Extracellular ATP Suppresses Perlecan Core Protein Synthesis via P2Y2 Receptor-Mediated Inhibition of Akt Signaling in Cultured Vascular Endothelial Cells
Abstract
1. Introduction
2. Results
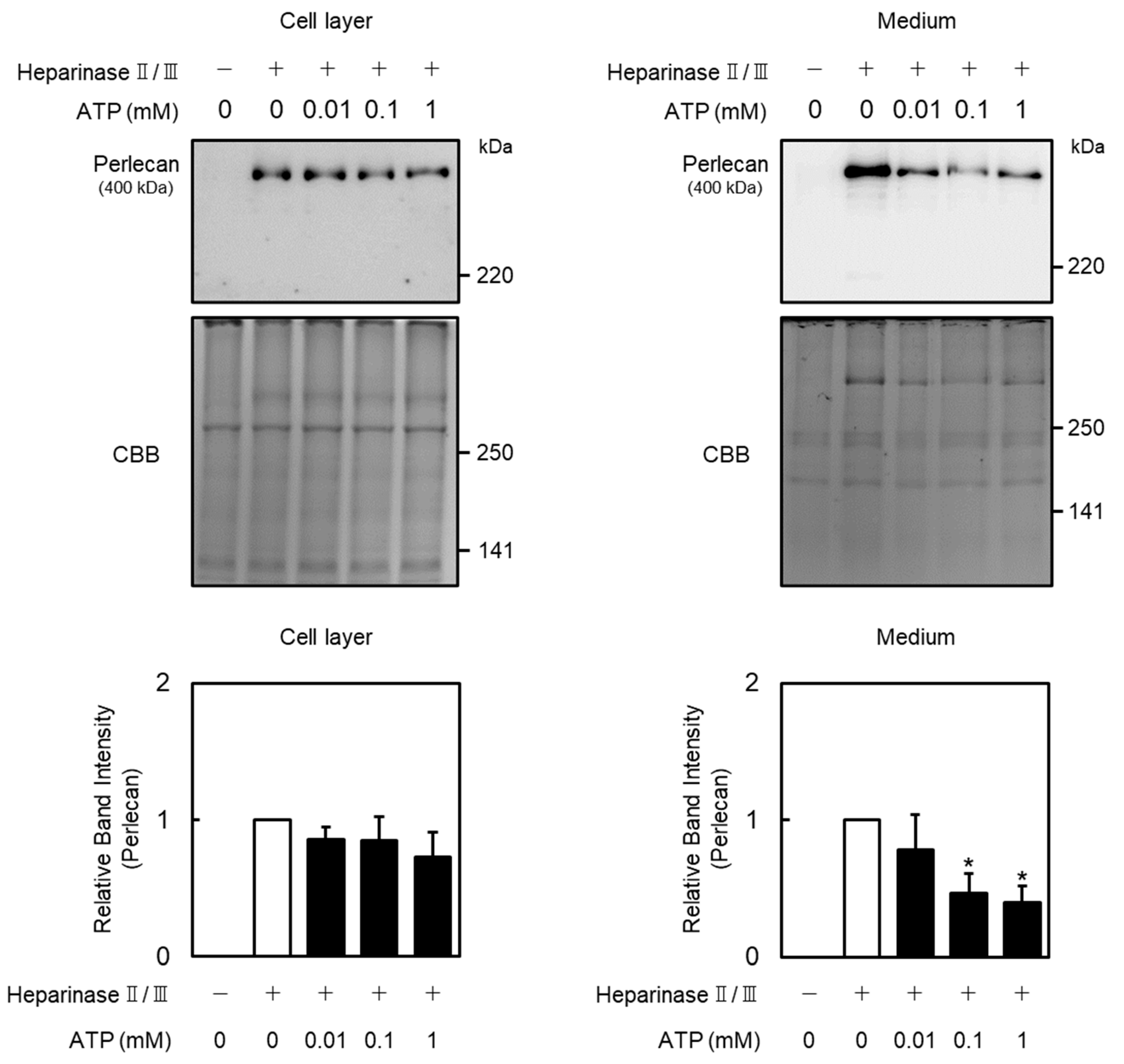
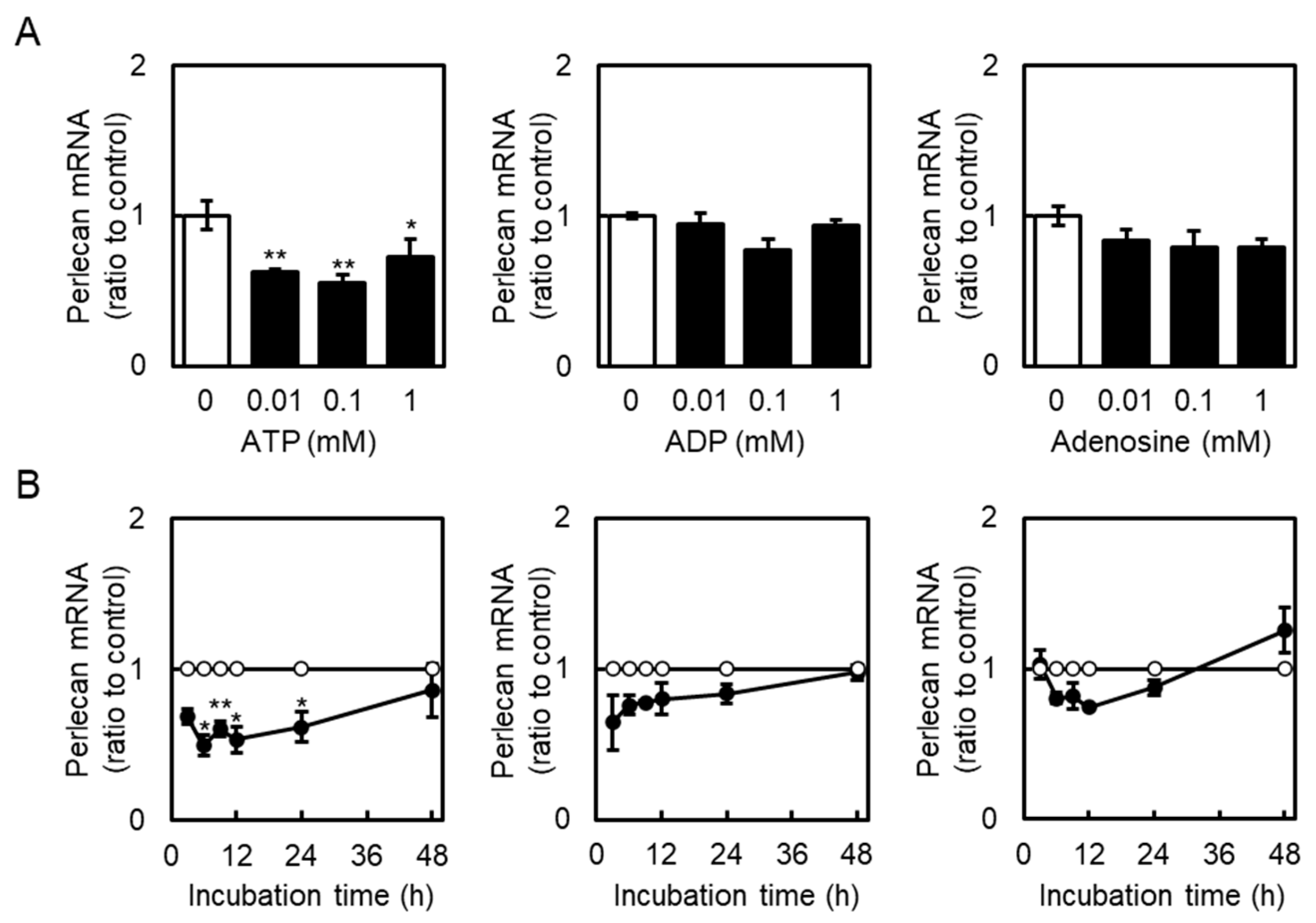
2.1. Effect of Extracellular ATP on Perlecan Expression in Vascular Endothelial Cells
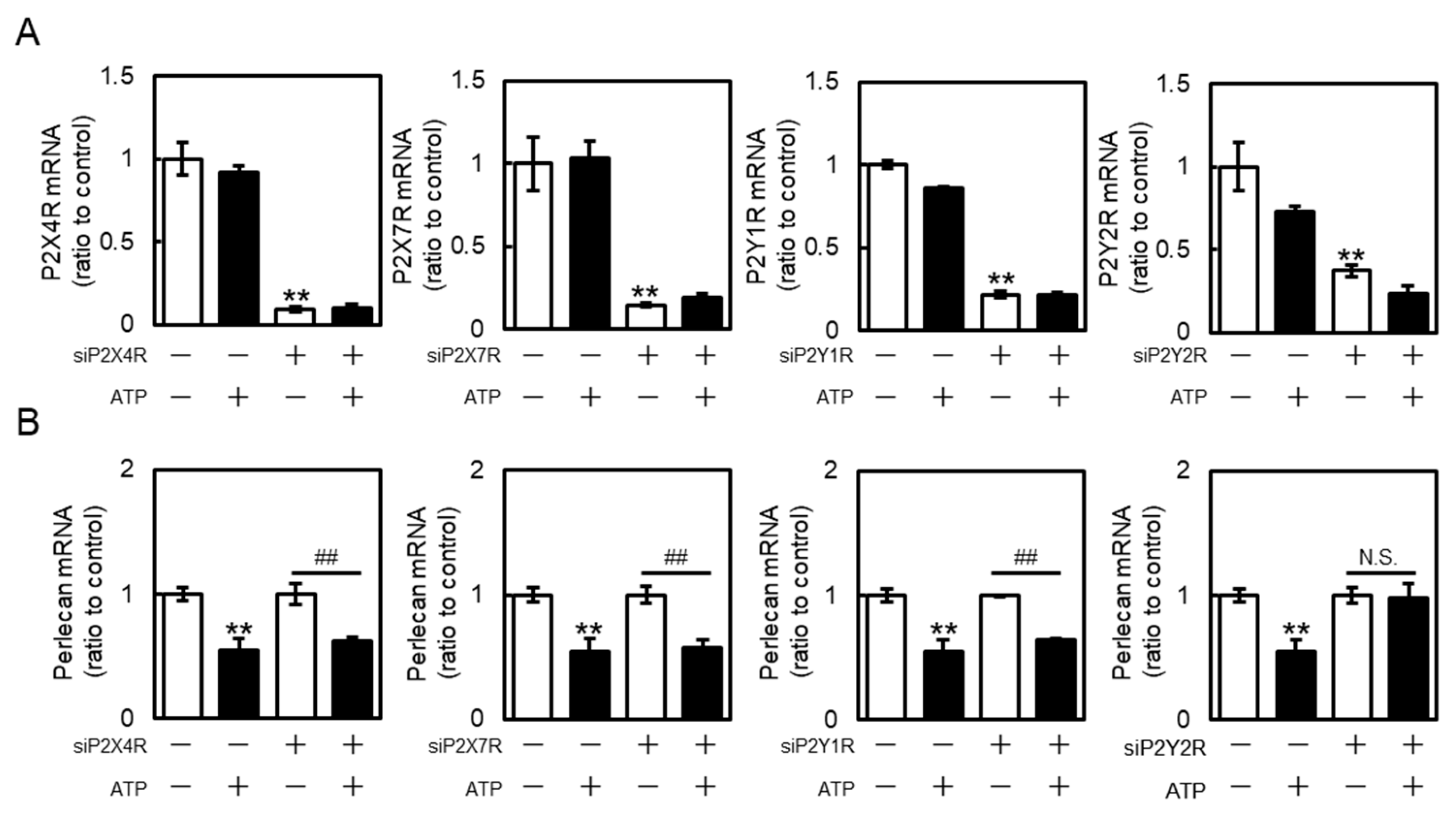
2.2. Involvement of P2 Receptors in the Suppression of Perlecan Expression by Extracellular ATP
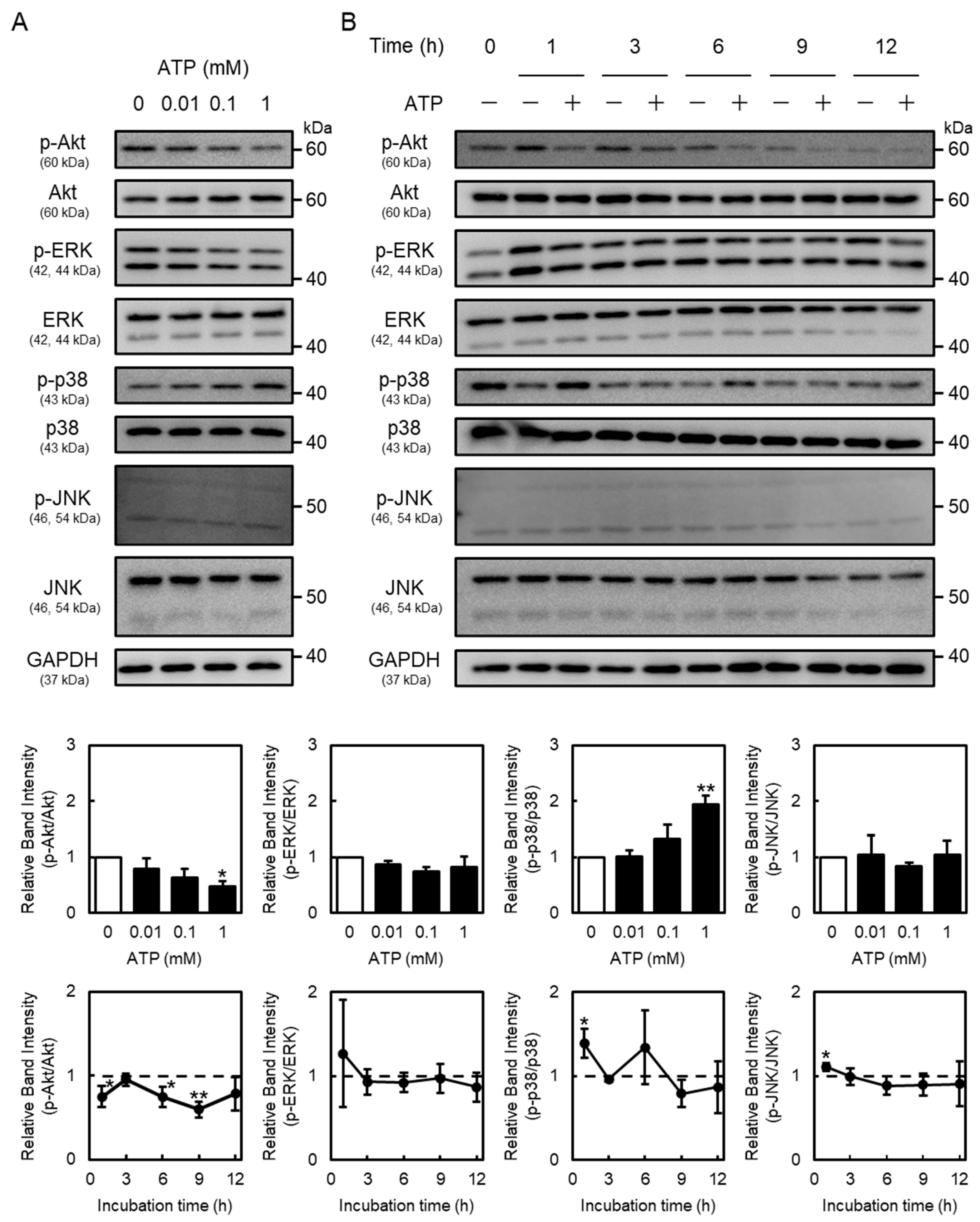
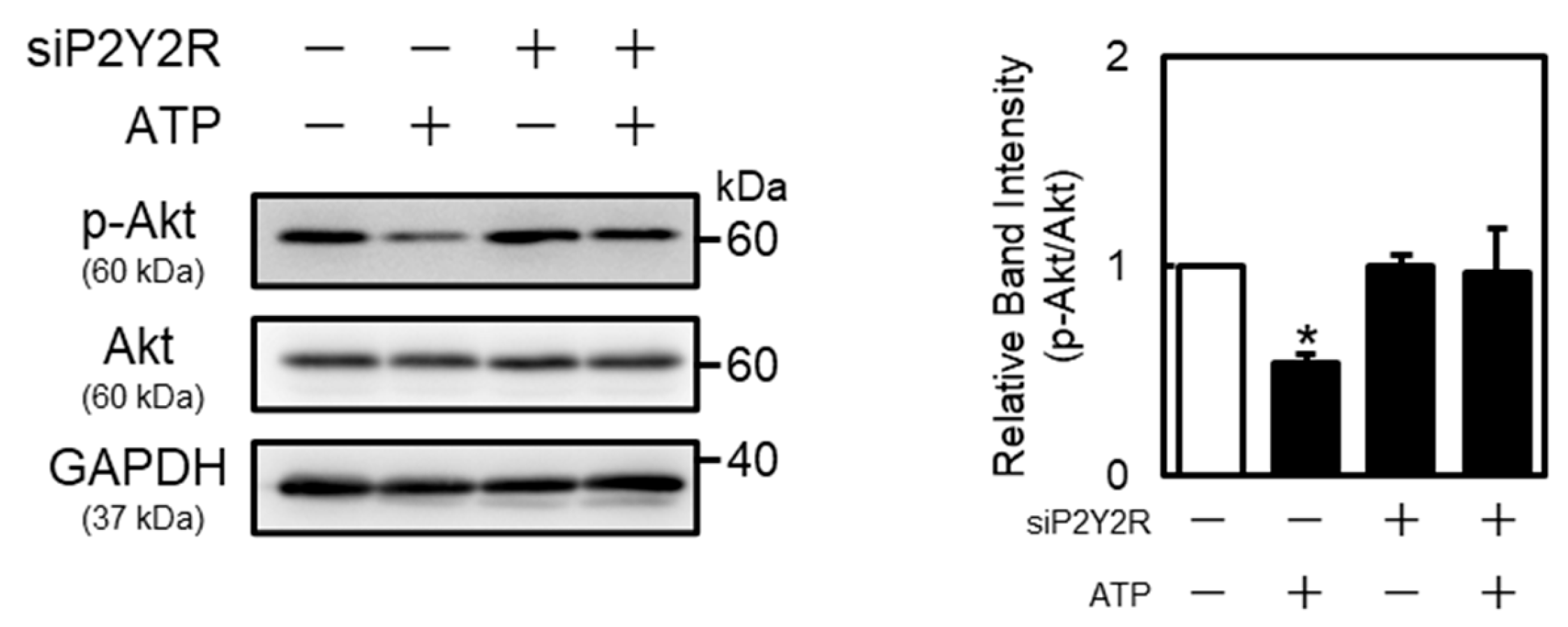
2.3. Suppression of Endothelial Akt Signaling by Extracellular ATP
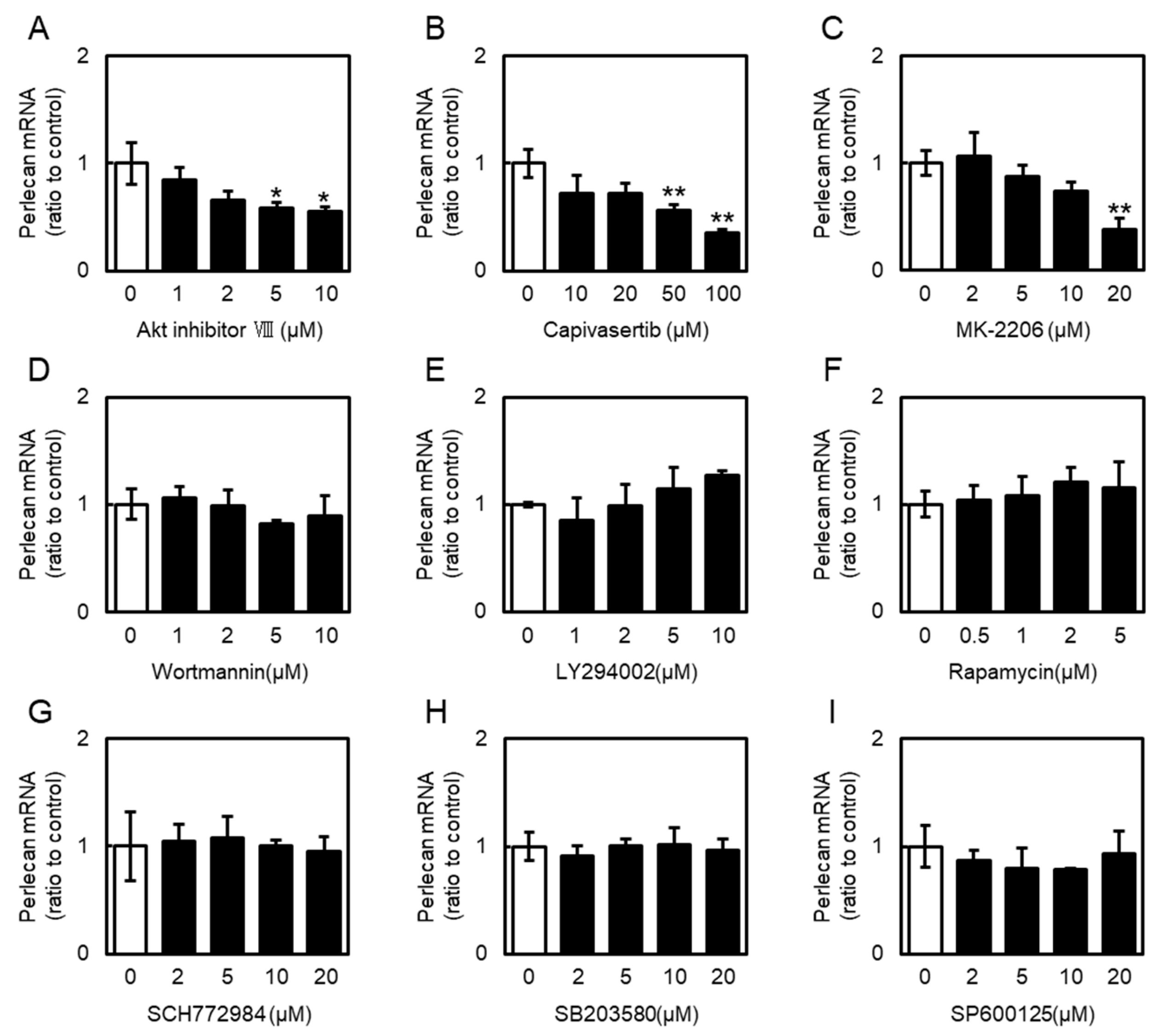
2.4. Roles of Phosphoinositide 3-Kinase (PI3K)/Akt and MAPK Signaling in Perlecan Expression
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Culture and Treatment
4.3. Small Interfering RNA (siRNA) Transfection
4.4. Reverse Transcription Polymerase Chain Reaction (RT-PCR)
4.5. Perlecan Core Protein Extraction
4.6. Western Blotting Analysis
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CBB | Coomassie brilliant blue |
| CMF-PBS | Calcium- and magnesium-free phosphate-buffered saline |
| DMEM | Dulbecco’s Modified Eagle Medium |
| EDTA | Ethylenediaminetetraacetic acid |
| ERK | Extracellular signal-regulated kinase |
| FBS | Fetal bovine serum |
| FGF-2 | Fibroblast growth factor-2 |
| GAPDH | Glyceraldehyde 3-phosphate dehydrogenase |
| HSPGs | Heparan sulfate proteoglycans |
| JNK | c-Jun N-terminal kinases |
| MAPK | Mitogen-activated protein kinase |
| mTORC1 | Mammalian target of rapamycin complex 1 |
| mTORC2 | Mammalian target of rapamycin complex 2 |
| NO | Nitric oxide |
| P2X4R | P2X4 receptor |
| P2X7R | P2X7 receptor |
| P2Y1R | P2Y1 receptor |
| P2Y2R | P2Y2 receptor |
| PI3K | Phosphoinositide 3-kinase |
| RT-PCR | Reverse transcription polymerase chain reaction |
