1. Introduction
It is recognized that the intake of dietary proteins exerts a greater suppressive effect on hunger and energy intake compared with an isoenergetic intake of fat and carbohydrates. This effect on satiety is partially dependent on the release of anorexigenic gut hormones in response to the detection of protein digestion products by the gut wall. To this regard, nutrient sensing by enteroendocrine cells facing the gut lumen is known to be mediated by specific cell surface receptors, mainly G-protein-coupled receptors and membrane transporters, so-called nutrient-sensing receptors.
The main peptidic hormones released into circulation by the enteroendocrine cells in the gut in response to an oral nutrient load include cholecystokinin (CCK), Tyrosine-Tyrosine (PYY), oxyntomodulin, glucagon-like-peptide-1 (GLP-1) and glucose dependent insulinotropic peptide (GIP). Distinct subtypes of enteroendocrine cells differ in the hormones they can release and in their localization along the GI tract. Among gut hormones, CCK is secreted by I cells in the proximal small intestine in response to luminal peptides and lipids and cooperates with the signaling networks in regulating appetite, food intake, gastric emptying, pancreatic enzyme secretion and gall bladder contraction [
1,
2,
3,
4].
In the last two decades, the mouse enteroendocrine cell line STC-1 has been widely used to investigate the effects of dietary proteins and peptides on CCK- and glucagon-like peptide-1 (GLP-1) secretion. These studies have also addressed the question of whether the specific structural features of a given peptide chain may be related to higher secretagogue activity with respect to others. A number of authors have reported on the isolation and identification of peptide fragments linked to a strong induction of hormone release, either CCK, or GLP-1 or both, between the products of enzymatic simulated gastrointestinal digestion (SGID) of distinct dietary proteins [
5,
6,
7,
8,
9,
10,
11,
12,
13,
14,
15,
16,
17]. Despite some studies highlighting a few structural characteristics shared by peptides endowed with high secretagogue activity, a detailed structure–activity relationship among CCK- or GLP-1-releasing peptides has not been reported yet. In fact, according to the current data available, this outcome may hardly be reachable.
In this regard, some factors may significantly contribute to expanding the heterogeneity of receptor ligands: briefly, a number of different nutrient-sensing receptors are involved in mediating the response of enteroendocrine cells to proteins and their digestion products; these receptors are able to detect both free amino acids and peptides; and finally, when limited to those observed using STC-1 cell cultures, the active concentrations of peptide ligands are relatively high (approx. 0.1–1 mM), suggesting low affinity ligand–receptor interactions [
1,
4,
18].
Nevertheless, research works reporting on food-protein-derived peptides acting as inducers of CCK release in STC-1 cell cultures in similar experimental conditions have accumulated in the last decade. These works make it possible to file a sizable number of peptide sequences acting as CCK-releasing factors, and to compare them to a set of peptide sequences which were revealed to be ineffective in the same trials [
5,
6,
7,
8,
9,
10,
11,
12,
13,
14,
15,
16].
The aim of this study was to list the currently available CCK-releasing peptide sequences identified using STC-1 cell cultures; to draw conclusions about the role played by peptide length, peptide amino acid composition and peptide amino acid sequence in differentiating their secretagogue activity; and to highlight the physicochemical properties and sequence motifs shared by the active peptides, if any, as well as any possible differential features between CCK-releasing peptides and ineffective peptides.
2. Results
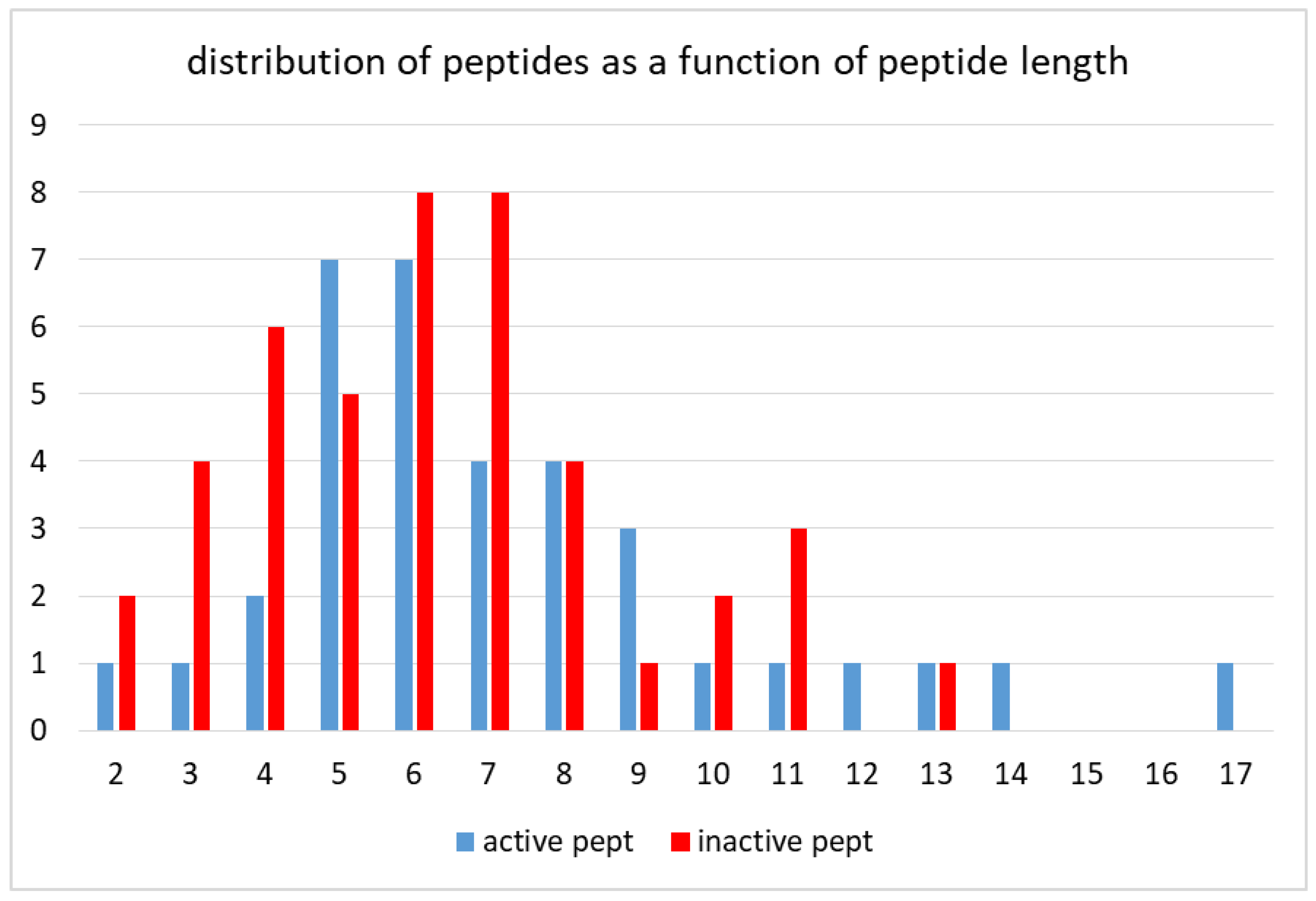
The “active peptide” and the “inactive peptide” set included 35- and 44-peptide sequences, respectively. The peptide length ranged from 2 amino acids up to 17 amino acids. The analysis of the frequency distribution as a function of peptide size (
Figure 1) revealed that most of the peptides were five to eight amino acids long: 62.85% of active peptides vs. 56.8% of inactive peptides. As to the two tails of the distribution, the relative amount of longer peptides (9 to 17 amino acids) was 25.7% of active peptides versus 15.9% of inactive peptides, whereas the relative amount of shorter peptides (2 to 4 amino acids) was 14.8% of active peptides versus 27.2% of inactive peptides.
