Three-Dimensional Models of the Dental Pulp: Bridging Fundamental Biology and Regenerative Therapy
Abstract
1. Introduction
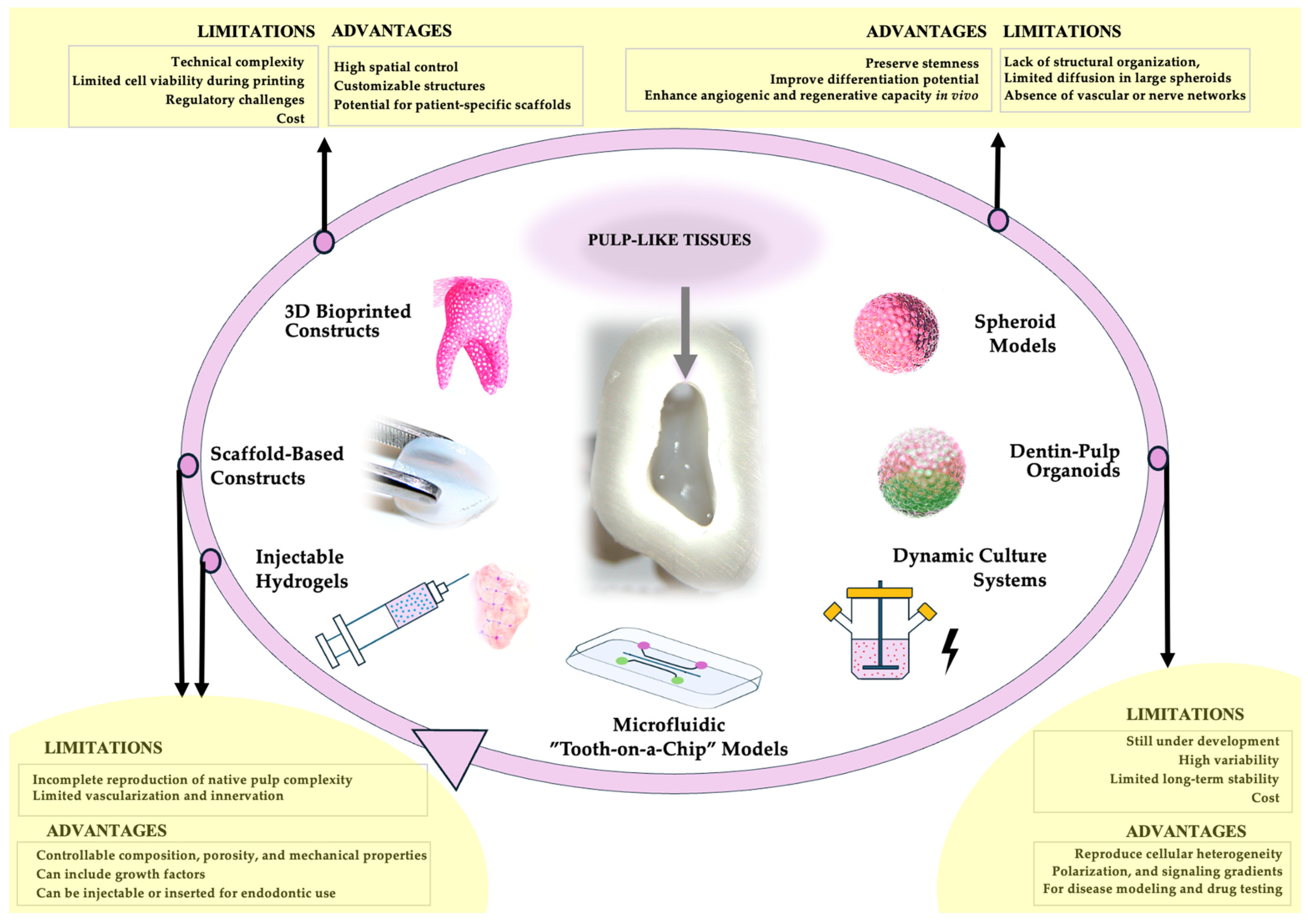
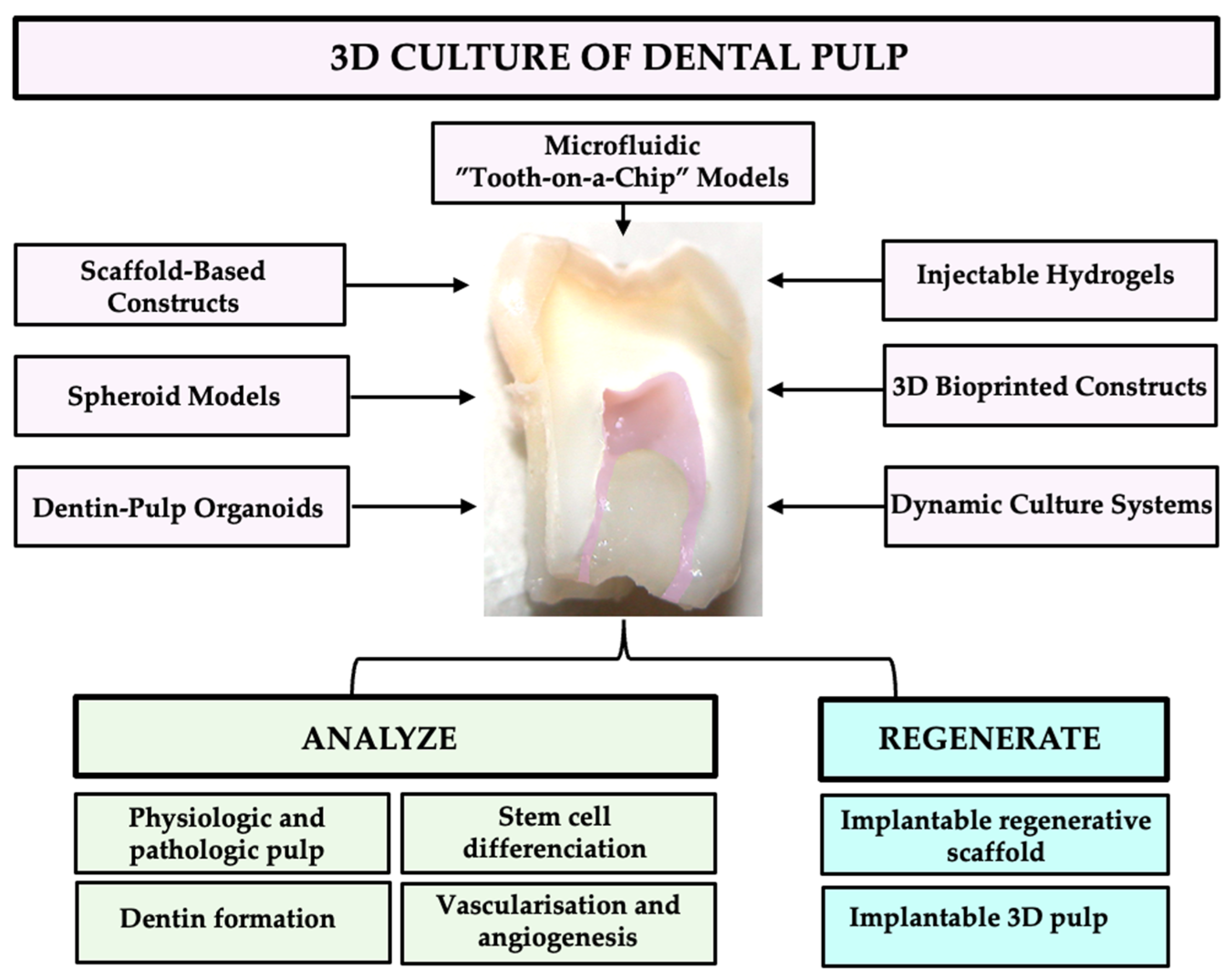
2. Architectures and Strategies in 3D Dental Pulp Modeling: Biomaterials, Cells, and Advanced Culture Systems
2.1. Composition
2.2. Scaffold-Based Constructs
2.3. Three-Dimensional Bioprinted Constructs
2.4. Injectable Hydrogels
2.5. Spheroid Models
2.6. Dentin-Pulp Organoids
2.7. Dynamic Culture Systems
2.8. Microfluidic “Tooth-on-a-Chip” Models
3. Harnessing 3D Pulp Models for Therapeutic Innovation
3.1. Modeling Physiologic Pulp Responses
3.2. Simulating Pathologic Pulp Conditions
3.3. Advancing Regenerative Endodontics
3.4. Tissue Engineering and Biofabrication of Pulp
4. Future Perspectives and Unmet Challenges in 3D Pulp Modeling
4.1. Harmonizing Stem Cell Sources and Culture Conditions
4.2. Replicating the Native Pulp Microenvironment
4.3. Engineering Vascularization and Functional Innervation
4.4. Addressing Reproducibility and Standardization
4.5. Integrating Multi-Cellular Complexity and Immune Interactions
4.6. Bridging Translation to the Clinic
4.7. Toward Next-Generation 3D Pulp Models
5. Advancing 3D Dental Pulp Models
6. Conclusions and Future Perspectives: Towards Predictive and Translational 3D Dental Pulp Models
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| 3D | three-dimensional |
| 2D | two-dimensional |
| DPSC | Dental pulp stem cells |
| SCAP | Stem cells from the apical papilla |
| PDLSC | Periodontal ligament stem cells |
| PEG | Polyethylene glycol |
| PCL | Polycaprolactone |
| PLGA | Poly(lactic-co-glycolic acid) |
| GelMA | Gelatin methacrylate |
| MSC | Mesenchymal stem cell |
| SHED | Stem cells from exfoliated deciduous teeth |
| HUVEC | Human umbilical vein endothelial cells |
| TGF-β | Transforming growth factor- β |
| VEGF | Vascular endothelial growth factor |
