Multiple Defects in Muscle Regeneration in the HSALR Mouse Model of RNA Toxicity
Abstract
1. Introduction
2. Results
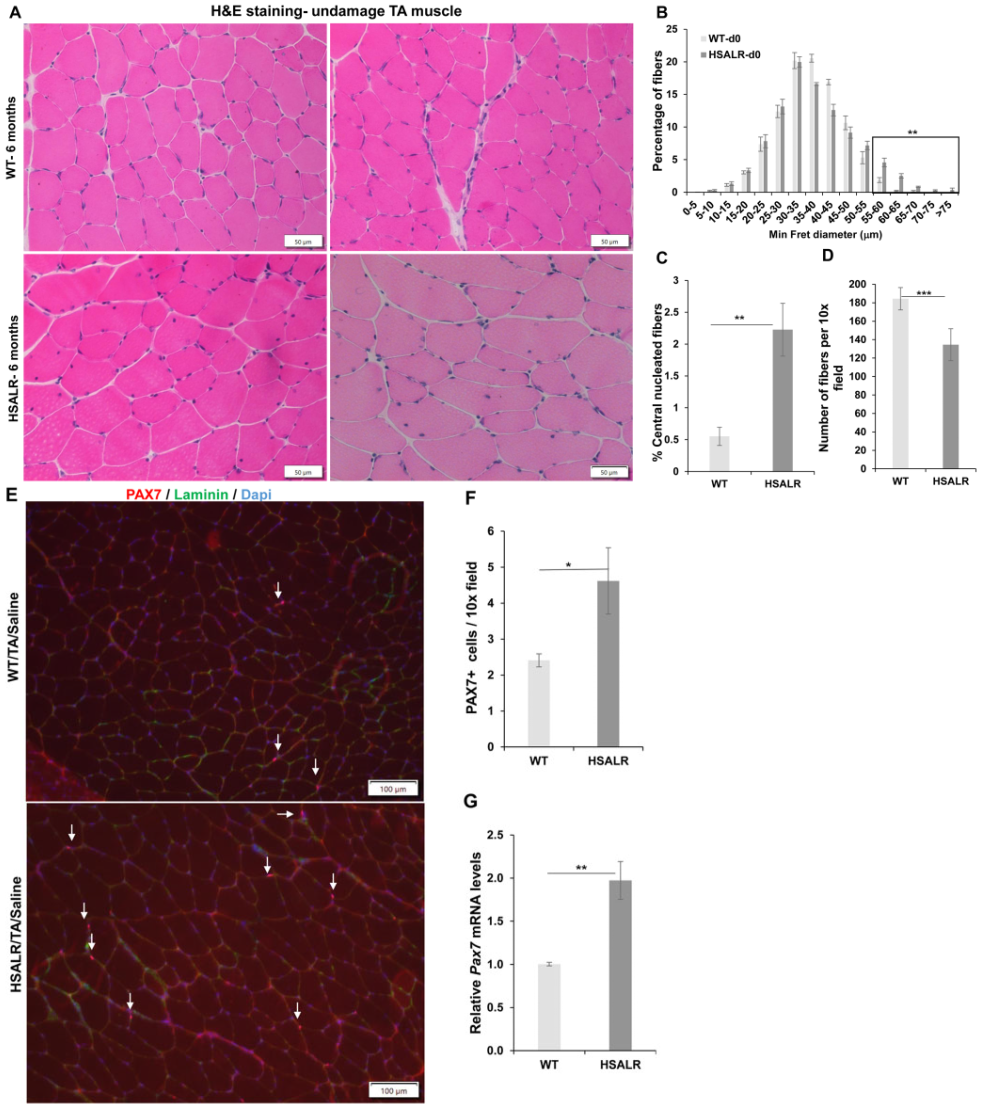
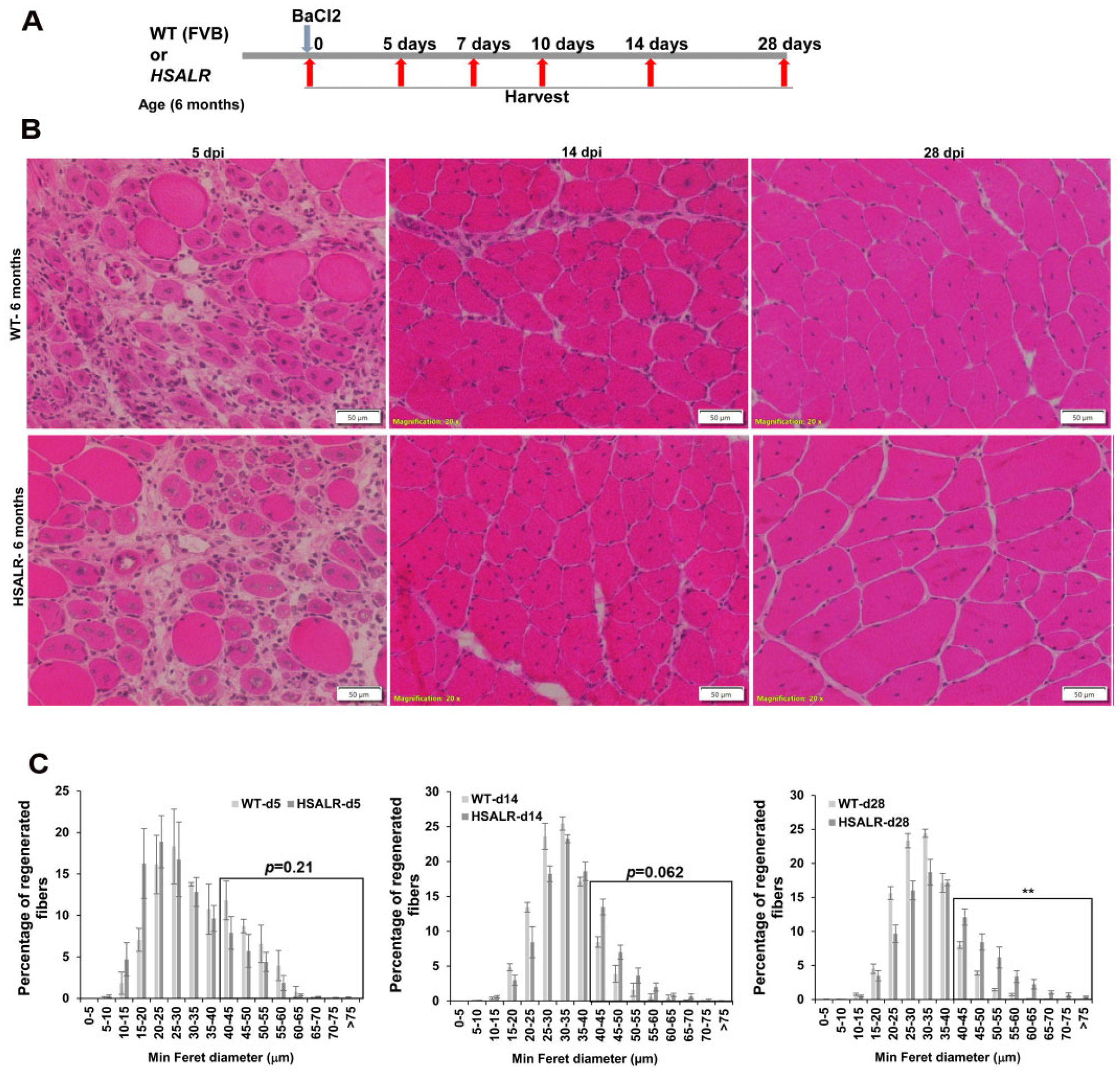
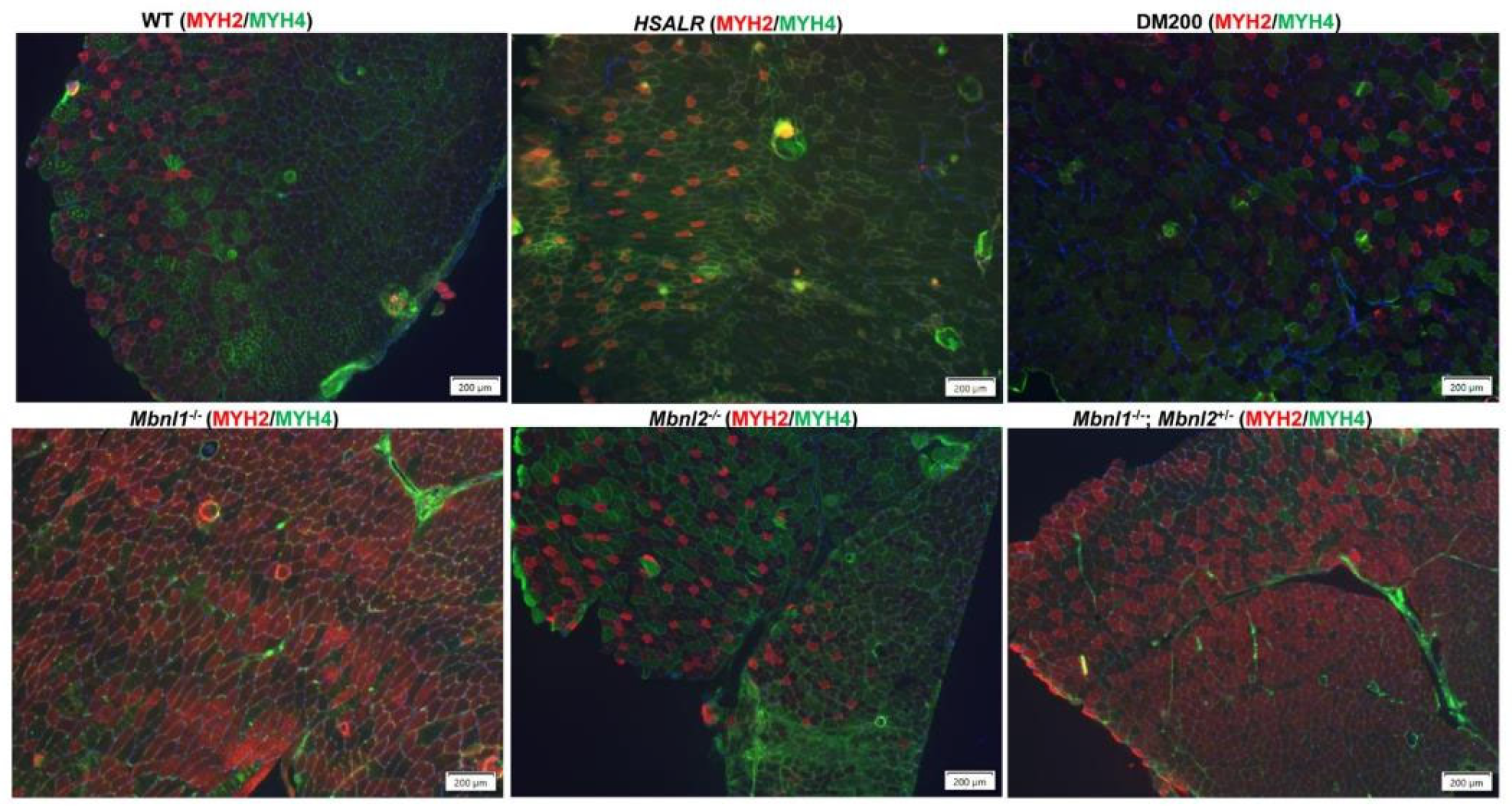
2.1. HSALR Muscle Histology and Fiber Size Analysis
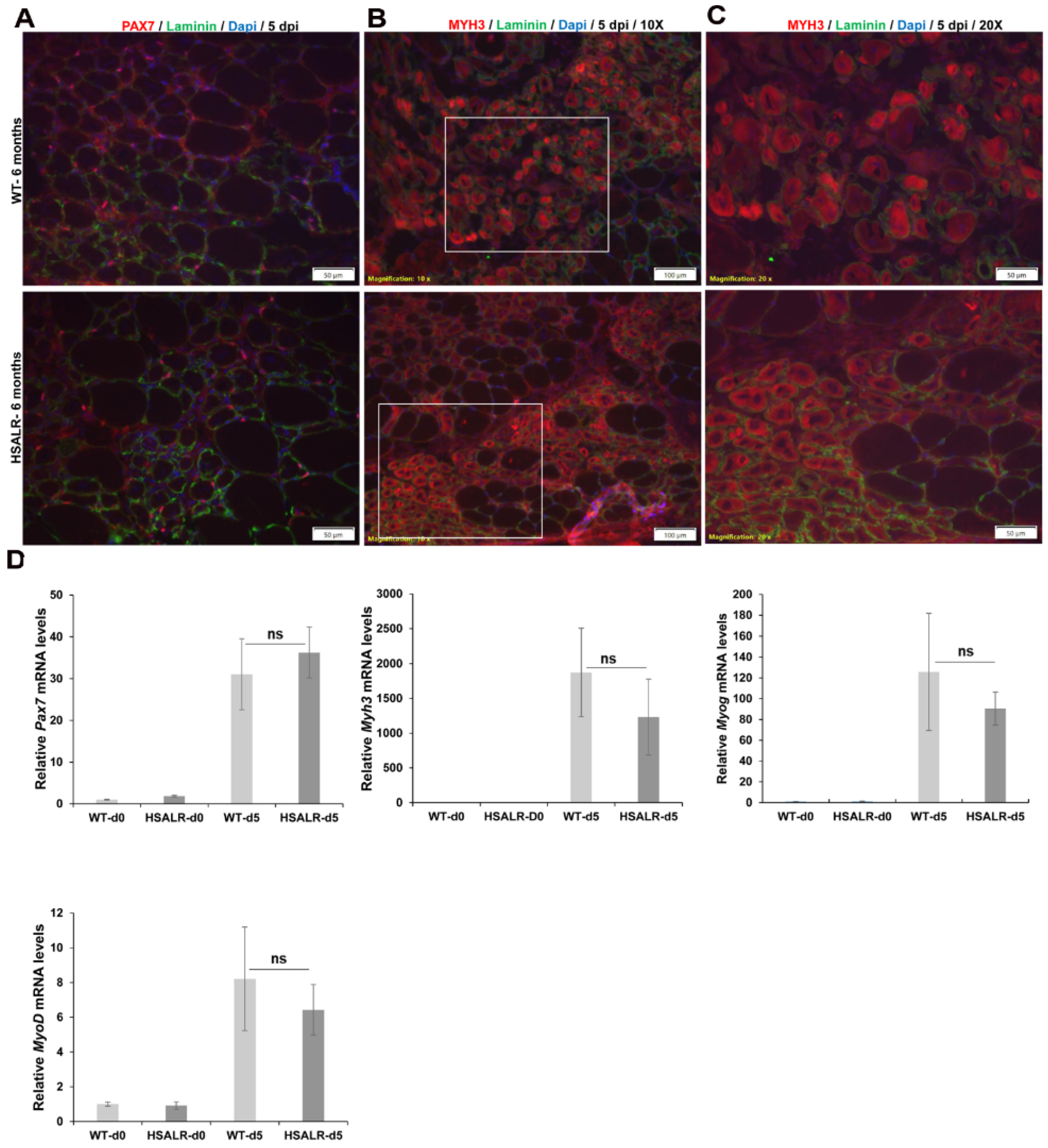
2.2. Muscle Regeneration Markers Are Similar in Wildtype and HSALR Mice
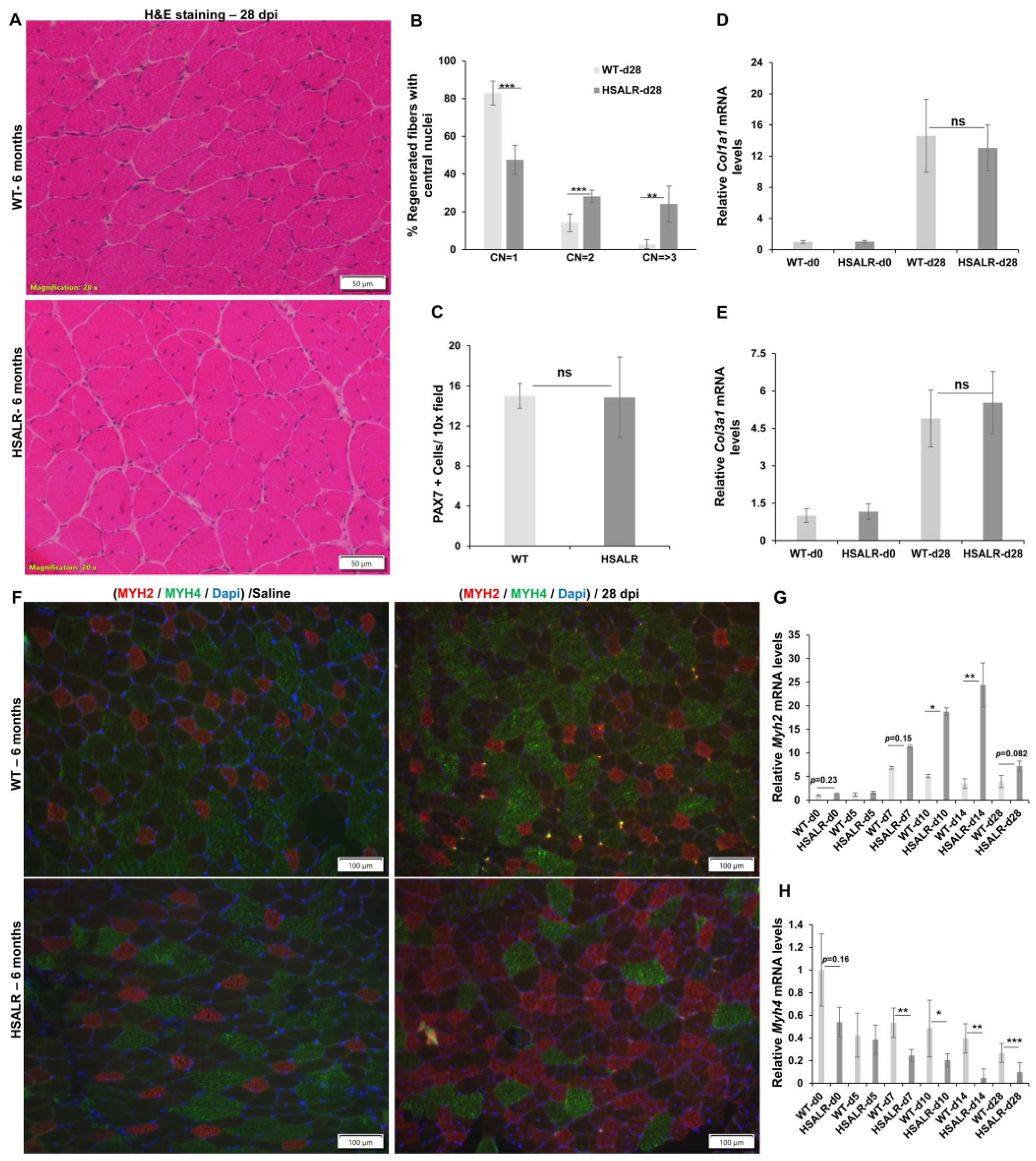
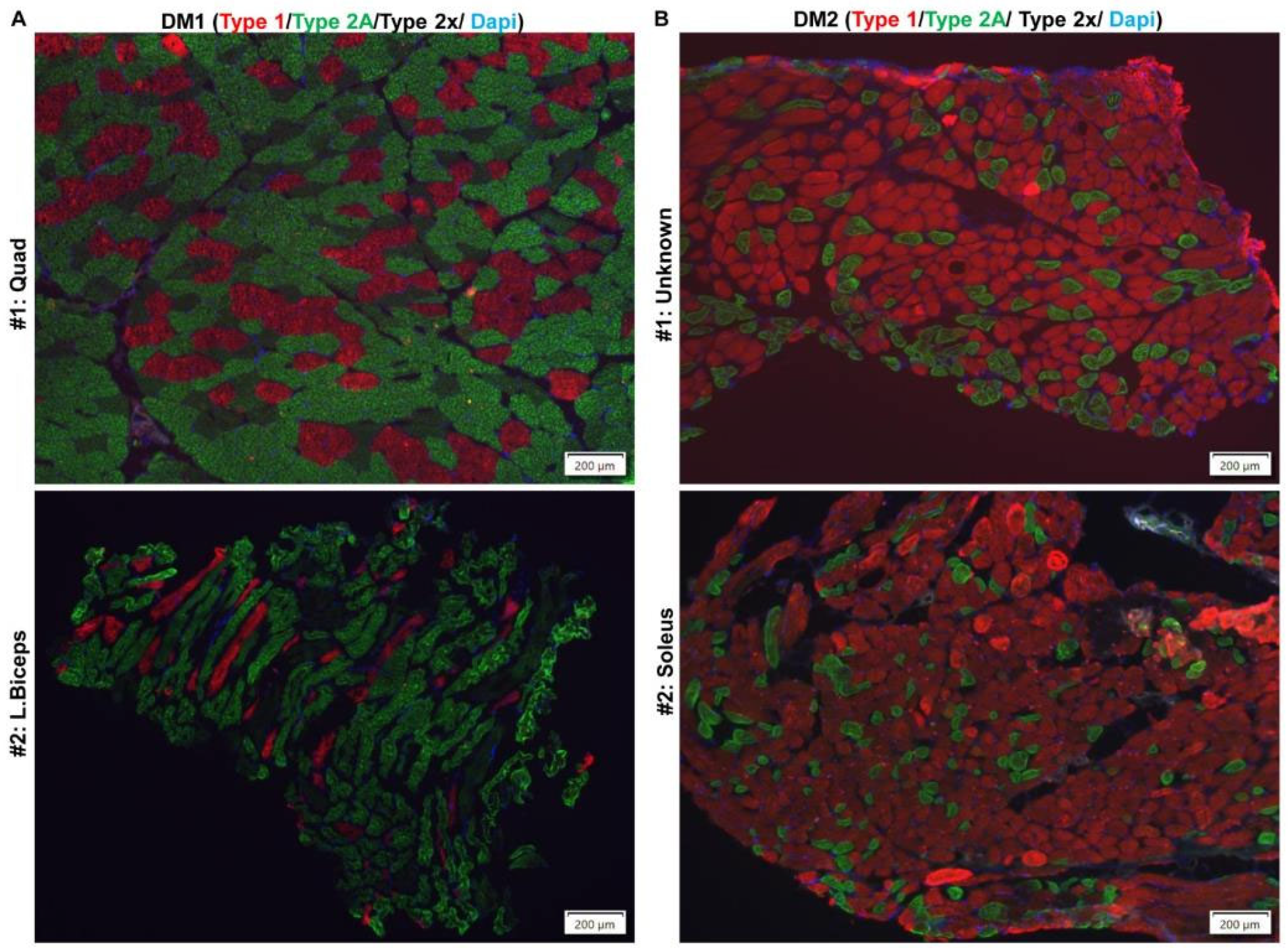
2.3. Histopathological Defects in Regenerated TA Muscles from HSALR Mice
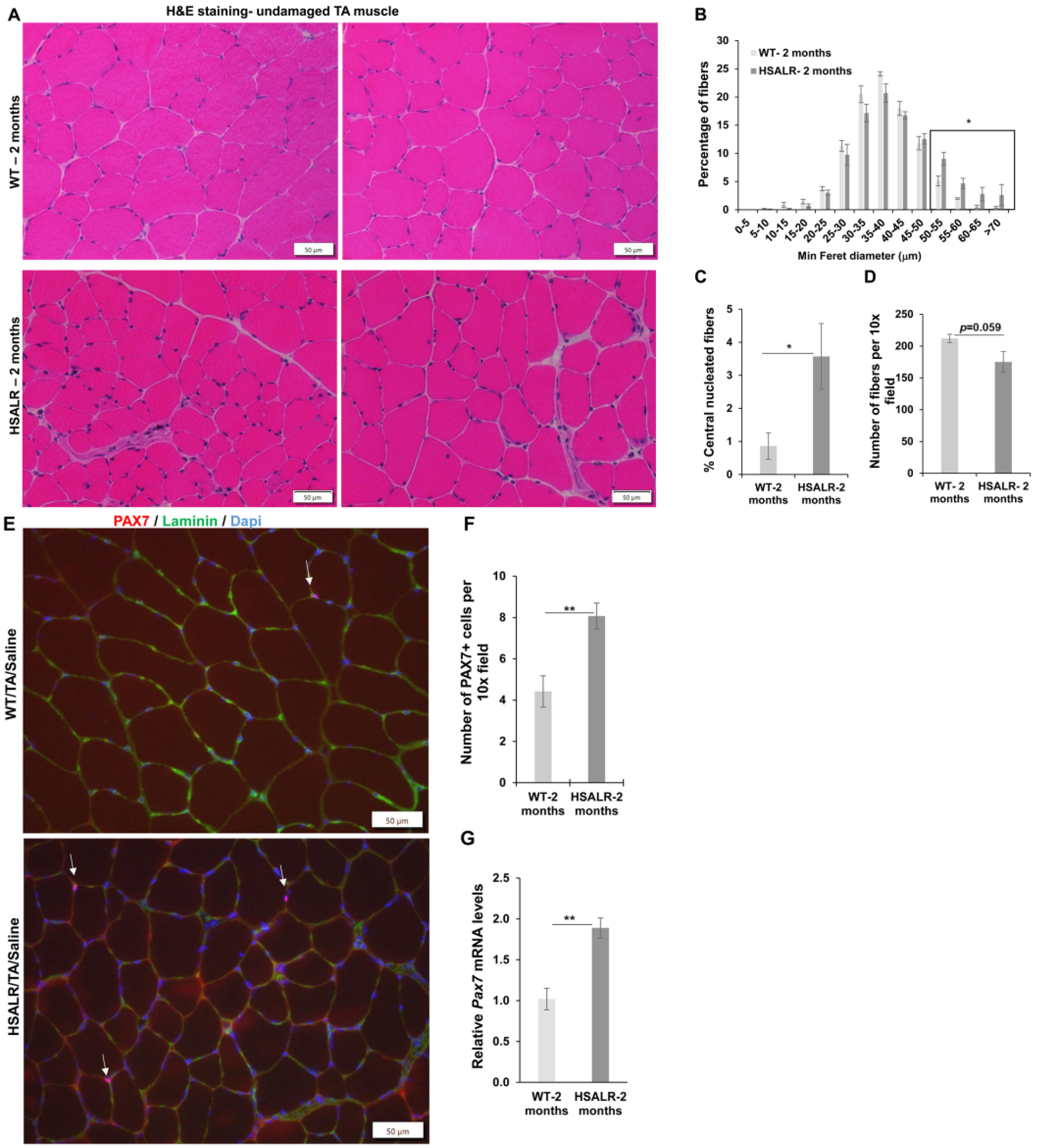
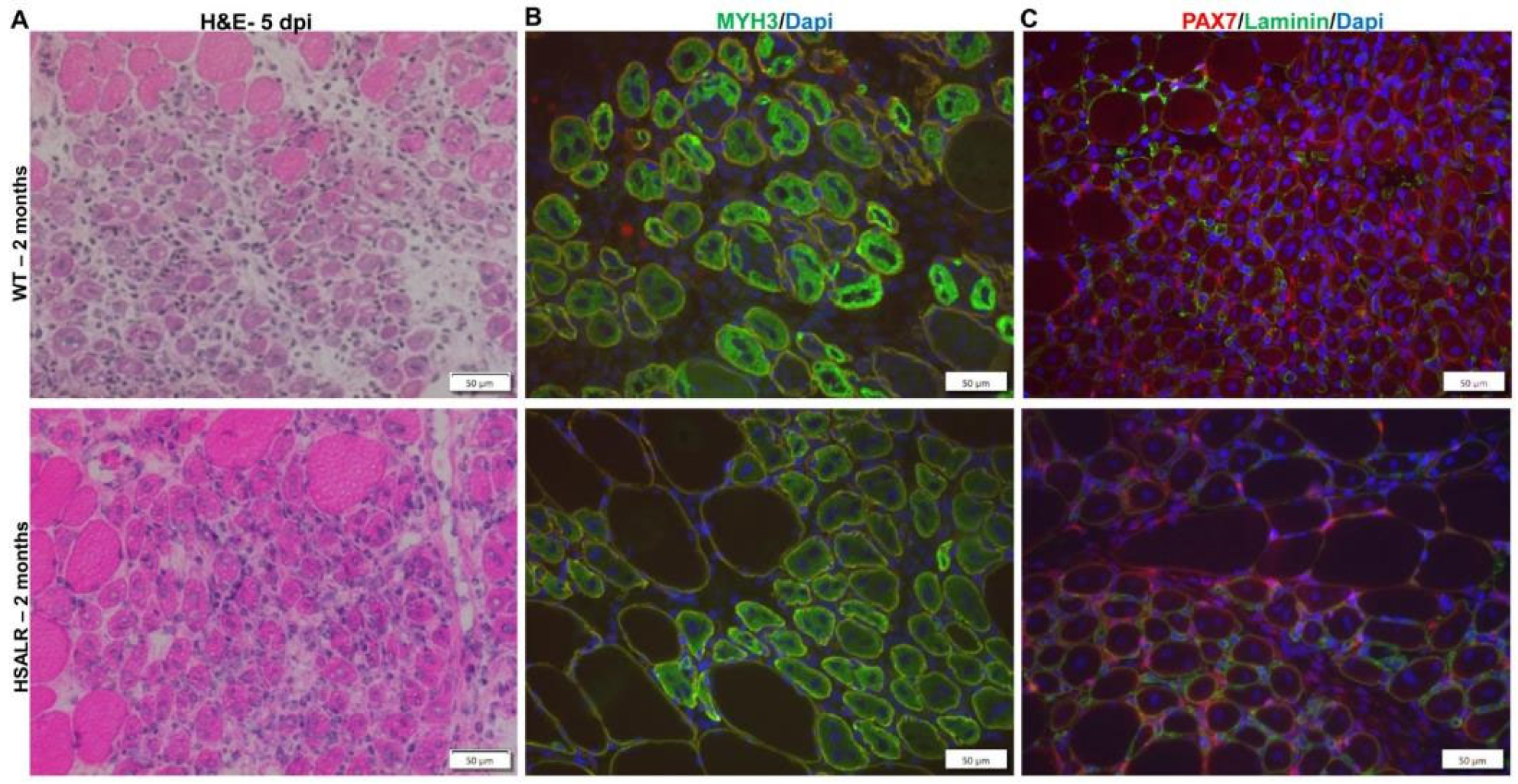
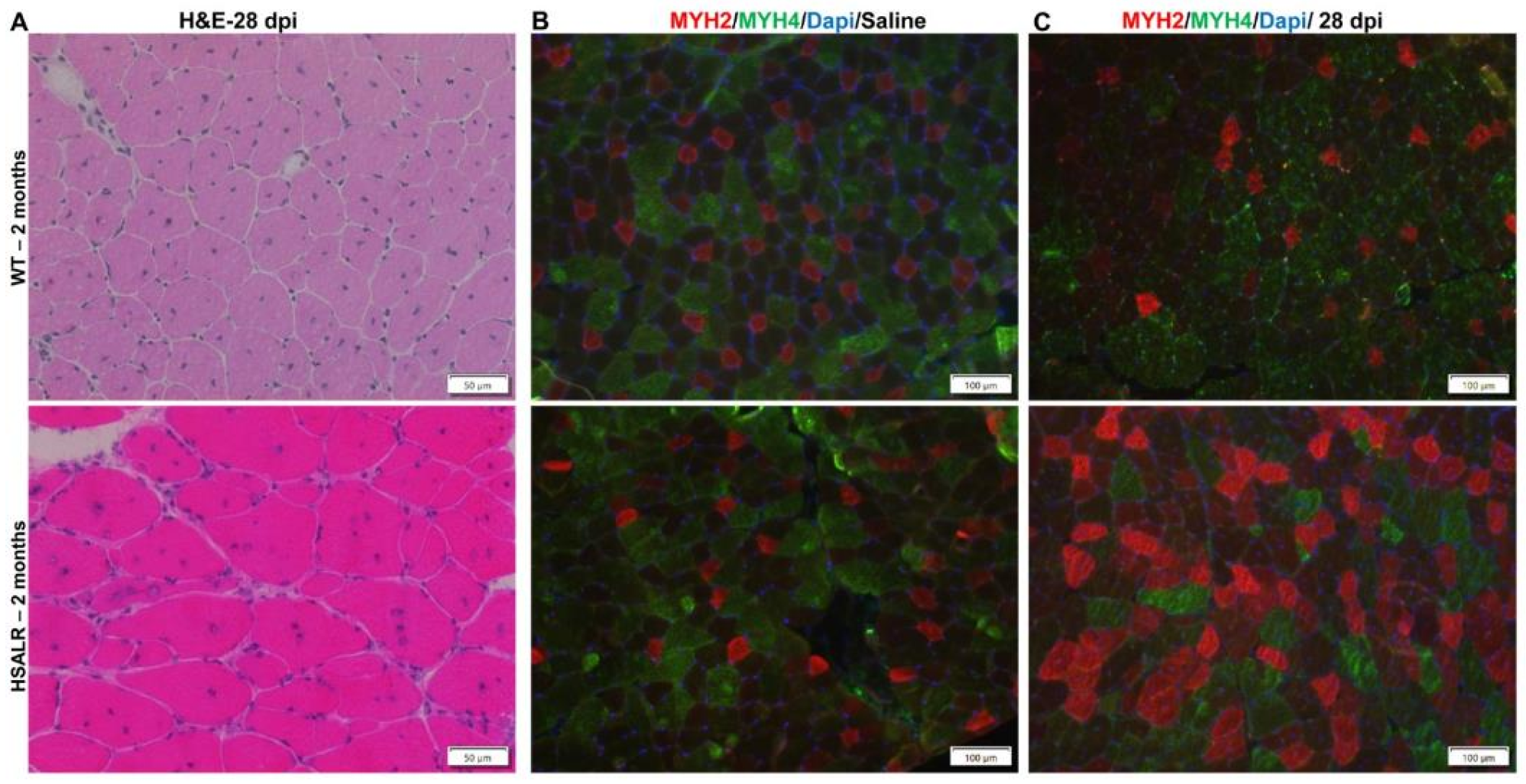
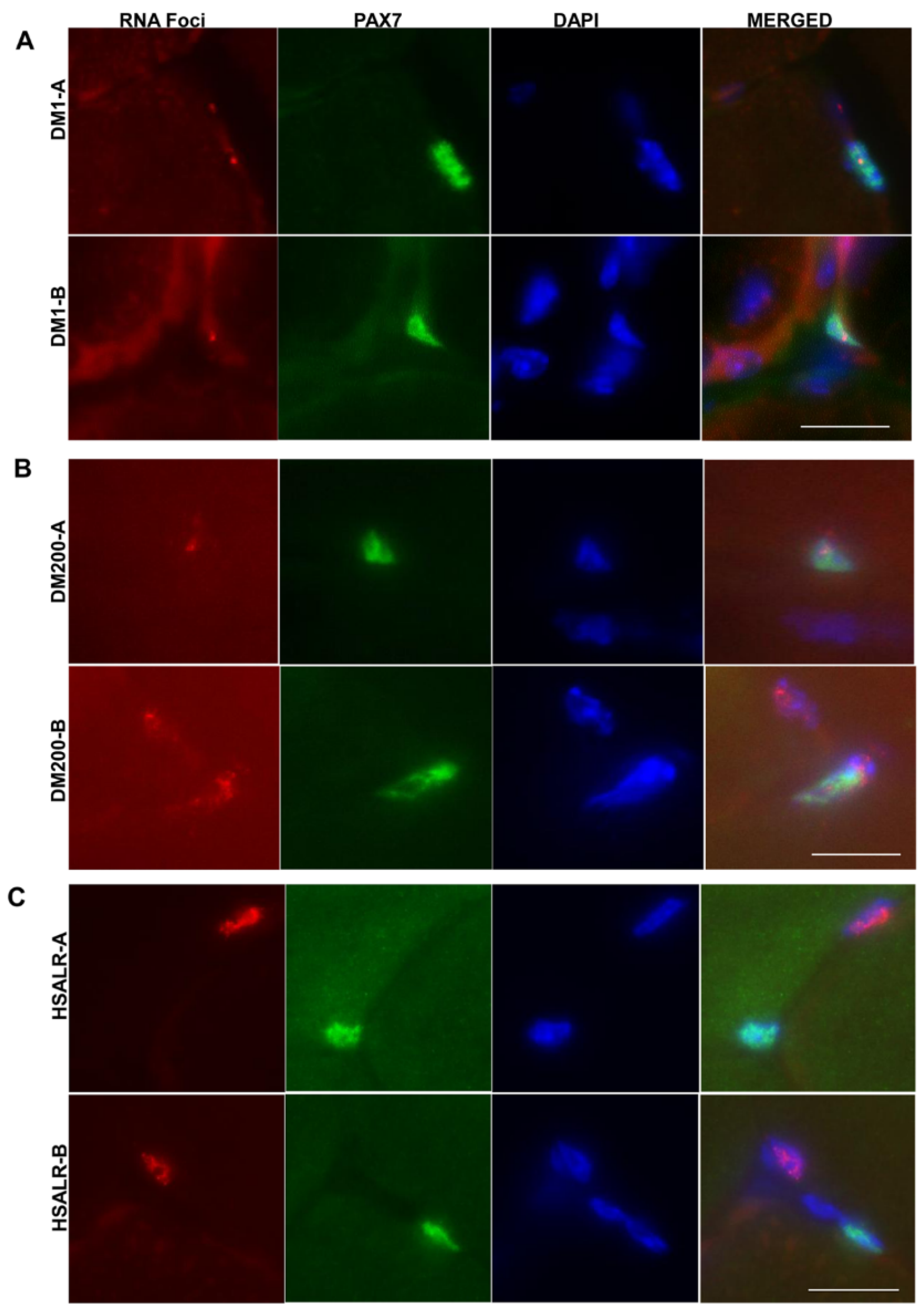
2.4. Study of HSALR Mice at 2 Months of Age
3. Discussion
4. Materials and Methods
4.1. Experimental Mice
4.2. Forelimb Grip Strength Test
4.3. Skeletal Muscle Injury
4.4. H&E Staining and Muscle Fiber Analysis of Skeletal Muscles
4.5. RNA Extraction, Quantitative Real-Time PCR (qRT-PCR) and Splicing Analysis
4.6. RNA-FISH and Immunofluorescence
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Johnson, N.E.; Butterfield, R.J.; Mayne, K.; Newcomb, T.; Imburgia, C.; Dunn, D.; Duval, B.; Feldkamp, M.L.; Weiss, R.B. Population-Based Prevalence of Myotonic Dystrophy Type 1 Using Genetic Analysis of Statewide Blood Screening Program. Neurology 2021, 96, e1045–e1053. [Google Scholar] [CrossRef] [PubMed]
- Liao, Q.; Zhang, Y.; He, J.; Huang, K. Global Prevalence of Myotonic Dystrophy: An Updated Systematic Review and Meta-Analysis. Neuroepidemiology 2022, 56, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Mahadevan, M.; Tsilfidis, C.; Sabourin, L.; Shutler, G.; Amemiya, C.; Jansen, G.; Neville, C.; Narang, M.; Barcelo, J.; O’Hoy, K.; et al. Myotonic dystrophy mutation: An unstable CTG repeat in the 3′ untranslated region of the gene. Science 1992, 255, 1253–1255. [Google Scholar] [CrossRef] [PubMed]
- Brook, J.D.; McCurrach, M.E.; Harley, H.G.; Buckler, A.J.; Church, D.; Aburatani, H.; Hunter, K.; Stanton, V.P.; Thirion, J.P.; Hudson, T.; et al. Molecular basis of myotonic dystrophy: Expansion of a trinucleotide (CTG) repeat at the 3′ end of a transcript encoding a protein kinase family member. Cell 1992, 68, 700–808, Erratum in Cell 1992, 69, 385. [Google Scholar] [CrossRef] [PubMed]