| S.E. | Standard error |
| siRNA | Small interfering RNA |
| UTP | Uridine-5′-triphosphate |
References
- Hurairah, H.; Ferro, A. The role of the endothelium in the control of vascular function. Int. J. Clin. Pract. 2004, 58, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Ross, R.; Glomset, J.; Harker, L. Response to injury and atherogenesis. Am. J. Pathol. 1977, 86, 675–684. [Google Scholar] [PubMed]
- Nedkoff, L.; Briffa, T.; Zemedikun, D.; Herrington, S.; Wright, F.L. Global Trends in Atherosclerotic Cardiovascular Disease. Clin. Ther. 2023, 45, 1087–1091. [Google Scholar] [CrossRef] [PubMed]
- Couchman, J.R.; Pataki, C.A. An introduction to proteoglycans and their localization. J. Histochem. Cytochem. 2012, 60, 885–897. [Google Scholar] [CrossRef]
- Melrose, J. Perlecan, a modular instructive proteoglycan with diverse functional properties. Int. J. Biochem. Cell Biol. 2020, 128, 105849. [Google Scholar] [CrossRef]
- Aviezer, D.; Hecht, D.; Safran, M.; Eisinger, M.; David, G.; Yayon, A. Perlecan, basal lamina proteoglycan, promotes basic fibroblast growth factor-receptor binding, mitogenesis, and angiogenesis. Cell 1994, 79, 1005–1013. [Google Scholar] [CrossRef]
- Mertens, G.; Cassiman, J.J.; Van den Berghe, H.; Vermylen, J.; David, G. Cell surface heparan sulfate proteoglycans from human vascular endothelial cells. Core protein characterization and antithrombin III binding properties. J. Biol. Chem. 1992, 267, 20435–20443. [Google Scholar] [CrossRef]
- Kaji, T.; Yamada, A.; Miyajima, S.; Yamamoto, C.; Fujiwara, Y.; Wight, T.N.; Kinsella, M.G. Cell density-dependent regulation of proteoglycan synthesis by transforming growth factor-β1 in cultured bovine aortic endothelial cells. J. Biol. Chem. 2000, 275, 1463–1470. [Google Scholar] [CrossRef]
