Mitochondrial Quality Control and Cell Death
Abstract
1. Introduction
2. Structure of Mitochondria
2.1. Outer Membrane
2.2. Inner Membrane
2.3. Outer Lumen
2.4. Mitochondrial Matrix
3. Mitochondrial Quality Control
3.1. Mitochondrial Biogenesis
3.2. Mitochondrial Division
3.3. Mitochondrial Fusion
3.4. Mitochondrial Autophagy
| Quality Control Processes | Proteins/Molecules | Molecular Mechanism | Reference |
|---|---|---|---|
| mitochondrial biogenesis | NRF1, NRF2 | Key transcription factor, regulates mitochondrial gene expression | [46] |
| PGC-1α | Activation of NRF1 and NRF2 for transcription of mitochondrial genes | [47] | |
| AMPK | Sensing cellular energy status, upregulating PGC-1α expression upon activation, and promoting mitochondrial biogenesis | [48] | |
| mitochondrial division | Drp1 | Core driver that binds to receptor proteins such as Fis1, Mff, MiD49, and MiD51, causing mitochondrial membrane rupture | [16] |
| Fis1 | Mitochondrial outer membrane receptor protein that recruits Drp1 and promotes its multimerization | [55] | |
| Mff | Aggregates at mitochondrial contractions and promotes mitochondrial division | [56] | |
| MiD49, MiD51 | Interacts with Drp1 to promote its oligomerization and GTPase activity and facilitates division | [57] | |
| AMPK | Phosphorylation of Ser155 and Ser173 sites of Drp1 promotes mitochondrial division | [60] | |
| Erk | Phosphorylation of the S616 site of Drp1 promotes mitochondrial division | [61] | |
| mitochondrial fusion | Mfn1 | Core factor of outer membrane fusion, mediates outer membrane contact and fusion | [65] |
| Mfn2 | Core factor of endothelial fusion, involved in endothelial fusion, more active than Mfn1 | [65] | |
| OPA1 | A key protein in endosomal fusion that promotes endosomal fusion through conformational changes | [66] | |
| S-OPA1 | Promotes OPA1-CL binding and membrane fusion | [72] | |
| mitochondrial autophagy | PINK1 | Accumulates on damaged mitochondria, activates E3 ubiquitin ligase activity, and recruits Parkin | [80] |
| Parkin | E3 ubiquitin ligase, ubiquitinates mitochondrial proteins and promotes their degradation | [82] | |
| p62/SQSTM1 | Mediates entry of damaged mitochondria into autophagosomes | [78] | |
| NIX | Ubiquitination aggregates at the outer mitochondrial membrane, induces depolarization and promotes mitochondrial autophagy | [86] | |
| BNIP3 | Interacts with BCL-2 family proteins to inhibit their anti-apoptotic function and promote damaged mitochondrial clearance | [87] | |
| ULK1 | Activation phosphorylates downstream substrates to promote autophagy complex formation and mitochondrial autophagy | [88] | |
| AMPK | Sensing energy states, activating ULK1, and promoting mitochondrial autophagy | [88] | |
| ROS, JNK | Trigger mitochondrial autophagy | [88] |
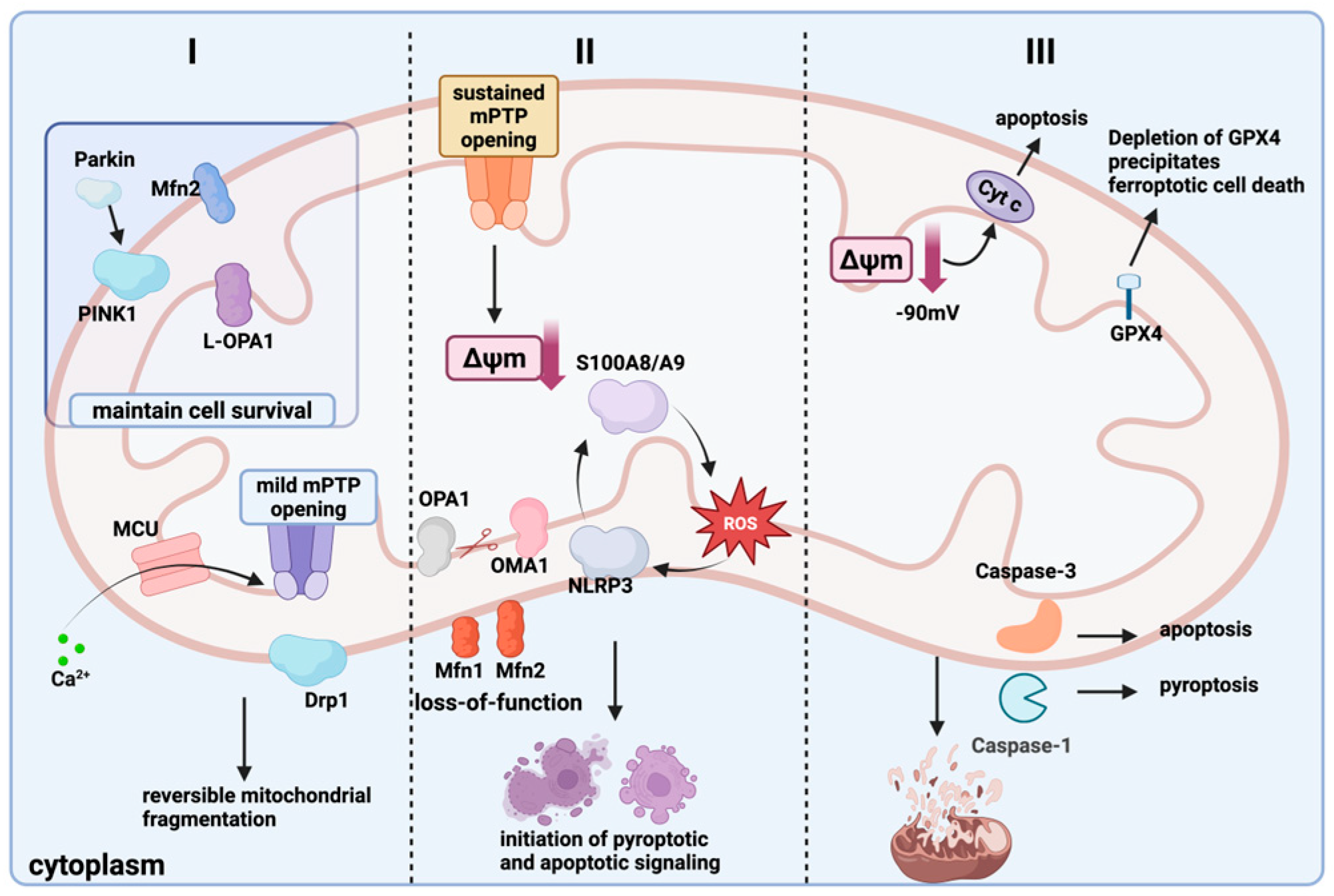
4. Regulation of Mitochondrial Mass and Cell Death
4.1. Mitochondrial Quality Control and Cell Pyroptosis
4.2. Mitochondrial Quality Control and Ferroptosis
4.3. Regulation of Mitochondrial Mass and Apoptosis
| Type of Cell Death | Quality Control Processes | Mechanism of Action | Proteins and Pathways | Reference |
|---|---|---|---|---|
| cellular pyroptosis | Increased outer membrane permeability Increased mitochondrial fission and decreased fusion Inhibition of mitochondrial autophagy | Promote cellular pyroptosis Isolates and repairs damaged mitochondria Loss of mitochondrial cristae and increased membrane permeability | GSDMD, Caspase-1, NLRP3, IL-1β, Mitochondrial calcium overload | [88,89,90] |
| iron death | Mitochondria decrease in size, cristae disappear Rupture of the outer mitochondrial membrane Inhibition of mitochondrial autophagy and activation of innate immune response | Lipid peroxidation, ROS accumulation Loss of membrane potential Induced occurrence of iron death | Erastin, RSL3, GPX4, SOD2, MGST1, mitochondrial membrane potential, STING/TLR9 | [99,100,103,104] |
| apoptosis | Enhanced mitochondrial division, reduced fusion, fragmentation Mitochondria become larger, cristae increase, then undergo vacuolization and change from tubular to granular MPT-induced calcium imbalance | Decreased ATP synthesis and increased ROS content Cytochrome C release Calcium overload; opening of the MPTP; loss of membrane potential; release of cytochrome c | Bcl-2, Bcl-XL, Caspase9, Caspase3, Drp1, Fis1, OPA1 | [106,107,109,110] |
