Marf- and Opa1-Dependent Formation of Mitochondrial Network Structure Is Required for Cell Growth and Subsequent Meiosis in Drosophila Males
Abstract
1. Introduction
2. Results
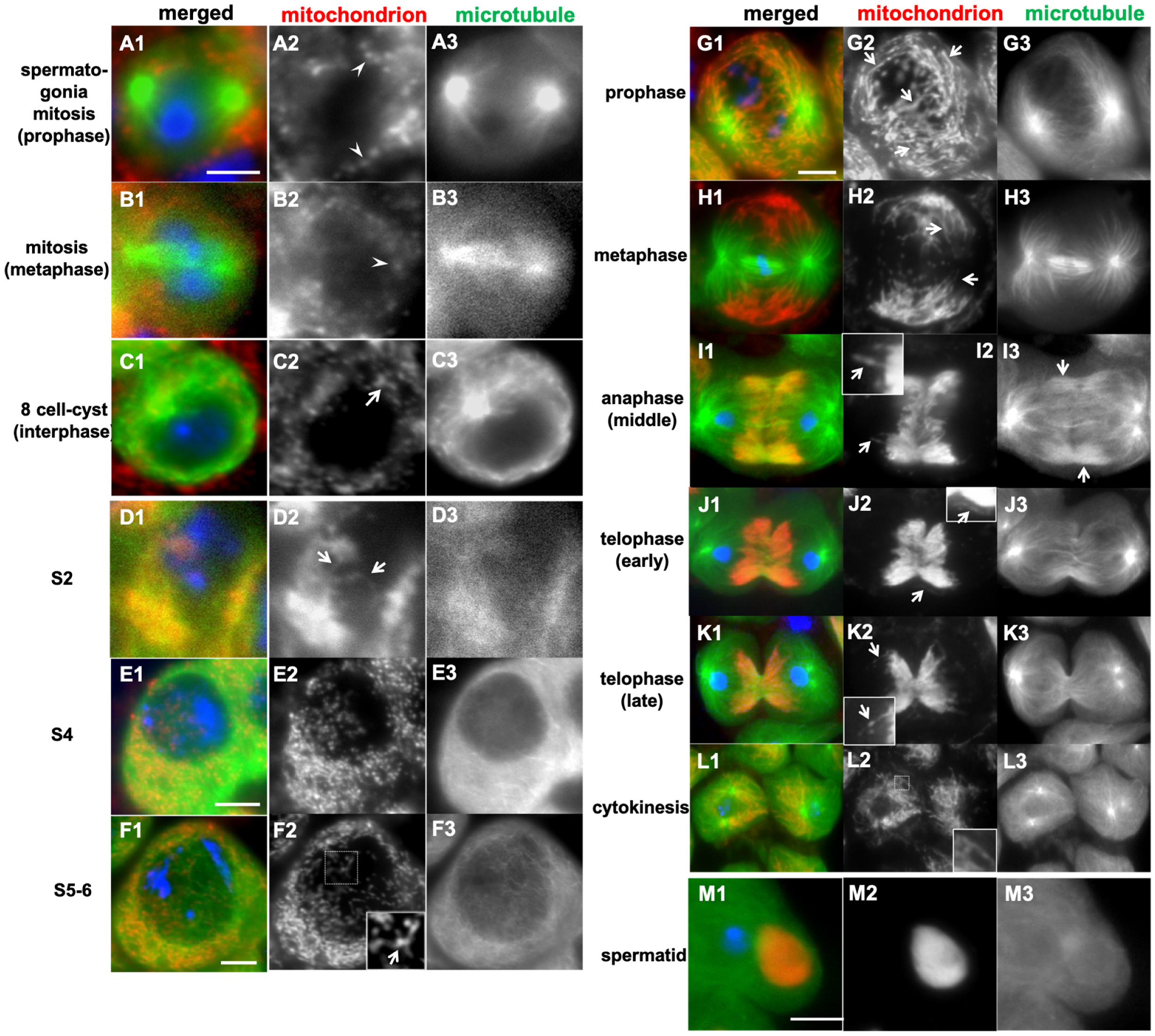
2.1. Dynamics of Mitochondrial Morphology in Drosophila Early Spermatogenesis and Meiotic Division
2.2. The Impact of Microtubule and F-Actin Depolymerization on Mitochondrial Network Formation and Distribution
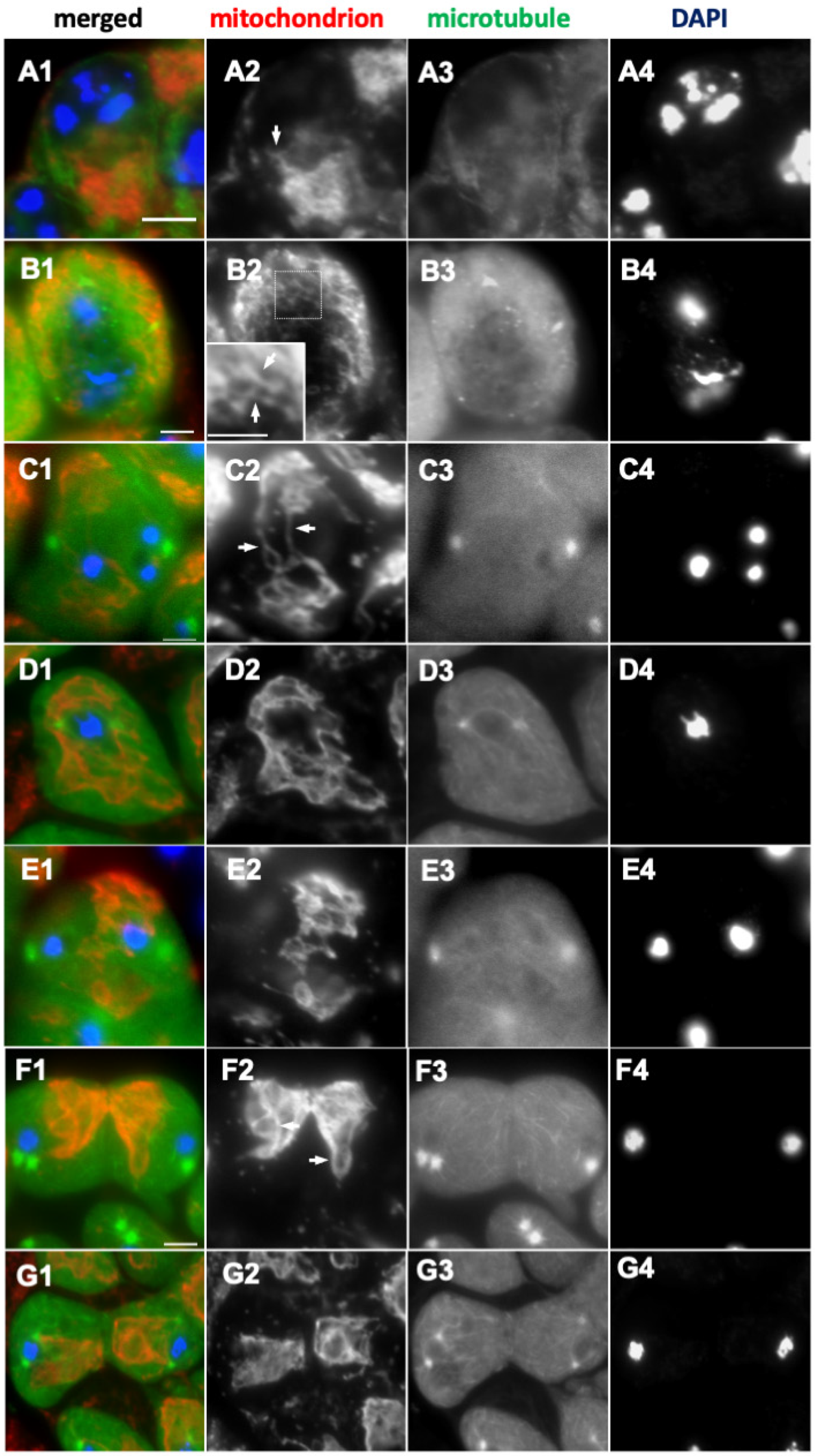
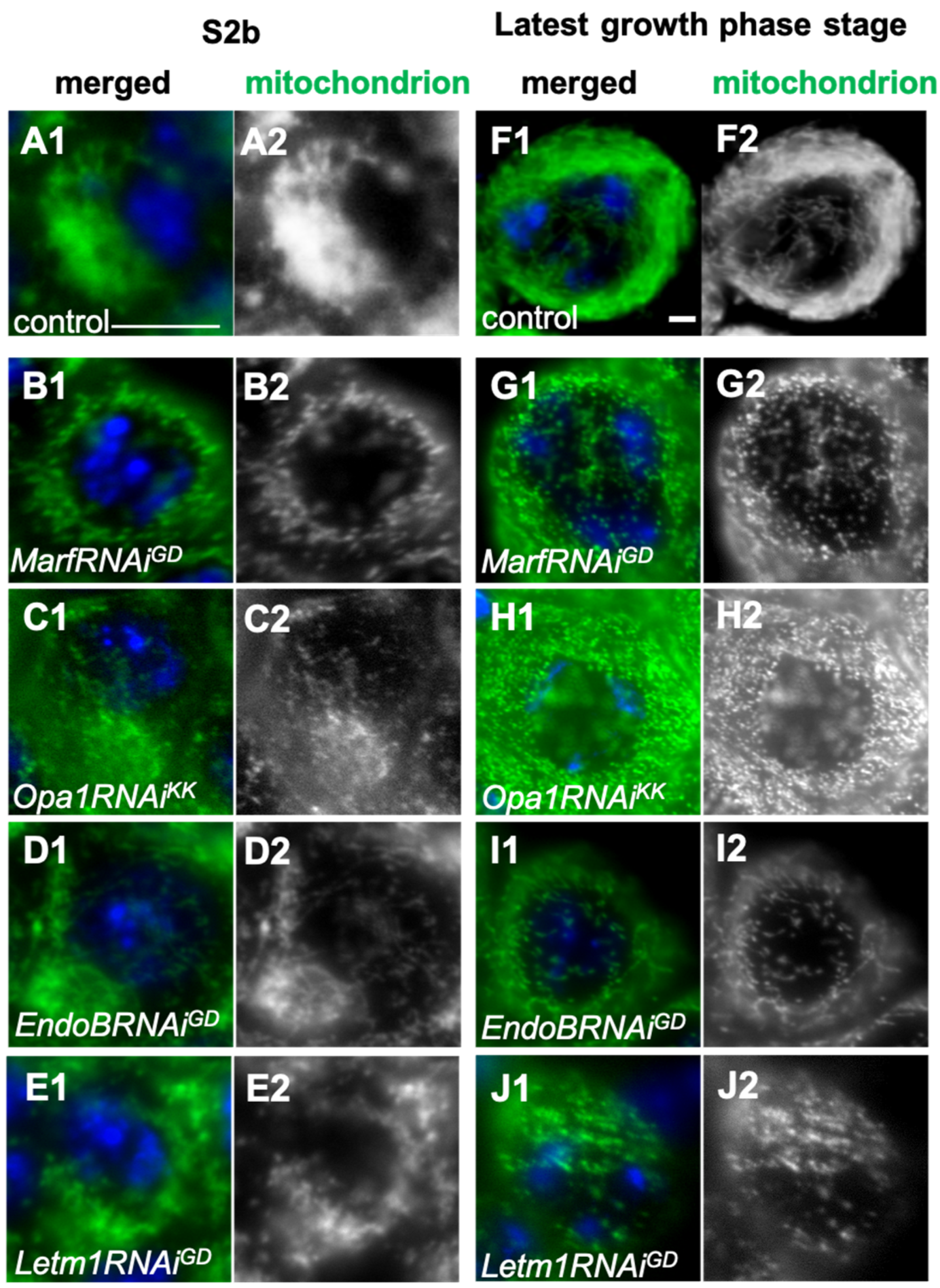
2.3. Formation of the Elongated Mitochondria Was Inhibited by the Knockdown of the Fusion Factors and the Mitochondrial Morphology Proteins
2.4. Knockdown of the Fusion Factors and the Morphology Proteins Inhibited ATP Synthesis in the Spermatocytes
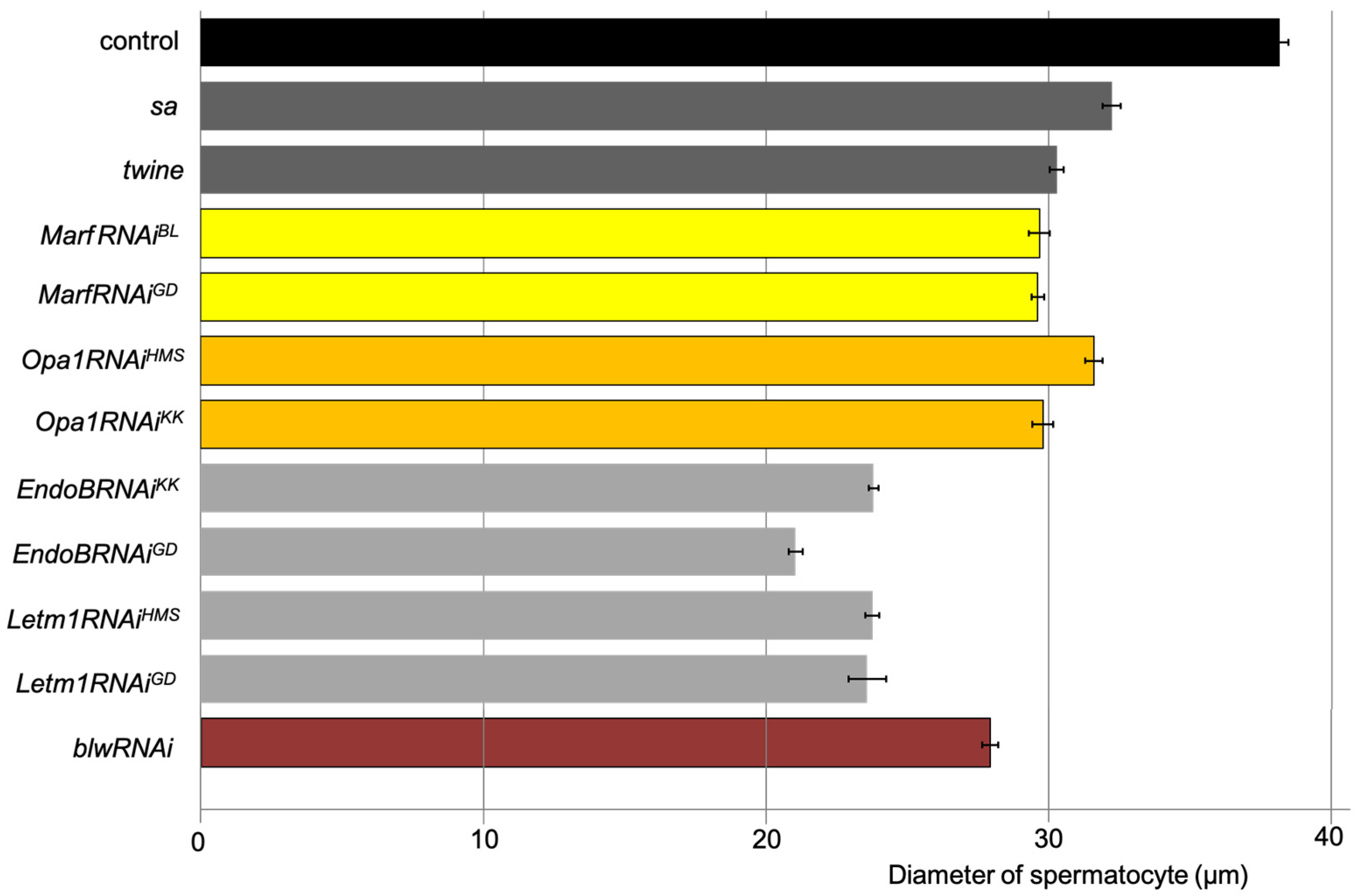
2.5. Spermatocyte Growth Was Affected by the Knockdown of Mitochondrial Fusion Factors and the Morphology Proteins
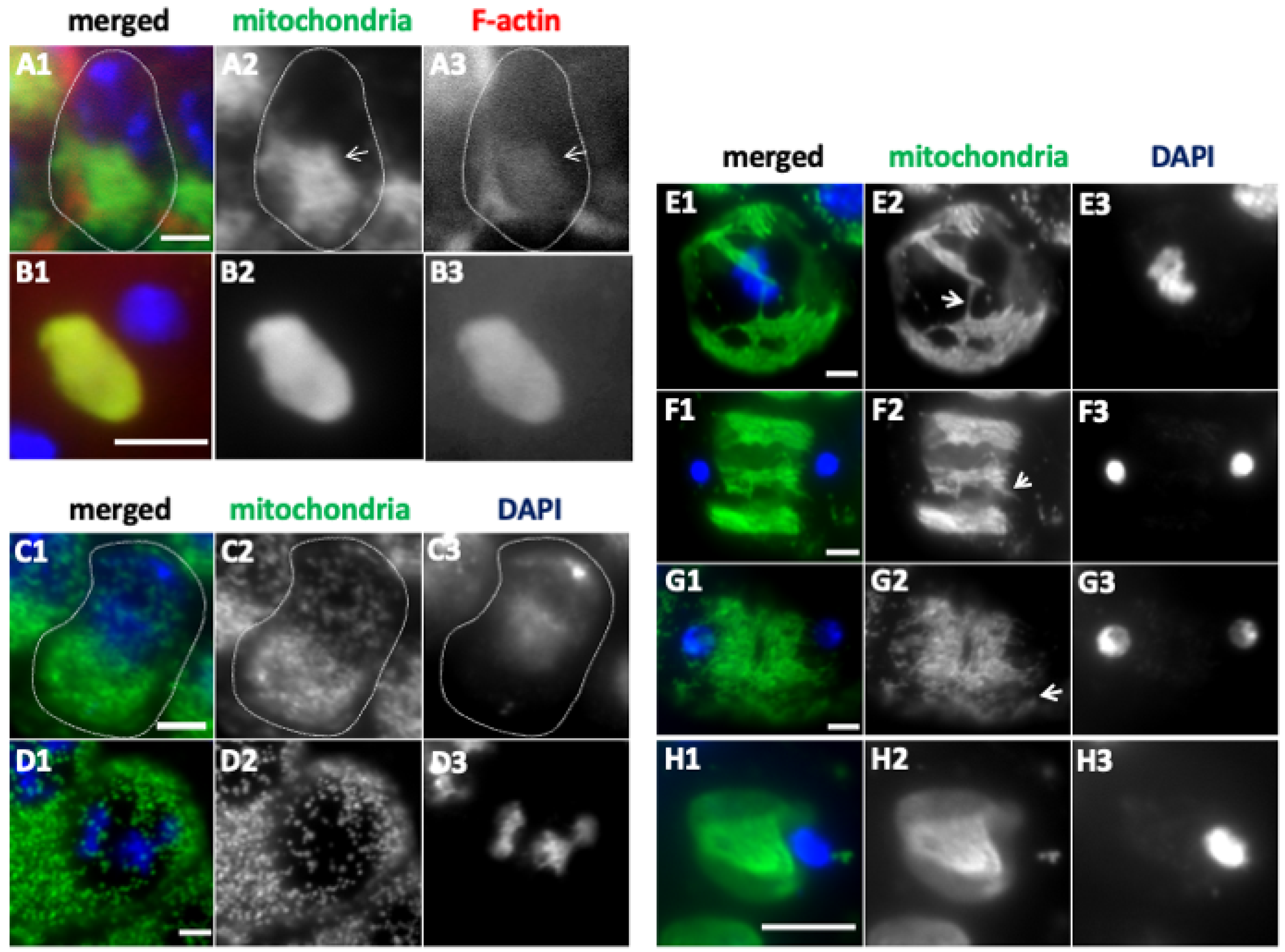
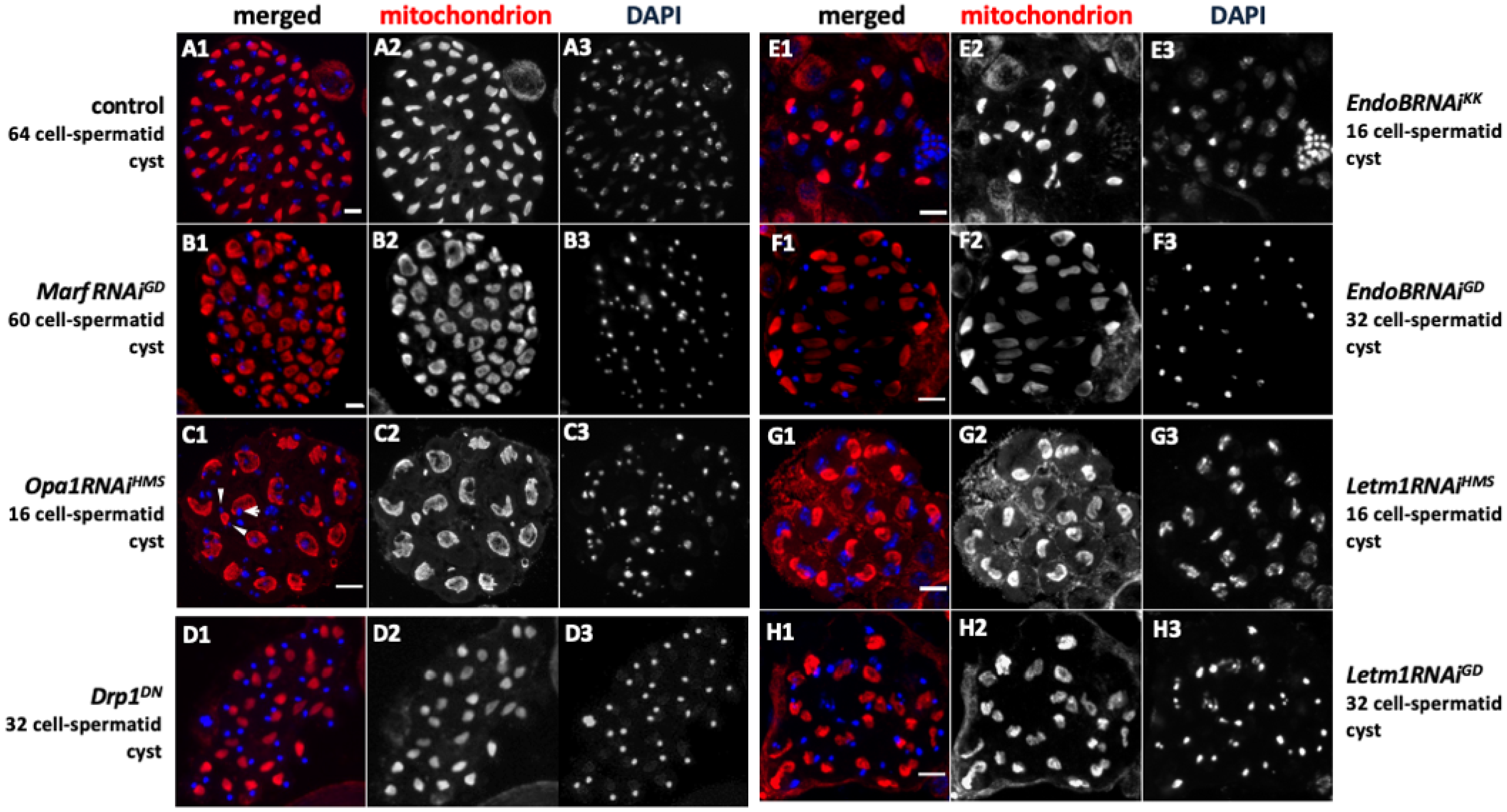
2.6. Spermatid Phenotype Arising from Abnormal Chromosome Segregation During Meiosis Owing to Knockdown of Fusion/Fission Factors and Morphogenetic Proteins
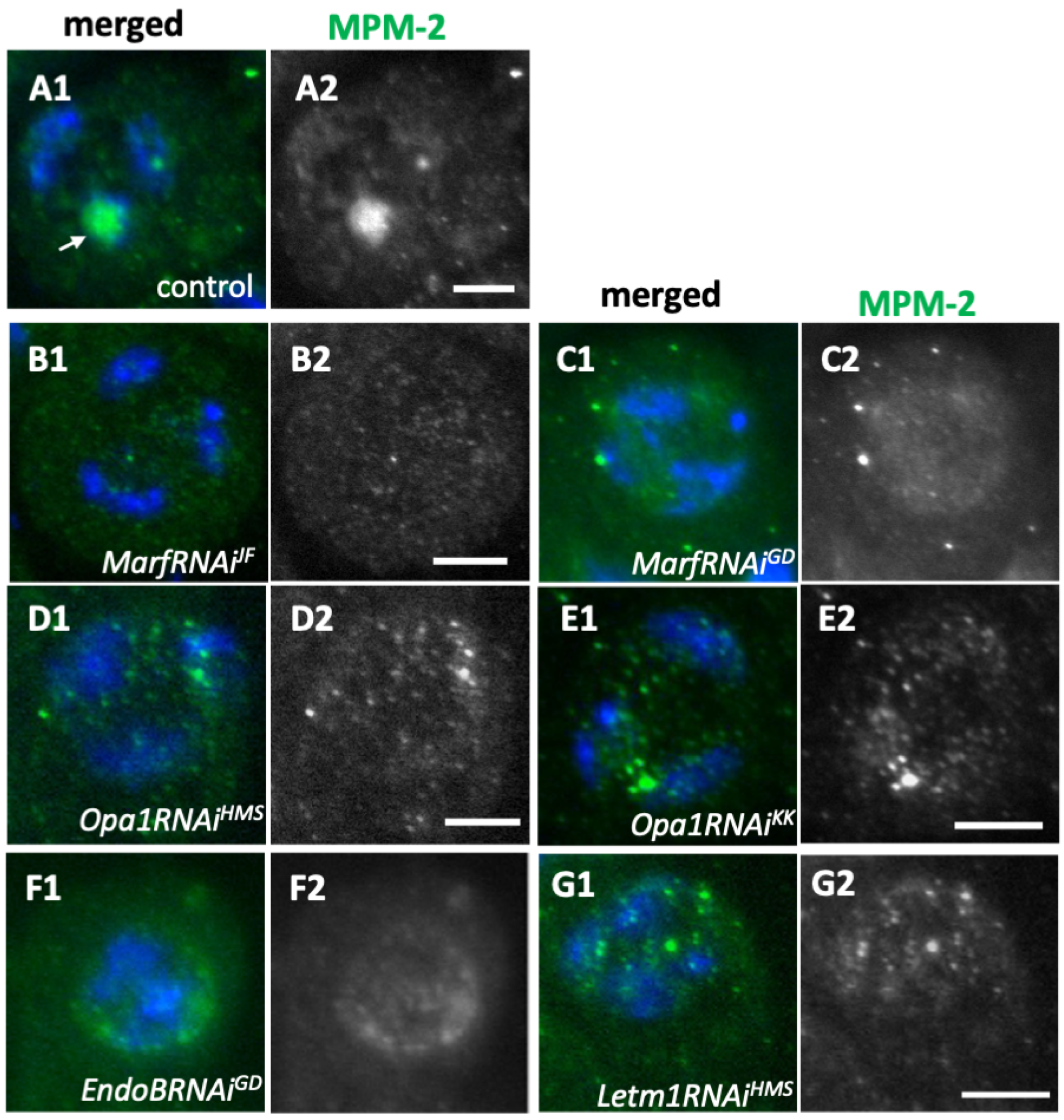
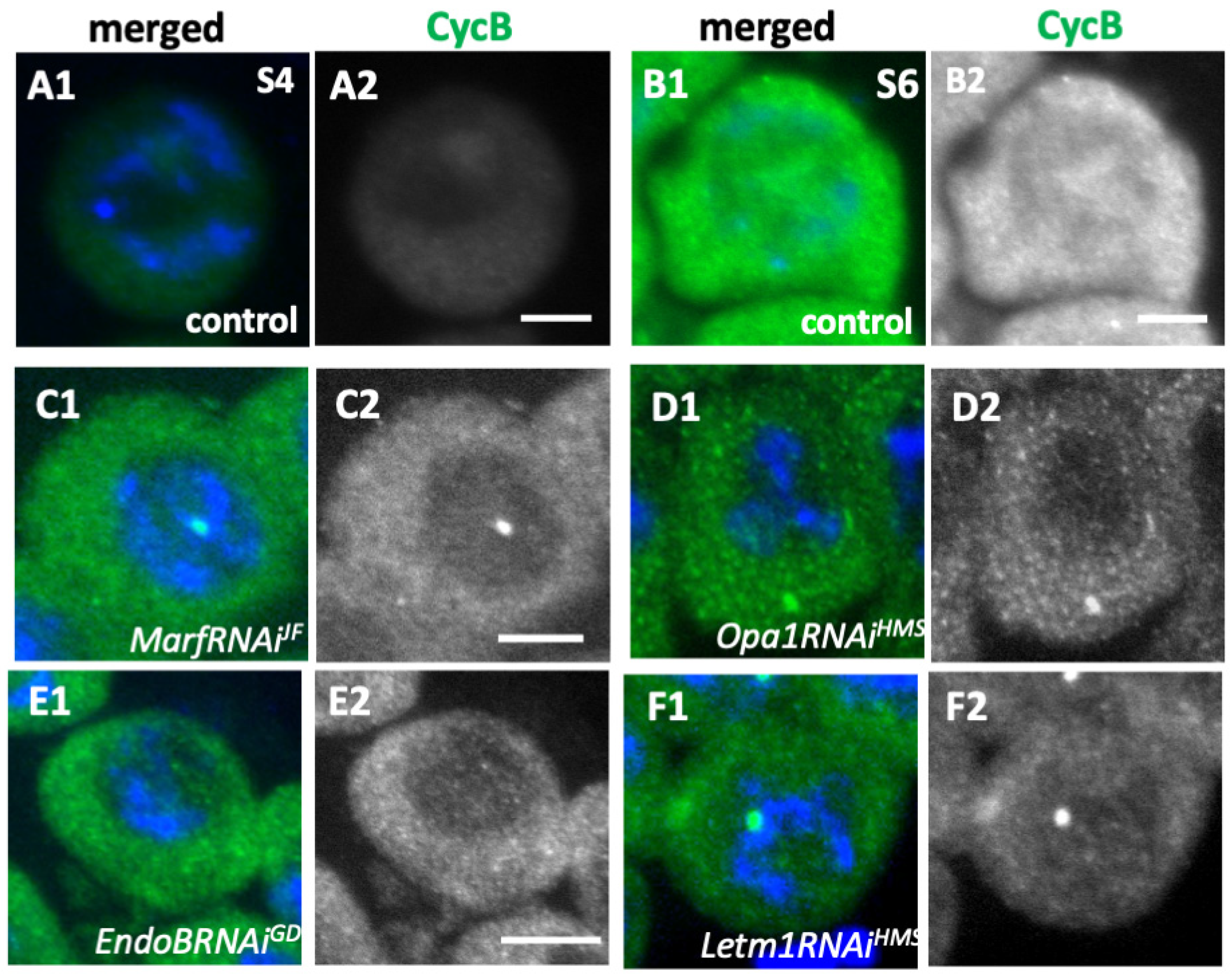
2.7. Knockdown of the Fusion Factors and the Morphology Proteins Inhibited Cdk1 Activation Before the Onset of Meiosis
2.8. Inhibition of Meiotic Initiation Caused by Knockdown of the Fusion Factors and the Morphology Proteins Was Partially Rescued by Overexpression of Cyclin B
2.9. Knockdown of the Fusion and Fission Factors and the Morphology Proteins Caused Abnormalities in Nebenkern Formation
3. Discussion
3.1. Mitochondria in Drosophila Spermatocytes Undergo Stage-Specific Changes Between a Shortened Form and an Interconnected Network Structure
3.2. Requirement of Fusion Factors, Microtubules, and F-Actin for the Formation of Elongated Mitochondrial Networks Constructed Before and During Male Meiosis
3.3. Requirement of the Mitochondrial Network to Be Formed via Fusion Factors for the Cell Growth of Spermatocytes Before Meiosis
3.4. Elongated Mitochondrial Networks Are Transferred to Daughter Cells While Maintaining the Structures During Male Meiosis
3.5. A Possible Checkpoint Mechanism That Monitors Mitochondrial Morphology and/or Function and Prevents the Progression of Meiosis in the Presence of Abnormalities
3.6. Conclusions
4. Materials and Methods