| BMP | Bone morphogenetic protein |
| DSPP | Dentin sialophosphoprotein |
| DMP-1 | Dentin matrix acidic phosphoprotein 1 |
| ALP | Alkaline phosphatase protein |
| Runx2 | Runt-related transcription factor 2 |
| LPS | Lipopolysaccharides |
| SDF-1 | Stromal cell-derived factor 1 |
| bFGF | Fibroblast growth factor 2 |
| ECM | Extracellular matrix |
| GMP | Good Manufacturing Practices |
| HIF-1α | Hypoxia-inducible factor 1-alpha |
| DNMT | DNA methyltransferase |
| iPSCs | Induced pluripotent stem cells |
| ATMP | Advanced Therapy Medicinal Product |
| GLP | Good Laboratory Practice |
| DPGC | Dental pulp guidance construct |
| IL-6 | Interleukin-6 |
| IL-1β TNF-α | Interleukin-1 beta Tumor necrosis factor-alpha |
| β-TCP | Beta-tricalcium phosphate |
| PLA | Polylactic acid |
| HA | Hydroxyapatite |
| DDM | Demineralized dentin matrix |
| TDM | Treated dentin matrix |
| MTA | Mineral trioxide aggregate |
| nHA | Nano-hydroxyapatite |
| GPTMS | (3- Glycidyloxypropyl)trimethoxysilane |
| scRNA-seq | Single-cell RNA sequencing |
References
- Keller, L.; Offner, D.; Schwinté, P.; Morand, D.; Wagner, Q.; Gros, C.; Bornert, F.; Bahi, S.; Musset, A.-M.; Benkirane-Jessel, N.; et al. Active Nanomaterials to Meet the Challenge of Dental Pulp Regeneration. Materials 2015, 8, 7461–7471. [Google Scholar] [CrossRef] [PubMed]
- Fioretti, F.; Mendoza-Palomares, C.; Avoaka-Boni, M.C.; Ramaroson, J.; Bahi, S.; Richert, L.; Granier, F.; Benkirane-Jessel, N.; Haikel, Y. Nano-Odontology: Nanostructured Assemblies for Endodontic Regeneration. J. Biomed. Nanotechnol. 2011, 7, 471–475. [Google Scholar] [CrossRef]
- Seck, A.; Zein, N.; Nounsi, A.; Harmouch, E.; Vidal Varbanova, A.; Fernandez De Grado, G.; Offner, D.; Lutz, J.-C.; Benkirane-Jessel, N.; Fioretti, F. Nanomaterials for Endodontic Regeneration. In Stem Cells and Regenerative Medicine; IOS Press: Amsterdam, The Netherlands, 2021; pp. 88–92. [Google Scholar]