5. Summary
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yu, L.; Zhao, Z.; Yao, G. Research of progress of mitochondria in the pathogenesis of sepsis. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 2023, 35, 669–672. [Google Scholar]
- Wal, P.; Wal, A.; Vig, H.; Mahmood, D.; Khan, M.M.U. Potential Applications of Mitochondrial Therapy with a Focus on Parkinson’s Disease and Mitochondrial Transplantation. Adv. Pharm. Bull. 2024, 14, 147–160. [Google Scholar] [CrossRef]
- Sherratt, H.S. Mitochondria: Structure and function. Rev. Neurol. 1991, 147, 417–430. [Google Scholar]
- Duarte, F.V.; Ciampi, D.; Duarte, C.B. Mitochondria as central hubs in synaptic modulation. Cell. Mol. Life Sci. 2023, 80, 173. [Google Scholar] [CrossRef]
- Sheng, Z.-H.; Cai, Q. Mitochondrial transport in neurons: Impact on synaptic homeostasis and neurodegeneration. Nat. Rev. Neurosci. 2012, 13, 77–93. [Google Scholar] [CrossRef]
- Meiliana, A.; Dewi, N.M.; Wijaya, A. Mitochondria: Master Regulator of Metabolism, Homeostasis, Stress, Aging and Epigenetics. Indones. Biomed. J. 2021, 13, 221–241. [Google Scholar] [CrossRef]
- Bonora, M.; Giorgi, C.; Pinton, P. Molecular mechanisms and consequences of mitochondrial permeability transition. Nat. Rev. Mol. Cell Biol. 2022, 23, 266–285. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Wang, Y.; Zhang, J.; Hu, C.; Jiang, J.; Li, Y.; Peng, Z. ROS-induced lipid peroxidation modulates cell death outcome: Mechanisms behind apoptosis, autophagy, and ferroptosis. Arch. Toxicol. 2023, 97, 1439–1451. [Google Scholar] [CrossRef] [PubMed]
- Araiso, Y.; Imai, K.; Endo, T. Role of the TOM Complex in Protein Import into Mitochondria: Structural Views. Annu. Rev. Biochem. 2022, 91, 679–703. [Google Scholar] [CrossRef]
- Arbogast, F.; Gros, F. Lymphocyte Autophagy in Homeostasis, Activation, and Inflammatory Diseases. Front. Immunol. 2018, 9, 1801, Erratum in Front. Immunol. 2018, 9, 2627. [Google Scholar] [CrossRef] [PubMed]
- McArthur, K.; Whitehead, L.W.; Heddleston, J.M.; Li, L.; Padman, B.S.; Oorschot, V.; Geoghegan, N.D.; Chappaz, S.; Davidson, S.; Chin, H.S.; et al. BAK/BAX macropores facilitate mitochondrial herniation and mtDNA efflux during apoptosis. Science 2018, 359, eaao6047. [Google Scholar] [CrossRef]
- Behl, T.; Kaur, D.; Sehgal, A.; Singh, S.; Sharma, N.; Zengin, G.; Andronie-Cioara, F.L.; Toma, M.M.; Bungau, S.; Bumbu, A.G. Role of Monoamine Oxidase Activity in Alzheimer’s Disease: An Insight into the Therapeutic Potential of Inhibitors. Molecules 2021, 26, 3724. [Google Scholar] [CrossRef] [PubMed]
- Eura, Y.; Ishihara, N.; Oka, T.; Mihara, K. Identification of a novel protein that regulates mitochondrial fusion by modulating mitofusin (Mfn) protein function. J. Cell Sci. 2006, 119, 4913–4925. [Google Scholar] [CrossRef]
- Qu, Y.; Sun, Y.; Yang, Z.; Ding, C. Calcium Ions Signaling: Targets for Attack and Utilization by Viruses. Front. Microbiol. 2022, 13, 889374. [Google Scholar] [CrossRef]
- Cui, X.; Zhou, Z.; Tu, H.; Wu, J.; Zhou, J.; Yi, Q.; Liu, O.; Dai, X. Mitophagy in fibrotic diseases: Molecular mechanisms and therapeutic applications. Front. Physiol. 2024, 15, 1430230. [Google Scholar] [CrossRef]
- Kraus, F.; Roy, K.; Pucadyil, T.J.; Ryan, M.T. Function and regulation of the divisome for mitochondrial fission. Nature 2021, 590, 57–66. [Google Scholar] [CrossRef]
- Liu, J.; Liu, W.; Li, R.; Yang, H. Mitophagy in Parkinson’s Disease: From Pathogenesis to Treatment. Cells 2019, 8, 712. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, D.; He, X.; Huang, Y.; Shao, H. Transport of Calcium Ions into Mitochondria. Curr. Genom. 2016, 17, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Pickrell, A.M.; Youle, R.J. The roles of PINK1, parkin, and mitochondrial fidelity in Parkinson’s disease. Neuron 2015, 85, 257–273. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Yan, M.; Chen, J.; Li, H.; Zhang, Y. MicroRNA-129-1-3p attenuates autophagy-dependent cell death by targeting MCU in granulosa cells of laying hens under H(2)O(2)-induced oxidative stress. Poult. Sci. 2023, 102, 103006. [Google Scholar] [CrossRef]
