNPs | CRISPR Ribonucleoproteins |
| cAMP | Cyclic Adenosine Monophosphate |
| DAG | Diacylglycerol |
| DAGL | Diacylglycerol Lipase |
| E/I | Excitation/Inhibition |
| ECS | Endocannabinoid System |
| EHC | Endocannabinoid Homeostasis Context |
| EMR | Electromagnetic Radiation |
| ERK1/2 | Extracellular Signal-Regulated Kinase 1/2 |
| FAAH | Fatty Acid Amide Hydrolase |
| FAAH-OUT | FAAH-Associated Noncoding RNA Gene |
| GALT | Gut-Associated Lymphoid Tissue |
| GI | Gastrointestinal |
| GLP-1 | Glucagon-Like Peptide 1 |
| GPCR | G-Protein-Coupled Receptor |
| GPR18 | G-Protein-Coupled Receptor 18 |
| GPR55 | G-Protein-Coupled Receptor 55 |
| HPA | Hypothalamic–Pituitary–Adrenal (Axis) |
| HDAC | Histone Deacetylase |
| IBD | Inflammatory Bowel Disease |
| IRAK1 | Interleukin-1 Receptor-Associated Kinase 1 |
| JNK | c-Jun N-Terminal Kinase |
| LPS | Lipopolysaccharide |
| LTD | Long-Term Depression |
| LTP | Long-Term Potentiation |
| MAGL | Monoacylglycerol Lipase |
| MAPK | Mitogen-Activated Protein Kinase |
| miRNA/miR- | MicroRNA (e.g., miR-146a, miR-155) |
| ML | Machine Learning |
| MS | Multiple Sclerosis |
| NAPE | N-Arachidonoyl-Phosphatidylethanolamine |
| NAPE-PLD | NAPE-Specific Phospholipase D |
| NF-κB | Nuclear Factor Kappa-Light-Chain-Enhancer of Activated B Cells |
| NPY | Neuropeptide Y |
| PD | Parkinson’s Disease |
| PKA | Protein Kinase A |
| PTSD | Post-Traumatic Stress Disorder |
| PYY | Peptide YY |
| SCFA | Short-Chain Fatty Acid |
| SLE | Systemic Lupus Erythematosus |
| SPM | Specialized Pro-Resolving Mediator |
| THC | Δ9-Tetrahydrocannabinol |
| TRAF6 | TNF Receptor-Associated Factor 6 |
| TRPV | Transient Receptor Potential Vanilloid (channel family) |
| TRPV1 | Transient Receptor Potential Vanilloid Type 1 |
| VGCCs | Voltage-Gated Calcium Channels |
References
- Ernst, J.; Grabiec, U.; Falk, K.; Dehghani, F.; Schaedlich, K. The Endocrine Disruptor DEHP and the ECS: Analysis of a Possible Crosstalk. Endocr. Connect. 2020, 9, 101–110. [Google Scholar] [CrossRef]
- Ibsen, M.S.; Finlay, D.B.; Patel, M.; Javitch, J.A.; Glass, M.; Grimsey, N.L. Cannabinoid CB1 and CB2 Receptor-Mediated Arrestin Translocation: Species, Subtype, and Agonist-Dependence. Front. Pharmacol. 2019, 10, 350. [Google Scholar] [CrossRef]
- Kosar, M.; Sarott, R.C.; Sykes, D.A.; Viray, A.E.G.; Vitale, R.M.; Tomašević, N.; Li, X.; Ganzoni, R.L.Z.; Kicin, B.; Reichert, L.; et al. Flipping the GPCR Switch: Structure-Based Development of Selective Cannabinoid Receptor 2 Inverse Agonists. ACS Cent. Sci. 2024, 10, 956–968. [Google Scholar] [CrossRef] [PubMed]
- Akimov, M.G.; Gamisonia, A.M.; Dudina, P.V.; Gretskaya, N.M.; Gaydaryova, A.A.; Kuznetsov, A.S.; Zinchenko, G.N.; Bezuglov, V.V. GPR55 Receptor Activation by the N-Acyl Dopamine Family Lipids Induces Apoptosis in Cancer Cells via the Nitric Oxide Synthase (nNOS) Over-Stimulation. Int. J. Mol. Sci. 2021, 22, 622. [Google Scholar] [CrossRef] [PubMed]
- Farah, S.I.; Hilston, S.; Tran, N.; Zvonok, N.; Makriyannis, A. 1-, 2- and 3-AG as Substrates of the Endocannabinoid Enzymes and Endogenous Ligands of the Cannabinoid Receptor 1. Biochem. Biophys. Res. Commun. 2022, 591, 31–36. [Google Scholar] [CrossRef]
- Simard, M.; Archambault, A.-S.; Lavoie, J.-P.C.; Dumais, É.; Di Marzo, V.; Flamand, N. Biosynthesis and Metabolism of Endocannabinoids and Their Congeners from the Monoacylglycerol and N-Acyl-Ethanolamine Families. Biochem. Pharmacol. 2022, 205, 115261. [Google Scholar] [CrossRef]
- Fagundo, A.B.; de la Torre, R.; Jiménez-Murcia, S.; Agüera, Z.; Pastor, A.; Casanueva, F.F.; Granero, R.; Baños, R.; Botella, C.; del Pino-Gutierrez, A.; et al. Modulation of the Endocannabinoids N-Arachidonoylethanolamine (AEA) and 2-Arachidonoylglycerol (2-AG) on Executive Functions in Humans. PLoS ONE 2013, 8, e66387. [Google Scholar] [CrossRef] [PubMed]
- Guida, M.; Ligresti, A.; De Filippis, D.; D’Amico, A.; Petrosino, S.; Cipriano, M.; Bifulco, G.; Simonetti, S.; Orlando, P.; Insabato, L.; et al. The Levels of the Endocannabinoid Receptor CB2 and Its Ligand 2-Arachidonoylglycerol Are Elevated in Endometrial Carcinoma. Endocrinology 2010, 151, 921–928. [Google Scholar] [CrossRef]
- Demaili, A.; Portugalov, A.; Maroun, M.; Akirav, I.; Braun, K.; Bock, J. Early Life Stress Induces Decreased Expression of CB1R and FAAH and Epigenetic Changes in the Medial Prefrontal Cortex of Male Rats. Front. Cell. Neurosci. 2024, 18, 1474992. [Google Scholar] [CrossRef]
- Jacobson, A.; Yang, D.; Vella, M.; Chiu, I.M. The Intestinal Neuro-Immune Axis: Crosstalk between Neurons, Immune Cells, and Microbes. Mucosal Immunol. 2021, 14, 555–565. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, T.; Meng, F.; Jiang, M.; Wu, S.; Xu, H. The Endocannabinoid System in the Brain Undergoes Long-Lasting Changes Following Neuropathic Pain. iScience 2024, 27, 111409. [Google Scholar] [CrossRef]
- Park, Y.; Watkins, B.A. Endocannabinoids and Aging-Inflammation, Neuroplasticity, Mood and Pain. Vitam. Horm. 2021, 115, 129–172. [Google Scholar] [CrossRef]
- Liu, Y.; Xing, H.; Zhang, Y.; Song, Y. The Endocannabinoid System in Alzheimer’s Disease: A Network Meta-Analysis. J. Neurosci. Res. 2024, 102, e25380. [Google Scholar] [CrossRef] [PubMed]
- Paloczi, J.; Varga, Z.V.; Hasko, G.; Pacher, P. Neuroprotection in Oxidative Stress-Related Neurodegenerative Diseases: Role of Endocannabinoid System Modulation. Antioxid. Redox Signal. 2018, 29, 75. [Google Scholar] [CrossRef]
- Wildsmith, K.R.; Holley, M.; Savage, J.C.; Skerrett, R.; Landreth, G.E. Evidence for Impaired Amyloid β Clearance in Alzheimer’s Disease. Alzheimers Res. Ther. 2013, 5, 33. [Google Scholar] [CrossRef] [PubMed]
- Steel, R.W.J.; Miller, J.H.; Sim, D.A.; Day, D.J. Delta-9-Tetrahydrocannabinol Disrupts Hippocampal Neuroplasticity and Neurogenesis in Trained, but Not Untrained Adolescent Sprague-Dawley Rats. Brain Res. 2014, 1548, 12–19. [Google Scholar] [CrossRef]
- Meunier, C.N.J.; Chameau, P.; Fossier, P.M. Modulation of Synaptic Plasticity in the Cortex Needs to Understand All the Players. Front. Synaptic Neurosci. 2017, 9, 2. [Google Scholar] [CrossRef] [PubMed]
- Rusconi, F.; Rubino, T.; Battaglioli, E. Endocannabinoid-Epigenetic Cross-Talk: A Bridge toward Stress Coping. Int. J. Mol. Sci. 2020, 21, 6252. [Google Scholar] [CrossRef]
- Micale, V.; Drago, F. Endocannabinoid System, Stress and HPA Axis. Eur. J. Pharmacol. 2018, 834, 230–239. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, Y.; Zhao, X.; Shang, C.; Xiang, M.; Li, L.; Cui, X. Microbiota-Derived Short-Chain Fatty Acids: Implications for Cardiovascular and Metabolic Disease. Front. Cardiovasc. Med. 2022, 9, 900381. [Google Scholar] [CrossRef]
- Forsythe, P.; Kunze, W.; Bienenstock, J. Moody Microbes or Fecal Phrenology: What Do We Know about the Microbiota-Gut-Brain Axis? BMC Med. 2016, 14, 58. [Google Scholar] [CrossRef] [PubMed]
- Mousa, W.K.; Chehadeh, F.; Husband, S. Microbial Dysbiosis in the Gut Drives Systemic Autoimmune Diseases. Front. Immunol. 2022, 13, 906258. [Google Scholar] [CrossRef]
- Sharkey, K.A.; Wiley, J.W. The Role of the Endocannabinoid System in the Brain-Gut Axis. Gastroenterology 2016, 151, 252. [Google Scholar] [CrossRef]
- Parada Venegas, D.; De la Fuente, M.K.; Landskron, G.; González, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.M.; Faber, K.N.; Hermoso, M.A. Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Front. Immunol. 2019, 10, 277, Erratum in Front Immunol. 2019, 10, 1486. [Google Scholar] [CrossRef]
- Suryavanshi, S.V.; Zaiachuk, M.; Pryimak, N.; Kovalchuk, I.; Kovalchuk, O. Cannabinoids Alleviate the LPS-Induced Cytokine Storm via Attenuating NLRP3 Inflammasome Signaling and TYK2-Mediated STAT3 Signaling Pathways In Vitro. Cells 2022, 11, 1391. [Google Scholar] [CrossRef]
- Reynoso-Moreno, I.; Tietz, S.; Vallini, E.; Engelhardt, B.; Gertsch, J.; Chicca, A. Selective Endocannabinoid Reuptake Inhibitor WOBE437 Reduces Disease Progression in a Mouse Model of Multiple Sclerosis. ACS Pharmacol. Transl. Sci. 2021, 4, 765–779. [Google Scholar] [CrossRef]
- Katchan, V.; David, P.; Shoenfeld, Y. Cannabinoids and Autoimmune Diseases: A Systematic Review. Autoimmun. Rev. 2016, 15, 513–528. [Google Scholar] [CrossRef]
- Lehmann, C.; Burkovskiy, I.; Kuethe, J.; Zhou, J.; Caldwell, C.; Kelly, M.E.M. Inhibition of the Cannabinoid 2 Receptor in CNS-Injury Induced Immunodeficiency Syndrome. Med. Hypotheses 2014, 82, 736–739. [Google Scholar] [CrossRef]
- Guillot, A.; Hamdaoui, N.; Bizy, A.; Zoltani, K.; Souktani, R.; Zafrani, E.-S.; Mallat, A.; Lotersztajn, S.; Lafdil, F. Cannabinoid Receptor 2 Counteracts Interleukin-17-Induced Immune and Fibrogenic Responses in Mouse Liver. Hepatology 2014, 59, 296. [Google Scholar] [CrossRef] [PubMed]
- Britzen-Laurent, N.; Weidinger, C.; Stürzl, M. Contribution of Blood Vessel Activation, Remodeling and Barrier Function to Inflammatory Bowel Diseases. Int. J. Mol. Sci. 2023, 24, 5517. [Google Scholar] [CrossRef] [PubMed]
- Perisetti, A.; Rimu, A.H.; Khan, S.A.; Bansal, P.; Goyal, H. Role of Cannabis in Inflammatory Bowel Diseases. Ann. Gastroenterol. 2020, 33, 134. [Google Scholar] [CrossRef]