- Kaji, T.; Yamamoto, C.; Oh-i, M.; Fujiwara, Y.; Yamazaki, Y.; Morita, T.; Plaas, A.H.; Wight, T.N. The vascular endothelial growth factor VEGF165 induces perlecan synthesis via VEGF receptor-2 in cultured human brain microvascular endothelial cells. Biochim. Biophys. Acta 2006, 1760, 1465–1474. [Google Scholar] [CrossRef]
- Fujii, N.; Kaji, T.; Akai, T.; Koizumi, F. Thrombin reduces large heparan sulfate proteoglycan molecules in cultured vascular endothelial cell layers through inhibition of core protein synthesis. Thromb. Res. 1997, 88, 299–307. [Google Scholar] [CrossRef]
- Corriden, R.; Insel, P.A. Basal release of ATP: An autocrine-paracrine mechanism for cell regulation. Sci. Signal. 2010, 3, re1. [Google Scholar] [CrossRef]
- Choi, H.W.; Ferrara, K.W.; Barakat, A.I. Modulation of ATP/ADP concentration at the endothelial surface by shear stress: Effect of flow recirculation. Ann. Biomed. Eng. 2007, 35, 505–516. [Google Scholar] [CrossRef] [PubMed]
- Bodin, P.; Burnstock, G. Increased release of ATP from endothelial cells during acute inflammation. Inflamm. Res. 1998, 47, 351–354. [Google Scholar] [CrossRef] [PubMed]
- Bodin, P.; Bailey, D.; Burnstock, G. Increased flow-induced ATP release from isolated vascular endothelial cells but not smooth muscle cells. Br. J. Pharmacol. 1991, 103, 1203–1205. [Google Scholar] [CrossRef] [PubMed]
- Holmsen, H.; Weiss, H.J. Secretable storage pools in platelets. Annu. Rev. Med. 1979, 30, 119–134. [Google Scholar] [CrossRef]
- North, R.A. P2X receptors. Phil. Trans. R. Soc. B 2016, 371, 20150427. [Google Scholar] [CrossRef]
- von Kügelgen, I.; Hoffmann, K. Pharmacology and structure of P2Y receptors. Neuropharmacology 2016, 104, 50–61. [Google Scholar] [CrossRef]
- Yamamoto, K.; Korenaga, R.; Kamiya, A.; Qi, Z.; Sokabe, M.; Ando, J. P2X4 receptors mediate ATP-induced calcium influx in human vascular endothelial cells. Am. J. Physiol. Heart Cric. Physiol. 2000, 279, H285–H292. [Google Scholar] [CrossRef]
