Inside the Matrix: Integrated Cytology and Molecular Testing of Thyroid FNAC Samples Using a Commercial Synthetic 3D Scaffold
Abstract
1. Introduction
2. Results
2.1. Study Population
2.2. Histopathology Outcomes
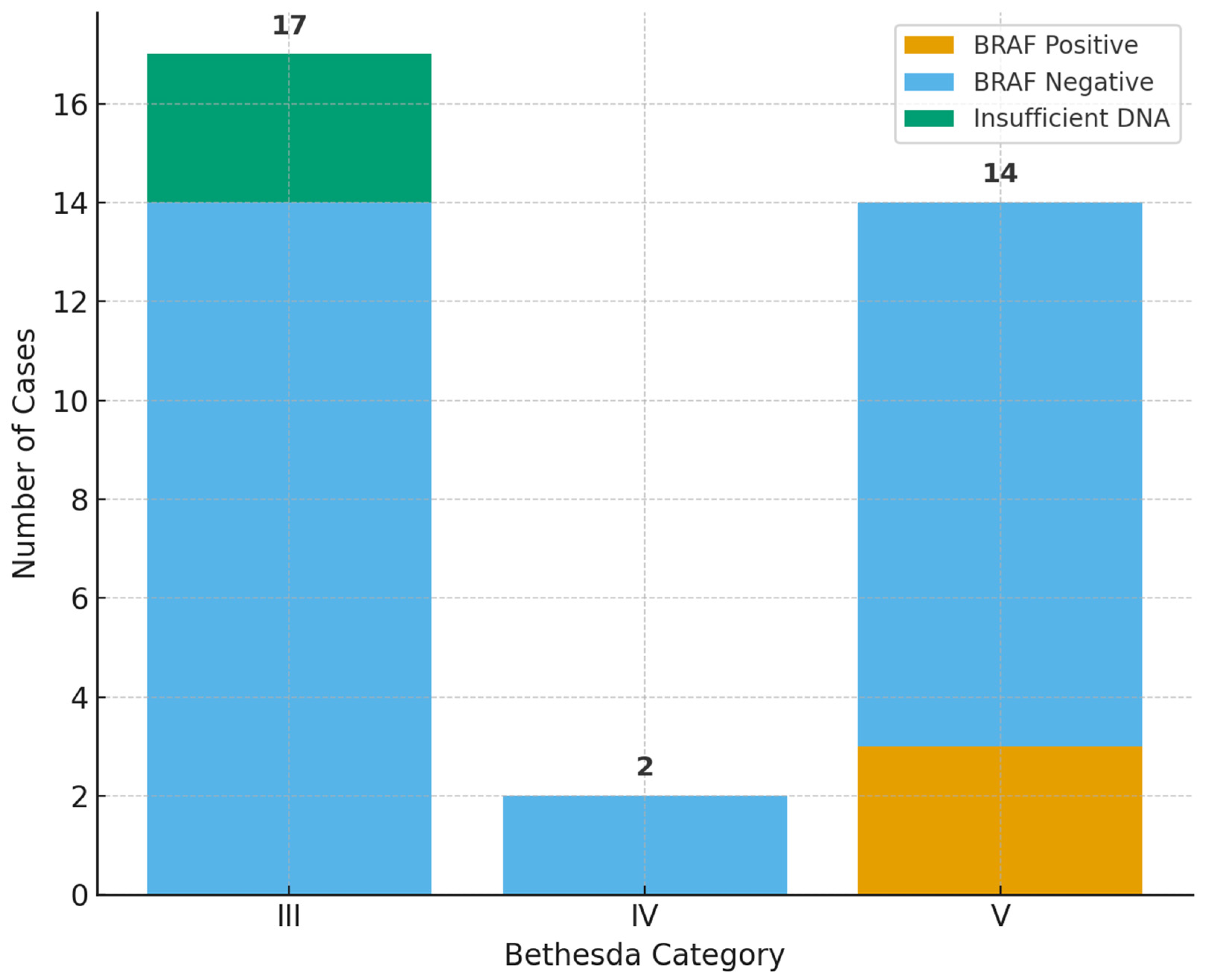
2.3. BRAF V600E Results
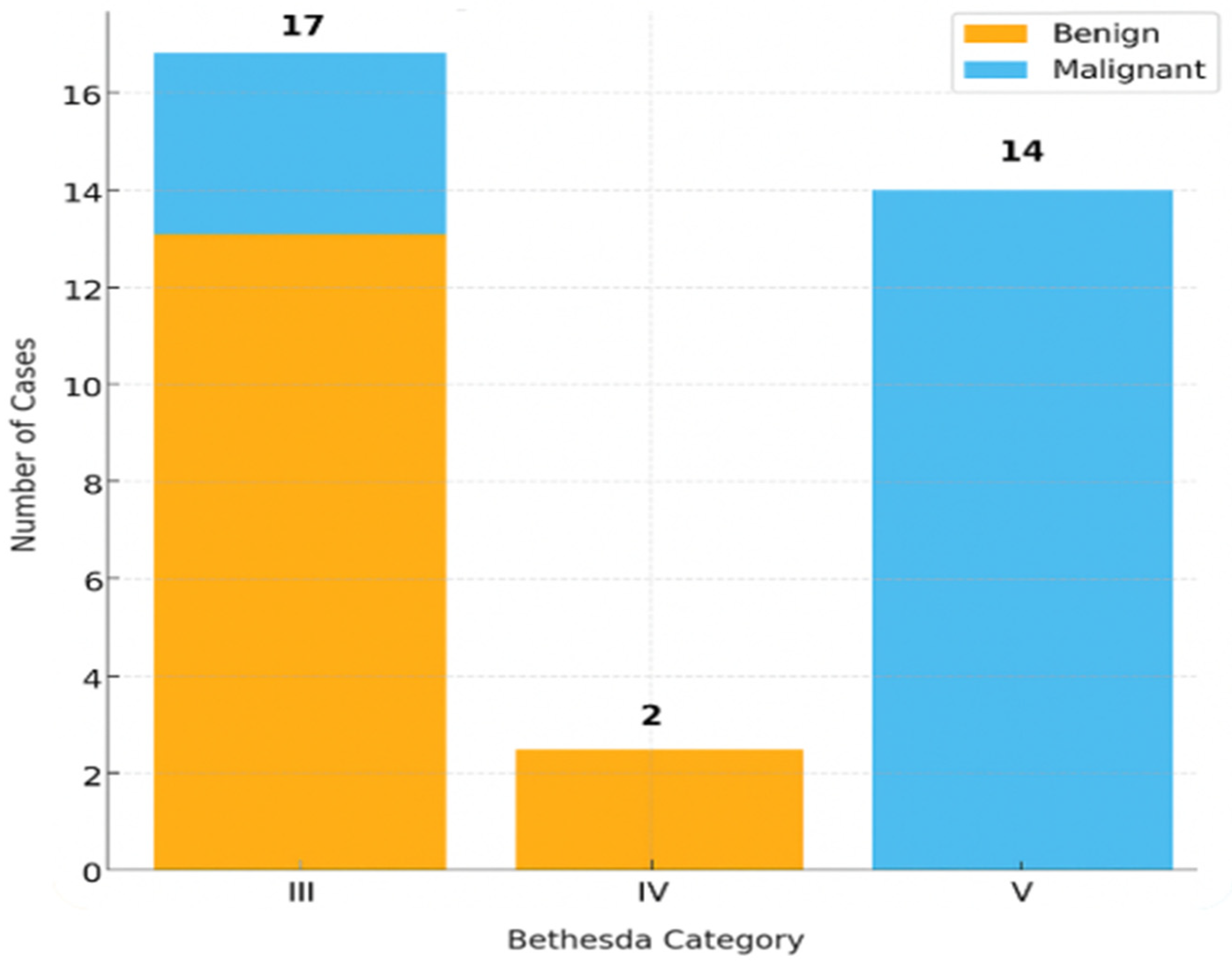
2.4. Bethesda Category Distribution and Patterns
2.5. DNA Quantity in Relation to Outcomes
3. Discussion
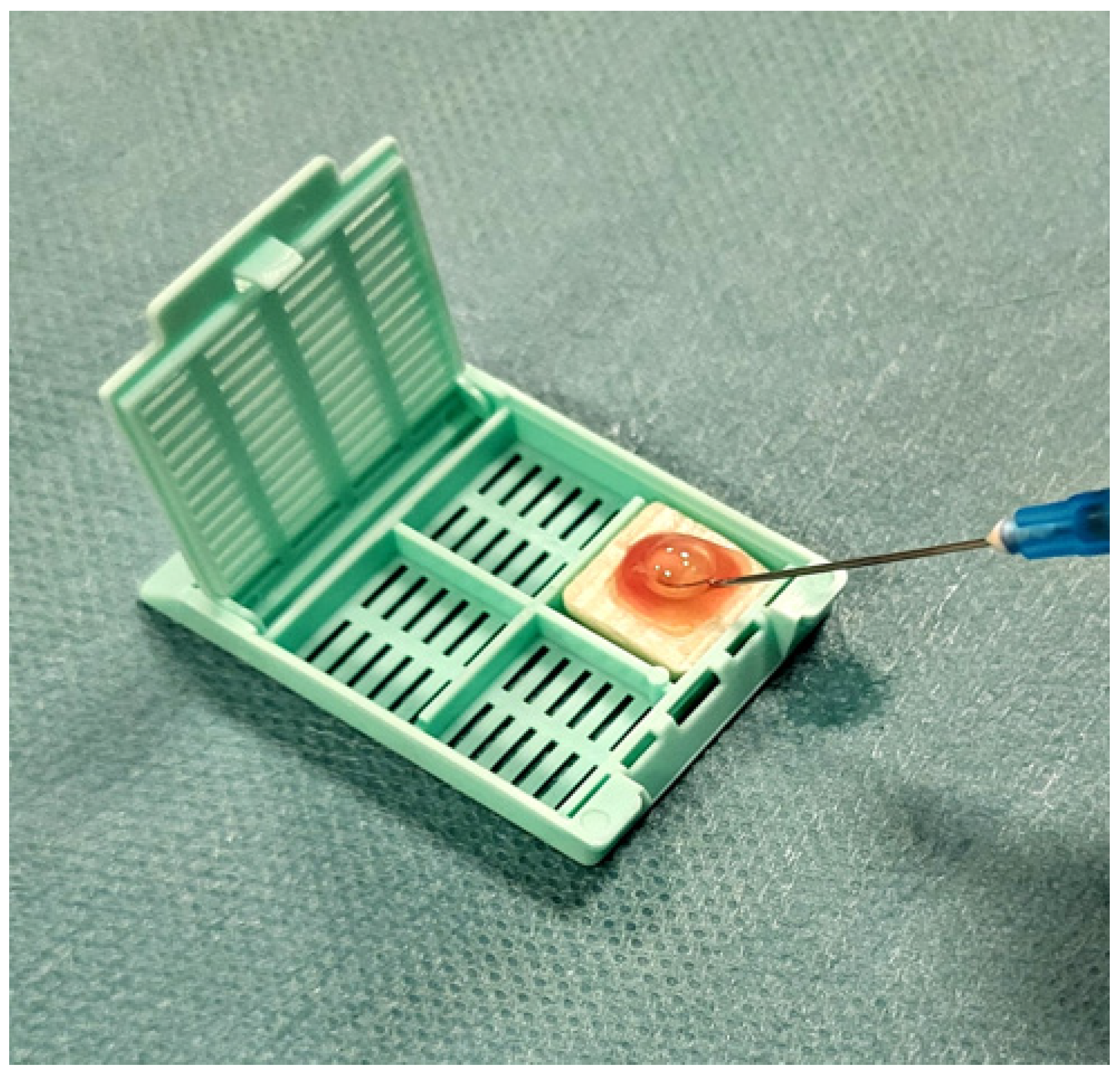
3.1. CytoMatrix as a Technical Solution to Cytology Limitations
3.2. BRAF V600E as a Proof of the Concept
3.3. Comparison with Previous Literature
3.4. Clinical Implications
3.5. Integration with Bethesda System
3.6. Strengths and Limitations
3.7. Future Perspectives
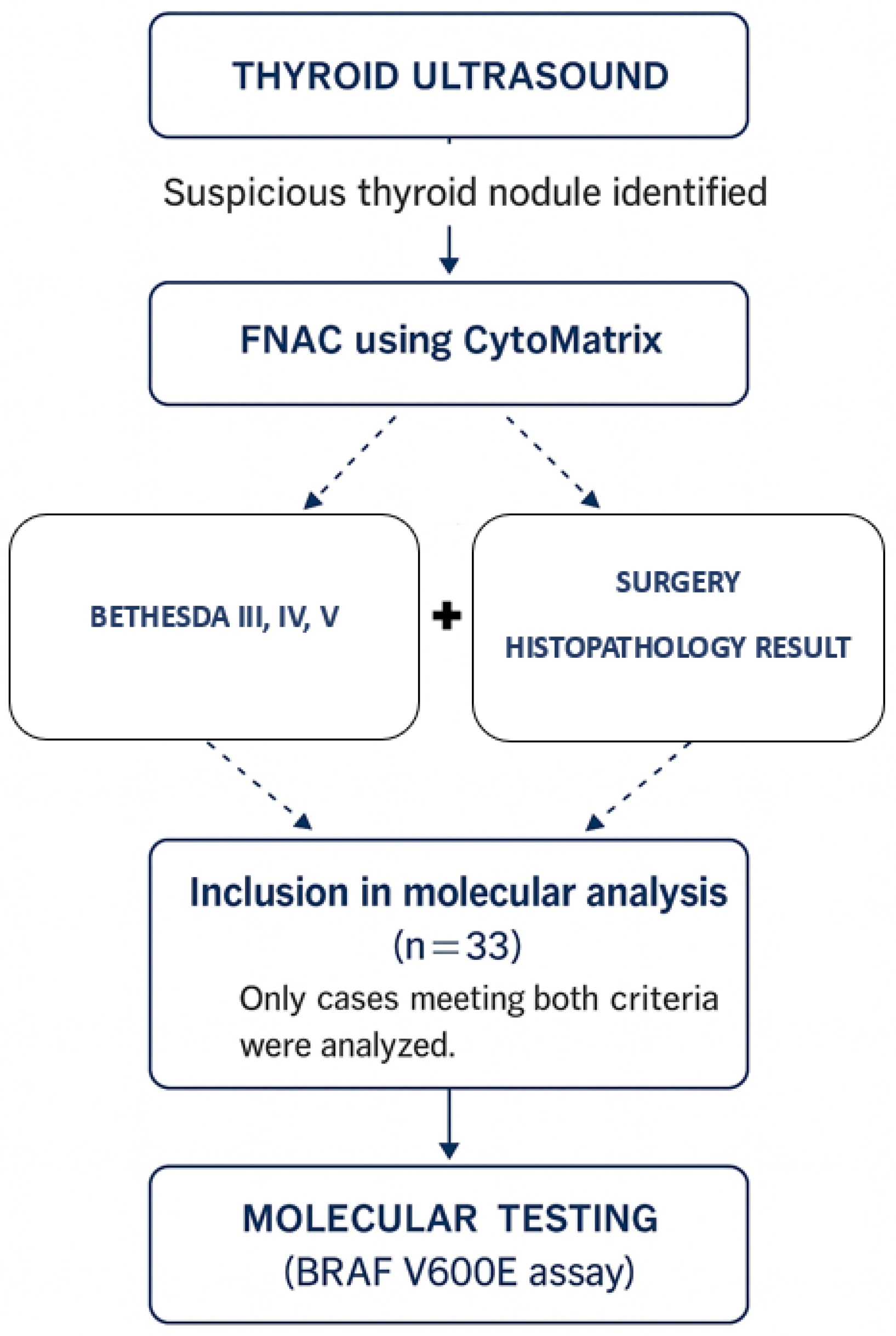
4. Materials and Methods
4.1. Study Design and Population
4.2. Inclusion and Exclusion Criteria
4.3. Sample Processing and Cytological Classification
4.4. DNA Extraction and Molecular Testing
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| EU-TIRADS | European Thyroid Imaging Reporting and Data System |
| DNA | Deoxyribonucleic Acid |
| FNAC | Fine-Needle Aspiration Cytology |
| PCR | Polymerase Chain Reaction |
| FFPE | Formalin-Fixed Paraffin-Embedded |
| PTC | Papillary Thyroid Carcinoma |
| FVPTC | Follicular Variant of Papillary Thyroid Carcinoma |
| ESMO | European Society for Medical Oncology |
| WHO | World Health Organization |
References
