Novel Aurone Derivative Ameliorates MASH Lipid Metabolism via the AMPK-ACC-PPARα Axis
Abstract
1. Introduction
2. Result
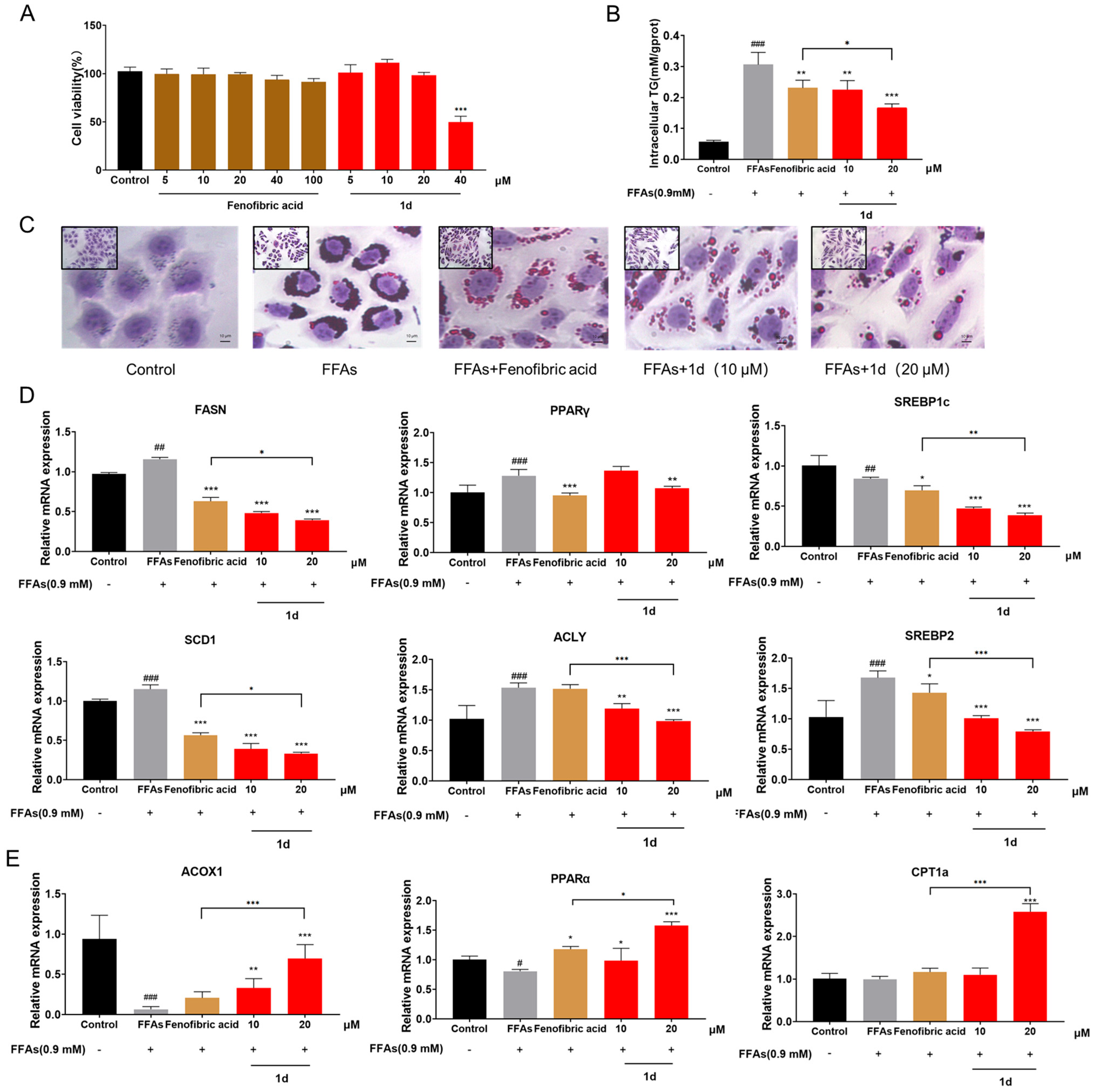
2.1. 1d Ameliorates FFAs-Induced Lipid Accumulation in L02 Hepatocytes
2.2. 1d Ameliorates Mitochondrial Dysfunction Induced by FFAs in L02 Cells
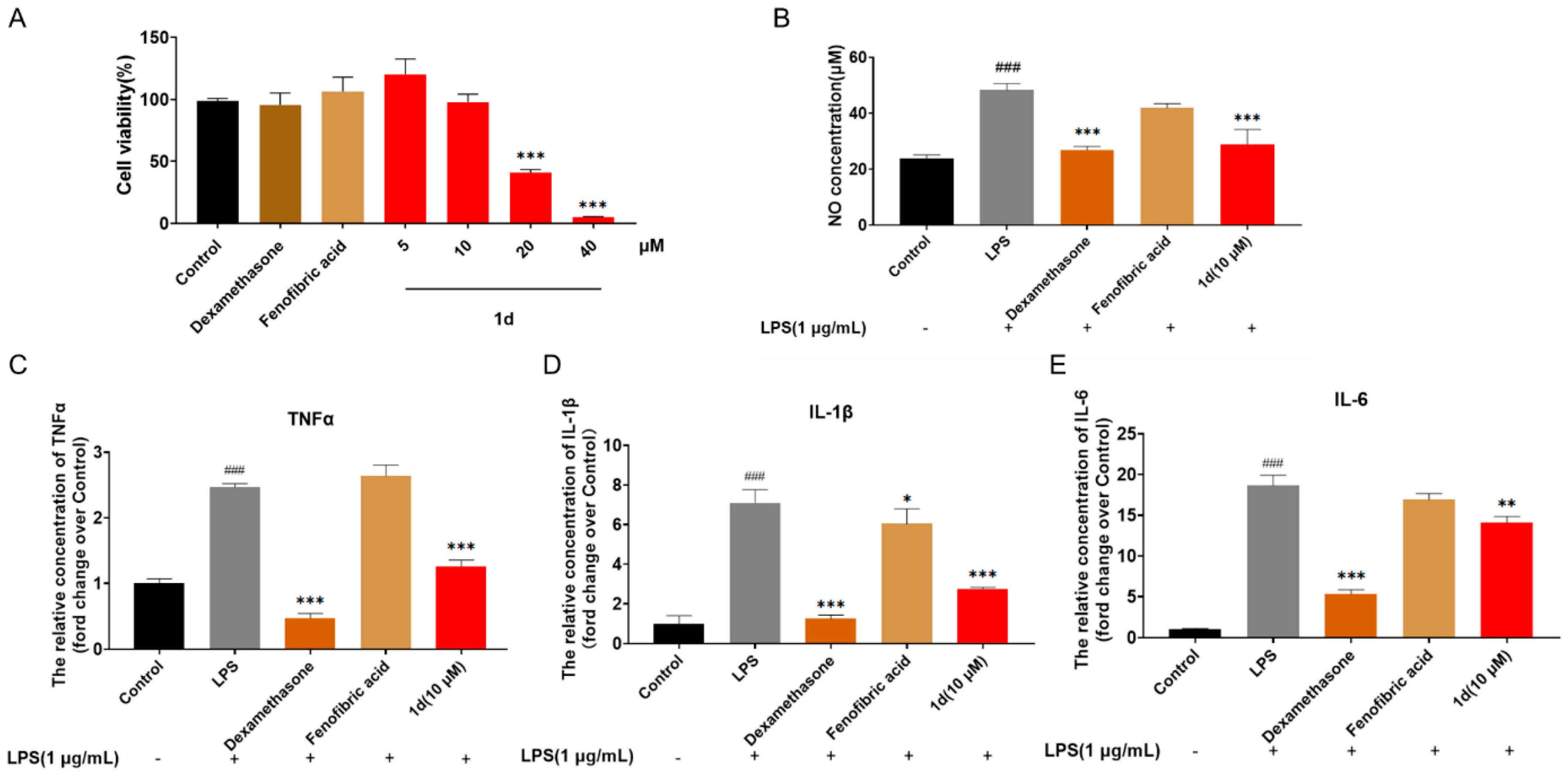
2.3. 1d Attenuates LPS-Induced Inflammatory Response in RAW264.7 Macrophages
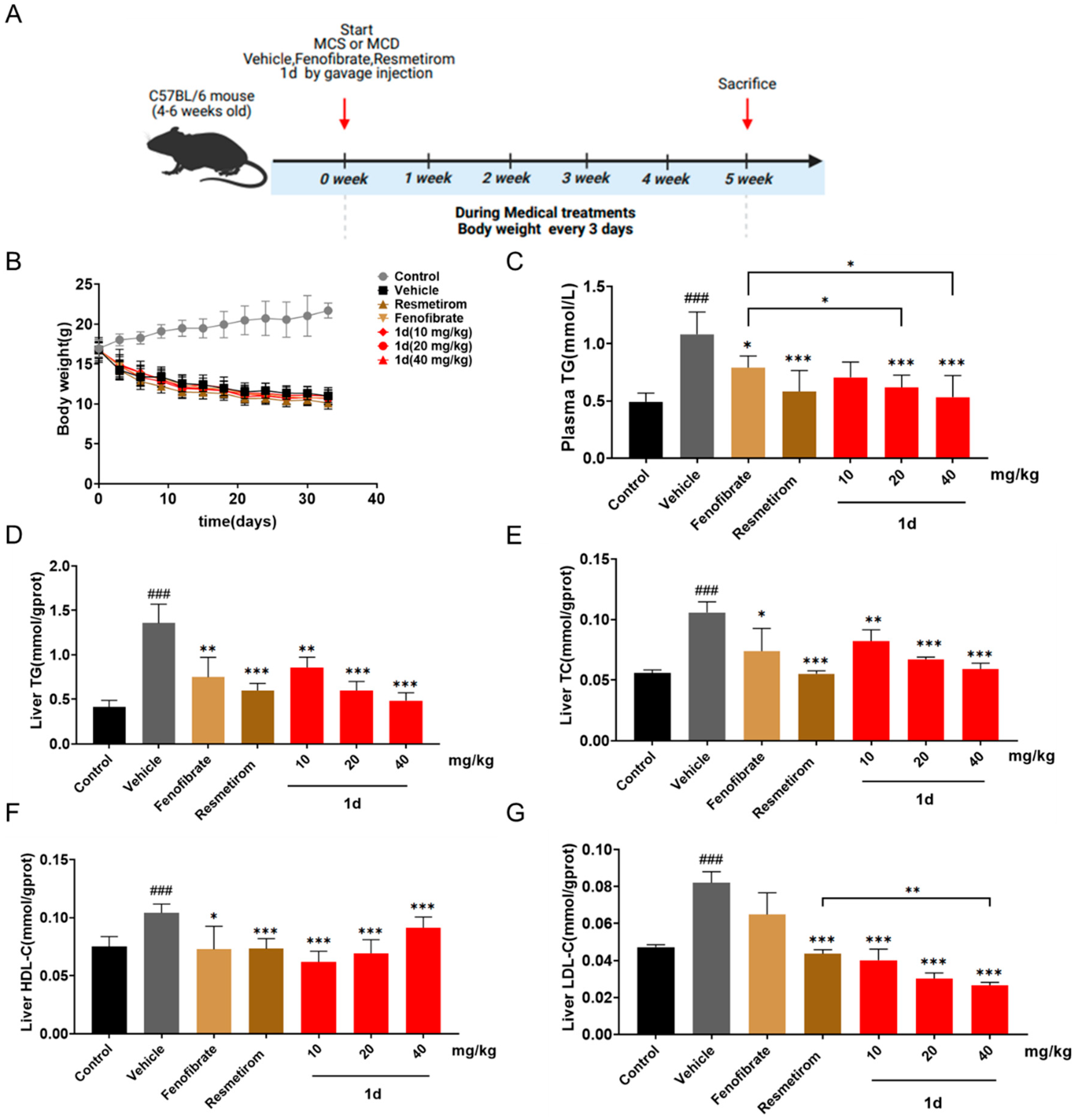
2.4. 1d Ameliorates Lipid Metabolism in MCD Diet-Induced MASH Mice
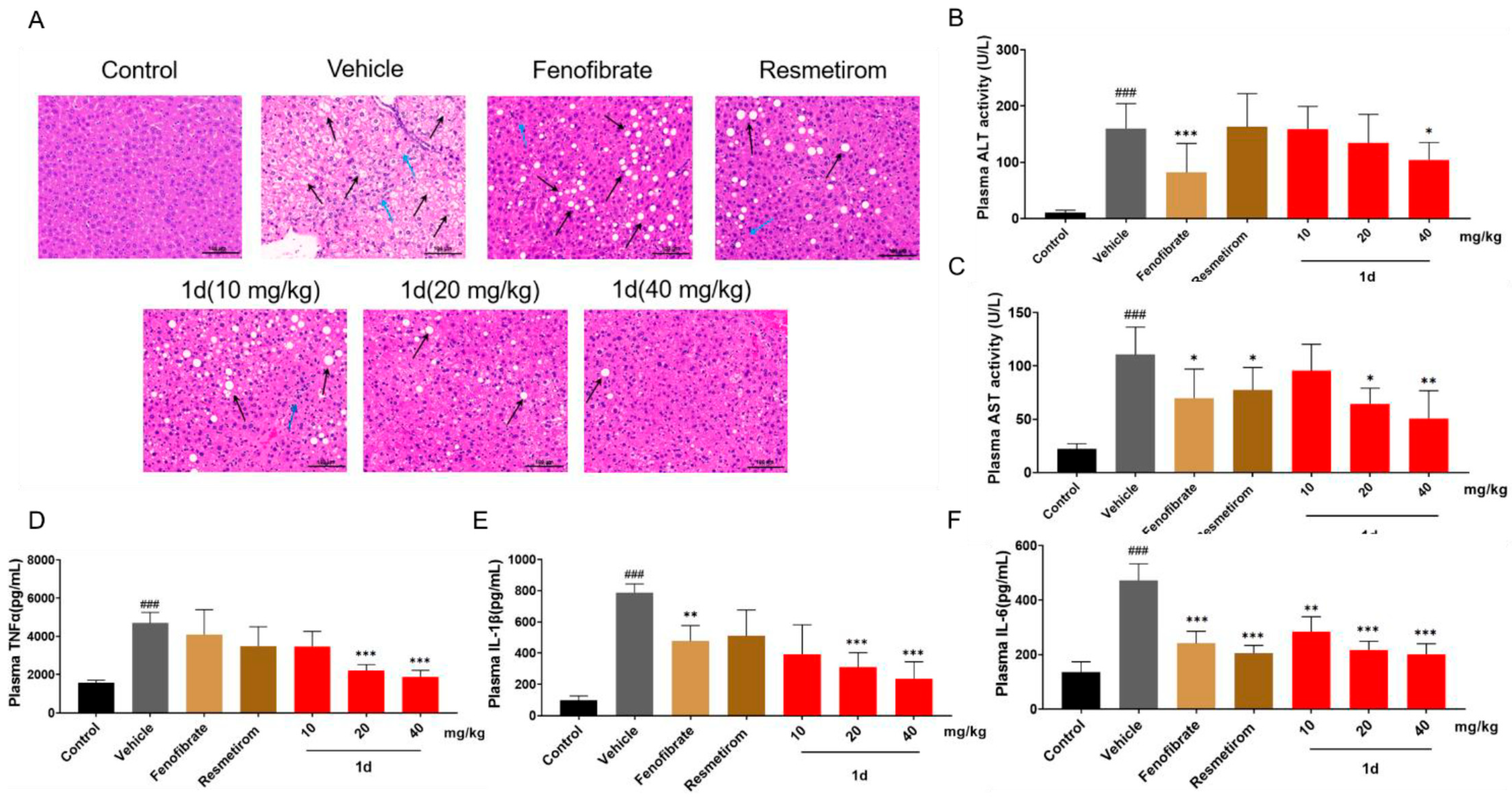
2.5. 1d Ameliorates Inflammation and Liver Histopathological Features in MCD Diet-Induced MASH Mice
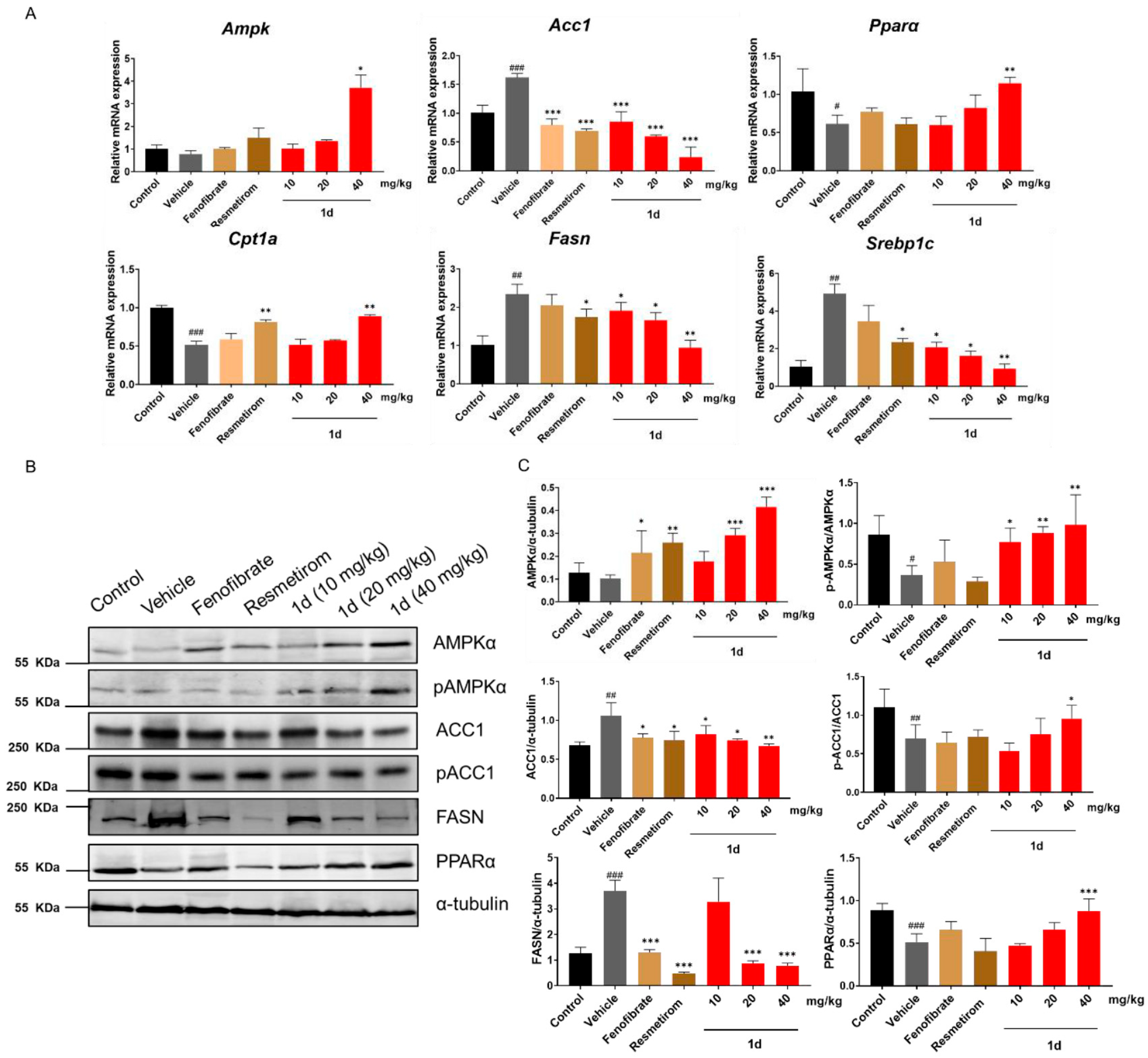
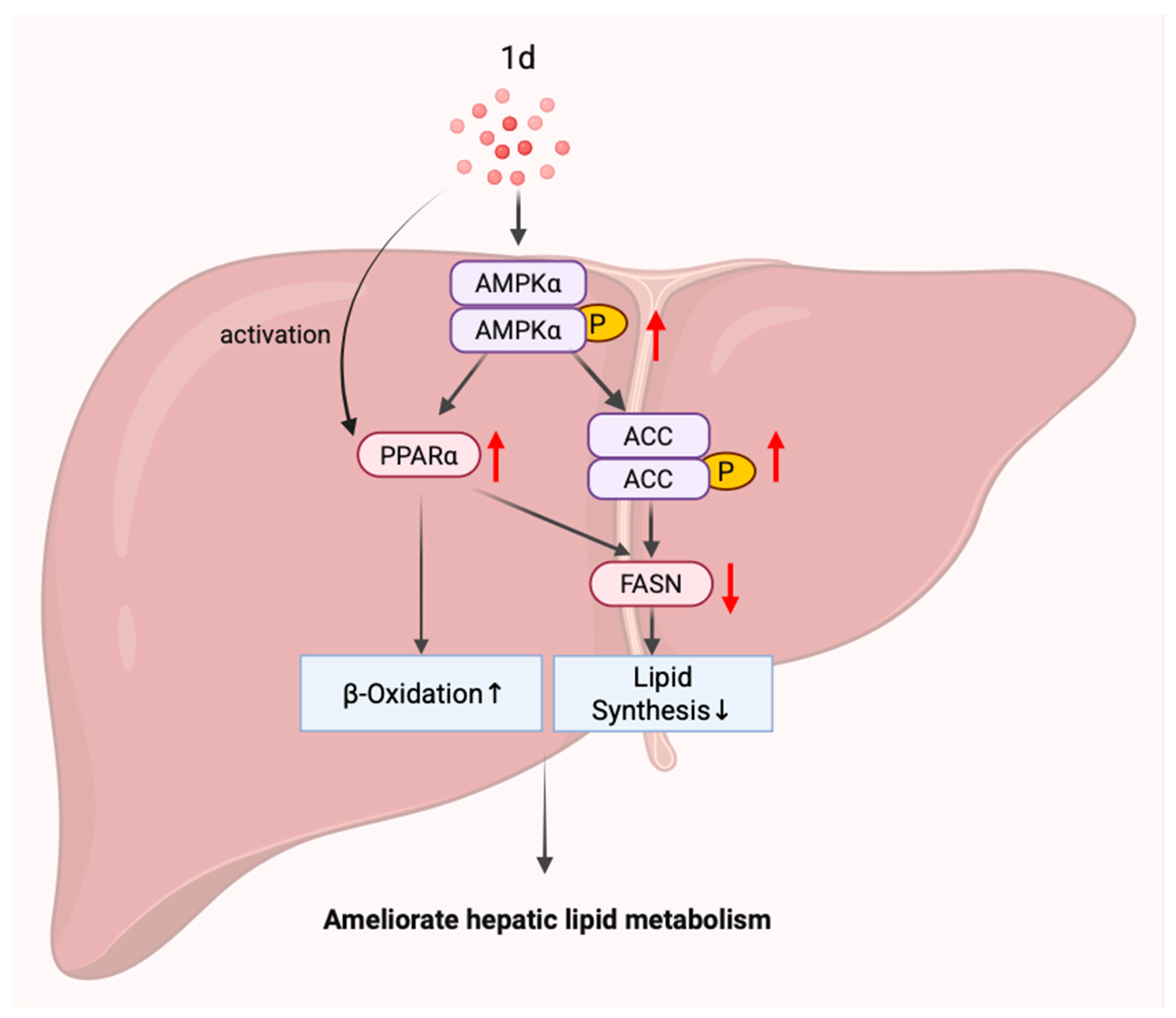
2.6. 1d Ameliorates Hepatic Lipid Metabolism in MCD Diet-Induced MASH Mice by Modulating the AMPK-ACC-PPARα Axis
3. Discussion
4. Materials and Methods
4.1. Cell Model
4.2. Cytotoxicity Assessment by MTT Assay
4.3. ATP Content Quantification
4.4. RNA Extraction and Quantitative Real-Time PCR Analysis
4.5. In Vivo Experimental and Tissue Collection
4.6. Plasma and Hepatic Biochemical Analyses
4.7. Protein Isolation and Western Blot Analysis
4.8. Hepatic Histopathological Examination
4.9. Statistical Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MASH | Metabolic dysfunction-associated steatohepatitis |
| MASLD | Metabolic dysfunction-Associated Steatotic Liver Disease |
| FFAs | Free Fatty Acids |
| MCD | Methionine-Choline Deficient |
| ATP | Adenosine Triphosphate |
| SFL | Simple Fatty Liver |
| HCC | Hepatocellular carcinoma |
| TG | Triglyceride |
| TC | Total Cholesterol |
| LDL-C | Low-Density Lipoprotein Cholesterol |
| THR-β | Thyroid Hormone Receptor-β |
| PPARs | Peroxisome proliferator-activated receptors |
| T2DM | Type 2 Diabetes Mellitus |
| LPS | Lipopolysaccharide |
| ROS | Reactive Oxygen Species |
| BSA | Bovine Serum Albumin |
| OA | Oleic Acid |
| PA | Palmitic Acid |
| TNFα | Tumor Necrosis Factor-α |
| IL-1β | Interleukin-1β |
| IL-6 | Interleukin-6 |
| AMPK | AMP-activated protein kinase |
| NO | Nitric Oxide |
| ACC | Acetyl-CoA carboxylase |
| CPT1 | Carnitine palmitoyltransferase 1 |
| FASN | Fatty Acid Synthase |
| CCCP | Carbonyl cyanide-chlorophenylhydrazone |
| ALT | Alanine Aminotransferase |
| AST | Aspartate Aminotransferase |
References
- Wong, V.W.; Ekstedt, M.; Wong, G.L.; Hagstrom, H. Changing epidemiology, global trends and implications for outcomes of NAFLD. J. Hepatol. 2023, 79, 842–852. [Google Scholar] [CrossRef]
- Cusi, K.; Sanyal, A.J.; Zhang, S.; Hartman, M.L.; Bue-Valleskey, J.M.; Hoogwerf, B.J.; Haupt, A. Non-alcoholic fatty liver disease (NAFLD) prevalence and its metabolic associations in patients with type 1 diabetes and type 2 diabetes. Diabetes Obes. Metab. 2017, 19, 1630–1634. [Google Scholar] [CrossRef]
