Swiss Cheese Gene Is Important for Intestinal Barrier, Microbiome, and Lipid Metabolism Regulation in Drosophila Gut
Abstract
1. Introduction
2. Results and Discussion
2.1. Knockout of the sws Gene Results in Altered Midgut Morphology Due to Disruption of the Septate Junction Structure
2.2. Disruption of the Septate Junction’s Structure Leads to Permeability of the Intestinal Barrier in the sws1 Mutant
2.3. Ectopic Expression of the Human PNPLA6 Gene Against the Background of the sws1 Mutation (Rescue Experiments)
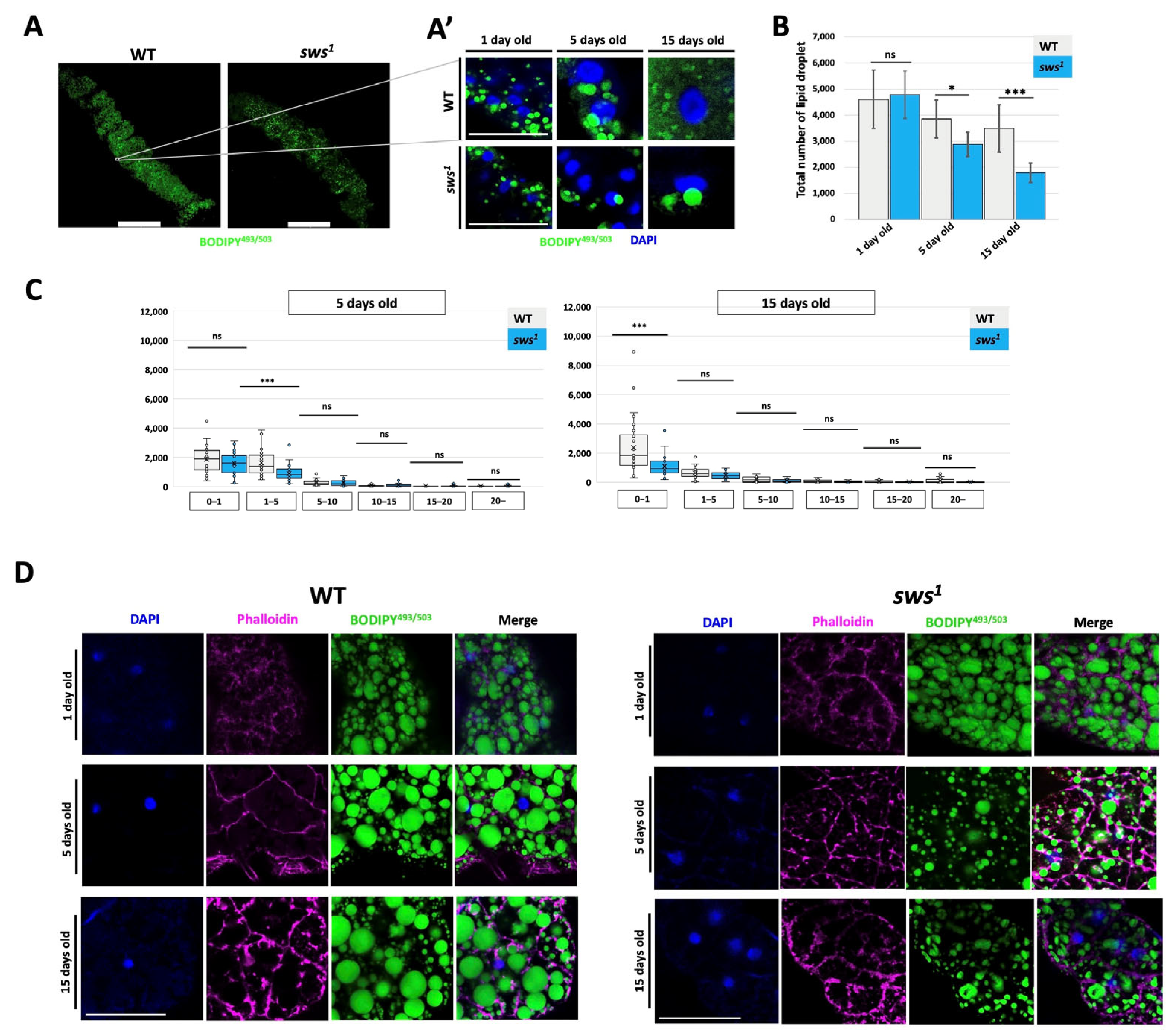
2.4. Dysfunction of the sws Gene Leads to a Disruption of the Supply of Neutral Lipids in Enterocytes, but Does Not Affect the Absorptive Function
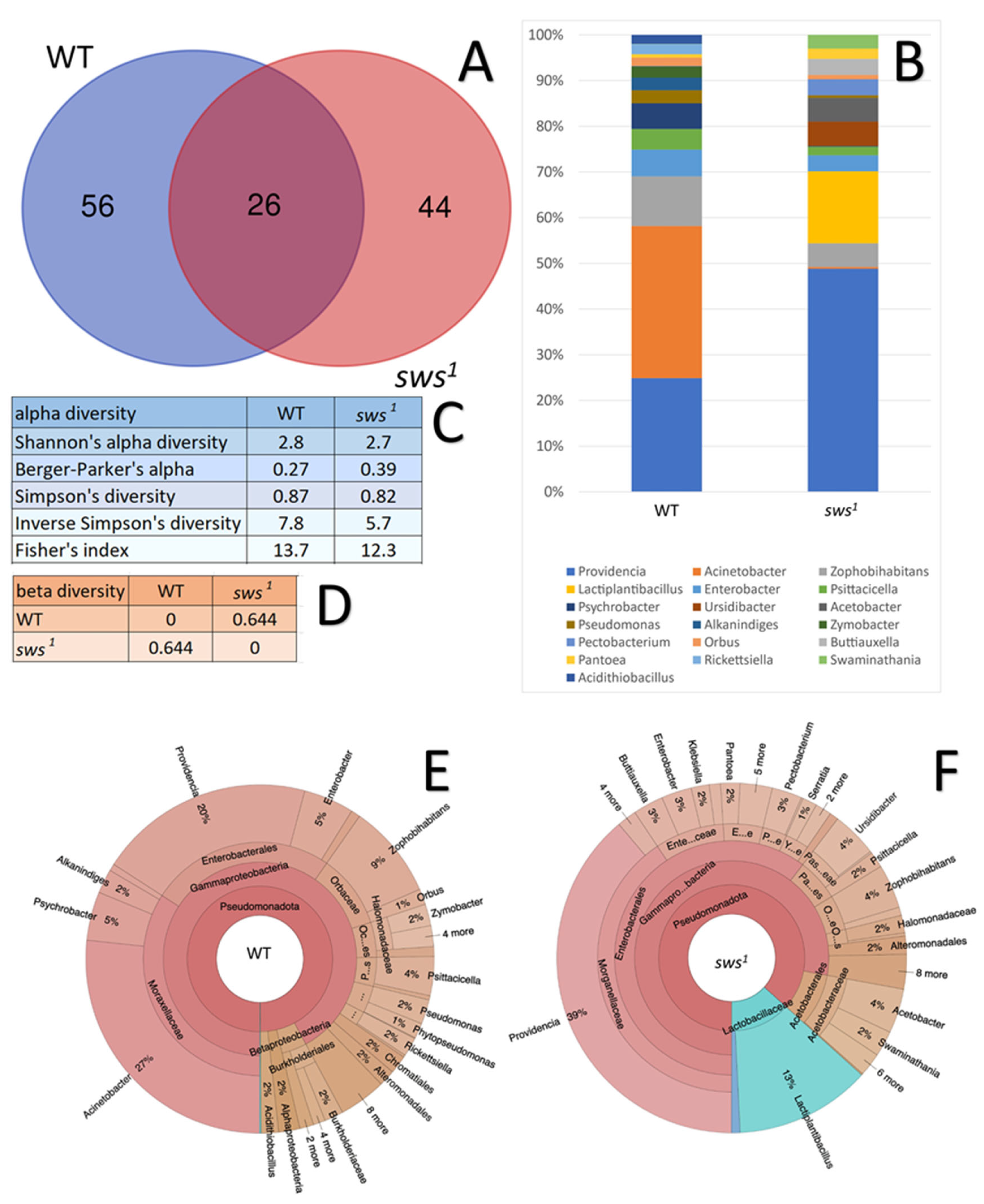
2.5. The Impact of sws Gene Dysfunction on the Gut Microbiome of Drosophila melanogaster
3. Materials and Methods
3.1. Drosophila Stocks and Feeding
3.2. Quantitative Analysis of mRNA Level
3.3. Permeability Assay
3.4. Immunohistochemical Staining and Microscopy
3.5. Lipid (FFA) and Glucose Quantification
3.6. Lipid Droplet Visualization in Enterocytes and Adipocytes
3.7. Oxidative Particle Measurement
3.8. 16S rRNA Sequencing and Analysis
3.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh, A.; Dawson, T.M.; Kulkarni, S. Neurodegenerative Disorders and Gut-Brain Interactions. J. Clin. Investig. 2021, 131, e143775. [Google Scholar] [CrossRef]
- Madhogaria, B.; Bhowmik, P.; Kundu, A. Correlation Between Human Gut Microbiome and Diseases. Infect. Med. 2022, 1, 180–191. [Google Scholar] [CrossRef]
- Adawi, M. The Role of Gut Microbiota in Autoimmune Disease Progression and Therapy: A Comprehensive Synthesis. Front. Microbiomes 2025, 4, 1553243. [Google Scholar] [CrossRef]
- Peelaerts, W.; Bousset, L.; Van Der Perren, A.; Moskalyuk, A.; Pulizzi, R.; Giugliano, M.; Van Den Haute, C.; Melki, R.; Baekelandt, V. α-Synuclein Strains Cause Distinct Synucleinopathies after Local and Systemic Administration. Nature 2015, 522, 340–344. [Google Scholar] [CrossRef] [PubMed]
- Patrick, K.L.; Bell, S.L.; Weindel, C.G.; Watson, R.O. Exploring the “Multiple-Hit Hypothesis” of Neurodegenerative Disease: Bacterial Infection Comes Up to Bat. Front. Cell. Infect. Microbiol. 2019, 9, 138. [Google Scholar] [CrossRef] [PubMed]
- Kanavin, Ø.J.; Fjermestad, K.W. Gastrointestinal and Urinary Complaints in Adults with Hereditary Spastic Paraparesis. Orphanet J. Rare Dis. 2018, 13, 58. [Google Scholar] [CrossRef] [PubMed]
- Mühlig-Versen, M.; Da Cruz, A.B.; Tschäpe, J.-A.; Moser, M.; Büttner, R.; Athenstaedt, K.; Glynn, P.; Kretzschmar, D. Loss of Swiss Cheese/Neuropathy Target Esterase Activity Causes Disruption of Phosphatidylcholine Homeostasis and Neuronal and Glial Death in Adult Drosophila. J. Neurosci. 2005, 25, 2865–2873. [Google Scholar] [CrossRef]
- Kmoch, S.; Majewski, J.; Ramamurthy, V.; Cao, S.; Fahiminiya, S.; Ren, H.; MacDonald, I.M.; Lopez, I.; Sun, V.; Keser, V.; et al. Mutations in PNPLA6 Are Linked to Photoreceptor Degeneration and Various Forms of Childhood Blindness. Nat. Commun. 2015, 6, 5614. [Google Scholar] [CrossRef]