- Taneja, K.L.; McCurrach, M.; Schalling, M.; Housman, D.; Singer, R.H. Foci of trinucleotide repeat transcripts in nuclei of myotonic dystrophy cells and tissues. J. Cell Biol. 1995, 128, 995–1002. [Google Scholar] [CrossRef] [PubMed]
- Davis, B.M.; McCurrach, M.E.; Taneja, K.L.; Singer, R.H.; Housman, D.E. Expansion of a CUG trinucleotide repeat in the 3′ untranslated region of myotonic dystrophy protein kinase transcripts results in nuclear retention of transcripts. Proc. Natl. Acad. Sci. USA 1997, 94, 7388–7393. [Google Scholar] [CrossRef]
- Mankodi, A.; Takahashi, M.P.; Jiang, H.; Beck, C.L.; Bowers, W.J.; Moxley, R.T.; Cannon, S.C.; Thornton, C.A. Expanded CUG repeats trigger aberrant splicing of ClC-1 chloride channel pre-mRNA and hyperexcitability of skeletal muscle in myotonic dystrophy. Mol. Cell 2002, 10, 35–44. [Google Scholar] [CrossRef]
- Osborne, R.J.; Thornton, C.A. RNA-dominant diseases. Hum. Mol. Genet. 2006, 15, R162–R169. [Google Scholar] [CrossRef]
- Jones, K.; Wei, C.; Iakova, P.; Bugiardini, E.; Schneider-Gold, C.; Meola, G.; Woodgett, J.; Killian, J.; Timchenko, N.A.; Timchenko, L.T. GSK3β mediates muscle pathology in myotonic dystrophy. J. Clin. Investig. 2012, 122, 4461–4472. [Google Scholar] [CrossRef]
- Kuyumcu-Martinez, N.M.; Wang, G.S.; Cooper, T.A. Increased steady-state levels of CUGBP1 in myotonic dystrophy 1 are due to PKC-mediated hyperphosphorylation. Mol. Cell 2007, 28, 68–78. [Google Scholar] [CrossRef]
- Ravel-Chapuis, A.; Belanger, G.; Yadava, R.S.; Mahadevan, M.S.; DesGroseillers, L.; Cote, J.; Jasmin, B.J. The RNA-binding protein Staufen1 is increased in DM1 skeletal muscle and promotes alternative pre-mRNA splicing. J. Cell Biol. 2012, 196, 699–712. [Google Scholar] [CrossRef] [PubMed]
- Brockhoff, M.; Rion, N.; Chojnowska, K.; Wiktorowicz, T.; Eickhorst, C.; Erne, B.; Frank, S.; Angelini, C.; Furling, D.; Ruegg, M.A.; et al. Targeting deregulated AMPK/mTORC1 pathways improves muscle function in myotonic dystrophy type I. J. Clin. Investig. 2017, 127, 549–563. [Google Scholar] [CrossRef] [PubMed]
- Yadava, R.S.; Foff, E.P.; Yu, Q.; Gladman, J.T.; Zheng, T.S.; Mahadevan, M.S. TWEAK Regulates Muscle Functions in a Mouse Model of RNA Toxicity. PLoS ONE 2016, 11, e0150192, Erratum in PLoS ONE 2018, 13, e0192816. [Google Scholar] [CrossRef] [PubMed]