4.1. Drosophila Stocks
4.2. Preparation of Post-Meiotic Spermatid Cysts
4.3. Administration of a Drug to the Testis Cells
4.4. Immunostaining of Testis Cells
4.5. ATP Assay
4.6. Transmission Electron Microscope Observation of Adult Testes
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bereiter-Hahn, J.; Vöth, M. Dynamics of mitochondria in living cells: Shape changes, dislocations, fusion, and fission of mitochondria. Microsc. Res. Tech. 1994, 27, 198–219. [Google Scholar] [CrossRef]
- Labbé, K.; Murley, A.; Nunnari, J. Determinants and functions of mitochondrial behavior. Annu. Rev. Cell Dev. Biol. 2014, 30, 357–391. [Google Scholar] [CrossRef]
- Hinton, A., Jr.; Katti, P.; Christensen, T.A.; Mungai, M.; Shao, J.; Zhang, L.; Trushin, S.; Alghanem, A.; Jaspersen, A.; Geroux, R.E.; et al. A comprehensive approach to sample preparation for electron microscopy and the assessment of mitochondrial morphology in tissue and cultured cells. Adv. Biol. 2023, 7, e2200202. [Google Scholar] [CrossRef] [PubMed]
- Stephan, T.; Ilgen, P.; Jakobs, S. Visualizing mitochondrial dynamics at the nanoscale. Light Sci. Appl. 2024, 13, 244. [Google Scholar] [CrossRef] [PubMed]
- Sauvanet, C.; Duvezin-Caubet, S.; di Rago, J.-P.; Rojo, M. Energetic requirements and bioenergetic modulation of mitochondrial morphology and dynamics. Semin. Cell Dev. Biol. 2010, 21, 558–565. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Vermulst, M.; Wang, Y.E.; Chomyn, A.; Prolla, T.A.; McCaffery, J.M.; Chan, D.C. Mitochondrial fusion is required for mtDNA stability in skeletal muscle and tolerance of mtDNA mutations. Cell 2010, 141, 280–289. [Google Scholar] [CrossRef]
- Madan, S.; Uttekar, B.; Chowdhary, S.; Rikhy, R. Mitochondria Lead the Way: Mitochondrial Dynamics and Function in Cellular Movements in Development and Disease. Front. Cell Dev. Biol. 2022, 9, 781933. [Google Scholar] [CrossRef]
- Okamoto, K.; Shaw, J.M. Mitochondrial morphology and dynamics in yeast and multicellular eukaryotes. Annu. Rev. Genet. 2005, 39, 503–536. [Google Scholar] [CrossRef]
- Hales, K.G.; Fuller, M.T. Developmentally regulated mitochondrial fusion mediated by a conserved, novel, predicted GTPase. Cell 1997, 90, 121–129. [Google Scholar] [CrossRef]
- Eura, Y.; Ishihara, N.; Yokota, S.; Mihara, K. Two Mitofusin proteins, mammalian homologues of FZO, with distinct functions are both required for mitochondrial fusion. J. Biochem. 2003, 134, 333–344. [Google Scholar] [CrossRef]