- Endlicher, R.; Drahota, Z.; Stefkova, K.; Cervinkova, Z.; Kucera, O. The Mitochondrial Permeability Transition Pore-Current Knowledge of Its Structure, Function, and Regulation, and Optimized Methods for Evaluating Its Functional State. Cells 2023, 12, 1273. [Google Scholar] [CrossRef]
- Bernardi, P.; Gerle, C.; Halestrap, A.P.; Jonas, E.A.; Karch, J.; Mnatsakanyan, N.; Pavlov, E.; Sheu, S.S.; Soukas, A.A. Identity, structure, and function of the mitochondrial permeability transition pore: Controversies, consensus, recent advances, and future directions. Cell Death Differ. 2023, 30, 1869–1885. [Google Scholar] [CrossRef]
- Sreejit, G.; Abdel-Latif, A.; Athmanathan, B.; Annabathula, R.; Dhyani, A.; Noothi, S.K.; Quaife-Ryan, G.A.; Al-Sharea, A.; Pernes, G.; Dragoljevic, D.; et al. Neutrophil-Derived S100A8/A9 Amplify Granulopoiesis After Myocardial Infarction. Circulation 2020, 141, 1080–1094. [Google Scholar] [CrossRef]
- Wu, H.; Wang, F.; Ta, N.; Zhang, T.; Gao, W. The Multifaceted Regulation of Mitochondria in Ferroptosis. Life 2021, 11, 222. [Google Scholar] [CrossRef]
- Pernas, L.; Scorrano, L. Mito-Morphosis: Mitochondrial Fusion, Fission, and Cristae Remodeling as Key Mediators of Cellular Function. Annu. Rev. Physiol. 2016, 78, 505–531. [Google Scholar] [CrossRef]
- Zerbes, R.M.; van der Klei, I.J.; Veenhuis, M.; Pfanner, N.; van der Laan, M.; Bohnert, M. Mitofilin complexes: Conserved organizers of mitochondrial membrane architecture. Biol. Chem. 2012, 393, 1247–1261. [Google Scholar] [CrossRef] [PubMed]
- Wolf, D.M.; Segawa, M.; Kondadi, A.K.; Anand, R.; Bailey, S.T.; Reichert, A.S.; van der Bliek, A.M.; Shackelford, D.B.; Liesa, M.; Shirihai, O.S. Individual cristae within the same mitochondrion display different membrane potentials and are functionally independent. EMBO J. 2019, 38, e101056. [Google Scholar] [CrossRef]
- Gilkerson, R.W.; Selker, J.M.L.; Capaldi, R.A. The cristal membrane of mitochondria is the principal site of oxidative phosphorylation. Febs Lett. 2003, 546, 355–358. [Google Scholar] [CrossRef] [PubMed]
- Camara, A.K.S.; Lesnefsky, E.J.; Stowe, D.F. Potential Therapeutic Benefits of Strategies Directed to Mitochondria. Antioxid. Redox Signal. 2010, 13, 279–347. [Google Scholar] [CrossRef]
- Rieger, B.; Arroum, T.; Borowski, M.T.; Villalta, J.; Busch, K.B. Mitochondrial F(1) F(O) ATP synthase determines the local proton motive force at cristae rims. EMBO Rep. 2021, 22, e52727. [Google Scholar] [CrossRef] [PubMed]
- Kondadi, A.K.; Anand, R.; Reichert, A.S. Cristae Membrane Dynamics—A Paradigm Change. Trends Cell Biol. 2020, 30, 923–936. [Google Scholar] [CrossRef] [PubMed]
- Scotti, P.A.; Urbanus, M.L.; Brunner, J.; de Gier, J.W.L.; von Heijne, G.; van der Does, C.; Driessen, A.J.M.; Oudega, B.; Luirink, J. YidC, the Escherichia coli homologue of mitochondrial Oxa1p, is a component of the Sec translocase. Embo J. 2000, 19, 542–549. [Google Scholar] [CrossRef] [PubMed]
- Prokisch, H.; Scharfe, C.; Camp, D.G.; Xiao, W.Z.; David, L.; Andreoli, C.; Monroe, M.E.; Moore, R.J.; Gritsenko, M.A.; Kozany, C.; et al. Integrative analysis of the mitochondrial proteome in yeast. PLoS Biol. 2004, 2, 795–804. [Google Scholar] [CrossRef]
- Utsumi, T.; Matsuzaki, K.; Kiwado, A.; Tanikawa, A.; Kikkawa, Y.; Hosokawa, T.; Otsuka, A.; Iuchi, Y.; Kobuchi, H.; Moriya, K. Identification and characterization of protein N-myristoylation occurring on four human mitochondrial proteins, SAMM50, TOMM40, MIC19, and MIC25. PLoS ONE 2018, 13, e0206355. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Q.; Guo, F.; Fu, L.; Dong, Y.; Xie, S.; Ding, X.; Hu, S.; Zhou, X.D.; Jiang, Y.; Zhou, H.; et al. 1-Deoxynojirimycin promotes cardiac function and rescues mitochondrial cristae in mitochondrial hypertrophic cardiomyopathy. J. Clin. Investig. 2023, 133, e164660. [Google Scholar] [CrossRef]
- Wenz, L.-S.; Opalinski, L.; Schuler, M.-H.; Ellenrieder, L.; Ieva, R.; Boettinger, L.; Qiu, J.; van der Laan, M.; Wiedemann, N.; Guiard, B.; et al. The presequence pathway is involved in protein sorting to the mitochondrial outer membrane. Embo Rep. 2014, 15, 678–685. [Google Scholar] [CrossRef]