- Yadava, R.S.; Foff, E.P.; Yu, Q.; Gladman, J.T.; Kim, Y.K.; Bhatt, K.S.; Thornton, C.A.; Zheng, T.S.; Mahadevan, M.S. TWEAK/Fn14, a pathway and novel therapeutic target in myotonic dystrophy. Hum. Mol. Genet. 2015, 24, 2035–2048. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Langlois, M.A.; Lee, K.B.; Riggs, A.D.; Puymirat, J.; Rossi, J.J. HnRNP H inhibits nuclear export of mRNA containing expanded CUG repeats and a distal branch point sequence. Nucleic Acids Res. 2005, 33, 3866–3874. [Google Scholar] [CrossRef]
- Pettersson, O.J.; Aagaard, L.; Andrejeva, D.; Thomsen, R.; Jensen, T.G.; Damgaard, C.K. DDX6 regulates sequestered nuclear CUG-expanded DMPK-mRNA in dystrophia myotonica type 1. Nucleic Acids Res. 2014, 42, 7186–7200. [Google Scholar] [CrossRef]
- Laurent, F.X.; Sureau, A.; Klein, A.F.; Trouslard, F.; Gasnier, E.; Furling, D.; Marie, J. New function for the RNA helicase p68/DDX5 as a modifier of MBNL1 activity on expanded CUG repeats. Nucleic Acids Res. 2012, 40, 3159–3171. [Google Scholar] [CrossRef]
- Llamusi, B.; Bargiela, A.; Fernandez-Costa, J.M.; Garcia-Lopez, A.; Klima, R.; Feiguin, F.; Artero, R. Muscleblind, BSF and TBPH are mislocalized in the muscle sarcomere of a Drosophila myotonic dystrophy model. Dis. Model. Mech. 2013, 6, 184–196. [Google Scholar] [CrossRef]
- Ozimski, L.L.; Sabater-Arcis, M.; Bargiela, A.; Artero, R. The hallmarks of myotonic dystrophy type 1 muscle dysfunction. Biol. Rev. Camb. Philos. Soc. 2021, 96, 716–730. [Google Scholar] [CrossRef]
- Mankodi, A.; Logigian, E.; Callahan, L.; McClain, C.; White, R.; Henderson, D.; Krym, M.; Thornton, C.A. Myotonic dystrophy in transgenic mice expressing an expanded CUG repeat. Science 2000, 289, 1769–1773. [Google Scholar] [CrossRef]
- Orengo, J.P.; Chambon, P.; Metzger, D.; Mosier, D.R.; Snipes, G.J.; Cooper, T.A. Expanded CTG repeats within the DMPK 3′ UTR causes severe skeletal muscle wasting in an inducible mouse model for myotonic dystrophy. Proc. Natl. Acad. Sci. USA 2008, 105, 2646–2651. [Google Scholar] [CrossRef] [PubMed]