- Deng, H.; Dodson, M.W.; Huang, H.; Guo, M. The Parkinson’s disease genes pink1 and parkin promote mitochondrial fission and/or inhibit fusion in Drosophila. Proc. Natl. Acad. Sci. USA 2008, 105, 14503–14508. [Google Scholar] [CrossRef]
- Nakamura, S.; Matsui, A.; Akabane, S.; Tamura, Y.; Hatano, A.; Miyano, Y.; Omote, H.; Kajikawa, M.; Maenaka, K.; Moriyama, Y.; et al. The mitochondrial inner membrane protein LETM1 modulates cristae organization through its LETM domain. Commun. Biol. 2020, 3, 99. [Google Scholar] [CrossRef] [PubMed]
- Karbowski, M.; Jeong, S.-Y.; Youle, R.J. Endophilin B1 is required for the maintenance of mitochondrial morphology. J. Cell Biol. 2004, 166, 1027–1039. [Google Scholar] [CrossRef]
- Zhao, J.; Lendahl, U.; Nistér, M. Regulation of mitochondrial dynamics: Convergences and divergences between yeast and vertebrates. Cell. Mol. Life Sci. 2013, 70, 951–976. [Google Scholar] [CrossRef]
- Chen, H.; Detmer, S.A.; Ewald, A.J.; Griffin, E.E.; Fraser, S.E.; Chan, D.C. Mitofusins Mfn1 and Mfn2 coordinately regulate mitochondrial fusion and are essential for embryonic development. J. Cell Biol. 2003, 160, 189–200. [Google Scholar] [CrossRef]
- Davies, V.J.; Hollins, A.J.; Piechota, M.J.; Yip, W.; Davies, J.R.; White, K.E.; Nicols, P.P.; Boulton, M.E.; Votruba, M. Opa1 deficiency in a mouse model of autosomal dominant optic atrophy impairs mitochondrial morphology, optic nerve structure and visual function. Hum. Mol. Genet. 2007, 16, 1307–1318. [Google Scholar] [CrossRef]
- Ishihara, N.; Nomura, M.; Jofuku, A.; Kato, H.; Suzuki, S.O.; Masuda, K.; Otera, H.; Nakanishi, Y.; Nonaka, I.; Goto, Y. Mitochondrial fission factor Drp1 is essential for embryonic development and synapse formation in mice. Nat. Cell Biol. 2009, 11, 958–966. [Google Scholar] [CrossRef] [PubMed]
- Fuller, M.T. Spermatogenesis. In The Development of Drosophila Melanogaster; Bate, M., Martinez Arias, A., Eds.; Cold Spring Harbor Laboratory Press: New York, NY, USA, 1993; pp. 71–147. [Google Scholar]
- Ichihara, K.; Shimizu, H.; Taguchi, O.; Yamaguchi, M.; Inoue, Y.H. A Drosophila orthologue of Larp protein family is required for multiple processes in male meiosis. Cell Struct. Funct. 2007, 32, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Inoue, Y.H.; Miyauchi, C.; Ogata, T.; Kitazawa, D. Dynamic alteration of cellular component of male meiosis in Drosophila. In Meiosis; Swan, A., Ed.; IntechOpen: London, UK, 2012; pp. 67–86. [Google Scholar]