- Limber, E.R.; Bresnick, G.H.; Lebovitz, R.M.; Appen, R.E.; Gilbert-Barness, E.F.; Pauli, R.M. Spinocerebellar Ataxia, Hypogonadotropic Hypogonadism, and Choroidal Dystrophy (Boucher-Neuhäuser Syndrome. Am. J. Med. Genet. 1989, 33, 409–414. [Google Scholar] [CrossRef]
- Rainier, S.; Bui, M.; Mark, E.; Thomas, D.; Tokarz, D.; Ming, L.; Delaney, C.; Richardson, R.J.; Albers, J.W.; Matsunami, N.; et al. Neuropathy Target Esterase Gene Mutations Cause Motor Neuron Disease. Am. J. Hum. Genet. 2008, 82, 780–785. [Google Scholar] [CrossRef]
- Deik, A.; Johannes, B.; Rucker, J.C.; Sánchez, E.; Brodie, S.E.; Deegan, E.; Landy, K.; Kajiwara, Y.; Scelsa, S.; Saunders-Pullman, R.; et al. Compound Heterozygous PNPLA6 Mutations Cause Boucher–Neuhäuser Syndrome with Late-Onset Ataxia. J. Neurol. 2014, 261, 2411–2423. [Google Scholar] [CrossRef]
- Topaloglu, A.K.; Lomniczi, A.; Kretzschmar, D.; Dissen, G.A.; Kotan, L.D.; McArdle, C.A.; Koc, A.F.; Hamel, B.C.; Guclu, M.; Papatya, E.D.; et al. Loss-of-Function Mutations in PNPLA6 Encoding Neuropathy Target Esterase Underlie Pubertal Failure and Neurological Deficits in Gordon Holmes Syndrome. J. Clin. Endocrinol. Metab. 2014, 99, E2067–E2075. [Google Scholar] [CrossRef]
- Fagerberg, L.; Hallström, B.M.; Oksvold, P.; Kampf, C.; Djureinovic, D.; Odeberg, J.; Habuka, M.; Tahmasebpoor, S.; Danielsson, A.; Edlund, K.; et al. Analysis of the Human Tissue-Specific Expression by Genome-Wide Integration of Transcriptomics and Antibody-Based Proteomics. Mol. Cell. Proteom. 2014, 13, 397–406. [Google Scholar] [CrossRef]
- Langdahl, J.H.; Frederiksen, A.L.; Nguyen, N.; Brusgaard, K.; Juhl, C.B. Boucher Neuhäuser Syndrome—A Rare Cause of Inherited Hypogonadotropic Hypogonadism. A Case of Two Adult Siblings with Two Novel Mutations in PNPLA6. Eur. J. Med. Genet. 2017, 60, 105–109. [Google Scholar] [CrossRef]
- Zheng, R.; Zhao, Y.; Wu, J.; Wang, Y.; Liu, J.-L.; Zhou, Z.-L.; Zhou, X.-T.; Chen, D.-N.; Liao, W.-H.; Li, J.-D. A Novel PNPLA6 Compound Heterozygous Mutation Identified in a Chinese Patient with Boucher-Neuhäuser Syndrome. Mol. Med. Rep. 2018, 18, 261–267. [Google Scholar] [CrossRef]
- Boulund, U.; Bastos, D.M.; Ferwerda, B.; Van Den Born, B.-J.; Pinto-Sietsma, S.-J.; Galenkamp, H.; Levin, E.; Groen, A.K.; Zwinderman, A.H.; Nieuwdorp, M. Gut Microbiome Associations with Host Genotype Vary Across Ethnicities and Potentially Influence Cardiometabolic Traits. Cell Host Microbe 2022, 30, 1464–1480.e6. [Google Scholar] [CrossRef] [PubMed]
- Lush, M.J.; Li, Y.; Read, D.J.; Willis, A.C.; Glynn, P. Neuropathy Target Esterase and a Homologous Drosophila Neurodegeneration-Associated Mutant Protein Contain a Novel Domain Conserved from Bacteria to Man. Biochem. J. 1998, 332, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Akassoglou, K.; Malester, B.; Xu, J.; Tessarollo, L.; Rosenbluth, J.; Chao, M.V. Brain-Specific Deletion of Neuropathy Target Esterase/Swisscheese Results in Neurodegeneration. Proc. Natl. Acad. Sci. USA 2004, 101, 5075–5080. [Google Scholar] [CrossRef]