- Targher, G.; Byrne, C.D.; Tilg, H. MASLD: A systemic metabolic disorder with cardiovascular and malignant complications. Gut 2024, 73, 691–702. [Google Scholar] [CrossRef]
- Byrne, C.D.; Targher, G. NAFLD: A multisystem disease. J. Hepatol. 2015, 62 (Suppl. S1), S47–S64. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Otgonsuren, M.; Henry, L.; Venkatesan, C.; Mishra, A.; Erario, M.; Hunt, S. Association of nonalcoholic fatty liver disease (NAFLD) with hepatocellular carcinoma (HCC) in the United States from 2004 to 2009. Hepatology 2015, 62, 1723–1730. [Google Scholar] [CrossRef]
- Li, Y.; Yang, P.; Ye, J.; Xu, Q.; Wu, J.; Wang, Y. Updated mechanisms of MASLD pathogenesis. Lipids Health Dis. 2024, 23, 117. [Google Scholar] [CrossRef]
- Machado, M.V.; Diehl, A.M. Pathogenesis of Nonalcoholic Steatohepatitis. Gastroenterology 2016, 150, 1769–1777. [Google Scholar] [CrossRef]
- Musso, G.; Gambino, R.; Cassader, M.; Pagano, G. Meta-analysis: Natural history of non-alcoholic fatty liver disease (NAFLD) and diagnostic accuracy of non-invasive tests for liver disease severity. Ann. Med. 2011, 43, 617–649. [Google Scholar] [CrossRef]
- Ekstedt, M.; Hagstrom, H.; Nasr, P.; Fredrikson, M.; Stal, P.; Kechagias, S.; Hultcrantz, R. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology 2015, 61, 1547–1554. [Google Scholar] [CrossRef]
- Ertle, J.; Dechene, A.; Sowa, J.P.; Penndorf, V.; Herzer, K.; Kaiser, G.; Schlaak, J.F.; Gerken, G.; Syn, W.K.; Canbay, A. Non-alcoholic fatty liver disease progresses to hepatocellular carcinoma in the absence of apparent cirrhosis. Int. J. Cancer 2011, 128, 2436–2443. [Google Scholar] [CrossRef]
- Starley, B.Q.; Calcagno, C.J.; Harrison, S.A. Nonalcoholic fatty liver disease and hepatocellular carcinoma: A weighty connection. Hepatology 2010, 51, 1820–1832. [Google Scholar] [CrossRef]
- Stickel, F.; Hellerbrand, C. Non-alcoholic fatty liver disease as a risk factor for hepatocellular carcinoma: Mechanisms and implications. Gut 2010, 59, 1303–1307. [Google Scholar] [CrossRef]
- Perla, F.M.; Prelati, M.; Lavorato, M.; Visicchio, D.; Anania, C. The Role of Lipid and Lipoprotein Metabolism in Non-Alcoholic Fatty Liver Disease. Children 2017, 4, 46. [Google Scholar] [CrossRef]
- Jarmakiewicz-Czaja, S.; Sokal-Dembowska, A.; Filip, R. Effects of Selected Food Additives on the Gut Microbiome and Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD). Medicina 2025, 61, 192. [Google Scholar] [CrossRef]
- Berlanga, A.; Guiu-Jurado, E.; Porras, J.A.; Auguet, T. Molecular pathways in non-alcoholic fatty liver disease. Clin. Exp. Gastroenterol. 2014, 7, 221–239. [Google Scholar] [CrossRef]
