Transmetalation in Cancer Pharmacology
Abstract
1. Introduction
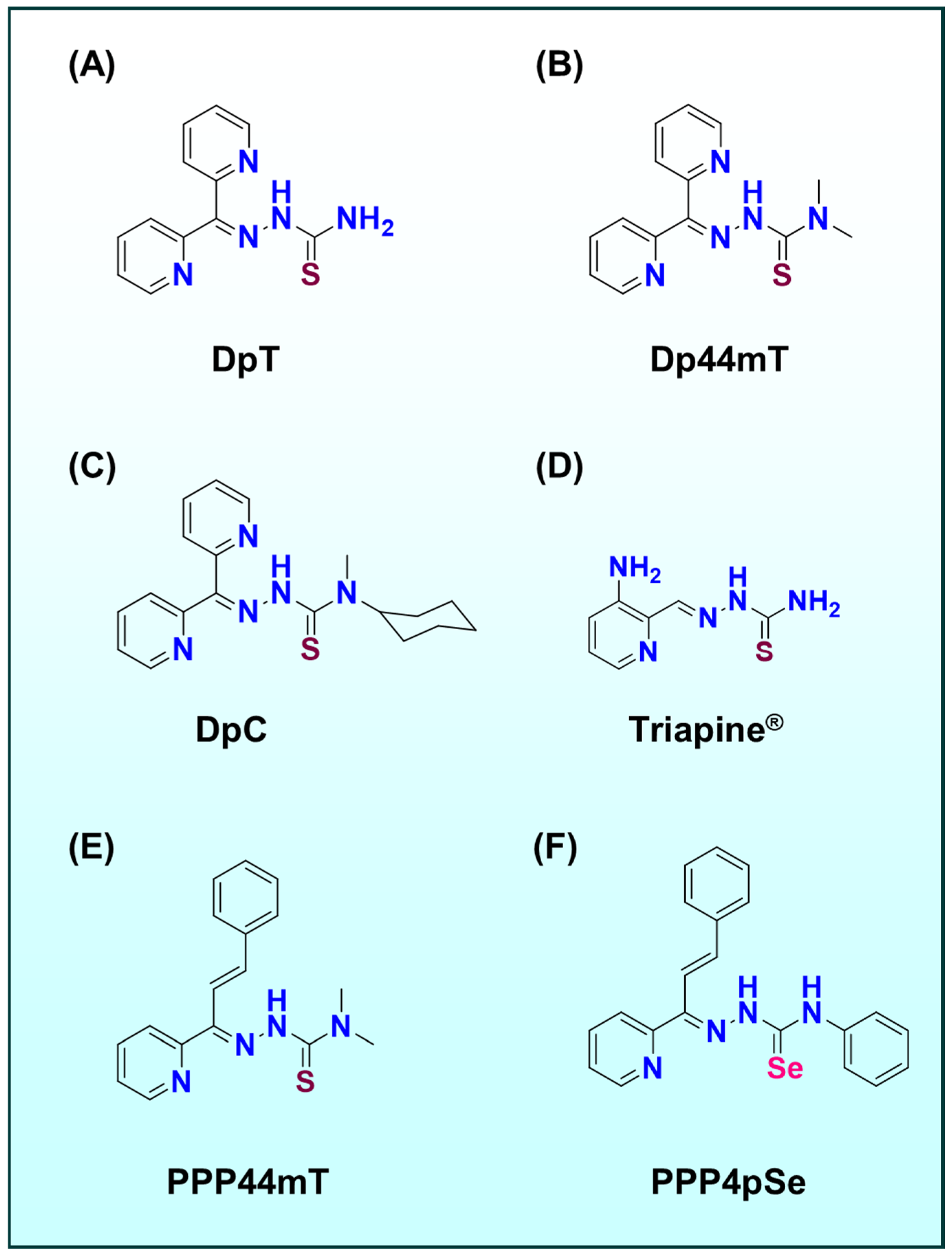
2. Metal-Based Ligand Systems in Cancer Therapy: Thiosemicarbazones and Beyond
2.1. Beyond TSCs Other Metal–Ligand Systems
2.2. Transmetalation as a Design Principle
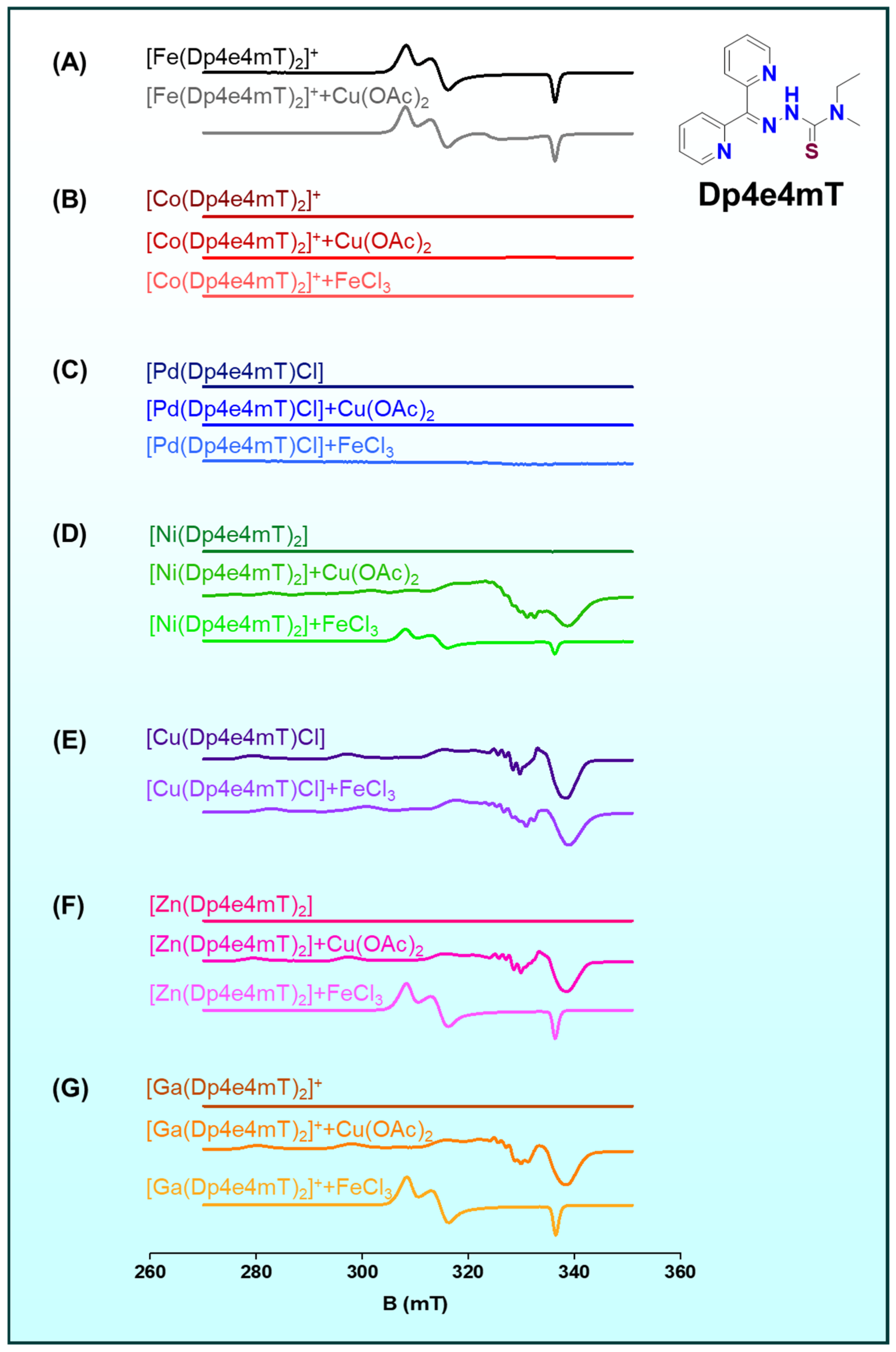
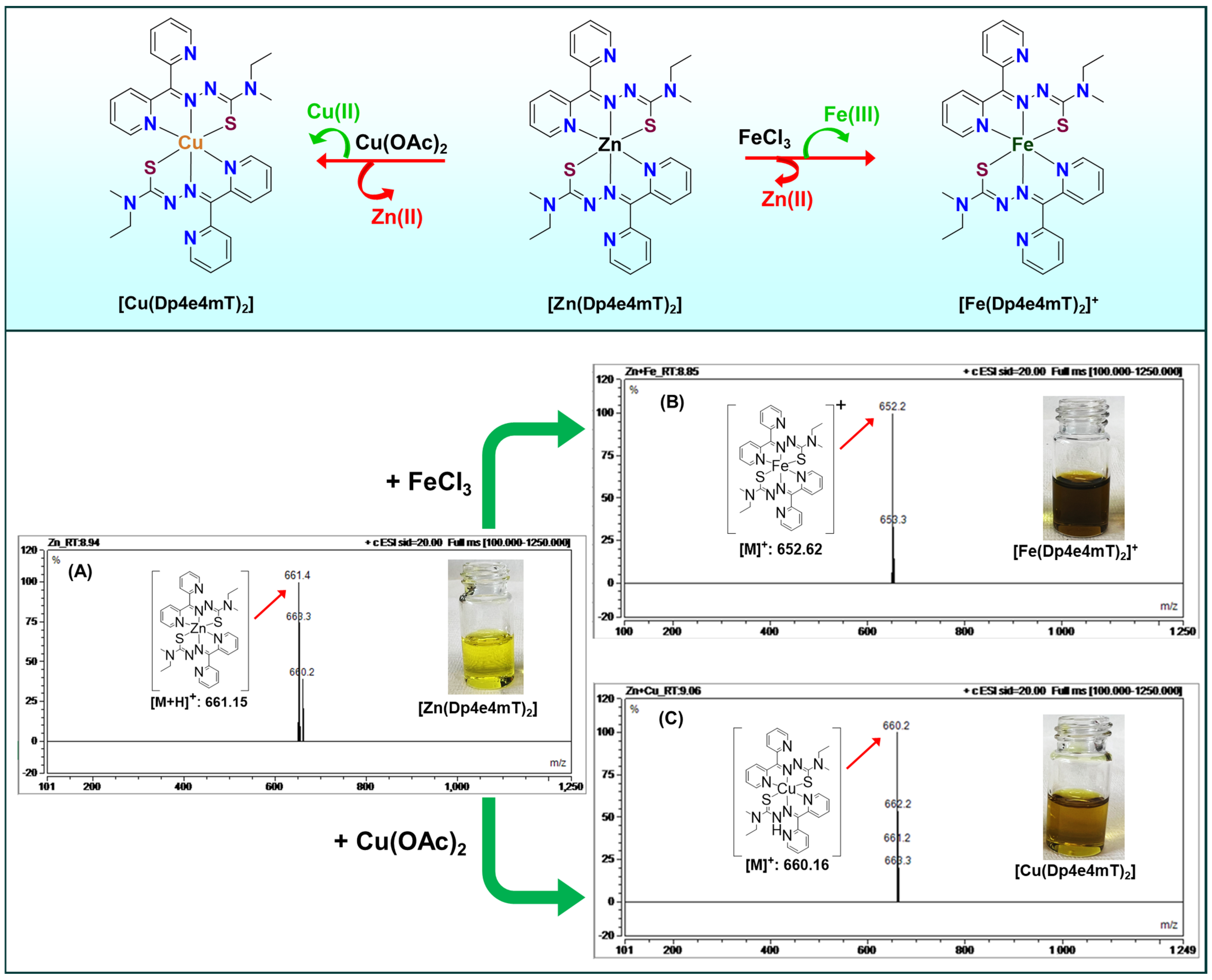
2.3. Transmetalation Mechanisms Among Fe(III), Cu(II), Zn(II), and Ti(IV)
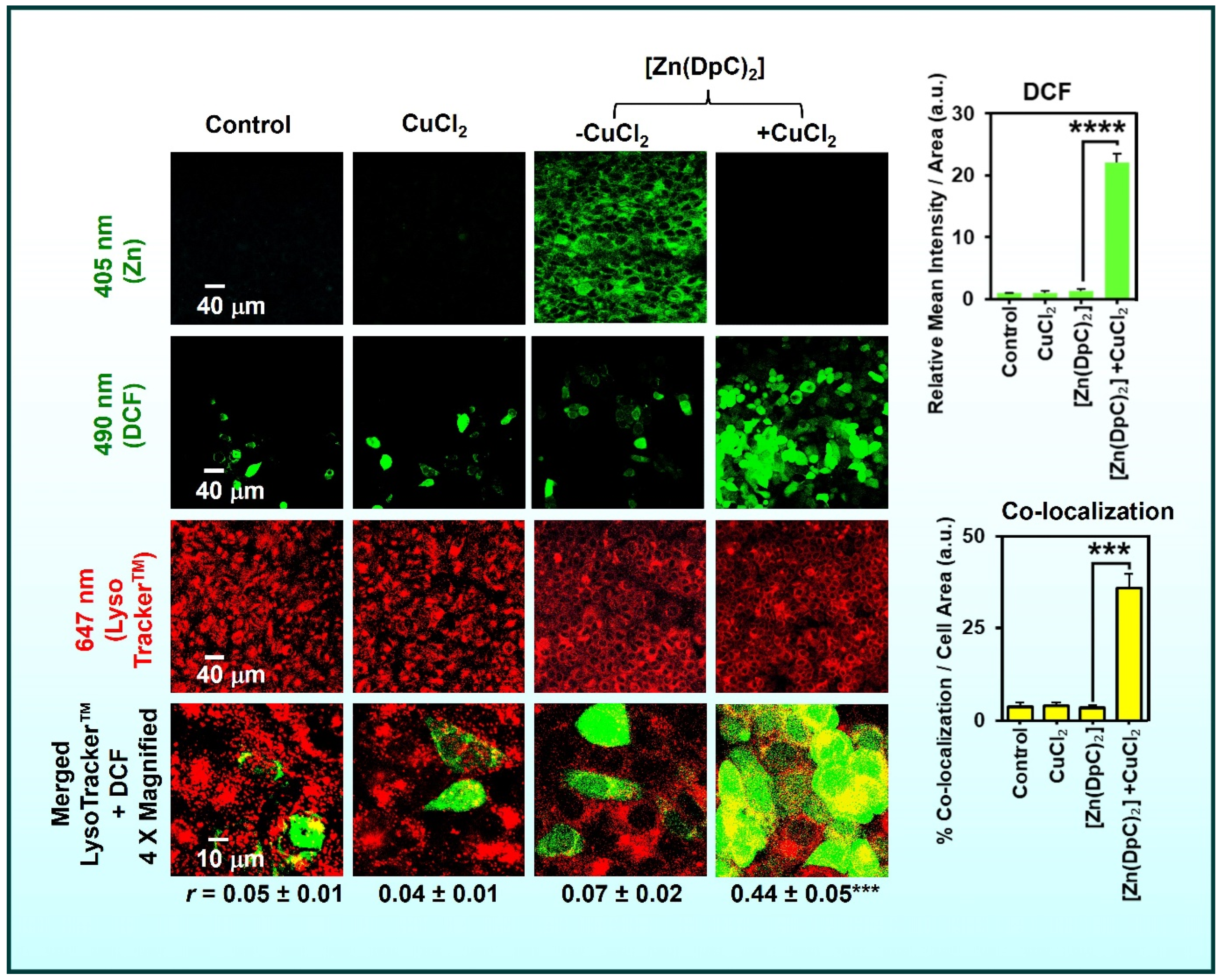
2.3.1. Transmetalation of Zn(II) to Cu(II)
2.3.2. Transmetalation of Fe(III)/Fe(II) to Cu(II)
2.3.3. Transmetalation of Ti(IV) to Fe(III)
2.3.4. Transmetalation of Zn(II) to Fe(III)
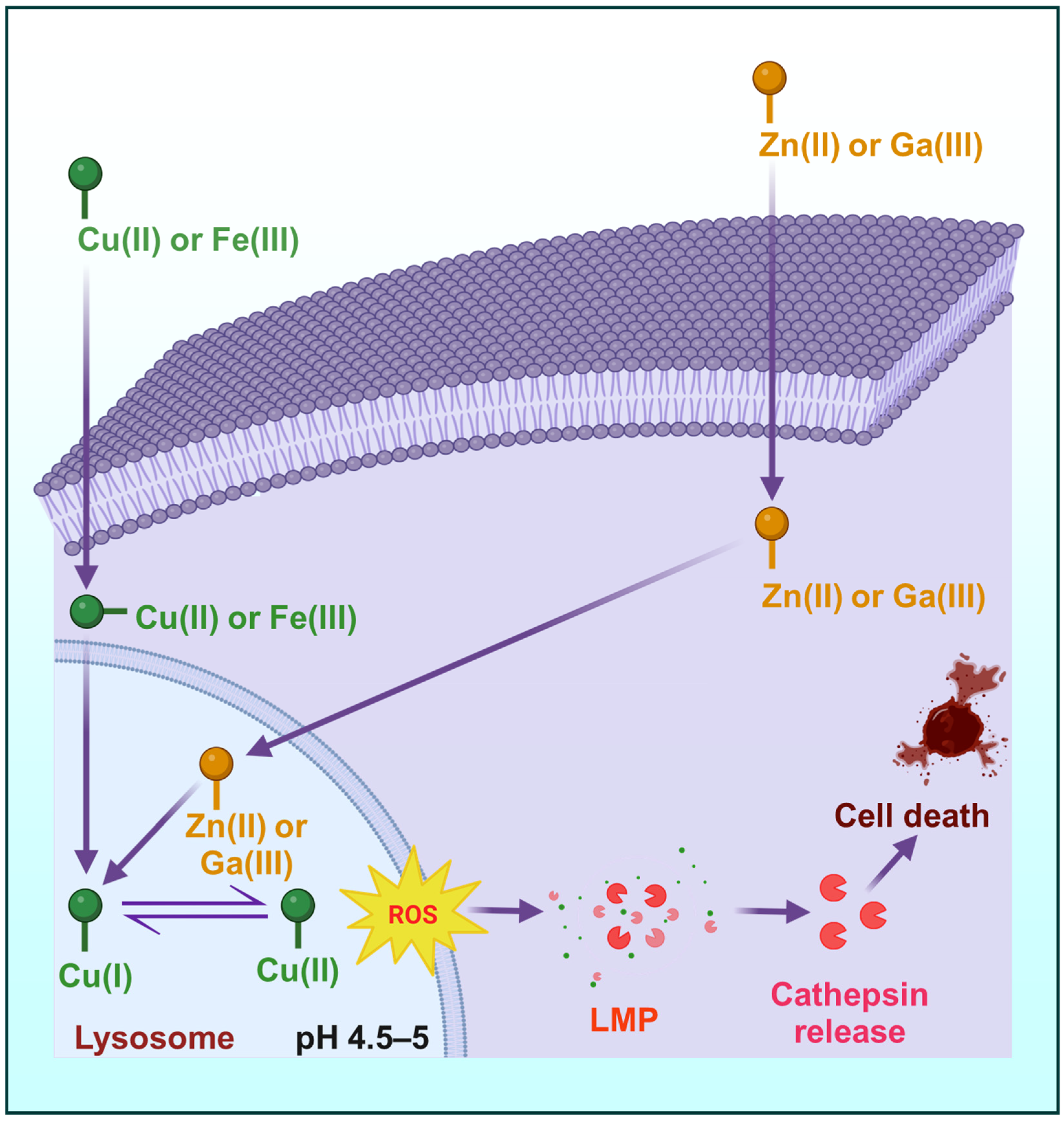
3. Impact of Transmetalation on Redox Activity, Metal Trafficking, Tumor Selectivity and ROS Generation
3.1. Metal Trafficking and Homeostasis
3.2. Tumor Selectivity
3.2.1. Elevated Intracellular Metals in Tumors
3.2.2. Acidic and Reducing Tumor Compartments
3.2.3. Differential Signaling Responses
4. Redox-Active Versus Redox-Inert Complexes: Efficacy and Off-Target Toxicity
4.1. Redox-Active Complexes
4.2. Redox-Inert Complexes
5. Advances in Ligand Design for Transmetalation Control and Efficacy
5.1. Steric Tuning (Hindrance and Bulk)
5.2. Electronic Tuning (Donor Strength and Redox Potential)
5.3. Backbone Rigidity and Conformation
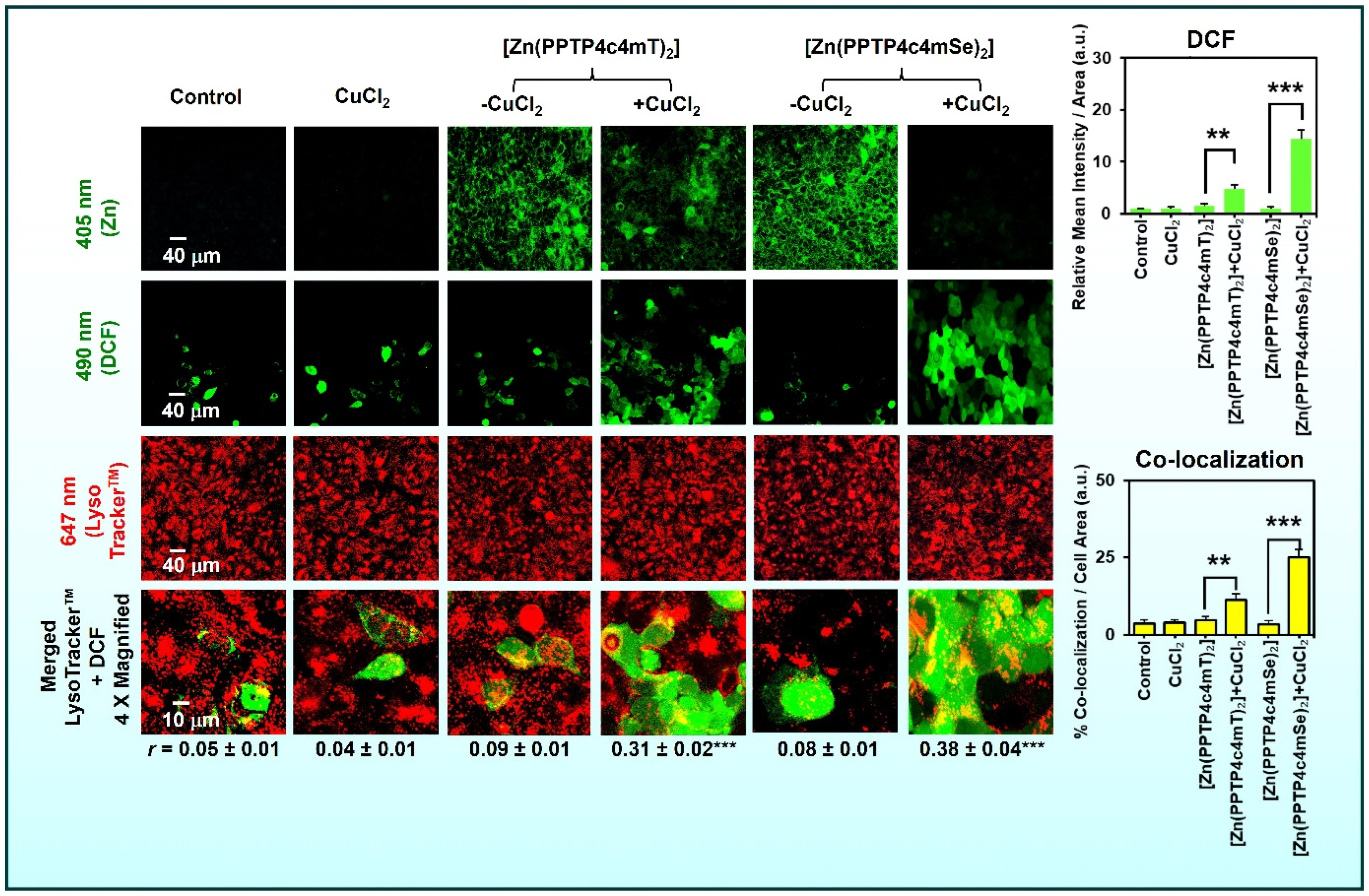
5.4. Isosteric Substitution (Sulfur Versus Selenium and Beyond)
5.5. Multi-Functional Ligand Design (Dual Chelator)
6. Biological Implications: Lysosomal Targeting, ROS, and Cell Death Pathways
6.1. Lysosomal Trapping and Activation
6.2. ROS Generation and Oxidative Damage
6.3. Apoptosis and Cell Cycle Effects
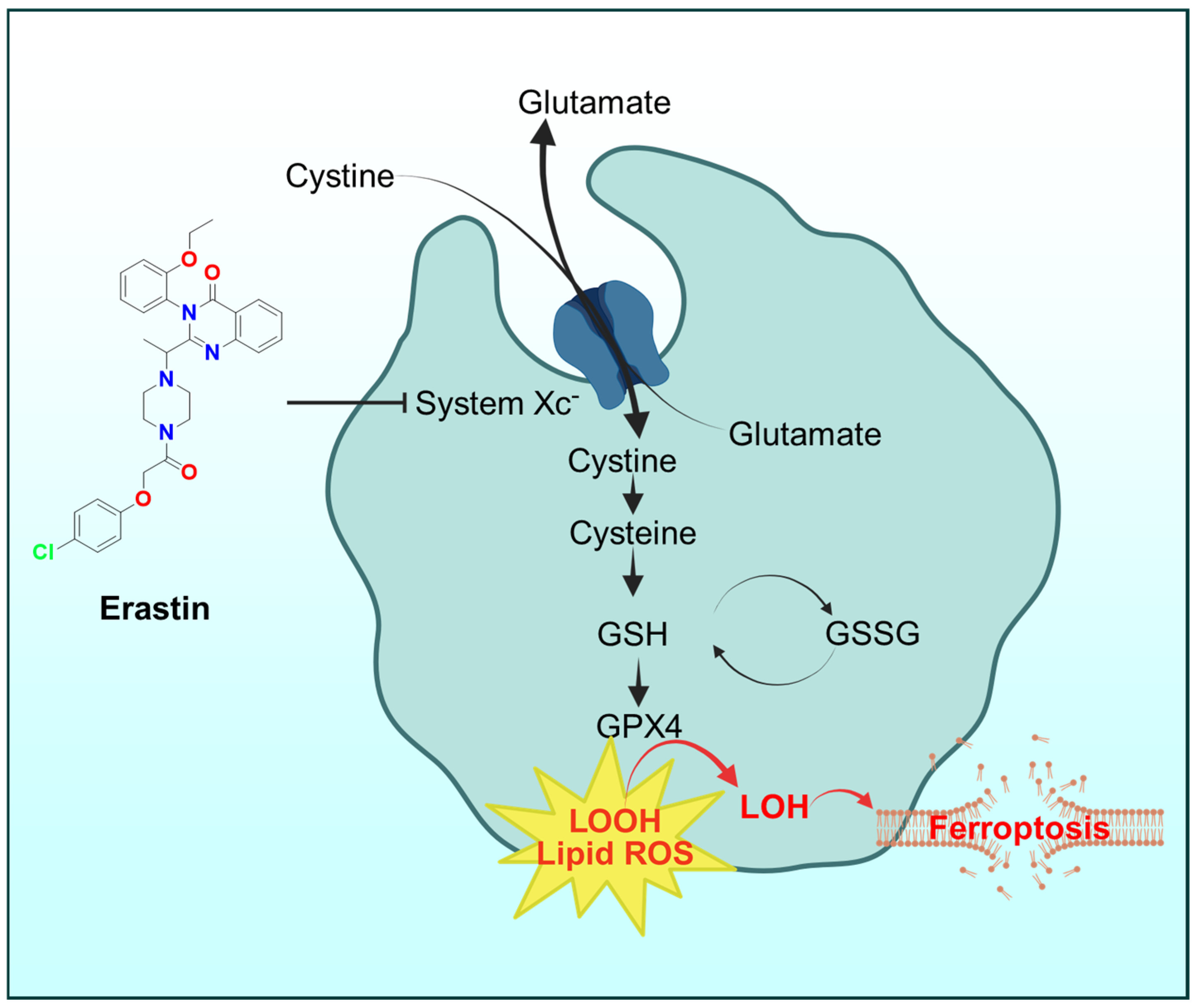
6.4. Ferroptosis
6.5. Inhibition of Oncogenic Signaling
7. Translational Considerations and Clinical Outlook
7.1. Preclinical Efficacy vs. Toxicity
7.2. Pharmacokinetics and Formulation
7.3. Clinical Trial Design and Patient Stratification
7.4. Regulatory and Manufacturing Considerations
7.5. Emerging Candidates and Future Directions
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AKT | Protein kinase B (from PI3K/AKT signaling pathway) |
| ATP | Adenosine Triphosphate |
| DefNEtTrp | Deferasirox N-ethyleneamine Triapine (dual chelator conjugate) |
| DFO | Desferrioxamine |
| DFX | Deferasirox (iron chelator) |
| DNA | Deoxyribonucleic Acid |
| Dp44mT | Di-2-pyridylketone-4,4-dimethyl-3-thiosemicarbazone |
| DpC | Di-2-pyridylketone-4-cyclohexyl-4-methyl-3-thiosemicarbazone |
| DpT | Di-2-pyridylketone thiosemicarbazone |
| EGFR | Epidermal Growth Factor Receptor |
| EPR | Enhanced Permeability and Retention effect |
| EPR | Electron Paramagnetic Resonance |
| GPX4 | Glutathione Peroxidase 4 |
| GSH | Glutathione |
| HER2 | Human Epidermal Growth Factor Receptor 2 |
| HIF-1α | Hypoxia-Inducible Factor-1 alpha |
| IGF1R | Insulin-like Growth Factor 1 Receptor |
| JNK | c-Jun N-terminal Kinase |
| LMP | Lysosomal Membrane Permeabilization |
| LOOH/LO• | Lipid hydroperoxide/Lipid radical |
| MAPK | Mitogen-Activated Protein Kinase |
| MET | mesenchymal–epithelial transition factor |
| MRI | Magnetic Resonance Imaging |
| NAT(s) | N-Acridine Thiosemicarbazone(s) |
| NDRG1 | N-myc Downregulated Gene-1 (metastasis suppressor) |
| PI3K | Phosphoinositide 3-Kinase |
| PPP44mT | (E)-3-Phenyl-1-(2-pyridinyl)-2-propen-1-one-4,4-dimethyl-3-thiosemicarbazone |
| PPP4pSe | (E)-3-Phenyl-1-(2-pyridinyl)-2-propen-1-one-4-phenyl-3-selenosemicarbazone |
| PPTP4c4mT | (1-(pyridin-2-yl)-3-(p-tolyl)prop-2-en-1-one-4-cyclohexyl-4-methyl-3-thiosemicarbazone |
| PPTP4c4mT | (1-(pyridin-2-yl)-3-(p-tolyl)prop-2-en-1-one-4-cyclohexyl-4-methyl-3-selenosemicarbazone |
| RNR | Ribonucleotide Reductase |
| ROS | Reactive Oxygen Species |
| Triapine® | 3-Aminopyridine-2-carboxaldehyde Thiosemicarbazone |
References
- Mahendiran, D.; Kumar, R.S.; Viswanathan, V.; Velmurugan, D.; Rahiman, A.K. Targeting of DNA molecules, BSA/c-Met tyrosine kinase receptors and anti-proliferative activity of bis(terpyridine)copper(ii) complexes. Dalton Trans. 2016, 45, 7794–7814. [Google Scholar] [CrossRef] [PubMed]
- Mahendiran, D.; Gurumoorthy, P.; Gunasekaran, K.; Kumar, R.S.; Rahiman, A.K. Structural modeling, in vitro antiproliferative activity, and the effect of substituents on the DNA fastening and scission actions of heteroleptic copper (II) complexes with terpyridines and naproxen. New J. Chem. 2015, 39, 7895–7911. [Google Scholar] [CrossRef]
- Mahendiran, D.; Amuthakala, S.; Bhuvanesh, N.S.P.; Kumar, R.S.; Rahiman, A.K. Copper complexes as prospective anticancer agents: In vitro and in vivo evaluation, selective targeting of cancer cells by DNA damage and S phase arrest. RSC Adv. 2018, 8, 16973–16990. [Google Scholar] [CrossRef] [PubMed]
- Mahendiran, D.; Kumar, R.S.; Viswanathan, V.; Velmurugan, D.; Rahiman, A.K. In vitro and in vivo anti-proliferative evaluation of bis(4′-(4-tolyl)-2,2′:6′,2″-terpyridine)copper(II) complex against Ehrlich ascites carcinoma tumors. J. Biol. Inorg. Chem. 2017, 22, 1109–1122. [Google Scholar] [CrossRef]
- Dharmasivam, M.; Kaya, B.; Wijesinghe, T.P.; Richardson, V.; Harmer, J.R.; Gonzalvez, M.A.; Lewis, W.; Azad, M.G.; Bernhardt, P.V.; Richardson, D.R. Differential transmetallation of complexes of the anti-cancer thiosemicarbazone, Dp4e4mT: Effects on anti-proliferative efficacy, redox activity, oxy-myoglobin and oxy-hemoglobin oxidation. Chem. Sci. 2024, 15, 974–990. [Google Scholar] [CrossRef]
- Ranasinghe, R.; Mathai, M.L.; Zulli, A. Cisplatin for cancer therapy and overcoming chemoresistance. Heliyon 2022, 8, e10608. [Google Scholar] [CrossRef]
- Ndagi, U.; Mhlongo, N.; Soliman, M.E. Metal complexes in cancer therapy—An update from drug design perspective. Drug Des. Devel. Ther. 2017, 11, 599–616. [Google Scholar] [CrossRef]
- Dharmasivam, M.; Kaya, B.; Wijesinghe, T.; Gholam Azad, M.; Gonzalvez, M.A.; Hussaini, M.; Chekmarev, J.; Bernhardt, P.V.; Richardson, D.R. Designing tailored thiosemicarbazones with bespoke properties: The styrene moiety imparts potent activity, inhibits heme center oxidation, and results in a novel “Stealth Zinc (II) Complex”. J. Med. Chem. 2023, 66, 1426–1453. [Google Scholar] [CrossRef]
- Kaya, B.; Gholam Azad, M.; Suleymanoglu, M.; Harmer, J.R.; Wijesinghe, T.P.; Richardson, V.; Zhao, X.; Bernhardt, P.V.; Dharmasivam, M.; Richardson, D.R. Isosteric Replacement of Sulfur to Selenium in a Thiosemicarbazone: Promotion of Zn(II) Complex Dissociation and Transmetalation to Augment Anticancer Efficacy. J. Med. Chem. 2024, 67, 12155–12183. [Google Scholar] [CrossRef]
- Kaya, B.; Smith, H.; Chen, Y.; Azad, M.G.; Russell, T.M.; Richardson, V.; Dharmasivam, M.; Richardson, D.R. Innovative N-Acridine Thiosemicarbazones and Their Zn(II) Complexes Transmetallate with Cu(II): Redox Activity and Suppression of Detrimental Oxy-Myoglobin Oxidation. Inorg. Chem. 2024, 63, 20840–20858. [Google Scholar] [CrossRef]
- Lovejoy, D.B.; Jansson, P.J.; Brunk, U.T.; Wong, J.; Ponka, P.; Richardson, D.R. Antitumor activity of metal-chelating compound Dp44mT is mediated by formation of a redox-active copper complex that accumulates in lysosomes. Cancer Res. 2011, 71, 5871–5880. [Google Scholar] [CrossRef]
- Jansson, P.J.; Sharpe, P.C.; Bernhardt, P.V.; Richardson, D.R. Novel thiosemicarbazones of the ApT and DpT series and their copper complexes: Identification of pronounced redox activity and characterization of their antitumor activity. J. Med. Chem. 2010, 53, 5759–5769. [Google Scholar] [CrossRef]
- Dharmasivam, M.; Zhang, S.; Zhao, X.; Richardson, V.; Wijesinghe, T.P.; Suleymanoglu, M.; Gholam Azad, M.; Bernhardt, P.V.; Kaya, B.; Richardson, D.R. Advantages of Novel Anti-cancer Selenosemicarbazones: Preferential Reactivity of Their Fe(III), Cu(II), and Zn(II) Complexes with Key Physiological Reductants/Ligands Versus Isosteric Thiosemicarbazones. J. Med. Chem. 2025, 68, 9594–9622. [Google Scholar] [CrossRef] [PubMed]
- Stacy, A.E.; Palanimuthu, D.; Bernhardt, P.V.; Kalinowski, D.S.; Jansson, P.J.; Richardson, D.R. Zinc(II)-Thiosemicarbazone Complexes Are Localized to the Lysosomal Compartment Where They Transmetallate with Copper Ions to Induce Cytotoxicity. J. Med. Chem. 2016, 59, 4965–4984. [Google Scholar] [CrossRef]