- Grevel, A.; Pfanner, N.; Becker, T. Coupling of import and assembly pathways in mitochondrial protein biogenesis. Biol. Chem. 2020, 401, 117–129. [Google Scholar] [CrossRef]
- Pagura, L.; Dumoulin, P.C.; Ellis, C.C.; Mendes, M.T.; Estevao, I.L.; Almeida, I.C.; Burleigh, B.A. Fatty acid elongases 1-3 have distinct roles in mitochondrial function, growth, and lipid homeostasis in Trypanosoma cruzi. J. Biol. Chem. 2023, 299, 104715. [Google Scholar] [CrossRef]
- Wang, J.; Edmondson, D.E. Topological Probes of Monoamine Oxidases A and B in Rat Liver Mitochondria: Inhibition by TEMPO-Substituted Pargyline Analogues and Inactivation by Proteolysis. Biochemistry 2011, 50, 2499–2505. [Google Scholar] [CrossRef]
- Walker, A.K.; Kavelaars, A.; Heijnen, C.J.; Dantzer, R. Neuroinflammation and Comorbidity of Pain and Depression. Pharmacol. Rev. 2014, 66, 80–101. [Google Scholar] [CrossRef]
- Karbowski, M.; Youle, R.J. Regulating mitochondrial outer membrane proteins by ubiquitination and proteasomal degradation. Curr. Opin. Cell Biol. 2011, 23, 476–482. [Google Scholar] [CrossRef]
- Sabbir, M.G.; Dar, N.J.; Bhat, S.A.; Alanazi, H.H.; Perry, J. Editorial: Proteins and protein-complexes underlying mitochondrial structure-function and metabolism: Implications in diseases. Front. Cell Dev. Biol. 2024, 12, 1386787. [Google Scholar] [CrossRef]
- Kuo, C.-L.; Babuharisankar, A.P.; Lin, Y.-C.; Lien, H.-W.; Lo, Y.K.; Chou, H.-Y.; Tangeda, V.; Cheng, L.-C.; Cheng, A.N.; Lee, A.Y.-L. Mitochondrial oxidative stress in the tumor microenvironment and cancer immunoescape: Foe or friend? J. Biomed. Sci. 2022, 29, 74. [Google Scholar] [CrossRef]
- Zhao, F.; Zou, M.-H. Role of the Mitochondrial Protein Import Machinery and Protein Processing in Heart Disease. Front. Cardiovasc. Med. 2021, 8, 749756. [Google Scholar] [CrossRef]
- Piantadosi, C.A.; Suliman, H.B. Mitochondrial transcription factor A induction by redox activation of nuclear respiratory factor 1. J. Biol. Chem. 2006, 281, 324–333. [Google Scholar] [CrossRef] [PubMed]
- Jamwal, S.; Blackburn, J.K.; Elsworth, J.D. PPARγ/PGC1α signaling as a potential therapeutic target for mitochondrial biogenesis in neurodegenerative disorders. Pharmacol. Ther. 2021, 219, 107705. [Google Scholar] [CrossRef]
- Rabinovitch, R.C.; Samborska, B.; Faubert, B.; Ma, E.H.; Gravel, S.-P.; Andrzejewski, S.; Raissi, T.C.; Pause, A.; St-Pierre, J.; Jones, R.G. AMPK Maintains Cellular Metabolic Homeostasis through Regulation of Mitochondrial Reactive Oxygen Species. Cell Rep. 2017, 21, 1–9. [Google Scholar] [CrossRef]
- Yamada, Y.; Takano, Y.; Satrialdi; Abe, J.; Hibino, M.; Harashima, H. Therapeutic Strategies for Regulating Mitochondrial Oxidative Stress. Biomolecules 2020, 10, 83. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Liu, S.; Wang, C.; Wang, Y.; Wan, M.; Liu, F.; Gong, M.; Yuan, Y.; Chen, Y.; Cheng, J.; et al. Mesenchymal Stem Cell-Derived Extracellular Vesicles Attenuate Mitochondrial Damage and Inflammation by Stabilizing Mitochondrial DNA. Acs Nano 2021, 15, 1519–1538, Erratum in Acs Nano 2021, 15, 20692. [Google Scholar] [CrossRef] [PubMed]
- Seo, B.J.; Choi, J.; La, H.; Habib, O.; Choi, Y.; Hong, K.; Do, J.T. Role of mitochondrial fission-related genes in mitochondrial morphology and energy metabolism in mouse embryonic stem cells. Redox Biol. 2020, 36, 101599. [Google Scholar] [CrossRef]
- Meyer, J.N.; Leuthner, T.C.; Luz, A.L. Mitochondrial fusion, fission, and mitochondrial toxicity. Toxicology 2017, 391, 42–53. [Google Scholar] [CrossRef]
- Anzell, A.R.; Maizy, R.; Przyklenk, K.; Sanderson, T.H. Mitochondrial Quality Control and Disease: Insights into Ischemia-Reperfusion Injury. Mol. Neurobiol. 2018, 55, 2547–2564. [Google Scholar] [CrossRef]
- Radi, E.; Formichi, P.; Battisti, C.; Federico, A. Apoptosis and Oxidative Stress in Neurodegenerative Diseases. J. Alzheimers Dis. 2014, 42, S125–S152. [Google Scholar] [CrossRef] [PubMed]