- Vedelek, V.; Jankovics, F.; Zádori, J.; Sinka, R. Mitochondrial Differentiation during Spermatogenesis: Lessons from Drosophila melanogaster. Int. J. Mol. Sci. 2024, 25, 3980. [Google Scholar] [CrossRef]
- McQuibban, G.A.; Lee, J.R.; Zheng, L.; Juusola, M.; Freeman, M. Normal mitochondrial dynamics requires rhomboid-7 and affects Drosophila lifespan and neuronal function. Curr. Biol. 2006, 16, 982–989. [Google Scholar] [CrossRef]
- Aldridge, A.C.; Benson, L.P.; Siegenthaler, M.M.; Whigham, B.T.; Stowers, R.S.; Hales, K.G. Roles for Drp1, a dynamin-related protein, and milton, a kinesin-associated protein, in mitochondrial segregation, unfurling and elongation during Drosophila spermatogenesis. Fly 2007, 1, 38–46. [Google Scholar] [CrossRef]
- White-Cooper, H.; Alphey, L.; Glover, D.M. The cdc25 homologue twine is required for only some aspects of the entry into meiosis in Drosophila. J. Cell Sci. 1993, 106, 1035–1044. [Google Scholar] [CrossRef]
- Taguchi, N.; Ishihara, N.; Jofuku, A.; Oka, T.; Mihara, K. Mitotic phosphorylation of dynamin-related GTPase Drp1 participates in mitochondrial fission. J. Biol. Chem. 2007, 282, 11521–11529. [Google Scholar] [CrossRef]
- Cenci, G.; Bonaccorsi, S.; Pisano, C.; Verni, F.; Gatti, M. Chromatin and microtubule organization during premeiotic, meiotic and early postmeiotic stages of Drosophila melanogaster spermatogenesis. J. Cell Sci. 1994, 107, 3521–3534. [Google Scholar] [CrossRef]
- Hirusaki, K.; Yokoyama, K.; Cho, K.; Ohta, Y. Temporal depolarization of mitochondria during M phase. Sci. Rep. 2017, 7, 16044. [Google Scholar] [CrossRef]
- Westermann, B. Molecular machinery of mitochondrial fusion and fission. J. Biol. Chem. 2008, 283, 13501–13505. [Google Scholar] [CrossRef]
- Trevisan, T.; Pendin, D.; Montagna, A.; Bova, S.; Ghelli, A.M.; Daga, A. Manipulation of Mitochondria Dynamics Reveals Separate Roles for Form and Function in Mitochondria Distribution. Cell Rep. 2018, 23, 1742–1753. [Google Scholar] [CrossRef]
- Jones, M.D.; Naylor, K. Simple to Complex: The Role of Actin and Microtubules in Mitochondrial Dynamics in Amoeba, Yeast, and Mammalian Cells. Int. J. Mol. Sci. 2022, 23, 9402. [Google Scholar] [CrossRef] [PubMed]
