Hematological Biomarkers for Early Detection of Lung Cancer: Evaluating the Diagnostic Potential of Circulating Interleukin Levels
Abstract
1. Introduction
2. Results
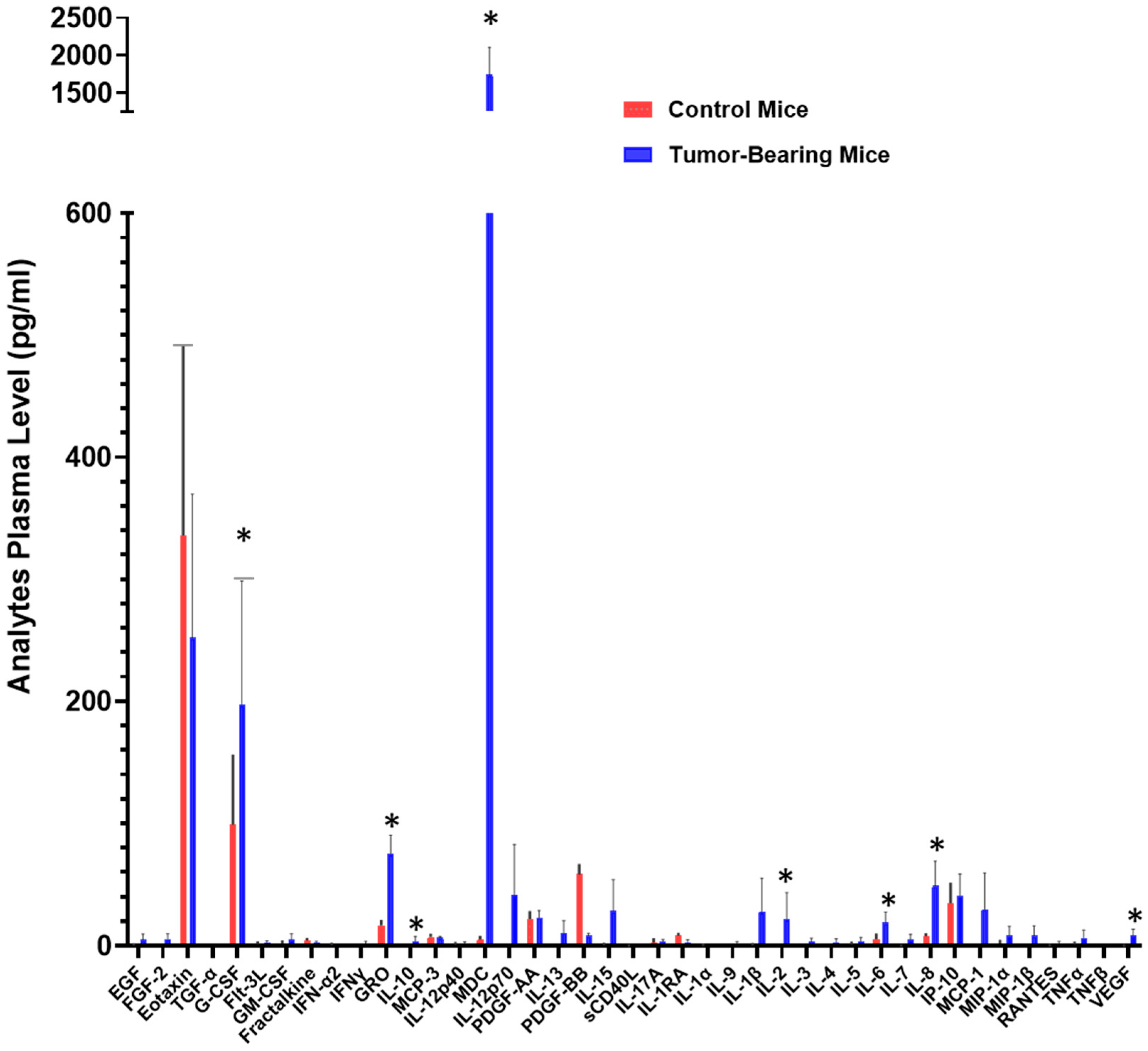
2.1. Circulating Signaling Protein Blood Levels Are Increased in a Preclinical Human NSCLC Xenograft Model
2.2. Interleukin Plasma Levels Are Significantly Elevated in Early-Stage NSCLC Patients
2.3. Comparable Plasma Levels of IL-1A, IL-6, IL-8, IL-10, and IL-17A Between Adenocarcinomas and Squamous Cell Carcinoma, the 2 Major NSCLC Pathological Subtypes of NSCLC
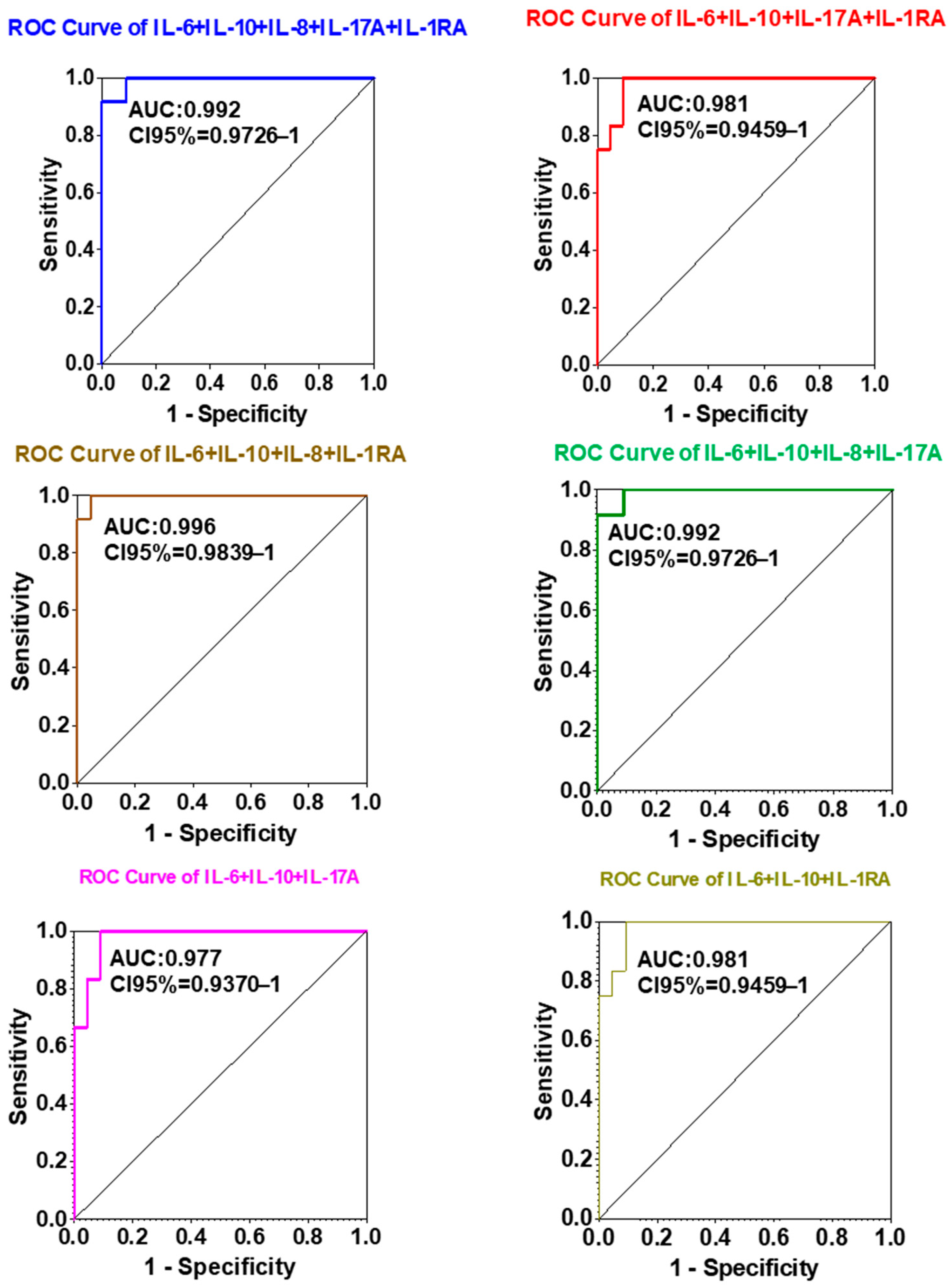
2.4. Diagnostic Efficacy of IL-1A, IL-6, IL-8, IL-10, and IL-17A Expression in Lung Cancer
3. Discussion
Study Limitations
4. Materials and Methods
4.1. Plasma Samples
4.2. Xenograft Mouse Models for Plasma Collection
4.3. Multiplex Cytokine Quantification
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AUC | area under the ROC curve |
| COPD | chronic obstructive pulmonary disease |
| LDCT | low-dose computed tomography |
| ctDNA | circulating tumor DNA |
| ELISA | Enzyme-Linked Immuno Assay |
| NSCLC | non-small cell lung cancer |
| ROC | Receiving operating characteristic |
References
- Yang, D.; Liu, Y.; Bai, C.; Wang, X.; Powell, C.A. Epidemiology of Lung Cancer and Lung Cancer Screening Programs in China and the United States. Cancer Lett. 2020, 468, 82–87. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Dizon, D.S.; Kamal, A.H. Cancer Statistics 2024: All Hands on Deck. CA Cancer J. Clin. 2024, 74, 8–9. [Google Scholar] [CrossRef]
- Nierengarten, M.B. Cancer Statistics 2024: Deaths Drop, Incidences Increase, Prevention Needed. Cancer 2024, 130, 1904. [Google Scholar] [CrossRef]
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer Statistics, 2024. CA Cancer J. Clin. 2024, 74, 12–49, https://doi.org/10.3322/caac.21820. Erratum in CA Cancer J. Clin. 2024, 74, 203. [Google Scholar] [CrossRef]