2.1. First Subset: Peptide Length from Two to Four Amino Acids
The pI values ranged from 3.7 to 9.1 in active peptides, and 3.8 up to 8.6 in inactive peptides. The comparison of the amino acid composition between the two groups revealed that L-Phe was exclusively present in active peptides (two out of four peptides), whereas the acidic amino acid L-Glu was found only in inactive peptides (four out of twelve peptides). The sole acidic peptide promoting CCK release (pI 3.7) contained L-Asp in place of L-Glu (
Table 1).
Apart from the presence of specific amino acids, the secretagogue activity may be related to physicochemical properties conferred by the side chains and shared by some amino acids. To this end, the color scheme used in this work (see Section should help with visualizing the similarities and differences between the two groups of peptides in terms of amino acid composition by highlighting the presence of aliphatic/aromatic, polar (with amide or hydroxyl groups), charged (basic or acidic), Sulfur-containing, or peculiar side chains.
The analysis showed that the active and the inactive peptides differ significantly in their N-terminal ends, with large hydrophobic (aliphatic) side chains in the second group versus L-Arg and L-Pro in the first group.
As to the comparison of the amino acid sequences, the alignment between the two sequences RALG (active) and RYLG (inactive) showed that the presence of L-Arg is not enough to confer CCK-releasing activity. Specifically, Santos-Hernandez and coworkers showed that one amino acid change within this short sequence (tiny-aliphatic replaced by large-aromatic amino acid with higher level of hydrophilicity), can cause a loss of activity [
8]. Finally, both groups of peptides contained L-Pro, which was located at the N-terminus in active peptides versus inner positions or the C-terminus in inactive peptides (
Table 1).
Table 2 shows the alignment between a subgroup of active peptide sequences and the sequence RFYGPV. This hexapeptide matches the C-terminal end of human osteocalcin and is an allosteric ligand to GPRC6A receptor, a nutrient-sensing receptor in enteroendocrine cells [
19]. The comparison revealed a similarity in amino acid composition (the shared amino acid being L-Arg, L-Phe, L-gly and L-Pro), the presence of L-Arg at the N-terminus and/or L-Phe in second position, and an aliphatic amino acid at the C-terminal end.
2.2. Second Subset: Peptide Length from Nine to Seventeen Amino Acids
The pI values ranged from 3.29 to 12.98 in active peptides, and 5.07 to 10.82 in inactive peptides. The H
R values ranged from 5.32 to 37.3, and 7.5 to 26.23, respectively, (
Table 3).
In order to compare the amino acid composition between the two groups of peptides, we considered the total amount of each amino acid in the active sequences versus the inactive sequences, expressed as a percentage of the sum of all amino acid moles in the respective group.
The comparison suggested a higher content of L-Leu, L-Val, and L-Asp in the active sequence pool versus a higher content of L-Gln and L-Pro in the inactive sequence pool. Specifically, the presence of multiple units of L-Pro scattered along the sequence and flanked by L-Gln and/or L-Gly, was the distinctive trait of four out of seven inactive peptides. Remarkably, L-Pro and L-Gly are both regarded as conformationally special amino acids. The content of aromatic amino acids was low in both groups of peptides. Finally, all the active peptides but one contained L-Asp and/or L-Glu whereas five out of seven inactive peptides did not contain any acidic amino acid (
Table 4).
The analysis suggested the separation of the active peptides into two subgroups: the first one includes five peptides with low pI value joined with a high HR value. The second subgroup includes four peptides containing two, three or five basic amino acids, respectively, scattered along the sequence.
Among the acidic peptides, three peptides contained multiple L-Glu and/or L-Asp residues (five or six residues in each peptide). The alignments of these peptides showed extensive sequence similarities. Apart from the clusters of acidic amino acids, the common traits included one L-Thr, an aromatic amino acid (L-Phe or L-Tyr) and, finally, amino acids with aliphatic side chains (mainly L-Leu and L-Val) flanking the acidic clusters. The alignment of the remaining two peptides, which contained one and two acidic residues, respectively, showed a scrambled sequence of six amino acids, including L-Glu, setting aside the insertion of a second acidic residue in one peptide (
Table 5).
Among basic peptides, the longest sequence matched the soy-beta conglycinin 51–63 peptide [
14]. The alignments showed some similarities between this sequence and two other distinct sequences, so that they all may be gathered into a subgroup named as soy-beta conglycinin 51–63 peptide-related subgroup. The shared traits were: aliphatic amino acid at the N-terminus (L-Val or L-Leu); basic amino acids (L-Arg or L-Lys) scattered along the sequence; prevalence of amino acids with hydrophilic side chains (polar- or charged-) in the C-terminal half of the peptide; and presence of amino acids with a hydroxyl group in their side chains at the C-terminus or in the second position starting from the C-terminus. Finally, a second alignment analysis highlighted the presence of an inverted sequence of five amino acids shared by the peptides endowed with pI values of 8.63 and 8.03, respectively, (
Table 6).