- Keam, S.J. Resmetirom: First Approval. Drugs 2024, 84, 729–735. [Google Scholar] [CrossRef]
- Taub, R.; Chiang, E.; Chabot-Blanchet, M.; Kelly, M.J.; Reeves, R.A.; Guertin, M.C.; Tardif, J.C. Lipid lowering in healthy volunteers treated with multiple doses of MGL-3196, a liver-targeted thyroid hormone receptor-beta agonist. Atherosclerosis 2013, 230, 373–380. [Google Scholar] [CrossRef]
- Harrison, S.A.; Bashir, M.R.; Guy, C.D.; Zhou, R.; Moylan, C.A.; Frias, J.P.; Alkhouri, N.; Bansal, M.B.; Baum, S.; Neuschwander-Tetri, B.A.; et al. Resmetirom (MGL-3196) for the treatment of non-alcoholic steatohepatitis: A multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet 2019, 394, 2012–2024. [Google Scholar] [CrossRef]
- Harrison, S.A.; Bedossa, P.; Guy, C.D.; Schattenberg, J.M.; Loomba, R.; Taub, R.; Labriola, D.; Moussa, S.E.; Neff, G.W.; Rinella, M.E.; et al. A Phase 3, Randomized, Controlled Trial of Resmetirom in NASH with Liver Fibrosis. N. Engl. J. Med. 2024, 390, 497–509. [Google Scholar] [CrossRef]
- Sinha, R.A.; Bruinstroop, E.; Singh, B.K.; Yen, P.M. Nonalcoholic Fatty Liver Disease and Hypercholesterolemia: Roles of Thyroid Hormones, Metabolites, and Agonists. Thyroid 2019, 29, 1173–1191. [Google Scholar] [CrossRef]
- Issemann, I.; Green, S. Activation of a member of the steroid hormone receptor superfamily by peroxisome proliferators. Nature 1990, 347, 645–650. [Google Scholar] [CrossRef]
- Braissant, O.; Foufelle, F.; Scotto, C.; Dauca, M.; Wahli, W. Differential expression of peroxisome proliferator-activated receptors (PPARs): Tissue distribution of PPAR-alpha, -beta, and -gamma in the adult rat. Endocrinology 1996, 137, 354–366. [Google Scholar] [CrossRef]
- Kliewer, S.A.; Forman, B.M.; Blumberg, B.; Ong, E.S.; Borgmeyer, U.; Mangelsdorf, D.J.; Umesono, K.; Evans, R.M. Differential expression and activation of a family of murine peroxisome proliferator-activated receptors. Proc. Natl. Acad. Sci. USA 1994, 91, 7355–7359. [Google Scholar] [CrossRef]
- Auboeuf, D.; Rieusset, J.; Fajas, L.; Vallier, P.; Frering, V.; Riou, J.P.; Staels, B.; Auwerx, J.; Laville, M.; Vidal, H. Tissue distribution and quantification of the expression of mRNAs of peroxisome proliferator-activated receptors and liver X receptor-alpha in humans: No alteration in adipose tissue of obese and NIDDM patients. Diabetes 1997, 46, 1319–1327. [Google Scholar] [CrossRef]
- Abbott, B.D. Review of the expression of peroxisome proliferator-activated receptors alpha (PPAR alpha), beta (PPAR beta), and gamma (PPAR gamma) in rodent and human development. Reprod. Toxicol. 2009, 27, 246–257. [Google Scholar] [CrossRef]