- Mahadevan, M.S.; Yadava, R.S.; Yu, Q.; Balijepalli, S.; Frenzel-McCardell, C.D.; Bourne, T.D.; Phillips, L.H. Reversible model of RNA toxicity and cardiac conduction defects in myotonic dystrophy. Nat. Genet. 2006, 38, 1066–1070. [Google Scholar] [CrossRef] [PubMed]
- Ho, T.H.; Bundman, D.; Armstrong, D.L.; Cooper, T.A. Transgenic mice expressing CUG-BP1 reproduce splicing mis-regulation observed in myotonic dystrophy. Hum. Mol. Genet. 2005, 14, 1539–1547. [Google Scholar] [CrossRef] [PubMed]
- Kanadia, R.N.; Johnstone, K.A.; Mankodi, A.; Lungu, C.; Thornton, C.A.; Esson, D.; Timmers, A.M.; Hauswirth, W.W.; Swanson, M.S. A muscleblind knockout model for myotonic dystrophy. Science 2003, 302, 1978–1980. [Google Scholar] [CrossRef]
- Timchenko, N.A.; Patel, R.; Iakova, P.; Cai, Z.J.; Quan, L.; Timchenko, L.T. Overexpression of CUG triplet repeat-binding protein, CUGBP1, in mice inhibits myogenesis. J. Biol. Chem. 2004, 279, 13129–13139. [Google Scholar] [CrossRef]
- Ward, A.J.; Rimer, M.; Killian, J.M.; Dowling, J.J.; Cooper, T.A. CUGBP1 overexpression in mouse skeletal muscle reproduces features of myotonic dystrophy type 1. Hum. Mol. Genet. 2010, 19, 3614–3622. [Google Scholar] [CrossRef]
- Seznec, H.; Agbulut, O.; Sergeant, N.; Savouret, C.; Ghestem, A.; Tabti, N.; Willer, J.C.; Ourth, L.; Duros, C.; Brisson, E.; et al. Mice transgenic for the human myotonic dystrophy region with expanded CTG repeats display muscular and brain abnormalities. Hum. Mol. Genet. 2001, 10, 2717–2726. [Google Scholar] [CrossRef]
- Ciciliot, S.; Rossi, A.C.; Dyar, K.A.; Blaauw, B.; Schiaffino, S. Muscle type and fiber type specificity in muscle wasting. Int. J. Biochem. Cell Biol. 2013, 45, 2191–2199. [Google Scholar] [CrossRef]
- Casar, J.C.; McKechnie, B.A.; Fallon, J.R.; Young, M.F.; Brandan, E. Transient up-regulation of biglycan during skeletal muscle regeneration: Delayed fiber growth along with decorin increase in biglycan-deficient mice. Dev. Biol. 2004, 268, 358–371. [Google Scholar] [CrossRef]
- Hardy, D.; Besnard, A.; Latil, M.; Jouvion, G.; Briand, D.; Thepenier, C.; Pascal, Q.; Guguin, A.; Gayraud-Morel, B.; Cavaillon, J.M.; et al. Comparative Study of Injury Models for Studying Muscle Regeneration in Mice. PLoS ONE 2016, 11, e0147198. [Google Scholar] [CrossRef]
- Caldwell, C.J.; Mattey, D.L.; Weller, R.O. Role of the basement membrane in the regeneration of skeletal muscle. Neuropathol. Appl. Neurobiol. 1990, 16, 225–238. [Google Scholar] [CrossRef] [PubMed]