- Khatami, F.; Tavangar, S.M. A Review of Driver Genetic Alterations in Thyroid Cancers. Iran. J. Pathol. 2018, 13, 125–135. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Popa, R.-M.; Mustață, D.-M.; Ionel, I.; Balogh, R.-M. Metal Element Traces Sampled from Peri-Urban Road Verge Particulate Matter. Appl. Sci. 2023, 13, 11649. [Google Scholar] [CrossRef]
- Choi, W.J.; Kim, J. Dietary factors and the risk of thyroid cancer: A review. Clin. Nutr. Res. 2014, 3, 75–88. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mustață, D.M.; Ionel, I.; Bisorca, D.; Balogh, R.M. Roof up or Down: Exploring Particulate Matter and Noise Pollution Dynamics in Convertible Vehicles. In CONAT 2024 International Congress of Automotive and Transport Engineering; Proceedings in Automotive Engineering; Springer: Berlin, Germany. [CrossRef]
- Zahid, A.; Shafiq, W.; Nasir, K.S.; Loya, A.; Raza, S.A.; Sohail, S.; Azmat, U. Malignancy rates in thyroid nodules classified as Bethesda categories III and IV; a subcontinent perspective. J. Clin. Transl. Endocrinol. 2021, 23, 100250. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Latia, M.; Borlea, A.; Mihuta, M.S.; Neagoe, O.C.; Stoian, D. Impact of ultrasound elastography in evaluating Bethesda category IV thyroid nodules with histopathological correlation. Front. Endocrinol. 2024, 15, 1393982. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Feroci, F.; Perini, D.; Giordano, A.; Romoli, L.; Guagni, T.; Coppola, A.; Giani, I.; Checchi, S.; Petrucci, A.; Sarno, A.; et al. ACR TI-RADS Score combined with cytopathology classification improves the risk stratification of indeterminate thyroid nodules. Minerva Endocrinol. 