- Staels, B.; Dallongeville, J.; Auwerx, J.; Schoonjans, K.; Leitersdorf, E.; Fruchart, J.C. Mechanism of action of fibrates on lipid and lipoprotein metabolism. Circulation 1998, 98, 2088–2093. [Google Scholar] [CrossRef]
- Pyper, S.R.; Viswakarma, N.; Yu, S.; Reddy, J.K. PPARalpha: Energy combustion, hypolipidemia, inflammation and cancer. Nucl. Recept. Signal 2010, 8, e002. [Google Scholar] [CrossRef]
- Qiu, Y.Y.; Zhang, J.; Zeng, F.Y.; Zhu, Y.Z. Roles of the peroxisome proliferator-activated receptors (PPARs) in the pathogenesis of nonalcoholic fatty liver disease (NAFLD). Pharmacol. Res. 2023, 192, 106786. [Google Scholar] [CrossRef]
- Gross, B.; Pawlak, M.; Lefebvre, P.; Staels, B. PPARs in obesity-induced T2DM, dyslipidaemia and NAFLD. Nat. Rev. Endocrinol. 2017, 13, 36–49. [Google Scholar] [CrossRef]
- Chen, H.; Tan, H.; Wan, J.; Zeng, Y.; Wang, J.; Wang, H.; Lu, X. PPAR-gamma signaling in nonalcoholic fatty liver disease: Pathogenesis and therapeutic targets. Pharmacol. Ther. 2023, 245, 108391. [Google Scholar] [CrossRef]
- Pawlak, M.; Lefebvre, P.; Staels, B. Molecular mechanism of PPARalpha action and its impact on lipid metabolism, inflammation and fibrosis in non-alcoholic fatty liver disease. J. Hepatol. 2015, 62, 720–733. [Google Scholar] [CrossRef]
- Ezhilarasan, D. Deciphering the molecular pathways of saroglitazar: A dual PPAR alpha/gamma agonist for managing metabolic NAFLD. Metabolism 2024, 155, 155912. [Google Scholar] [CrossRef] [PubMed]
- Souza, M.; Al-Sharif, L.; Antunes, V.L.J.; Huang, D.Q.; Loomba, R. Comparison of pharmacological therapies in metabolic dysfunction-associated steatohepatitis for fibrosis regression and MASH resolution: Systematic review and network meta-analysis. Hepatology 2025, 82, 1523–1533. [Google Scholar] [CrossRef]
- Sun, Y.; Wu, C.; Xin, G.; Zhong, B.; Wu, X.; Liu, Y.; Shi, J.; Zhang, Q.; Zhao, Y.; Gao, Y.; et al. Chiglitazar in MASLD with hypertriglyceridemia and insulin resistance: A phase II, randomized, double-blind, placebo-controlled study. Hepatology 2025. [Google Scholar] [CrossRef]
- Herzig, S.; Shaw, R.J. AMPK: Guardian of metabolism and mitochondrial homeostasis. Nat. Rev. Mol. Cell Biol. 2018, 19, 121–135. [Google Scholar] [CrossRef]
- Carling, D. AMPK signalling in health and disease. Curr. Opin. Cell Biol. 2017, 45, 31–37. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, S.; Zhai, A.; Zhang, B.; Tian, G. AMPK-Mediated Regulation of Lipid Metabolism by Phosphorylation. Biol. Pharm. Bull. 2018, 41, 985–993. [Google Scholar] [CrossRef]
- Zhang, L.; Zhou, X.; Chen, H.; You, L.; Zhang, T.; Cheng, M.; Yao, Y.; Pan, X.; Yang, X. Mulberry extract ameliorates T2DM-related symptoms via AMPK pathway in STZ-HFD-induced C57BL/6J mice. J. Ethnopharmacol. 2023, 313, 116475. [Google Scholar]
