Repurposing Antimalarials for Oral Cancer: Selective Efficacy of Hydroxychloroquine on Gingival Squamous Cell Carcinoma
Abstract
1. Introduction
2. Results
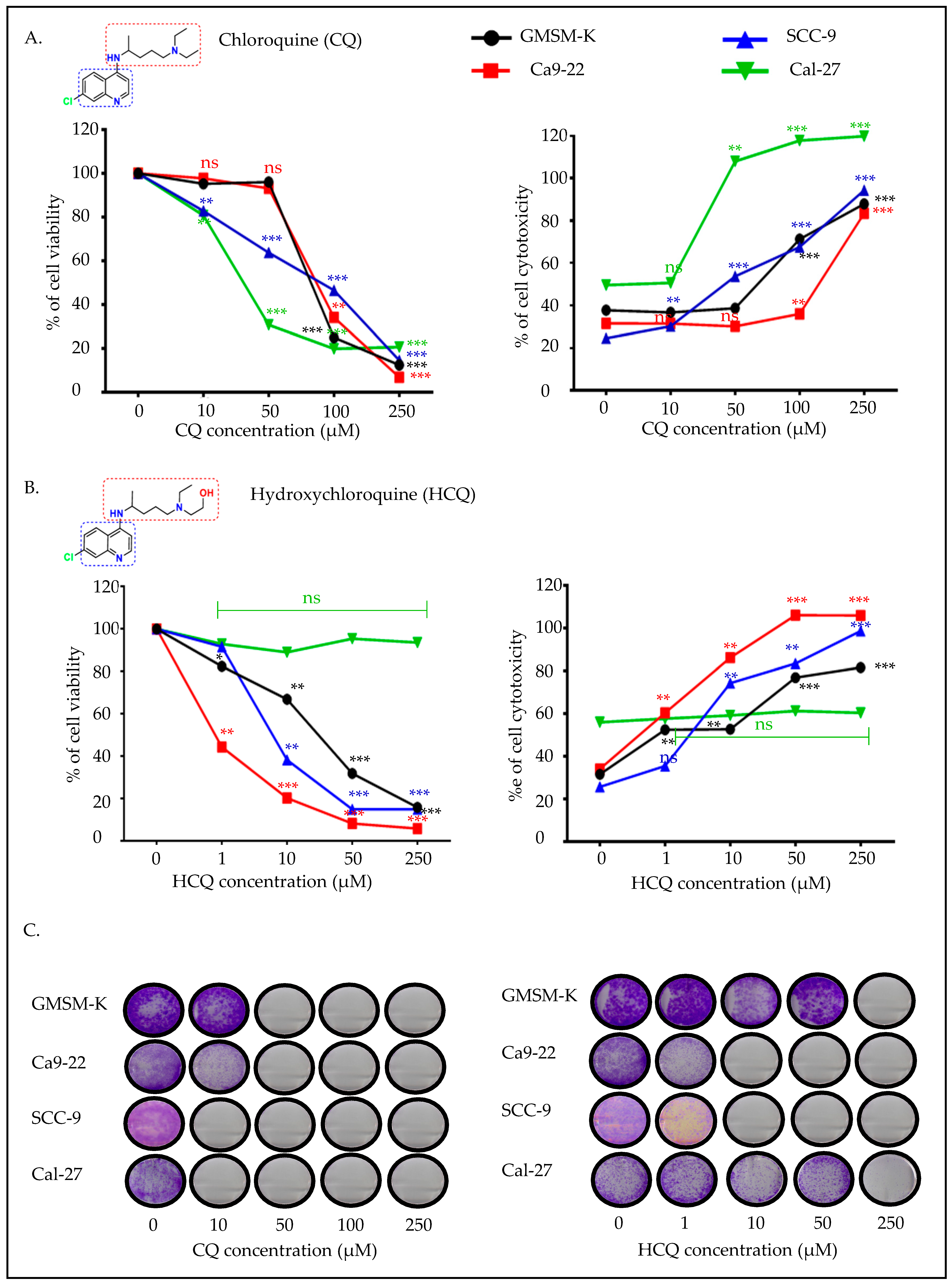
2.1. CQ Presents High Efficacy Against Tongue Carcinoma, While HCQ Is More Selective for Gingival Carcinoma
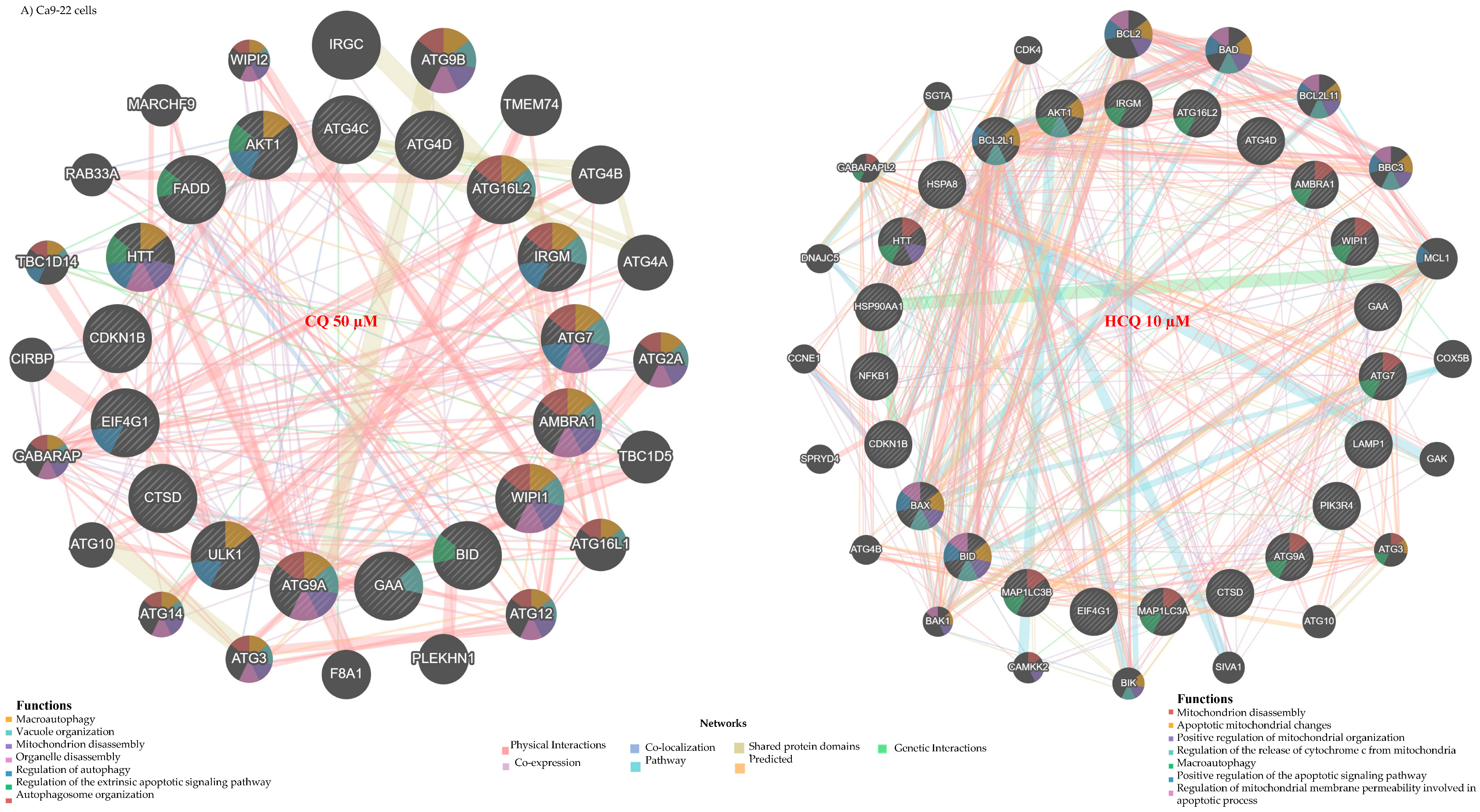
2.2. CQ and HCQ Inhibit Autophagy, with Gingival Carcinoma Being More Sensitive to HCQ than Tongue Carcinoma
2.3. CQ and HCQ Caused Moderate Accumulation of Autophagosomes, Accompanied by Up-Regulation of Autophagy-Related Genes, Indicating Compensatory Feedback Activation via TFEB
2.4. CQ and HCQ Reduce Mitochondrial Membrane Potential, with Gingival Carcinoma Being More Sensitive to HCQ than Tongue Carcinoma
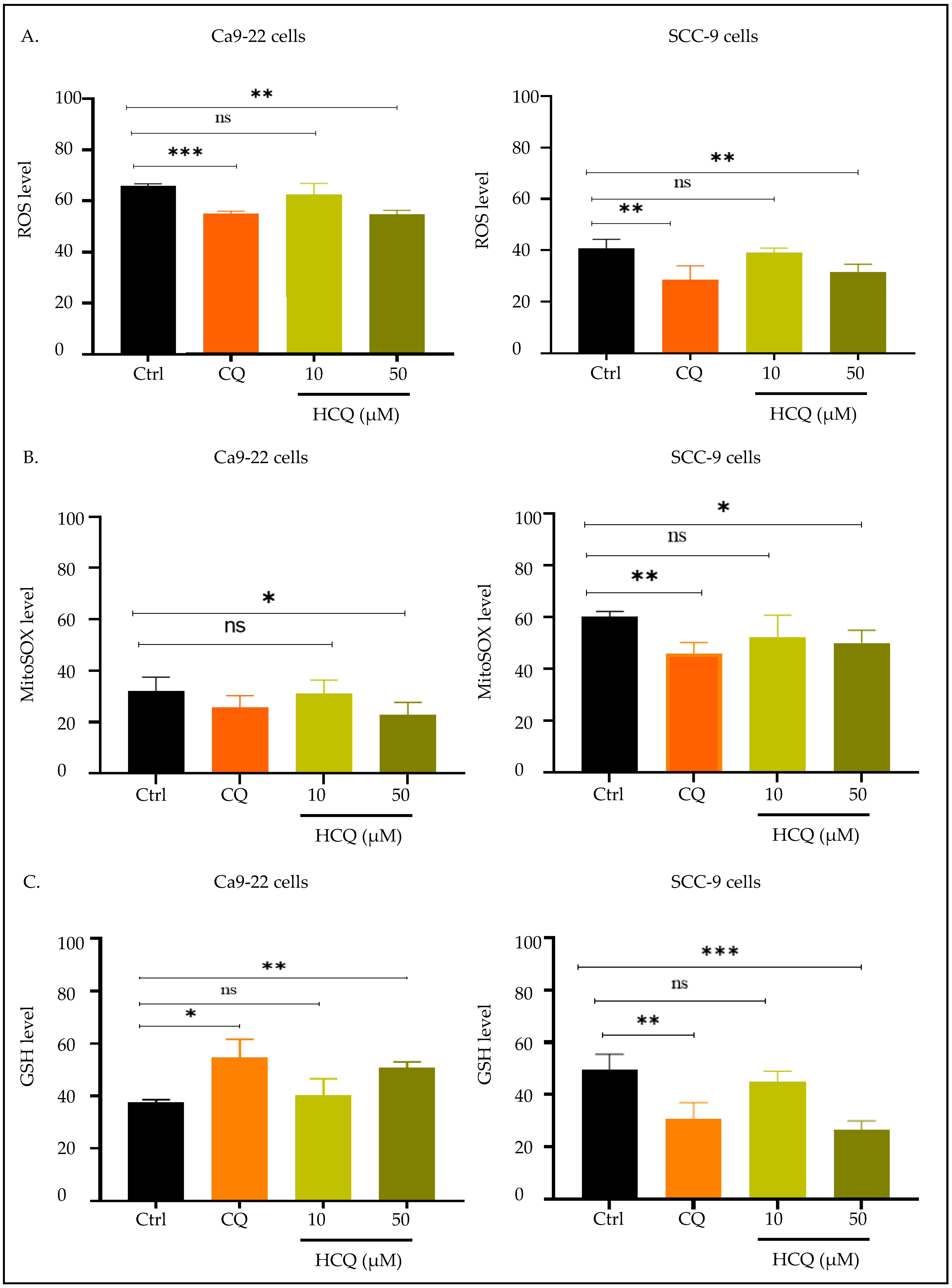
2.5. CQ and HCQ Modulate GSH Levels Differently Across Tongue and Gingival Cell Lines
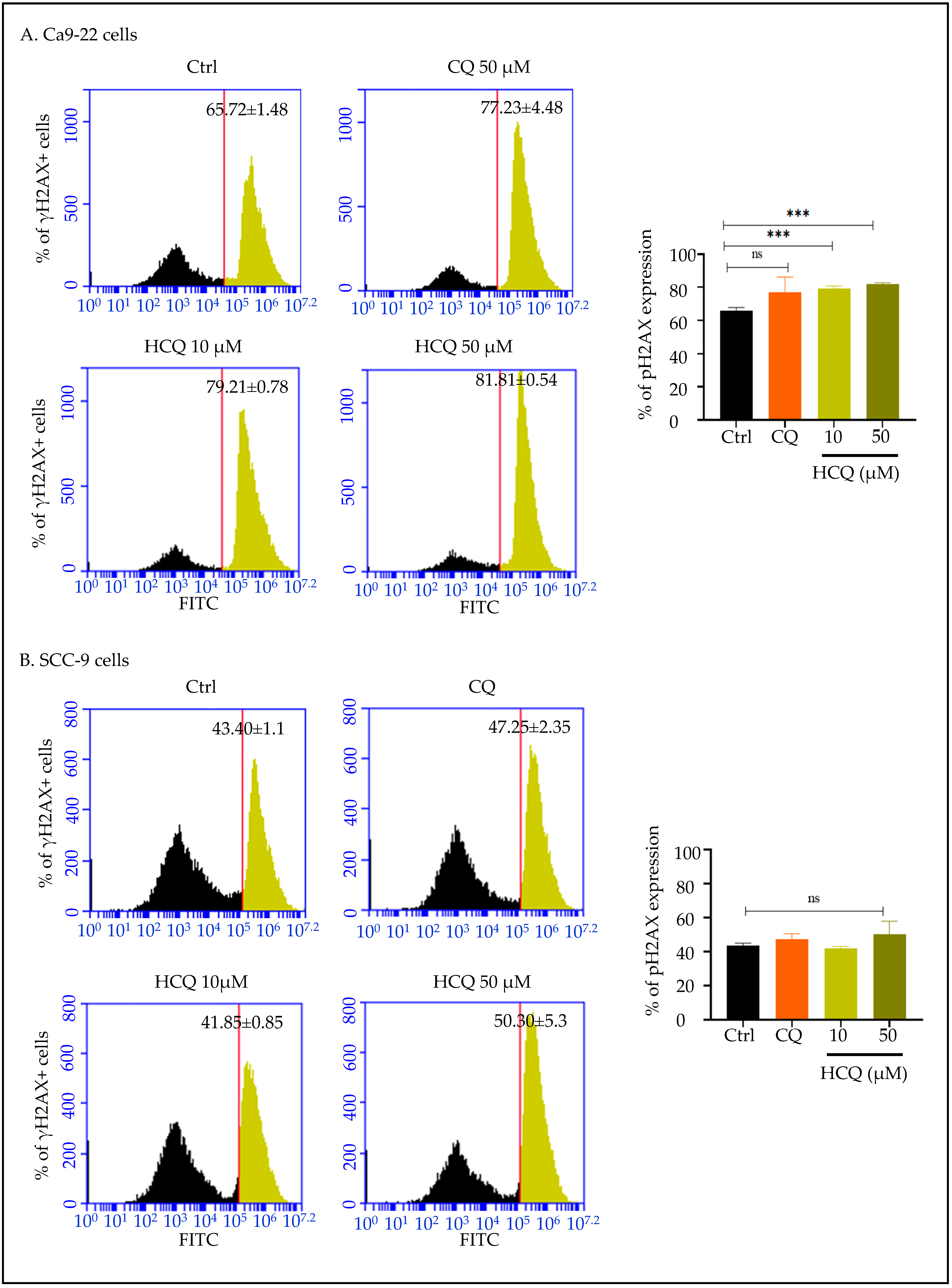
2.6. HCQ Triggers DNA Damage in Gingival Cells, with No Detectable Effect in Tongue Carcinoma
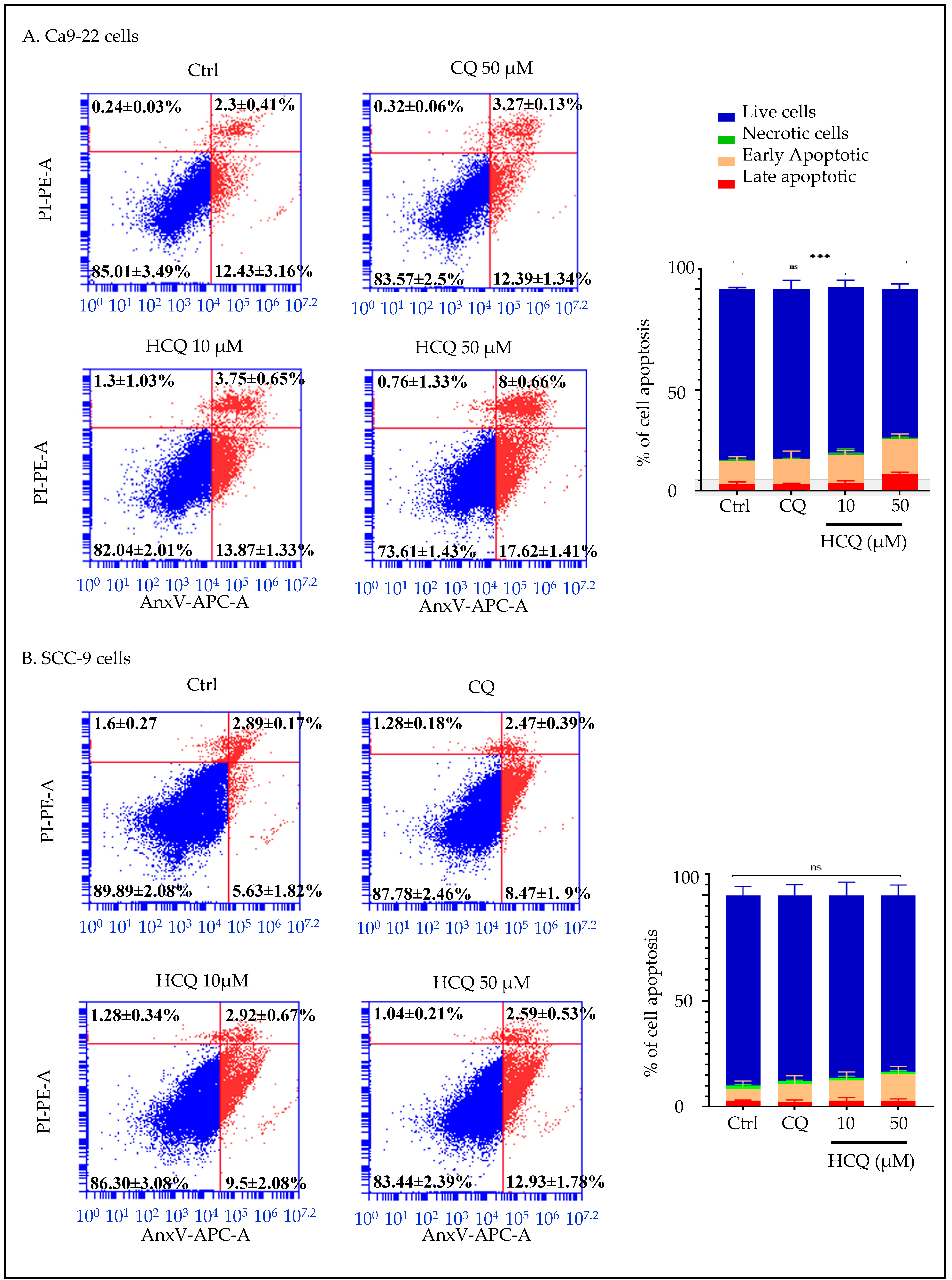
2.7. HCQ Selectively Induces Apoptosis in Human Gingival Carcinoma Cells
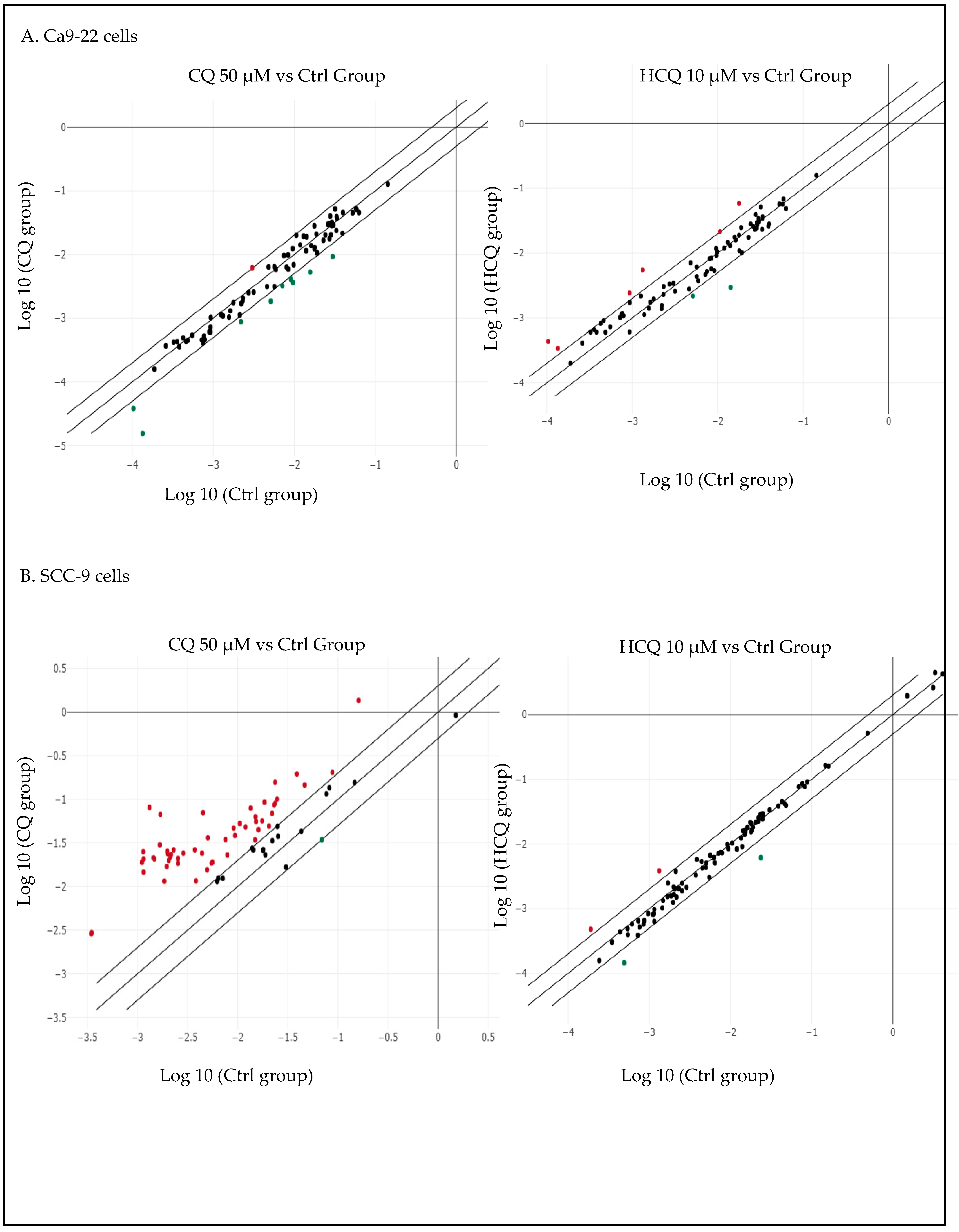
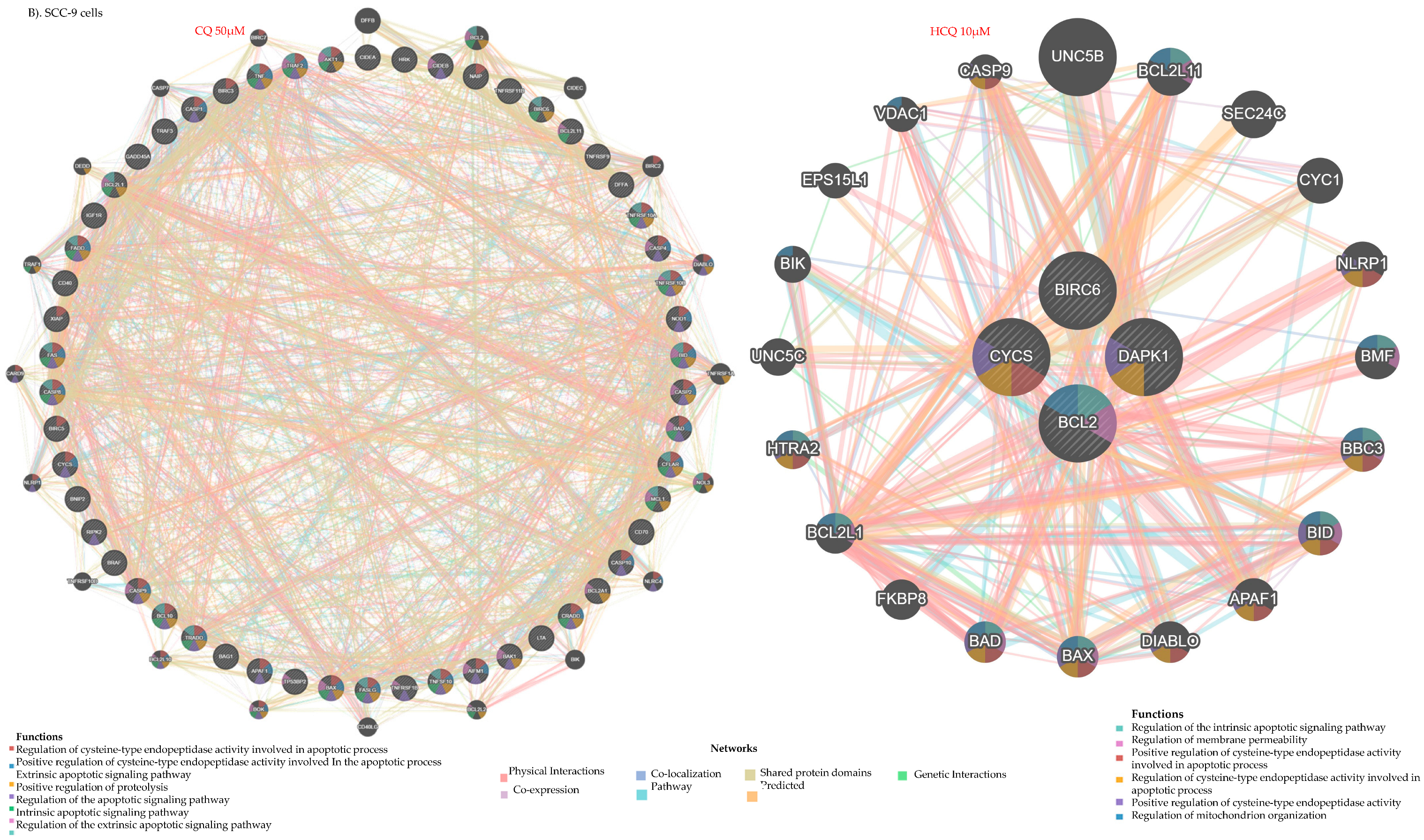
2.8. HCQ and CQ Differentially Modulate the Expression of Apoptosis-Related Genes Depending on the Type of OC Cells
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Treatment Regimen
4.3. MTT Cell Viability Assay
4.4. LDH Cell Cytotoxicity Assay
4.5. Clonogenic Test
4.6. Cell Autophagy Assay
4.7. Gene Expression Profiles and Interaction Networks
4.8. Mitochondrial Membrane Potential (∆Ψm) Assay
4.9. Oxidative Stress: Total ROS, MitoSOX, and GSH
4.10. DNA Damage Analysis (γH2AX Detection)
4.11. Cell Apoptosis Assay
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AnxV | Annexin V |
| Cal-27 | Human tongue carcinoma cell line |
| Ca9-22 | Human gingival squamous cell carcinoma line |
| CQ | Chloroquine |
| GMSM-K | Non-oncogenic oral epithelial cells |
| HCQ | Hydroxychloroquine |
| LDH | lactate dehydrogenase |
| MitoSOX | Red Mitochondrial Superoxide |
| OC | Oral cancer |
| OSCC | Oral squamous cell carcinoma |
| PI | Propidium Iodide |
| SCC-9 | Human tongue carcinoma cell line |
| 5-FU | 5-fluorouracil |
| Δψm | Mitochondrial membrane potential |
References
- Di Spirito, F.; Amato, A.; Romano, A.; Dipalma, G.; Xhajanka, E.; Baroni, A.; Serpico, R.; Inchingolo, F.; Contaldo, M. Analysis of Risk Factors of Oral Cancer and Periodontitis from a Sex- and Gender-Related Perspective: Gender Dentistry. Appl. Sci. 2022, 12, 9135. [Google Scholar] [CrossRef]
- Bray, F.; Laversanne, M.; Sung, H.Y.A.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Ca Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Kawecki, M.M.; Nedeva, I.R.; Iloya, J.; Macfarlane, T.V. Mouth Cancer Awareness in General Population: Results from Grampian Region of Scotland, United Kingdom. J. Oral Maxillofac. Res. 2019, 10, e3. [Google Scholar] [CrossRef]
- Gupta, B.; Bray, F.; Kumar, N.; Johnson, N.W. Associations between oral hygiene habits, diet, tobacco and alcohol and risk of oral cancer: A case-control study from India. Cancer Epidemiol. 2017, 51, 7–14. [Google Scholar] [CrossRef]
- Kijowska, J.; Grzegorczyk, J.; Gliwa, K.; Jedras, A.; Sitarz, M. Epidemiology, Diagnostics, and Therapy of Oral Cancer-Update Review. Cancers 2024, 16, 3156. [Google Scholar] [CrossRef]