- Fioretti, F.; Mendoza-Palomares, C.; Helms, M.; Al Alam, D.; Richert, L.; Arntz, Y.; Rinckenbach, S.; Garnier, F.; Haïkel, Y.; Gangloff, S.C.; et al. Nanostructured Assemblies for Dental Application. ACS Nano 2010, 4, 3277–3287. [Google Scholar] [CrossRef]
- Piglionico, S.S.; Pons, C.; Romieu, O.; Cuisinier, F.; Levallois, B.; Panayotov, I.V. In Vitro, Ex Vivo, and in Vivo Models for Dental Pulp Regeneration. J. Mater. Sci. Mater. Med. 2023, 34, 15. [Google Scholar] [CrossRef]
- Hadjichristou, C.; Papachristou, E.; Bonovolias, I.; Bakopoulou, A. Three-Dimensional Tissue Engineering-Based Dentin/Pulp Tissue Analogue as Advanced Biocompatibility Evaluation Tool of Dental Restorative Materials. Dent. Mater. 2020, 36, 229–248. [Google Scholar] [CrossRef]
- Hsiao, H.-Y.; Nien, C.-Y.; Hong, H.-H.; Cheng, M.-H.; Yen, T.-H. Application of Dental Stem Cells in Three-Dimensional Tissue Regeneration. World J. Stem Cells 2021, 13, 1610–1624. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Li, X.; Liu, H.; Yang, X.; Li, R.; Zhao, H.; Shang, Z. Organoids in the oral and maxillofacial region: Present and future. Int. J. Oral Sci. 2024, 16, 61, Erratum in Int. J. Oral Sci. 2025, 17, 43. https://doi.org/10.1038/s41368-025-00377-5. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ohlsson, E.; Galler, K.M.; Widbiller, M. A Compilation of Study Models for Dental Pulp Regeneration. Int. J. Mol. Sci. 2022, 23, 14361. [Google Scholar] [CrossRef] [PubMed]
- Zein, N.; Harmouch, E.; Lutz, J.; De Grado, G.; Kuchler-Bopp, S.; Clauss, F.; Offner, D.; Hua, G.; Benkirane-Jessel, N.; Fioretti, F. Polymer-Based Instructive Scaffolds for Endodontic Regeneration. Materials 2019, 12, 2347. [Google Scholar] [CrossRef]
- Aguilar, A.; Zein, N.; Harmouch, E.; Hafdi, B.; Bornert, F.; Offner, D.; Clauss, F.; Fioretti, F.; Huck, O.; Benkirane-Jessel, N.; et al. Application of Chitosan in Bone and Dental Engineering. Molecules 2019, 24, 3009. [Google Scholar] [CrossRef]