- Miranda, K.; Mehrpouya-Bahrami, P.; Nagarkatti, P.S.; Nagarkatti, M. Cannabinoid Receptor 1 Blockade Attenuates Obesity and Adipose Tissue Type 1 Inflammation Through miR-30e-5p Regulation of Delta-Like-4 in Macrophages and Consequently Downregulation of Th1 Cells. Front. Immunol. 2019, 10, 1049. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, B.L.F. The Role of CB1 in Immune Modulation by Cannabinoids. Pharmacol. Ther. 2013, 137, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Vučković, S.; Srebro, D.; Vujović, K.S.; Vučetić, Č.; Prostran, M. Cannabinoids and Pain: New Insights From Old Molecules. Front. Pharmacol. 2018, 9, 1259. [Google Scholar] [CrossRef] [PubMed]
- Starowicz, K.; Malek, N.; Przewlocka, B. Cannabinoid Receptors and Pain. Wiley Interdiscip. Rev. Membr. Transp. Signal. 2013, 2, 121–132. [Google Scholar] [CrossRef]
- Bagot, R.C.; Labonté, B.; Peña, C.J.; Nestler, E.J. Epigenetic Signaling in Psychiatric Disorders: Stress and Depression. Dialogues Clin. Neurosci. 2014, 16, 281–295. [Google Scholar] [CrossRef]
- Jia, Y.; Qi, D.; Wang, T.; Zhang, Y.; Chen, X.; Deng, H.; Meng, D. The Role of Pre-Onset Hair Hormone in Predicting the Prognosis of Patients with Severe Pneumonia and Acute COVID-19 Outbreak. Heliyon 2024, 10, e30636. [Google Scholar] [CrossRef]
- Naidu, S.A.G.; Wallace, T.C.; Davies, K.J.A.; Naidu, A.S. Lactoferrin for Mental Health: Neuro-Redox Regulation and Neuroprotective Effects across the Blood-Brain Barrier with Special Reference to Neuro-COVID-19. J. Diet. Suppl. 2023, 20, 218–253. [Google Scholar] [CrossRef]
- Ertl, N.; Freeman, T.P.; Mokrysz, C.; Ofori, S.; Borissova, A.; Petrilli, K.; Curran, H.V.; Lawn, W.; Wall, M.B. Acute Effects of Different Types of Cannabis on Young Adult and Adolescent Resting-State Brain Networks. Neuropsychopharmacology 2024, 49, 1640–1651. [Google Scholar] [CrossRef]
- Gao, K.; Mu, C.; Farzi, A.; Zhu, W. Tryptophan Metabolism: A Link Between the Gut Microbiota and Brain. Adv. Nutr. 2020, 11, 709–723. [Google Scholar] [CrossRef]
- Herman, J.P.; McKlveen, J.M.; Ghosal, S.; Kopp, B.; Wulsin, A.; Makinson, R.; Scheimann, J.; Myers, B. Regulation of the Hypothalamic-Pituitary-Adrenocortical Stress Response. Compr. Physiol. 2016, 6, 603–621. [Google Scholar] [CrossRef]
- Giselle, C.; Wong, M.S.C.; Johanna, M.; Montgomery, P.H.D.; Michael, W.; Taylor, P.H.D. The Gut-Microbiota-Brain Axis in Autism Spectrum Disorder. Exon Publ. 2021, 95–113. [Google Scholar] [CrossRef]
- Frerichs, N.M.; de Meij, T.G.J.; Niemarkt, H.J. Microbiome and Its Impact on Fetal and Neonatal Brain Development: Current Opinion in Pediatrics. Curr. Opin. Clin. Nutr. Metab. Care 2024, 27, 297. [Google Scholar] [CrossRef]
- Fakhfouri, G.; Mijailović, N.R.; Rahimian, R. Psychiatric Comorbidities of Inflammatory Bowel Disease: It Is a Matter of Microglia’s Gut Feeling. Cells 2024, 13, 177. [Google Scholar] [CrossRef]
- Vuic, B.; Milos, T.; Tudor, L.; Konjevod, M.; Nikolac Perkovic, M.; Jazvinscak Jembrek, M.; Nedic Erjavec, G.; Svob Strac, D. Cannabinoid CB2 Receptors in Neurodegenerative Proteinopathies: New Insights and Therapeutic Potential. Biomedicines 2022, 10, 3000. [Google Scholar] [CrossRef]
- Wu, K.; Liu, Y.; Shao, S.; Song, W.; Chen, X.; Dong, Y.; Zhang, Y. The Microglial Innate Immune Receptors TREM-1 and TREM-2 in the Anterior Cingulate Cortex (ACC) Drive Visceral Hypersensitivity and Depressive-like Behaviors Following DSS-Induced Colitis. Brain. Behav. Immun. 2023, 112, 96–117. [Google Scholar] [CrossRef]
- Tudorancea, I.M.; Ciorpac, M.; Stanciu, G.D.; Caratașu, C.; Săcărescu, A.; Ignat, B.; Burlui, A.; Rezuș, E.; Creangă, I.; Alexa-Stratulat, T.; et al. The Therapeutic Potential of the Endocannabinoid System in Age-Related Diseases. Biomedicines 2022, 10, 2492. [Google Scholar] [CrossRef] [PubMed]
- Boczek, T.; Zylinska, L. Receptor-Dependent and Independent Regulation of Voltage-Gated Ca2+ Channels and Ca2+-Permeable Channels by Endocannabinoids in the Brain. Int. J. Mol. Sci. 2021, 22, 8168. [Google Scholar] [CrossRef] [PubMed]
- Maroto, I.B.; Costas-Insua, C.; Berthoux, C.; Moreno, E.; Ruiz-Calvo, A.; Montero-Fernández, C.; Macías-Camero, A.; Martín, R.; García-Font, N.; Sánchez-Prieto, J.; et al. Control of a Hippocampal Recurrent Excitatory Circuit by Cannabinoid Receptor-Interacting Protein Gap43. Nat. Commun. 2023, 14, 2303. [Google Scholar] [CrossRef] [PubMed]
- Aguiar, A.F.L.; Campos, R.M.P.; Isaac, A.R.; Paes-Colli, Y.; Carvalho, V.M.; Sampaio, L.S.; de Melo Reis, R.A. Long-Term Treatment with Cannabidiol-Enriched Cannabis Extract Induces Synaptic Changes in the Adolescent Rat Hippocampus. Int. J. Mol. Sci. 2023, 24, 11775. [Google Scholar] [CrossRef]
- Azarfarin, M.; Ghadiri, T.; Dadkhah, M.; Sahab-Negah, S. The Interaction between Cannabinoids and Long-Term Synaptic Plasticity: A Survey on Memory Formation and Underlying Mechanisms. Cell Biochem. Funct. 2024, 42, e4100. [Google Scholar] [CrossRef]
- Elrassas, H.H.; Elsayed, Y.A.R.; Abdeen, M.S.; Mohamed, A.T.; El Nagar, Z.M. Synthetic Cannabinoids Impact on Cognitive Functions. Egypt. J. Neurol. Psychiatry Neurosurg. 2023, 59, 151. [Google Scholar] [CrossRef]
- Crowley, K.; Kiraga, Ł.; Miszczuk, E.; Skiba, S.; Banach, J.; Latek, U.; Mendel, M.; Chłopecka, M. Effects of Cannabinoids on Intestinal Motility, Barrier Permeability, and Therapeutic Potential in Gastrointestinal Diseases. Int. J. Mol. Sci. 2024, 25, 6682. [Google Scholar] [CrossRef]
- Komorowska-Müller, J.A.; Schmöle, A.-C. CB2 Receptor in Microglia: The Guardian of Self-Control. Int. J. Mol. Sci. 2021, 22, 19. [Google Scholar] [CrossRef]
- Rakotoarivelo, V.; Mayer, T.Z.; Simard, M.; Flamand, N.; Di Marzo, V. The Impact of the CB2 Cannabinoid Receptor in Inflammatory Diseases: An Update. Molecules 2024, 29, 3381. [Google Scholar] [CrossRef]
- Kibret, B.G.; Roberts, A.; Kneebone, A.; Embaby, S.; Fernandez, J.; Liu, Q.-R.; Onaivi, E.S. Cannabinoid CB2 Receptors Modulate Alcohol Induced Behavior, and Neuro-Immune Dysregulation in Mice. Behav. Brain Res. 2023, 448, 114439. [Google Scholar] [CrossRef]
- Bietar, B.; Tanner, S.; Lehmann, C. Neuroprotection and Beyond: The Central Role of CB1 and CB2 Receptors in Stroke Recovery. Int. J. Mol. Sci. 2023, 24, 16728. [Google Scholar] [CrossRef] [PubMed]
- Abd-Nikfarjam, B.; Dolati-Somarin, A.; Baradaran Rahimi, V.; Askari, V.R. Cannabinoids in Neuroinflammatory Disorders: Focusing on Multiple Sclerosis, Parkinsons, and Alzheimers Diseases. BioFactors 2023, 49, 560–583. [Google Scholar] [CrossRef] [PubMed]
- Scott, C.; Hall, S.; Zhou, J.; Lehmann, C. Cannabinoids and the Endocannabinoid System in Early SARS-CoV-2 Infection and Long COVID-19—A Scoping Review. J. Clin. Med. 2024, 13, 227. [Google Scholar] [CrossRef]
- Al-Kuraishy, H.M.; Al-Gareeb, A.I.; Alkazmi, L.; El-Bouseary, M.M.; Hamad, R.S.; Abdelhamid, M.; Batiha, G.E.-S. The Potential Nexus between Helminths and SARS-CoV-2 Infection: A Literature Review. J. Immunol. Res. 2023, 2023, 5544819. [Google Scholar] [CrossRef]
- Zamith Cunha, R.; Zannoni, A.; Salamanca, G.; De Silva, M.; Rinnovati, R.; Gramenzi, A.; Forni, M.; Chiocchetti, R. Expression of Cannabinoid (CB1 and CB2) and Cannabinoid-Related Receptors (TRPV1, GPR55, and PPARα) in the Synovial Membrane of the Horse Metacarpophalangeal Joint. Front. Vet. Sci. 2023, 10, 1045030. [Google Scholar] [CrossRef]
- Zamith Cunha, R.; Salamanca, G.; Mille, F.; Delprete, C.; Franciosi, C.; Piva, G.; Gramenzi, A.; Chiocchetti, R. Endocannabinoid System Receptors at the Hip and Stifle Joints of Middle-Aged Dogs: A Novel Target for the Therapeutic Use of Cannabis sativa Extract in Canine Arthropathies. Animals 2023, 13, 2833. [Google Scholar] [CrossRef] [PubMed]
- Honkisz-Orzechowska, E.; Łażewska, D.; Baran, G.; Kieć-Kononowicz, K. Uncovering the Power of GPR18 Signalling: How RvD2 and Other Ligands Could Have the Potential to Modulate and Resolve Inflammation in Various Health Disorders. Molecules 2024, 29, 1258. [Google Scholar] [CrossRef] [PubMed]
- Lipscomb, M.; Walis, S.; Marinello, M.; Mena, H.A.; MacNamara, K.C.; Spite, M.; Fredman, G. Resolvin D2 Limits Atherosclerosis Progression via Myeloid Cell-GPR18. FASEB J. 2024, 38, e23555. [Google Scholar] [CrossRef]
- Farrell, P.; Farrell, L.; Farrell, M.K. The Approach to the Management of a Child with Chronic Abdominal Pain. Curr. Treat. Options Pediatr. 2024, 10, 64–78. [Google Scholar] [CrossRef]
- Lian, J.; Casari, I.; Falasca, M. Modulatory Role of the Endocannabinoidome in the Pathophysiology of the Gastrointestinal Tract. Pharmacol. Res. 2022, 175, 106025. [Google Scholar] [CrossRef]
- Hu, J.; Fan, W.; Xu, Y.; Li, X.; Zhang, H.; Li, S.; Xue, L. Maladaptive Changes in the Homeostasis of AEA-TRPV1/CB1R Induces Pain-Related Hyperactivity of Nociceptors after Spinal Cord Injury. Cell Biosci. 2025, 15, 2. [Google Scholar] [CrossRef]
- Dörnyei, G.; Vass, Z.; Juhász, C.B.; Nádasy, G.L.; Hunyady, L.; Szekeres, M. Role of the Endocannabinoid System in Metabolic Control Processes and in the Pathogenesis of Metabolic Syndrome: An Update. Biomedicines 2023, 11, 306. [Google Scholar] [CrossRef]