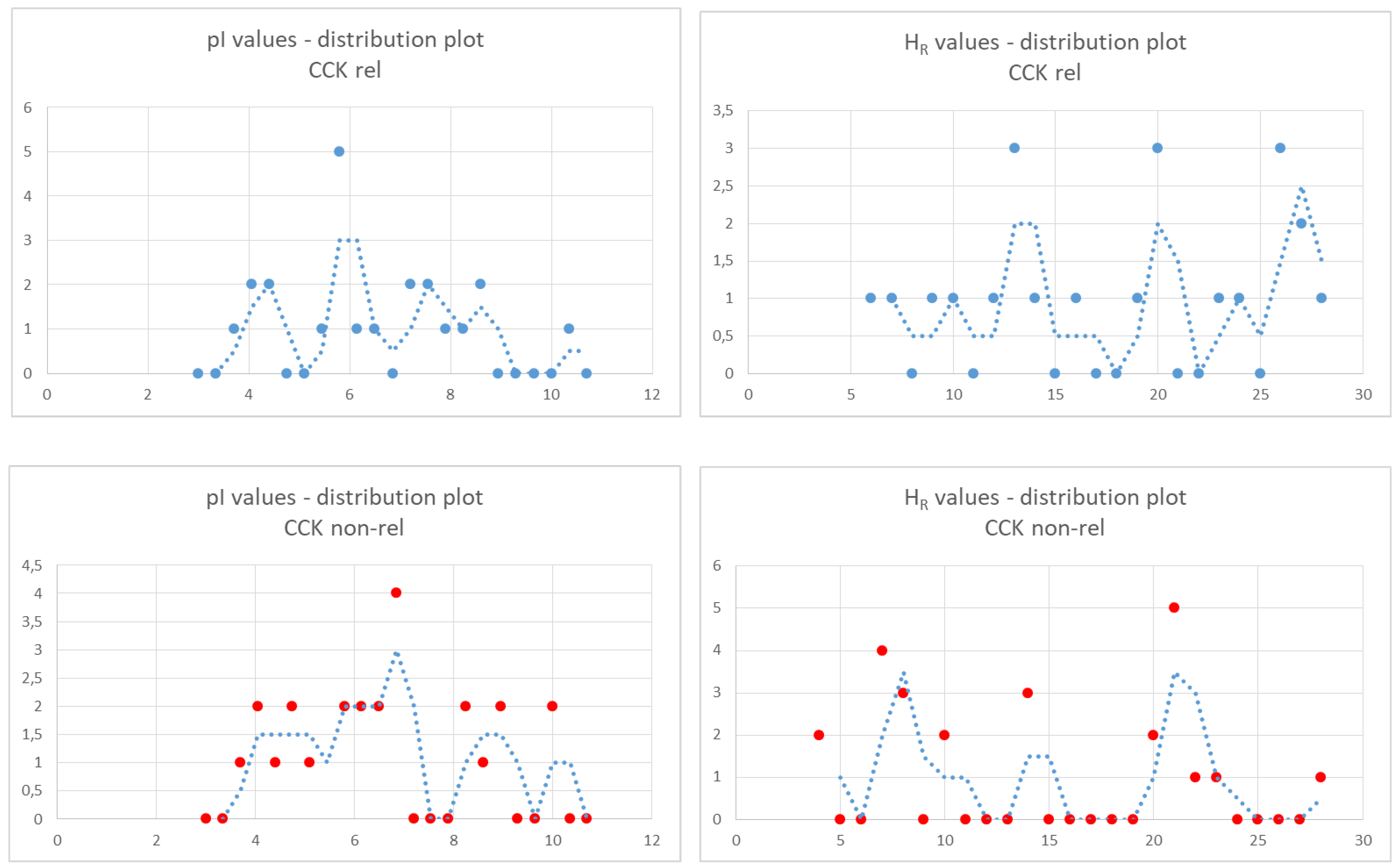
2.3. Third Subset: Peptide Length from Five to Eight Amino Acids
The number of peptides in the two groups (22 active vs. 25 inactive peptides) (
Table 7 and
Table 8, respectively) made it possible to analyze the distribution of pI and H
R values. To this end, we grouped the values into bins and displayed the number of elements in each bin by using dots in the distribution plots. The bin sizes were 0.35 and 1.0 units for the pI plot and H
R plot, respectively, and were calculated as follows: (max value − min value)/(number of values). Trend lines are also shown. The distribution plots of the pI values were similar between the two groups. On the other hand, the distribution of H
R values showed a greater number of values lower than 10 in the inactive peptide group versus a greater number of values higher than 25 in the active peptide group, suggesting that a marked hydrophobicity of a five- to eight-amino-acid-long peptide might be a positive marker of CCK-releasing activity (
Figure 2).
Finally, assuming normal distribution of values, we calculated the mean values of pI and HR in the two groups. We observed similar mean pI values, whereas the mean HR value tended to be lower in inactive peptides compared to active peptides (pI: 6.2 ± 1.7 vs. 6 ± 2; HR: 17.9 ± 7.6 vs. 14.1 ± 7.4, as the mean ± SD of 22 vs. 25 values; unpaired t test, p < 0.1), in agreement with the conclusion of the distribution analysis.
We used a bioinformatic tool for peptide solubility prediction, wherein solubility was defined in PROSO II as a sequence that was transfectable, expressible, secretable, separable and soluble in an
E. coli system (see
Section 4 for details). Most peptides were predicted as weakly soluble (59% of active vs. 68% of inactive peptides). In total, 23% of active peptides and 20% of inactive peptides were predicted as unlikely to be soluble. The remaining peptides were likely soluble (18% of active vs. 8% of inactive peptides).
The comparison of the cumulative amino acid composition between active and inactive peptides suggested a higher relative content of L-Leu, L-Asp, L-Arg and L-Tyr and a lower relative content of L-Gln, L-Gly and L-Thr in the active peptide sequence group versus the inactive sequence group (
Table 9). Moreover, we might want to remark that the active peptides in this subset contained a greater percentage of aromatic amino acids and L-Pro and a lower percentage of L-Lys, L-Glu and L-Thr compared to the active peptides in the second subset (meaning active peptides longer than nine amino acids) (
Table 9 versus
Table 4).
The relative content of L-His, L-Met, L-Phe, L Val, L-Ile, L-Ser, L-Lys and L-Pro was similar in active versus inactive peptides. Restricted to these amino acids, we examined their flanking amino acids within all the active peptide sequences, and then we listed the C-terminal and N-terminal dipeptides and the tripeptides containing each amino acid (
Table 10). We did not include the di- or tripeptides that were also found in inactive peptide sequences. The outcome was a list of short amino acid motifs which may be related to CCK-releasing activity. L-His was found at the C-terminal end or flanked by amino acids with hydrophobic side chains in tripeptides. L-Phe was mainly found at internal positions, whereas L-Ser was at terminal positions (N-terminal or C-terminal end, indifferently). L-Ile was linked to an aromatic amino acid and/or L-Arg in tripeptides, and to L-Pro in N-terminal or C-terminal dipeptides. Finally, the relative content of L-Pro and L-Val was high in both active and inactive peptide sequences and the related amino acid motifs were heterogeneous in both groups.
In order to analyze the amino acid sequences to find possible elements shared by active peptides and possible differential elements between active and inactive peptides, the sequences were separated into three subgroups according to their pI values: low pI values (acidic), high pI values (basic), and intermediate pI values.