- Bernhardt, P.V.; Sharpe, P.C.; Islam, M.; Lovejoy, D.B.; Kalinowski, D.S.; Richardson, D.R. Iron chelators of the dipyridylketone thiosemicarbazone class: Precomplexation and transmetalation effects on anticancer activity. J. Med. Chem. 2009, 52, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Barbanente, A.; Kopecka, J.; Vitone, D.; Niso, M.; Rizzi, R.; Cuocci, C.; Abatematteo, F.S.; Mastropasqua, F.; Colabufo, N.A.; Margiotta, N.; et al. First-In-Class Thiosemicarbazone Metal Complexes Targeting the Sigma-2 Receptor (S2R) as an Innovative Strategy against Pancreatic Cancer. J. Med. Chem. 2024, 67, 20118–20134. [Google Scholar] [CrossRef]
- Holland, J.P.; Aigbirhio, F.I.; Betts, H.M.; Bonnitcha, P.D.; Burke, P.; Christlieb, M.; Churchill, G.C.; Cowley, A.R.; Dilworth, J.R.; Donnelly, P.S.; et al. Functionalized bis(thiosemicarbazonato) complexes of zinc and copper: Synthetic platforms toward site-specific radiopharmaceuticals. Inorg. Chem. 2007, 46, 465–485. [Google Scholar] [CrossRef] [PubMed]
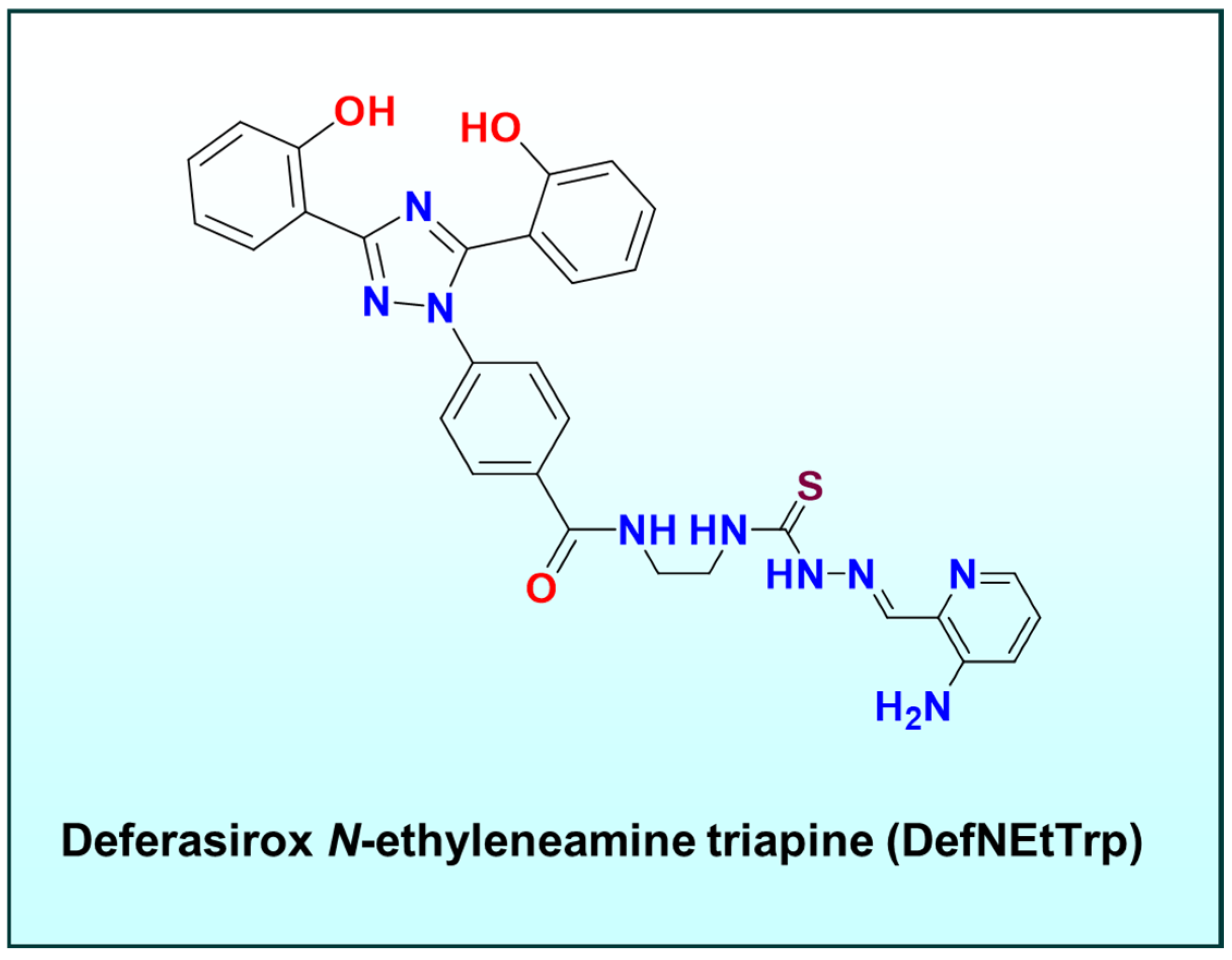
- Rodriguez, I.; Acosta, C.; Nieves-Escobar, C.; Strangmark, E.; Claudio-Ares, O.; Vargas Figueroa, A.I.; Soto-Millan, A.M.; Orta-Rivera, A.M.; Astashkin, A.V.; Tinoco, A.D. DefNEtTrp: An Iron Dual Chelator Approach for Anticancer Application. JACS Au 2024, 4, 4799–4808. [Google Scholar] [CrossRef] [PubMed]
- Santoro, A.; Vileno, B.; Palacios, Ò.; Peris-Díaz, M.D.; Riegel, G.; Gaiddon, C.; Krężel, A.; Faller, P. Reactivity of Cu(II)–, Zn(II)–and Fe(II)–thiosemicarbazone complexes with glutathione and metallothionein: From stability to dissociation to transmetallation. Metallomics 2019, 11, 994–1004. [Google Scholar] [CrossRef]
- Dharmasivam, M.; Azad, M.G.; Afroz, R.; Richardson, V.; Jansson, P.J.; Richardson, D.R. The thiosemicarbazone, DpC, broadly synergizes with multiple anti-cancer therapeutics and demonstrates temperature- and energy-dependent uptake by tumor cells. Biochim. Biophys. Acta Gen. Subj. 2022, 1866, 130152. [Google Scholar] [CrossRef]
- Bormio Nunes, J.H.; Hager, S.; Mathuber, M.; Posa, V.; Roller, A.; Enyedy, E.A.; Stefanelli, A.; Berger, W.; Keppler, B.K.; Heffeter, P.; et al. Cancer Cell Resistance Against the Clinically Investigated Thiosemicarbazone COTI-2 Is Based on Formation of Intracellular Copper Complex Glutathione Adducts and ABCC1-Mediated Efflux. J. Med. Chem. 2020, 63, 13719–13732. [Google Scholar] [CrossRef]
- Ishiguro, K.; Lin, Z.; Rutherford, T.; Ratner, E. Antitumor activity of the ribonucleotide reductase inhibitor Triapine alone or in combination with paclitaxel. Gynecol. Oncol. 2015, 137, 79. [Google Scholar] [CrossRef]
- Popovic-Bijelic, A.; Kowol, C.R.; Lind, M.E.; Luo, J.; Himo, F.; Enyedy, E.A.; Arion, V.B.; Graslund, A. Ribonucleotide reductase inhibition by metal complexes of Triapine (3-aminopyridine-2-carboxaldehyde thiosemicarbazone): A combined experimental and theoretical study. J. Inorg. Biochem. 2011, 105, 1422–1431. [Google Scholar] [CrossRef]
- Kunos, C.A.; Chu, E.; Makower, D.; Kaubisch, A.; Sznol, M.; Ivy, S.P. Phase I Trial of Triapine-Cisplatin-Paclitaxel Chemotherapy for Advanced Stage or Metastatic Solid Tumor Cancers. Front. Oncol. 2017, 7, 62. [Google Scholar] [CrossRef]
- Plamthottam, S.; Sun, D.; Van Valkenburgh, J.; Valenzuela, J.; Ruehle, B.; Steele, D.; Poddar, S.; Marshalik, M.; Hernandez, S.; Radu, C.G.; et al. Activity and electrochemical properties: Iron complexes of the anticancer drug triapine and its analogs. J. Biol. Inorg. Chem. 2019, 24, 621–632. [Google Scholar] [CrossRef] [PubMed]
- Murillo, M.I.; Gaiddon, C.; Le Lagadec, R. Targeting of the intracellular redox balance by metal complexes towards anticancer therapy. Front. Chem. 2022, 10, 967337. [Google Scholar] [CrossRef] [PubMed]
- Gaur, K.; Perez Otero, S.C.; Benjamin-Rivera, J.A.; Rodriguez, I.; Loza-Rosas, S.A.; Vazquez Salgado, A.M.; Akam, E.A.; Hernandez-Matias, L.; Sharma, R.K.; Alicea, N.; et al. Iron Chelator Transmetalative Approach to Inhibit Human Ribonucleotide Reductase. JACS Au 2021, 1, 865–878. [Google Scholar] [CrossRef]
- Murren, J.; Modiano, M.; Clairmont, C.; Lambert, P.; Savaraj, N.; Doyle, T.; Sznol, M. Phase I and pharmacokinetic study of triapine, a potent ribonucleotide reductase inhibitor, administered daily for five days in patients with advanced solid tumors. Clin. Cancer Res. 2003, 9, 4092–4100. [Google Scholar]
- Lin, Z.P.; Belcourt, M.F.; Carbone, R.; Eaton, J.S.; Penketh, P.G.; Shadel, G.S.; Cory, J.G.; Sartorelli, A.C. Excess ribonucleotide reductase R2 subunits coordinate the S phase checkpoint to facilitate DNA damage repair and recovery from replication stress. Biochem. Pharmacol. 2007, 73, 760–772. [Google Scholar] [CrossRef]
- Chung, M.H.; Aimaier, R.; Yu, Q.; Li, H.; Li, Y.; Wei, C.; Gu, Y.; Wang, W.; Guo, Z.; Long, M.; et al. RRM2 as a novel prognostic and therapeutic target of NF1-associated MPNST. Cell. Oncol. 2023, 46, 1399–1413. [Google Scholar] [CrossRef]
- Besleaga, I.; Stepanenko, I.; Petrasheuskaya, T.V.; Darvasiova, D.; Breza, M.; Hammerstad, M.; Marc, M.A.; Prado-Roller, A.; Spengler, G.; Popovic-Bijelic, A.; et al. Triapine Analogues and Their Copper(II) Complexes: Synthesis, Characterization, Solution Speciation, Redox Activity, Cytotoxicity, and mR2 RNR Inhibition. Inorg. Chem. 2021, 60, 11297–11319. [Google Scholar] [CrossRef]
- Jansson, P.J.; Yamagishi, T.; Arvind, A.; Seebacher, N.; Gutierrez, E.; Stacy, A.; Maleki, S.; Sharp, D.; Sahni, S.; Richardson, D.R. Di-2-pyridylketone 4,4-dimethyl-3-thiosemicarbazone (Dp44mT) overcomes multidrug resistance by a novel mechanism involving the hijacking of lysosomal P-glycoprotein (Pgp). J. Biol. Chem. 2015, 290, 9588–9603. [Google Scholar] [CrossRef]
- Kovacevic, Z.; Chikhani, S.; Lovejoy, D.B.; Richardson, D.R. Novel thiosemicarbazone iron chelators induce up-regulation and phosphorylation of the metastasis suppressor N-myc down-stream regulated gene 1: A new strategy for the treatment of pancreatic cancer. Mol. Pharmacol. 2011, 80, 598–609. [Google Scholar] [CrossRef]
- Geleta, B.; Park, K.C.; Jansson, P.J.; Sahni, S.; Maleki, S.; Xu, Z.; Murakami, T.; Pajic, M.; Apte, M.V.; Richardson, D.R.; et al. Breaking the cycle: Targeting of NDRG1 to inhibit bi-directional oncogenic cross-talk between pancreatic cancer and stroma. FASEB J. 2021, 35, e21347. [Google Scholar] [CrossRef]
- Shehadeh-Tout, F.; Milioli, H.H.; Roslan, S.; Jansson, P.J.; Dharmasivam, M.; Graham, D.; Anderson, R.; Wijesinghe, T.; Azad, M.G.; Richardson, D.R.; et al. Innovative thiosemicarbazones that induce multi-modal mechanisms to down-regulate estrogen-, progesterone-, androgen- and prolactin-receptors in breast cancer. Pharmacol. Res. 2023, 193, 106806. [Google Scholar] [CrossRef]
- Lovejoy, D.B.; Sharp, D.M.; Seebacher, N.; Obeidy, P.; Prichard, T.; Stefani, C.; Basha, M.T.; Sharpe, P.C.; Jansson, P.J.; Kalinowski, D.S.; et al. Novel second-generation di-2-pyridylketone thiosemicarbazones show synergism with standard chemotherapeutics and demonstrate potent activity against lung cancer xenografts after oral and intravenous administration in vivo. J. Med. Chem. 2012, 55, 7230–7244. [Google Scholar] [CrossRef] [PubMed]
- Quach, P.; Gutierrez, E.; Basha, M.T.; Kalinowski, D.S.; Sharpe, P.C.; Lovejoy, D.B.; Bernhardt, P.V.; Jansson, P.J.; Richardson, D.R. Methemoglobin formation by triapine, di-2-pyridylketone-4,4-dimethyl-3-thiosemicarbazone (Dp44mT), and other anticancer thiosemicarbazones: Identification of novel thiosemicarbazones and therapeutics that prevent this effect. Mol. Pharmacol. 2012, 82, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Mies, K.A.; Gebhardt, P.; Möllmann, U.; Crumbliss, A.L. Synthesis, siderophore activity and iron (III) chelation chemistry of a novel mono-hydroxamate, bis-catecholate siderophore mimic: Nα,-Nε-Bis [2,3-dihydroxybenzoyl]-l-lysyl-(γ-N-methyl-N-hydroxyamido)-l-glutamic acid. J. Inorg. Biochem. 2008, 102, 850–861. [Google Scholar] [CrossRef] [PubMed]
- Adjimani, J.P.; Asare, P. Antioxidant and free radical scavenging activity of iron chelators. Toxicol. Rep. 2015, 2, 721–728. [Google Scholar] [CrossRef]
- Liu, Z.D.; Hider, R.C. Design of iron chelators with therapeutic application. Coord. Chem. Rev. 2002, 232, 151–171. [Google Scholar] [CrossRef]