- Yadava, R.S.; Mandal, M.; Giese, J.M.; Rigo, F.; Bennett, C.F.; Mahadevan, M.S. Modeling muscle regeneration in RNA toxicity mice. Hum. Mol. Genet. 2021, 30, 1111–1130. [Google Scholar] [CrossRef] [PubMed]
- Yadava, R.S.; Mandal, M.; Mahadevan, M.S. Studying the Effect of MBNL1 and MBNL2 Loss in Skeletal Muscle Regeneration. Int. J. Mol. Sci. 2024, 25, 2687. [Google Scholar] [CrossRef] [PubMed]
- Andersen, G.; Orngreen, M.C.; Preisler, N.; Colding-Jorgensen, E.; Clausen, T.; Duno, M.; Jeppesen, T.D.; Vissing, J. Muscle phenotype in patients with myotonic dystrophy type 1. Muscle Nerve 2013, 47, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Bassez, G.; Chapoy, E.; Bastuji-Garin, S.; Radvanyi-Hoffman, H.; Authier, F.J.; Pellissier, J.F.; Eymard, B.; Gherardi, R.K. Type 2 myotonic dystrophy can be predicted by the combination of type 2 muscle fiber central nucleation and scattered atrophy. J. Neuropathol. Exp. Neurol. 2008, 67, 319–325. [Google Scholar] [CrossRef]
- Augusto, V.; Padovani, C.; Eduardo, G.; Campos, R. Skeletal muscle fiber types in C57BL6J mice. J. Morphol. Sci. 2004, 21, 89–94. [Google Scholar]
- Mauro, A. Satellite cell of skeletal muscle fibers. J. Biophys. Biochem. Cytol. 1961, 9, 493–495. [Google Scholar] [CrossRef]
- von Maltzahn, J.; Jones, A.E.; Parks, R.J.; Rudnicki, M.A. Pax7 is critical for the normal function of satellite cells in adult skeletal muscle. Proc. Natl. Acad. Sci. USA 2013, 110, 16474–16479. [Google Scholar] [CrossRef]
- Sahgal, V.; Bernes, S.; Sahgal, S.; Lischwey, C.; Subramani, V. Skeletal muscle in preterm infants with congenital myotonic dystrophy. Morphologic and histochemical study. J. Neurol. Sci. 1983, 59, 47–55. [Google Scholar] [CrossRef]
- Thornell, L.E.; Lindstom, M.; Renault, V.; Klein, A.; Mouly, V.; Ansved, T.; Butler-Browne, G.; Furling, D. Satellite cell dysfunction contributes to the progressive muscle atrophy in myotonic dystrophy type 1. Neuropathol. Appl. Neurobiol. 2009, 35, 603–613. [Google Scholar] [CrossRef]
- Yadava, R.S.; Kim, Y.K.; Mandal, M.; Mahadevan, K.; Gladman, J.T.; Yu, Q.; Mahadevan, M.S. MBNL1 overexpression is not sufficient to rescue the phenotypes in a mouse model of RNA toxicity. Hum. Mol. Genet. 2019, 28, 2330–2338. [Google Scholar] [CrossRef] [PubMed]