- Jakowiecki, J.; Abel, R.; Orzeł, U.; Pasznik, P.; Preissner, R.; Filipek, S. Allosteric Modulation of the CB1 Cannabinoid Receptor by Cannabidiol—A Molecular Modeling Study of the N-Terminal Domain and the Allosteric-Orthosteric Coupling. Molecules 2021, 26, 2456. [Google Scholar] [CrossRef]
- Lai, Y.-H.; Wu, T.-C.; Tsai, B.-Y.; Hung, Y.-P.; Lin, H.-J.; Tsai, Y.-S.; Ko, W.-C.; Tsai, P.-J. Peroxisome Proliferator-Activated Receptor-γ as the Gatekeeper of Tight Junction in Clostridioides Difficile Infection. Front. Microbiol. 2022, 13, 986457. [Google Scholar] [CrossRef]
- Chakaroun, R.M.; Massier, L.; Kovacs, P. Gut Microbiome, Intestinal Permeability, and Tissue Bacteria in Metabolic Disease: Perpetrators or Bystanders? Nutrients 2020, 12, 1082. [Google Scholar] [CrossRef]
- Malek, A.; Ahmadi Badi, S.; Karimi, G.; Bizouarn, T.; Irian, S.; Siadat, S.D. The Effect of Bacteroides Fragilis and Its Postbiotics on the Expression of Genes Involved in the Endocannabinoid System and Intestinal Epithelial Integrity in Caco-2 Cells. J. Diabetes Metab. Disord. 2023, 22, 1417–1424. [Google Scholar] [CrossRef] [PubMed]
- Liśkiewicz, P.; Kaczmarczyk, M.; Misiak, B.; Wroński, M.; Bąba-Kubiś, A.; Skonieczna-Żydecka, K.; Marlicz, W.; Bieńkowski, P.; Misera, A.; Pełka-Wysiecka, J.; et al. Analysis of Gut Microbiota and Intestinal Integrity Markers of Inpatients with Major Depressive Disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry 2021, 106, 110076. [Google Scholar] [CrossRef] [PubMed]
- Behl, T.; Makkar, R.; Sehgal, A.; Singh, S.; Makeen, H.A.; Albratty, M.; Alhazmi, H.A.; Meraya, A.M.; Bungau, S. Exploration of Multiverse Activities of Endocannabinoids in Biological Systems. Int. J. Mol. Sci. 2022, 23, 5734. [Google Scholar] [CrossRef]
- Dallabrida, K.G.; de Oliveira Bender, J.M.; Chade, E.S.; Rodrigues, N.; Sampaio, T.B. Endocannabinoid System Changes throughout Life: Implications and Therapeutic Potential for Autism, ADHD, and Alzheimer’s Disease. Brain Sci. 2024, 14, 592. [Google Scholar] [CrossRef]
- Cao, Y.; Li, R.; Bai, L. Vagal Sensory Pathway for the Gut-Brain Communication. Semin. Cell Dev. Biol. 2024, 156, 228–243. [Google Scholar] [CrossRef]
- López-Gómez, L.; Szymaszkiewicz, A.; Zielińska, M.; Abalo, R. The Enteric Glia and Its Modulation by the Endocannabinoid System, a New Target for Cannabinoid-Based Nutraceuticals? Molecules 2022, 27, 6773. [Google Scholar] [CrossRef]
- Fried, S.; Wemelle, E.; Cani, P.D.; Knauf, C. Interactions between the Microbiota and Enteric Nervous System during Gut-Brain Disorders. Neuropharmacology 2021, 197, 108721. [Google Scholar] [CrossRef]
- de Sá, M.C.I.; Castor, M.G.M. Therapeutic Use of Palmitoylethanolamide as an Anti-Inflammatory and Immunomodulator. Future Pharmacol. 2023, 3, 951–977. [Google Scholar] [CrossRef]
- Yasmeen, N.; Selvaraj, H.; Lakhawat, S.S.; Datta, M.; Sharma, P.K.; Jain, A.; Khanna, R.; Srinivasan, J.; Kumar, V. Possibility of Averting Cytokine Storm in SARS-COV 2 Patients Using Specialized pro-Resolving Lipid Mediators. Biochem. Pharmacol. 2023, 209, 115437. [Google Scholar] [CrossRef]
- Ahmed, I.; Rehman, S.U.; Shahmohamadnejad, S.; Zia, M.A.; Ahmad, M.; Saeed, M.M.; Akram, Z.; Iqbal, H.M.N.; Liu, Q. Therapeutic Attributes of Endocannabinoid System against Neuro-Inflammatory Autoimmune Disorders. Molecules 2021, 26, 3389. [Google Scholar] [CrossRef]
- Sheik, A.; Farani, M.R.; Kim, E.; Kim, S.; Gupta, V.K.; Kumar, K.; Huh, Y.S. Therapeutic Targeting of the Tumor Microenvironments with Cannabinoids and Their Analogs: Update on Clinical Trials. Environ. Res. 2023, 231, 115862. [Google Scholar] [CrossRef]
- Silva, N.R.; Arjmand, S.; Domingos, L.B.; Chaves-Filho, A.M.; Mottin, M.; Real, C.C.; Waszkiewicz, A.L.; Gobira, P.H.; Ferraro, A.N.; Landau, A.M.; et al. Modulation of the Endocannabinoid System by (S)-Ketamine in an Animal Model of Depression. Pharmacol. Res. 2025, 211, 107545. [Google Scholar] [CrossRef]
- Speranza, L.; Filiz, K.D.; Lippiello, P.; Ferraro, M.G.; Pascarella, S.; Miniaci, M.C.; Volpicelli, F. Enduring Neurobiological Consequences of Early-Life Stress: Insights from Rodent Behavioral Paradigms. Biomedicines 2024, 12, 1978. [Google Scholar] [CrossRef] [PubMed]
- Bellia, F.; Girella, A.; Annunzi, E.; Benatti, B.; Vismara, M.; Priori, A.; Festucci, F.; Fanti, F.; Compagnone, D.; Adriani, W.; et al. Selective Alterations of Endocannabinoid System Genes Expression in Obsessive Compulsive Disorder. Transl. Psychiatry 2024, 14, 118. [Google Scholar] [CrossRef]
- Stachowicz, K. Deciphering the Mechanisms of Reciprocal Regulation or Interdependence at the Cannabinoid CB1 Receptors and Cyclooxygenase-2 Level: Effects on Mood, Cognitive Implications, and Synaptic Signaling. Neurosci. Biobehav. Rev. 2023, 155, 105439. [Google Scholar] [CrossRef]
- Szanda, G.; Jourdan, T.; Wisniewski, É.; Cinar, R.; Godlewski, G.; Rajki, A.; Liu, J.; Chedester, L.; Szalai, B.; Tóth, A.D.; et al. Cannabinoid Receptor Type 1 (CB1R) Inhibits Hypothalamic Leptin Signaling via β-Arrestin1 in Complex with TC-PTP and STAT3. iScience 2023, 26, 107207. [Google Scholar] [CrossRef] [PubMed]
- Capozzi, A.; Caissutti, D.; Mattei, V.; Gado, F.; Martellucci, S.; Longo, A.; Recalchi, S.; Manganelli, V.; Riitano, G.; Garofalo, T.; et al. Anti-Inflammatory Activity of a CB2 Selective Cannabinoid Receptor Agonist: Signaling and Cytokines Release in Blood Mononuclear Cells. Molecules 2022, 27, 64. [Google Scholar] [CrossRef] [PubMed]
- Shuba, Y.M. Beyond Neuronal Heat Sensing: Diversity of TRPV1 Heat-Capsaicin Receptor-Channel Functions. Front. Cell. Neurosci. 2021, 14, 612480. [Google Scholar] [CrossRef]
- Csekő, K.; Beckers, B.; Keszthelyi, D.; Helyes, Z. Role of TRPV1 and TRPA1 Ion Channels in Inflammatory Bowel Diseases: Potential Therapeutic Targets? Pharmaceuticals 2019, 12, 48. [Google Scholar] [CrossRef]
- Busquets-García, A.; Bolaños, J.P.; Marsicano, G. Metabolic Messengers: Endocannabinoids. Nat. Metab. 2022, 4, 848–855. [Google Scholar] [CrossRef]
- Matheson, J.; Zhou, X.M.M.; Bourgault, Z.; Le Foll, B. Potential of Fatty Acid Amide Hydrolase (FAAH), Monoacylglycerol Lipase (MAGL), and Diacylglycerol Lipase (DAGL) Enzymes as Targets for Obesity Treatment: A Narrative Review. Pharmaceuticals 2021, 14, 1316. [Google Scholar] [CrossRef]
- van Egmond, N.; Straub, V.M.; van der Stelt, M. Targeting Endocannabinoid Signaling: FAAH and MAG Lipase Inhibitors. Annu. Rev. Pharmacol. Toxicol. 2021, 61, 441–463. [Google Scholar] [CrossRef] [PubMed]
- Della Pietra, A.; Krivoshein, G.; Ivanov, K.; Giniatullina, R.; Jyrkkänen, H.-K.; Leinonen, V.; Lehtonen, M.; van den Maagdenberg, A.M.J.M.; Savinainen, J.; Giniatullin, R. Potent Dual MAGL/FAAH Inhibitor AKU-005 Engages Endocannabinoids to Diminish Meningeal Nociception Implicated in Migraine Pain. J. Headache Pain 2023, 24, 38. [Google Scholar] [CrossRef] [PubMed]
- Nayak, K.; Gopan, A.; Priyanka; Muraleedharan, A.; Varghese, A.; Patil, A.; Manokaran, K. Gut Microbiota and Geriatric Health. Rev. Res. Med. Microbiol. 2024, 36, 69–79. [Google Scholar] [CrossRef]
- Kaszyńska, A.A. Cannabinoids: Potential for Modulation and Enhancement When Combined with Vitamin B12 in Case of Neurodegenerative Disorders. Pharmaceuticals 2024, 17, 813. [Google Scholar] [CrossRef]
- Wreven, E.; Ruiz de Adana, M.S.; Hardivillé, S.; Gmyr, V.; Kerr-Conte, J.; Chetboun, M.; Pasquetti, G.; Delalleau, N.; Thévenet, J.; Coddeville, A.; et al. Pharmaceutical Targeting of the Cannabinoid Type 1 Receptor Impacts the Crosstalk between Immune Cells and Islets to Reduce Insulitis in Humans. Diabetologia 2024, 67, 1877–1896, Correction in Diabetologia 2025, 68, 902. [Google Scholar] [CrossRef]
- Geyer, F.; Geyer, M.; Klapproth, S.; Wolff, K.-D.; Nieberler, M. Protocol for Generating Monoclonal CRISPR-Cas9-Mediated Knockout Cell Lines Using RNPs and Lipofection in HNSCC Cells. STAR Protoc. 2023, 4, 102366. [Google Scholar] [CrossRef]
- Banerjee, S.; Saha, D.; Sharma, R.; Jaidee, W.; Puttarak, P.; Chaiyakunapruk, N.; Chaoroensup, R. Phytocannabinoids in Neuromodulation: From Omics to Epigenetics. J. Ethnopharmacol. 2024, 330, 118201. [Google Scholar] [CrossRef]
- Navarro, D.; Gasparyan, A.; Navarrete, F.; Torregrosa, A.B.; Rubio, G.; Marín-Mayor, M.; Acosta, G.B.; Garcia-Gutiérrez, M.S.; Manzanares, J. Molecular Alterations of the Endocannabinoid System in Psychiatric Disorders. Int. J. Mol. Sci. 2022, 23, 4764. [Google Scholar] [CrossRef] [PubMed]
- Su, T.; Yan, Y.; Li, Q.; Ye, J.; Pei, L. Endocannabinoid System Unlocks the Puzzle of Autism Treatment via Microglia. Front. Psychiatry 2021, 12, 734837. [Google Scholar] [CrossRef] [PubMed]
- Camberos-Barraza, J.; Camacho-Zamora, A.; Bátiz-Beltrán, J.C.; Osuna-Ramos, J.F.; Rábago-Monzón, Á.R.; Valdez-Flores, M.A.; Angulo-Rojo, C.E.; Guadrón-Llanos, A.M.; Picos-Cárdenas, V.J.; Calderón-Zamora, L.; et al. Sleep, Glial Function, and the Endocannabinoid System: Implications for Neuroinflammation and Sleep Disorders. Int. J. Mol. Sci. 2024, 25, 3160. [Google Scholar] [CrossRef]
- Monory, K.; Polack, M.; Remus, A.; Lutz, B.; Korte, M. Cannabinoid CB1 Receptor Calibrates Excitatory Synaptic Balance in the Mouse Hippocampus. J. Neurosci. Off. J. Soc. Neurosci. 2015, 35, 3842–3850. [Google Scholar] [CrossRef]