- Lazaridou, M.; Christodoulou, E.; Nerantzaki, M.; Kostoglou, M.; Lambropoulou, D.A.; Katsarou, A.; Pantopoulos, K.; Bikiaris, D.N. Formulation and In-Vitro Characterization of Chitosan-Nanoparticles Loaded with the Iron Chelator Deferoxamine Mesylate (DFO). Pharmaceutics 2020, 12, 238. [Google Scholar] [CrossRef]
- Bellotti, D.; Remelli, M. Deferoxamine B: A Natural, Excellent and Versatile Metal Chelator. Molecules 2021, 26, 3255. [Google Scholar] [CrossRef] [PubMed]
- Kontoghiorghe, C.N.; Kontoghiorghes, G.J. Efficacy and safety of iron-chelation therapy with deferoxamine, deferiprone, and deferasirox for the treatment of iron-loaded patients with non-transfusion-dependent thalassemia syndromes. Drug Des. Devel. Ther. 2016, 10, 465–481. [Google Scholar] [CrossRef] [PubMed]
- Umemura, M.; Kim, J.H.; Aoyama, H.; Hoshino, Y.; Fukumura, H.; Nakakaji, R.; Sato, I.; Ohtake, M.; Akimoto, T.; Narikawa, M.; et al. The iron chelating agent, deferoxamine detoxifies Fe(Salen)-induced cytotoxicity. J. Pharmacol. Sci. 2017, 134, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Raymond, K.N.; Dertz, E.A.; Kim, S.S. Enterobactin: An archetype for microbial iron transport. Proc. Natl. Acad. Sci. USA 2003, 100, 3584–3588. [Google Scholar] [CrossRef]
- Saha, P.; Yeoh, B.S.; Xiao, X.; Golonka, R.M.; Kumarasamy, S.; Vijay-Kumar, M. Enterobactin, an iron chelating bacterial siderophore, arrests cancer cell proliferation. Biochem. Pharmacol. 2019, 168, 71–81. [Google Scholar] [CrossRef]
- Zhang, Q.; Jin, B.; Shi, Z.; Wang, X.; Liu, Q.; Lei, S.; Peng, R. Novel enterobactin analogues as potential therapeutic chelating agents: Synthesis, thermodynamic and antioxidant studies. Sci. Rep. 2016, 6, 34024. [Google Scholar] [CrossRef]
- Raymond, K.N.; Allred, B.E.; Sia, A.K. Coordination Chemistry of Microbial Iron Transport. Acc. Chem. Res. 2015, 48, 2496–2505. [Google Scholar] [CrossRef]
- Abergel, R.J.; Warner, J.A.; Shuh, D.K.; Raymond, K.N. Enterobactin Protonation and Iron Release: Structural Characterization of the Salicylate Coordination Shift in Ferric Enterobactin1. J. Am. Chem. Soc. 2006, 128, 8920–8931. [Google Scholar] [CrossRef]
- Jakupec, M.A.; Keppler, B.K. Gallium in cancer treatment. Curr. Top. Med. Chem. 2004, 4, 1575–1583. [Google Scholar] [CrossRef]
- Timerbaev, A.R. Advances in developing tris(8-quinolinolato)gallium(iii) as an anticancer drug: Critical appraisal and prospects. Metallomics 2009, 1, 193–198. [Google Scholar] [CrossRef]
- Darwesh, A.M.F.; Imberti, C.; Bartnicka, J.J.; Al-Salemee, F.; Blower, J.E.; Rigby, A.; Bordoloi, J.; Griffiths, A.; Ma, M.T.; Blower, P.J. In Vivo Trafficking of the Anticancer Drug Tris(8-Quinolinolato) Gallium (III) (KP46) by Gallium-68/67 PET/SPECT Imaging. Molecules 2023, 28, 7217. [Google Scholar] [CrossRef]
- Bernstein, L.R.; JM van der Hoeven, J.; Boer, R.O. Hepatocellular carcinoma detection by gallium scan and subsequent treatment by gallium maltolate: Rationale and case study. Anti-Cancer Agents Med. Chem. 2011, 11, 585–590. [Google Scholar] [CrossRef] [PubMed]
- Chua, M.S.; Bernstein, L.R.; Li, R.; So, S.K. Gallium maltolate is a promising chemotherapeutic agent for the treatment of hepatocellular carcinoma. Anticancer. Res. 2006, 26, 1739–1743. [Google Scholar] [PubMed]
- Abeysinghe, P.M.; Harding, M.M. Antitumour bis(cyclopentadienyl) metal complexes: Titanocene and molybdocene dichloride and derivatives. Dalton Trans. 2007, 32, 3474–3482. [Google Scholar] [CrossRef]
- Deally, A.; Hackenberg, F.; Lally, G.; Müller-Bunz, H.; Tacke, M. Synthesis and Cytotoxicity Studies of Silyl-Substituted Titanocene Dichloride Derivatives. Organometallics 2012, 31, 5782–5790. [Google Scholar] [CrossRef]
- Gao, L.M.; Hernandez, R.; Matta, J.; Melendez, E. Synthesis, Ti(IV) intake by apotransferrin and cytotoxic properties of functionalized titanocene dichlorides. J. Biol. Inorg. Chem. 2007, 12, 959–967. [Google Scholar] [CrossRef]
- Koleros, E.; Stamatatos, T.C.; Psycharis, V.; Raptopoulou, C.P.; Perlepes, S.P.; Klouras, N. In Search for Titanocene Complexes with Improved Cytotoxic Activity: Synthesis, X-Ray Structure, and Spectroscopic Study of Bis (η5-cyclopentadienyl) difluorotitanium (IV). Bioinorg. Chem. Appl. 2010, 2010, 914580. [Google Scholar] [CrossRef]
- Causey, P.W.; Baird, M.C.; Cole, S.P. Synthesis, characterization, and assessment of cytotoxic properties of a series of titanocene dichloride derivatives. Organometallics 2004, 23, 4486–4494. [Google Scholar] [CrossRef]
- Andres, S.A.; Bajaj, K.; Vishnosky, N.S.; Peterson, M.A.; Mashuta, M.S.; Buchanan, R.M.; Bates, P.J.; Grapperhaus, C.A. Synthesis, Characterization, and Biological Activity of Hybrid Thiosemicarbazone-Alkylthiocarbamate Metal Complexes. Inorg. Chem. 2020, 59, 4924–4935. [Google Scholar] [CrossRef]
- Brown, O.C.; Baguna Torres, J.; Holt, K.B.; Blower, P.J.; Went, M.J. Copper complexes with dissymmetrically substituted bis(thiosemicarbazone) ligands as a basis for PET radiopharmaceuticals: Control of redox potential and lipophilicity. Dalton Trans. 2017, 46, 14612–14630. [Google Scholar] [CrossRef]
- Pascu, S.I.; Waghorn, P.A.; Conry, T.D.; Betts, H.M.; Dilworth, J.R.; Churchill, G.C.; Pokrovska, T.; Christlieb, M.; Aigbirhio, F.I.; Warren, J.E. Designing Zn(II) and Cu(II) derivatives as probes for in vitro fluorescence imaging. Dalton Trans. 2007, 62, 4988–4997. [Google Scholar] [CrossRef]
- Donnelly, P.S.; Caragounis, A.; Du, T.; Laughton, K.M.; Volitakis, I.; Cherny, R.A.; Sharples, R.A.; Hill, A.F.; Li, Q.X.; Masters, C.L.; et al. Selective intracellular release of copper and zinc ions from bis(thiosemicarbazonato) complexes reduces levels of Alzheimer disease amyloid-beta peptide. J. Biol. Chem. 2008, 283, 4568–4577. [Google Scholar] [CrossRef]
- Cortezon-Tamarit, F.; Song, K.; Kuganathan, N.; Arrowsmith, R.L.; Mota Merelo de Aguiar, S.R.; Waghorn, P.A.; Brookfield, A.; Shanmugam, M.; Collison, D.; Ge, H.; et al. Structural and Functional Diversity in Rigid Thiosemicarbazones with Extended Aromatic Frameworks: Microwave-Assisted Synthesis and Structural Investigations. ACS Omega 2023, 8, 16047–16079. [Google Scholar] [CrossRef]
- Lim, S.; Price, K.A.; Chong, S.F.; Paterson, B.M.; Caragounis, A.; Barnham, K.J.; Crouch, P.J.; Peach, J.M.; Dilworth, J.R.; White, A.R.; et al. Copper and zinc bis(thiosemicarbazonato) complexes with a fluorescent tag: Synthesis, radiolabelling with copper-64, cell uptake and fluorescence studies. J. Biol. Inorg. Chem. 2010, 15, 225–235. [Google Scholar] [CrossRef]
- McAllum, E.J.; Roberts, B.R.; Hickey, J.L.; Dang, T.N.; Grubman, A.; Donnelly, P.S.; Liddell, J.R.; White, A.R.; Crouch, P.J. ZnII (atsm) is protective in amyotrophic lateral sclerosis model mice via a copper delivery mechanism. Neurobiol. Dis. 2015, 81, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Chaudhary, A.; Sonker, H.; Subhadarshini, S.; Jolly, M.K.; Singh, R.G. Zinc(II) Complexes of SIRTi1/2 Analogues Transmetallating with Copper Ions and Inducing ROS Mediated Paraptosis. ACS Org. Inorg. Au 2024, 4, 319–328. [Google Scholar] [CrossRef]
- Martínez-Camarena, Á.; Sour, A.; Faller, P. Impact of human serum albumin on Cu II and Zn II complexation by ATSM (diacetyl-bis (N 4-methylthiosemicarbazone)) and a water soluble analogue. Dalton Trans. 2023, 52, 13758–13768. [Google Scholar] [CrossRef] [PubMed]
- Hickey, J.L.; James, J.L.; Henderson, C.A.; Price, K.A.; Mot, A.I.; Buncic, G.; Crouch, P.J.; White, J.M.; White, A.R.; Smith, T.A.; et al. Intracellular distribution of fluorescent copper and zinc bis(thiosemicarbazonato) complexes measured with fluorescence lifetime spectroscopy. Inorg. Chem. 2015, 54, 9556–9567. [Google Scholar] [CrossRef] [PubMed]
- Arrowsmith, R.L.; Waghorn, P.A.; Jones, M.W.; Bauman, A.; Brayshaw, S.K.; Hu, Z.; Kociok-Kohn, G.; Mindt, T.L.; Tyrrell, R.M.; Botchway, S.W.; et al. Fluorescent gallium and indium bis(thiosemicarbazonates) and their radiolabelled analogues: Synthesis, structures and cellular confocal fluorescence imaging investigations. Dalton Trans. 2011, 40, 6238–6252. [Google Scholar] [CrossRef]