- Lococo, F.; Chiappetta, M.; Cesario, A.; Margaritora, S. Non-Small-Cell Lung Cancer with Pathological Complete Response after Induction Therapy Followed by Surgical Resection: Which Is the Pattern of Failure and Which Are the Future Perspectives? Eur. J. Cardio-Thorac. Surg. 2020, 58, 407. [Google Scholar] [CrossRef]
- Pfannschmidt, J.; Muley, T.; Hoffmann, H.; Bülzebruck, H.; Dienemann, H. Prognosis after Complete Surgical Resection for Non-Small Cell Lung Cancer Based on the Staging Classification. Dtsch. Med. Wochenschr. 2006, 131, 2643–2648. [Google Scholar] [CrossRef]
- Roszkowski-Śliż, K. Adjuvant radiotherapy after complete surgical resection in non-small cell lung cancer. Pneumonol. Alergol. Pol. 2012, 80, 95–98. [Google Scholar]
- Nguyen, D.; Gharagozloo, F.; Tempesta, B.; Meyer, M.; Gruessner, A. Long-Term Results of Robotic Anatomical Segmentectomy for Early-Stage Non-Small-Cell Lung Cancer. Eur. J. Cardio-Thorac. Surg. 2019, 55, 427–433. [Google Scholar] [CrossRef]
- Baran, K.; Waśko, J.; Kryczka, J.; Boncela, J.; Jabłoński, S.; Kolesińska, B.; Brzeziańska-Lasota, E.; Kordiak, J. The Comparison of Serum Exosome Protein Profile in Diagnosis of NSCLC Patients. Int. J. Mol. Sci. 2023, 24, 13669. [Google Scholar] [CrossRef]
- Ohara, S.; Suda, K.; Tsutani, Y. Utility and Future Perspectives of Circulating Tumor DNA Analysis in Non-Small Cell Lung Cancer Patients in the Era of Perioperative Chemo-Immunotherapy. Cells 2025, 14, 1312. [Google Scholar] [CrossRef]
- Lamorte, D.; De Luca, L.; Tartarone, A.; Trino, S.; Giulivo, I.; De Stradis, A.; Maietti, M.; Caivano, A.; Laurenzana, I. Serum Extracellular Vesicle microRNAs as Potential Biomarkers to Predict Pembrolizumab Response and Prognosis in Metastatic Non-Small Cell Lung Cancer Patients. Front. Immunol. 2025, 16, 1540906. [Google Scholar] [CrossRef]
- Aries, A.; Drénou, B.; Lahlil, R. Liquid Biopsy and Epigenetic Signatures in AML, ALL, and CNS Tumors: Diagnostic and Monitoring Perspectives. Int. J. Mol. Sci. 2025, 26, 7547. [Google Scholar] [CrossRef]
- Nikanjam, M.; Kato, S.; Kurzrock, R. Liquid Biopsy: Current Technology and Clinical Applications. J. Hematol. Oncol. 2022, 15, 131. [Google Scholar] [CrossRef]