- Pizza, F.X.; Buckley, K.H. Regenerating Myofibers after an Acute Muscle Injury: What Do We Really Know about Them? Int. J. Mol. Sci. 2023, 24, 12545. [Google Scholar] [CrossRef] [PubMed]
- Fukada, S.I.; Higashimoto, T.; Kaneshige, A. Differences in muscle satellite cell dynamics during muscle hypertrophy and regeneration. Skelet. Muscle 2022, 12, 17. [Google Scholar] [CrossRef] [PubMed]
- Cutler, A.A.; Pawlikowski, B.; Wheeler, J.R.; Betta, N.D.; Elston, T.; O’Rourke, R.; Jones, K.; Olwin, B.B. The regenerating skeletal muscle niche drives satellite cell return to quiescence. iScience 2022, 25, 104444. [Google Scholar] [CrossRef] [PubMed]
- Buckley, K.H.; Nestor-Kalinoski, A.L.; Pizza, F.X. Positional Context of Myonuclear Transcription During Injury-Induced Muscle Regeneration. Front. Physiol. 2022, 13, 845504. [Google Scholar] [CrossRef]
- Hojfeldt, G.; Sorenson, T.; Gonzales, A.; Kjaer, M.; Andersen, J.L.; Mackey, A.L. Fusion of myofibre branches is a physiological feature of healthy human skeletal muscle regeneration. Skelet. Muscle. 2023, 13, 13. [Google Scholar] [CrossRef]
- Wada, K.; Katsuta, S.; Soya, H. Formation process and fate of the nuclear chain after injury in regenerated myofiber. Anat. Rec. 2008, 291, 122–128. [Google Scholar] [CrossRef]
- Meyer, G.A. Evidence of induced muscle regeneration persists for years in the mouse. Muscle Nerve 2018, 58, 858–862. [Google Scholar] [CrossRef]
- Gómez-Oca, R.; Cowling, B.S.; Laporte, J. Common Pathogenic Mechanisms in Centronuclear and Myotubular Myopathies and Latest Treatment Advances. Int. J. Mol. Sci. 2021, 22, 11377. [Google Scholar] [CrossRef]
- Fugier, C.; Klein, A.F.; Hammer, C.; Vassilopoulos, S.; Ivarsson, Y.; Toussaint, A.; Tosch, V.; Vignaud, A.; Ferry, A.; Messaddeq, N.; et al. Misregulated alternative splicing of BIN1 is associated with T tubule alterations and muscle weakness in myotonic dystrophy. Nat. Med. 2011, 17, 720–725. [Google Scholar] [CrossRef]
- Kimura, T.; Nakamori, M.; Lueck, J.D.; Pouliquin, P.; Aoike, F.; Fujimura, H.; Dirksen, R.T.; Takahashi, M.P.; Dulhunty, A.F.; Sakoda, S.; et al. Altered mRNA splicing of the skeletal muscle ryanodine receptor and sarcoplasmic/endoplasmic reticulum Ca2+-ATPase in myotonic dystrophy type 1. Hum. Mol. Genet. 2005, 14, 2189–2200. [Google Scholar] [CrossRef] [PubMed]