- Holland, J.P.; Barnard, P.J.; Bayly, S.R.; Betts, H.M.; Churchill, G.C.; Dilworth, J.R.; Edge, R.; Green, J.C.; Hueting, R. Synthesis, Radiolabelling and Confocal Fluorescence Microscopy of Styrene-Derivatised Bis(thiosemicarbazonato) Zinc and-Copper Complexes; Wiley Online Library: Hoboken, NJ, USA, 2008. [Google Scholar]
- Falcone, E.; Ritacca, A.G.; Hager, S.; Schueffl, H.; Vileno, B.; El Khoury, Y.; Hellwig, P.; Kowol, C.R.; Heffeter, P.; Sicilia, E.; et al. Copper-Catalyzed Glutathione Oxidation is Accelerated by the Anticancer Thiosemicarbazone Dp44mT and Further Boosted at Lower pH. J. Am. Chem. Soc. 2022, 144, 14758–14768. [Google Scholar] [CrossRef]
- Basha, M.T.; Chartres, J.D.; Pantarat, N.; Ali, M.A.; Mirza, A.H.; Kalinowski, D.S.; Richardson, D.R.; Bernhardt, P.V. Heterocyclic dithiocarbazate iron chelators: Fe coordination chemistry and biological activity. Dalton Trans. 2012, 41, 6536–6548. [Google Scholar] [CrossRef]
- Claudio-Ares, O.; Luciano-Rodriguez, J.; Del Valle-Gonzalez, Y.L.; Schiavone-Chamorro, S.L.; Pastor, A.J.; Rivera-Reyes, J.O.; Metzler, C.L.; Dominguez-Orona, L.M.; Vargas-Perez, B.L.; Skouta, R.; et al. Exploring the Use of Intracellular Chelation and Non-Iron Metals to Program Ferroptosis for Anticancer Application. Inorganics 2024, 12, 26. [Google Scholar] [CrossRef] [PubMed]
- Bernhardt, P.V.; Martinez, M.; Rodriguez, C.; Vazquez, M. Biologically active thiosemicarbazone Fe chelators and their reactions with ferrioxamine B and ferric EDTA; a kinetic study. Dalton Trans. 2012, 41, 2122–2130. [Google Scholar] [CrossRef] [PubMed]
- Gonzalvez, M.A.; Algarra, A.G.; Basallote, M.G.; Bernhardt, P.V.; Fernandez-Trujillo, M.J.; Martinez, M. Proton-assisted air oxidation mechanisms of iron(ii) bis-thiosemicarbazone complexes at physiological pH: A kinetico-mechanistic study. Dalton Trans. 2019, 48, 16578–16587. [Google Scholar] [CrossRef]
- Summers, K.L. A Structural Chemistry Perspective on the Antimalarial Properties of Thiosemicarbazone Metal Complexes. Mini Rev. Med. Chem. 2019, 19, 569–590. [Google Scholar] [CrossRef]
- Mrozek-Wilczkiewicz, A.; Malarz, K.; Rams-Baron, M.; Serda, M.; Bauer, D.; Montforts, F.P.; Ratuszna, A.; Burley, T.; Polanski, J.; Musiol, R. Iron Chelators and Exogenic Photosensitizers. Synergy through Oxidative Stress Gene Expression. J. Cancer 2017, 8, 1979–1987. [Google Scholar] [CrossRef] [PubMed]
- Timoshnikov, V.A.; Selyutina, O.Y.; Polyakov, N.E.; Didichenko, V.; Kontoghiorghes, G.J. Mechanistic Insights of Chelator Complexes with Essential Transition Metals: Antioxidant/Pro-Oxidant Activity and Applications in Medicine. Int. J. Mol. Sci. 2022, 23, 1247. [Google Scholar] [CrossRef]
- Balakrishnan, N.; Haribabu, J.; Dhanabalan, A.K.; Swaminathan, S.; Sun, S.; Dibwe, D.F.; Bhuvanesh, N.; Awale, S.; Karvembu, R. Thiosemicarbazone(s)-anchored water soluble mono- and bimetallic Cu(ii) complexes: Enzyme-like activities, biomolecular interactions, anticancer property and real-time live cytotoxicity. Dalton Trans. 2020, 49, 9411–9424. [Google Scholar] [CrossRef]
- Babak, M.V.; Ahn, D. Modulation of Intracellular Copper Levels as the Mechanism of Action of Anticancer Copper Complexes: Clinical Relevance. Biomedicines 2021, 9, 852. [Google Scholar] [CrossRef]
- Raviprolu, V.T.; Farias, P.; Carta, V.; Harman, H.; Lavallo, V. When the Ferrocene Analogy Breaks Down: Metallocene Transmetallation Chemistry. Angew. Chem. Int. Ed. Engl. 2023, 62, e202308359. [Google Scholar] [CrossRef]
- Loza-Rosas, S.A.; Vazquez-Salgado, A.M.; Rivero, K.I.; Negron, L.J.; Delgado, Y.; Benjamin-Rivera, J.A.; Vazquez-Maldonado, A.L.; Parks, T.B.; Munet-Colon, C.; Tinoco, A.D. Expanding the Therapeutic Potential of the Iron Chelator Deferasirox in the Development of Aqueous Stable Ti(IV) Anticancer Complexes. Inorg. Chem. 2017, 56, 7788–7802. [Google Scholar] [CrossRef]
- Choudhary, N.; Scheiber, H.; Zhang, J.; Patrick, B.O.; de Guadalupe Jaraquemada-Peláez, M.; Orvig, C. H4HBEDpa: Octadentate Chelate after AE Martell. Inorg. Chem. 2021, 60, 12855–12869. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, I.; Fernández-Vega, L.; Maser-Figueroa, A.N.; Sang, B.; González-Pagán, P.; Tinoco, A.D. Exploring titanium (IV) complexes as potential antimicrobial compounds. Antibiotics 2022, 11, 158. [Google Scholar] [CrossRef]
- Jomova, K.; Baros, S.; Valko, M. Redox active metal-induced oxidative stress in biological systems. Transit. Met. Chem. 2012, 37, 127–134. [Google Scholar] [CrossRef]
- Bonaccorso, C.; Marzo, T.; La Mendola, D. Biological Applications of Thiocarbohydrazones and Their Metal Complexes: A Perspective Review. Pharmaceuticals 2019, 13, 4. [Google Scholar] [CrossRef]
- Domingo, J.L.; Semelka, R.C. Gadolinium toxicity: Mechanisms, clinical manifestations, and nanoparticle role. Arch. Toxicol. 2025, 99, 3897–3916. [Google Scholar] [CrossRef]
- Petering, D.H. Reactions of the Zn Proteome with Cd2+ and Other Xenobiotics: Trafficking and Toxicity. Chem. Res. Toxicol. 2017, 30, 189–202. [Google Scholar] [CrossRef] [PubMed]
- Maret, W.; Li, Y. Coordination dynamics of zinc in proteins. Chem. Rev. 2009, 109, 4682–4707. [Google Scholar] [CrossRef]
- Lawson, M.K.; Valko, M.; Cronin, M.T.; Jomová, K. Chelators in iron and copper toxicity. Curr. Pharmacol. Rep. 2016, 2, 271–280. [Google Scholar] [CrossRef]
- Rychtarcikova, Z.; Lettlova, S.; Tomkova, V.; Korenkova, V.; Langerova, L.; Simonova, E.; Zjablovskaja, P.; Alberich-Jorda, M.; Neuzil, J.; Truksa, J. Tumor-initiating cells of breast and prostate origin show alterations in the expression of genes related to iron metabolism. Oncotarget 2016, 8, 6376. [Google Scholar] [CrossRef]
- Wang, Y.F.; Zhang, J.; Su, Y.; Shen, Y.Y.; Jiang, D.X.; Hou, Y.Y.; Geng, M.Y.; Ding, J.; Chen, Y. G9a regulates breast cancer growth by modulating iron homeostasis through the repression of ferroxidase hephaestin. Nat. Commun. 2017, 8, 274. Correction in Nat. Commun. 2020, 11, 3789. https://doi.org/10.1038/s41467-020-17413-z. [CrossRef] [PubMed]
- Merlot, A.M.; Kalinowski, D.S.; Richardson, D.R. Novel chelators for cancer treatment: Where are we now? Antioxid. Redox Signal. 2013, 18, 973–1006. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, L.; Ding, J.; Chen, Y. Iron Metabolism in Cancer. Int. J. Mol. Sci. 2018, 20, 95. [Google Scholar] [CrossRef] [PubMed]
- Hsu, M.Y.; Mina, E.; Roetto, A.; Porporato, P.E. Iron: An Essential Element of Cancer Metabolism. Cells 2020, 9, 2591. [Google Scholar] [CrossRef]
- Wang, J.; Li, J.; Liu, J.; Chan, K.Y.; Lee, H.S.; Lin, K.N.; Wang, C.C.; Lau, T.S. Interplay of Ferroptosis and Cuproptosis in Cancer: Dissecting Metal-Driven Mechanisms for Therapeutic Potentials. Cancers 2024, 16, 512. [Google Scholar] [CrossRef]
- Zhou, L.; Zhao, B.; Zhang, L.; Wang, S.; Dong, D.; Lv, H.; Shang, P. Alterations in Cellular Iron Metabolism Provide More Therapeutic Opportunities for Cancer. Int. J. Mol. Sci. 2018, 19, 1545. [Google Scholar] [CrossRef]
- Xu, L.; Peng, M.; Gao, T.; Wang, D.; Lian, X.; Sun, H.; Shi, J.; Wang, Y.; Wang, P. Nanoenabled Intracellular Metal Ion Homeostasis Regulation for Tumor Therapy. Adv. Sci. 2024, 11, e2306203. [Google Scholar] [CrossRef]
- Leitao, M.; Morais, T.S. Tailored Metal-Based Catalysts: A New Platform for Targeted Anticancer Therapies. J. Med. Chem. 2024, 67, 16967–16990. [Google Scholar] [CrossRef]
- Halcrow, P.; Datta, G.; Ohm, J.E.; Soliman, M.L.; Chen, X.; Geiger, J.D. Role of endolysosomes and pH in the pathogenesis and treatment of glioblastoma. Cancer Rep. 2019, 2, e1177. [Google Scholar] [CrossRef] [PubMed]
- Polishchuk, E.V.; Polishchuk, R.S. The emerging role of lysosomes in copper homeostasis. Metallomics 2016, 8, 853–862. [Google Scholar] [CrossRef]
- Kontoghiorghes, G.J. New Iron Metabolic Pathways and Chelation Targeting Strategies Affecting the Treatment of All Types and Stages of Cancer. Int. J. Mol. Sci. 2022, 23, 13990. [Google Scholar] [CrossRef]
- Anthony, E.J.; Bolitho, E.M.; Bridgewater, H.E.; Carter, O.W.L.; Donnelly, J.M.; Imberti, C.; Lant, E.C.; Lermyte, F.; Needham, R.J.; Palau, M.; et al. Metallodrugs are unique: Opportunities and challenges of discovery and development. Chem. Sci. 2020, 11, 12888–12917. [Google Scholar] [CrossRef]
- Aye, Y.; Long, M.J.C.; Stubbe, J. Mechanistic studies of semicarbazone triapine targeting human ribonucleotide reductase in vitro and in mammalian cells: Tyrosyl radical quenching not involving reactive oxygen species. J. Biol. Chem. 2012, 287, 35768–35778. [Google Scholar] [CrossRef]