- Ding, K.; Yi, M.; Li, L.; Zhang, Y. Interleukin Polymorphisms and Protein Levels Associated with Lung Cancer Susceptibility and Phenotypes. Expert Rev. Clin. Immunol. 2021, 17, 1029–1040. [Google Scholar] [CrossRef]
- Kaanane, H.; Senhaji, N.; Berradi, H.; Benchakroun, N.; Benider, A.; Karkouri, M.; El Attar, H.; Flores, O.; Khyatti, M.; Nadifi, S. The Influence of Interleukin-6, Interleukin-8, Interleukin-10, Interleukin-17, TNF-A, MIF, STAT3 on Lung Cancer Risk in Moroccan Population. Cytokine 2022, 151, 155806. [Google Scholar] [CrossRef]
- Vahl, J.M.; Friedrich, J.; Mittler, S.; Trump, S.; Heim, L.; Kachler, K.; Balabko, L.; Fuhrich, N.; Geppert, C.-I.; Trufa, D.I.; et al. Interleukin-10-Regulated Tumour Tolerance in Non-Small Cell Lung Cancer. Br. J. Cancer 2017, 117, 1644–1655. [Google Scholar] [CrossRef]
- Granville, C.A.; Dennis, P.A. An Overview of Lung Cancer Genomics and Proteomics. Am. J. Respir. Cell Mol. Biol. 2005, 32, 169–176. [Google Scholar] [CrossRef]
- Mulshine, J.L.; Sullivan, D.C. Clinical Practice. Lung cancer screening. N. Engl. J. Med. 2005, 352, 2714–2720. [Google Scholar] [CrossRef]
- Cai, D.; Xu, Y.; Ding, R.; Qiu, K.; Zhang, R.; Wang, H.; Huang, L.; Xie, X.; Yan, H.; Deng, Y.; et al. Extensive Serum Biomarker Analysis in Patients with Non-Small-Cell Lung Carcinoma. Cytokine 2020, 126, 154868. [Google Scholar] [CrossRef]
- Li, F.; Cao, Y.; Li, J.; Gao, C.; Dong, X.; Ren, P.; Meng, C.; Chen, C. The Clinical Significance of Serum Adipocytokines Level in Patients with Lung Cancer. J. Thorac. Dis. 2019, 11, 3547–3555. [Google Scholar] [CrossRef]
- Xie, H.; Chen, J.; Lv, X.; Zhang, L.; Wu, J.; Ge, X.; Yang, Q.; Zhang, D.; Chen, J. Clinical Value of Serum and Exhaled Breath Condensate miR-186 and IL-1beta Levels in Non-Small Cell Lung Cancer. Technol. Cancer Res. Treat. 2020, 19, 1533033820947490. [Google Scholar] [CrossRef]
- Yan, X.; Han, L.; Zhao, R.; Fatima, S.; Zhao, L.; Gao, F. Prognosis Value of IL-6, IL-8, and IL-1beta in Serum of Patients with Lung Cancer: A Fresh Look at Interleukins as a Biomarker. Heliyon 2022, 8, e09953. [Google Scholar] [CrossRef]