- Kim, K.; Lee, S.-G.; Kegelman, T.P.; Su, Z.-Z.; Das, S.K.; Dash, R.; Dasgupta, S.; Barral, P.M.; Hedvat, M.; Diaz, P.; et al. Role of Excitatory Amino Acid Transporter-2 (EAAT2) and Glutamate in Neurodegeneration: Opportunities for Developing Novel Therapeutics. J. Cell. Physiol. 2011, 226, 2484–2493. [Google Scholar] [CrossRef]
- Wang, S.; Wang, Y.; Zou, S. A Glance at the Molecules That Regulate Oligodendrocyte Myelination. Curr. Issues Mol. Biol. 2022, 44, 2194–2216. [Google Scholar] [CrossRef] [PubMed]
- Pallarés-Moratalla, C.; Bergers, G. The Ins and Outs of Microglial Cells in Brain Health and Disease. Front. Immunol. 2024, 15, 1305087. [Google Scholar] [CrossRef] [PubMed]
- Lively, S.; Schlichter, L.C. Microglia Responses to Pro-Inflammatory Stimuli (LPS, IFNγ + TNFα) and Reprogramming by Resolving Cytokines (IL-4, IL-10). Front. Cell. Neurosci. 2018, 12, 215. [Google Scholar] [CrossRef]
- Li, J.; Wang, H.; Liu, D.; Li, X.; He, L.; Pan, J.; Shen, Q.; Peng, Y. CB2R Activation Ameliorates Late Adolescent Chronic Alcohol Exposure-Induced Anxiety-like Behaviors during Withdrawal by Preventing Morphological Changes and Suppressing NLRP3 Inflammasome Activation in Prefrontal Cortex Microglia in Mice. Brain. Behav. Immun. 2023, 110, 60–79. [Google Scholar] [CrossRef]
- Sharon, N.; Yarmolinsky, L.; Khalfin, B.; Fleisher-Berkovich, S.; Ben-Shabat, S. Cannabinoids’ Role in Modulating Central and Peripheral Immunity in Neurodegenerative Diseases. Int. J. Mol. Sci. 2024, 25, 6402. [Google Scholar] [CrossRef]
- Korem, N.; Bassir Nia, A.; Hillmer, A.T.; D’Souza, D.; Nabulsi, N.; Ropchan, J.; Huang, Y.; Cosgrove, K.; Levy, I.; Pietrzak, R.H.; et al. Cannabinoid 1 Receptor Availability in Posttraumatic Stress Disorder: A Positron Emission Tomography Study. Transl. Psychiatry 2025, 15, 310. [Google Scholar] [CrossRef]
- Pandey, S.; Kashif, S.; Youssef, M.; Sarwal, S.; Zraik, H.; Singh, R.; Rutkofsky, I.H. Endocannabinoid System in Irritable Bowel Syndrome and Cannabis as a Therapy. Complement. Ther. Med. 2020, 48, 102242. [Google Scholar] [CrossRef]
- Miao, Y.; Zhao, F.; Guan, W. A Novel Insight into the Antidepressant Effect of Cannabidiol: Possible Involvement of the 5-HT1A, CB1, GPR55, and PPARγ Receptors. Int. J. Neuropsychopharmacol. 2024, 28, pyae064. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Sharma, A.; Taliyan, R.; Urmera, M.T.; Herrera-Calderon, O.; Heinbockel, T.; Rahman, S.; Goyal, R. Orchestration of the Circadian Clock and Its Association with Alzheimer’s Disease: Role of Endocannabinoid Signaling. Ageing Res. Rev. 2022, 73, 101533. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Paramanik, V. DNA Methylation, Histone Acetylation in the Regulation of Memory and Its Modulation during Aging. Front. Aging 2024, 5, 1480932. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Arellano, J.; Canseco-Alba, A.; Cutler, S.J.; León, F. The Polypharmacological Effects of Cannabidiol. Molecules 2023, 28, 3271. [Google Scholar] [CrossRef]
- Jiang, L.; Han, D.; Hao, Y.; Song, Z.; Sun, Z.; Dai, Z. Linking Serotonin Homeostasis to Gut Function: Nutrition, Gut Microbiota and Beyond. Crit. Rev. Food Sci. Nutr. 2024, 64, 7291–7310. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, J.; Wu, X.; Jing, H.; Zhang, S.; Hu, Z.; Rao, L.; Chang, Q.; Wang, L.; Zhang, Z. Gut Microbiota Alteration after Cholecystectomy Contributes to Post-Cholecystectomy Diarrhea via Bile Acids Stimulating Colonic Serotonin. Gut Microbes 2023, 15, 2168101. [Google Scholar] [CrossRef]
- Rusch, J.A.; Layden, B.T.; Dugas, L.R. Signalling Cognition: The Gut Microbiota and Hypothalamic-Pituitary-Adrenal Axis. Front. Endocrinol. 2023, 14, 1130689. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, Z.; Chen, X.; Chao, G.; Zhang, S. The Role of Gut Microbiota and Metabolites in Regulating the Immune Response in Drug-Induced Enteritis. J. Appl. Microbiol. 2023, 134, lxad032. [Google Scholar] [CrossRef]
- Cuddihey, H.; MacNaughton, W.K.; Sharkey, K.A. Role of the Endocannabinoid System in the Regulation of Intestinal Homeostasis. Cell. Mol. Gastroenterol. Hepatol. 2022, 14, 947–963. [Google Scholar] [CrossRef]
- Brazil, J.; Azcutia, V.; Kelm, M.; Cummings, R.; Parkos, C. CD11B/CD18 Sialylation Regulates Neutrophil Trafficking and Inflammatory Function in the Intestine. Inflamm. Bowel Dis. 2024, 30, S65. [Google Scholar] [CrossRef]
- Haspula, D.; Clark, M.A. Cannabinoid Receptors: An Update on Cell Signaling, Pathophysiological Roles and Therapeutic Opportunities in Neurological, Cardiovascular, and Inflammatory Diseases. Int. J. Mol. Sci. 2020, 21, 7693. [Google Scholar] [CrossRef]
- Di Tommaso, N.; Santopaolo, F.; Gasbarrini, A.; Ponziani, F.R. The Gut–Vascular Barrier as a New Protagonist in Intestinal and Extraintestinal Diseases. Int. J. Mol. Sci. 2023, 24, 1470. [Google Scholar] [CrossRef]
- He, C.; Chen, Z.; Huang, J.; Gan, R.; Wang, J.; Wang, L.; Li, D.; Yao, J. Interleukin-22 Ameliorates Dextran Sulfate Sodium-Induced Colitis through the Upregulation of lncRNA-UCL to Accelerate Claudin-1 Expression via Sequestering miR-568 in Mice. Oxid. Med. Cell. Longev. 2022, 2022, 8543720. [Google Scholar] [CrossRef] [PubMed]
- Knoop, K.A.; Newberry, R.D. Goblet Cells: Multifaceted Players in Immunity at Mucosal Surfaces. Mucosal Immunol. 2018, 11, 1551–1557. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Zhou, Y.; Sun, Y.; Wang, Q. Intestinal Fatty Acid Binding Protein: A Rising Therapeutic Target in Lipid Metabolism. Prog. Lipid Res. 2022, 87, 101178. [Google Scholar] [CrossRef]
- Xu, B.; Chen, L.; Zhan, Y.; Marquez, K.N.S.; Zhuo, L.; Qi, S.; Zhu, J.; He, Y.; Chen, X.; Zhang, H.; et al. The Biological Functions and Regulatory Mechanisms of Fatty Acid Binding Protein 5 in Various Diseases. Front. Cell Dev. Biol. 2022, 10, 857919. [Google Scholar] [CrossRef]
- Dai, D.; Qiu, K.; Zhang, H.; Wu, S.; Han, Y.; Wu, Y.; Qi, G.; Wang, J. Organic Acids as Alternatives for Antibiotic Growth Promoters Alter the Intestinal Structure and Microbiota and Improve the Growth Performance in Broilers. Front. Microbiol. 2021, 11, 618144. [Google Scholar] [CrossRef] [PubMed]
- Wiącek, J.; Podgórski, T.; Kusy, K.; Łoniewski, I.; Skonieczna-Żydecka, K.; Karolkiewicz, J. Evaluating the Impact of Probiotic Therapy on the Endocannabinoid System, Pain, Sleep and Fatigue: A Randomized, Double-Blind, Placebo-Controlled Trial in Dancers. Int. J. Mol. Sci. 2024, 25, 5611. [Google Scholar] [CrossRef]
- Yin, Z.; Wang, K.; Liu, Y.; Li, Y.; He, F.; Yin, J.; Tang, W. Lactobacillus Johnsonii Improves Intestinal Barrier Function and Reduces Post-Weaning Diarrhea in Piglets: Involvement of the Endocannabinoid System. Animals 2024, 14, 493. [Google Scholar] [CrossRef]
- He, Z.; Liu, Y.; Li, Z.; Sun, T.; Li, Z.; Liu, C.; Xiang, H. Gut Microbiota-Mediated Alterations of Hippocampal CB1R Regulating the Diurnal Variation of Cognitive Impairment Induced by Hepatic Ischemia–Reperfusion Injury in Mice. Neurochem. Res. 2024, 49, 2165–2178. [Google Scholar] [CrossRef]
- Parksepp, M.; Haring, L.; Kilk, K.; Koch, K.; Uppin, K.; Kangro, R.; Zilmer, M.; Vasar, E. The Expanded Endocannabinoid System Contributes to Metabolic and Body Mass Shifts in First-Episode Schizophrenia: A 5-Year Follow-Up Study. Biomedicines 2022, 10, 243. [Google Scholar] [CrossRef] [PubMed]
- Lowe, H.; Toyang, N.; Steele, B.; Bryant, J.; Ngwa, W. The Endocannabinoid System: A Potential Target for the Treatment of Various Diseases. Int. J. Mol. Sci. 2021, 22, 9472. [Google Scholar] [CrossRef] [PubMed]
- Shafiei-Jahani, P.; Yan, S.; Kazemi, M.H.; Li, X.; Akbari, A.; Sakano, K.; Sakano, Y.; Hurrell, B.P.; Akbari, O. CB2 Stimulation of Adipose Resident ILC2s Orchestrates Immune Balance and Ameliorates Type 2 Diabetes Mellitus. Cell Rep. 2024, 43, 114434. [Google Scholar] [CrossRef]
- Gojani, E.G.; Wang, B.; Li, D.-P.; Kovalchuk, O.; Kovalchuk, I. Anti-Inflammatory Effects of Minor Cannabinoids CBC, THCV, and CBN in Human Macrophages. Molecules 2023, 28, 6487. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Wu, D.; Li, J.; Wu, M.; Li, Q.; Che, Z.; Cheng, X.; Cheng, Q.; Yin, F.; Zhang, H.; et al. Myeloid Beta-Arrestin 2 Depletion Attenuates Metabolic Dysfunction-Associated Steatohepatitis via the Metabolic Reprogramming of Macrophages. Cell Metab. 2024, 36, 2281–2297.e7. [Google Scholar] [CrossRef]
- Wu, Q.; Ma, Y.; Liu, Y.; Wang, N.; Zhao, X.; Wen, D. CB2R Agonist JWH-133 Attenuates Chronic Inflammation by Restraining M1 Macrophage Polarization via Nrf2/HO-1 Pathway in Diet-Induced Obese Mice. Life Sci. 2020, 260, 118424, Correction in Life Sci. 2021, 286, 120102. [Google Scholar] [CrossRef]
- Tan, K.B.C.; Alexander, H.D.; Linden, J.; Murray, E.K.; Gibson, D.S. Anti-Inflammatory Effects of Phytocannabinoids and Terpenes on Inflamed Tregs and Th17 Cells in Vitro. Exp. Mol. Pathol. 2024, 139, 104924. [Google Scholar] [CrossRef]
- Wei, C.; Huang, L.; Zheng, Y.; Cai, X. Selective Activation of Cannabinoid Receptor 2 Regulates Treg/Th17 Balance to Ameliorate Neutrophilic Asthma in Mice. Ann. Transl. Med. 2021, 9, 1015. [Google Scholar] [CrossRef]