- Lopez-Verdin, S.; Lavalle-Carrasco, J.; Carreon-Burciaga, R.G.; Serafin-Higuera, N.; Molina-Frechero, N.; Gonzalez-Gonzalez, R.; Bologna-Molina, R. Molecular Markers of Anticancer Drug Resistance in Head and Neck Squamous Cell Carcinoma: A Literature Review. Cancers 2018, 10, 376. [Google Scholar] [CrossRef]
- Johnson, D.E.; Burtness, B.; Leemans, C.R.; Lui, V.W.Y.; Bauman, J.E.; Grandis, J.R. Head and neck squamous cell carcinoma. Nat. Rev. Dis. Primers 2020, 6, 92. [Google Scholar]
- Rivera, C. Essentials of oral cancer. Int. J. Clin. Exp. Pathol. 2015, 8, 11884–11894. [Google Scholar]
- Muller, S.; Tilakaratne, W.M. Update from the 5th Edition of the World Health Organization Classification of Head and Neck Tumors: Tumours of the Oral Cavity and Mobile Tongue. Head Neck Pathol. 2022, 16, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Eskander, A.; Dziegielewski, P.T.; Patel, M.R.; Jethwa, A.R.; Pai, P.S.; Silver, N.L.; Sajisevi, M.; Sanabria, A.; Doweck, I.; Khariwala, S.S.; et al. Oral Cavity Cancer Surgical and Nodal Management: A Review From the American Head and Neck Society. JAMA Otolaryngol. Head Neck Surg. 2024, 150, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Yang, J.Y.; Lee, D.M.; Lee, J.Y.; Hwang, D.S.; Ryu, M.H.; Kim, U.K. Retrospective analysis on prognosis of oral cancer patients according to surgical approaches for effective cancer ablation: Swing approach versus visor approach. Maxillofac. Plast. Reconstr. Surg. 2024, 46, 15. [Google Scholar] [CrossRef]
- Nandini, D.B.; Rao, R.S.; Hosmani, J.; Khan, S.; Patil, S.; Awan, K.H. Novel therapies in the management of oral cancer: An update. Dis. Mon. 2020, 66, 101036. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.S.; Kim, Y.K.; Yun, P.Y. Cisplatin Plus Cetuximab Inhibits Cisplatin-Resistant Human Oral Squamous Cell Carcinoma Cell Migration and Proliferation but Does Not Enhance Apoptosis. Int. J. Mol. Sci. 2021, 22, 8167. [Google Scholar] [CrossRef] [PubMed]
- Krishnamurthy, S.; Ahmed, I.; Bhise, R.; Mohanti, B.K.; Sharma, A.; Rieckmann, T.; Paterson, C.; Bonomo, P. The dogma of Cetuximab and Radiotherapy in head and neck cancer—A dawn to dusk journey. Clin. Transl. Radiat. Oncol. 2022, 34, 75–81. [Google Scholar] [CrossRef]
- Naruse, T.; Yanamoto, S.; Matsushita, Y.; Sakamoto, Y.; Morishita, K.; Ohba, S.; Shiraishi, T.; Yamada, S.I.; Asahina, I.; Umeda, M. Cetuximab for the treatment of locally advanced and recurrent/metastatic oral cancer: An investigation of distant metastasis. Mol. Clin. Oncol. 2016, 5, 246–252. [Google Scholar] [CrossRef]
- Ju, W.T.; Liu, Y.; Wang, L.Z.; Li, J.; Ren, G.X.; Sun, J.; Tu, W.Y.; Hu, Y.J.; Ji, T.; Yang, W.J.; et al. Phase III trial of docetaxel cisplatin 5-fluorouracil induction chemotherapy for resectable oral cancer suggests favorable pathological response as a surrogate endpoint for good therapeutic outcome. Cancer Commun. 2021, 41, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Le, X.N.; Hanna, E.Y. Optimal regimen of cisplatin in squamous cell carcinoma of head and neck yet to be determined. Ann. Transl. Med. 2018, 6, 229. [Google Scholar] [CrossRef]
- Hartner, L. Chemotherapy for Oral Cancer. Dent. Clin. N. Am. 2018, 62, 87–97. [Google Scholar] [CrossRef]
- Longley, D.B.; Harkin, D.P.; Johnston, P.G. 5-Fluorouracil: Mechanisms of action and clinical strategies. Nat. Rev. Cancer 2003, 3, 330–338. [Google Scholar] [CrossRef]