- Zhou, J.; Diao, X.; Wang, S.; Yao, Y. Diagnosis Value of Combined Detection of Serum SF, CEA and CRP in Non-Small Cell Lung Cancer. Cancer Manag. Res. 2020, 12, 8813–8819. [Google Scholar] [CrossRef]
- Guo, M.; Jeon, W.J.; Joung, B.; Tai, D.; Gavralidis, A.; Elliott, A.; Baca, Y.; de Semir, D.; Liu, S.V.; Reeves, M.; et al. Prognostic Significance and Emerging Predictive Potential of Interleukin-1β Expression in Oncogene-Driven NSCLC. Cancers 2025, 17, 2895. [Google Scholar] [CrossRef]
- Niu, K.; Tang, J.; Zhong, J.; Zheng, L.; Zhu, Y.; Ling, H.; Liu, Y.; Xu, Q.; Qiu, P.; Chen, L. Connection between Circulating Inflammatory Cytokines and Non-Small Cell Lung Cancer: Integrated Mendelian Randomization and Gene Function Analysis. Discov. Oncol. 2025, 16, 1005. [Google Scholar] [CrossRef]
- Fishbein, A.; Wang, W.; Yang, H.; Yang, J.; Hallisey, V.M.; Deng, J.; Verheul, S.M.L.; Hwang, S.H.; Gartung, A.; Wang, Y.; et al. Resolution of Eicosanoid/Cytokine Storm Prevents Carcinogen and Inflammation-Initiated Hepatocellular Cancer Progression. Proc. Natl. Acad. Sci. USA 2020, 117, 21576–21587. [Google Scholar] [CrossRef]
- Sandfeld-Paulsen, B.; Meldgaard, P.; Sorensen, B.S.; Safwat, A.; Aggerholm-Pedersen, N. The Prognostic Role of Inflammation-Scores on Overall Survival in Lung Cancer Patients. Acta Oncol. 2019, 58, 371–376. [Google Scholar] [CrossRef]
- Winther-Larsen, A.; Aggerholm-Pedersen, N.; Sandfeld-Paulsen, B. Inflammation Scores as Prognostic Biomarkers in Small Cell Lung Cancer: A Systematic Review and Meta-Analysis. Syst. Rev. 2021, 10, 40. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, J.; Zhang, Q.; Zhang, J.; Lou, Y.; Yang, J.; Chen, Y.; Wei, T.; Zhang, J.; Fu, Q.; et al. Tumour Cell-Derived Debris and IgG Synergistically Promote Metastasis of Pancreatic Cancer by Inducing Inflammation via Tumour-Associated Macrophages. Br. J. Cancer 2019, 121, 786–795. [Google Scholar] [CrossRef]
- Mantovani, A.; Barajon, I.; Garlanda, C. IL-1 and IL-1 Regulatory Pathways in Cancer Progression and Therapy. Immunol. Rev. 2018, 281, 57–61. [Google Scholar] [CrossRef]