- Gentili, M.; Ronchetti, S.; Ricci, E.; Di Paola, R.; Gugliandolo, E.; Cuzzocrea, S.; Bereshchenko, O.; Migliorati, G.; Riccardi, C. Selective CB2 Inverse Agonist JTE907 Drives T Cell Differentiation towards a Treg Cell Phenotype and Ameliorates Inflammation in a Mouse Model of Inflammatory Bowel Disease. Pharmacol. Res. 2019, 141, 21–31. [Google Scholar] [CrossRef]
- Kalani, M.; Hodjati, H.; Ghoddusi Johari, H.; Doroudchi, M. Memory T Cells of Patients with Abdominal Aortic Aneurysm Differentially Expressed Micro RNAs 21, 92a, 146a, 155, 326 and 663 in Response to Helicobacter Pylori and Lactobacillus Acidophilus. Mol. Immunol. 2021, 130, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Gilyazova, I.; Ivanova, E.; Pavlov, V.; Khasanova, G.; Khasanova, A.; Izmailov, A.; Asadullina, D.; Gilyazova, G.; Wang, G.; Gareev, I.; et al. Exosomal miRNA-155 and miRNA-146a Are Promising Prognostic Biomarkers of the Severity of Hemorrhagic Fever with Renal Syndrome. Non-Coding RNA Res. 2023, 8, 75–82. [Google Scholar] [CrossRef]
- Li, H.; Wang, Y.; Han, X. ESP-B4 Promotes Nasal Epithelial Cell-Derived Extracellular Vesicles Containing miR-146a-5p to Modulate Smad3/GATA-3 Thus Relieving Allergic Rhinitis: ESP-B4/miR-146a-5p in AR. Phytomedicine 2023, 108, 154516. [Google Scholar] [CrossRef]
- Young, A.P.; Denovan-Wright, E.M. The Dynamic Role of Microglia and the Endocannabinoid System in Neuroinflammation. Front. Pharmacol. 2022, 12, 806417. [Google Scholar] [CrossRef]
- Xue, T.; Ma, R.-H.; Xu, C.; Sun, B.; Yan, D.-F.; Liu, X.-M.; Gao, D.; Li, Z.-H.; Gao, Y.; Wang, C.-Z. The Endocannabinoid System Is Involved in the Anxiety-like Behavior Induced by Dual-Frequency 2.65/0.8 GHz Electromagnetic Radiation in Mice. Front. Mol. Neurosci. 2024, 17, 1366855. [Google Scholar] [CrossRef]
- Mikaeili, H.; Habib, A.M.; Yeung, C.W.-L.; Santana-Varela, S.; Luiz, A.P.; Panteleeva, K.; Zuberi, S.; Athanasiou-Fragkouli, A.; Houlden, H.; Wood, J.N.; et al. Molecular Basis of FAAH-OUT-Associated Human Pain Insensitivity. Brain J. Neurol. 2023, 146, 3851–3865. [Google Scholar] [CrossRef]
- Papa, A.; Pasquini, S.; Galvani, F.; Cammarota, M.; Contri, C.; Carullo, G.; Gemma, S.; Ramunno, A.; Lamponi, S.; Gorelli, B.; et al. Development of Potent and Selective FAAH Inhibitors with Improved Drug-like Properties as Potential Tools to Treat Neuroinflammatory Conditions. Eur. J. Med. Chem. 2023, 246, 114952. [Google Scholar] [CrossRef]
- Dasram, M.H.; Naidoo, P.; Walker, R.B.; Khamanga, S.M. Targeting the Endocannabinoid System Present in the Glioblastoma Tumour Microenvironment as a Potential Anti-Cancer Strategy. Int. J. Mol. Sci. 2024, 25, 1371. [Google Scholar] [CrossRef]
- Kalkan, H.; Panza, E.; Pagano, E.; Ercolano, G.; Moriello, C.; Piscitelli, F.; Sztretye, M.; Capasso, R.; Di Marzo, V.; Iannotti, F.A. Dysfunctional Endocannabinoid CB1 Receptor Expression and Signaling Contribute to Skeletal Muscle Cell Toxicity Induced by Simvastatin. Cell Death Dis. 2023, 14, 544. [Google Scholar] [CrossRef]
- Anderson, L.L.; Doohan, P.T.; Hawkins, N.A.; Bahceci, D.; Thakur, G.A.; Kearney, J.A.; Arnold, J.C. The Endocannabinoid System Impacts Seizures in a Mouse Model of Dravet Syndrome. Neuropharmacology 2022, 205, 108897. [Google Scholar] [CrossRef] [PubMed]
- Köfalvi, A.; Moreno, E.; Cordomí, A.; Cai, N.-S.; Fernández-Dueñas, V.; Ferreira, S.G.; Guixà-González, R.; Sánchez-Soto, M.; Yano, H.; Casadó-Anguera, V.; et al. Control of Glutamate Release by Complexes of Adenosine and Cannabinoid Receptors. BMC Biol. 2020, 18, 9. [Google Scholar] [CrossRef]
- Migliaro, M.; Sánchez-Zavaleta, R.; Soto-Tinoco, E.; Ruiz-Contreras, A.E.; Méndez-Díaz, M.; Herrera-Solís, A.; Pérez de la Mora, M.; Prospéro-García, O.E. Dominance Status Is Associated with a Variation in Cannabinoid Receptor 1 Expression and Amphetamine Reward. Pharmacol. Biochem. Behav. 2022, 221, 173483. [Google Scholar] [CrossRef]
- Fang, S.; Kang, W.-T.; Li, H.; Cai, Q.; Liang, W.; Zeng, M.; Yu, Q.; Zhong, R.; Tao, Y.; Liu, S.; et al. Development of Cannabidiol Derivatives as Potent Broad-Spectrum Antibacterial Agents with Membrane-Disruptive Mechanism. Eur. J. Med. Chem. 2024, 266, 116149. [Google Scholar] [CrossRef]
- Chang, H.; Perkins, M.H.; Novaes, L.S.; Qian, F.; Zhang, T.; Neckel, P.H.; Scherer, S.; Ley, R.E.; Han, W.; de Araujo, I.E. Stress-Sensitive Neural Circuits Change the Gut Microbiome via Duodenal Glands. Cell 2024, 187, 5393–5412.e30. [Google Scholar] [CrossRef]
- Shan, R.; Zhang, Y.; Shi, Y.; Wang, X.; Wang, X.; Ma, G.; Li, Q. Activation of Cannabinoid Type 2 Receptor in Microglia Reduces Neuroinflammation through Inhibiting Aerobic Glycolysis to Relieve Hypertension. Biomolecules 2024, 14, 333. [Google Scholar] [CrossRef] [PubMed]
- Adams-Huet, B.; Jialal, I. Correlates of Insulin Resistance in Nascent Metabolic Syndrome. Clin. Med. Insights Endocrinol. Diabetes 2023, 16, 11795514231168279. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Xu, Z.; Carey, L.; Romero, J.; Makriyannis, A.; Hillard, C.J.; Ruggiero, E.; Dockum, M.; Houk, G.; Mackie, K.; et al. A Peripheral CB2 Cannabinoid Receptor Mechanism Suppresses Chemotherapy-Induced Peripheral Neuropathy: Evidence from a CB2 Reporter Mouse. Pain 2022, 163, 834. [Google Scholar] [CrossRef] [PubMed]
- Amersfoort, J.; Eelen, G.; Carmeliet, P. Immunomodulation by Endothelial Cells—Partnering up with the Immune System? Nat. Rev. Immunol. 2022, 22, 576–588. [Google Scholar] [CrossRef]
- Lopez Trinidad, L.M.; Martinez, R.; Kapravelou, G.; Galisteo, M.; Aranda, P.; Porres, J.M.; Lopez-Jurado, M. Caloric Restriction, Physical Exercise, and CB1 Receptor Blockade as an Efficient Combined Strategy for Bodyweight Control and Cardiometabolic Status Improvement in Male Rats. Sci. Rep. 2021, 11, 4286. [Google Scholar] [CrossRef]
- Gallardo, A.A.; Gutierrez, M.R.; Gomez, L.A.J.; Delos Reyes, P.A.O.; Dones, S.A.A.; Dumbrique, M.M.U.; Lim, H.E.; Liquido, M.I.H.; Macawile, M.M.L.; Maglaqui, E.D.A.B.; et al. A Comparative Analysis on the Potential Anticancer Properties of Tetrahydrocannabinol, Cannabidiol, and Tetrahydrocannabivarin Compounds Through In Silico Approach. Asian Pac. J. Cancer Prev. APJCP 2024, 25, 839–856. [Google Scholar] [CrossRef]
- Carnevali, L.; Statello, R.; Vacondio, F.; Ferlenghi, F.; Spadoni, G.; Rivara, S.; Mor, M.; Sgoifo, A. Antidepressant-like Effects of Pharmacological Inhibition of FAAH Activity in Socially Isolated Female Rats. Eur. Neuropsychopharmacol. 2020, 32, 77–87. [Google Scholar] [CrossRef]
- Menéndez-Pérez, C.; Rivas-Santisteban, R.; del Valle, E.; Tolivia, J.; Navarro, A.; Franco, R.; Martínez-Pinilla, E. Heteromers Formed by GPR55 and Either Cannabinoid CB1 or CB2 Receptors Are Upregulated in the Prefrontal Cortex of Multiple Sclerosis Patients. Int. J. Mol. Sci. 2024, 25, 4176. [Google Scholar] [CrossRef]
- Gustavsen, S.; Olsson, A.; Oturai, A.B.; Linnet, K.; Thomsen, R.; Rasmussen, B.S.; Jørgensen, C.F.; Langkilde, A.R.; Sorensen, P.S.; Sellebjerg, F.; et al. The Peripheral Endocannabinoid System and Its Association with Biomarkers of Inflammation in Untreated Patients with Multiple Sclerosis. Eur. J. Neurol. 2023, 30, 3212–3220. [Google Scholar] [CrossRef] [PubMed]
- Cristino, L.; Bisogno, T.; Di Marzo, V. Cannabinoids and the Expanded Endocannabinoid System in Neurological Disorders. Nat. Rev. Neurol. 2020, 16, 9–29. [Google Scholar] [CrossRef]
- Yang, J.; Wang, L.; Mei, M.; Guo, J.; Yang, X.; Liu, S. Electroacupuncture Repairs Intestinal Barrier by Upregulating CB1 through Gut Microbiota in DSS-Induced Acute Colitis. Chin. Med. 2023, 18, 24. [Google Scholar] [CrossRef]
- Wiley, M.B.; DiPatrizio, N.V. Diet-Induced Gut Barrier Dysfunction Is Exacerbated in Mice Lacking Cannabinoid 1 Receptors in the Intestinal Epithelium. Int. J. Mol. Sci. 2022, 23, 10549. [Google Scholar] [CrossRef]
- Di Vincenzo, F.; Del Gaudio, A.; Petito, V.; Lopetuso, L.R.; Scaldaferri, F. Gut Microbiota, Intestinal Permeability, and Systemic Inflammation: A Narrative Review. Intern. Emerg. Med. 2024, 19, 275–293. [Google Scholar] [CrossRef]
- Zhang, Y.; Ding, X.; Zhang, X.; Li, Y.; Xu, R.; Li, H.-J.; Zuo, D.; Chen, G. Unveiling the Contribution of Tumor-Associated Macrophages in Driving Epithelial-Mesenchymal Transition: A Review of Mechanisms and Therapeutic Strategies. Front. Pharmacol. 2024, 15, 1404687. [Google Scholar] [CrossRef]
- Huang, Y.; Wu, G.; Bi, G.; Cheng, L.; Liang, J.; Li, M.; Zhang, H.; Shan, G.; Hu, Z.; Chen, Z.; et al. Unveiling Chemotherapy-Induced Immune Landscape Remodeling and Metabolic Reprogramming in Lung Adenocarcinoma by scRNA-Sequencing. eLife 2024, 13, RP95988. [Google Scholar] [CrossRef] [PubMed]
- Bunsick, D.A.; Matsukubo, J.; Szewczuk, M.R. Cannabinoids Transmogrify Cancer Metabolic Phenotype via Epigenetic Reprogramming and a Novel CBD Biased G Protein-Coupled Receptor Signaling Platform. Cancers 2023, 15, 1030. [Google Scholar] [CrossRef] [PubMed]
- Iozzo, M.; Sgrignani, G.; Comito, G.; Chiarugi, P.; Giannoni, E. Endocannabinoid System and Tumour Microenvironment: New Intertwined Connections for Anticancer Approaches. Cells 2021, 10, 3396. [Google Scholar] [CrossRef] [PubMed]
- Kienzl, M.; Kargl, J.; Schicho, R. The Immune Endocannabinoid System of the Tumor Microenvironment. Int. J. Mol. Sci. 2020, 21, 8929. [Google Scholar] [CrossRef]