- Tan, Y.H.; Wang, Z.H.; Xu, M.T.; Li, B.W.; Huang, Z.; Qin, S.Y.; Nice, E.C.; Tang, J.; Huang, C.H. Oral squamous cell carcinomas: State of the field and emerging directions. Int. J. Oral Sci. 2023, 15, 44. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, S.; Yue, K.; Wang, X.D. The recurrence and survival of oral squamous cell carcinoma: A report of 275 cases. Chin. J. Cancer 2013, 32, 614–618. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Sun, K.R.; Gan, R.H.; Yan, Y.X.; Zhang, C.C.; Zheng, D.L.; Lu, Y.G. WNT3 promotes chemoresistance to 5-Fluorouracil in oral squamous cell carcinoma via activating the canonical β-catenin pathway. BMC Cancer 2024, 24, 564. [Google Scholar] [CrossRef]
- Kucharski, D.J.; Jaszczak, M.K.; Boratynski, P.J. A Review of Modifications of Quinoline Antimalarials: Mefloquine and (hydroxy)Chloroquine. Molecules 2022, 27, 1003. [Google Scholar] [CrossRef]
- Axfors, C.; Schmitt, A.M.; Janiaud, P.; van’t Hooft, J.; Abd-Elsalam, S.; Abdo, E.F.; Abella, B.S.; Akram, J.; Amaravadi, R.K.; Angus, D.C.; et al. Mortality outcomes with hydroxychloroquine and chloroquine in COVID-19 from an international collaborative meta-analysis of randomized trials. Nat. Commun. 2021, 12, 2349, Erratum in Nat. Commun. 2012, 12, 3001. [Google Scholar] [CrossRef] [PubMed]
- Cortegiani, A.; Ingoglia, G.; Ippolito, M.; Giarratano, A.; Einav, S. A systematic review on the efficacy and safety of chloroquine for the treatment of COVID-19. J. Crit. Care 2020, 57, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.C.; Lin, J.K.; Chen, S.S.L. Inhibition of HIV-1 tat-mediated transactivation by quinacrine and chloroquine. Biochem. Biophys. Res. Commun. 1996, 226, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Martinez, G.P.; Zabaleta, M.E.; Di Giulio, C.; Charris, J.E.; Mijares, M.R. The Role of Chloroquine and Hydroxychloroquine in Immune Regulation and Diseases. Curr. Pharm. Des. 2020, 26, 4467–4485. [Google Scholar] [CrossRef]
- Martinson, J.A.; Montoya, C.J.; Usuga, X.; Ronquillo, R.; Landay, A.L.; Desai, S.N. Chloroquine Modulates HIV-1-Induced Plasmacytoid Dendritic Cell Alpha Interferon: Implication for T-Cell Activation. Antimicrob. Agents Chemother. 2010, 54, 871–881. [Google Scholar] [CrossRef]
- Park, T.Y.; Jang, Y.; Kim, W.; Shin, J.; Toh, H.T.; Kim, C.H.; Yoon, H.S.; Leblanc, P.; Kim, K.S. Chloroquine modulates inflammatory autoimmune responses through Nurr1 in autoimmune diseases. Sci. Rep. 2019, 9, 15559. [Google Scholar] [CrossRef]
- Al-Bari, M.A.A. Chloroquine analogues in drug discovery: New directions of uses, mechanisms of actions and toxic manifestations from malaria to multifarious diseases. J. Antimicrob. Chemother. 2015, 70, 1608–1621. [Google Scholar] [CrossRef]
- Degan, S.; May, B.L.; Jin, Y.J.; Ben Hammoda, M.; Sun, H.Y.; Zhang, G.Q.; Wang, Y.; Erdmann, D.; Warren, W.; Zhang, J.Y. Co-Treatment of Chloroquine and Trametinib Inhibits Melanoma Cell Proliferation and Decreases Immune Cell Infiltration. Front. Oncol. 2022, 12, 782877, Erratum in Front. Oncol. 2024, 14, 1418213. [Google Scholar] [CrossRef] [PubMed]
- Enzenmüller, S.; Gonzalez, P.; Debatin, K.M.; Fulda, S. Chloroquine overcomes resistance of lung carcinoma cells to the dual PI3K/mTOR inhibitor PI103 by lysosome-mediated apoptosis. Anti-Cancer Drugs 2013, 24, 14–19. [Google Scholar] [CrossRef]