- Yigit, M.; Degirmencioglu, S.; Ugurlu, E.; Yaren, A. Effect of Serum Interleukin-1 Receptor Antagonist Level on Survival of Patients with Non-Small Cell Lung Cancer. Mol. Clin. Oncol. 2017, 6, 708–712. [Google Scholar] [CrossRef]
- Kuen, D.S.; Kim, B.S.; Chung, Y. IL-17-Producing Cells in Tumor Immunity: Friends or Foes? Immune Netw. 2020, 20, e6. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Liu, R.; Wei, C.; Li, D.; Gao, X. The Role of IL-17 in Lung Cancer Growth. Cytokine 2023, 169, 156265. [Google Scholar] [CrossRef]
- Raskova, M.; Lacina, L.; Kejik, Z.; Venhauerová, A.; Skaličková, M.; Kolář, M.; Jakubek, M.; Rosel, D.; Smetana, K.; Brábek, J. The Role of IL-6 in Cancer Cell Invasiveness and Metastasis-Overview and Therapeutic Opportunities. Cells 2022, 11, 3698. [Google Scholar] [CrossRef]
- Pine, S.R.; Mechanic, L.E.; Enewold, L.; Chaturvedi, A.K.; Katki, H.A.; Zheng, Y.-L.; Bowman, E.D.; Engels, E.A.; Caporaso, N.E.; Harris, C.C. Increased Levels of Circulating Interleukin 6, Interleukin 8, C-Reactive Protein, and Risk of Lung Cancer. J. Natl. Cancer Inst. 2011, 103, 1112–1122. [Google Scholar] [CrossRef]
- Guo, Y.; Xu, F.; Lu, T.; Duan, Z.; Zhang, Z. Interleukin-6 Signaling Pathway in Targeted Therapy for Cancer. Cancer Treat. Rev. 2012, 38, 904–910. [Google Scholar] [CrossRef]
- Alfaro, C.; Sanmamed, M.F.; Rodriguez-Ruiz, M.E.; Teijeira, Á.; Oñate, C.; González, Á.; Ponz, M.; Schalper, K.A.; Pérez-Gracia, J.L.; Melero, I. Interleukin-8 in Cancer Pathogenesis, Treatment and Follow-Up. Cancer Treat. Rev. 2017, 60, 24–31. [Google Scholar] [CrossRef]
- Fousek, K.; Horn, L.A.; Palena, C. Interleukin-8: A Chemokine at the Intersection of Cancer Plasticity, Angiogenesis, and Immune Suppression. Pharmacol. Ther. 2021, 219, 107692. [Google Scholar] [CrossRef]
- Fernando, R.I.; Hamilton, D.H.; Dominguez, C.; David, J.M.; McCampbell, K.K.; Palena, C. IL-8 Signaling Is Involved in Resistance of Lung Carcinoma Cells to Erlotinib. Oncotarget 2016, 7, 42031–42044. [Google Scholar] [CrossRef]
- Zhu, Y.M.; Webster, S.J.; Flower, D.; Woll, P.J. Interleukin-8/CXCL8 Is a Growth Factor for Human Lung Cancer Cells. Br. J. Cancer 2004, 91, 1970–1976. [Google Scholar] [CrossRef] [PubMed]