- Yamamoto, K.; Sokabe, T.; Matsumoto, T.; Yoshimura, K.; Shibata, M.; Ohura, N.; Fukuda, T.; Sato, T.; Sekine, K.; Kato, S.; et al. Impaired flow-dependent control of vascular tone and remodeling in P2X4-deficient mice. Nat. Med. 2006, 12, 133–137. [Google Scholar] [CrossRef]
- Cabou, C.; Martinez, L.O. The Interplay of Endothelial P2Y Receptors in Cardiovascular Health: From Vascular Physiology to Pathology. Int. J. Mol. Sci. 2022, 23, 5883. [Google Scholar] [CrossRef]
- Ding, L.; Ma, W.; Littmann, T.; Camp, R.; Shen, J. The P2Y2 nucleotide receptor mediates tissue factor expression in human coronary artery endothelial cells. J. Biol. Chem. 2011, 286, 27027–27038. [Google Scholar] [CrossRef]
- Peikert, A.; König, S.; Suchanek, D.; Rofa, K.; Schäfer, I.; Dimanski, D.; Karnbrock, L.; Bulatova, K.; Engelmann, J.; Hoppe, N.; et al. P2X4 deficiency reduces atherosclerosis and plaque inflammation in mice. Sci. Rep. 2022, 12, 2801. [Google Scholar] [CrossRef] [PubMed]
- Strassheim, D.; Verin, A.; Batori, R.; Nijmeh, H.; Burns, N.; Kovacs-Kasa, A.; Umapathy, N.S.; Kotamarthi, J.; Gokhale, Y.S.; Karoor, V.; et al. P2Y Purinergic Receptors, Endothelial Dysfunction, and Cardiovascular Diseases. Int. J. Mol. Sci. 2020, 21, 6855. [Google Scholar] [CrossRef] [PubMed]
- Ikeuchi, L.; Hara, T.; Kitabatake, K.; Uchiumi, F.; Yamamoto, C.; Tsukimoto, M.; Fujie, T.; Kaji, T. Expression profiles of purinergic P1 and P2 receptors in cultured bovine aortic endothelial cells, bovine aortic smooth muscle cells, and human vascular endothelial EA.hy926 cells. J. Toxicol. Sci. 2025, 50, 583–591. [Google Scholar] [CrossRef] [PubMed]
- Ralevic, V.; Burnstock, G. Receptors for purines and pyrimidines. Pharmacol. Rev. 1998, 50, 413–492. [Google Scholar] [CrossRef]