2025, 50, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Hannoush, Z.C.; Ruiz-Cordero, R.; Jara, M.; Kargi, A.Y. Current State of Molecular Cytology in Thyroid Nodules: Platforms and Their Diagnostic and Theranostic Utility. J. Clin. Med. 2024, 13, 1759. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bonucci, M.; Minelli, S.; Castro, C.L.; Camponi, C.; Scimeca, M.; Scipioni, A.; Spugnini, E.; Baldi, A. Cytomatrix: A new procedure to enhance the diagnostic usefulness of fine-needle aspirates. Ann. Res. Oncol. 2021, 1, 192–198. [Google Scholar] [CrossRef]
- Patel, J.; Klopper, J.; Cottrill, E.E. Molecular diagnostics in the evaluation of thyroid nodules: Current use and prospective opportunities. Front. Endocrinol. 2023, 14, 1101410. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chong, Y.; Ji, S.-J.; Kang, C.S.; Lee, E.J. Can liquid-based preparation substitute for conventional smear in thyroid fine-needle aspiration? A systematic review based on meta-analysis. Endocr. Connect. 2017, 6, 817–829. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Malukani, K.; Matreja, S.S.; Nandedkar, S.S.; Varma, A.V.; Saxena, A.; Ajmera, A. Comparison of efficacy of cell block versus conventional smear study in exudative fluids. Niger. Postgrad. Med. J. 2017, 24, 245–249. [Google Scholar] [CrossRef] [PubMed]