The relative amount of acidic peptide sequences was 22.73% of active peptides versus 20% of inactive peptides, respectively. The H
R values of the inactive peptides did not exceed 10, whereas four out of five active peptides showed H
R values higher than 13 and two values that were near to 20, suggesting that a low pI value along with hydrophobicity may be a positive marker of CCK-releasing activity (
Table 7 and
Table 8).
The alignment of the five active sequences highlighted some common traits: specifically, the presence of a core made of aliphatic amino acids (mainly, L-Leu) and L-Asp (in one peptide replaced by L-Glu) was joined with charged amino acids at the C-terminal end. Moreover, the alignment of the active peptide DLVDK with the inactive peptide LGVDE suggested that the insertion of L-Gly might be related to a reduction in CCK-releasing activity. It is worth remarking that the pI and H
R values of these peptides were quite similar (
Table 11).
The relative amount of basic peptide sequences was 18.2% of active peptides versus 28% of inactive peptides, respectively. In both groups, the pI values ranged approximately from 8 to 10. The H
R values varied significantly in both subgroups, although hydrophobicity was the prevailing character in active peptides (
Table 7 and
Table 8).
The alignment of four active sequences revealed a common structural motif centered on L-Arg and an aromatic amino acid (L-Tyr or L-Trp) at the N-terminal side of the peptide sequence (
Table 12, panel A). Then, in order to identify possible differential amino acid motifs between active and inactive peptides, we examined pairs of peptide sequences endowed with similar pI and HR values. The alignments shown in
Table 12 (panel B) confirmed that the simultaneous presence of an aromatic amino acid and L-Arg at the N-terminal side was necessary for CCK-releasing activity. Indeed, the peptide YPWQRF was inactive, despite being the richest one in aromatic amino acids at the N-terminal end, and the removal of the N-terminal L-Trp from WIRGCRL caused a loss of activity despite the N-terminal L-Arg in the truncated peptide (see also ref. [
8]). However, the presence of L-Lys as a basic amino acid and L-Phe as an aromatic amino acid at the N-terminal side was not related to any secretagogue activity in basic peptides (
Table 12, panel B). It is worth remarking that the latter note has not been extended to all the subsets of five- to eight-amino-acid-long peptides.
The relative amount of peptides endowed with intermediate pI values was 59% of active peptides versus 48% of inactive peptides. The pI values ranged from 5.4 to 7.6 in the active peptide subset and 5.0 to 6.8 in the inactive peptide subset. The number of peptides in both subsets made it possible to analyze the peptides with pI higher than 6.0 (neutral) and the peptides with pI lower than 5.9 (mildly acidic) separately.
Among the neutral peptides (
Table 13), the pI values of active sequences ranged from 6.5 to 7.6 and exceeded 7.2 in four out of six sequences, whereas the pI value of inactive sequences never exceeded 6.8. The related H
R values ranged from 11 to 27 units in active sequences and 7 to 23 in inactive sequences, so that the most hydrophobic sequences were active, whereas the most hydrophilic sequence was inactive. The cumulative amino acid composition was similar in neutral active peptides versus neutral inactive peptides. The main differences were the higher amount of polar amino acids with amide groups in inactive sequences, and the presence of L-Met and L-Tyr only in active sequences. These remarks suggest that CCK-releasing activity was most likely related to the presence of differential amino acid sequence motifs rather than specific amino acid residues. Specifically, the two groups differed significantly in the molecular size/degree of hydrophilicity of the N-terminal residues and the third residue starting from the N-terminal end. Finally, the alignment of the active peptide sequences revealed two coupled structural elements found across four sequences: L-His at position 5 or 6 joined with an aliphatic branched-chain amino acid at position 3 or 2 from the N-terminal end (L-Leu in three peptides and L-Val in the octapeptide). In three out of four sequences, L-His was the C-terminal amino acid residue (
Table 13).
As to the mildly acidic peptides, the pI values ranged from 5.4 to 5.8 for active sequences and 5.1 to 6.0 for inactive sequences. The H
R values of three active peptides exceeded 27 units, whereas 24 units was the highest value among the inactive peptides. Both groups contained two peptides with higher degrees of hydrophilicity and similar H
R values (lower than 10 units). The main differences in the cumulative amino acid composition were the higher contents of polar amino acids with an amide group in the inactive sequences, the same as was observed in neutral peptides, and the presence of L-Arg, L-His, L-Tyr and L-Trp only in the active sequences (
Table 14). Finally, the alignment of the active peptide sequences did not show any common trait shared by the majority of peptides; nevertheless, it highlighted some amino acid motifs shared by coupled peptides and not found in the inactive peptide sequences: L-Pro-L-Leu-aromatic amino acid (L-Trp or L-Phe) versus L-Pro-L-Leu-L-Asn in the inactive peptide; L-Val-L-Thr-(L-Gly)-L-Val versus L-Val-L-Thr-L-Gln in the inactive peptide; L-Arg (N-terminal)-...-...-...-...-...-L-Asp (or L-Glu), not found in inactive peptides (
Table 14).