- Abbass, M.M.S.; El-Rashidy, A.A.; Sadek, K.M.; Moshy, S.E.; Radwan, I.A.; Rady, D.; Dörfer, C.E.; Fawzy El-Sayed, K.M. Hydrogels and Dentin-Pulp Complex Regeneration: From the Benchtop to Clinical Translation. Polymers 2020, 12, 2935. [Google Scholar] [CrossRef] [PubMed]
- Eap, S.; Keller, L.; Schiavi, J.; Huck, O.; Jacomine, L.; Fioretti, F.; Gauthier, C.; Sebastian, V.; Schwinté, P.; Benkirane-Jessel, N. A Living Thick Nanofibrous Implant Bifunctionalized with Active Growth Factor and Stem Cells for Bone Regeneration. Int. J. Nanomed. 2015, 10, 1061–1075. [Google Scholar] [CrossRef]
- Mendoza-Palomares, C.; Ferrand, A.; Facca, S.; Fioretti, F.; Ladam, G.; Kuchler-Bopp, S.; Regnier, T.; Mainard, D.; Benkirane-Jessel, N. Smart Hybrid Materials Equipped by Nanoreservoirs of Therapeutics. ACS Nano 2012, 6, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Duarte Campos, D.F.; Zhang, S.; Kreimendahl, F.; Köpf, M.; Fischer, H.; Vogt, M.; Blaeser, A.; Apel, C.; Esteves-Oliveira, M. Hand-Held Bioprinting for de Novo Vascular Formation Applicable to Dental Pulp Regeneration. Connect. Tissue Res. 2020, 61, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Zhou, N.; Zhu, S.; Wei, X.; Liao, X.; Wang, Y.; Xu, Y.; Bai, L.; Wan, H.; Liu, L.; Zhang, J.; et al. 3D-Bioprinted Hydrogels with Instructive Niches for Dental Pulp Regeneration. Int. J. Bioprint. 2024, 10, 1790. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, X.; Song, W.; Pan, T.; Wang, H.; Ning, T.; Wei, Q.; Xu, H.H.K.; Wu, B.; Ma, D. Effects of 3-Dimensional Bioprinting Alginate/Gelatin Hydrogel Scaffold Extract on Proliferation and Differentiation of Human Dental Pulp Stem Cells. J. Endod. 2019, 45, 706–715. [Google Scholar] [CrossRef]
- Nowwarote, N.; Petit, S.; Ferre, F.C.; Dingli, F.; Laigle, V.; Loew, D.; Osathanon, T.; Fournier, B.P.J. Extracellular Matrix Derived from Dental Pulp Stem Cells Promotes Mineralization. Front. Bioeng. Biotechnol. 2021, 9, 740712. [Google Scholar] [CrossRef]