- Oktay, M.H.; Adler, E.; Hakima, L.; Grunblatt, E.; Pieri, E.; Seymour, A.; Khader, S.; Cajigas, A.; Suhrland, M.; Goswami, S. The Application of Molecular Diagnostics to Stained Cytology Smears. J. Mol. Diagn. 2016, 18, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Saharti, S. The diagnostic value of add-on thyroid cell block in the evaluation of thyroid lesions. Cytojournal 2023, 20, 3. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nikiforov, Y.E.; Steward, D.L.; Robinson-Smith, T.M.; Haugen, B.R.; Klopper, J.P.; Zhu, Z.; Fagin, J.A.; Falciglia, M.; Weber, K.; Nikiforova, M.N. Molecular testing for mutations in improving the fine-needle aspiration diagnosis of thyroid nodules. J. Clin. Endocrinol. Metab. 2009, 94, 2092–2098. [Google Scholar] [CrossRef] [PubMed]
- Rossi, E.D.; Vielh, P. Thyroid and Molecular Testing. Advances in Thyroid Molecular Cytopathology. J. Mol. Pathol. 2021, 2, 77–92. [Google Scholar] [CrossRef]
- Alzumaili, B.; Sadow, P.M. Update on Molecular Diagnostics in Thyroid Pathology: A Review. Genes 2023, 14, 1314. [Google Scholar] [CrossRef]
- Streinu, D.-R.; Neagoe, O.C.; Borlea, A.; Icma, I.; Derban, M.; Stoian, D. Enhancing diagnostic precision in thyroid nodule assessment: Evaluating the efficacy of a novel cell preservation technique in fine-needle aspiration cytology. Front. Endocrinol. 2024, 15, 1438063. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- La Nuova Frontiera Della Citologia. Available online: https://Cytomatrix.It/ (accessed on 3 September 2025).