2.4. Description of Physicochemical Properties of Peptides Using Two Sets of Amino Acid Scalar Descriptors
Peptide Length from Five to Eight Amino Acids
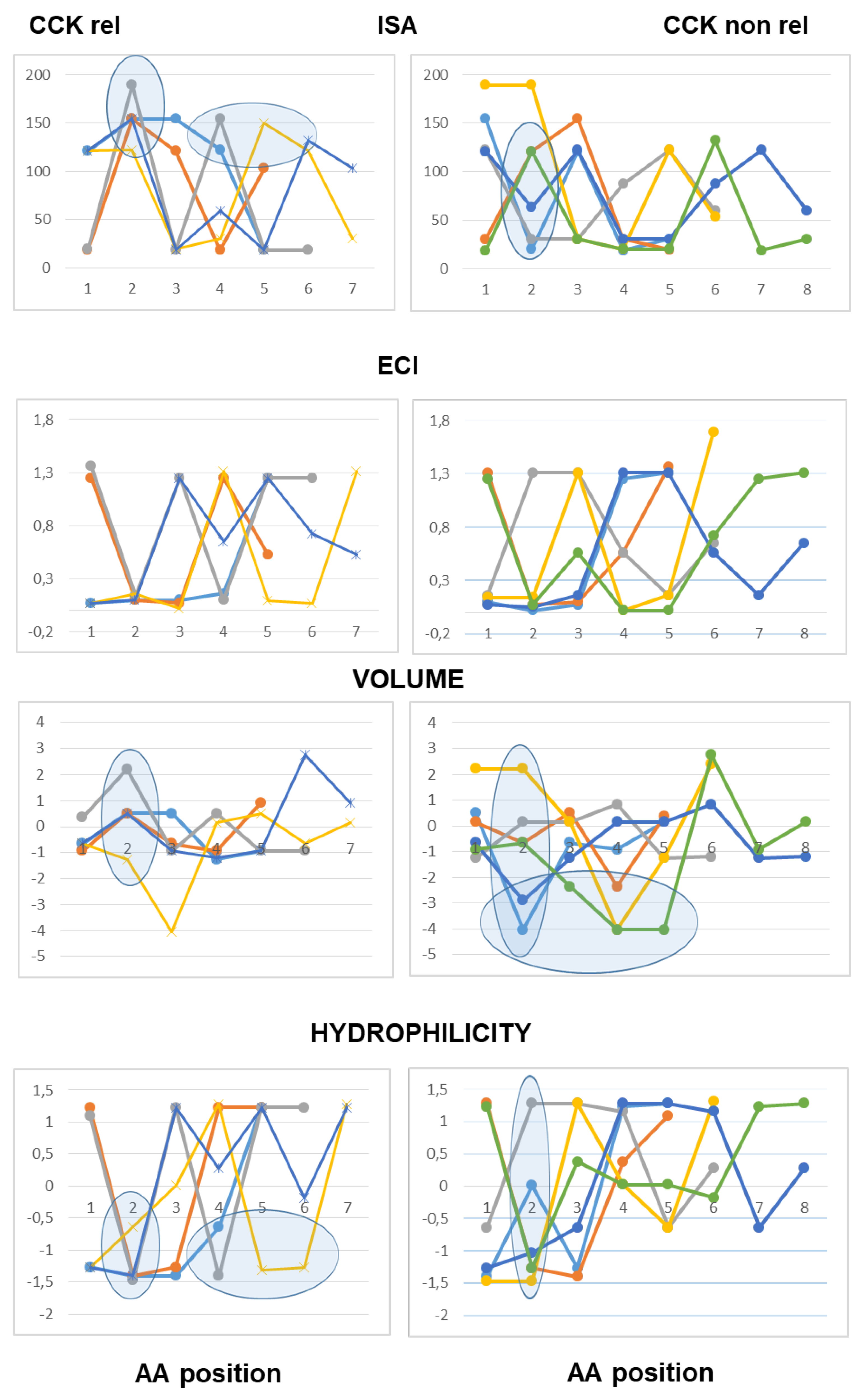
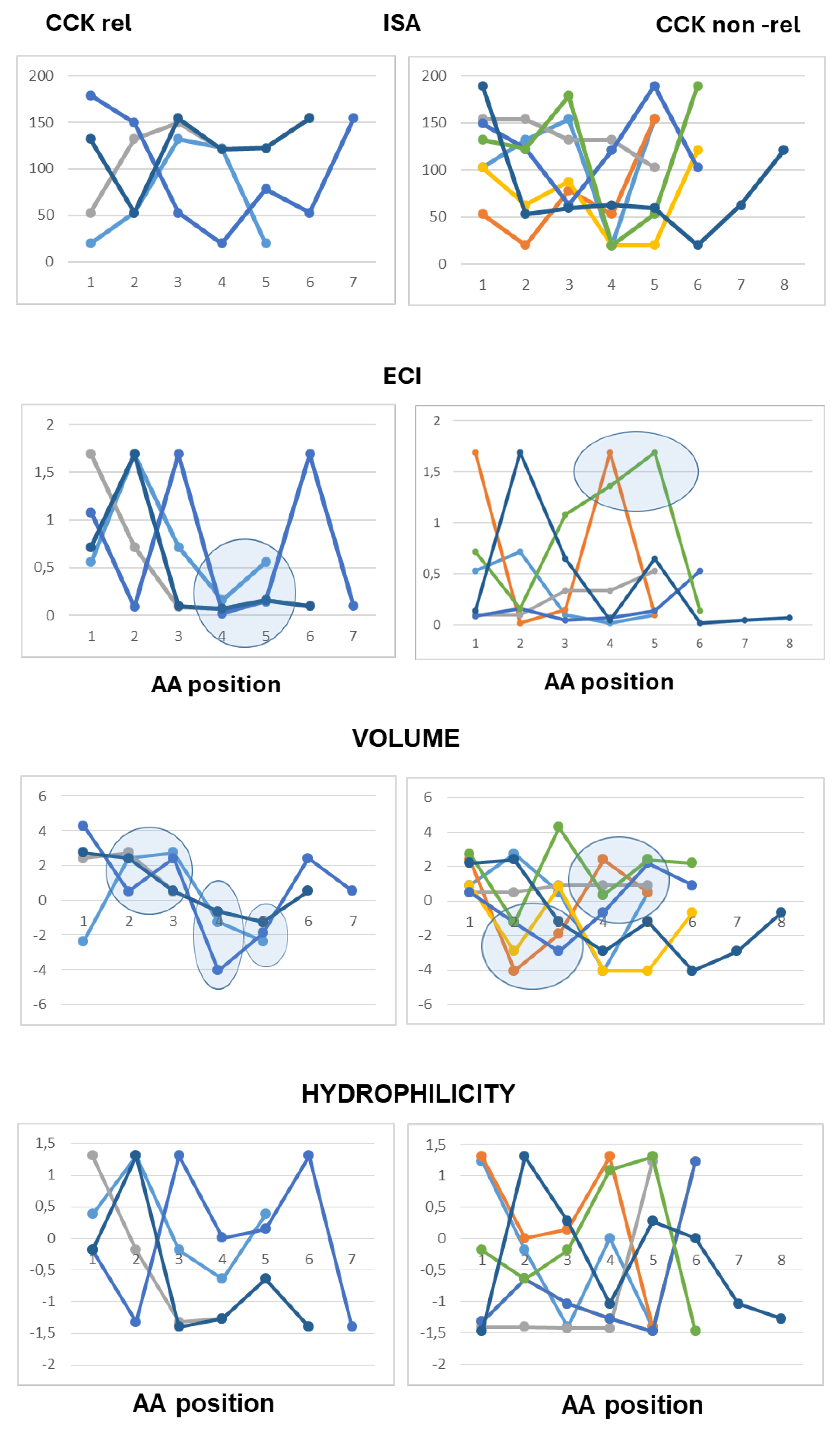
The use of line graphs helped to visualize differences between active and inactive peptides in scalar descriptors related to hydrophobic character, charge concentration and volume of the amino acid residues from the N-terminal to the C-terminal end.