- Kleele, T.; Rey, T.; Winter, J.; Zaganelli, S.; Mahecic, D.; Lambert, H.P.; Ruberto, F.P.; Nemir, M.; Wai, T.; Pedrazzini, T.; et al. Distinct fission signatures predict mitochondrial degradation or biogenesis. Nature 2021, 593, 435–439. [Google Scholar] [CrossRef]
- Zerihun, M.; Sukumaran, S.; Qvit, N. The Drp1-Mediated Mitochondrial Fission Protein Interactome as an Emerging Core Player in Mitochondrial Dynamics and Cardiovascular Disease Therapy. Int. J. Mol. Sci. 2023, 24, 5785. [Google Scholar] [CrossRef]
- Jin, Q.; Li, R.; Hu, N.; Xin, T.; Zhu, P.; Hu, S.; Ma, S.; Zhu, H.; Ren, J.; Zhou, H. DUSP1 alleviates cardiac ischemia/reperfusion injury by suppressing the Mff-required mitochondrial fission and Bnip3-related mitophagy via the JNK pathways. Redox Biol. 2018, 14, 576–587. [Google Scholar] [CrossRef]
- Ding, M.; Feng, N.; Tang, D.; Feng, J.; Li, Z.; Jia, M.; Liu, Z.; Gu, X.; Wang, Y.; Fu, F.; et al. Melatonin prevents Drp1-mediated mitochondrial fission in diabetic hearts through SIRT1-PGC1α pathway. J. Pineal Res. 2018, 65, e12491. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.-y.; Wei, X.-x.; Zhi, X.-l.; Wang, X.-h.; Meng, D. Drp1-dependent mitochondrial fission in cardiovascular disease. Acta Pharmacol. Sin. 2021, 42, 655–664. [Google Scholar] [CrossRef]
- Toyama, E.Q.; Herzig, S.; Courchet, J.; Lewis, T.L., Jr.; Loson, O.C.; Hellberg, K.; Young, N.P.; Chen, H.; Polleux, F.; Chan, D.C.; et al. AMP-activated protein kinase mediates mitochondrial fission in response to energy stress. Science 2016, 351, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Xin, T.; Li, D.; Wang, C.; Zhu, H.; Zhou, H. Therapeutic effect of Sirtuin 3 on ameliorating nonalcoholic fatty liver disease: The role of the ERK-CREB pathway and Bnip3-mediated mitophagy. Redox Biol. 2018, 18, 229–243. [Google Scholar] [CrossRef]
- Guardia-Laguarta, C.; Area-Gomez, E.; Rueb, C.; Liu, Y.; Magrane, J.; Becker, D.; Voos, W.; Schon, E.A.; Przedborski, S. α-Synuclein Is Localized to Mitochondria-Associated ER Membranes. J. Neurosci. 2014, 34, 249–259. [Google Scholar] [CrossRef]
- Deng, Y.; Ngo, D.T.M.; Holien, J.K.; Lees, J.G.; Lim, S.Y. Mitochondrial Dynamin-Related Protein Drp1: A New Player in Cardio-oncology. Curr. Oncol. Rep. 2022, 24, 1751–1763. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Xiao, L.; Zhang, Z.; Wang, Y.; Kouis, P.; Rasmussen, L.J.; Dai, F. Effects of reactive oxygen species and mitochondrial dysfunction on reproductive aging. Front. Cell Dev. Biol. 2024, 12, 1347286. [Google Scholar] [CrossRef]
- Xie, L.; Zhou, T.; Xie, Y.; Bode, A.M.; Cao, Y. Mitochondria-Shaping Proteins and Chemotherapy. Front. Oncol. 2021, 11, 769036. [Google Scholar] [CrossRef]
- Iqbal, S.; Hood, D.A. The role of mitochondrial fusion and fission in skeletal muscle function and dysfunction. Front. Biosci.-Landmark 2015, 20, 157–172. [Google Scholar]
- Song, Z.; Ghochani, M.; McCaffery, J.M.; Frey, T.G.; Chan, D.C. Mitofusins and OPA1 Mediate Sequential Steps in Mitochondrial Membrane Fusion. Mol. Biol. Cell 2009, 20, 3525–3532. [Google Scholar] [CrossRef]
- Yang, Y.; Lei, W.; Zhao, L.; Wen, Y.; Li, Z. Insights Into Mitochondrial Dynamics in Chlamydial Infection. Front. Cell. Infect. Microbiol. 2022, 12, 835181. [Google Scholar] [CrossRef]
- Heath-Engel, H.M.; Shore, G.C. Mitochondrial membrane dynamics, cristae remodelling and apoptosis. Biochim. Biophys. Acta-Mol. Cell Res. 2006, 1763, 549–560. [Google Scholar] [CrossRef]
- Kanova, M.; Kohout, P. Molecular Mechanisms Underlying Intensive Care Unit-Acquired Weakness and Sarcopenia. Int. J. Mol. Sci. 2022, 23, 8396. [Google Scholar] [CrossRef] [PubMed]
- Suen, D.-F.; Norris, K.L.; Youle, R.J. Mitochondrial dynamics and apoptosis. Genes. Dev. 2008, 22, 1577–1590. [Google Scholar] [CrossRef]
- Ning, P.; Jiang, X.; Yang, J.; Zhang, J.; Yang, F.; Cao, H. Mitophagy: A potential therapeutic target for insulin resistance. Front. Physiol. 2022, 13, 957968. [Google Scholar] [CrossRef]
- Tang, Y.-C.; Tian, H.-X.; Yi, T.; Chen, H.-B. The critical roles of mitophagy in cerebral ischemia. Protein Cell 2016, 7, 699–713. [Google Scholar] [CrossRef]
- Martinez-Carreres, L.; Nasrallah, A.; Fajas, L. Cancer: Linking Powerhouses to Suicidal Bags. Front. Oncol. 2017, 7, 204. [Google Scholar] [CrossRef]