- Montella, M.; Cozzolino, I.; Marino, F.Z.; Clery, E.; Carraturo, E.; Brancaccio, G.; Argenziano, G.; Troiani, T.; Savarese, G.; Berretta, M.; et al. Application of CytoMatrix for the diagnosis of melanoma metastases on FNA cytology samples: Performance of a novel cell block method. Cancer Cytopathol. 2023, 131, 516–525. [Google Scholar] [CrossRef] [PubMed]
- Verri, M.; Scarpino, S.; Naciu, A.M.; Lopez, G.; Tabacco, G.; Taffon, C.; Pilozzi, E.; Palermo, A.; Crescenzi, A. Real-Time Evaluation of Thyroid Cytology Using New Digital Microscopy Allows for Sample Adequacy Assessment, Morphological Classification, and Supports Molecular Analysis. Cancers 2023, 15, 4215. [Google Scholar] [CrossRef]
- De Leo, A.; Serban, D.; Maloberti, T.; Sanza, V.; Coluccelli, S.; Altimari, A.; Gruppioni, E.; Chiarucci, F.; Corradini, A.G.; Repaci, A.; et al. Expanding the Spectrum of BRAF Non-V600E Mutations in Thyroid Nodules: Evidence-Based Data from a Tertiary Referral Centre. Int. J. Mol. Sci. 2023, 24, 4057. [Google Scholar] [CrossRef] [PubMed]
- Forma, A.; Kłodnicka, K.; Pająk, W.; Flieger, J.; Teresińska, B.; Januszewski, J.; Baj, J. Thyroid Cancer: Epidemiology, Classification, Risk Factors, Diagnostic and Prognostic Markers, and Current Treatment Strategies. Int. J. Mol. Sci. 2025, 26, 5173. [Google Scholar] [CrossRef]
- Attia, A.S.; Hussein, M.; Issa, P.P.; Elnahla, A.; Farhoud, A.; Magazine, B.M.; Youssef, M.R.; Aboueisha, M.; Shama, M.; Toraih, E.; et al. Association of BRAFV600E Mutation with the Aggressive Behavior of Papillary Thyroid Microcarcinoma: A Meta-Analysis of 33 Studies. Int. J. Mol. Sci. 2022, 23, 15626. [Google Scholar] [CrossRef]
- Cantara, S.; Marzocchi, C.; Pilli, T.; Cardinale, S.; Forleo, R.; Castagna, M.G.; Pacini, F. Molecular Signature of Indeterminate Thyroid Lesions: Current Methods to Improve Fine Needle Aspiration Cytology (FNAC) Diagnosis. Int. J. Mol. Sci. 2017, 18, 775. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- George, N.; Chu, S.; Manning, S.; Lim, K.-Z.; Mond, M.; Tay, E.; Yellapu, B.; Jones, K.; Fellowes, A.; Kumar, B.; et al. Tiered approach to molecular testing of thyroid fine needle aspiration samples may improve preoperative diagnosis. Eur. J. Surg. Oncol. 2025, 51, 110082. [Google Scholar] [CrossRef] [PubMed]
- Al-Shobaki, T.; Al-Najjar, H.; Iskanderian, R.; Younis, E.; Abdallah, N.; Tbakhi, A.; Haddad, H.; Al-Masri, M.; Obeid, Z.; Jarrar, A. BRAF V600E mutation in papillary thyroid carcinoma: It’s relation to clinical features and oncologic outcomes in a single cancer centre experience. Endocr. Connect. 2021, 10, 1531–1537. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bruschini, S.; di Martino, S.; Pisanu, M.E.; Fattore, L.; De Vitis, C.; Laquintana, V.; Buglioni, S.; Tabbì, E.; Cerri, A.; Visca, P.; et al. CytoMatrix for a reliable and simple characterization of lung cancer stem cells from malignant pleural effusions. J. Cell. Physiol. 2020, 235, 1877–1887. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Generi Biotech. ONCO-BRAF Real-Time PCR Detection Kit Product Information. Generi Biotech s.r.o. Available online: https://www.generi-biotech.com/catalogue.php?p=1474&lang=en&type=0 (accessed on 5 October 2025).