- Lin, Y.C.; Lin, J.F.; Wen, S.I.; Yang, S.C.; Tsai, T.F.; Chen, H.E.; Chou, K.Y.; Hwang, T.I.S. Chloroquine and hydroxychloroquine inhibit bladder cancer cell growth by targeting basal autophagy and enhancing apoptosis. Kaohsiung J. Med. Sci. 2017, 33, 215–223. [Google Scholar] [CrossRef]
- Li, X.Y.; Zhou, Y.; Li, Y.S.; Yang, L.; Ma, Y.B.; Peng, X.Q.; Yang, S.; Liu, J.G.; Li, H.Y. Autophagy: A novel mechanism of chemoresistance in cancers. Biomed. Pharmacother. 2019, 119, 109415. [Google Scholar] [CrossRef]
- Yang, B.W.; Li, G.Z.; Wang, S.Q.; Zheng, Y.F.; Zhang, J.P.; Pan, B.; Wang, N.; Wang, Z.Y. Tumor-associated macrophages/C-X-C motif chemokine ligand 1 promotes breast cancer autophagy-mediated chemoresistance via IGF1R/STAT3/HMGB1 signaling. Cell Death Dis. 2024, 15, 743. [Google Scholar] [CrossRef]
- Yu, H.; Zhuang, J.T.; Zhou, Z.J.; Song, Q.; Lv, J.C.; Yang, X.; Yang, H.W.; Lu, Q. METTL16 suppressed the proliferation and cisplatin-chemoresistance of bladder cancer by degrading PMEPA1 mRNA in a m6A manner through autophagy pathway. Int. J. Biol. Sci. 2024, 20, 1471–1491. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.; Pu, H.J.; Zhang, X.; Yang, Q.; Wang, W.M.; Li, W.L.; Li, Z.Y. CLMP increases 5-fluorouracil sensitivity in colorectal cancer through the inhibition of autophagy. Tissue Cell 2025, 93, 102771. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Jiang, C.W.; Zhang, W.L.; Yang, Z.Y.; Sun, G. Targeting oncogenic MAGEA6 sensitizes triple negative breast cancer to doxorubicin through its autophagy and ferroptosis by stabling AMPKα1. Cell Death Discov. 2024, 10, 430. [Google Scholar] [CrossRef]
- Chen, H.E.; Lin, J.F.; Lin, Y.C.; Wen, S.I.; Yang, S.C.; Tsai, T.F.; Chou, K.Y.; Hwang, I.S.T. Chloroquine induces lysosomal membrane permeability-mediated cell death in bladder cancer cells. Formos. J. Surg. 2018, 51, 133–141. [Google Scholar] [CrossRef]
- Cocco, S.; Leone, A.; Roca, M.S.; Lombardi, R.; Piezzo, M.; Caputo, R.; Ciardiello, C.; Costantini, S.; Bruzzese, F.; Sisalli, M.J.; et al. Inhibition of autophagy by chloroquine prevents resistance to PI3K/AKT inhibitors and potentiates their antitumor effect in combination with paclitaxel in triple negative breast cancer models. J. Transl. Med. 2022, 20, 290. [Google Scholar] [CrossRef]
- Loehberg, C.R.; Strissel, P.L.; Dittrich, R.; Strick, R.; Dittmer, J.; Dittmer, A.; Fabry, B.; Kalender, W.A.; Koch, T.; Wachter, D.L.; et al. Akt and p53 are potential mediators of reduced mammary tumor growth by Chloroquine and the mTOR inhibitor RAD001. Biochem. Pharmacol. 2012, 83, 480–488. [Google Scholar] [CrossRef]
- Wang, D.; Li, Y.G.; Chang, W.L.; Feng, M.N.; Yang, Y.M.; Zhu, X.X.; Liu, Z.B.; Fu, Y. CircSEC24B activates autophagy and induces chemoresistance of colorectal cancer via OTUB1-mediated deubiquitination of SRPX2. Cell Death Dis. 2024, 15, 693. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.L.; Yuan, L.; Tang, Y.C.; Xu, Z.Y.; Xu, H.D.; Cheng, X.D.; Qin, J.J. The Role of Autophagy in Gastric Cancer Chemoresistance: Friend or Foe? Front. Cell Dev. Biol. 2020, 8, 621428. [Google Scholar] [CrossRef]
- Liu, J.; Cao, R.Y.; Xu, M.Y.; Wang, X.; Zhang, H.Y.; Hu, H.R.; Li, Y.F.; Hu, Z.H.; Zhong, W.; Wang, M.L. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov. 2020, 6, 16. [Google Scholar] [CrossRef]
- Ren, L.; Xu, W.; Overton, J.L.; Yu, S.D.; Chiamvimonvat, N.; Thai, P.N. Assessment of Chloroquine and Hydroxychloroquine Safety Profiles: A Systematic Review and Meta-Analysis. Front. Pharmacol. 2020, 11, 562777. [Google Scholar] [CrossRef]