- Gerber, H.P.; McMurtrey, A.; Kowalski, J.; Yan, M.; Keyt, B.A.; Dixit, V.; Ferrara, N. Vascular endothelial growth factor regulates endothelial cell survival through the phosphatidylinositol 3′-kinase/Akt signal transduction pathway. Requirement for Flk-1/KDR activation. J. Biol. Chem. 1998, 273, 30336–30343. [Google Scholar] [CrossRef]
- Gustafsson, E.; Almonte-Becerril, M.; Bloch, W.; Costell, M. Perlecan maintains microvessel integrity in vivo and modulates their formation in vitro. PLoS ONE 2013, 8, e53715. [Google Scholar] [CrossRef]
- Tran, P.K.; Tran-Lundmark, K.; Soininen, R.; Tryggvason, K.; Thyberg, J.; Hedin, U. Increased intimal hyperplasia and smooth muscle cell proliferation in transgenic mice with heparan sulfate-deficient perlecan. Circ. Res. 2004, 94, 550–558. [Google Scholar] [CrossRef]
- Nugent, M.A.; Nugent, H.M.; Iozzo, R.V.; Sanchack, K.; Edelman, E.R. Perlecan is required to inhibit thrombosis after deep vascular injury and contributes to endothelial cell-mediated inhibition of intimal hyperplasia. Proc. Natl. Acad. Sci. USA 2000, 97, 6722–6727. [Google Scholar] [CrossRef]
- Brown, J.C.; Sasaki, T.; Göhring, W.; Yamada, Y.; Timpl, R. The C-terminal domain V of perlecan promotes β1 integrin-mediated cell adhesion, binds heparin, nidogen and fibulin-2 and can be modified by glycosaminoglycans. Eur. J. Biochem. 1997, 250, 39–46. [Google Scholar] [CrossRef]
- Hayashi, K.; Madri, J.A.; Yurchenco, P.D. Endothelial cells interact with the core protein of basement membrane perlecan through beta 1 and beta 3 integrins: An adhesion modulated by glycosaminoglycan. J. Cell Biol. 1992, 119, 945–959. [Google Scholar] [CrossRef]
- Woodley, D.T.; Rao, C.N.; Hassell, J.R.; Liotta, L.A.; Martin, G.R.; Kleinman, H.K. Interactions of basement membrane components. Biochim. Biophys. Acta 1983, 761, 278–283. [Google Scholar] [CrossRef] [PubMed]
- Whitelock, J.M.; Murdoch, A.D.; Iozzo, R.V.; Underwood, P.A. The degradation of human endothelial cell-derived perlecan and release of bound basic fibroblast growth factor by stromelysin, collagenase, plasmin, and heparanases. J. Biol. Chem. 1996, 271, 10079–10086. [Google Scholar] [CrossRef] [PubMed]