- Cheng, L.Y.; Haydu, L.E.; Song, P.; Nie, J.; Tetzlaff, M.T.; Kwong, L.N.; Gershenwald, J.E.; Davies, M.A.; Zhang, D.Y. High sensitivity sanger sequencing detection of BRAF mutations in metastatic melanoma FFPE tissue specimens. Sci. Rep. 2021, 11, 9043. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zhao, N.; Chen, D.; Wei, K.; Su, N.; Huang, J.-F.; Xu, H.-Q.; Duan, G.-J.; Fu, W.-L.; Huang, Q. Improved detection of BRAF V600E using allele-specific PCR coupled with external and internal controllers. Sci. Rep. 2017, 7, 13817. [Google Scholar] [CrossRef]
- Rindi, G.; Mete, O.; Uccella, S.; Basturk, O.; La Rosa, S.; Brosens, L.A.A.; Ezzat, S.; de Herder, W.W.; Klimstra, D.S.; Papotti, M.; et al. Overview of the 2022 WHO Classification of Neuroendocrine Neoplasms. Endocr. Pathol. 2022, 33, 115–154. [Google Scholar] [CrossRef] [PubMed]
- Angell, T.E.; Lechner, M.G.; Jang, J.K.; Correa, A.J.; LoPresti, J.S.; Epstein, A.L. BRAF V600E in papillary thyroid carcinoma is associated with increased programmed death ligand 1 expression and suppressive immune cell infiltration. Thyroid 2014, 24, 1385–1393. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gupta, S.; Dey, P. Diagnostic challenges in the gray-zone lesions of fine-needle aspiration cytology. Cytojournal 2021, 18, 23. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Khan, S.; Omar, T.; Michelow, P. Effectiveness of the cell block technique in diagnostic cytopathology. J. Cytol. 2012, 29, 177–182. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lu, H.-Z.; Qiu, T.; Ying, J.-M.; Lyn, N. Association between BRAFV600E mutation and the clinicopathological features of solitary papillary thyroid microcarcinoma. Oncol. Lett. 2017, 13, 1595–1600. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Howell, G.M.; Hodak, S.P.; Yip, L. RAS mutations in thyroid cancer. Oncologist 2013, 18, 926–932. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Accardo, G.; Conzo, G.; Esposito, D.; Gambardella, C.; Mazzella, M.; Castaldo, F.; Di Donna, C.; Polistena, A.; Avenia, N.; Colantuoni, V.; et al. Genetics of medullary thyroid cancer: An overview. Int. J. Surg. 2017, 41 (Suppl. S1), S2–S6. [Google Scholar] [CrossRef] [PubMed]
- Nikiforova, M.N.; Mercurio, S.; Wald, A.I.; de Moura, M.B.; Callenberg, K.; Santana-Santos, L.; Gooding, W.E.; Yip, L.; Ferris, R.L.; Nikiforov, Y.E. Analytical performance of the ThyroSeq v3 genomic classifier for cancer diagnosis in thyroid nodules. Cancer 2018, 124, 1682–1690. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vignali, P.; Macerola, E.; Poma, A.M.; Sparavelli, R.; Basolo, F. Indeterminate Thyroid Nodules: From Cytology to Molecular Testing. Diagnostics 2023, 13, 3008. [Google Scholar] [CrossRef]
- Oh, M.Y.; Choi, H.M.; Jang, J.; Son, H.; Park, S.S.; Song, M.; Kim, Y.H.; Cho, S.W.; Chai, Y.J.; Chung, W.; et al. Small Multi-Gene DNA Panel Can Aid in Reducing the Surgical Resection Rate and Predicting the Malignancy Risk of Thyroid Nodules. Endocrinol. Metab. 2024, 39, 777–792. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Capezzone, M.; Rossi, M.; Bardi, S.; Morabito, E.M.; Dalmazio, G.; Iapichino, G.; Galassi, S.; Seralessandri, S.; Torregrossa, L.; Tosti Balducci, M.; et al. Diagnostic Performance of Next-Generation Sequencing (NGS) in Indeterminate Thyroid Nodules: A Single Hospital Experience. Int. J. Mol. Sci. 2025, 26, 4225. [Google Scholar] [CrossRef]
- Cabané, P.; Correa, C.; Bode, I.; Aguilar, R.; Elorza, A.A. Biomarkers in Thyroid Cancer: Emerging Opportunities from Non-Coding RNAs and Mitochondrial Space. Int. J. Mol. Sci. 2024, 25, 6719. [Google Scholar] [CrossRef]
- Bass, B.P.; Engel, K.B.; Greytak, S.R.; Moore, H.M. A review of preanalytical factors affecting molecular, protein, and morphological analysis of formalin-fixed, paraffin-embedded (FFPE) tissue: How well do you know your FFPE specimen? Arch. Pathol. Lab. Med. 2014, 138, 1520–1530. [Google Scholar] [CrossRef] [PubMed]