- Vona, R.; Mileo, A.M.; Matarrese, P. Microtubule-Based Mitochondrial Dynamics as a Valuable Therapeutic Target in Cancer. Cancers 2021, 13, 5812. [Google Scholar] [CrossRef]
- Vargas, J.N.S.; Hamasaki, M.; Kawabata, T.; Youle, R.J.; Yoshimori, T. The mechanisms and roles of selective autophagy in mammals. Nat. Rev. Mol. Cell Biol. 2023, 24, 167–185. [Google Scholar] [CrossRef]
- Peng, X.; Hou, R.; Yang, Y.; Luo, Z.; Cao, Y. Current Studies of Mitochondrial Quality Control in the Preeclampsia. Front. Cardiovasc. Med. 2022, 9, 836111. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Wei, L.; Li, M. Progress in regulation of mitochondrial dynamics and mitochondrial autophagy. Sheng Li Xue Bao [Acta Physiol. Sin.] 2020, 72, 475–487. [Google Scholar]
- Zhang, Y.; Weng, J.; Huan, L.; Sheng, S.; Xu, F. Mitophagy in atherosclerosis: From mechanism to therapy. Front. Immunol. 2023, 14, 1165507. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.-X.; Ni, H.-M.; Li, M.; Liao, Y.; Chen, X.; Stolz, D.B.; Dorn, G.W., II; Yin, X.-M. Nix Is Critical to Two Distinct Phases of Mitophagy, Reactive Oxygen Species-mediated Autophagy Induction and Parkin-Ubiquitin-p62-mediated Mitochondrial Priming. J. Biol. Chem. 2010, 285, 27879–27890. [Google Scholar] [CrossRef]
- Zhang, J.; Yuan, G.; Liang, T.; Pan, P.; Li, X.; Li, H.; Shen, H.; Wang, Z.; Chen, G. Nix Plays a Neuroprotective Role in Early Brain Injury After Experimental Subarachnoid Hemorrhage in Rats. Front. Neurosci. 2020, 14, 245. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-S.; Koentjoro, B.; Sue, C.M. Commentary: Nix restores mitophagy and mitochondrial function to protect against PINK1/Parkin-related Parkinson’s disease. Front. Mol. Neurosci. 2017, 10, 297. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, A.; Xue, Z.; Liu, J.; He, Y.; Liu, G.; Zhao, Z.; Li, W.; Zhang, Q.; Chen, A.; et al. BCL2L13 promotes mitophagy through DNM1L-mediated mitochondrial fission in glioblastoma. Cell Death Dis. 2023, 14, 585. [Google Scholar] [CrossRef] [PubMed]
- Poole, L.P.; Bock-Hughes, A.; Berardi, D.E.; Macleod, K.F. ULK1 promotes mitophagy via phosphorylation and stabilization of BNIP3. Sci. Rep. 2021, 11, 20526. [Google Scholar] [CrossRef]
- Kim, H.; Jeon, B.T.; Kim, I.M.; Bennett, S.J.; Lorch, C.M.; Viana, M.P.; Myers, J.F.; Trupp, C.J.; Whipps, Z.T.; Kundu, M.; et al. Sestrin2 Phosphorylation by ULK1 Induces Autophagic Degradation of Mitochondria Damaged by Copper-Induced Oxidative Stress. Int. J. Mol. Sci. 2020, 21, 6130. [Google Scholar] [CrossRef]
- Kumar, M.; Papaleo, E. A pan-cancer assessment of alterations of the kinase domain of ULK1, an upstream regulator of autophagy. Sci. Rep. 2020, 10, 14874. [Google Scholar] [CrossRef]
- Wu, Y.; Zhao, Z.; Ma, M.; Zhang, W.; Liu, W.; Liang, X.; Zhao, T.; Luo, Y.; Wang, Y.; Li, M.; et al. Ultrasound-activated erythrocyte membrane-camouflaged Pt (II) layered double hydroxide enhances PD-1 inhibitor efficacy in triple-negative breast cancer through cGAS-STING pathway-mediated immunogenic cell death. Theranostics 2025, 15, 1456–1477. [Google Scholar] [CrossRef]
- Maryanovich, M.; Gross, A. A ROS rheostat for cell fate regulation. Trends Cell Biol. 2013, 23, 129–134. [Google Scholar] [CrossRef]
- An, H.; Heo, J.S.; Kim, P.; Lian, Z.; Lee, S.; Park, J.; Hong, E.; Pang, K.; Park, Y.; Ooshima, A.; et al. Tetraarsenic hexoxide enhances generation of mitochondrial ROS to promote pyroptosis by inducing the activation of caspase-3/GSDME in triple-negative breast cancer cells. Cell Death Dis. 2021, 12, 159. [Google Scholar] [CrossRef] [PubMed]
- Bhatti, R.; Sato, P.Y. Exploring the role of pyroptosis in the pathogenicity of heart disease. Front. Physiol. 2024, 15, 1357285. [Google Scholar] [CrossRef]
- Liu, D.; Zhong, X.; Cao, W.; Chen, L. Research progress in effects of pyroptosis on intestinal inflammatory injury. Zhong Nan Da Xue Xue BaoYi Xue Ban J. Cent. South Univ. Med. Sci. 2023, 48, 252–259. [Google Scholar]
- Wang, J.; Su, H.; Wang, M.; Ward, R.; An, S.; Xu, T.-R. Pyroptosis and the fight against lung cancer. Med. Res. Rev. 2025, 45, 5–28. [Google Scholar] [CrossRef]