- Song, Y.; Wang, M.; Mao, F.; Shao, M.; Zhao, B.; Song, Z.; Shao, C.; Gong, Y. Knockdown of Pnpla6 Protein Results in Motor Neuron Defects in Zebrafish. Dis. Models Mech. 2013, 6, 404–413. [Google Scholar] [CrossRef] [PubMed]
- Winrow, C.J.; Hemming, M.L.; Allen, D.M.; Quistad, G.B.; Casida, J.E.; Barlow, C. Loss of Neuropathy Target Esterase in Mice Links Organophosphate Exposure to Hyperactivity. Nat. Genet. 2003, 33, 477–485. [Google Scholar] [CrossRef]
- Gallazzini, M.; Ferraris, J.D.; Kunin, M.; Morris, R.G.; Burg, M.B. Neuropathy Target Esterase Catalyzes Osmoprotective Renal Synthesis of Glycerophosphocholine in Response to High NaCl. Proc. Natl. Acad. Sci. USA 2006, 103, 15260–15265. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-X.; Xu, L.-L.; Mei, J.-H.; Yu, X.-B.; Kuang, H.-B.; Liu, H.-Y.; Wu, Y.-J.; Wang, J.-L. Involvement of Neuropathy Target Esterase in Tri-Ortho-Cresyl Phosphate-Induced Testicular Spermatogenesis Failure and Growth Inhibition of Spermatogonial Stem Cells in Mice. Toxicol. Lett. 2012, 211, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Moser, M.; Stempfl, T.; Li, Y.; Glynn, P.; Büttner, R.; Kretzschmar, D. Cloning and Expression of the Murine Sws/NTE Gene. Mech. Dev. 2000, 90, 279–282. [Google Scholar] [CrossRef]
- McFerrin, J.; Patton, B.L.; Sunderhaus, E.R.; Kretzschmar, D. NTE/PNPLA6 Is Expressed in Mature Schwann Cells and Is Required for Glial Ensheathment of Remak Fibers. Glia 2017, 65, 804–816. [Google Scholar] [CrossRef]
- Read, D.J.; Li, Y.; Chao, M.V.; Cavanagh, J.B.; Glynn, P. Neuropathy Target Esterase Is Required for Adult Vertebrate Axon Maintenance. J. Neurosci. 2009, 29, 11594–11600. [Google Scholar] [CrossRef]
- Moser, M.; Li, Y.; Vaupel, K.; Kretzschmar, D.; Kluge, R.; Glynn, P.; Buettner, R. Placental Failure and Impaired Vasculogenesis Result in Embryonic Lethality for Neuropathy Target Esterase-Deficient Mice. Mol. Cell. Biol. 2004, 24, 1667–1679. [Google Scholar] [CrossRef]
- Kretzschmar, D.; Hasan, G.; Sharma, S.; Heisenberg, M.; Benzer, S. The Swiss Cheese Mutant Causes Glial Hyperwrapping and Brain Degeneration in Drosophila. J. Neurosci. 1997, 17, 7425–7432. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Rieche, F.; Eckl, N.; Duch, C.; Kretzschmar, D. Glial Expression of Swiss-Cheese (SWS), the Drosophila Orthologue of Neuropathy Target Esterase, Is Required for Neuronal Ensheathment and Function. Dis. Models Mech. 2016, 9, 283–294. [Google Scholar] [CrossRef]
- Ryabova, E.V.; Melentev, P.A.; Komissarov, A.E.; Surina, N.V.; Ivanova, E.A.; Matiytsiv, N.; Shcherbata, H.R.; Sarantseva, S.V. Morpho-Functional Consequences of Swiss Cheese Knockdown in Glia of Drosophila melanogaster. Cells 2021, 10, 529. [Google Scholar] [CrossRef]
- Melentev, P.A.; Ryabova, E.V.; Surina, N.V.; Zhmujdina, D.R.; Komissarov, A.E.; Ivanova, E.A.; Boltneva, N.P.; Makhaeva, G.F.; Sliusarenko, M.I.; Yatsenko, A.S.; et al. Loss of Swiss Cheese in Neurons Contributes to Neurodegeneration with Mitochondria Abnormalities, Reactive Oxygen Species Acceleration and Accumulation of Lipid Droplets in Drosophila Brain. Int. J. Mol. Sci. 2021, 22, 8275. [Google Scholar] [CrossRef]