| Characteristics | NSCLC (n = 22) | COPD (n = 6) | Normal (n = 12) |
|---|---|---|---|
| Age (years) | 72 (61, 84) | 64 (62, 67) | 63 (60, 69) |
| Gender | |||
| Male | 11 | 3 | 8 |
| Female | 11 | 3 | 4 |
| Pathological types | |||
| Squamous cell carcinoma | 11 | - | - |
| Adenocarcinoma | 11 | - | - |
| Clinical stage | |||
| Stage IA | 11 | - | - |
| Stage IB | 7 | - | - |
| Stage IIA | 4 | - | - |
| Pathological Types | n | IL-1RA | IL-6 | IL-8 | IL-10 | IL-17A |
|---|---|---|---|---|---|---|
| Squamous cell carcinoma | 11 | 38.35 ± 10.41 | 31.75 ± 12.20 | 52.87 ± 23.06 | 65.71 ± 30.31 | 64.91 ± 40.72 |
| Adenocarcinoma | 11 | 128.00 ± 62.04 | 20.14 ± 6.17 | 49.08 ± 26.73 | 86.66 ± 31.02 | 28.32 ± 9.49 |
| p-value | 0.20 | 0.41 | 0.92 | 0.72 | 0.40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morjani, O.; Yang, Y.-W.; Lahlil, R.; Lakhiari, H.; Alaoui, H. Hematological Biomarkers for Early Detection of Lung Cancer: Evaluating the Diagnostic Potential of Circulating Interleukin Levels. Int. J. Mol. Sci. 2025, 26, 11014. https://doi.org/10.3390/ijms262211014
Morjani O, Yang Y-W, Lahlil R, Lakhiari H, Alaoui H. Hematological Biomarkers for Early Detection of Lung Cancer: Evaluating the Diagnostic Potential of Circulating Interleukin Levels. International Journal of Molecular Sciences. 2025; 26(22):11014. https://doi.org/10.3390/ijms262211014
Chicago/Turabian StyleMorjani, Ouafaa, Yi-Wei Yang, Rachid Lahlil, Hamid Lakhiari, and Hassan Alaoui. 2025. "Hematological Biomarkers for Early Detection of Lung Cancer: Evaluating the Diagnostic Potential of Circulating Interleukin Levels" International Journal of Molecular Sciences 26, no. 22: 11014. https://doi.org/10.3390/ijms262211014
APA StyleMorjani, O., Yang, Y.-W., Lahlil, R., Lakhiari, H., & Alaoui, H. (2025). Hematological Biomarkers for Early Detection of Lung Cancer: Evaluating the Diagnostic Potential of Circulating Interleukin Levels. International Journal of Molecular Sciences, 26(22), 11014. https://doi.org/10.3390/ijms262211014