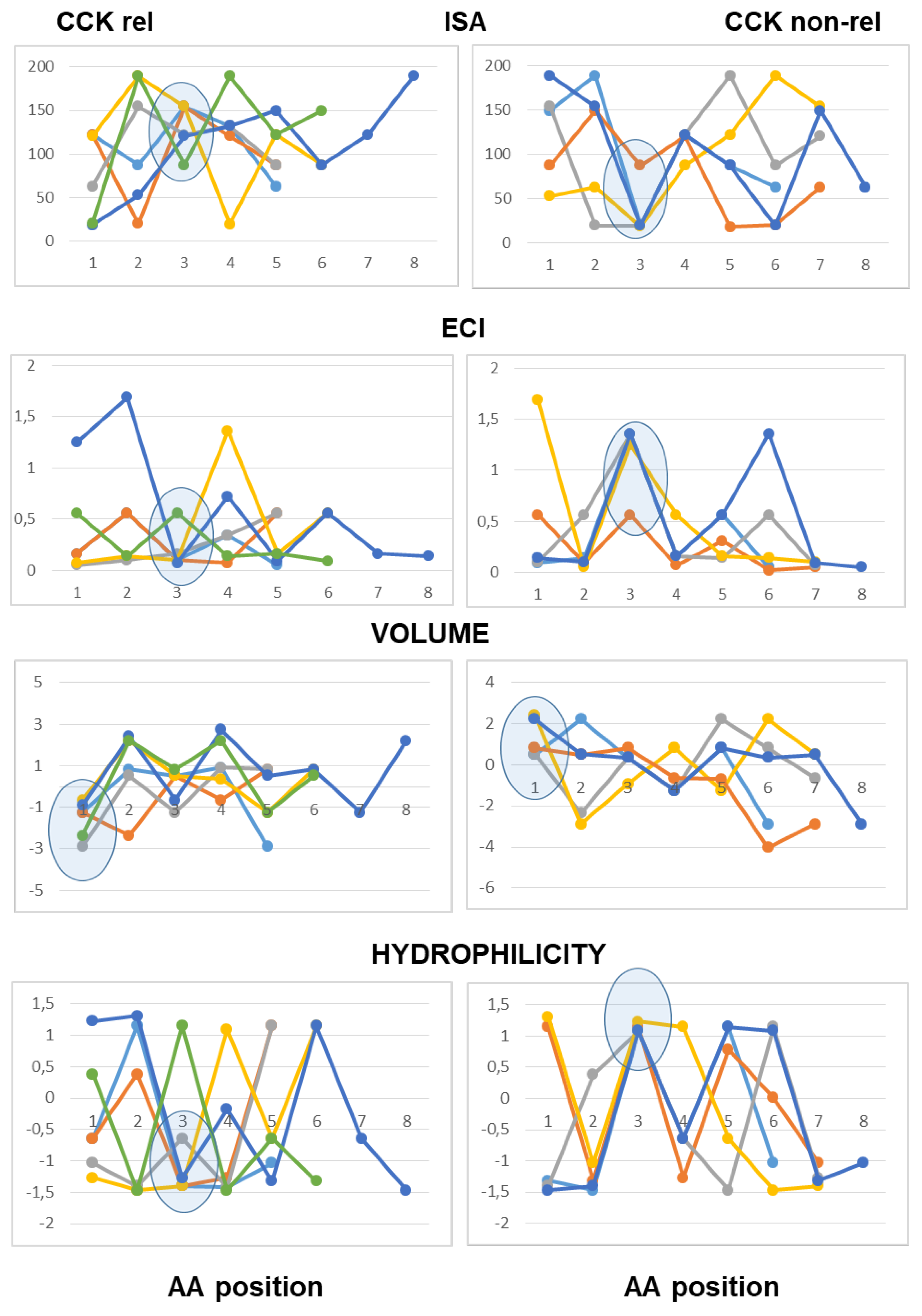
As to peptides with neutral pI values (6.1–7.9), the analysis highlighted a difference at the third position from the N-terminal end according to both scales of descriptors, with hydrophobic side chains and reduced charge concentration in active peptides versus polar or charged side chains in inactive peptides. According to the Barley scale, active peptides also differed from inactive peptides in the molecular size of N-terminal amino acids (
Figure 3).
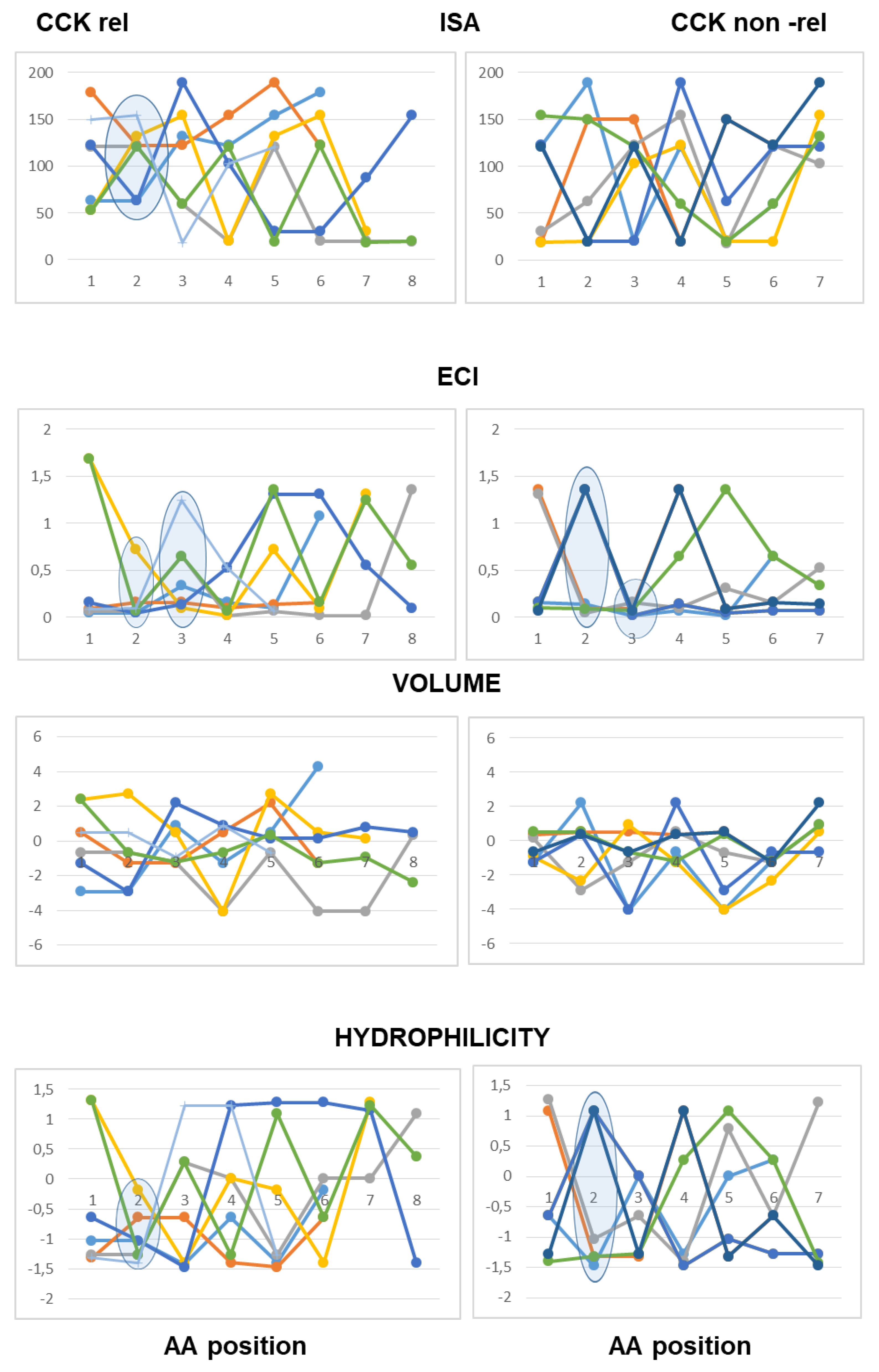
As to the peptides endowed with pI values lower than 6.0 (mildly acidic), the use of the Collantes and Dunn scalar descriptors highlighted some structural features found only in inactive peptide sequences: specifically, the occurrence of amino acids with very high or very low ISA values (out of the range of 60 to 130) at the second position from the N-terminus, and the occurrence of amino acids with high ECI values (higher than 0.72) at the second position joined with amino acids with very low ECI values (lower than 0.1) at the third position from the N-terminus. The use of the Barley scalar descriptors confirmed the relevance of the hydrophobic character of the side chain at position 2, in agreement with the previous scale, whereas it did not suggest a distinct trait related to the molecular size of active peptides versus inactive peptides (
Figure 4).