- Böhm, J.; Vasli, N.; Maurer, M.; Cowling, B.S.; Shelton, G.D.; Kress, W.; Toussaint, A.; Prokic, I.; Schara, U.; Anderson, T.J.; et al. Altered splicing of the BIN1 muscle-specific exon in humans and dogs with highly progressive centronuclear myopathy. PLoS Genet. 2013, 9, e1003430, Erratum in PLoS Genet. 2013, 9. [Google Scholar] [CrossRef] [PubMed]
- Prokic, I.; Cowling, B.S.; Kutchukian, C.; Kretz, C.; Tasfaout, H.; Gache, V.; Hergueux, J.; Wendling, O.; Ferry, A.; Toussaint, A.; et al. Differential physiological roles for BIN1 isoforms in skeletal muscle development, function and regeneration. Dis. Model. Mech. 2020, 13, dmm044354. [Google Scholar] [CrossRef] [PubMed]
- Hintze, S.; Knaier, L.; Limmer, S.; Schoser, B.; Meinke, P. Nuclear Envelope Transmembrane Proteins in Myotonic Dystrophy Type 1. Front. Physiol. 2018, 9, 1532. [Google Scholar] [CrossRef] [PubMed]
- Todorow, V.; Hintze, S.; Schoser, B.; Meinke, P. Nuclear envelope transmembrane proteins involved in genome organization are misregulated in myotonic dystrophy type 1 muscle. Front. Cell Dev. Biol. 2022, 10, 1007331. [Google Scholar] [CrossRef] [PubMed]
- Apel, E.D.; Lewis, R.M.; Grady, R.M.; Sanes, J.R. Syne-1, a dystrophin- and Klarsicht-related protein associated with synaptic nuclei at the neuromuscular junction. J. Biol. Chem. 2000, 275, 31986–31995. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, R.; Zhu, B.; Yang, X.; Ding, X.; Duan, S.; Xu, T.; Zhuang, Y.; Han, M. Syne-1 and Syne-2 play crucial roles in myonuclear anchorage and motor neuron innervation. Development 2007, 134, 901–908. [Google Scholar] [CrossRef]
- Nadaj-Pakleza, A.; Lusakowska, A.; Sułek-Piątkowska, A.; Krysa, W.; Rajkiewicz, M.; Kwieciński, H.; Kamińska, A. Muscle pathology in myotonic dystrophy: Light and electron microscopic investigation in eighteen patients. Folia Morphol. 2011, 70, 121–129. [Google Scholar]
- Schoser, B.G.; Schneider-Gold, C.; Kress, W.; Goebel, H.H.; Reilich, P.; Koch, M.C.; Pongratz, D.E.; Toyka, K.V.; Lochmuller, H.; Ricker, K. Muscle pathology in 57 patients with myotonic dystrophy type 2. Muscle Nerve 2004, 29, 275–281. [Google Scholar] [CrossRef]