- Evanko, S.P.; Raines, E.W.; Ross, R.; Gold, L.I.; Wight, T.N. Proteoglycan distribution in lesions of atherosclerosis depends on lesion severity, structural characteristics, and the proximity of platelet-derived growth factor and transforming growth factor-beta. Am. J. Pathol. 1998, 152, 533–546. [Google Scholar] [PubMed]
- Burnstock, G.; Knight, G.E. Cellular distribution and functions of P2 receptor subtypes in different systems. Int. Rev. Cytol. 2004, 240, 31–304. [Google Scholar] [CrossRef]
- Yegutkin, G.G. Nucleotide- and nucleoside-converting ectoenzymes: Important modulators of purinergic signalling cascade. Biochim. Biophys. Acta 2008, 1783, 673–694. [Google Scholar] [CrossRef]
- von Kügelgen, I. Pharmacological profiles of cloned mammalian P2Y-receptor subtypes. Pharmacol. Ther. 2006, 110, 415–432. [Google Scholar] [CrossRef]
- Alessi, D.R.; James, S.R.; Downes, C.P.; Holmes, A.B.; Gaffney, P.R.; Reese, C.B.; Cohen, P. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Balpha. Curr. Biol. 1997, 7, 261–269. [Google Scholar] [CrossRef]
- Sarbassov, D.D.; Guertin, D.A.; Ali, S.M.; Sabatini, D.M. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science 2005, 307, 1098–1101. [Google Scholar] [CrossRef]
- Campbell, R.B.; Liu, F.; Ross, A.H. Allosteric activation of PTEN phosphatase by phosphatidylinositol 4,5-bisphosphate. J. Biol. Chem. 2003, 278, 33617–33620. [Google Scholar] [CrossRef]
- Maehama, T.; Dixon, J.E. The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J. Biol. Chem. 1998, 273, 13375–13378. [Google Scholar] [CrossRef]
- Coover, R.A.; Healy, T.E.; Guo, L.; Chaney, K.E.; Hennigan, R.F.; Thomson, C.S.; Aschbacher-Smith, L.E.; Jankowski, M.P.; Ratner, N. Tonic ATP-mediated growth suppression in peripheral nerve glia requires arrestin-PP2 and is evaded in NF1. Acta Neuropathol. Commun. 2018, 6, 127. [Google Scholar] [CrossRef]