- Kim, B.G.; Kim, B.R.; Kim, D.Y.; Kim, W.Y.; Kang, S.; Lee, S.I.; Oh, S.C. Cannabidiol Enhances Atezolizumab Efficacy by Upregulating PD-L1 Expression via the cGAS-STING Pathway in Triple-Negative Breast Cancer Cells. Cancer Immunol. Res. 2024, 12, 1796–1807. [Google Scholar] [CrossRef]
- Li, D.; Cao, D.; Sun, Y.; Cui, Y.; Zhang, Y.; Jiang, J.; Cao, X. The Roles of Epigallocatechin Gallate in the Tumor Microenvironment, Metabolic Reprogramming, and Immunotherapy. Front. Immunol. 2024, 15, 1331641. [Google Scholar] [CrossRef] [PubMed]
- Bachari, A.; Nassar, N.; Telukutla, S.; Zomer, R.; Piva, T.J.; Mantri, N. Evaluating the Mechanism of Cell Death in Melanoma Induced by the Cannabis Extract PHEC-66. Cells 2024, 13, 268. [Google Scholar] [CrossRef]
- Bachari, A.; Nassar, N.; Schanknecht, E.; Telukutla, S.; Piva, T.J.; Mantri, N. Rationalizing a Prospective Coupling Effect of Cannabinoids with the Current Pharmacotherapy for Melanoma Treatment. WIREs Mech. Dis. 2024, 16, e1633. [Google Scholar] [CrossRef]
- Barker, H.; Ferraro, M.J. Exploring the Versatile Roles of the Endocannabinoid System and Phytocannabinoids in Modulating Bacterial Infections. Infect. Immun. 2024, 92, e00020-24. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, X.; Tang, N.; Guo, M.; Ai, D. Boosting Clear Cell Renal Carcinoma-Specific Drug Discovery Using a Deep Learning Algorithm and Single-Cell Analysis. Int. J. Mol. Sci. 2024, 25, 4134. [Google Scholar] [CrossRef]
- Akhilesh; Menon, A.; Agrawal, S.; Chouhan, D.; Gadepalli, A.; Das, B.; Kumar, R.; Singh, N.; Tiwari, V. Virtual Screening and Molecular Dynamics Investigations Using Natural Compounds against Autotaxin for the Treatment of Chronic Pain. J. Biomol. Struct. Dyn. 2024, 43, 5372–5392. [Google Scholar] [CrossRef]
- Ji, S.; You, Y.; Peng, B.; Zhong, T.; Kuang, Y.; Li, S.; Du, L.; Chen, L.; Sun, X.; Dai, J.; et al. Multi-Omics Analysis Reveals the Metabolic Regulators of Duodenal Low-Grade Inflammation in a Functional Dyspepsia Model. Front. Immunol. 2022, 13, 944591. [Google Scholar] [CrossRef] [PubMed]
- Granata, A. Functional Genomics in Stroke: Current and Future Applications of iPSCs and Gene Editing to Dissect the Function of Risk Variants. BMC Cardiovasc. Disord. 2023, 23, 223. [Google Scholar] [CrossRef]
- Borkowski, K.; Seyfried, N.T.; Arnold, M.; Lah, J.J.; Levey, A.I.; Hales, C.M.; Dammer, E.B.; Blach, C.; Louie, G.; Kaddurah-Daouk, R.; et al. Integration of Plasma and CSF Metabolomics with CSF Proteomic Reveals Novel Associations between Lipid Mediators and Central Nervous System Vascular and Energy Metabolism. Sci. Rep. 2023, 13, 13752. [Google Scholar] [CrossRef]
- Sánchez-Sanz, A.; Posada-Ayala, M.; Sabín-Muñoz, J.; Fernández-Miranda, I.; Aladro-Benito, Y.; Álvarez-Lafuente, R.; Royuela, A.; García-Hernández, R.; la Fuente, O.R.-D.; Romero, J.; et al. Endocannabinoid Levels in Peripheral Blood Mononuclear Cells of Multiple Sclerosis Patients Treated with Dimethyl Fumarate. Sci. Rep. 2022, 12, 20300. [Google Scholar] [CrossRef]
- Bresciani, G.; Manai, F.; Davinelli, S.; Tucci, P.; Saso, L.; Amadio, M. Novel Potential Pharmacological Applications of Dimethyl Fumarate—An Overview and Update. Front. Pharmacol. 2023, 14, 1264842. [Google Scholar] [CrossRef]
- Sun, J.; Chen, F.; Wu, G. Potential Effects of Gut Microbiota on Host Cancers: Focus on Immunity, DNA Damage, Cellular Pathways, and Anticancer Therapy. ISME J. 2023, 17, 1535–1551. [Google Scholar] [CrossRef]
- Facchin, S.; Bertin, L.; Bonazzi, E.; Lorenzon, G.; De Barba, C.; Barberio, B.; Zingone, F.; Maniero, D.; Scarpa, M.; Ruffolo, C.; et al. Short-Chain Fatty Acids and Human Health: From Metabolic Pathways to Current Therapeutic Implications. Life 2024, 14, 559. [Google Scholar] [CrossRef]
- Francavilla, F.; Intranuovo, F.; La Spada, G.; Lacivita, E.; Catto, M.; Graps, E.A.; Altomare, C.D. Inflammaging and Immunosenescence in the Post-COVID Era: Small Molecules, Big Challenges. ChemMedChem 2024, 20, e202400672. [Google Scholar] [CrossRef] [PubMed]
- Gojani, E.G.; Wang, B.; Li, D.-P.; Kovalchuk, O.; Kovalchuk, I. The Impact of Psilocybin on High Glucose/Lipid-Induced Changes in INS-1 Cell Viability and Dedifferentiation. Genes 2024, 15, 183. [Google Scholar] [CrossRef] [PubMed]
- Ishiguro, H.; Kibret, B.G.; Horiuchi, Y.; Onaivi, E.S. Potential Role of Cannabinoid Type 2 Receptors in Neuropsychiatric and Neurodegenerative Disorders. Front. Psychiatry 2022, 13, 828895. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, N.A.; Mahmoud, H.E.; El-Nikhely, N.A.; Hussein, A.A.; El-Khordagui, L.K. Carbon Dots Labeled Lactiplantibacillus Plantarum: A Fluorescent Multifunctional Biocarrier for Anticancer Drug Delivery. Front. Bioeng. Biotechnol. 2023, 11, 1166094. [Google Scholar] [CrossRef]
- Golchin, A.; Ranjbarvan, P.; Parviz, S.; Shokati, A.; Naderi, R.; Rasmi, Y.; Kiani, S.; Moradi, F.; Heidari, F.; Saltanatpour, Z.; et al. The Role of Probiotics in Tissue Engineering and Regenerative Medicine. Regen. Med. 2023, 18, 635–657. [Google Scholar] [CrossRef] [PubMed]
- Brierley, S.M.; Greenwood-Van Meerveld, B.; Sarnelli, G.; Sharkey, K.A.; Storr, M.; Tack, J. Targeting the Endocannabinoid System for the Treatment of Abdominal Pain in Irritable Bowel Syndrome. Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 5–25. [Google Scholar] [CrossRef]
- Gyires, K.; Zádori, Z.S. Role of Cannabinoids in Gastrointestinal Mucosal Defense and Inflammation. Curr. Neuropharmacol. 2016, 14, 935. [Google Scholar] [CrossRef] [PubMed]
- Silva, H.-H.; Tavares, V.; Neto, B.V.; Cerqueira, F.; Medeiros, R.; Silva, M.-R.G. FAAH Rs324420 Polymorphism: Biological Pathways, Impact on Elite Athletic Performance and Insights for Sport Medicine. Genes 2023, 14, 1946. [Google Scholar] [CrossRef]
- Hosseinzadeh Anvar, L.; Ahmadalipour, A. Fatty Acid Amide Hydrolase C385A Polymorphism Affects Susceptibility to Various Diseases. BioFactors 2023, 49, 62–78. [Google Scholar] [CrossRef]
- Pacher, P.; Bátkai, S.; Kunos, G. The Endocannabinoid System as an Emerging Target of Pharmacotherapy. Pharmacol. Rev. 2006, 58, 389–462. [Google Scholar] [CrossRef]
- Basavarajappa, B.S.; Subbanna, S. Molecular Insights into Epigenetics and Cannabinoid Receptors. Biomolecules 2022, 12, 1560. [Google Scholar] [CrossRef]
- Maciocha, F.; Suchanecka, A.; Chmielowiec, K.; Chmielowiec, J.; Ciechanowicz, A.; Boroń, A. Correlations of the CNR1 Gene with Personality Traits in Women with Alcohol Use Disorder. Int. J. Mol. Sci. 2024, 25, 5174. [Google Scholar] [CrossRef]
- Reece, A.S.; Hulse, G.K. Perturbation of 3D Nuclear Architecture, Epigenomic Aging and Dysregulation, and Cannabinoid Synaptopathy Reconfigures Conceptualization of Cannabinoid Pathophysiology: Part 2—Metabolome, Immunome, Synaptome. Front. Psychiatry 2023, 14, 1182536. [Google Scholar] [CrossRef]
- Mikulska, J.; Juszczyk, G.; Gawrońska-Grzywacz, M.; Herbet, M. HPA Axis in the Pathomechanism of Depression and Schizophrenia: New Therapeutic Strategies Based on Its Participation. Brain Sci. 2021, 11, 1298. [Google Scholar] [CrossRef] [PubMed]
- Maciak, K.; Dziedzic, A.; Miller, E.; Saluk-Bijak, J. miR-155 as an Important Regulator of Multiple Sclerosis Pathogenesis. A Review. Int. J. Mol. Sci. 2021, 22, 4332. [Google Scholar] [CrossRef]
- Donati Zeppa, S.; Ferrini, F.; Agostini, D.; Amatori, S.; Barbieri, E.; Piccoli, G.; Sestili, P.; Stocchi, V. Nutraceuticals and Physical Activity as Antidepressants: The Central Role of the Gut Microbiota. Antioxidants 2022, 11, 236. [Google Scholar] [CrossRef]
- Armeli, F.; Bonucci, A.; Maggi, E.; Pinto, A.; Businaro, R. Mediterranean Diet and Neurodegenerative Diseases: The Neglected Role of Nutrition in the Modulation of the Endocannabinoid System. Biomolecules 2021, 11, 790. [Google Scholar] [CrossRef]
- Pucci, M.; D’Addario, C.; Micioni Di Bonaventura, E.; Mercante, F.; Annunzi, E.; Fanti, F.; Sergi, M.; Botticelli, L.; Einaudi, G.; Cifani, C.; et al. Endocannabinoid System Regulation in Female Rats with Recurrent Episodes of Binge Eating. Int. J. Mol. Sci. 2022, 23, 15228. [Google Scholar] [CrossRef]
- Bloch Priel, S.; Yitzhaky, A.; Gurwitz, D.; Hertzberg, L. Cannabinoid Receptor Gene CNR1 Is Downregulated in Subcortical Brain Samples and Upregulated in Blood Samples of Individuals with Schizophrenia: A Participant Data Systematic Meta-Analysis. Eur. J. Neurosci. 2023, 58, 3540–3554. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Chen, T.; Yu, J.; Wan, L.; Zhang, J.; Chen, J.; Wei, W.; Li, X. Insights into the Regulatory Role of Epigenetics in Moyamoya Disease: Current Advances and Future Prospectives. Mol. Ther. Nucleic Acids 2024, 35, 102281. [Google Scholar] [CrossRef]
- Gómez-Pascual, A.; Naccache, T.; Xu, J.; Hooshmand, K.; Wretlind, A.; Gabrielli, M.; Lombardo, M.T.; Shi, L.; Buckley, N.J.; Tijms, B.M.; et al. Paired Plasma Lipidomics and Proteomics Analysis in the Conversion from Mild Cognitive Impairment to Alzheimer’s Disease. Comput. Biol. Med. 2024, 176, 108588. [Google Scholar] [CrossRef] [PubMed]