- Tsap, M.I.; Yatsenko, A.S.; Hegermann, J.; Beckmann, B.; Tsikas, D.; Shcherbata, H.R. Unraveling the Link Between Neuropathy Target Esterase NTE/SWS, Lysosomal Storage Diseases, Inflammation, Abnormal Fatty Acid Metabolism, and Leaky Brain Barrier. eLife 2024, 13, e98020. [Google Scholar] [CrossRef]
- Sunderhaus, E.R.; Law, A.D.; Kretzschmar, D. ER Responses Play a Key Role in Swiss-Cheese/Neuropathy Target Esterase-Associated Neurodegeneration. Neurobiol. Dis. 2019, 130, 104520. [Google Scholar] [CrossRef]
- Jena, B. Membrane Fusion: Role of SNAREs and Calcium. Protein Pept. Lett. 2009, 16, 712–717. [Google Scholar] [CrossRef]
- Shin, L.; Wang, S.; Lee, J.; Flack, A.; Mao, G.; Jena, B.P. Lysophosphatidylcholine Inhibits Membrane-Associated SNARE Complex Disassembly. J. Cell. Mol. Med. 2012, 16, 1701–1708. [Google Scholar] [CrossRef]
- Hedger, G.; Yen, H.-Y. The Influence of Phosphoinositide Lipids in the Molecular Biology of Membrane Proteins: Recent Insights from Simulations. J. Mol. Biol. 2025, 437, 168937. [Google Scholar] [CrossRef]
- Melentev, P.A.; Sharapenkov, E.G.; Surina, N.V.; Ivanova, E.A.; Ryabova, E.V.; Sarantseva, S.V. Drosophila Lysophospholipase Gene Swiss Cheese Is Required for Survival and Reproduction. Insects 2021, 13, 14. [Google Scholar] [CrossRef] [PubMed]
- Izumi, Y.; Furuse, K.; Furuse, M. Septate Junctions Regulate Gut Homeostasis Through Regulation of Stem Cell Proliferation and Enterocyte Behavior in Drosophila. J. Cell Sci. 2019, 132, jcs.232108. [Google Scholar] [CrossRef] [PubMed]
- Hochmuth, C.E.; Biteau, B.; Bohmann, D.; Jasper, H. Redox Regulation by Keap1 and Nrf2 Controls Intestinal Stem Cell Proliferation in Drosophila. Cell Stem Cell 2011, 8, 188–199. [Google Scholar] [CrossRef]
- Loftus, L.V.; Amend, S.R.; Pienta, K.J. Interplay between Cell Death and Cell Proliferation Reveals New Strategies for Cancer Therapy. Int. J. Mol. Sci. 2022, 23, 4723. [Google Scholar] [CrossRef]
- Izumi, Y.; Furuse, K.; Furuse, M. The Novel Membrane Protein Hoka Regulates Septate Junction Organization and Stem Cell Homeostasis in the Drosophila Gut. J. Cell Sci. 2021, 134, jcs257022. [Google Scholar] [CrossRef] [PubMed]
- Resnik-Docampo, M.; Cunningham, K.M.; Ruvalcaba, S.M.; Choi, C.; Sauer, V.; Jones, D.L. Neuroglian Regulates Drosophila Intestinal Stem Cell Proliferation Through Enhanced Signaling via the Epidermal Growth Factor Receptor. Stem Cell Rep. 2021, 16, 1584–1597. [Google Scholar] [CrossRef]
- Izumi, Y.; Yanagihashi, Y.; Furuse, M. A Novel Protein Complex, Mesh–Ssk, Is Required for Septate Junction Formation in the Drosophila Midgut. J. Cell Sci. 2012, 125, 4923–4933. [Google Scholar] [CrossRef]
- Schuler, M.-H.; Di Bartolomeo, F.; Mårtensson, C.U.; Daum, G.; Becker, T. Phosphatidylcholine Affects Inner Membrane Protein Translocases of Mitochondria. J. Biol. Chem. 2016, 291, 18718–18729. [Google Scholar] [CrossRef]
- Xu, C.; Tang, H.-W.; Hung, R.-J.; Hu, Y.; Ni, X.; Housden, B.E.; Perrimon, N. The Septate Junction Protein Tsp2A Restricts Intestinal Stem Cell Activity via Endocytic Regulation of aPKC and Hippo Signaling. Cell Rep. 2019, 26, 670–688.e6. [Google Scholar] [CrossRef]
- Chatterjee, N.; Perrimon, N. What Fuels the Fly: Energy Metabolism in Drosophila and Its Application to the Study of Obesity and Diabetes. Sci. Adv. 2021, 7, eabg4336. [Google Scholar] [CrossRef] [PubMed]
- Heier, C.; Kühnlein, R.P. Triacylglycerol Metabolism in Drosophila melanogaster. Genetics 2018, 210, 1163–1184. [Google Scholar] [CrossRef]
- Matthews, M.K.; Wilcox, H.; Hughes, R.; Veloz, M.; Hammer, A.; Banks, B.; Walters, A.; Schneider, K.J.; Sexton, C.E.; Chaston, J.M. Genetic Influences of the Microbiota on the Life Span of Drosophila melanogaster. Appl. Environ. Microbiol. 2020, 86, e00305-20. [Google Scholar] [CrossRef] [PubMed]
- Wesseltoft, J.B.; Danielsen, C.D.; Andersen, A.M.; De Jonge, N.; Olsen, A.; Rohde, P.D.; Kristensen, T.N. Feeding Drosophila Gut Microbiomes from Young and Old Flies Modifies the Microbiome. Sci. Rep. 2024, 14, 7799. [Google Scholar] [CrossRef] [PubMed]
- Cassol, I.; Ibañez, M.; Bustamante, J.P. Key Features and Guidelines for the Application of Microbial Alpha Diversity Metrics. Sci. Rep. 2025, 15, 622. [Google Scholar] [CrossRef]
- Anderson, M.J.; Crist, T.O.; Chase, J.M.; Vellend, M.; Inouye, B.D.; Freestone, A.L.; Sanders, N.J.; Cornell, H.V.; Comita, L.S.; Davies, K.F.; et al. Navigating the Multiple Meanings of β Diversity: A Roadmap for the Practicing Ecologist: Roadmap for Beta Diversity. Ecol. Lett. 2011, 14, 19–28. [Google Scholar] [CrossRef]
- Kers, J.G.; Saccenti, E. The Power of Microbiome Studies: Some Considerations on Which Alpha and Beta Metrics to Use and How to Report Results. Front. Microbiol. 2022, 12, 796025. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.J.; Jandová, A.; Jeffs, C.T.; Higgie, M.; Nováková, E.; Lewis, O.T.; Hrček, J. Microbiome Structure of a Wild Drosophila Community Along Tropical Elevational Gradients and Comparison to Laboratory Lines. Appl. Environ. Microbiol. 2023, 89, e00099-23. [Google Scholar] [CrossRef]
- Arias-Rojas, A.; Arifah, A.Q.; Angelidou, G.; Alshaar, B.; Schombel, U.; Forest, E.; Frahm, D.; Brinkmann, V.; Paczia, N.; Beisel, C.L.; et al. MprF-Mediated Immune Evasion Is Necessary for Lactiplantibacillus plantarum Resilience in the Drosophila Gut During Inflammation. PLoS Pathog. 2024, 20, e1012462. [Google Scholar] [CrossRef]
- Phillips, L.E.; Sotelo, K.L.; Moran, N.A. Characterization of Gut Symbionts from Wild-Caught Drosophila and Other Diptera: Description of Utexia brackfieldae Gen. Nov., Sp. Nov., Orbus sturtevantii sp. Nov., Orbus wheelerorum sp. Nov, and Orbus mooreae Sp. Nov. Int. J. Syst. Evol. Microbiol. 2024, 74, 006516. [Google Scholar] [CrossRef]
- De Cock, M.; Virgilio, M.; Vandamme, P.; Augustinos, A.; Bourtzis, K.; Willems, A.; De Meyer, M. Impact of Sample Preservation and Manipulation on Insect Gut Microbiome Profiling. A Test Case with Fruit Flies (Diptera, Tephritidae). Front. Microbiol. 2019, 10, 2833. [Google Scholar] [CrossRef]
- Park, R.; Dzialo, M.C.; Spaepen, S.; Nsabimana, D.; Gielens, K.; Devriese, H.; Crauwels, S.; Tito, R.Y.; Raes, J.; Lievens, B.; et al. Microbial Communities of the House Fly Musca Domestica Vary with Geographical Location and Habitat. Microbiome 2019, 7, 147. [Google Scholar] [CrossRef]
- Ioannou, P.; Ziogou, A.; Giannakodimos, A.; Giannakodimos, I.; Tsantes, A.G.; Samonis, G. Psychrobacter Infections in Humans—A Narrative Review of Reported Cases. Antibiotics 2025, 14, 140. [Google Scholar] [CrossRef]
- Téfit, M.A.; Leulier, F. Lactobacillus plantarum Favors the Early Emergence of Fit and Fertile Adult Drosophila upon Chronic Undernutrition. J. Exp. Biol. 2017, 220, 900–907. [Google Scholar] [CrossRef]
- Grenier, T.; Consuegra, J.; Ferrarini, M.G.; Akherraz, H.; Bai, L.; Dusabyinema, Y.; Rahioui, I.; Da Silva, P.; Gillet, B.; Hughes, S.; et al. Intestinal GCN2 Controls Drosophila Systemic Growth in Response to Lactiplantibacillus Plantarum Symbiotic Cues Encoded by r/tRNA Operons. eLife 2023, 12, e76584. [Google Scholar] [CrossRef] [PubMed]
- Gallo, M.; Vento, J.M.; Joncour, P.; Quagliariello, A.; Maritan, E.; Silva-Soares, N.F.; Battistolli, M.; Beisel, C.L.; Martino, M.E. Beneficial Commensal Bacteria Promote Drosophila Growth by Downregulating the Expression of Peptidoglycan Recognition Proteins. iScience 2022, 25, 104357. [Google Scholar] [CrossRef] [PubMed]
- Storelli, G.; Defaye, A.; Erkosar, B.; Hols, P.; Royet, J.; Leulier, F. Lactobacillus Plantarum Promotes Drosophila Systemic Growth by Modulating Hormonal Signals Through TOR-Dependent Nutrient Sensing. Cell Metab. 2011, 14, 403–414. [Google Scholar] [CrossRef]
- Al Atrouni, A.; Joly-Guillou, M.-L.; Hamze, M.; Kempf, M. Reservoirs of Non-Baumannii Acinetobacter Species. Front. Microbiol. 2016, 7, 49. [Google Scholar] [CrossRef]
- Ashe, E.C.; Comeau, A.M.; Zejdlik, K.; O’Connell, S.P. Characterization of Bacterial Community Dynamics of the Human Mouth Throughout Decomposition via Metagenomic, Metatranscriptomic, and Culturing Techniques. Front. Microbiol. 2021, 12, 689493. [Google Scholar] [CrossRef]
- Qadir, M.; Hussain, A.; Hamayun, M.; Shah, M.; Iqbal, A.; Irshad, M.; Ahmad, A.; Lodhi, M.A.; Lee, I.-J. Phytohormones Producing Acinetobacter bouvetii P1 Mitigates Chromate Stress in Sunflower by Provoking Host Antioxidant Response. Antioxidants 2021, 10, 1868. [Google Scholar] [CrossRef]
- Raittz, R.T.; Reginatto De Pierri, C.; Maluk, M.; Bueno Batista, M.; Carmona, M.; Junghare, M.; Faoro, H.; Cruz, L.M.; Battistoni, F.; Souza, E.D.; et al. Comparative Genomics Provides Insights into the Taxonomy of Azoarcus and Reveals Separate Origins of Nif Genes in the Proposed Azoarcus and Aromatoleum Genera. Genes 2021, 12, 71. [Google Scholar] [CrossRef] [PubMed]
- Aromatoleum toluvorans. Available online: https://www.amibase.org/detail_data.php?page=overview&country=Thailand&scientific_name=Aromatoleum_toluvorans (accessed on 30 September 2025).
- Rodríguez-Blanco, A.; Vetion, G.; Escande, M.-L.; Delille, D.; Ghiglione, J.-F. Gallaecimonas pentaromativorans Gen. Nov., sp. Nov., a Bacterium Carrying 16S rRNA Gene Heterogeneity and Able to Degrade High-Molecular-Mass Polycyclic Aromatic Hydrocarbons. Int. J. Syst. Evol. Microbiol. 2010, 60, 504–509. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.Y.; Dutta, A.; Tan, T.K.; Hari, R.; Othman, R.Y.; Choo, S.W. Comprehensive Genome Analysis of a Pangolin-Associated Paraburkholderia fungorum Provides New Insights into Its Secretion Systems and Virulence. PeerJ 2020, 8, e9733. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Orduña, F.N.; Pineda-Mendoza, R.M.; Vega-Correa, B.; López, M.F.; Cano-Ramírez, C.; Zhang, X.X.; Chen, W.F.; Zúñiga, G. A Polyphasic Taxonomy Analysis Reveals the Presence of an Ecotype of Rahnella Contaminans Associated with the Gut of Dendroctonus-Bark Beetles. Front. Microbiol. 2023, 14, 1171164. [Google Scholar] [CrossRef]
- Clark, R.I.; Salazar, A.; Yamada, R.; Fitz-Gibbon, S.; Morselli, M.; Alcaraz, J.; Rana, A.; Rera, M.; Pellegrini, M.; Ja, W.W.; et al. Distinct Shifts in Microbiota Composition During Drosophila Aging Impair Intestinal Function and Drive Mortality. Cell Rep. 2015, 12, 1656–1667. [Google Scholar] [CrossRef]
- Rera, M.; Clark, R.I.; Walker, D.W. Intestinal Barrier Dysfunction Links Metabolic and Inflammatory Markers of Aging to Death in Drosophila. Proc. Natl. Acad. Sci. USA 2012, 109, 21528–21533. [Google Scholar] [CrossRef]
- Guo, L.; Karpac, J.; Tran, S.L.; Jasper, H. PGRP-SC2 Promotes Gut Immune Homeostasis to Limit Commensal Dysbiosis and Extend Lifespan. Cell 2014, 156, 109–122. [Google Scholar] [CrossRef] [PubMed]
- Nehme, N.T.; Liégeois, S.; Kele, B.; Giammarinaro, P.; Pradel, E.; Hoffmann, J.A.; Ewbank, J.J.; Ferrandon, D. A Model of Bacterial Intestinal Infections in Drosophila melanogaster. PLoS Pathog. 2007, 3, e173. [Google Scholar] [CrossRef]
- Simões, M.L.; Gonçalves, L.; Silveira, H. Hemozoin Activates the Innate Immune System and Reduces Plasmodium Berghei Infection in Anopheles Gambiae. Parasit Vectors 2015, 8, 12. [Google Scholar] [CrossRef]
- Miguel-Aliaga, I.; Jasper, H.; Lemaitre, B. Anatomy and Physiology of the Digestive Tract of Drosophila melanogaster. Genetics 2018, 210, 357–396. [Google Scholar] [CrossRef]
- Bai, S.; Yao, Z.; Raza, M.F.; Cai, Z.; Zhang, H. Regulatory Mechanisms of Microbial Homeostasis in Insect Gut. Insect Sci. 2021, 28, 286–301. [Google Scholar] [CrossRef]
- Khan, S.A.; Kojour, M.A.M.; Han, Y.S. Recent Trends in Insect Gut Immunity. Front. Immunol. 2023, 14, 1272143. [Google Scholar] [CrossRef]
- Robertson, S.J.; Goethel, A.; Girardin, S.E.; Philpott, D.J. Innate Immune Influences on the Gut Microbiome: Lessons from Mouse Models. Trends Immunol. 2018, 39, 992–1004. [Google Scholar] [CrossRef]
- Liu, J.; Yang, W.; Liao, W.; Huang, Y.; Chen, W.; Bu, X.; Huang, S.; Jiang, W.; Swevers, L. Immunological function of Bombyx Toll9-2 in the silkworm (Bombyx mori) larval midgut: Activation by Escherichia coli/lipopolysaccharide and regulation of growth. Arch. Insect Biochem. Physiol. 2024, 116, e22130. [Google Scholar] [CrossRef]
- Lemaitre, B.; Reichhart, J.-M.; Hoffmann, J.A. Drosophila Host Defense: Differential Induction of Antimicrobial Peptide Genes after Infection by Various Classes of Microorganisms. Proc. Natl. Acad. Sci. USA 1997, 94, 14614–14619. [Google Scholar] [CrossRef] [PubMed]