- Erb, L.; Liu, J.; Ockerhausen, J.; Kong, Q.; Garrad, R.C.; Griffin, K.; Neal, C.; Krugh, B.; Santiago-Pérez, L.I.; González, F.A.; et al. An RGD sequence in the P2y2 receptor interacts with αVβ3 integrins and is required for Go-mediated signal transduction. J. Cell Biol. 2001, 153, 491–501. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.H.; Chang, C.C.; Yang, N.J.; Lee, Y.H.; Juan, S.H. RhoA-mediated inhibition of vascular endothelial cell mobility: Positive feedback through reduced cytosolic p21 and p27. J. Cell. Physiol. 2014, 229, 1455–1465. [Google Scholar] [CrossRef] [PubMed]
- Hara, T.; Kojima, T.; Matsuzaki, H.; Nakamura, T.; Yoshida, E.; Fujiwara, Y.; Yamamoto, C.; Saito, S.; Kaji, T. Induction of Syndecan-4 by Organic-Inorganic Hybrid Molecules with a 1,10-Phenanthroline Structure in Cultured Vascular Endothelial Cells. Int. J. Mol. Sci. 2017, 18, 352. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Met. 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Jungmann, O.; Nikolovska, K.; Stock, C.; Schulz, J.N.; Eckes, B.; Riethmüller, C.; Owens, R.T.; Iozzo, R.V.; Seidler, D.G. The dermatan sulfate proteoglycan decorin modulates α2β1 integrin and the vimentin intermediate filament system during collagen synthesis. PLoS ONE 2012, 7, e50809. [Google Scholar] [CrossRef]
- Empere, M.; Wang, X.; Prein, C.; Aspberg, A.; Moser, M.; Oohashi, T.; Clausen-Schaumann, H.; Aszodi, A.; Alberton, P. Aggrecan governs intervertebral discs development by providing critical mechanical cues of the extracellular matrix. Front. Bioeng. Biotechnol. 2023, 11, 1128587. [Google Scholar] [CrossRef]
| Target | Forward | Reverse |
|---|---|---|
| HSPG2 (perlecan) | 5′-ATGGCAGCGATGAAGCGGAC-3′ | 5′-TTGTGGACACGCAGCGGAAC-3′ |