- Ghai, M.K.; Khatri, A.; Kumar, K.; Thakur, I.S. Multi-Omics and Advance Technologies in Biodegradation of Emerging Contaminants and Eco-Estrogens in Environmental Waste. Total Environ. Adv. 2024, 11, 200113. [Google Scholar] [CrossRef]
- Tyrakis, P.; Agridi, C.; Kourti, M. A Comprehensive Exploration of the Multifaceted Neuroprotective Role of Cannabinoids in Alzheimer’s Disease across a Decade of Research. Int. J. Mol. Sci. 2024, 25, 8630. [Google Scholar] [CrossRef]
- Zieneldien, T.; Kim, J.; Cao, C. The Multifaceted Role of Neuroprotective Plants in Alzheimer’s Disease Treatment. Geriatrics 2022, 7, 24. [Google Scholar] [CrossRef]
- Khaspekov, L.G.; Illarioshkin, S.N. Therapeutic Application of Modulators of Endogenous Cannabinoid System in Parkinson’s Disease. Int. J. Mol. Sci. 2024, 25, 8520. [Google Scholar] [CrossRef]
- Yu, X.; Jia, Y.; Dong, Y. Research Progress on the Cannabinoid Type-2 Receptor and Parkinson’s Disease. Front. Aging Neurosci. 2024, 15, 1298166. [Google Scholar] [CrossRef]
- Basile, M.S.; Mazzon, E. The Role of Cannabinoid Type 2 Receptors in Parkinson’s Disease. Biomedicines 2022, 10, 2986. [Google Scholar] [CrossRef]
- Kolesarova, M.; Simko, P.; Urbanska, N.; Kiskova, T. Exploring the Potential of Cannabinoid Nanodelivery Systems for CNS Disorders. Pharmaceutics 2023, 15, 204. [Google Scholar] [CrossRef]
- Kibret, B.G.; Ishiguro, H.; Horiuchi, Y.; Onaivi, E.S. New Insights and Potential Therapeutic Targeting of CB2 Cannabinoid Receptors in CNS Disorders. Int. J. Mol. Sci. 2022, 23, 975. [Google Scholar] [CrossRef]
- Vasincu, A.; Rusu, R.-N.; Ababei, D.-C.; Larion, M.; Bild, W.; Stanciu, G.D.; Solcan, C.; Bild, V. Endocannabinoid Modulation in Neurodegenerative Diseases: In Pursuit of Certainty. Biology 2022, 11, 440. [Google Scholar] [CrossRef]
- Khan, H.; Ghori, F.K.; Ghani, U.; Javed, A.; Zahid, S. Cannabinoid and Endocannabinoid System: A Promising Therapeutic Intervention for Multiple Sclerosis. Mol. Biol. Rep. 2022, 49, 5117–5131. [Google Scholar] [CrossRef] [PubMed]
- Al-Khazaleh, A.K.; Jaye, K.; Chang, D.; Münch, G.W.; Bhuyan, D.J. Buds and Bugs: A Fascinating Tale of Gut Microbiota and Cannabis in the Fight against Cancer. Int. J. Mol. Sci. 2024, 25, 872. [Google Scholar] [CrossRef] [PubMed]
- Vijay, A.; Kouraki, A.; Gohir, S.; Turnbull, J.; Kelly, A.; Chapman, V.; Barrett, D.A.; Bulsiewicz, W.J.; Valdes, A.M. The Anti-Inflammatory Effect of Bacterial Short Chain Fatty Acids Is Partially Mediated by Endocannabinoids. Gut Microbes 2021, 13, 1997559. [Google Scholar] [CrossRef] [PubMed]
- Suryavanshi, S.V.; Kovalchuk, I.; Kovalchuk, O. Cannabinoids as Key Regulators of Inflammasome Signaling: A Current Perspective. Front. Immunol. 2021, 11, 613613. [Google Scholar] [CrossRef]
- Rodríguez Mesa, X.M.; Moreno Vergara, A.F.; Contreras Bolaños, L.A.; Guevara Moriones, N.; Mejía Piñeros, A.L.; Santander González, S.P. Therapeutic Prospects of Cannabinoids in the Immunomodulation of Prevalent Autoimmune Diseases. Cannabis Cannabinoid Res. 2021, 6, 196–210. [Google Scholar] [CrossRef]
- Tamminga, S.J.; Emal, L.M.; Boschman, J.S.; Levasseur, A.; Thota, A.; Ruotsalainen, J.H.; Schelvis, R.M.; Nieuwenhuijsen, K.; Molen, H.F. Individual-level Interventions for Reducing Occupational Stress in Healthcare Workers. Cochrane Database Syst. Rev. 2023, 2023, CD002892. [Google Scholar] [CrossRef]
- Reuveni, N.; Carlson, C.A.; Schwartz, S.; Meter, D.; Barrett, T.S.; Freeman, S.M. The Antidepressant and Anxiolytic Effects of Cannabinoids in Chronic Unpredictable Stress: A Preclinical Systematic Review and Meta-Analysis. Transl. Psychiatry 2022, 12, 217. [Google Scholar] [CrossRef]
- Kwee, C.M.B.; van der Flier, F.E.; Duits, P.; van Balkom, A.J.L.M.; Cath, D.C.; Baas, J.M.P. Effects of Cannabidiol on Fear Conditioning in Anxiety Disorders: Decreased Threat Expectation during Retention, but No Enhanced Fear Re-Extinction. Psychopharmacology 2024, 241, 833–847. [Google Scholar] [CrossRef]
- Portugalov, A.; Peled, G.; Zorin, S.; Akirav, I. Cannabidiol Modulates Neuroinflammatory Markers in a PTSD Model Conducted on Female Rats. Biomolecules 2024, 14, 1384. [Google Scholar] [CrossRef]
- Shen, S.; Wu, C.; Lin, G.; Yang, X.; Zhou, Y.; Zhao, C.; Miao, Z.; Tian, X.; Wang, K.; Yang, Z.; et al. Structure-Based Identification of a G Protein–Biased Allosteric Modulator of Cannabinoid Receptor CB1. Proc. Natl. Acad. Sci. USA 2024, 121, e2321532121. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Yin, S.; He, Y.; Cao, Y.; Jiang, C. Biomaterials and Encapsulation Techniques for Probiotics: Current Status and Future Prospects in Biomedical Applications. Nanomaterials 2023, 13, 2185. [Google Scholar] [CrossRef] [PubMed]
- Jansma, J.; Brinkman, F.; van Hemert, S.; El Aidy, S. Targeting the Endocannabinoid System with Microbial Interventions to Improve Gut Integrity. Prog. Neuropsychopharmacol. Biol. Psychiatry 2021, 106, 110169. [Google Scholar] [CrossRef]
- Ji, S.; Xiong, M.; Chen, H.; Liu, Y.; Zhou, L.; Hong, Y.; Wang, M.; Wang, C.; Fu, X.; Sun, X. Cellular Rejuvenation: Molecular Mechanisms and Potential Therapeutic Interventions for Diseases. Signal Transduct. Target. Ther. 2023, 8, 116. [Google Scholar] [CrossRef] [PubMed]
- Brugnatelli, V.; Turco, F.; Freo, U.; Zanette, G. Irritable Bowel Syndrome: Manipulating the Endocannabinoid System as First-Line Treatment. Front. Neurosci. 2020, 14, 371. [Google Scholar] [CrossRef]
- Sihag, J.; Di Marzo, V. (Wh)Olistic (E)Ndocannabinoidome-Microbiome-Axis Modulation through (N)Utrition (WHEN) to Curb Obesity and Related Disorders. Lipids Health Dis. 2022, 21, 9. [Google Scholar] [CrossRef]
- Kandpal, M.; Indari, O.; Baral, B.; Jakhmola, S.; Tiwari, D.; Bhandari, V.; Pandey, R.K.; Bala, K.; Sonawane, A.; Jha, H.C. Dysbiosis of Gut Microbiota from the Perspective of the Gut–Brain Axis: Role in the Provocation of Neurological Disorders. Metabolites 2022, 12, 1064. [Google Scholar] [CrossRef]
- Aso, E.; Ferrer, I. CB2 Cannabinoid Receptor As Potential Target against Alzheimer’s Disease. Front. Neurosci. 2016, 10, 243. [Google Scholar] [CrossRef]
- Meng, E.X.; Verne, G.N.; Zhou, Q. Macrophages and Gut Barrier Function: Guardians of Gastrointestinal Health in Post-Inflammatory and Post-Infection Responses. Int. J. Mol. Sci. 2024, 25, 9422. [Google Scholar] [CrossRef]
- Martinez Ramirez, C.E.; Ruiz-Pérez, G.; Stollenwerk, T.M.; Behlke, C.; Doherty, A.; Hillard, C.J. Endocannabinoid Signaling in the Central Nervous System. Glia 2023, 71, 5–35. [Google Scholar] [CrossRef]
- Vasincu, A.; Rusu, R.-N.; Ababei, D.-C.; Neamțu, M.; Arcan, O.D.; Macadan, I.; Beșchea Chiriac, S.; Bild, W.; Bild, V. Exploring the Therapeutic Potential of Cannabinoid Receptor Antagonists in Inflammation, Diabetes Mellitus, and Obesity. Biomedicines 2023, 11, 1667. [Google Scholar] [CrossRef] [PubMed]
- Almogi-Hazan, O.; Or, R. Cannabis, the Endocannabinoid System and Immunity—The Journey from the Bedside to the Bench and Back. Int. J. Mol. Sci. 2020, 21, 4448. [Google Scholar] [CrossRef] [PubMed]
- Shaman, J.A. The Future of Pharmacogenomics: Integrating Epigenetics, Nutrigenomics, and Beyond. J. Pers. Med. 2024, 14, 1121. [Google Scholar] [CrossRef]
- Vora, L.K.; Gholap, A.D.; Jetha, K.; Thakur, R.R.S.; Solanki, H.K.; Chavda, V.P. Artificial Intelligence in Pharmaceutical Technology and Drug Delivery Design. Pharmaceutics 2023, 15, 1916. [Google Scholar] [CrossRef]
| References | Focus Area | Key Findings and Implications | ECS Components | Mechanisms Explored | Relevance to Therapeutics |
|---|---|---|---|---|---|
| Neuroscience and Neuroprotection | |||||
| [13] | Neurodegeneration | ECS modulation reduces neuroinflammation and supports cognitive function | CB1, CB2 | Microglial phagocytosis, neuroprotection | Neuroprotective strategies in Alzheimer’s disease |
| [14] | Neuroprotection | ECS reduces neurodegeneration and slows MS progression | CB2 | Immune modulation, neuroprotection | Neuroprotective approaches in multiple sclerosis |
| [15] | Neurodegenerative Disease | CB2 enhances amyloid-beta clearance and limits inflammation | CB2 | Microglial activation control | Alzheimer’s disease therapy |
| [16] | Brain Development | CB1 influences neural progenitor differentiation and cortical patterning | CB1 | Neurodevelopmental signaling, Wnt/β-catenin pathway | Developmental neurobiology insights |
| [17] | Synaptic Plasticity | ECS modulates synaptic strength and supports LTP/LTD balance | CB1 | Synaptic transmission modulation | Cognitive enhancement and memory therapies |
| [18] | Cognitive Health | ECS improves cognitive flexibility and reduces stress-induced impairment | CB1 | HPA axis regulation, synaptic modulation | Stress resilience and cognitive therapy |
| [19] | Mood Regulation | CB1 regulates emotional response and stress resilience | CB1 | HPA axis modulation, amygdala activity | ECS-based anxiolytic and antidepressant therapies |
| Microbiota–Gut–Brain Axis | |||||
| [20] | Microbiota–Gut–Brain | SCFAs modulate ECS signaling, enhancing gut–brain communication | CB1, CB2 | SCFA–ECS cross-talk | Gut–brain therapies for mental health |
| [21] | Gut–Brain Axis | ECS regulates gut motility and visceral inflammation | CB1 | Enteric signaling modulation | Gut–brain axis-targeted therapies |