- Yang, J.L.; Guo, Z.Y.; Liu, X.; Liu, Q.; Wu, M.; Yao, X.T.; Liu, Y.; Cui, C.; Li, H.Y.; Song, C.L.; et al. Cytotoxicity Evaluation of Chloroquine and Hydroxychloroquine in Multiple Cell Lines and Tissues by Dynamic Imaging System and Physiologically Based Pharmacokinetic Model. Front. Pharmacol. 2020, 11, 574720. [Google Scholar] [CrossRef]
- Dragowska, W.H.; Weppler, S.A.; Wang, J.C.; Wong, L.Y.; Kapanen, A.I.; Rawji, J.S.; Warburton, C.; Qadir, M.A.; Donohue, E.; Roberge, M.; et al. Induction of Autophagy is an Early Response to Gefitinib and a Potential Therapeutic Target in Breast Cancer. PLoS ONE 2013, 8, e76503. [Google Scholar] [CrossRef]
- Wolpin, B.M.; Rubinson, D.A.; Wang, X.X.; Chan, J.A.; Cleary, J.M.; Enzinger, P.C.; Fuchs, C.S.; McCleary, N.J.; Meyerhardt, J.A.; Ng, K.; et al. Phase II and Pharmacodynamic Study of Autophagy Inhibition Using Hydroxychloroquine in Patients With Metastatic Pancreatic Adenocarcinoma. Oncologist 2014, 19, 637–638. [Google Scholar] [CrossRef]
- Xu, R.; Ji, Z.Y.; Xu, C.; Zhu, J. The clinical value of using chloroquine or hydroxychloroquine as autophagy inhibitors in the treatment of cancers A systematic review and meta-analysis. Medicine 2018, 97, e12912. [Google Scholar] [CrossRef]
- Anand, K.; Niravath, P.; Patel, T.; Ensor, J.; Rodriguez, A.; Boone, T.; Wong, S.T.; Chang, J.C. A Phase II Study of the Efficacy and Safety of Chloroquine in Combination With Taxanes in the Treatment of Patients With Advanced or Metastatic Anthracycline-refractory Breast Cancer. Clin. Breast Cancer 2021, 21, 199–204. [Google Scholar] [CrossRef]
- Lakhter, A.J.; Sahu, R.P.; Sun, Y.; Kaufmann, W.K.; Androphy, E.J.; Travers, J.B.; Naidu, S.R. Chloroquine Promotes Apoptosis in Melanoma Cells by Inhibiting BH3 Domain-Mediated PUMA Degradation. J. Investig. Dermatol. 2013, 133, 2247–2254. [Google Scholar] [CrossRef] [PubMed]
- Samaras, P.; Tusup, M.; Nguyen-Kim, T.D.L.; Seifert, B.; Bachmann, H.; von Moos, R.; Knuth, A.; Pascolo, S. Phase I study of a chloroquine-gemcitabine combination in patients with metastatic or unresectable pancreatic cancer. Cancer Chemother. Pharm. 2017, 80, 1005–1012. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhu, Y.; Gu, L.; Li, K.; Ma, A.; Liu, L.; Meng, Y.; Zhang, J.; Shen, S.; Shi, Q.; et al. Chloroquine Suppresses Colorectal Cancer Progression via Targeting CHKA and PFKM to inhibit the PI3K/AKT Pathway and the Warburg Effect. Int. J. Biol. Sci. 2025, 21, 1619–1631. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.Q.; Wang, S.B.; Shao, Y.F.; Shi, J.N.; Wang, W.; Chen, W.Y.; Ye, Z.Q.; Jiang, J.Y.; Fang, Q.X.; Zhang, G.B.; et al. Hydroxychloroquine potentiates the anti-cancer effect of bevacizumab on glioblastoma via the inhibition of autophagy. Biomed. Pharmacother. 2019, 118, 109339. [Google Scholar] [CrossRef]
- Mauthe, M.; Orhon, I.; Rocchi, C.; Zhou, X.; Luhr, M.; Hijlkema, K.J.; Coppes, R.P.; Engedal, N.; Mari, M.; Reggiori, F. Chloroquine inhibits autophagic flux by decreasing autophagosome-lysosome fusion. Autophagy 2018, 14, 1435–1455. [Google Scholar] [CrossRef]
- Doeppner, T.R.; Coman, C.; Burdusel, D.; Ancuta, D.L.; Brockmeier, U.; Pirici, D.N.; Yaoyun, K.; Hermann, D.M.; Popa-Wagner, A. Long-term treatment with chloroquine increases lifespan in middle-aged male mice possibly via autophagy modulation, proteasome inhibition and glycogen metabolism. Aging 2022, 14, 4195–4210. [Google Scholar] [CrossRef]