As to the acidic peptides (pI lower than 5.0), the comparison of the line graphs obtained using the Barley scalar descriptors suggested remarking on some peculiar traits that may contribute to differentiating active and inactive peptides. The volume values varied over a wider range in the inactive peptide sequences compared to the active peptide sequences. Specifically, the insertion of tiny amino acids (L-Gly, L-Ala and L-Ser) along the sequence was related to lack of CCK-releasing activity. Moreover, according to both scales of descriptors, the presence of amino acids with large hydrophobic side chains (aromatic or aliphatic) at position 2 from the N-terminal end was required to confer CCK-releasing activity, in that small side chains and/or hydrophilic side chains at the second position were seen only in inactive peptides. Of course, this trait was not enough to confer activity (
Figure 5).
As to basic peptides (pI higher than 8.0), four active peptide sequences were available versus seven inactive peptide sequences. The comparison of the line graphs obtained using both scales did not suggest any differential trait related to the hydrophobicity of the amino acid residues between the two groups, in that the scalar descriptor values varied over a wide range from position 1 to position 5 in both active and inactive peptide sequences. On the other hand, the Barley’s volume scalar descriptor varied over a narrow range at positions 2, 3, 4 and 5 of the active peptides, so that the line graphs suggested a peculiar structural feature linked to the molecular size of the amino acid side chains not seen in the inactive peptides: specifically, large amino acid side chains at both positions 2 and 3 followed by small or tiny amino acids at both positions 4 and 5. Finally, as to the Collantes and Dunn scale, the trend of ECI values along the amino acid sequence suggested that a high charge concentration of amino acid side chains at positions 1, 2 and 3 was a preferential character of active peptides, whereas a high charge concentration at positions 4 and 5 was related to a lack of activity. In other words, the presence of L-Arg (not so L-Lys), at or near the N-terminal end may be favorable to CCK-releasing activity, whereas L-Arg near to the C-terminal end makes the peptide basic, but it is unfavorable to CCK-releasing activity (
Figure 6).
3. Discussion
The knowledge of the structural features underlying the secretagogue effect of protein digestion products on enteroendocrine cells in the gut may help to predict the effects of distinct dietary proteins on this branch of the hormonal control of food intake and energy homeostasis, starting from the current availability of a number of Peptidomics studies with long lists of peptides found in gastrointestinal digestion or among the products of in vitro simulated gastrointestinal digestion of food proteins as well as the availability of bioinformatic tools for the analysis of dietary protein-related full-length amino acid sequences [
20,
21,
22]. This knowledge is still limited, but it would help to design special foods and ingredients producing an adequate metabolic response.
In recent years, articles reporting on the isolation of dietary protein-derived peptide fragments that were able to induce CCK and/or GLP-1 release from enteroendocrine cells in vitro have accumulated. In some studies, the authors also tried to point out some distinctive structural features of these peptides related to their secretagogue activity and to elucidate which receptor might be involved. Briefly, after protein digestion in the gut, both free amino acids and peptides can induce the release of hormones from enteroendocrine cells. Actually, there is evidence supporting the prevailing role of peptidic fragments versus free amino acids, despite it still being under debate [
4,
5,
6,
7,
8,
9,
10,
11,
12,
13,
14,
15,
16]. This consideration does not diminish the role played by the free amino acid peak in the plasma after meals in the control of food intake, energy metabolism and anabolic and catabolic functions in tissues, i.e., in skeletal muscle tissue, through multiple direct and indirect actions [
3]. Focusing on peptides, the features related to the induction of CCK and/or GLP-1 release may include size, hydrophobicity, net charge, specific amino acid content and the position of the selected amino acid in the chain. It is important to remark that some features may be shared by both CCK- and GLP-1-releasing peptides, but some features may also be distinctive or have been associated with the release of one hormone in experimental studies and cannot be regarded as common to both classes of peptides [
1].
A great number of CCK-releasing peptide sequences have been published in the last two decades. If we might want to briefly summarize the conclusions drawn by the authors of related articles, possible distinctive structural features of CCK-releasing peptides include a peptide length in the range 4 to 11 amino acids, an isoelectric point higher than 6.8, high hydrophobicity, and a content of at least one aromatic amino acid. Actually, these characteristics should not be regarded as strictly necessary or, on the other hand, as enough to confer CCK-releasing activity to a peptide. Indeed, a few peptides containing alphatic amino acids in place of aromatic amino acids or peptides endowed with marked hydrophilicity or negative net charge were revealed to be active in vitro. Moreover, despite most of the studies agreeing that a major role is played by peptide size and peptide composition versus specific amino acid sequence motifs in conferring secretagogue activity, in a few studies the removal of one or more selected amino acids from the N-terminal or the C-terminal end of an active peptide was shown to cause a loss of activity without significantly altering the hydrophobicity index or the isoelectric point: specifically, the removal of the N-terminal L-Ser from SRYPS and the removal of YLE from the C-terminus of RYLGYLE [
8].
Now, the availability of published CCK-releasing peptide sequences identified and characterized by using quite similar in vitro experimental conditions has suggested the relevance of carrying out a comparative analysis of their primary structure and their physicochemical properties with the aim of finding structural features shared by the secretagogue peptides and, in addition, to highlight possible distinctive features of these peptides compared to peptides that were found to be completely ineffective in the same studies.