- Anvari, M.S.; Gharib, A.; Abolhasani, M.; Azari-Yaam, A.; Gharalari, F.H.; Safavi, M.; Zare-Mirzaie, A.; Vasei, M. Pre-analytical Practices in the Molecular Diagnostic Tests, A Concise Review. Iran. J. Pathol. 2021, 16, 1–19. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wong, S.Q.; Li, J.; Tan, A.Y.; Vedururu, R.; Pang, J.M.; Do, H.; Ellul, J.; Doig, K.; Bell, A.; MacArthur, G.A.; et al. CANCER 2015 Cohort. Sequence artefacts in a prospective series of formalin-fixed tumours tested for mutations in hotspot regions by massively parallel sequencing. BMC Med. Genom. 2014, 7, 23. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- A Steiert, T.; Parra, G.; Gut, M.; Arnold, N.; Trotta, J.-R.; Tonda, R.; Moussy, A.; Gerber, Z.; Abuja, P.M.; Zatloukal, K.; et al. A critical spotlight on the paradigms of FFPE-DNA sequencing. Nucleic Acids Res. 2023, 51, 7143–7162. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lu, H.; Qiu, T.; Ying, J.; Guo, C.; Lyn, N. Correlation between BRAF V600E mutation and clinicopathologic features of papillary thyroid carcinoma. Chin. J. Pathol. 2014, 43, 794–798. (In Chinese) [Google Scholar] [PubMed]
- Goh, X.; Lum, J.; Yang, S.P.; Chionh, S.B.; Koay, E.; Chiu, L.; Parameswaran, R.; Ngiam, K.Y.; Loh, T.K.S.; Nga, M.E.; et al. BRAF mutation in papillary thyroid cancer-Prevalence and clinical correlation in a South-East Asian cohort. Clin. Otolaryngol. 2019, 44, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Uno, D.; Endo, K.; Yoshikawa, T.; Hirai, N.; Kobayashi, E.; Nakanishi, Y.; Kondo, S.; Yoshizaki, T. Correlation between gene mutations and clinical characteristics in papillary thyroid cancer: A retrospective analysis of BRAF mutations and RET rearrangements. Thyroid Res. 2024, 17, 21. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cho, Y.Y.; Park, S.Y.; Shin, J.H.; Oh, Y.L.; Choe, J.-H.; Kim, J.-H.; Kim, J.S.; Yim, H.S.; Kim, Y.-L.; Ki, C.-S.; et al. Highly Sensitive and Specific Molecular Test for Mutations in the Diagnosis of Thyroid Nodules: A Prospective Study of BRAF-Prevalent Population. Int. J. Mol. Sci. 2020, 21, 5629. [Google Scholar] [CrossRef]
- Jinih, M.; Foley, N.; Osho, O.; Houlihan, L.; Toor, A.; Khan, J.; Achakzai, A.; Redmond, H. BRAFV600E mutation as a predictor of thyroid malignancy in indeterminate nodules: A systematic review and meta-analysis. Eur. J. Surg. Oncol. 2017, 43, 1219–1227. [Google Scholar] [CrossRef] [PubMed]
- Valderrabano, P.; McIver, B. Evaluation and Management of Indeterminate Thyroid Nodules: The Revolution of Risk Stratification Beyond Cytological Diagnosis. Cancer Control. 2017, 24, 1073274817729231. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rossi, E.D.; Larocca, L.M.; Pantanowitz, L. Ancillary molecular testing of indeterminate thyroid nodules. Cancer Cytopathol. 2018, 126 (Suppl. S8), 654–671. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, R.; Hier, J.; Payne, K.E.; Abdulhaleem, M.; Dimitstein, O.; Eisenbach, N.; Forest, V.-I.; Payne, R.J. Impact of Molecular Testing on Surgical Decision-Making in Indeterminate Thyroid Nodules: A Systematic Review and Meta-Analysis of Recent Advancements. Cancers 2025, 17, 1156. [Google Scholar] [CrossRef]
- Fumagalli, C.; Serio, G. Molecular testing in indeterminate thyroid nodules: An additional tool for clinical decision-making. Pathologica 2023, 115, 205–216. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hier, J.; Avior, G.; Pusztaszeri, M.; Krasner, J.R.; Alyouha, N.; Forest, V.-I.; Hier, M.P.; Mlynarek, A.; Richardson, K.; Sadeghi, N.; et al. Molecular testing for cytologically suspicious and malignant (Bethesda V and VI) thyroid nodules to optimize the extent of surgical intervention: A retrospective chart review. J. Otolaryngol. Head Neck Surg. 2021, 50, 29. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tessler, I.; Morand, G.B.; Gecel, N.A.; Dotan, A.; Yamin, T.; Alon, E.E.; Payne, R.J.; Avior, G. Guiding Management of Bethesda V Thyroid Nodules: The Role of Molecular Testing. Clin. Endocrinol. 2025. Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Guo, X.; Yang, M.; Wang, K.; Cao, G.; Liu, Y.; Hou, X.; Chen, L.; Liang, K. BRAFV600E genetic testing should be recommended for Bethesda III or V thyroid nodules based on fine-needle aspiration. Sci. Rep. 2023, 13, 17129. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Filetti, S.; Durante, C.; Hartl, D.; Leboulleux, S.; Locati, L.D.; Newbold, K.; Papotti, M.G.; Berruti, A.; ESMO Guidelines Committee. Thyroid cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up†. Ann. Oncol. 2019, 30, 1856–1883. [Google Scholar] [CrossRef] [PubMed]
- Scarpino, S.; Taccogna, S.; Pepe, G.; Papini, E.; D’angelo, M.; Cascone, F.; Nicoletti, D.; Guglielmi, R.; Palermo, A.; Trombetta, M.; et al. Morphological and Molecular Assessment in Thyroid Cytology Using Cell-Capturing Scaffolds. Horm. Metab. Res. 2020. Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.Z.; Baloch, Z.W.; Cochand-Priollet, B.; Schmitt, F.C.; Vielh, P.; VanderLaan, P.A. The 2023 Bethesda System for Reporting Thyroid Cytopathology. Thyroid 2023, 33, 1039–1044. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.-T.; Lee, C.-H. BRAF mutation in papillary thyroid carcinoma: Pathogenic role and clinical implications. J. Chin. Med. Assoc. 2010, 73, 113–128. [Google Scholar] [CrossRef] [PubMed]
- Czarniecka, A.; Oczko-Wojciechowska, M.; Barczyński, M. BRAF V600E mutation in prognostication of papillary thyroid cancer (PTC) recurrence. Gland. Surg. 2016, 5, 495–505. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Asbl, O.B.O.I.P.; Deans, Z.C.; Costa, J.L.; Cree, I.; Dequeker, E.; Edsjö, A.; Henderson, S.; Hummel, M.; Ligtenberg, M.J.; Loddo, M.; et al. Integration of next-generation sequencing in clinical diagnostic molecular pathology laboratories for analysis of solid tumours; an expert opinion on behalf of IQN Path ASBL. Virchows Arch. 2017, 470, 5–20. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vestergaard, L.K.; Mikkelsen, N.S.; Oliveira, D.V.N.P.; Poulsen, T.S.; Hoegdall, E.V. Rescue of Low-Yield DNA Samples for Next-Generation Sequencing Using Vacuum Centrifugal Concentration in a Clinical Workflow. Reports 2023, 6, 23. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yip, L.; Farris, C.; Kabaker, A.S.; Hodak, S.P.; Nikiforova, M.N.; McCoy, K.L.; Stang, M.T.; Smith, K.J.; Nikiforov, Y.E.; Carty, S.E. Cost Impact of Molecular Testing for Indeterminate Thyroid Nodule Fine-Needle Aspiration Biopsies. J. Clin. Endocrinol. Metab. 2012, 97, 1905–1912. [Google Scholar] [CrossRef] [PubMed]
- Pinki, P.; Alok, D.; Ranjan, A.; Chand, M.N. Fine Needle Aspiration Cytology versus Fine Needle Capillary Sampling in Cytological Diagnosis of Thyroid Lesions. Iran. J. Pathol. 2015, 10, 47–53. [Google Scholar] [PubMed] [PubMed Central]
- Tauro, L.F.; Lobo, G.J.; Fernandes, H.; George, C.; Aithala, P.S.; Shenoy, D.; Shetty, P. A Comparative Study on Fine Needle Aspiration Cytology versus Fine Needle Capillary Cytology in Thyroid Nodules. Oman Med. J. 2012, 27, 151–156. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- QIAamp DNA FFPE Advanced Kit – FFPE Tissue DNA Extraction. Available online: https://www.qiagen.com/es/products/discovery-and-translational-research/dna-rna-purification/dna-purification/genomic-dna/qiaamp-dna-ffpe-advanced-kits (accessed on 5 October 2025).

| Variable | Result |
| Age | 23–74 years |
| Sex | 26 F (78.8%), 7 M (21.2%) |
| DNA Concentration (ng/μL) | 5.68 ± 3.48 (range 1.6–13.7) |
| Sufficient DNA | 30/33 (90%) |
| BRAF V600E-Positive | 3 |
| BRAF V600E-Negative | 27 |
| Diagnosis | n | % |
| Benign Nodular Goiter | 11 | 36.7 |
| Papillary Thyroid Carcinoma | 10 | 33.3 |
| Follicular Variant of PTC (FV-PTC) | 6 | 20.0 |
| Follicular Adenoma | 2 | 6.7 |
| Medullary Carcinoma | 1 | 3.3 |
| Mutation | Malignant | Benign | Total |
| BRAF-Positive | 3 | 0 | 3 |
| BRAF-Negative | 14 | 13 | 27 |
| Total | 17 | 13 | 30 |
| Bethesda | Malignant | Benign | Total |
| III | 4 | 13 | 17 |
| IV | 0 | 2 | 2 |
| V | 14 | 0 | 14 |
| Bethesda | BRAF-Positive | BRAF-Negative | Insufficient DNA | Total |
| III | 0 | 14 | 3 | 17 |
| IV | 0 | 2 | 0 | 2 |
| V | 3 | 11 | 0 | 14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Streinu, D.R.; Stoian, D.L.; Neagoe, O.C.; Derban, M.; Ciordas, P.D.; Marian, C. Inside the Matrix: Integrated Cytology and Molecular Testing of Thyroid FNAC Samples Using a Commercial Synthetic 3D Scaffold. Int. J. Mol. Sci. 2025, 26, 11100. https://doi.org/10.3390/ijms262211100
Streinu DR, Stoian DL, Neagoe OC, Derban M, Ciordas PD, Marian C. Inside the Matrix: Integrated Cytology and Molecular Testing of Thyroid FNAC Samples Using a Commercial Synthetic 3D Scaffold. International Journal of Molecular Sciences. 2025; 26(22):11100. https://doi.org/10.3390/ijms262211100
Chicago/Turabian StyleStreinu, Diana Raluca, Dana Liana Stoian, Octavian Constantin Neagoe, Mihnea Derban, Paula Diana Ciordas, and Catalin Marian. 2025. "Inside the Matrix: Integrated Cytology and Molecular Testing of Thyroid FNAC Samples Using a Commercial Synthetic 3D Scaffold" International Journal of Molecular Sciences 26, no. 22: 11100. https://doi.org/10.3390/ijms262211100
APA StyleStreinu, D. R., Stoian, D. L., Neagoe, O. C., Derban, M., Ciordas, P. D., & Marian, C. (2025). Inside the Matrix: Integrated Cytology and Molecular Testing of Thyroid FNAC Samples Using a Commercial Synthetic 3D Scaffold. International Journal of Molecular Sciences, 26(22), 11100. https://doi.org/10.3390/ijms262211100