- Sestak, V.; Stariat, J.; Cermanova, J.; Potuckova, E.; Chladek, J.; Roh, J.; Bures, J.; Jansova, H.; Prusa, P.; Sterba, M.; et al. Novel and potent anti-tumor and anti-metastatic di-2-pyridylketone thiosemicarbazones demonstrate marked differences in pharmacology between the first and second generation lead agents. Oncotarget 2015, 6, 42411–42428. [Google Scholar] [CrossRef] [PubMed]
- Jomova, K.; Valko, M. Advances in metal-induced oxidative stress and human disease. Toxicology 2011, 283, 65–87. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Morris, H.; Cronin, M.T. Metals, toxicity and oxidative stress. Curr. Med. Chem. 2005, 12, 1161–1208. [Google Scholar] [CrossRef] [PubMed]
- Ercal, N.; Gurer-Orhan, H.; Aykin-Burns, N. Toxic metals and oxidative stress part I: Mechanisms involved in metal-induced oxidative damage. Curr. Top. Med. Chem. 2001, 1, 529–539. [Google Scholar] [CrossRef]
- Uriu-Adams, J.Y.; Keen, C.L. Copper, oxidative stress, and human health. Mol. Aspects Med. 2005, 26, 268–298. [Google Scholar] [CrossRef]
- Joyce, P.; Allen, C.J.; Alonso, M.J.; Ashford, M.; Bradbury, M.S.; Germain, M.; Kavallaris, M.; Langer, R.; Lammers, T.; Peracchia, M.T. A translational framework to DELIVER nanomedicines to the clinic. Nat. Nanotechnol. 2024, 19, 1597–1611. [Google Scholar] [CrossRef]
- Stielow, M.; Witczyńska, A.; Kubryń, N.; Fijałkowski, Ł.; Nowaczyk, J.; Nowaczyk, A. The bioavailability of drugs—The current state of knowledge. Molecules 2023, 28, 8038. [Google Scholar] [CrossRef]
- Sharma, S. Nanotheranostics in evidence based personalized medicine. Curr. Drug Targets 2014, 15, 915–930. [Google Scholar] [CrossRef]
- Peng, X.X.; Gao, S.; Zhang, J.L. Gallium (III) complexes in cancer chemotherapy. Eur. J. Inorg. Chem. 2022, 2022, e202100953. [Google Scholar] [CrossRef]
- Qi, J.; Qian, K.; Tian, L.; Cheng, Z.; Wang, Y. Gallium (iii)–2-benzoylpyridine-thiosemicarbazone complexes promote apoptosis through Ca2+ signaling and ROS-mediated mitochondrial pathways. New Chem. 2018, 42, 10226–10233. [Google Scholar] [CrossRef]
- Kostova, I.; Balkansky, S. Metal complexes of biologically active ligands as potential antioxidants. Curr. Med. Chem. 2013, 20, 4508–4539. [Google Scholar] [CrossRef]
- Chassaing, S.; Collin, F.; Dorlet, P.; Gout, J.; Hureau, C.; Faller, P. Copper and heme-mediated Abeta toxicity: Redox chemistry, Abeta oxidations and anti-ROS compounds. Curr. Top. Med. Chem. 2012, 12, 2573–2595. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Liu, Z.; Lei, H.; Miao, Y.B.; Chen, J. Programmable Nanomodulators for Precision Therapy, Engineering Tumor Metabolism to Enhance Therapeutic Efficacy. Adv. Healthc. Mater. 2025, 14, e2403019. [Google Scholar] [CrossRef] [PubMed]
- Chitambar, C.R.; Antholine, W.E. Iron-targeting antitumor activity of gallium compounds and novel insights into triapine((R))-metal complexes. Antioxid. Redox Signal. 2013, 18, 956–972. [Google Scholar] [CrossRef]
- Chitambar, C.R. Gallium-containing anticancer compounds. Future Med. Chem. 2012, 4, 1257–1272. [Google Scholar] [CrossRef]
- Jakupec, M.A.; Keppler, B.K. Gallium and other main group metal compounds as antitumor agents. Met. Ions Biol. Syst. 2004, 42, 425–462. [Google Scholar]
- Munteanu, C.R.; Suntharalingam, K. Advances in cobalt complexes as anticancer agents. Dalton Trans. 2015, 44, 13796–13808. [Google Scholar] [CrossRef]
- Jana, A.; Aher, A.; Brandao, P.; Bera, P.; Sharda, S.; Phadikar, U.; Manna, S.K.; Mahapatra, A.K.; Bera, P. Evaluation of the anticancer activities with various ligand substituents in Co(II/III)-picolyl phenolate derivatives: Synthesis, characterization, DFT, DNA cleavage, and molecular docking studies. Dalton Trans. 2022, 51, 2346–2363. [Google Scholar] [CrossRef]
- Zhang, P.; Sadler, P.J. Redox-active metal complexes for anticancer therapy. Eur. J. Inorg. Chem. 2017, 2017, 1541–1548. [Google Scholar] [CrossRef]
- Batinic-Haberle, I.; Tovmasyan, A.; Spasojevic, I. Mn Porphyrin-Based Redox-Active Drugs: Differential Effects as Cancer Therapeutics and Protectors of Normal Tissue Against Oxidative Injury. Antioxid. Redox Signal. 2018, 29, 1691–1724. [Google Scholar] [CrossRef]
- Tovmasyan, A.; Bueno-Janice, J.C.; Jaramillo, M.C.; Sampaio, R.S.; Reboucas, J.S.; Kyui, N.; Benov, L.; Deng, B.; Huang, T.T.; Tome, M.E.; et al. Radiation-Mediated Tumor Growth Inhibition Is Significantly Enhanced with Redox-Active Compounds That Cycle with Ascorbate. Antioxid. Redox Signal. 2018, 29, 1196–1214. [Google Scholar] [CrossRef] [PubMed]
- Guan, Q.X.; Yu, L.B.; Wang, P.; Hu, Q.Y.; Tan, C.P. Mitochondria-Targeted Titanium Complex Exerts Potent Anticancer Activity by Disturbing Iron Homeostasis. ACS Pharmacol. Transl. Sci. 2025, 8, 1804–1813. [Google Scholar] [CrossRef]
- Gaur, K.; Vazquez-Salgado, A.M.; Duran-Camacho, G.; Dominguez-Martinez, I.; Benjamin-Rivera, J.A.; Fernandez-Vega, L.; Sarabia, L.C.; Garcia, A.C.; Perez-Deliz, F.; Mendez Roman, J.A.; et al. Iron and Copper Intracellular Chelation as an Anticancer Drug Strategy. Inorganics 2018, 6, 126. [Google Scholar] [CrossRef]
- Estrada-Montaño, A.S.; Ryabov, A.D.; Gries, A.; Gaiddon, C.; Le Lagadec, R. Iron (III) pincer complexes as a strategy for anticancer studies. Eur. J. Inorg. Chem. 2017, 2017, 1673–1678. [Google Scholar] [CrossRef]
- Kaya, B.; Smith, H.; Chen, Y.; Azad, M.G.; Russell, T.M.; Richardson, V.; Bernhardt, P.V.; Dharmasivam, M.; Richardson, D.R. Targeting lysosomes by design: Novel N-acridine thiosemicarbazones that enable direct detection of intracellular drug localization and overcome P-glycoprotein (Pgp)-mediated resistance. Chem. Sci. 2024, 15, 15109–15124. [Google Scholar] [CrossRef] [PubMed]
- Ahrland, S. Thermodynamics of complex formation between hard and soft acceptors and donors. In Structure and Bonding; Springer: Berlin/Heidelberg, Germany, 2008; pp. 118–149. [Google Scholar]
- Xu, H.; Xu, D.C.; Wang, Y. Natural Indices for the Chemical Hardness/Softness of Metal Cations and Ligands. ACS Omega 2017, 2, 7185–7193. [Google Scholar] [CrossRef]
- Braunstein, P.; Danopoulos, A.A. Transition Metal Chain Complexes Supported by Soft Donor Assembling Ligands. Chem. Rev. 2021, 121, 7346–7397. [Google Scholar] [CrossRef] [PubMed]
- Augustine, L.J.; Kasper, J.M.; Forbes, T.Z.; Mason, S.E.; Batista, E.R.; Yang, P. Influencing Bonding Interactions of the Neptunyl (V, VI) Cations with Electron-Donating and-Withdrawing Groups. Inorg. Chem. 2023, 62, 6055–6064. [Google Scholar] [CrossRef] [PubMed]
- Strautmann, J.B.; George, S.D.; Bothe, E.; Bill, E.; Weyhermuller, T.; Stammler, A.; Bogge, H.; Glaser, T. Molecular and electronic structures of mononuclear iron complexes using strongly electron-donating ligands and their oxidized forms. Inorg. Chem. 2008, 47, 6804–6824. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Zhang, Y.; Li, A.Y. Effects of Electron-Withdrawing and-Donating Substituents in N-Donor Scorpionate Ligands and the Metal 5 f/4 f Orbitals on Am (III)/Eu (III) Complexation and Separation. ChemistrySelect 2022, 7, e202203622. [Google Scholar] [CrossRef]
- McGrady, J.E.; Lovell, T.; Stranger, R.; Humphrey, M.G. Bonding of η1-acetylide ligands to electron-rich ruthenium centers: Can electron-withdrawing ligands induce significant metal-to-ligand back-bonding? Organometallics 1997, 16, 4004–4011. [Google Scholar] [CrossRef]
- Hannah, T.J.; Chitnis, S.S. Ligand-enforced geometric constraints and associated reactivity in p-block compounds. Chem. Soc. Rev. 2024, 53, 764–792. [Google Scholar] [CrossRef]
- Power, P.P. Stable two-coordinate, open-shell (d1–d9) transition metal complexes. Chem. Rev. 2012, 112, 3482–3507. [Google Scholar] [CrossRef]
- Carnes, M.E.; Collins, M.S.; Johnson, D.W. Transmetalation of self-assembled, supramolecular complexes. Chem. Soc. Rev. 2014, 43, 1825–1834. [Google Scholar] [CrossRef]
- Tamoradi, T.; Navaee, A.; Salimi, A.; Mousavi, S.M.; Ghadermazi, M.; Veisi, H. Magnetic nanoparticles supported Cu2+ and Ce3+ complexes: Toward the chemical and electrochemical oxidation of alcohol and sulfide derivatives. Res. Chem. Intermed. 2019, 45, 4517–4530. [Google Scholar] [CrossRef]