| [22] | Gut–Immune Interaction | ECS mediates immune tolerance and reduces dysbiosis-driven inflammation | CB2 | Microbiota–immune signaling | Microbiota-based immune therapies |
| [23] | Gut Motility | CB1 activation regulates peristalsis and gut microbial composition | CB1 | Enteric nervous system signaling | GI motility and IBS interventions |
| [24] | Gut–Immune Balance | SCFAs induce CB2 upregulation, supporting immune tolerance | CB2 | SCFA–CB2 interaction | Gut-microbiota-linked immune regulation |
| Immunology and Autoimmune Regulation | |||||
| [25] | Immunology | Cannabinoids modulate T cell polarization and cytokine balance | CB2 | Cytokine modulation, immunosuppression | Autoimmune and inflammatory disease therapies |
| [26] | Autoimmune Disorders | CB2 reduces immune cell infiltration and promotes myelin protection | CB2 | T cell migration control | Multiple sclerosis treatment pathways |
| [27] | Autoimmunity | CB2 activation suppresses T cell activity and inflammatory cytokines | CB2 | Immune regulation | Autoimmune disease intervention |
| [28] | Autoimmunity | CB2 downregulates inflammatory cytokines and restores immune tolerance | CB2 | Cytokine modulation | Therapeutic immune regulation |
| [29] | Immune Regulation | CB2 stabilizes cytokine networks and maintains immune homeostasis | CB2 | Cytokine balance mechanisms | Anti-inflammatory therapy design |
| Gastrointestinal and Metabolic Health | |||||
| [30] | Gut Barrier Function | CB1/CB2 maintain epithelial integrity and reduce permeability | CB1, CB2 | Tight junction stabilization, anti-inflammatory signaling | Barrier reinforcement in IBD |
| [31] | Inflammatory Bowel Disease | ECS signaling suppresses inflammatory cytokines and restores mucosal health | CB1, CB2 | TNF-α/IL-1β inhibition | Novel IBD therapies |
| [32] | Metabolic Health | CB1 modulates adipogenesis and metabolic regulation | CB1 | Lipid metabolism control | Metabolic syndrome treatment |
| [33] | Obesity and Metabolism | CB1 influences lipid storage and insulin sensitivity | CB1 | Lipogenesis and glucose regulation | Anti-obesity pharmacological targets |
| Pain, Stress, and Epigenetic Modulation | |||||
| [34] | Pain Management | CB1–TRPV1 co-activation reduces chronic pain perception | CB1, TRPV1 | Pain desensitization, neurotransmission control | Chronic pain relief |
| [35] | Chronic Pain | TRPV1–CB1 interaction mitigates neuropathic pain | CB1, TRPV1 | Receptor cross-talk modulation | Neuropathic pain treatment |
| [36] | Epigenetic Modulation | Stress-induced CB1 methylation alters ECS adaptability | CB1 | DNA methylation, histone modification | Epigenetic strategies in stress disorders |
| References | Focus | Methods | Key Findings | Implications |
|---|---|---|---|---|
| [145] | ECS in anxiety-like behavior due to EMR | Mouse model, dual-frequency EMR exposure, CB1 agonist/antagonist treatment, corticosterone/CRH levels. | Dual-frequency EMR reduced CB1 expression, lowered 2-AG levels, and induced anxiety; CB1 agonist alleviated anxiety. | CB1 agonists may counteract EMR-induced anxiety disorders. |
| [146] | FAAH-OUT and pain insensitivity | Genetic analysis, endocannabinoid level measurements, BDNF expression in fibroblasts. | FAAH-OUT deletion reduced FAAH, increased anandamide, and elevated BDNF expression. | FAAH-OUT deletion underpins pain insensitivity; potential for new pain treatments. |
| [147] | Endocannabinoid reuptake inhibitors | Chemical probe synthesis, in vivo behavioral assays, ECS activity measurement. | Novel inhibitors increased ECS signaling and produced cannabimimetic effects in mice. | Promising ECS modulators for treating ECS-related disorders. |
| [148] | ECS drug discovery | Screening ECS-targeting compounds for potency, receptor selectivity, and therapeutic effects. | Identified selective ECS modulators with minimal off-target effects. | New ECS drugs for inflammation, pain, and metabolic conditions. |
| [149] | Guineensine as an ECS uptake inhibitor | Pharmacological profiling, uptake inhibition assays, behavioral tests in mice. | Guineensine effectively inhibited endocannabinoid uptake, enhancing ECS signaling and producing cannabimimetic behavioral effects in mice. | Potential new lead for ECS-targeted therapy. |
| [150] | ECS in epilepsy | Mouse epilepsy models, CB1/CB2 pharmacological modulation, ECS component profiling. | ECS modulates seizure thresholds; CB1 activation reduced seizures, while CB2 regulated inflammation. | ECS-targeted therapies may improve epilepsy management. |
| [151] | Caffeine–CB1 receptor interaction | Animal models, CB1 receptor assays, striatum neurotransmitter analysis. | Caffeine increased CB1 activity and altered striatal neurotransmitter levels. | Insights into caffeine’s impact on ECS-mediated neural signaling. |
| [152] | CB1 and social behavior | CB1 expression in starling brains, behavioral observation, correlation analysis. | CB1 expression correlated with social dominance and behavior. | ECS involvement in social hierarchies and behavior regulation. |
| [153] | Antimicrobial properties of ECS compounds | Screening cannabis-derived compounds for bacterial inhibition, including antibiotic-resistant strains. | Cannabis compounds showed potent antimicrobial effects, especially on resistant strains. | Basis for developing ECS-derived antibiotics. |
| [154] | ECS and gut microbiota interactions | Fecal microbiota transplantation in mice, ECS modulation analysis, microbiome composition measurement. | ECS activity modulated gut microbiota, influencing stress responses and intestinal inflammation. | ECS–microbiota interplay as a therapeutic target for gut and mental health disorders. |
| [155] | ECS modulation in neuroinflammation | In vitro studies on microglial cells, CB2 receptor agonist treatment, inflammatory cytokine measurement. | CB2 activation reduced pro-inflammatory cytokine release in microglial cells. | Targeting CB2 receptors may offer therapeutic strategies for neuroinflammatory conditions. |
| [156] | ECS involvement in metabolic syndrome | Clinical study measuring endocannabinoid levels in patients with metabolic syndrome, correlation analysis. | Elevated endocannabinoid levels correlated with increased insulin resistance and adiposity. | ECS modulation could be a potential therapeutic approach for managing metabolic syndrome. |
| [157] | Role of ECS in osteoarthritis pain | Animal model of osteoarthritis, intra-articular injection of CB1/CB2 agonists, pain behavior assessment. | Activation of CB2 receptors reduced pain behaviors in osteoarthritic animals. | CB2 receptor agonists may serve as novel analgesics for osteoarthritis pain management. |
| [158] | ECS and stress-induced immunosuppression | Human study assessing endocannabinoid levels before and after acute stress, immune cell activity measurement. | Acute stress increased endocannabinoid levels, which correlated with reduced immune cell activity. | ECS plays a role in stress-induced immunosuppression; potential target for stress-related immune dysfunction. |
| [159] | ECS regulation of appetite in obesity | Obese mouse model, administration of CB1 receptor antagonist, food intake and weight monitoring. | CB1 antagonist reduced food intake and body weight in obese mice. | CB1 receptor antagonists may be effective in controlling appetite and weight in obesity treatment. |
| [160] | ECS and cancer cell proliferation | In vitro study on cancer cell lines, treatment with CB1/CB2 agonists, cell proliferation assays. | CB1 activation promoted, while CB2 activation inhibited, cancer cell proliferation. | Differential roles of CB1 and CB2 in cancer cell growth suggest receptor-specific therapeutic strategies. |
| [161] | ECS involvement in neuropathic pain | Rat model of nerve injury, administration of FAAH inhibitor, pain sensitivity assessment. | FAAH inhibition increased endocannabinoid levels and reduced neuropathic pain behaviors. | FAAH inhibitors may be potential treatments for neuropathic pain. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Șerban, M.; Toader, C.; Covache-Busuioc, R.-A. The Endocannabinoid System in Human Disease: Molecular Signaling, Receptor Pharmacology, and Therapeutic Innovation. Int. J. Mol. Sci. 2025, 26, 11132. https://doi.org/10.3390/ijms262211132
Șerban M, Toader C, Covache-Busuioc R-A. The Endocannabinoid System in Human Disease: Molecular Signaling, Receptor Pharmacology, and Therapeutic Innovation. International Journal of Molecular Sciences. 2025; 26(22):11132. https://doi.org/10.3390/ijms262211132
Chicago/Turabian StyleȘerban, Matei, Corneliu Toader, and Răzvan-Adrian Covache-Busuioc. 2025. "The Endocannabinoid System in Human Disease: Molecular Signaling, Receptor Pharmacology, and Therapeutic Innovation" International Journal of Molecular Sciences 26, no. 22: 11132. https://doi.org/10.3390/ijms262211132
APA StyleȘerban, M., Toader, C., & Covache-Busuioc, R.-A. (2025). The Endocannabinoid System in Human Disease: Molecular Signaling, Receptor Pharmacology, and Therapeutic Innovation. International Journal of Molecular Sciences, 26(22), 11132. https://doi.org/10.3390/ijms262211132