- Halcrow, P.W.; Geiger, J.D.; Chen, X. Overcoming Chemoresistance: Altering pH of Cellular Compartments by Chloroquine and Hydroxychloroquine. Front. Cell Dev. Biol. 2021, 9, 627639. [Google Scholar] [CrossRef]
- Begum, H.M.; Shen, K. Intracellular and microenvironmental regulation of mitochondrial membrane potential in cancer cells. WIREs Mech. Dis. 2023, 15, e1595. [Google Scholar] [CrossRef]
- Begum, H.M.; Ta, H.P.; Zhou, H.; Ando, Y.; Kang, D.; Nemes, K.; Mariano, C.F.; Hao, J.; Yu, M.; Shen, K. Spatial Regulation of Mitochondrial Heterogeneity by Stromal Confinement in Micropatterned Tumor Models. Sci. Rep. 2019, 9, 11187. [Google Scholar] [CrossRef]
- Javaid, H.M.A.; Lim, H.; Shin, S.; Huh, J.Y. Inhibition of autophagy with chloroquine dysregulates mitochondrial quality control and energetics in adipocytes. Arch. Pharm. Res. 2022, 45, 731–742. [Google Scholar] [CrossRef]
- Boya, P.; Gonzalez-Polo, R.A.; Poncet, D.; Andreau, K.; Vieira, H.L.A.; Roumier, T.; Perfettini, J.L.; Kroemer, G. Mitochondrial membrane permeabilization is a critical step of lysosome-initiated apoptosis induced by hydroxychloroquine. Oncogene 2003, 22, 3927–3936. [Google Scholar] [CrossRef] [PubMed]
- Ushio-Fukai, M.; Alexander, R.W. Reactive oxygen species as mediators of angiogenesis signaling—Role of NAD(P)H oxidase. Mol. Cell Biochem. 2004, 264, 85–97. [Google Scholar] [CrossRef]
- Ushio-Fukai, M.; Nakamura, Y. Reactive oxygen species and angiogenesis: NADPH oxidase as target for cancer therapy. Cancer Lett. 2008, 266, 37–52. [Google Scholar] [CrossRef]
- Marullo, R.; Werner, E.; Degtyareva, N.; Moore, B.; Altavilla, G.; Ramalingam, S.S.; Doetsch, P.W. Cisplatin Induces a Mitochondrial-ROS Response That Contributes to Cytotoxicity Depending on Mitochondrial Redox Status and Bioenergetic Functions. PLoS ONE 2013, 8, e81162. [Google Scholar] [CrossRef]
- Jung, K.H.; Lee, J.H.; Park, J.W.; Moon, S.H.; Cho, Y.S.; Choe, Y.S.; Lee, K.H. Effects of curcumin on cancer cell mitochondrial function and potential monitoring with F-FDG uptake. Oncol. Rep. 2016, 35, 861–868. [Google Scholar] [CrossRef]
- Cao, L.; Liu, J.B.; Zhang, L.; Xiao, X.; Li, W. Curcumin inhibits HO-induced invasion and migration of human pancreatic cancer via suppression of the ERK/NF-κB pathway. Oncol. Rep. 2016, 36, 2245–2251. [Google Scholar] [CrossRef] [PubMed]
- Vessoni, A.T.; Quinet, A.; de Andrade-Lima, L.C.; Martins, D.J.; Garcia, C.C.M.; Rocha, C.R.R.; Vieira, D.B.; Menck, C.F.M. Chloroquine-induced glioma cells death is associated with mitochondrial membrane potential loss, but not oxidative stress. Free Radic. Biol. Med. 2016, 90, 91–100. [Google Scholar] [CrossRef]
- Benbelkacem, M.; Moulai, N.; Chader, H.; Ouahioune, W.; Bourouba, M. Dichloroacetate and chloroquine in combination with arsenite suppress ROS-induced oral squamous cell carcinoma (OSCC) development and improve BALB/c mice survival. Free Radic. Biol. Med. 2025, 227, 593–607. [Google Scholar] [CrossRef]
- Chen, M.Y.; Yadav, V.K.; Chu, Y.C.; Ong, J.R.; Huang, T.Y.; Lee, K.F.; Lee, K.H.; Yeh, C.T.; Lee, W.H. Hydroxychloroquine (HCQ) Modulates Autophagy and Oxidative DNA Damage Stress in Hepatocellular Carcinoma to Overcome Sorafenib Resistance via TLR9/SOD1/hsa-miR-30a-5p/Beclin-1 Axis. Cancers 2021, 13, 3227, Correction in Cancers 2023, 15, 1028. https://doi.org/10.3390/cancers15041028. [Google Scholar] [CrossRef] [PubMed]