The outcome was the fractionation of the available CCK-releasing peptide sequences into distinct subsets according primarily to their size and then to their isoelectric point. Each subset was further analyzed to find the similarities, if any, in the degree of hydrophobicity/hydrophilicity, amino acid composition and amino acid sequence (referring to selected amino acids in selected positions in the chain) between peptides. In this way, for each subset we reported on a combination of structural features which can be regarded as related to CCK-releasing activity in vitro and, where appropriate, we also suggested a reference peptide. Actually, our analysis confirmed the heterogeneity of CCK-releasing peptides from food proteins in their primary structure and their physicochemical characteristics. The relatively lower occurrence of L-Glu and L-Gln in active versus inactive peptides was a striking and unexpected finding.
As mentioned in the Introduction, some factors may account for the heterogeneity of CCK-releasing peptides: i.e., the involvement of multiple distinct nutrient-sensing receptors in mediating the response of enteroendocrine cells to the products of the enzymatic digestion of proteins in both free amino acids and peptides; for each receptor, the possible occurrence of more binding sites for orthosteric and allosteric ligands, with the range of the effective concentrations of peptides as reported in the cited references suggesting low-affinity receptor–ligand interactions.
Before drawing a few conclusions, it is also worth remarking on some limits which are intrinsic to the in vitro research strategy underlying the identification of all the active and inactive peptides mentioned in this study. STC-1 cells are regarded as a valid tool for the investigation of the secretagogue activity of dietary-derived compounds on enteroendocrine cells. Nevertheless, these cells do not express all the receptors and transporters that are believed to play a role in the detection of amino acids and peptides by enteroendocrine cells in the gut wall—so-called nutrient-sensing receptors. Moreover, before being exposed to peptides in buffered balanced salt solution, the cells had long been cultured in growth medium (DMEM). According to the manufacturers, DMEM contains fifteen distinct amino acids, and their concentrations range from 0.1 mM to 0.9 mM except that of glutamine (Q) (over 2 mM). The medium lacks glutamic acid (E), aspartic acid (D), proline (P), alanine (A) and asparagine (N). It would be important to investigate whether the medium composition and the intracellular content of a given amino acid at time 0 may affect the cellular response following exposure to peptides differing in size (di- or tripeptides versus longer peptides) and to peptides endowed with differential amino acid compositions, i.e., high content of aliphatic amino acids or aromatic amino acids or L-glutamine and so on. Furthermore, the fate of a given peptide after being dissolved in aqueous culture medium may affect its biological activities in cells, including secretagogue activity. Peptides may differ in their tendency to form aggregates, from dimers to oligomers, in their low-affinity interactions with the cell surface and adhesion to culture plates and in their passive diffusion, if any, across the lipid bilayer. As to the peptide sequences reviewed in this work, we cannot discuss these issues. We might just add that the predicted hydrophobicity/hydrophilicity and the predicted solubility as defined in PROSO II do not seem to be major determinants in differentiating between CCK-releasing and non-CCK-releasing peptides in vitro.
In vitro analysis is aimed at investigating hormone secretion by enteroendocrine cells in response to direct nutrient sensing, including peptide sensing. In reality, multiple pathways cooperate in the regulation of hormone secretion in vivo. Products derived from food protein digestion can affect the enteroendocrine cell activity acting directly at the apical or basolateral membrane, but they may also play a role after absorption into circulation, acting through multiple indirect pathways as metabolic substrates and precursors of signaling molecules in different tissues. To this end, we have previously mentioned the role played by free amino acid peaks in the plasma after meals. In summary, the ingestion of food proteins is expected to affect the neuroendocrine network underlying energy homeostasis at multiple levels, but direct nutrient sensing may surely have a significant role in the short term.
In conclusion, the currently available set of CCK-releasing peptide sequences and the data on their in vitro activity as reported in the studies reviewed in the present work may not allow a quantitative structure–activity relationship analysis but are fine to use to perform a qualitative analysis of their primary structure and to predict their physicochemical properties using bioinformatics tools. To this end, a method was applied consisting of the fractionation of peptides into subsets and the comparison between paired subsets of active and inactive peptides. For each subset, a few distinctive structural features related to CCK-releasing activity were highlighted. Additionally, the analysis provided evidence that it may be that minor changes in the primary structure make up the difference between active and inactive peptides, as previously suggested by studies addressing the effects of one amino acid change or deletion on CCK- and GLP-1-releasing activity [
8]. Hence, the chance to predict the activity of a peptide never tested in vitro using reference structures has to still be considered to be low. Nevertheless, the identification of primary sequence elements related to receptor-mediated biological activity may also be the base for further steps forward addressing the three-dimensional structure of active peptides in molecular docking simulations. This kind of analysis is beyond the aim of the present study, but it could lead to a better understanding of which amino acid changes can be permitted in terms of secretagogue activity. Actually, it is worth remarking that predicting the well-defined spatial structure of short peptides like those included in the third subset of CCK-releasing peptides may be challenging.
Current research on bioactive peptides encrypted in dietary protein sequences aims to exploit the possible functional properties of specific protein sources with different outcomes, including recommendations for healthy diets, development of functional foods and novel dietary supplements with health-related benefits and the development of pharmaceuticals. Success in this applied research field basically depends on technologies enabling large-scale peptide production and delivery systems and increasing peptide bioavailability after oral administration. Current chemical and biotechnological strategies for peptide synthesis and delivery are beyond the aim of this work, and some references may be found elsewhere [
4,
23]. Briefly, three primary methods are currently used for peptide production: chemical synthesis; enzymatic digestion of proteins using plant, microbial or animal proteases and DNA recombinant technologies. As to improving peptide bioavailability in the gastrointestinal tract, some strategies have been suggested and investigated, including peptide encapsulation and the addition of enhancers for permeation across the intestinal epithelial layer. Focusing on dietary recommendations and claims of health-related benefits of a given food, it is worth remarking that the processing technologies applied to food (i.e., heating techniques in dairy industry), may significantly vary the release of bioactive compounds from a given protein compared to what is expected based on its native structure by altering the digestion rate and the protein susceptibility to proteases or inducing reactions which involve the amino acid side chains. Finally, characterization of the in vitro activity of peptides, peptide isolation and sequencing and bioinformatic analysis of primary peptide structures are necessary steps to conduct applied research in the bioactive peptide field. Then, once peptides of interest have been identified, a more accurate determination of their physicochemical properties by in vitro biochemical analysis is mandatory to optimize large-scale production and delivery. To this end, peptide aggregation and peptide solubility, along with peptide stability, are often a major challenge in peptide application and are difficult to predict. At present, the biochemical characterization of the CCK-releasing peptides discussed in this article was beyond the aim of the study.