- D’Angelo, D.; Reane, D.V.; Raffaello, A. Neither too much nor too little: Mitochondrial calcium concentration as a balance between physiological and pathological conditions. Front. Mol. Biosci. 2023, 10, 1336416. [Google Scholar] [CrossRef]
- Cozac, D.A.; Halatiu, V.B.; Scridon, A. The alarmin tandem: Unraveling the complex effect of S100A8/A9—From atherosclerosis to cardiac arrhythmias. Front. Immunol. 2025, 16, 1630410. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, Y.; Shao, Y.; Lu, X.; Zhang, H.; Miao, C. S100A8/A9(hi) neutrophils induce mitochondrial dysfunction and PANoptosis in endothelial cells via mitochondrial complex I deficiency during sepsis. Cell Death Dis. 2024, 15, 462. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Wei, X.; Yi, X.; Jiang, D.-S. Mitophagy-related regulated cell death: Molecular mechanisms and disease implications. Cell Death Dis. 2024, 15, 505. [Google Scholar] [CrossRef]
- Osellame, L.D.; Rahim, A.A.; Hargreaves, I.P.; Gegg, M.E.; Richard-Londt, A.; Brandner, S.; Waddington, S.N.; Schapira, A.H.V.; Duchen, M.R. Mitochondria and Quality Control Defects in a Mouse Model of Gaucher Disease—Links to Parkinson’s Disease. Cell Metab. 2013, 17, 941–953. [Google Scholar] [CrossRef]
- Huang, P.; Zhang, Z.; Zhang, P.; Feng, J.; Xie, J.; Zheng, Y.; Liang, X.; Zhu, B.; Chen, Z.; Feng, S.; et al. TREM2 Deficiency Aggravates NLRP3 Inflammasome Activation and Pyroptosis in MPTP-Induced Parkinson’s Disease Mice and LPS-Induced BV2 Cells. Mol. Neurobiol. 2024, 61, 2590–2605. [Google Scholar] [CrossRef]
- Karbowski, M.; Youle, R.J. Dynamics of mitochondrial morphology in healthy cells and during apoptosis. Cell Death Differ. 2003, 10, 870–880. [Google Scholar] [CrossRef]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An Iron-Dependent Form of Nonapoptotic Cell Death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Cao, F.; Yin, H.-L.; Huang, Z.-J.; Lin, Z.-T.; Mao, N.; Sun, B.; Wang, G. Ferroptosis: Past, present and future. Cell Death Dis. 2020, 11, 88. [Google Scholar] [CrossRef] [PubMed]
- Diao, J.; Jia, Y.; Dai, E.; Liu, J.; Kang, R.; Tang, D.; Han, L.; Zhong, Y.; Meng, L. Ferroptotic therapy in cancer: Benefits, side effects, and risks. Mol. Cancer 2024, 23, 89. [Google Scholar] [CrossRef]
- Xie, L.-H.; Fefelova, N.; Pamarthi, S.H.; Gwathmey, J.K. Molecular Mechanisms of Ferroptosis and Relevance to Cardiovascular Disease. Cells 2022, 11, 2726. [Google Scholar] [CrossRef]
- Gan, B. How erastin assassinates cells by ferroptosis revealed. Protein Cell 2023, 14, 84–86. [Google Scholar] [CrossRef]
- Fu, C.; Cao, N.; Zeng, S.; Zhu, W.; Fu, X.; Liu, W.; Fan, S. Role of mitochondria in the regulation of ferroptosis and disease. Front. Med. 2023, 10, 1301822. [Google Scholar] [CrossRef]
- Ren, W.; Ge, X.; Li, M.; Sun, J.; Li, S.; Gao, S.; Shan, C.; Gao, B.; Xi, P. Visualization of cristae and mtDNA interactions via STED nanoscopy using a low saturation power probe. Light-Sci. Appl. 2024, 13, 116, Correction in Light-Sci. Appl. 2024, 13, 235. [Google Scholar] [CrossRef]
- Chen, G.Y.; Nunez, G. Sterile inflammation: Sensing and reacting to damage. Nat. Rev. Immunol. 2010, 10, 826–837. [Google Scholar] [CrossRef]
- Colglazier, K.A.; Mukherjee, N.; Contreras, C.J.; Templin, A.T. RISING STARS: Evidence for established and emerging forms of β-cell death. J. Endocrinol. 2024, 262, e230378. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Deng, J.; Zhou, H.; Tan, W.; Lin, L.; Yang, J. Programmed Cell Death in Sepsis Associated Acute Kidney Injury. Front. Med. 2022, 9, 883028. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Tang, L.; Zhang, J.; He, D.; Tan, J.; Li, X. Research progress on the role of mitochondrial dynamics disorder in sepsis-associated acute kidney injury. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 2024, 36, 1117–1120. [Google Scholar] [PubMed]
- Gencpinar, T.; Bilen, C.; Kemahli, B.; Kacar, K.; Akokay, P.; Bayrak, S.; Erdal, C. Effects of rivaroxaban on myocardial mitophagy in the rat heart. Turk. Gogus Kalp Damar Cerrahisi Derg.-Turk. J. Thorac. Cardiovasc. Surg. 2023, 31, 301–308. [Google Scholar] [CrossRef]
- Estaquier, J.; Arnoult, D. Inhibiting Drp1-mediated mitochondrial fission selectively prevents the release of cytochrome c during apoptosis. Cell Death Differ. 2007, 14, 1086–1094. [Google Scholar] [CrossRef] [PubMed]