- Tan, Q.; Cao, Y.; Zheng, X.; Peng, M.; Huang, E.; Wang, J. BMP4-Regulated Human Dental Pulp Stromal Cells Promote Pulp-like Tissue Regeneration in a Decellularized Dental Pulp Matrix Scaffold. Odontology 2021, 109, 895–903. [Google Scholar] [CrossRef]
- Zhao, F.; Zhang, Z.; Guo, W. The 3-Dimensional Printing for Dental Tissue Regeneration: The State of the Art and Future Challenges. Front. Bioeng. Biotechnol. 2024, 12, 1356580. [Google Scholar] [CrossRef]
- Yudaev, P.A.; Chistyakov, E.M. Progress in Dental Materials: Application of Natural Ingredients. Russ. Chem. Rev. 2024, 93, RCR5108. [Google Scholar] [CrossRef]
- Jin, G.; Ravichandran, V.; Shim, M.S.; Kim, J.-E. Incorporating an Artificially Synthesized Fluoride Complex into Urethane-Acrylate-Based 3D Printing Resin: Effects on Mechanical Properties, Cytotoxicity, Antimicrobial Actions, and Its Long-Term Fluoride-Releasing Properties. J. Dent. 2024, 150, 105363. [Google Scholar] [CrossRef] [PubMed]
- Antibiotics in Dentistry: A Narrative Review of the Evidence Beyond the Myth. Available online: https://colab.ws/articles/10.3390%2Fijerph20116025 (accessed on 3 November 2025).
- Effect of Calcium Fluoride Nanoparticles in Prevention of Demineralization During Orthodontic Fixed Appliance Treatment: A Randomized Clinical Trial. Available online: https://colab.ws/articles/10.1093%2Fejo%2Fcjac055 (accessed on 3 November 2025).
- Application of Antimicrobial Nanoparticles in Dentistry. Available online: https://colab.ws/articles/10.3390%2Fmolecules24061033 (accessed on 3 November 2025).
- Herbs Used in Dentistry: Need of the New Era. Available online: https://colab.ws/articles/10.4103%2Fjpcdoh.jpcdoh_7_20 (accessed on 3 November 2025).
- Plant Extracts: Antimicrobial Properties, Mechanisms of Action and Applications. Available online: https://colab.ws/articles/10.1007%2F978-981-15-7098-8_11 (accessed on 3 November 2025).