- Bosco-Drayon, V.; Poidevin, M.; Boneca, I.G.; Narbonne-Reveau, K.; Royet, J.; Charroux, B. Peptidoglycan Sensing by the Receptor PGRP-LE in the Drosophila Gut Induces Immune Responses to Infectious Bacteria and Tolerance to Microbiota. Cell Host Microbe 2012, 12, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chen, W.; Situ, J.; Li, J.; Chen, J.; Lai, M.; Huang, F.; Li, B. BmToll9-1 Is a Positive Regulator of the Immune Response in the Silkworm Bombyx mori. Insects 2024, 15, 643. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Severiano, F.; Santamaría, A.; Pedraza-Chaverri, J.; Medina-Campos, O.N.; Ríos, C.; Segovia, J. Increased Formation of Reactive Oxygen Species, but No Changes in Glutathione Peroxidase Activity, in Striata of Mice Transgenic for the Huntington’s Disease Mutation. Neurochem. Res. 2004, 29, 729–733. [Google Scholar] [CrossRef] [PubMed]
- Martin, M. Cutadapt Removes Adapter Sequences from High-Throughput Sequencing Reads. EMBnet J. 2011, 17, 10. [Google Scholar] [CrossRef]
- Epi2me-Labs/Fastcat 2025. Available online: https://github.com/epi2me-labs/fastcat (accessed on 30 June 2025).
- Wood, D.E.; Lu, J.; Langmead, B. Improved Metagenomic Analysis with Kraken 2. Genome Biol 2019, 20, 257. [Google Scholar] [CrossRef]
- Index of /Blast/Db. Available online: https://ftp.ncbi.nlm.nih.gov/blast/db/ (accessed on 30 September 2025).
- Lu, J.; Breitwieser, F.P.; Thielen, P.; Salzberg, S.L. Bracken: Estimating Species Abundance in Metagenomics Data. PeerJ Comput. Sci. 2017, 3, e104. [Google Scholar] [CrossRef]
- Lu, J.; Rincon, N.; Wood, D.E.; Breitwieser, F.P.; Pockrandt, C.; Langmead, B.; Salzberg, S.L.; Steinegger, M. Metagenome Analysis Using the Kraken Software Suite. Nat. Protoc. 2022, 17, 2815–2839. [Google Scholar] [CrossRef]
- Ondov, B.D.; Bergman, N.H.; Phillippy, A.M. Interactive Metagenomic Visualization in a Web Browser. BMC Bioinform. 2011, 12, 385. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ivanova, E.A.; Ryabova, E.V.; Komissarov, A.E.; Slepneva, E.E.; Stulov, A.A.; Bulat, S.A.; Sarantseva, S.V. Swiss Cheese Gene Is Important for Intestinal Barrier, Microbiome, and Lipid Metabolism Regulation in Drosophila Gut. Int. J. Mol. Sci. 2025, 26, 11085. https://doi.org/10.3390/ijms262211085
Ivanova EA, Ryabova EV, Komissarov AE, Slepneva EE, Stulov AA, Bulat SA, Sarantseva SV. Swiss Cheese Gene Is Important for Intestinal Barrier, Microbiome, and Lipid Metabolism Regulation in Drosophila Gut. International Journal of Molecular Sciences. 2025; 26(22):11085. https://doi.org/10.3390/ijms262211085
Chicago/Turabian StyleIvanova, Ekaterina A., Elena V. Ryabova, Artem E. Komissarov, Elizaveta E. Slepneva, Anton A. Stulov, Sergey A. Bulat, and Svetlana V. Sarantseva. 2025. "Swiss Cheese Gene Is Important for Intestinal Barrier, Microbiome, and Lipid Metabolism Regulation in Drosophila Gut" International Journal of Molecular Sciences 26, no. 22: 11085. https://doi.org/10.3390/ijms262211085
APA StyleIvanova, E. A., Ryabova, E. V., Komissarov, A. E., Slepneva, E. E., Stulov, A. A., Bulat, S. A., & Sarantseva, S. V. (2025). Swiss Cheese Gene Is Important for Intestinal Barrier, Microbiome, and Lipid Metabolism Regulation in Drosophila Gut. International Journal of Molecular Sciences, 26(22), 11085. https://doi.org/10.3390/ijms262211085