- Chand, A.; Sahoo, D.K.; Rana, A.; Jena, S.; Biswal, H.S. The Prodigious Hydrogen Bonds with Sulfur and Selenium in Molecular Assemblies, Structural Biology, and Functional Materials. Acc. Chem. Res. 2020, 53, 1580–1592. [Google Scholar] [CrossRef]
- Lu, T.; Chen, R.; Liu, Q.; Zhong, Y.; Lei, F.; Zeng, Z. Unveiling the Nature and Strength of Selenium-Centered Chalcogen Bonds in Binary Complexes of SeO2 with Oxygen-/Sulfur-Containing Lewis Bases: Insights from Theoretical Calculations. Int. J. Mol. Sci. 2024, 25, 5609. [Google Scholar] [CrossRef]
- Iwaoka, M.; Arai, K. From sulfur to selenium. A new research arena in chemical biology and biological chemistry. Curr. Chem. Biol. 2013, 7, 2–24. [Google Scholar] [CrossRef]
- Krezel, A.; Maret, W. The Bioinorganic Chemistry of Mammalian Metallothioneins. Chem. Rev. 2021, 121, 14594–14648. [Google Scholar] [CrossRef]
- Pavan, F.R.; Maia, P.I.d.S.; Leite, S.R.; Deflon, V.M.; Batista, A.A.; Sato, D.N.; Franzblau, S.G.; Leite, C.Q. Thiosemicarbazones, semicarbazones, dithiocarbazates and hydrazide/hydrazones: Anti-Mycobacterium tuberculosis activity and cytotoxicity. Eur. J. Med. Chem. 2010, 45, 1898–1905. [Google Scholar] [CrossRef]
- Hammad, M.; Raftari, M.; Cesario, R.; Salma, R.; Godoy, P.; Emami, S.N.; Haghdoost, S. Roles of Oxidative Stress and Nrf2 Signaling in Pathogenic and Non-Pathogenic Cells: A Possible General Mechanism of Resistance to Therapy. Antioxidants 2023, 12, 1371. [Google Scholar] [CrossRef]
- Phan, L.M.; Rezaeian, A.H. ATM: Main Features, Signaling Pathways, and Its Diverse Roles in DNA Damage Response, Tumor Suppression, and Cancer Development. Genes 2021, 12, 845. [Google Scholar] [CrossRef]
- Xie, X.; Zhang, Y.; Wang, Z.; Wang, S.; Jiang, X.; Cui, H.; Zhou, T.; He, Z.; Feng, H.; Guo, Q.; et al. ATM at the crossroads of reactive oxygen species and autophagy. Int. J. Biol. Sci. 2021, 17, 3080–3090. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Sorrell, M.; Berman, Z. Functional interplay between ATM/ATR-mediated DNA damage response and DNA repair pathways in oxidative stress. Cell Mol. Life Sci. 2014, 71, 3951–3967. [Google Scholar] [CrossRef]
- Jomova, K.; Raptova, R.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Reactive oxygen species, toxicity, oxidative stress, and antioxidants: Chronic diseases and aging. Arch. Toxicol. 2023, 97, 2499–2574. [Google Scholar] [CrossRef]
- Kołodziej, U.; Maciejczyk, M.; Zalewska, A. Oxidative stress–repair systems of oxidatively damaged biomolecules. Prog. Health Sci. 2018, 8, 141–150. [Google Scholar]
- Marchenko, N.D.; Moll, U.M. Mitochondrial death functions of p53. Mol. Cell Oncol. 2014, 1, e955995. [Google Scholar] [CrossRef]
- Wang, H.; Guo, M.; Wei, H.; Chen, Y. Targeting p53 pathways: Mechanisms, structures, and advances in therapy. Signal Transduct. Target. Ther. 2023, 8, 92. [Google Scholar] [CrossRef]
- Ritacca, A.G.; Falcone, E.; Doumi, I.; Vileno, B.; Faller, P.; Sicilia, E. Dual Role of Glutathione as a Reducing Agent and Cu-Ligand Governs the ROS Production by Anticancer Cu-Thiosemicarbazone Complexes. Inorg. Chem. 2023, 62, 3957–3964. [Google Scholar] [CrossRef]
- Carter, A.; Racey, S.; Veuger, S. The role of iron in DNA and genomic instability in cancer, a target for iron chelators that can induce ROS. Appl. Sci. 2022, 12, 10161. [Google Scholar] [CrossRef]
- Caillot, M.; Dakik, H.; Mazurier, F.; Sola, B. Targeting Reactive Oxygen Species Metabolism to Induce Myeloma Cell Death. Cancers 2021, 13, 2411. [Google Scholar] [CrossRef] [PubMed]
- Fujii, J. Redox remodeling of central metabolism as a driving force for cellular protection, proliferation, differentiation, and dysfunction. Free Radic. Res. 2024, 58, 606–629. [Google Scholar] [CrossRef]
- Senapati, S.; Mahanta, A.K.; Kumar, S.; Maiti, P. Controlled drug delivery vehicles for cancer treatment and their performance. Signal Transduct. Target. Ther. 2018, 3, 7. [Google Scholar] [CrossRef]
- Peng, K.; Zheng, Y.; Xia, W.; Mao, Z.W. Organometallic anti-tumor agents: Targeting from biomolecules to dynamic bioprocesses. Chem. Soc. Rev. 2023, 52, 2790–2832. [Google Scholar] [CrossRef] [PubMed]
- Hunsaker, E.W.; Franz, K.J. Emerging Opportunities To Manipulate Metal Trafficking for Therapeutic Benefit. Inorg. Chem. 2019, 58, 13528–13545. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, M. Quinones as photosensitizer for photodynamic therapy: ROS generation, mechanism and detection methods. Photodiagn. Photodyn. Ther. 2016, 13, 175–187. [Google Scholar] [CrossRef]
- Kwong, W.L.; Lok, C.N.; Tse, C.W.; Wong, E.L.; Che, C.M. Anti-cancer iron(II) complexes of pentadentate N-donor ligands: Cytotoxicity, transcriptomics analyses, and mechanisms of action. Chemistry 2015, 21, 3062–3072. [Google Scholar] [CrossRef]
- Ganguly, A.; Basu, S.; Chakraborty, P.; Chatterjee, S.; Sarkar, A.; Chatterjee, M.; Choudhuri, S.K. Targeting mitochondrial cell death pathway to overcome drug resistance with a newly developed iron chelate. PLoS ONE 2010, 5, e11253. [Google Scholar] [CrossRef]
- Shao, J.; Ma, Z.Y.; Li, A.; Liu, Y.H.; Xie, C.Z.; Qiang, Z.Y.; Xu, J.Y. Thiosemicarbazone Cu(II) and Zn(II) complexes as potential anticancer agents: Syntheses, crystal structure, DNA cleavage, cytotoxicity and apoptosis induction activity. J. Inorg. Biochem. 2014, 136, 13–23. [Google Scholar] [CrossRef]
- Matesanz, A.I.; Jimenez-Faraco, E.; Ruiz, M.C.; Balsa, L.M.; Navarro-Ranninger, C.; León, I.E.; Quiroga, A.G. Mononuclear Pd (II) and Pt (II) complexes with an α-N-heterocyclic thiosemicarbazone: Cytotoxicity, solution behaviour and interaction versus proven models from biological media. Inorg. Chem. Front. 2018, 5, 73–83. [Google Scholar] [CrossRef]
- Frigerio, C.; Galli, M.; Castelli, S.; Da Prada, A.; Clerici, M. Control of Replication Stress Response by Cytosolic Fe-S Cluster Assembly (CIA) Machinery. Cells 2025, 14, 442. [Google Scholar] [CrossRef]
- Richardson, D.R. Molecular mechanisms of iron uptake by cells and the use of iron chelators for the treatment of cancer. Curr. Med. Chem. 2005, 12, 2711–2729. [Google Scholar] [CrossRef]
- Saletta, F.; Rahmanto, Y.S.; Noulsri, E.; Richardson, D.R. Iron chelator-mediated alterations in gene expression: Identification of novel iron-regulated molecules that are molecular targets of hypoxia-inducible factor-1α and p53. Mol. Pharmacol. 2010, 77, 443–458. [Google Scholar] [CrossRef]
- Weinreb, O.; Amit, T.; Mandel, S.; Kupershmidt, L.; Youdim, M.B. Neuroprotective multifunctional iron chelators: From redox-sensitive process to novel therapeutic opportunities. Antioxid. Redox Signal. 2010, 13, 919–949. [Google Scholar] [CrossRef]
- Cai, J.; Yang, J.; Jones, D.P. Mitochondrial control of apoptosis: The role of cytochrome c. Biochim. Biophys. Acta 1998, 1366, 139–149. [Google Scholar] [CrossRef]
- Caroppi, P.; Sinibaldi, F.; Fiorucci, L.; Santucci, R. Apoptosis and human diseases: Mitochondrion damage and lethal role of released cytochrome C as proapoptotic protein. Curr. Med. Chem. 2009, 16, 4058–4065. [Google Scholar] [CrossRef]
- Garrido, C.; Galluzzi, L.; Brunet, M.; Puig, P.E.; Didelot, C.; Kroemer, G. Mechanisms of cytochrome c release from mitochondria. Cell Death Differ. 2006, 13, 1423–1433. [Google Scholar] [CrossRef]
- Zec, M.; Srdic-Rajic, T.; Krivokuca, A.; Jankovic, R.; Todorovic, T.; Andelkovic, K.; Radulovic, S. Novel selenosemicarbazone metal complexes exert anti-tumor effect via alternative, caspase-independent necroptotic cell death. Med. Chem. 2014, 10, 759–771. [Google Scholar] [CrossRef]
- Bisceglie, F.; Alinovi, R.; Pinelli, S.; Galetti, M.; Pioli, M.; Tarasconi, P.; Mutti, A.; Goldoni, M.; Pelosi, G. Autophagy and apoptosis: Studies on the effects of bisthiosemicarbazone copper(ii) complexes on p53 and p53-null tumour cell lines. Metallomics 2016, 8, 1255–1265. [Google Scholar] [CrossRef]
- Hancock, C.N.; Stockwin, L.H.; Han, B.; Divelbiss, R.D.; Jun, J.H.; Malhotra, S.V.; Hollingshead, M.G.; Newton, D.L. A copper chelate of thiosemicarbazone NSC 689534 induces oxidative/ER stress and inhibits tumor growth in vitro and in vivo. Free Radic. Biol. Med. 2011, 50, 110–121. [Google Scholar] [CrossRef]