- Jourdain, I.; Gachet, Y.; Hyams, J.S. The dynamin related protein Dnm1 fragments mitochondria in a microtubule-dependent manner during the fission yeast cell cycle. Cell Motil. Cytoskelet. 2009, 66, 509–523. [Google Scholar] [CrossRef] [PubMed]
- Mehta, K.; Chacko, L.A.; Chug, M.K.; Jhunjhunwala, S.; Ananthanarayanan, V. Association of mitochondria with microtubules inhibits mitochondrial fission by precluding assembly of the fission protein Dnm1. J. Biol. Chem. 2019, 294, 3385–3396. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.J.; Kim, Y.J.; Yu, W.D.; Kim, Y.S.; Lee, J.H. Microtubule Integrity Is Associated with the Functional Activity of Mitochondria in HEK293. Cells 2021, 10, 3600. [Google Scholar] [CrossRef]
- Li, S.; Wu, Z.; Li, Y.; Tantray, I.; De Stefani, D.; Mattarei, A.; Krishnan, G.; Gao, F.B.; Vogel, H.; Lu, B. Altered MICOS Morphology and Mitochondrial Ion Homeostasis Contribute to Poly(GR) Toxicity Associated with C9-ALS/FTD. Cell Rep. 2020, 32, 107989. [Google Scholar] [CrossRef]
- Mattenberger, Y.; James, D.I.; Martinou, J.-C. Fusion of mitochondria in mammalian cells is dependent on the mitochondrial inner membrane potential and independent of microtubules or actin. FEBS Lett. 2003, 538, 53–59. [Google Scholar] [CrossRef]
- Gatti, P.; Schiavon, C.; Cicero, J.; Manor, U.; Germain, M. Mitochondria- and ER-associated actin are required for mitochondrial fusion. Nat. Commun. 2025, 16, 451. [Google Scholar] [CrossRef] [PubMed]
- Mitra, K.; Rikhy, R.; Lilly, M.; Lippincott-Schwartz, J. DRP1-dependent mitochondrial fission initiates follicle cell differentiation during Drosophila oogenesis. J. Cell Biol. 2012, 197, 487–497. [Google Scholar] [CrossRef]
- Inoki, K.; Zhu, T.; Guan, K.-L. TSC2 Mediates cellular energy response to control cell growth and survival. Cell 2003, 115, 577–590. [Google Scholar] [CrossRef] [PubMed]
- Ueishi, S.; Shimizu, H.; Inoue, Y.H. Male germline stem cell division and spermatocyte growth require insulin signaling in Drosophila. Cell Struct. Funct. 2009, 34, 61–69. [Google Scholar] [CrossRef]
- Wong, E.D.; Wagner, J.A.; Gorsich, S.W.; McCaffery, J.M.; Shaw, J.M.; Nunnari, J. The Dynamin-related GTPase, Mgm1p, is an intermembrane space protein required for maintenance of fusion competent mitochondria. J. Cell Biol. 2000, 151, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Parone, P.A.; Da Cruz, S.; Tondera, D.; Mattenberger, Y.; James, D.I.; Maechler, P.; Barja, F.; Martinou, J.-C. Preventing mitochondrial fission impairs mitochondrial function and leads to loss of mitochondrial DNA. PLoS ONE 2008, 3, e3257. [Google Scholar] [CrossRef]