- Dawood, A.; Marti, B.M.; Sauret-Jackson, V.; Darwood, A. 3D Printing in Dentistry. Br. Dent. J. 2015, 219, 521–529, Erratum in Br. Dent. J. 2016, 220, 86. [Google Scholar] [CrossRef]
- Yue, J.; Zhao, P.; Gerasimov, J.Y.; van de Lagemaat, M.; Grotenhuis, A.; Rustema-Abbing, M.; van der Mei, H.C.; Busscher, H.J.; Herrmann, A.; Ren, Y. 3D-Printable Antimicrobial Composite Resins. Adv. Funct. Mater. 2015, 25, 6756–6767. [Google Scholar] [CrossRef]
- Li, Z.; Wang, C.; Qiu, W.; Liu, R. Antimicrobial Thiol–Ene–Acrylate Photosensitive Resins for DLP 3D Printing. Photochem. Photobiol. 2019, 95, 1219–1229. [Google Scholar] [CrossRef]
- Comparison in Terms of Accuracy Between DLP and LCD Printing Technology for Dental Model Printing. Available online: https://www.mdpi.com/2304-6767/10/10/181 (accessed on 3 November 2025).
- Segneanu, A.-E.; Bejenaru, L.E.; Bejenaru, C.; Blendea, A.; Mogoşanu, G.D.; Biţă, A.; Boia, E.R. Advancements in Hydrogels: A Comprehensive Review of Natural and Synthetic Innovations for Biomedical Applications. Polymers 2025, 17, 2026. [Google Scholar] [CrossRef] [PubMed]
- Athirasala, A.; Tahayeri, A.; Thrivikraman, G.; França, C.M.; Monteiro, N.; Tran, V.; Ferracane, J.; Bertassoni, L.E. A Dentin-Derived Hydrogel Bioink for 3D Bioprinting of Cell Laden Scaffolds for Regenerative Dentistry. Biofabrication 2018, 10, 024101. [Google Scholar] [CrossRef] [PubMed]
- Dissanayaka, W.L.; Zhu, L.; Hargreaves, K.M.; Jin, L.; Zhang, C. Scaffold-Free Prevascularized Microtissue Spheroids for Pulp Regeneration. J. Dent. Res. 2014, 93, 1296–1303. [Google Scholar] [CrossRef]
- Favreau, H.; Pijnenburg, L.; Seitlinger, J.; Fioretti, F.; Keller, L.; Scipioni, D.; Adriaensen, H.; Kuchler-Bopp, S.; Ehlinger, M.; Mainard, D.; et al. Osteochondral Repair Combining Therapeutics Implant with Mesenchymal Stem Cells Spheroids. Nanomed. Nanotechnol. Biol. Med. 2020, 29, 102253. [Google Scholar] [CrossRef]
- Liu, F.; Xiao, J.; Chen, L.-H.; Pan, Y.-Y.; Tian, J.-Z.; Zhang, Z.-R.; Bai, X.-C. Self-Assembly of Differentiated Dental Pulp Stem Cells Facilitates Spheroid Human Dental Organoid Formation and Prevascularization. World J. Stem Cells 2024, 16, 287–304. [Google Scholar] [CrossRef]
- Jeong, S.Y.; Lee, S.; Choi, W.H.; Jee, J.H.; Kim, H.-R.; Yoo, J. Fabrication of Dentin-Pulp-Like Organoids Using Dental-Pulp Stem Cells. Cells 2020, 9, 642. [Google Scholar] [CrossRef]
- Xu, X.; Li, Z.; Ai, X.; Tang, Y.; Yang, D.; Dou, L. Human Three-Dimensional Dental Pulp Organoid Model for Toxicity Screening of Dental Materials on Dental Pulp Cells and Tissue. Int. Endod. J. 2022, 55, 79–88. [Google Scholar] [CrossRef]
- Torizal, F.G.; Noorintan, S.T.; Gania, Z. Bioengineering Tooth and Periodontal Organoids from Stem and Progenitor Cells. Organoids 2024, 3, 247–265. [Google Scholar] [CrossRef]
- Li, F.-C.; Kishen, A. 3D Organoids for Regenerative Endodontics. Biomolecules 2023, 13, 900. [Google Scholar] [CrossRef] [PubMed]
- Farshbaf, A.; Mottaghi, M.; