Trigger Points of Necroptosis (RIPK1, RIPK3, and MLKL)—Promising Horizon or Blind Alley in Therapy of Colorectal Cancer?
Abstract
1. Introduction
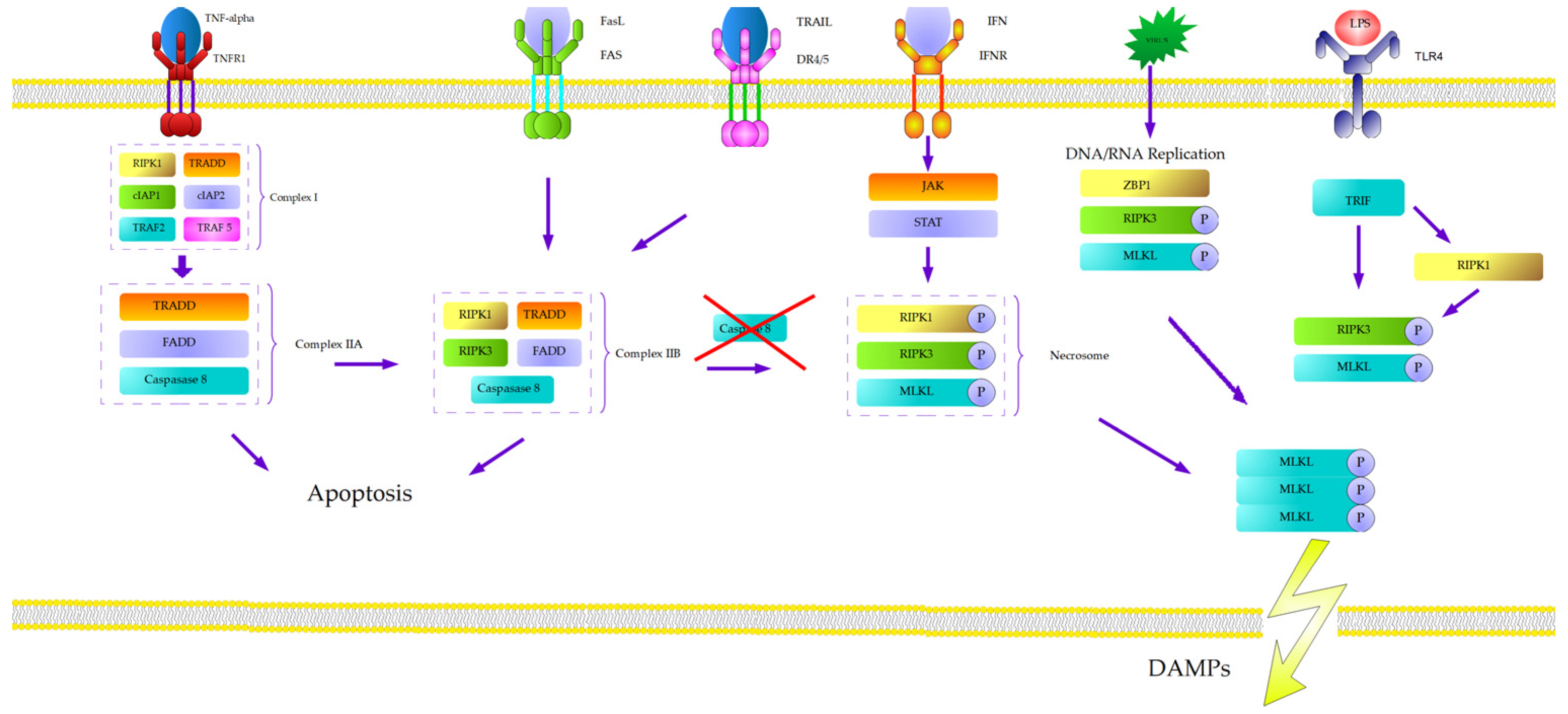
2. Natural History of Necroptosis
3. Molecular Mechanism of Necroptosis
4. RIPK 1—Receptor-Interacting Serine/Threonine Kinase 1
5. RIPK 3—Receptor-Interacting Serine/Threonine Kinase 3
6. MLKL—Mixed Lineage Kinase Domain-Like Pseudokinase
7. Other Agents
8. Potential Necroptotic Agents in Therapy of Colorectal Cancer
9. Conclusions
10. Materials and Methods
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CRC | colorectal cancer |
| EGFR | epidermal growth factor receptor |
| EMT | endothelial–mesenchymal transition |
| VEGF | vascular endothelial growth factor |
| TNF | tumor necrosis factor |
| TRAIL | tumor necrosis factor-associated apoptosis-inducing ligand |
| FasL | Fas ligand |
| RIPK 1 | receptor-interacting protein kinase 1 |
| RIPK3 | receptor-interacting protein kinase 3 |
| MLKL | mixed lineage kinase domain-like pseudokinase |
| RCD | regulated cell death |
| TLR | toll-like receptor |
| TNFR-1 | tumor necroptosis factor receptor-1 |
| TRADD | tumor necrosis factor receptor type 1- associated DEATH domain protein |
| TRAF2 | TNF receptor-associated factor 5 |
| TRAF5 | TNF receptor-associated factor 5 |
| cIAP1 | cellular inhibitor of apoptosis protein 1 |
| cIAP2 | cellular inhibitor of apoptosis protein 2 |
| IKK | IκB kinase α and β |
| FADD | Fas-associated protein with death domain |
| CYLD | deubiquitinating enzyme cylindromatosis |
| TNFAIP3 | ubiquitin-modifying TNF alpha-induced protein 3 |
| LUBAC | linear ubiquitin chain assembly complex |
| RHIM | RIP homotypic interaction motif |
| HSP90 | heat shock protein 90 |
| CDC37 | cell division cycle 37 homolog |
| PPM1B | protein phosphatase Mg2+/Mn2+-dependent 1B |
| Stub1 | protein Chip |
| PIP | phosphatidylinositol phosphate |
| DAMP | damage-associated nuclear pattern |
| LPS | Lipopolysaccharide |
| TRIF | TIR-domain-containing adaptor-inducing interferon-β |
| ZBP1 | z-DNA binding protein 1 |
| RIP | receptor-interacting protein |
| IAP | inhibitor of apoptosis proteins |
| FLIP | FLICE-like inhibitory protein |
| MCU | mitochondrial Ca2+ uniporter |
| FMRP | fragile X messenger ribonucleoprotein |
| OS | overall survival |
| CXCL1 | chemokine (C-X-C motif) ligand 1 |
| DAMPs | damage-associated molecular patterns |
| CAT | colitis-associated tumorigenesis |
| DCs | dendritic cells |
| GLTP | Glycolipid transfer protein |
| GSL | Glycosphingolipid |
| CDK2 | cyclin-dependent kinase-2 |
| CDK4 | cyclin-dependent kinase-4 |
| pMLKL | phosphorylation of human mixed lineage kinase domain-like protein |
| PRMT1 | protein arginine N-methyltransferase 1 |
| MDSC | myeloid-derived immune suppressor cells |
| RIP3ADMA | RIP3 R486 di-methylation specific antibody |
| POD | cationic peroxidase |
| PmPOD | cationic peroxidase purified from proso millets |
| Egt | ergothioneine |
| MAM | 2-methoxy-6-acetyl-7-methyljuglone |
| ROS | reactive oxygen species |
| TNF-ƒΏ | tumor necrosis factor ƒΏ |
| GSK3 | glycogen synthase kinase 3 |
| PCD | programmed cell death |
| CQ | Chloroquine |
| LMP | lysosomal membrane permeabilization |
| NRG | Necroptosis related genes |
References
- Siegel, R.L.; Wagle, N.S.; Cercek, A.; Smith, R.A.; Jemal, A. Colorectal cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 233–254. [Google Scholar] [CrossRef] [PubMed]
- Baidoun, F.; Elshiwy, K.; Elkeraie, Y.; Merjaneh, Z.; Khoudari, G.; Sarmini, M.T.; Gad, M.; Al-Husseini, M.; Saad, A. Colorectal cancer epidemiology: Recent trends and impact on outcomes. Curr. Drug Targets 2021, 22, 998–1009. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, D.; Humblet, Y.; Siena, S.; Khayat, D.; Bleiberg, H.; Santoro, A.; Bets, D.; Mueser, M.; Harstrick, A.; Verslype, C.; et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractorymetastatic colorectal cancer. N. Engl. J. Med. 2004, 351, 337–345. [Google Scholar] [CrossRef]
- Peeters, M.; Price, T.J.; Cervantes, A.; Sobrero, A.F.; Ducreux, M.; Hotko, Y.; André, T.; Chan, E.; Lordick, F.; Punt, C.J.; et al. Randomized phase III study of panitumumab with fluorouracil, leucovorin, and irinotecan (FOLFIRI) compared with FOLFIRI alone as second-line treatment in patients with metastatic colorectal cancer. J. Clin. Oncol. 2010, 28, 4706–4713. [Google Scholar] [CrossRef]
- Van Cutsem, E.; Tabernero, J.; Lakomy, R.; Prenen, H.; Prausová, J.; Macarulla, T.; Ruff, P.; van Hazel, G.A.; Moiseyenko, V.; Ferry, D.; et al. Addition of aflibercept to fluorouracil, leucovorin, and irinotecan improves survival in a phase III randomized trial in patients with metastatic colorectal cancer previously treated with an oxaliplatin-based regimen. J. Clin. Oncol. 2012, 30, 3499–3506. [Google Scholar] [CrossRef]
- Giantonio, B.J.; Catalano, P.J.; Meropol, N.J.; O’Dwyer, P.J.; Mitchell, E.P.; Alberts, S.R.; Schwartz, M.A.; Benson, A.B., III. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: Results from the Eastern Cooperative Oncology Group Study E3200. J. Clin. Oncol. 2007, 25, 1539–1544. [Google Scholar] [CrossRef]
- Heinemann, V.; von Weikersthal, L.F.; Decker, T.; Kiani, A.; Vehling-Kaiser, U.; Al-Batran, S.E.; Heintges, T.; Lerchenmüller, C.; Kahl, C.; Seipelt, G.; et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): A randomised, open-label, phase 3 trial. Lancet Oncol. 2014, 15, 1065–1075. [Google Scholar] [CrossRef] [PubMed]
- Grothey, A.; Van Cutsem, E.; Sobrero, A.; Siena, S.; Falcone, A.; Ychou, M.; Humblet, Y.; Bouché, O.; Mineur, L.; Barone, C.; et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): An international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 2013, 381, 303–312. [Google Scholar] [CrossRef]
- Mayer, R.J.; Van Cutsem, E.; Falcone, A.; Yoshino, T.; Garcia-Carbonero, R.; Mizunuma, N.; Yamazaki, K.; Shimada, Y.; Tabernero, J.; Komatsu, Y.; et al. Randomized trial of TAS-102 for refractory metastatic colorectal cancer. N. Engl. J. Med. 2015, 372, 1909–1919. [Google Scholar] [CrossRef]
- André, T.; Shiu, K.K.; Kim, T.W.; Jensen, B.V.; Jensen, L.H.; Punt, C.; Smith, D.; Garcia-Carbonero, R.; Benavides, M.; Gibbs, P.; et al. Pembrolizumab in microsatellite-instability-high advanced colorectal cancer. N. Engl. J. Med. 2020, 383, 2207–2218. [Google Scholar] [CrossRef] [PubMed]
- André, T.; Lonardi, S.; Wong, K.Y.M.; Lenz, H.-J.; Gelsomino, F.; Aglietta, M.; Morse, M.; Van Cutsem, E.; McDermott, R.; Hill, A.; et al. Nivolumab plus low-dose ipilimumab in previously treated patients with microsatellite instability-high/mismatch repair-deficient metastatic colorectal cancer: 4-year follow-up from CheckMate 142. Ann. Oncol. 2022, 33, 1052–1060. [Google Scholar] [CrossRef]
- Andre, T.; Elez, E.; Van Cutsem, E.; Jensen, L.H.; Bennouna, J.; Mendez, G.; Schenker, M.; de la Fouchardiere, C.; Limon, M.L.; Yoshino, T.; et al. Nivolumab plus Ipilimumab in Microsatellite-Instability-High Metastatic Colorectal Cancer. N. Engl. J. Med. 2024, 391, 2014–2026. [Google Scholar] [CrossRef]
- Zhu, T.; Wu, B.W. Recognition of necroptosis: From molecular mechanisms to detection methods. Biomed. Pharmacother. 2024, 178, 117196. [Google Scholar] [CrossRef]
- Saeed, W.K.; Jun, D.W.; Jang, K.; Koh, D.H. Necroptosis signaling in liver diseases: An update. Pharmacol. Res. 2019, 148, 104439. [Google Scholar] [CrossRef]
- Belavgeni, A.; Meyer, C.; Stumpf, J.; Hugo, C.; Linkermann, A. Ferroptosis and Necroptosis in the Kidney. Cell Chem. Biol. 2020, 27, 448–462. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Tang, M.B.; Luo, H.Y.; Shi, C.H.; Xu, Y.M. Necroptosis in neurodegenerative diseases: A potential therapeutic target. Cell Death Dis. 2017, 8, e2905. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Chen, Y.; Liu, Q. Necroptosis in heart disease: Molecular mechanisms and therapeutic implications. J. Mol. Cell. Cardiol. 2022, 169, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Sun, H.; Yu, Y.; Che, N.; Han, J.; Cheng, R.; Zhao, N.; Guo, Y.; Huang, C.; Zhang, D. RIPK1-dependent necroptosis promotes vasculogenic mimicry formation via eIF4E in triple-negative breast cancer. Cell Death Dis. 2023, 14, 335, Erratum in Cell Death Dis. 2023, 14, 607. https://doi.org/10.1038/s41419-023-06052-z. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sokołowski, M.; Łacina, P.; Bogunia-Kubik, K.; Mazur, G.; Butrym, A. Polymorphisms in Necroptosis Pathway Genes: Novel Prognostic Markers for Multiple Myeloma Treatment Outcomes. Int. J. Mol. Sci. 2025, 26, 5237. [Google Scholar] [CrossRef]
- Park, J.E.; Lee, J.H.; Lee, S.Y.; Hong, M.J.; Choi, J.E.; Park, S.; Jeong, J.Y.; Lee, E.B.; Choi, S.H.; Lee, Y.H.; et al. Expression of key regulatory genes in necroptosis and its effect on the prognosis in non-small cell lung cancer. J. Cancer 2020, 11, 5503–5510. [Google Scholar] [CrossRef]
- Laster, S.M.; Wood, J.G.; Gooding, L.R. Tumor necrosis factor can induce both apoptotic and necrotic forms of cell lysis. J. Immunol. 1988, 141, 2629–2634. [Google Scholar] [CrossRef]
- Vercammen, D.; Beyaert, R.; Denecker, G.; Goossens, V.; Van Loo, G.; Declercq, W.; Grooten, J.; Fiers, W.; Vandenabeele, P. Inhibition of caspases increases the sensitivity of L929 cells to necrosis mediated by tumor necrosis factor. J. Exp. Med. 1998, 187, 1477–1485. [Google Scholar] [CrossRef] [PubMed]
- Kawahara, A.; Ohsawa, Y.; Matsumura, H.; Uchiyama, Y.; Nagata, S. Caspase-independent cell killing by Fas-associatedprotein with death domain. J. Cell Biol. 1998, 143, 1353–1360. [Google Scholar] [CrossRef]
- Holler, N.; Zaru, R.; Micheau, O.; Thome, M.; Attinger, A.; Valitutti, S.; Bodmer, J.L.; Schneider, P.; Seed, B.; Tschopp, J. Fas triggers an alternative, caspase-8-independent cell death pathway using the kinase RIP as effector molecule. Nat. Immunol. 2000, 1, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Chan, F.K.; Shisler, J.; Bixby, J.G.; Felices, M.; Zheng, L.; Appel, M.; Orenstein, J.; Moss, B.; Lenardo, M.J. A role for tumor necrosis factor receptor-2 and receptor-interacting protein in programmed necrosis and antiviral responses. J. Biol. Chem. 2003, 278, 51613–51621. [Google Scholar] [CrossRef] [PubMed]
- Degterev, A.; Hitomi, J.; Germscheid, M.; Ch’en, I.L.; Korkina, O.; Teng, X.; Abbott, D.; Cuny, G.D.; Yuan, C.; Wagner, G.; et al. Identification of RIP1 kinase as a specific cellular target of necrostatins. Nat. Chem. Biol. 2008, 4, 313–321. [Google Scholar] [CrossRef]
- He, S.; Wang, L.; Miao, L.; Wang, T.; Du, F.; Zhao, L.; Wang, X. Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-alpha. Cell 2009, 137, 1100–1111. [Google Scholar] [CrossRef]
- Sun, L.; Wang, H.; Wang, Z.; He, S.; Chen, S.; Liao, D.; Wang, L.; Yan, J.; Liu, W.; Lei, X.; et al. Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell 2012, 148, 213–227. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Vitale, I.; Abrams, J.M.; Alnemri, E.S.; Baehrecke, E.H.; Blagosklonny, M.V.; Dawson, T.M.; Dawson, V.L.; El-Deiry, W.S.; Fulda, S.; et al. Molecular definitions of cell death subroutines: Recommendations of the Nomenclature Committee on Cell Death 2012. Cell Death Differ. 2012, 19, 107–120. [Google Scholar] [CrossRef]
- Galluzzi, L.; Vitale, I.; Aaronson, S.A.; Abrams, J.M.; Adam, D.; Agostinis, P.; Alnemri, E.S.; Altucci, L.; Amelio, I.; Andrews, D.W.; et al. Molecular mechanisms of cell death: Recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018, 25, 486–541. [Google Scholar] [CrossRef]
- Kaiser, W.J.; Sridharan, H.; Huang, C.; Mandal, P.; Upton, J.W.; Gough, P.J.; Sehon, C.A.; Marquis, R.W.; Bertin, J.; Mocarski, E.S. Toll-like receptor 3-mediated necrosis via TRIF, RIP3, and MLKL. J. Biol. Chem. 2013, 288, 31268–31279. [Google Scholar] [CrossRef] [PubMed]
- Vandenabeele, P.; Galluzzi, L. Molecular mechanisms of necroptosis: An ordered cellular explosion. Nat. Rev. Mol. Cell Biol. 2010, 11, 700–714. [Google Scholar] [CrossRef]
- Ea, C.K.; Deng, L. Activation of IKK by TNFα requires site-specific ubiquitination of RIP1 and polyubiquitin binding by NEMO. Mol. Cell 2006, 22, 245–257. [Google Scholar] [CrossRef]
- Micheau, O.; Tschopp, J. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell 2003, 114, 181–190. [Google Scholar] [CrossRef]
- Wegner, K.W.; Saleh, D.; Degterev, A. Complex Pathologic Roles of RIPK1 and RIPK3: Moving Beyond Necroptosis. Trends Pharmacol. Sci. 2017, 38, 202–225. [Google Scholar] [CrossRef]
- Annibaldi, A.; Wicky, J.S. Ubiquitin-Mediated Regulation of RIPK1 Kinase Activity Independent of IKK and MK2. Mol. Cell 2018, 69, 566–578. [Google Scholar] [CrossRef]
- Wu, X.N.; Yang, Z.H.; Wang, X.K.; Zhang, Y.; Wan, H.; Song, Y.; Chen, X.; Shao, J.; Han, J. Distinct roles of RIP1-RIP3 hetero- and RIP3-RIP3 homo-interaction in mediating necroptosis. Cell Death Differ. 2014, 21, 1709–1720. [Google Scholar] [CrossRef] [PubMed]
- Tummers, B.; Green, D.R. Caspase-8: Regulating life and death. Immunol. Rev. 2017, 277, 76–89. [Google Scholar] [CrossRef]
- Fritsch, M.; Günther, S.D.; Schwarzer, R.; Albert, M.-C.; Schorn, F.; Werthenbach, J.P.; Schiffmann, L.M.; Stair, N.; Stocks, H.; Seeger, J.M.; et al. Caspase-8 is the molecular switch for apoptosis, necroptosis and pyroptosis. Nature 2019, 575, 683–687. [Google Scholar] [CrossRef] [PubMed]
- Koike, A.; Hanatani, M.; Fujimori, K. Pan-caspase inhibitors induce necroptosis via ROS-mediated activation of mixed lineage kinase domain-like protein and p38 in classically activated macrophages. Exp. Cell Res. 2019, 380, 171–179. [Google Scholar] [CrossRef]
- Jin, H.S.; Lee, D.H.; Kim, D.H.; Chung, J.H.; Lee, S.J.; Lee, T.H. cIAP1, cIAP2, and XIAP act cooperatively via nonredundant pathways to regulate genotoxic stress-induced nuclear factor-kappaB activation. Cancer Res. 2009, 69, 1782–1791. [Google Scholar] [CrossRef]
- Hitomi, J.; Christofferson, D.E. Identification of a molecular signaling network that regulates a cellular necrotic cell death pathway. Cell 2008, 135, 1311–1323. [Google Scholar] [CrossRef]
- Moquin, D.M.; McQuade, T. CYLD deubiquitinates RIP1 in the TNFα-induced necrosome to facilitate kinase activation and programmed necrosis. PLoS ONE 2013, 8, e76841. [Google Scholar] [CrossRef]
- Donnell, M.A.; Perez-Jimenez, E. Caspase 8 inhibits programmed necrosis by processing CYLD. Nat. Cell Biol. 2011, 13, 1437–1442. [Google Scholar] [CrossRef]
- Li, D.; Xu, T. A cytosolic heat shock protein 90 and cochaperone CDC37 complex is required for RIP3 activation during necroptosis. Proc. Natl. Acad. Sci. USA 2015, 112, 5017–5022. [Google Scholar] [CrossRef]
- Li, J.; McQuade, T. The RIP1/RIP3 necrosome forms a functional amyloid signaling complex required for programmed necrosis. Cell 2012, 150, 339–350. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, D.A.; Weinlich, R. Characterization of RIPK3-mediated phosphorylation of the activation loop of MLKL duringnecroptosis. Cell Death Differ. 2016, 23, 76–88. [Google Scholar] [CrossRef]
- Chen, W.; Wu, J. Ppm1b negatively regulates necroptosis through dephosphorylating Rip3. Nat. Cell Biol. 2015, 17, 434–444. [Google Scholar] [CrossRef]
- Raninga, P.V.; Di Trapani, G. Targeted knockdown of DJ-1 induces multiple myeloma cell death via KLF6 upregulation. Apoptosis 2016, 12, 1422–1437. [Google Scholar] [CrossRef] [PubMed]
- Murphy, J.M.; Czabotar, P.E.; Hildebrand, J.M.; Lucet, I.S.; Zhang, J.-G.; Alvarez-Diaz, S.; Lewis, R.; Lalaoui, N.; Metcalf, D.; Webb, A.I.; et al. The pseudokinase MLKL mediates necroptosis via a molecular switch mechanism. Immunity 2013, 39, 443–453. [Google Scholar] [CrossRef] [PubMed]
- Quarato, G.; Guy, C.S. Sequential engagement of distinct MLKL phosphatidylinositol-binding sites executes necroptosis. Mol. Cell 2016, 61, 589–601. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Jitkaew, S.; Zhao, J.; Chiang, H.C.; Choksi, S.; Liu, J.; Ward, Y.; Wu, L.G.; Liu, Z.G. Plasma membrane translocation of trimerized MLKL protein is required for TNF-induced necroptosis. Nat. Cell Biol. 2014, 16, 55–65, Erratum in Nat Cell Biol. 2014, 16, 200. [Google Scholar] [CrossRef]
- Wada, N.; Kawano, Y. Shikonin dually functions as a proteasome inhibitor and a necroptosis inducer in multiple myeloma cells. Int. J. Oncol. 2015, 46, 963–972. [Google Scholar] [CrossRef]
- Lu, W.; Sun, J. Mitochondrial protein PGAM5 regulates mitophagic protection against cell necroptosis. PLoS ONE 2016, 11, 0147792. [Google Scholar] [CrossRef]
- Kaczmarek, A.; Vandenabeele, P. Necroptosis: The Release of Damage-Associated Molecular Patterns and Its Physiological Relevance. Immunity 2013, 38, 209–223. [Google Scholar] [CrossRef]
- Guerrache, A.; Micheau, O. TNF-Related Apoptosis-Inducing Ligand: Non-Apoptotic Signalling. Cells 2024, 13, 521. [Google Scholar] [CrossRef]
- Najjar, M.; Saleh, D.; Zelic, M.; Nogusa, S.; Shah, S.; Tai, A.; Finger, J.N.; Polykratis, A.; Gough, P.J.; Bertin, J.; et al. RIPK1 and RIPK3 Kinases Promote Cell-Death-Independent Inflammation by Toll-like Receptor 4. Immunity 2016, 45, 46–59. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, T.; Yin, C.; Boyd, D.F.; Quarato, G.; Ingram, J.P.; Shubina, M.; Ragan, K.B.; Ishizuka, T.; Crawford, J.C.; Tummers, B.; et al. Influenza Virus Z-RNAs Induce ZBP1-Mediated Necroptosis. Cell 2020, 180, 1115–1129.e13. [Google Scholar] [CrossRef] [PubMed]
- Jiao, H.; Wachsmuth, L.; Kumari, S.; Schwarzer, R.; Lin, J.; Eren, R.O.; Fisher, A.; Lane, R.; Young, G.R.; Kassiotis, G.; et al. Z-nucleic-acid sensing triggers ZBP1-dependent necroptosis and inflammation. Nature 2020, 580, 391–395, Erratum in Nature 2020, 580, E10. https://doi.org/10.1038/s41586-020-2207-y. [Google Scholar] [CrossRef]
- Thapa, R.J.; Nogusa, S.; Chen, P.; Maki, J.L.; Lerro, A.; Andrake, M.; Rall, G.F.; Degterev, A.; Balachandran, S. Interferon-induced RIP1/RIP3-mediated necrosis requires PKR and is licensed by FADD and caspases. Proc. Natl. Acad. Sci. USA 2013, 110, E3109–E3118. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Liang, Y.; Zhao, S.; Ding, Y.; Zhuang, Q.; Shi, Q.; Ai, T.; Wu, S.Q.; Han, J. ZBP1 mediates interferon-inducednecroptosis. Cell Mol. Immunol. 2020, 17, 356–368. [Google Scholar] [CrossRef]
- Lin, Y. RIP1-Mediated Signaling Pathways in Cell Survival and Death Control. In Necrotic Cell Death; Springer: New York, NY, USA, 2014; pp. 23–43. [Google Scholar]
- Stanger, B.Z.; Leder, P.; Lee, T.H.; Kim, E.; Seed, B. RIP: A novel protein containing a death domain that interacts withFas/APO-1 (CD95) in yeast and causes cell death. Cell 1995, 81, 513–523. [Google Scholar] [CrossRef]
- Clucas, J.; Meier, P. Roles of RIPK1 as a stress sentinel coordinating cell survival and immunogenic cell death. Nat. Rev. Mol.Cell Biol. 2023, 24, 835–852. [Google Scholar] [CrossRef] [PubMed]
- Vandenabeele, P.; Grootjans, S.; Callewaert, N.; Takahashi, N. Necrostatin-1 blocks both RIPK1 and IDO: Consequences for the studyof cell death in experimental disease models. Cell Death Differ. 2013, 20, 185–187. [Google Scholar] [CrossRef] [PubMed]
- Wagner, J.; Vredevoogd, D.; Yu, X.; Lu, D.; Peeper, D.S.; Hermanns, H.M.; Wang, J.; Wajant, H.; Siegmund, D. TRAF2 and RIPK1 redundantly mediate classical NFκB signaling by TNFR1 and CD95-type death receptors. Cell Death Dis. 2025, 16, 35. [Google Scholar] [CrossRef]
- Imai, T.; Lin, J.; Kaya, G.G.; Ju, E.; Kondylis, V.; Kelepouras, K.; Liccardi, G.; Kim, C.; Pasparakis, M. The RIPK1 deathdomain restrains ZBP1- and TRIF-mediated cell death and inflammation. Immunity 2024, 57, 1497–1513. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Wu, C.J.; Zhao, Y.; Ashwell, J.D. Optineurin negatively regulates TNFα-induced NF-κB activation by competing with NEMO for ubiquitinated RIP. Curr. Biol. 2007, 17, 1438–1443. [Google Scholar] [CrossRef]
- Kondylis, V.; Kumari, S.; Vlantis, K.; Pasparakis, M. The interplay of IKK, NF-κB and RIPK1 signaling in the regulation of cell death, tissue homeostasis and inflammation. Immunol Rev. 2017, 277, 113–127. [Google Scholar] [CrossRef]
- Zeng, F.; Chen, X.; Cui, W.; Wen, W.; Lu, F.; Sun, X.; Ma, D.; Yuan, Y.; Li, Z.; Hou, N.; et al. RIPK1 Binds MCU to Mediate Induction of Mitochondrial Ca2⁺ Uptake and Promotes Colorectal Oncogenesis. Cancer Res. 2018, 78, 2876–2885. [Google Scholar] [CrossRef]
- Di Grazia, A.; Marafini, I.; Pedini, G.; Di Fusco, D.; Laudisi, F.; Dinallo, V.; Rosina, E.; Stolfi, C.; Franzè, E.; Sileri, P.; et al. The Fragile X Mental Retardation Protein Regulates RIPK1 and Colorectal Cancer Resistance to Necroptosis. Cell Mol. Gastroenterol. Hepatol. 2021, 11, 639–658. [Google Scholar] [CrossRef]
- Lin, P.; Lin, C.; He, R.; Chen, H.; Teng, Z.; Yao, H.; Liu, S.; Hoffman, R.M.; Ye, J.; Zhu, G. TRAF6 regulates the abundance of RIPK1 and inhibits the RIPK1/RIPK3/MLKL necroptosis signaling pathway and affects the progression of colorectal cancer. Cell Death Dis. 2023, 14, 6. [Google Scholar] [CrossRef]
- Kang, A.R.; Kim, J.L.; Kim, Y.; Kang, S.; Oh, S.C.; Park, J.K. A novel RIP1-mediated canonical WNT signaling pathway that promotes colorectal cancer metastasis via β -catenin stabilization-induced EMT. Cancer Gene Ther. 2023, 30, 1403–1413. [Google Scholar] [CrossRef]
- Zhou, Y.; Xiang, Y.; Liu, S.; Li, C.; Dong, J.; Kong, X.; Ji, X.; Cheng, X.; Zhang, L. RIPK3 signaling and its role in regulated cell death and diseases. Cell Death Discov. 2024, 10, 200. [Google Scholar] [CrossRef]
- Conev, N.V.; Dimitrova, E.G.; Bogdanova, M.K.; Kashlov, Y.K.; Chaushev, B.G.; Radanova, M.A.; Petrov, D.P.; Georgiev, K.D.; Bachvarov, C.H.; Todorov, G.N.; et al. RIPK3 expression as a potential predictive and prognostic marker in metastatic colon cancer. Clin. Investig. Med. 2019, 42, E31–E38. [Google Scholar] [CrossRef] [PubMed]
- He, G.W.; Günther, C.; Thonn, V.; Yu, Y.Q.; Martini, E.; Buchen, B.; Neurath, M.F.; Stürzl, M.; Becker, C. Regression of apoptosis-resistant colorectal tumors by induction of necroptosis in mice. J. Exp. Med. 2017, 214, 1655–1662. [Google Scholar] [CrossRef] [PubMed]
- Bozec, D.; Iuga, A.C.; Roda, G.; Dahan, S.; Yeretssian, G. Critical function of the necroptosis adaptor RIPK3 in protecting from intestinal tumorigenesis. Oncotarget 2016, 7, 46384–46400. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.Y.; Zheng, M.; Li, Y.M.; Fan, X.Y.; Wang, J.C.; Li, Z.C.; Yang, H.J.; Yu, J.M.; Cui, J.; Jiang, J.L.; et al. RIP3 promotes colitis-associated colorectal cancer by controlling tumor cell proliferation and CXCL1-induced immune suppression. Theranostics 2019, 9, 3659–3673. [Google Scholar] [CrossRef]
- Xu, L.; Zhuang, C. Mixed Lineage Kinase Domain-Like Protein (MLKL): From Mechanisms to Therapeutic Opportunities. Adv. Sci. 2025, 12, e09277. [Google Scholar] [CrossRef]
- Zhao, Q.; Yu, X.; Li, M.; Liu, Y.; Han, Y.; Zhang, X.; Li, X.M.; Wu, X.; Qin, J.; Fang, J.; et al. MLKL attenuates colon inflammation and colitis-tumorigenesis via suppression of inflammatory responses. Cancer Lett. 2019, 459, 100–111. [Google Scholar] [CrossRef]
- Li, X.; Guo, J.; Ding, A.P.; Qi, W.W.; Zhang, P.H.; Lv, J.; Qiu, W.S.; Sun, Z.Q. Association of Mixed Lineage Kinase Domain-Like Protein Expression with Prognosis in Patients with Colon Cancer. Technol. Cancer Res. Treat. 2017, 16, 428–434. [Google Scholar] [CrossRef]
- O’Connell, E.; Reynolds, I.S.; Lindner, A.U.; Salvucci, M.; O’Grady, T.; Bacon, O.; Cho, S.; McDonough, E.; Longley, D.; Ginty, F.; et al. Apoptotic and Necroptotic Mediators are Differentially Expressed in Mucinous and Non-Mucinous Colorectal Cancer. Front. Oncol. 2022, 12, 815001. [Google Scholar] [CrossRef]
- Van Hoecke, L.; Van Lint, S.; Roose, K.; Van Parys, A.; Vandenabeele, P.; Grooten, J.; Tavernier, J.; De Koker, S.; Saelens, X. Treatment with mRNA coding for the necroptosis mediator MLKL induces antitumor immunity directed against neo-epitopes. Nat. Commun. 2018, 9, 3417. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Wu, B.; Guo, Y.S.; Zhou, Y.H.; Fu, Z.G.; Xu, B.Q.; Li, J.H.; Jing, L.; Jiang, J.L.; Tang, J.; et al. Necrostatin-1 reduces intestinal inflammation and colitis-associated tumorigenesis in mice. Am. J. Cancer Res. 2015, 5, 3174–3185. [Google Scholar]
- Mishra, S.K.; Stephenson, D.J.; Chalfant, C.E.; Brown, R.E. Upregulation of human glycolipid transfer protein (GLTP) induces necroptosis in colon carcinoma cells. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2019, 1864, 158–167. [Google Scholar] [CrossRef]
- Zhang, L.; He, Y.; Jiang, Y.; Wu, Q.; Liu, Y.; Xie, Q.; Zou, Y.; Wu, J.; Zhang, C.; Zhou, Z.; et al. PRMT1 reverts the immune escape of necroptotic colon cancer through RIP3 methylation. Cell Death Dis. 2023, 14, 233. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Wang, S.; Chang, W.; Zhang, X.; Deng, R.; Yan, H.; Zhu, W.; Wang, H.; Cai, Y.; Liu, Z.; et al. Role of necroptosis-related genes in immune activity and prognosis of colorectal cancer. Front. Immunol. 2025, 16, 1619749. [Google Scholar] [CrossRef]
- Cui, X.; Wang, R.; Wang, Z. Cationic peroxidase from proso millet induces human colon cancer cell necroptosis by regulating autocrine TNF-α and RIPK3 demethylation. Food Funct. 2018, 9, 1878–1888. [Google Scholar] [CrossRef]
- Onofrio, N.; Martino, E.; Balestrieri, A.; Mele, L.; Cautela, D.; Castaldo, D.; Balestrieri, M.L. Diet-derived ergothioneine induces necroptosis in colorectal cancer cells by activating the SIRT3/MLKL pathway. FEBS Lett. 2022, 596, 1313–1329. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Wu, X.; Gao, H.; Yu, J.; Zhao, W.; Lu, J.J.; Wang, J.; Du, G.; Chen, X. Cytosolic calcium mediates RIP1/RIP3 complex-dependent necroptosis through JNK activation and mitochondrial ROS production in human colon cancer cells. Free Radic. Biol. Med. 2017, 108, 433–444. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.Y.; Zhang, B. Cobalt chloride induces necroptosis in human colon cancer HT-29 cells. Asian Pac. J. Cancer Prev. 2015, 16, 2569–2574. [Google Scholar] [CrossRef]
- Metzig, M.O.; Fuchs, D.; Tagscherer, K.E.; Gröne, H.J.; Schirmacher, P.; Roth, W. Inhibition of caspases primes colon cancer cells for 5-fluorouracil-induced TNF-α-dependent necroptosis driven by RIP1 kinase and NF-κB. Oncogene 2016, 35, 3399–3409. [Google Scholar] [CrossRef]
- Grassilli, E.; Ianzano, L.; Bonomo, S.; Missaglia, C.; Cerrito, M.G.; Giovannoni, R.; Masiero, L.; Lavitrano, M. GSK3A isredundant with GSK3B in modulating drug resistance and chemotherapy-induced necroptosis. PLoS ONE 2014, 9, 100947. [Google Scholar] [CrossRef]
- Hou, X.; Yang, C.; Zhang, L.; Hu, T.; Sun, D.; Cao, H.; Yang, F.; Guo, G.; Gong, C.; Zhang, X.; et al. Killing colon cancer cells through PCD pathways by a novel hyaluronic acid-modified shell-core nanoparticle loaded with RIP3 in combination with chloroquine. Biomaterials 2017, 124, 195–210. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Xu, Y.; Chen, Y.; Xu, W.; Yao, M.; Ding, W. Development of a prognostic prediction model based on damage-associated molecular pattern for colorectal cancer applying bulk RNA-seq analysis. Sci. Rep. 2025, 15, 25792. [Google Scholar] [CrossRef]
- Valdeolivas, A.; Amberg, B.; Giroud, N.; Richardson, M.; Gálvez, E.J.C.; Badillo, S.; Julien-Laferrière, A.; Túrós, D.; Voith von Voithenberg, L.; Wells, I.; et al. Profiling the heterogeneity of colorectal cancer consensus molecular subtypes using spatial transcriptomics. npj Precis. Oncol. 2024, 8, 10. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xu, H.; Cui, H.; Weng, S.; Zhang, Y.; Wang, L.; Xing, Z.; Han, X.; Liu, Z. Crosstalk of cell death pathways unveils an autophagy-related gene AOC3 as a critical prognostic marker in colorectal cancer. Commun. Biol. 2024, 7, 296. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Moriwaki, K.; Bertin, J.; Gough, P.J.; Orlowski, G.M.; Chan, F.K. Differential roles of RIPK1 and RIPK3 in TNF-induced necroptosis and chemotherapeutic agent-induced cell death. Cell Death Dis. 2015, 6, 1636. [Google Scholar] [CrossRef]

| Mechanism | Experimental Model | Therapeutic Implications | Reference |
|---|---|---|---|
| Interaction with mitochondrial Ca2+ uniporter | Human CRC cells obtained from patients | Promotion of proliferation by increasing the mitochondrial Ca2+ | [70] |
| Analysis of mRNA transcription of fragile X messenger ribonucleoprotein (FMRP) | Human CRC cells obtained from patients | The FMRP takes part in controlling RIPK1 expression and necroptotic activation in CRC. | [71] |
| Interaction with TRAF-6 | SW480 and HCT116 human colon cancer cell lines, MC38 mouse colon cancer, HEK293T cell line | TRAF-6 promotes colorectal cell progression by inhibiting the RIPK1/RIPK3/MLKL necroptosis signaling pathway, | [72] |
| RIPK1 in controlling WNT/β-catenin canonical signaling | Colorectal cancer cell lines HCT116 and DLD-1 and mice in vivo models | RIPK1 plays role in the WNT3A–RIP1–β-catenin pathway in CRC cells (enhancing migration and invasion) | [73] |
| Mechanism | Experimental Model | Therapeutic Implications | Reference |
|---|---|---|---|
| Connection between RIPK3 expression to response for 5-fluorouracil therapy in metastatic CRC | Human CRC cells obtained from patients | The high expression of RIPK3 is associated with longer (p = 0.02) OS (p = 0.036) and lower risk of disease progression (p = 0.044) | [75] |
| Overcome cell death resistance in caspase-8-deficient colorectal cancer (CRC) and expression of RIPK3 | Mouse and human cell model |
Xenograft mouse cell model of caspase-8-deficiency leads to regression of tumors.
RIPK3 is highly expressed in mouse models of CRC and in a subset of human CRC cell lines | [76] |
| Role of RIPK3-deficiency in tumorogenensis by uncontrolled activation of NF-κB, STAT3, AKT, and Wnt-β-catenin pathways | Human CRC cells obtained from patients |
The expression of RIPK3 is reduced in tumors from patients with inflammatory bowel diseases.
The expression of RIPK3 is downregulated in human CRC and correlated with cancer progression. | [77] |
| Role of RIPK3 in the progression of colitis-associated cancer (CAC) | Human CAC cells obtained from patients and mouse CRC cells |
RIPK 3 expression was upregulated in mouse CAC and human colon cancer
High expression of RIPK3 enhances the proliferation of premalignant intestinal epithelial cells (IECs) and promotes myeloid-cell-induced adaptive immune suppression | [78] |
| Mechanism | Experimental Model | Therapeutic Implications | Reference |
|---|---|---|---|
| Role of MLKL in colitis and colitis-associated tumorigenesis | In vivo mouse model | MLKL plays a role in maintaining intestinal homeostasis, protecting against colitis and tumorigenesis. | [81] |
| Association between expression of MLKL and clinical prognosis | Human CRC cells obtained from patients | The low expression of MLKL is connected with shorter OS (p = 0.011), even in the subpopulation that received an adjuvant chemotherapy p = 0.005), and shorter PFS (p = 0.032) | [82] |
| Attempt to determine expression of apoptosis and necroptosis mediators in mucinous CRC by evaluating RNA gene expression | Mouse CRC cell lines-SW1463 (mucinous rectal), SW837 (non-mucinous rectal), LS174T (mucinous colon) and HCT116 (non-mucinous colon) |
Treatment with 5-FU did not significantly elevate cell death events in mucinous cells, while non-mucinous cells showed robust cell death responses.
5-fluorouracil-induced phosphorylation of MLKL in mucinous cancer cells. | [83] |
| Generic antitumor therapy based on the intratumor delivery of mRNA encoded MLKL | Mouse cell lines |
Inhibition of primary tumor growth and protects against distal metastasis.
Improve antitumor activity in combination with i mmune checkpoint inhibitor | [84] |
| Potential Therapeutic Target/Agent | Trigger Point | Reference |
|---|---|---|
| PmPOD (Proso millet Cationic Peroxidase) | RIPK1, RIPK3 | [88] |
| EGT (Ergothioneine) | RIPK1/RIPK3/MLKL and SIRT 3 | [89] |
| MAM (2-methoxy-6-acetyl-7-methyljuglone) | RIPK1/RIPK3 | [90] |
| Cobalt chloride | Unknown | [91] |
| Pan-caspase inhibitor IDN-731 | ƒΏ (TNF-ƒΏ) | [92] |
| GSK3 -glycogen synthase kinase 3 | Unknown | [93] |
| CQ-Chloroquine | RIPK3 | [94] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sokołowski, M.; Butrym, A. Trigger Points of Necroptosis (RIPK1, RIPK3, and MLKL)—Promising Horizon or Blind Alley in Therapy of Colorectal Cancer? Int. J. Mol. Sci. 2025, 26, 11101. https://doi.org/10.3390/ijms262211101
Sokołowski M, Butrym A. Trigger Points of Necroptosis (RIPK1, RIPK3, and MLKL)—Promising Horizon or Blind Alley in Therapy of Colorectal Cancer? International Journal of Molecular Sciences. 2025; 26(22):11101. https://doi.org/10.3390/ijms262211101
Chicago/Turabian StyleSokołowski, Marcin, and Aleksandra Butrym. 2025. "Trigger Points of Necroptosis (RIPK1, RIPK3, and MLKL)—Promising Horizon or Blind Alley in Therapy of Colorectal Cancer?" International Journal of Molecular Sciences 26, no. 22: 11101. https://doi.org/10.3390/ijms262211101
APA StyleSokołowski, M., & Butrym, A. (2025). Trigger Points of Necroptosis (RIPK1, RIPK3, and MLKL)—Promising Horizon or Blind Alley in Therapy of Colorectal Cancer? International Journal of Molecular Sciences, 26(22), 11101. https://doi.org/10.3390/ijms262211101