- Vihola, A.; Bassez, G.; Meola, G.; Zhang, S.; Haapasalo, H.; Paetau, A.; Mancinelli, E.; Rouche, A.; Hogrel, J.Y.; Laforet,, P.; et al. Histopathological differences of myotonic dystrophy type 1 (DM1) and PROMM/DM2. Neurology 2003, 60, 1854–1857. [Google Scholar] [CrossRef]
- Andre, L.M.; Ausems, C.R.; Wansink, D.G.; Wieringa, B. Abnormalities in Skeletal Muscle Myogenesis, Growth, and Regeneration in Myotonic Dystrophy. Front. Neurol. 2018, 9, 368. [Google Scholar] [CrossRef] [PubMed]
- Stringer, C.; Wang, T.; Michaelos, M.; Pachitariu, M. Cellpose: A generalist algorithm for cellular segmentation. Nat. Methods 2021, 18, 100–106. [Google Scholar] [CrossRef]
- Waisman, A.; Norris, A.M.; Elias Costa, M.; Kopinke, D. Automatic and unbiased segmentation and quantification of myofibers in skeletal muscle. Sci. Rep. 2021, 11, 11793. [Google Scholar] [CrossRef]
- Langlois, M.A.; Lee, N.S.; Rossi, J.J.; Puymirat, J. Hammerhead ribozyme-mediated destruction of nuclear foci in myotonic dystrophy myoblasts. Mol. Ther. 2003, 7, 670–680. [Google Scholar] [CrossRef]
- Mankodi, A.; Teng-Umnuay, P.; Krym, M.; Henderson, D.; Swanson, M.; Thornton, C.A. Ribonuclear inclusions in skeletal muscle in myotonic dystrophy types 1 and 2. Ann. Neurol. 2003, 54, 760–768. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yadava, R.S.; Zineddin, M.A.; Mahadevan, M.S. Multiple Defects in Muscle Regeneration in the HSALR Mouse Model of RNA Toxicity. Int. J. Mol. Sci. 2025, 26, 10985. https://doi.org/10.3390/ijms262210985
Yadava RS, Zineddin MA, Mahadevan MS. Multiple Defects in Muscle Regeneration in the HSALR Mouse Model of RNA Toxicity. International Journal of Molecular Sciences. 2025; 26(22):10985. https://doi.org/10.3390/ijms262210985
Chicago/Turabian StyleYadava, Ramesh S., Mira A. Zineddin, and Mani S. Mahadevan. 2025. "Multiple Defects in Muscle Regeneration in the HSALR Mouse Model of RNA Toxicity" International Journal of Molecular Sciences 26, no. 22: 10985. https://doi.org/10.3390/ijms262210985
APA StyleYadava, R. S., Zineddin, M. A., & Mahadevan, M. S. (2025). Multiple Defects in Muscle Regeneration in the HSALR Mouse Model of RNA Toxicity. International Journal of Molecular Sciences, 26(22), 10985. https://doi.org/10.3390/ijms262210985