- Pang, Y.; Xu, X.; Xiang, X.; Li, Y.; Zhao, Z.; Li, J.; Gao, S.; Liu, Q.; Mai, K.; Ai, Q. High Fat Activates O-GlcNAcylation and Affects AMPK/ACC Pathway to Regulate Lipid Metabolism. Nutrients 2021, 13, 1740. [Google Scholar] [CrossRef]
- Zang, L.; Kagotani, K.; Hayakawa, T.; Tsuji, T.; Okumura, K.; Shimada, Y.; Nishimura, N. The Hexane Extract of Citrus sphaerocarpa Ameliorates Visceral Adiposity by Regulating the PI3K/AKT/FoxO1 and AMPK/ACC Signaling Pathways in High-Fat-Diet-Induced Obese Mice. Molecules 2023, 28, 8026. [Google Scholar] [CrossRef]
- Li, Y.; Li, X.; Liu, J.; Jayavanth, P.; Bai, W.; Jiao, R. Vitisin A Outperforms Cyanidin-3-O-Glucoside in Triglyceride Reduction by Modulating Hepatic Lipogenesis and Fatty Acid beta-Oxidation. Int. J. Mol. Sci. 2025, 26, 1521. [Google Scholar] [CrossRef]
- Ni, J.; Shang, Y.; Wang, W.D.; Wang, C.; Wang, A.M.; Li, G.J.; Chen, S.Z. FASN Inhibitors Enhance Bestatin-Related Tumor Cell Apoptosis Through Upregulating PEPT1. Curr. Mol. Pharmacol. 2023, 16, 771–786. [Google Scholar]
- Dong, J.; Li, M.; Peng, R.; Zhang, Y.; Qiao, Z.; Sun, N. ACACA reduces lipid accumulation through dual regulation of lipid metabolism and mitochondrial function via AMPK- PPARalpha- CPT1A axis. J. Transl. Med. 2024, 22, 196. [Google Scholar] [CrossRef]
- Xu, G.; Huang, K.; Zhou, J. Hepatic AMP Kinase as a Potential Target for Treating Nonalcoholic Fatty Liver Disease: Evidence from Studies of Natural Products. Curr. Med. Chem. 2018, 25, 889–907. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, W.; Yang, L.; Zhao, W.; Liu, Z.; Wang, E.; Wang, J. Phytochemical gallic acid alleviates nonalcoholic fatty liver disease via AMPK-ACC-PPARa axis through dual regulation of lipid metabolism and mitochondrial function. Phytomedicine 2023, 109, 154589. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.; Zou, Y.; Li, Y.; Yang, W.; Liu, F.; Wang, J.; Qie, Z.; Liu, Z.; Yu, P.; Xiang, C.; et al. Design, synthesis and anti-diabetic evaluation of novel PPAR agonists based on functional group-oriented strategy. Eur. J. Med. Chem. 2025, 301, 118226. [Google Scholar] [CrossRef] [PubMed]
- Wen, H.; Deng, H.; Li, B.; Chen, J.; Zhu, J.; Zhang, X.; Yoshida, S.; Zhou, Y. Mitochondrial diseases: From molecular mechanisms to therapeutic advances. Signal Transduct. Target. Ther. 2025, 10, 9. [Google Scholar] [CrossRef]
- Sunny, N.E.; Bril, F.; Cusi, K. Mitochondrial Adaptation in Nonalcoholic Fatty Liver Disease: Novel Mechanisms and Treatment Strategies. Trends Endocrinol. Metab. 2017, 28, 250–260. [Google Scholar] [CrossRef]
- Fromenty, B.; Roden, M. Mitochondrial alterations in fatty liver diseases. J. Hepatol. 2023, 78, 415–429. [Google Scholar] [CrossRef]