- Ovejero-Sánchez, M.; Rubio-Heras, J.; de la Peña, M.D.V.; San-Segundo, L.; Pérez-Losada, J.; González-Sarmiento, R.; Herrero, A.B. Chloroquine-Induced DNA Damage Synergizes with Nonhomologous End Joining Inhibition to Cause Ovarian Cancer Cell Cytotoxicity. Int. J. Mol. Sci. 2022, 23, 7518. [Google Scholar] [CrossRef]
- Iglesias-Corral, D.; García-Valles, P.; Arroyo-Garrapucho, N.; Bueno-Martínez, E.; Ruiz-Robles, J.M.; Ovejero-Sánchez, M.; González-Sarmiento, R.; Herrero, A.B. Chloroquine-induced DNA damage synergizes with DNA repair inhibitors causing cancer cell death. Front. Oncol. 2024, 14, 1390518. [Google Scholar] [CrossRef] [PubMed]
- Prieto-Correa, J.R.; Bologna-Molina, R.; González-González, R.; Molina-Frechero, N.; Soto-Avila, J.J.; Isiordia-Espinoza, M.; Márquez, M.C.B.; Verdín, S.L. DNA oxidative damage in oral cancer: 8-hydroxy-2<acute accent>-deoxyguanosine immunoexpression assessment. Med. Oral Patol. Oral 2023, 28, E530–E538. [Google Scholar] [CrossRef]
- Hoque, M.M.; Iida, Y.; Kotani, H.; Kartika, I.D.; Harada, M. Hydroxychloroquine Promotes Bcl-xL Inhibition-induced Apoptosis in BxPC-3 Human Pancreatic Cancer Cells. Anticancer. Res. 2022, 42, 3495–3506. [Google Scholar] [CrossRef]
- Yao, C.J.; Chow, J.M.; Chuang, S.E.; Chang, C.L.; Yan, M.D.; Lee, H.L.; Lai, I.C.; Lin, P.C.; Lai, G.M. Induction of Forkhead Class box O3a and apoptosis by a standardized ginsenoside formulation, KG-135, is potentiated by autophagy blockade in A549 human lung cancer cells. J. Ginseng Res. 2017, 41, 247–256. [Google Scholar] [CrossRef]
- Wang, P.; Burikhanov, R.; Jayswal, R.; Weiss, H.L.; Arnold, S.M.; Villano, J.L.; Rangnekar, V.M. Neoadjuvant administration of hydroxychloroquine in a phase 1 clinical trial induced plasma Par-4 levels and apoptosis in diverse tumors. Genes. Cancer 2018, 9, 190–197. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Huang, Z.; Chen, X.; Zhang, B. The roles of osteoprotegerin in cancer, far beyond a bone player. Cell Death Discov. 2022, 8, 252. [Google Scholar] [CrossRef]
- Russmueller, G.; Moser, D.; Wurger, T.; Wrba, F.; Christopoulos, P.; Kostakis, G.; Seemann, R.; Stadler, V.; Wimmer, G.; Kornek, G.; et al. Upregulation of osteoprotegerin expression correlates with bone invasion and predicts poor clinical outcome in oral cancer. Oral Oncol. 2015, 51, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Vik, A.; Brodin, E.E.; Mathiesen, E.B.; Brox, J.; Jorgensen, L.; Njolstad, I.; Braekkan, S.K.; Hansen, J.B. Serum osteoprotegerin and future risk of cancer and cancer-related mortality in the general population: The Tromso study. Eur. J. Epidemiol. 2015, 30, 219–230. [Google Scholar] [CrossRef]
- Hammache, M.; Benchekroun, S.; Alamri, A.; Jalouli, M.; Mohamed, M.Y.A.; Boufahja, F.; Chahine, M.; Chandad, F.; Semlali, A. Modulation of signature cancer-related genes in oral cancer cells (Ca9-22) by anethole treatment: Insights into therapeutic potential. PLoS ONE 2024, 19, e0315085. [Google Scholar] [CrossRef] [PubMed]
- Issa, H.; Loubaki, L.; Al Amri, A.; Zibara, K.; Almutairi, M.H.; Rouabhia, M.; Semlali, A. Eugenol as a potential adjuvant therapy for gingival squamous cell carcinoma. Sci. Rep. 2024, 14, 10958. [Google Scholar] [CrossRef]
- Gassib, N.; Issa, H.; Loubaki, L.; Behaz, S.; Almutairi, M.H.; Rouabhia, M.; Semlali, A. Cellular mechanisms mediating the anti-cancer effects of carnosol on gingiva carcinoma. Sci. Rep. 2024, 14, 12266. [Google Scholar] [CrossRef]