- Paliwal, K.; Swain, A.; Mishra, D.P.; Kumar, M. Targeting Triple Negative Breast Cancer with a Dinuclear Copper(II) Thiocarbohydrazone Complex: Efficacy Evaluation and Cellular Response. ACS Omega 2025, 10, 41342–41358. [Google Scholar] [CrossRef]
- Li, A.; Huang, K.; Pan, W.; Wu, Y.; Liang, Y.; Zhang, Z.; Wu, D.; Ma, L.; Gou, Y. Thiosemicarbazone Mixed-Valence Cu(I/II) Complex against Lung Adenocarcinoma Cells through Multiple Pathways Involving Cuproptosis. J. Med. Chem. 2024, 67, 9091–9103. [Google Scholar] [CrossRef]
- Szymonik, J.; Wala, K.; Gornicki, T.; Saczko, J.; Pencakowski, B.; Kulbacka, J. The Impact of Iron Chelators on the Biology of Cancer Stem Cells. Int. J. Mol. Sci. 2021, 23, 89. [Google Scholar] [CrossRef]
- Vidanapathirana, G.; Islam, M.S.; Gamage, S.; Lam, A.K.; Gopalan, V. The Role of Iron Chelation Therapy in Colorectal Cancer: A Systematic Review on Its Mechanisms and Therapeutic Potential. Cancer Med. 2025, 14, e71019. [Google Scholar] [CrossRef]
- Malarz, K.; Mrozek-Wilczkiewicz, A.; Serda, M.; Rejmund, M.; Polanski, J.; Musiol, R. The role of oxidative stress in activity of anticancer thiosemicarbazones. Oncotarget 2018, 9, 17689–17710. [Google Scholar] [CrossRef]
- Ghosh, S.; Chakrabarty, R.; Paira, P. Harnessing photodynamic therapy for programmed cell death: The central role and contributions of metal complexes as next generation photosensitizers. RSC Med. Chem. 2025; online ahead of print. [Google Scholar] [CrossRef]
- Zhang, Y.; Doan, B.T.; Gasser, G. Metal-Based Photosensitizers as Inducers of Regulated Cell Death Mechanisms. Chem. Rev. 2023, 123, 10135–10155. [Google Scholar] [CrossRef]
- Baecker, D.; Sesli, O.; Knabl, L.; Huber, S.; Orth-Holler, D.; Gust, R. Investigating the antibacterial activity of salen/salophene metal complexes: Induction of ferroptosis as part of the mode of action. Eur. J. Med. Chem. 2021, 209, 112907. [Google Scholar] [CrossRef] [PubMed]
- Lv, M.; Zheng, Y.; Dai, X.; Zhao, J.; Hu, G.; Ren, M.; Shen, Z.; Su, Z.; Wu, C.; Liu, H.K.; et al. Ruthenium(ii)-Arene Complex Triggers Immunogenic Ferroptosis for Reversing Drug Resistance. J. Med. Chem. 2024, 67, 20156–20171. [Google Scholar] [CrossRef]
- Abeydeera, N. Recent Development of Exploring Ferroptosis-Inspired Effect of Iron as a Feasible Strategy for Combating Multidrug Resistant Bacterial Infections. Appl. Microbiol. 2025, 5, 73. [Google Scholar] [CrossRef]
- Su, L.J.; Zhang, J.H.; Gomez, H.; Murugan, R.; Hong, X.; Xu, D.; Jiang, F.; Peng, Z.Y. Reactive Oxygen Species-Induced Lipid Peroxidation in Apoptosis, Autophagy, and Ferroptosis. Oxid. Med. Cell. Longev. 2019, 2019, 5080843. [Google Scholar] [CrossRef]
- Maiorino, M.; Conrad, M.; Ursini, F. GPx4, Lipid Peroxidation, and Cell Death: Discoveries, Rediscoveries, and Open Issues. Antioxid. Redox Signal. 2018, 29, 61–74. [Google Scholar] [CrossRef]
- Wei, Y.; Lv, H.; Shaikh, A.B.; Han, W.; Hou, H.; Zhang, Z.; Wang, S.; Shang, P. Directly targeting glutathione peroxidase 4 may be more effective than disrupting glutathione on ferroptosis-based cancer therapy. Biochim. Biophys. Acta Gen. Subj. 2020, 1864, 129539. [Google Scholar] [CrossRef]
- Stockwell, B.R.; Jiang, X.; Gu, W. Emerging Mechanisms and Disease Relevance of Ferroptosis. Trends Cell Biol. 2020, 30, 478–490. [Google Scholar] [CrossRef] [PubMed]
- Mazhar, M.; Din, A.U.; Ali, H.; Yang, G.; Ren, W.; Wang, L.; Fan, X.; Yang, S. Implication of ferroptosis in aging. Cell Death Discov. 2021, 7, 149. [Google Scholar] [CrossRef]
- Liang, C.; Zhang, X.; Yang, M.; Dong, X. Recent Progress in Ferroptosis Inducers for Cancer Therapy. Adv. Mater. 2019, 31, e1904197. [Google Scholar] [CrossRef]
- Poltorack, C.D.; Dixon, S.J. Understanding the role of cysteine in ferroptosis: Progress & paradoxes. FEBS J. 2022, 289, 374–385. [Google Scholar] [PubMed]
- Otasevic, V.; Vucetic, M.; Grigorov, I.; Martinovic, V.; Stancic, A. Ferroptosis in Different Pathological Contexts Seen through the Eyes of Mitochondria. Oxid. Med. Cell. Longev. 2021, 2021, 5537330. [Google Scholar] [CrossRef] [PubMed]
- You, Y.; Qian, Z.; Jiang, Y.; Chen, L.; Wu, D.; Liu, L.; Zhang, F.; Ning, X.; Zhang, Y.; Xiao, J. Insights into the pathogenesis of gestational and hepatic diseases: The impact of ferroptosis. Front. Cell Dev. Biol. 2024, 12, 1482838. [Google Scholar] [CrossRef]
- Ibrahim, O.; O’Sullivan, J. Iron chelators in cancer therapy. BioMetals 2020, 33, 201–215. [Google Scholar] [CrossRef]
- PapeVeronika, F.; EnyedyÉva, A.; KepplerBernhard, K.; KowolChristian, R. Anticancer thiosemicarbazones: Chemical properties, interaction with iron metabolism, and resistance development. Antioxid. Redox Signal. 2019, 30, 1062–1082. [Google Scholar]
- Wang, Y.; Wei, Z.; Pan, K.; Li, J.; Chen, Q. The function and mechanism of ferroptosis in cancer. Apoptosis 2020, 25, 786–798. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, C.; Wang, J.; Hu, W.; Feng, Z. The Regulation of Ferroptosis by Tumor Suppressor p53 and its Pathway. Int. J. Mol. Sci. 2020, 21, 8387. [Google Scholar] [CrossRef] [PubMed]
- Hassannia, B.; Vandenabeele, P.; Vanden Berghe, T. Targeting Ferroptosis to Iron Out Cancer. Cancer Cell 2019, 35, 830–849. [Google Scholar] [CrossRef]
- Xu, Y.; Zhao, J.; Zhao, Y.; Zhou, L.; Qiao, H.; Xu, Q.; Liu, Y. The role of ferroptosis in neurodegenerative diseases. Mol. Biol. Rep. 2023, 50, 1655–1661. [Google Scholar] [CrossRef]
- Reichert, C.O.; de Freitas, F.A.; Sampaio-Silva, J.; Rokita-Rosa, L.; Barros, P.L.; Levy, D.; Bydlowski, S.P. Ferroptosis Mechanisms Involved in Neurodegenerative Diseases. Int. J. Mol. Sci. 2020, 21, 8765. [Google Scholar] [CrossRef]
- David, S.; Jhelum, P.; Ryan, F.; Jeong, S.Y.; Kroner, A. Dysregulation of Iron Homeostasis in the Central Nervous System and the Role of Ferroptosis in Neurodegenerative Disorders. Antioxid. Redox Signal. 2022, 37, 150–170. [Google Scholar] [CrossRef]
- Richardson, A.; Kovacevic, Z.; Richardson, D.R. Iron chelation: Inhibition of key signaling pathways in the induction of the epithelial mesenchymal transition in pancreatic cancer and other tumors. Crit. Rev. Oncog. 2013, 18, 409–434. [Google Scholar] [CrossRef] [PubMed]
- Abdelaal, G.; Veuger, S. Reversing oncogenic transformation with iron chelation. Oncotarget 2021, 12, 106–124. [Google Scholar] [CrossRef] [PubMed]
- Joshi, V.; Lakhani, S.R.; McCart Reed, A.E. NDRG1 in cancer: A suppressor, promoter, or both? Cancers 2022, 14, 5739. [Google Scholar] [CrossRef]
- Liu, W.; Kovacevic, Z.; Peng, Z.; Jin, R.; Wang, P.; Yue, F.; Zheng, M.; Huang, M.L.; Jansson, P.J.; Richardson, V.; et al. The molecular effect of metastasis suppressors on Src signaling and tumorigenesis: New therapeutic targets. Oncotarget 2015, 6, 35522–35541. [Google Scholar] [CrossRef] [PubMed]
- Macsek, P.; Skoda, J.; Krchniakova, M.; Neradil, J.; Veselska, R. Iron-Chelation Treatment by Novel Thiosemicarbazone Targets Major Signaling Pathways in Neuroblastoma. Int. J. Mol. Sci. 2021, 23, 376. [Google Scholar] [CrossRef]
- Moussa, R.S.; Kovacevic, Z.; Richardson, D.R. Differential targeting of the cyclin-dependent kinase inhibitor, p21CIP1/WAF1, by chelators with anti-proliferative activity in a range of tumor cell-types. Oncotarget 2015, 6, 29694–29711. [Google Scholar] [CrossRef]
- Yu, Y.; Kalinowski, D.S.; Kovacevic, Z.; Siafakas, A.R.; Jansson, P.J.; Stefani, C.; Lovejoy, D.B.; Sharpe, P.C.; Bernhardt, P.V.; Richardson, D.R. Thiosemicarbazones from the old to new: Iron chelators that are more than just ribonucleotide reductase inhibitors. J. Med. Chem. 2009, 52, 5271–5294. [Google Scholar] [CrossRef]
- Pena, Q.; Wang, A.; Zaremba, O.; Shi, Y.; Scheeren, H.W.; Metselaar, J.M.; Kiessling, F.; Pallares, R.M.; Wuttke, S.; Lammers, T. Metallodrugs in cancer nanomedicine. Chem. Soc. Rev. 2022, 51, 2544–2582. [Google Scholar] [CrossRef]
- Joseph, S.; Chakrabarty, R.; Paira, P. Advances in nano-drug delivery systems for metallic compounds in cancer therapy: Challenges and future perspectives. Dalton Trans. 2025, 54, 13820–13850. [Google Scholar] [CrossRef]
- Abdullah, K.M.; Sharma, G.; Singh, A.P.; Siddiqui, J.A. Nanomedicine in Cancer Therapeutics: Current Perspectives from Bench to Bedside. Mol. Cancer 2025, 24, 169. [Google Scholar] [CrossRef]
- Wang, A.; Walden, M.; Ettlinger, R.; Kiessling, F.; Gassensmith, J.J.; Lammers, T.; Wuttke, S.; Pena, Q. Biomedical Metal-Organic Framework Materials: Perspectives and Challenges. Adv. Funct. Mater. 2024, 34, 2308589. [Google Scholar] [CrossRef]