| P2X4R | 5′-CGCCTCCTTCTGCCTGCC-3′ | 5′-ATGATGTCAAAGCGGATGCCA-3′ |
| P2X7R | 5′-CGCAGTCAATGAATACTACTACAAGAAGA-3′ | 5′-GGGTGAGTCGTGAAGAGACAGATG-3′ |
| P2Y1R | 5′-CTTCTACTACTTCAATAAGACCGACTG-3′ | 5′-TGCCCACCACCACGATG-3′ |
| P2Y2R | 5′-CTATGGCGTGGTGTGCGTG-3′ | 5′-GCAGTAAAGGTTGGTGTAGAAGAGG-3′ |
| B2M (β2-microglobulin) | 5′-CCATCCAGCGTCCTCCAAAGA-3′ | 5′-TTCAATCTGGGGTGGATGGAA-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ikeuchi, L.; Hara, T.; Kitabatake, K.; Uchiumi, F.; Yamamoto, C.; Tsukimoto, M.; Fujie, T.; Kaji, T. Extracellular ATP Suppresses Perlecan Core Protein Synthesis via P2Y2 Receptor-Mediated Inhibition of Akt Signaling in Cultured Vascular Endothelial Cells. Int. J. Mol. Sci. 2025, 26, 10973. https://doi.org/10.3390/ijms262210973
Ikeuchi L, Hara T, Kitabatake K, Uchiumi F, Yamamoto C, Tsukimoto M, Fujie T, Kaji T. Extracellular ATP Suppresses Perlecan Core Protein Synthesis via P2Y2 Receptor-Mediated Inhibition of Akt Signaling in Cultured Vascular Endothelial Cells. International Journal of Molecular Sciences. 2025; 26(22):10973. https://doi.org/10.3390/ijms262210973
Chicago/Turabian StyleIkeuchi, Lihito, Takato Hara, Kazuki Kitabatake, Fumiaki Uchiumi, Chika Yamamoto, Mitsutoshi Tsukimoto, Tomoya Fujie, and Toshiyuki Kaji. 2025. "Extracellular ATP Suppresses Perlecan Core Protein Synthesis via P2Y2 Receptor-Mediated Inhibition of Akt Signaling in Cultured Vascular Endothelial Cells" International Journal of Molecular Sciences 26, no. 22: 10973. https://doi.org/10.3390/ijms262210973
APA StyleIkeuchi, L., Hara, T., Kitabatake, K., Uchiumi, F., Yamamoto, C., Tsukimoto, M., Fujie, T., & Kaji, T. (2025). Extracellular ATP Suppresses Perlecan Core Protein Synthesis via P2Y2 Receptor-Mediated Inhibition of Akt Signaling in Cultured Vascular Endothelial Cells. International Journal of Molecular Sciences, 26(22), 10973. https://doi.org/10.3390/ijms262210973