- Semlali, A.; Papadakos, S.; Contant, C.; Zouaoui, I.; Rouabhia, M. Rapamycin inhibits oral cancer cell growth by promoting oxidative stress and suppressing ERK1/2, NF-κB and beta-catenin pathways. Front. Oncol. 2022, 12, 873447. [Google Scholar] [CrossRef]
- Semlali, A.; Ajala, I.; Beji, S.; Al-Zharani, M.M.; Rouabhia, M. Synergistic Effect of Anethole and Platinum Drug Cisplatin against Oral Cancer Cell Growth and Migration by Inhibiting MAPKase, Beta-Catenin, and NF-κB Pathways. Pharmaceuticals 2023, 16, 700. [Google Scholar] [CrossRef]
- Semlali, A.; Beji, S.; Ajala, I.; Al-Zharani, M.; Rouabhia, M. Synergistic Effects of New Curcumin Analog (PAC) and Cisplatin on Oral Cancer Therapy. Curr. Issues Mol. Biol. 2023, 45, 5018–5035. [Google Scholar] [CrossRef]
- Papadakos, S.; Issa, H.; Alamri, A.; Alamri, A.; Semlali, A. Rapamycin as a Potential Alternative Drug for Squamous Cell Gingiva Carcinoma (Ca9-22): A Focus on Cell Cycle, Apoptosis and Autophagy Genetic Profile. Pharmaceuticals 2024, 17, 131. [Google Scholar] [CrossRef]
- Semlali, A.; Beji, S.; Ajala, I.; Rouabhia, M. Effects of tetrahydrocannabinols on human oral cancer cell proliferation, apoptosis, autophagy, oxidative stress, and DNA damage. Arch. Oral Biol. 2021, 129, 105200. [Google Scholar] [CrossRef] [PubMed]
- Semlali, A.; Contant, C.; Al-Otaibi, B.; Al-Jammaz, I.; Chandad, F. The curcumin analog (PAC) suppressed cell survival and induced apoptosis and autophagy in oral cancer cells. Sci. Rep. 2021, 11, 11701. [Google Scholar] [CrossRef] [PubMed]
- Contant, C.; Rouabhia, M.; Loubaki, L.; Chandad, F.; Semlali, A. Anethole induces anti-oral cancer activity by triggering apoptosis, autophagy and oxidative stress and by modulation of multiple signaling pathways. Sci. Rep. 2021, 11, 13087. [Google Scholar] [CrossRef] [PubMed]
- Loubaki, L.; Rouabhia, M.; Zahrani, M.A.; Amri, A.A.; Semlali, A. Oxidative Stress and Autophagy Mediate Anti-Cancer Properties of Cannabis Derivatives in Human Oral Cancer Cells. Cancers 2022, 14, 4924. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baroudi, S.; González Poleo, D.A.; Issa, H.; Almutairi, M.H.; Semlali, A. Repurposing Antimalarials for Oral Cancer: Selective Efficacy of Hydroxychloroquine on Gingival Squamous Cell Carcinoma. Int. J. Mol. Sci. 2025, 26, 10994. https://doi.org/10.3390/ijms262210994
Baroudi S, González Poleo DA, Issa H, Almutairi MH, Semlali A. Repurposing Antimalarials for Oral Cancer: Selective Efficacy of Hydroxychloroquine on Gingival Squamous Cell Carcinoma. International Journal of Molecular Sciences. 2025; 26(22):10994. https://doi.org/10.3390/ijms262210994
Chicago/Turabian StyleBaroudi, Sana, Diego Alejandro González Poleo, Hawraa Issa, Mikhlid H. Almutairi, and Abdelhabib Semlali. 2025. "Repurposing Antimalarials for Oral Cancer: Selective Efficacy of Hydroxychloroquine on Gingival Squamous Cell Carcinoma" International Journal of Molecular Sciences 26, no. 22: 10994. https://doi.org/10.3390/ijms262210994
APA StyleBaroudi, S., González Poleo, D. A., Issa, H., Almutairi, M. H., & Semlali, A. (2025). Repurposing Antimalarials for Oral Cancer: Selective Efficacy of Hydroxychloroquine on Gingival Squamous Cell Carcinoma. International Journal of Molecular Sciences, 26(22), 10994. https://doi.org/10.3390/ijms262210994