- Knox, J.J.; Hotte, S.J.; Kollmannsberger, C.; Winquist, E.; Fisher, B.; Eisenhauer, E.A. Phase II study of Triapine in patients with metastatic renal cell carcinoma: A trial of the National Cancer Institute of Canada Clinical Trials Group (NCIC IND.161). Investig. New Drugs 2007, 25, 471–477. [Google Scholar] [CrossRef]
- Synnott, N.C.; O’Connell, D.; Crown, J.; Duffy, M.J. COTI-2 reactivates mutant p53 and inhibits growth of triple-negative breast cancer cells. Breast Cancer Res. Treat. 2020, 179, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Rao, V.A.; Zhang, J.; Klein, S.R.; Espandiari, P.; Knapton, A.; Dickey, J.S.; Herman, E.; Shacter, E.B. The iron chelator Dp44mT inhibits the proliferation of cancer cells but fails to protect from doxorubicin-induced cardiotoxicity in spontaneously hypertensive rats. Cancer Chemother. Pharmacol. 2011, 68, 1125–1134. [Google Scholar] [CrossRef]
- Miglioli, F.; De Franco, M.; Bartoli, J.; Scaccaglia, M.; Pelosi, G.; Marzano, C.; Rogolino, D.; Gandin, V.; Carcelli, M. Anticancer activity of new water-soluble sulfonated thiosemicarbazone copper (II) complexes targeting disulfide isomerase. Eur. J. Med. Chem. 2024, 276, 116697. [Google Scholar] [CrossRef] [PubMed]
- Feizpour, S.; Hosseini-Yazdi, S.A.; Safarzadeh, E.; Baradaran, B.; Dusek, M.; Poupon, M. A novel water-soluble thiosemicarbazone Schiff base ligand and its complexes as potential anticancer agents and cellular fluorescence imaging. J. Biol. Inorg. Chem. 2023, 28, 457–472. [Google Scholar] [CrossRef] [PubMed]
- Kunos, C.A.; Andrews, S.J.; Moore, K.N.; Chon, H.S.; Ivy, S.P. Randomized Phase II Trial of Triapine-Cisplatin-Radiotherapy for Locally Advanced Stage Uterine Cervix or Vaginal Cancers. Front. Oncol. 2019, 9, 1067. [Google Scholar] [CrossRef]
- Moorthy, N.S.; Cerqueira, N.M.; Ramos, M.J.; Fernandes, P.A. Aryl-and Heteroaryl-Thiosemicarbazone Derivatives and Their Metal Complexes: A Template for Pharmacological Activities. In Topics in Anti-Cancer Research: Volume 3; Bentham Science Publishers: Sharjah, United Arab Emirates, 2014; pp. 446–483. [Google Scholar]
- Martin, L.K.; Grecula, J.; Jia, G.; Wei, L.; Yang, X.; Otterson, G.A.; Wu, X.; Harper, E.; Kefauver, C.; Zhou, B.S.; et al. A dose escalation and pharmacodynamic study of triapine and radiation in patients with locally advanced pancreas cancer. Int. J. Radiat. Oncol. Biol. Phys. 2012, 84, e475–e481. [Google Scholar] [CrossRef]
- Liu, X.; Jiang, J.; Meng, H. Transcytosis-An effective targeting strategy that is complementary to “EPR effect” for pancreatic cancer nano drug delivery. Theranostics 2019, 9, 8018. [Google Scholar] [CrossRef]
- Botter, E.; Caligiuri, I.; Rizzolio, F.; Visentin, F.; Scattolin, T. Liposomal Formulations of Metallodrugs for Cancer Therapy. Int. J. Mol. Sci. 2024, 25, 9337. [Google Scholar] [CrossRef]
- Dhaliwal, A.; Zheng, G. Improving accessibility of EPR-insensitive tumor phenotypes using EPR-adaptive strategies: Designing a new perspective in nanomedicine delivery. Theranostics 2019, 9, 8091–8108. [Google Scholar] [CrossRef]
- Gawali, P.; Saraswat, A.; Bhide, S.; Gupta, S.; Patel, K. Human solid tumors and clinical relevance of the enhanced permeation and retention effect: A ‘golden gate’ for nanomedicine in preclinical studies? Nanomedicine 2023, 18, 169–190. [Google Scholar] [CrossRef]
- Graf, N.; Lippard, S.J. Redox activation of metal-based prodrugs as a strategy for drug delivery. Adv. Drug Deliv. Rev. 2012, 64, 993–1004. [Google Scholar] [CrossRef] [PubMed]
- Wankhede, S.; Badule, A.; Chaure, S.; Damahe, A.; Damahe, M.; Porwal, O. Challenges and Strategies in Prodrug Design: A Comprehensive Review. J. Adv. Sci. Res. 2025, 16, 1–20. [Google Scholar] [CrossRef]
- Lowndes, S.A.; Harris, A.L. The role of copper in tumour angiogenesis. J. Mammary Gland. Biol. Neoplasia 2005, 10, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Antoniades, V.; Sioga, A.; Dietrich, E.M.; Meditskou, S.; Ekonomou, L.; Antoniades, K. Is copper chelation an effective anti-angiogenic strategy for cancer treatment? Med. Hypotheses 2013, 81, 1159–1163. [Google Scholar] [CrossRef]
- Xie, H.; Kang, Y.J. Role of copper in angiogenesis and its medicinal implications. Curr. Med. Chem. 2009, 16, 1304–1314. [Google Scholar] [CrossRef]
- Sedgwick, A.C.; Brewster, J.T.; Harvey, P.; Iovan, D.A.; Smith, G.; He, X.P.; Tian, H.; Sessler, J.L.; James, T.D. Metal-based imaging agents: Progress towards interrogating neurodegenerative disease. Chem. Soc. Rev. 2020, 49, 2886–2915. [Google Scholar] [CrossRef]
- Anderson, C.J.; Ferdani, R. Copper-64 radiopharmaceuticals for PET imaging of cancer: Advances in preclinical and clinical research. Cancer Biother. Radiopharm. 2009, 24, 379–393. [Google Scholar] [CrossRef] [PubMed]
- Lange, J.L. Copper and Gallium PET Imaging Agents for Applications in HIV and Cancer. Ph.D. Dissertation, Monash University, Melbourne, Australia, 2020. [Google Scholar]
- Bartholomä, M.D. Recent developments in the design of bifunctional chelators for metal-based radiopharmaceuticals used in Positron Emission Tomography. Inorg. Chim. Acta 2012, 389, 36–51. [Google Scholar] [CrossRef]
- Morales, M.; Xue, X. Targeting iron metabolism in cancer therapy. Theranostics 2021, 11, 8412–8429. [Google Scholar] [CrossRef]
- Agrawal, N.; Mishra, R.; Pathak, S.; Goyal, A.; Shah, K. Hydrazides and hydrazones: Robust scaffolds in neurological and neurodegenerative disorders. Lett. Org. Chem. 2023, 20, 123–136. [Google Scholar] [CrossRef]
- Georgieva, M.; Sharkov, M.; Mateev, E.; Tzankova, D.; Popov, G.; Manov, V.; Zlatkov, A.; Simeonova, R.; Kondeva-Burdina, M. Classical Paal-Knorr Cyclization for Synthesis of Pyrrole-Based Aryl Hydrazones and In Vitro/In Vivo Evaluation on Pharmacological Models of Parkinson’s Disease. Molecules 2025, 30, 3154. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Zhao, J.; Chen, G.; Han, H.; Hu, S.; Wang, N.; Wang, J.; Chen, Y.; Zhou, Z.; Dai, B.; et al. Design, synthesis, and evaluation of hydrazones as dual inhibitors of ryanodine receptors and acetylcholinesterases for Alzheimer’s disease. Bioorg. Chem. 2023, 133, 106432. [Google Scholar] [CrossRef] [PubMed]
- Potuckova, E.; Jansova, H.; Machacek, M.; Vavrova, A.; Haskova, P.; Tichotova, L.; Richardson, V.; Kalinowski, D.S.; Richardson, D.R.; Simunek, T. Quantitative analysis of the anti-proliferative activity of combinations of selected iron-chelating agents and clinically used anti-neoplastic drugs. PLoS ONE 2014, 9, e88754. [Google Scholar] [CrossRef]
- Zhang, L.; Fan, X.; Yi, X.; Lin, X.; Zhang, J. Coordination-Delayed-Hydrolysis Method for the Synthesis and Structural Modulation of Titanium-Oxo Clusters. Acc. Chem. Res. 2022, 55, 3150–3161. [Google Scholar] [CrossRef]



| Metal Couple | Half-Reaction (Reduction Direction) | E° vs. NHE (V) | Biological Relevance |
|---|---|---|---|
| Fe3+/Fe2+ | Fe3+ + e− → Fe2+ | +0.16 to +0.82 | Redox-active; drives Fenton-like ROS formation [8]. |
| Cu2+/Cu+ | Cu2+ + e− → Cu+ | +0.15 to −0.25 | Redox-active; supports ROS generation and redox cycling [8]. |
| Zn2+/Zn | Zn2+ + 2e− → Zn(s) | −1.2 to −1.4 | Redox-inert; not involved in Fenton-type reactions [8,13]. |
| Ga3+/Ga | Ga3+ + 3e− → Ga(s) | −0.53 | Redox-inert; mimics Fe(III) without ROS activity [5]. |
| Ti4+/Ti3+ | Ti4+ + e− → Ti3+ | −0.9 | Weakly redox-active; unlikely to drive Fenton chemistry [83]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dharmasivam, M.; Kaya, B. Transmetalation in Cancer Pharmacology. Int. J. Mol. Sci. 2025, 26, 11008. https://doi.org/10.3390/ijms262211008
Dharmasivam M, Kaya B. Transmetalation in Cancer Pharmacology. International Journal of Molecular Sciences. 2025; 26(22):11008. https://doi.org/10.3390/ijms262211008
Chicago/Turabian StyleDharmasivam, Mahendiran, and Busra Kaya. 2025. "Transmetalation in Cancer Pharmacology" International Journal of Molecular Sciences 26, no. 22: 11008. https://doi.org/10.3390/ijms262211008
APA StyleDharmasivam, M., & Kaya, B. (2025). Transmetalation in Cancer Pharmacology. International Journal of Molecular Sciences, 26(22), 11008. https://doi.org/10.3390/ijms262211008