- Chung, J.Y.; Steen, J.A.; Schwarz, T.L. Phosphorylation-Induced Motor Shedding Is Required at Mitosis for Proper Distribution and Passive Inheritance of Mitochondria. Cell Rep. 2016, 16, 2142–2155. [Google Scholar] [CrossRef]
- Lawrence, E.J.; Boucher, E.; Mandato, C.A. Mitochondria-cytoskeleton associations in mammalian cytokinesis. Cell Div. 2016, 11, 3. [Google Scholar] [CrossRef]
- Tanabe, K.; Awane, R.; Shoda, T.; Yamazoe, K.; Inoue, Y.H. Mutations in mxc tumor-suppressor gene induce chromosome instability in Drosophila male meiosis. Cell Struct. Funct. 2019, 44, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, R.; Yamazoe, K.; Inoue, Y.H. Nuclear Export of Cyclin B Mediated by the Nup62 Complex Is Required for Meiotic Initiation in Drosophila Males. Cells 2020, 9, 270. [Google Scholar] [CrossRef] [PubMed]
- Udagawa, O.; Ishihara, T.; Maeda, M.; Matsunaga, Y.; Tsukamoto, S.; Kawano, N.; Miyado, K.; Shitara, H.; Yokota, S.; Nomura, M.; et al. Mitochondrial fission factor Drp1 maintains oocyte quality via dynamic rearrangement of multiple organelles. Curr. Biol. 2014, 24, 2451–2458. [Google Scholar] [CrossRef] [PubMed]
- Baker, C.C.; Gim, B.S.; Fuller, M.T. Cell type-specific translational repression of Cyclin B during meiosis in males. Development 2015, 142, 3394–3402. [Google Scholar] [CrossRef]
- Sawyer, E.M.; Brunner, E.C.; Hwang, Y.; Ivey, L.E.; Brown, O.; Bannon, M.; Akrobetu, D.; Sheaffer, K.E.; Morgan, O.; Field, C.O.; et al. Testis-specific ATP synthase peripheral stalk subunits required for tissue-specific mitochondrial morphogenesis in Drosophila. BMC Cell Biol. 2017, 18, 6. [Google Scholar] [CrossRef]
- Kitazawa, D.; Yamaguchi, M.; Mori, H.; Inoue, Y.H. COPI-mediated membrane trafficking is required for cytokinesis in Drosophila male meiotic divisions. J. Cell Sci. 2012, 125, 3649–3660. [Google Scholar] [CrossRef]
- Park, J.; Lee, G.; Chung, J. The PINK1-Parkin pathway is involved in the regulation of mitochondrial remodeling process. Biochem. Biophys. Res. Commun. 2009, 37, 518–523. [Google Scholar] [CrossRef]
- Yamazoe, K.; Inoue, Y.H. Cyclin B Export to the Cytoplasm via the Nup62 Subcomplex and Subsequent Rapid Nuclear Import Are Required for the Initiation of Drosophila Male Meiosis. Cells 2023, 12, 2611. [Google Scholar] [CrossRef]
- Inoue, Y.H.; Savoian, M.S.; Suzuki, T.; Máthé, E.; Yamamoto, M.T.; Glover, D.M. Mutations in orbit/mast reveal that the central spindle is comprised of two microtubule populations, those that initiate cleavage and those that propagate furrow ingression. J. Cell Biol. 2004, 166, 49–60. [Google Scholar] [CrossRef]
- Hayashi, D.; Tanabe, K.; Katsube, H.; Inoue, Y.H. B-type nuclear lamin and the nuclear pore complex Nup107-160 influences maintenance of the spindle envelope required for cytokinesis in Drosophila male meiosis. Biol. Open 2016, 5, 1011–1021. [Google Scholar] [CrossRef]
- Oka, S.; Hirai, J.; Yasukawa, T.; Nakahara, Y.; Inoue, Y.H. A correlation of reactive oxygen species accumulation by depletion of superoxide dismutases with age-dependent impairment in the nervous system and muscles of Drosophila adults. Biogerontology 2015, 16, 485–501. [Google Scholar] [CrossRef]
- Tanabe, K.; Okazaki, R.; Kaizuka, K.; Inoue, Y.H. Time-lapse Observation of Chromosomes, Cytoskeletons and Cell Organelles during Male Meiotic Divisions in Drosophila. Bio Protoc. 2017, 7, e2225. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, M.; Le, T.D.; Inoue, Y.H. Downregulating mitochondrial DNA polymerase γ in the muscle stimulated autophagy, apoptosis, and muscle aging-related phenotypes in Drosophila adults. Biomolecules 2022, 12, 1105. [Google Scholar] [CrossRef] [PubMed]
- Azuma, M.; Ogata, T.; Yamazoe, K.; Tanaka, Y.; Inoue, Y.H. Heat shock cognate 70 genes contribute to Drosophila spermatocyte growth progression possibly through the insulin signaling pathway. Dev. Growth Differ. 2021, 63, 231–248. [Google Scholar] [CrossRef] [PubMed]

| Knockdown and Dominant Negative exp. | 16-Cell Cysts * | 17-31-Cell Cysts | 32-Cell Cysts | 33-63-Cell Cysts | 64-Cell Cysts (Normal) |
|---|---|---|---|---|---|
| control | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 68 (100) |
| MarfRNAiJF | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 50 (100) |
| MarfRNAiGD | 0 (0) | 0 (0) | 0 (0) | 8 (20.0) | 32 (80.0) |
| Opa1RNAiHMS | 3 (3.2) | 0 (0) | 2 (2.2) | 0 (0) | 88 (94.6) |
| Opa1RNAiKK | 97 (84.3) | 16 (15.7) | 0 (0) | 0 (0) | 0 (0) |
| Drp1RNAiJF | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 59 (100) |
| Drp1DN | 0 (0) | 0 (0) | 0 (0) | 30 (58.8) | 21 (41.2) |
| EndoBRNAiKK | 54 (100) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| EndoBRNAiGD | 42 (82.4) | 0 (0) | 9 (17.6) | 0 (0) | 0 (0) |
| Letm1RNAiHMS | 40 (100) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Letm1RNAiGD | 42 (84.0) | 0 (0) | 8 (16.0) | 0 (0) | 0 (0) |
| Knockdown | n | Number of Nuclei in a Spermatid (%) | Macro/Micro Nuclei (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Normal | Abnormal | ||||||||
| 1 | 0 | 2 | 3 | 4 | 5> | Total | |||
| control | 1290 | 99.5 | 0.4 | 0.1 | 0 | 0 | 0 | 0.5 | 0 |
| Opa1RNAiHMS | 1713 | 27.1 | 7 | 24.3 | 15.8 | 12.3 | 13.4 | 73.9 | 23.5 |
| Opa1RNAiKK | 952 | 70 | 29.9 | 12.2 | 11.6 | 4.6 | 1.5 | 30 | 32.4 |
| Knockdown and Ectopic Expression | 16 Cell- Cysts (%) *1 | 17~31 Cell -Cysts | 32 Cell -Cysts *2 | 33~63 Cell -Cysts | 64 Cell -Cysts *3 | Total Cysts |
|---|---|---|---|---|---|---|
| Opa1RNAiKK, mCherry | 91 (84.3) | 16 (14.8) | 1 (0.9) | 0 (0) | 0 (0) | 108 |
| Opa1RNAiKK, CycB | 67 (51.9) | 7 (5.4) | 33 (25.6) | 4 (3.1) | 18 (14.0) | 129 |
| EendoBRNAiKK, mCherry | 67 (62.6) | 26 (24.3) | 3 (2.8) | 11 (10.3) | 0 (0) | 107 |
| EndoBRNAiKK, CycB | 8 (7.6) | 17 (16.2) | 11 (10.5) | 40 (38.1) | 29 (27.6) | 105 |
| CycB | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 106 (100) | 106 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsuo, T.; Yamanaka, M.; Inoue, Y.H. Marf- and Opa1-Dependent Formation of Mitochondrial Network Structure Is Required for Cell Growth and Subsequent Meiosis in Drosophila Males. Int. J. Mol. Sci. 2025, 26, 9991. https://doi.org/10.3390/ijms26209991
Matsuo T, Yamanaka M, Inoue YH. Marf- and Opa1-Dependent Formation of Mitochondrial Network Structure Is Required for Cell Growth and Subsequent Meiosis in Drosophila Males. International Journal of Molecular Sciences. 2025; 26(20):9991. https://doi.org/10.3390/ijms26209991
Chicago/Turabian StyleMatsuo, Tatsuru, Mitsuki Yamanaka, and Yoshihiro H. Inoue. 2025. "Marf- and Opa1-Dependent Formation of Mitochondrial Network Structure Is Required for Cell Growth and Subsequent Meiosis in Drosophila Males" International Journal of Molecular Sciences 26, no. 20: 9991. https://doi.org/10.3390/ijms26209991
APA StyleMatsuo, T., Yamanaka, M., & Inoue, Y. H. (2025). Marf- and Opa1-Dependent Formation of Mitochondrial Network Structure Is Required for Cell Growth and Subsequent Meiosis in Drosophila Males. International Journal of Molecular Sciences, 26(20), 9991. https://doi.org/10.3390/ijms26209991