- Sookoian, S.; Pirola, C.J. Resmetirom for treatment of MASH. Cell 2024, 187, 2897–2897.e1. [Google Scholar] [CrossRef]
- Radosavljevic, T.; Brankovic, M.; Samardzic, J.; Djuretic, J.; Vukicevic, D.; Vucevic, D.; Jakovljevic, V. Altered Mitochondrial Function in MASLD: Key Features and Promising Therapeutic Approaches. Antioxidants 2024, 13, 906. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, H.; Li, Y.; Chen, H.; Wang, C.; Wong, V.K.W.; Jiang, Z.; Zhang, W. Phytotherapy using blueberry leaf polyphenols to alleviate non-alcoholic fatty liver disease through improving mitochondrial function and oxidative defense. Phytomedicine 2020, 69, 153209. [Google Scholar] [CrossRef] [PubMed]
- Boland, M.L.; Laker, R.C.; Mather, K.; Nawrocki, A.; Oldham, S.; Boland, B.B.; Lewis, H.; Conway, J.; Naylor, J.; Guionaud, S.; et al. Resolution of NASH and hepatic fibrosis by the GLP-1R/GcgR dual-agonist Cotadutide via modulating mitochondrial function and lipogenesis. Nat. Metab. 2020, 2, 413–431. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, G.R.; Carpentier, A.C.; Wang, D. MASH: The nexus of metabolism, inflammation, and fibrosis. J. Clin. Investig. 2025, 135, e186420. [Google Scholar] [CrossRef] [PubMed]
- Samuel, V.T.; Shulman, G.I. Nonalcoholic Fatty Liver Disease, Insulin Resistance, and Ceramides. N. Engl. J. Med. 2019, 381, 1866–1869. [Google Scholar] [CrossRef] [PubMed]
- Ballestri, S.; Zona, S.; Targher, G.; Romagnoli, D.; Baldelli, E.; Nascimbeni, F.; Roverato, A.; Guaraldi, G.; Lonardo, A. Nonalcoholic fatty liver disease is associated with an almost twofold increased risk of incident type 2 diabetes and metabolic syndrome. Evidence from a systematic review and meta-analysis. J. Gastroenterol. Hepatol. 2016, 31, 936–944. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Golabi, P.; de Avila, L.; Paik, J.M.; Srishord, M.; Fukui, N.; Qiu, Y.; Burns, L.; Afendy, A.; Nader, F. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: A systematic review and meta-analysis. J. Hepatol. 2019, 71, 793–801. [Google Scholar] [CrossRef]
- Mucinski, J.M.; Salvador, A.F.; Moore, M.P.; Fordham, T.M.; Anderson, J.M.; Shryack, G.; Cunningham, R.P.; Lastra, G.; Gaballah, A.H.; Diaz-Arias, A.; et al. Histological improvements following energy restriction and exercise: The role of insulin resistance in resolution of MASH. J. Hepatol. 2024, 81, 781–793. [Google Scholar] [CrossRef]
- Bansal, S.K.; Bansal, M.B. Pathogenesis of MASLD and MASH—Role of insulin resistance and lipotoxicity. Aliment. Pharmacol. Ther. 2024, 59 (Suppl. 1), S10–S22. [Google Scholar] [CrossRef]
- Ziamanesh, F.; Mohammadi, M.; Ebrahimpour, S.; Tabatabaei-Malazy, O.; Mosallanejad, A.; Larijani, B. Unraveling the link between insulin resistance and Non-alcoholic fatty liver disease (or metabolic dysfunction-associated steatotic liver disease): A Narrative Review. J. Diabetes Metab. Disord. 2023, 22, 1083–1094. [Google Scholar] [CrossRef]
- Pierantonelli, I.; Svegliati-Baroni, G. Nonalcoholic Fatty Liver Disease: Basic Pathogenetic Mechanisms in the Progression from NAFLD to NASH. Transplantation 2019, 103, e1–e13. [Google Scholar] [CrossRef]
- Konings, L.A.M.; Miguelanez-Matute, L.; Boeren, A.M.P.; van de Luitgaarden, I.A.T.; Dirksmeier, F.; de Knegt, R.J.; Tushuizen, M.E.; Grobbee, D.E.; Holleboom, A.G.; Cabezas, M.C. Pharmacological treatment options for metabolic dysfunction-associated steatotic liver disease in patients with type 2 diabetes mellitus: A systematic review. Eur. J. Clin. Investig. 2025, 55, e70003. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Yan, W.; Cui, X.; Liu, N.; Wei, X.; Sun, Y.; Fan, K.; Liu, J.; Zhu, Y.; Wang, Z.; et al. Liraglutide attenuates type 2 diabetes mellitus-associated non-alcoholic fatty liver disease by activating AMPK/ACC signaling and inhibiting ferroptosis. Mol. Med. 2023, 29, 132. [Google Scholar] [CrossRef] [PubMed]
- Stratina, E.; Stanciu, C.; Nastasa, R.; Zenovia, S.; Stafie, R.; Rotaru, A.; Cuciureanu, T.; Muzica, C.; Sfarti, C.; Girleanu, I.; et al. New Insights on Using Oral Semaglutide versus Dapagliflozin in Patients with Type 2 Diabetes and Metabolic Dysfunction-Associated Steatotic Liver Disease. Diagnostics 2024, 14, 1475. [Google Scholar] [CrossRef]
- An, H.; Jang, Y.; Choi, J.; Hur, J.; Kim, S.; Kwon, Y. New Insights into AMPK, as a Potential Therapeutic Target in Metabolic Dysfunction-Associated Steatotic Liver Disease and Hepatic Fibrosis. Biomol Ther. 2025, 33, 18–38. [Google Scholar] [CrossRef]
- Peng, C.; Jiang, H.; Jing, L.; Yang, W.; Guan, X.; Wang, H.; Yu, S.; Cao, Y.; Wang, M.; Ma, H.; et al. Macrophage SUCLA2 coupled glutaminolysis manipulates obesity through AMPK. Nat. Commun. 2025, 16, 1738. [Google Scholar] [CrossRef]
- Iwaki, M.; Kobayashi, T.; Nogami, A.; Ogawa, Y.; Imajo, K.; Sakai, E.; Nakada, Y.; Koyama, S.; Kurihashi, T.; Oza, N.; et al. Pemafibrate for treating MASLD complicated by hypertriglyceridaemia: A multicentre, open-label, randomised controlled trial study protocol. BMJ Open 2024, 14, e088862. [Google Scholar] [CrossRef]
- Cooreman, M.P.; Vonghia, L.; Francque, S.M. MASLD/MASH and type 2 diabetes: Two sides of the same coin? From single PPAR to pan-PPAR agonists. Diabetes Res. Clin. Pract. 2024, 212, 111688. [Google Scholar] [CrossRef]
- Li, N.; Wu, S.; Li, X.; Yan, M.; Ding, Y.; Zhang, L.; Brenner, D.A.; Liu, X.; Kisseleva, T. Peroxisome Proliferator-Activated Receptor Agonist IVA337 Alleviates Inflammation and Fibrosis in MASH by Restoring Lipid Homeostasis. Am. J. Pathol. 2025, 195, 1822–1838. [Google Scholar] [CrossRef] [PubMed]