- Pradeepkiran, J.A.; Reddy, P.H. Defective mitophagy in Alzheimer’s disease. Ageing Res. Rev. 2020, 64, 101191. [Google Scholar] [CrossRef] [PubMed]
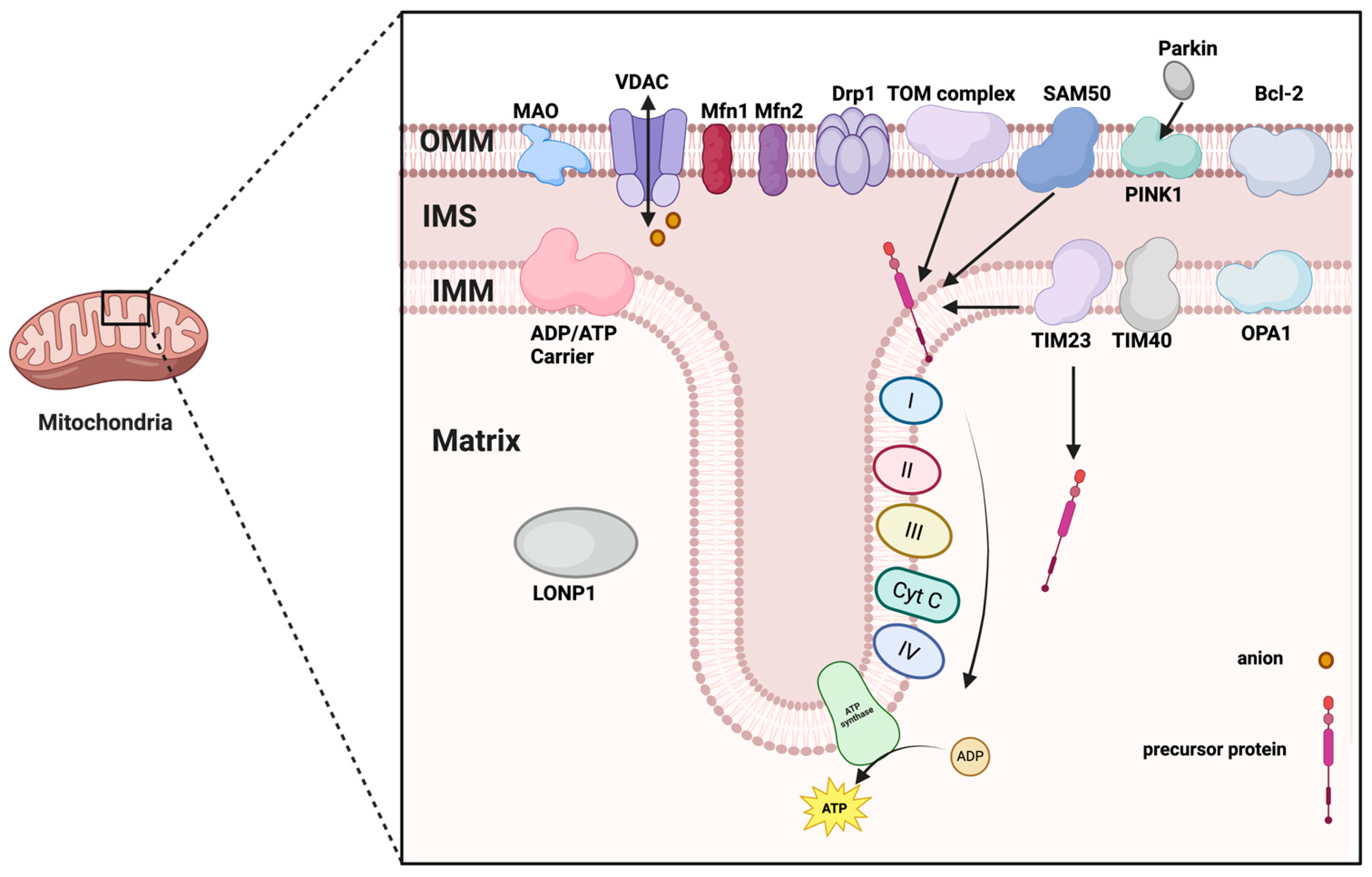
| Structure | Protein | Function | Reference |
|---|---|---|---|
| OMM | TOM complex | Transfer of specific proteins | [9] |
| BAX | Increased mitochondrial outer membrane permeability activates apoptotic program | [10] | |
| Monoamine oxidase | Catalytic oxidation and deamination of monoamines | [12] | |
| Mfn1/2 | Involved in the regulation of mitochondrial fusion | [13] | |
| VDAC | Transporting ions and small molecules | [14] | |
| Bcl-2 | Regulation of apoptosis | [11] | |
| NLRP3 | Involved in immune response and activation of inflammatory vesicles | [15] | |
| BNIP3 | Involved in the regulation of mitochondrial autophagy and apoptosis | [11] | |
| FUNDC1 | Involved in the transportation and storage of iron ions | [11] | |
| NDUFA9 | Closely related to the function of the mitochondrial electron transport chain | [11] | |
| Drp1 | Causes the rupture of the mitochondrial membrane | [16] | |
| PINK1 | Promotes Parkin recruitment and ubiquitination | [17] | |
| IMM | ATPase | Drive ATP synthesis | [32] |
| NADH dehydrogenase | Oxidizes NADH to NAD+ and generates electron flow | [32] | |
| Succinate dehydrogenase | Oxidizing succinic acid to fenugreek acid and transferring electrons | [32] | |
| Cytochrome C reductase | Accepts electrons from complex II and passes them to cytochrome C | [32] | |
| Cytochrome C oxidase | Accepts electrons delivered by cytochrome c and delivers them to oxygen to produce water | [32] | |
| Proton pump | Driving ATP synthesis through a proton gradient | [32] | |
| SecYEG complex | Transport polypeptide chains from the inner membrane to the outer membrane | [32] | |
| TOM complex | Transport of proteins synthesized by ribosomes in the inner mitochondrial membrane | [33] | |
| TIM complex | Involved in transmembrane transport of proteins | [33] | |
| CCT, SAM50 | Involved in protein folding, modification and quality control | [34] | |
| OPA1 | Promotes the fusion of the inner membrane | [35] | |
| Outer Lumen | signal-anchored | Involved in protein localization and transport | [36] |
| porin | Perform small molecule transport | [37] | |
| β-barrel protein | Bound to TIM for translocation to the matrix | [37] | |
| alpha helical transmembrane fragment protein | Embedded in the outer membrane, involved in protein transport, quality control and membrane dynamics | [37] | |
| fatty acid elongase | Lengthening of short-chain fatty acids into longer saturated fatty acids maintains cellular lipid homeostasis and energy metabolism | [38] | |
| adrenaline oxidase | Involved in the oxidative deamination of adrenaline | [39] | |
| tryptophan degrading enzyme | Catalyzes the breakdown of serine to 5-HT or kynurenine | [40] | |
| Mitochondrial Matrix | AAC | Regulation of energy homeostasis by exchange of ATP in the cytoplasm and ADP in the mitochondrial matrix | [42] |
| LONP1 | Involved in protein degradation and DNA quality control | [43] | |
| TIM23 | Transport of precursor proteins from the mitochondrial membrane space to the matrix | [44] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Zhang, M.; Jin, H.; Lv, S.; Li, Y.; Li, Y. Mitochondrial Quality Control and Cell Death. Int. J. Mol. Sci. 2025, 26, 11084. https://doi.org/10.3390/ijms262211084
Zhang Z, Zhang M, Jin H, Lv S, Li Y, Li Y. Mitochondrial Quality Control and Cell Death. International Journal of Molecular Sciences. 2025; 26(22):11084. https://doi.org/10.3390/ijms262211084
Chicago/Turabian StyleZhang, Zurui, Mengyuan Zhang, Hongchi Jin, Shuang Lv, Yilei Li, and Yanru Li. 2025. "Mitochondrial Quality Control and Cell Death" International Journal of Molecular Sciences 26, no. 22: 11084. https://doi.org/10.3390/ijms262211084
APA StyleZhang, Z., Zhang, M., Jin, H., Lv, S., Li, Y., & Li, Y. (2025). Mitochondrial Quality Control and Cell Death. International Journal of Molecular Sciences, 26(22), 11084. https://doi.org/10.3390/ijms262211084