- Koyama, Y.; Brenner, D.A. Liver inflammation and fibrosis. J. Clin. Investig. 2017, 127, 55–64. [Google Scholar] [CrossRef]
- Filozof, C.; Chow, S.C.; Dimick-Santos, L.; Chen, Y.F.; Williams, R.N.; Goldstein, B.J.; Sanyal, A. Clinical endpoints and adaptive clinical trials in precirrhotic nonalcoholic steatohepatitis: Facilitating development approaches for an emerging epidemic. Hepatol. Commun. 2017, 1, 577–585. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Park, J.S.; Lee, Y.S.; Bae, S.H. PERK prevents hepatic lipotoxicity by activating the p62-ULK1 axis-mediated noncanonical KEAP1-Nrf2 pathway. Redox Biol. 2022, 50, 102235. [Google Scholar] [CrossRef]
- Galaris, D.; Pantopoulos, K. Oxidative stress and iron homeostasis: Mechanistic and health aspects. Crit. Rev. Clin. Lab. Sci. 2008, 45, 1–23. [Google Scholar] [CrossRef]
- Bertolani, C.; Marra, F. The role of adipokines in liver fibrosis. Pathophysiology 2008, 15, 91–101. [Google Scholar] [CrossRef]
- Kumar, S.; Duan, Q.; Wu, R.; Harris, E.N.; Su, Q. Pathophysiological communication between hepatocytes and non-parenchymal cells in liver injury from NAFLD to liver fibrosis. Adv. Drug Deliv. Rev. 2021, 176, 113869. [Google Scholar] [CrossRef] [PubMed]
- Schattenberg, J.M.; Pares, A.; Kowdley, K.V.; Heneghan, M.A.; Caldwell, S.; Pratt, D.; Bonder, A.; Hirschfield, G.M.; Levy, C.; Vierling, J.; et al. A randomized placebo-controlled trial of elafibranor in patients with primary biliary cholangitis and incomplete response to UDCA. J. Hepatol. 2021, 74, 1344–1354. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, S.; Zou, Y.; Zhang, W.; Yu, J.; Qie, Z.; Liu, Z.; Yu, P.; Xiang, C.; Teng, Y. Novel Aurone Derivative Ameliorates MASH Lipid Metabolism via the AMPK-ACC-PPARα Axis. Int. J. Mol. Sci. 2025, 26, 11099. https://doi.org/10.3390/ijms262211099
Bai S, Zou Y, Zhang W, Yu J, Qie Z, Liu Z, Yu P, Xiang C, Teng Y. Novel Aurone Derivative Ameliorates MASH Lipid Metabolism via the AMPK-ACC-PPARα Axis. International Journal of Molecular Sciences. 2025; 26(22):11099. https://doi.org/10.3390/ijms262211099
Chicago/Turabian StyleBai, Sule, Yi Zou, Wenyi Zhang, Jiajia Yu, Zhenzhen Qie, Zhen Liu, Peng Yu, Cen Xiang, and Yuou Teng. 2025. "Novel Aurone Derivative Ameliorates MASH Lipid Metabolism via the AMPK-ACC-PPARα Axis" International Journal of Molecular Sciences 26, no. 22: 11099. https://doi.org/10.3390/ijms262211099
APA StyleBai, S., Zou, Y., Zhang, W., Yu, J., Qie, Z., Liu, Z., Yu, P., Xiang, C., & Teng, Y. (2025). Novel Aurone Derivative Ameliorates MASH Lipid Metabolism via the AMPK-ACC-PPARα Axis. International Journal of Molecular Sciences, 26(22), 11099. https://doi.org/10.3390/ijms262211099





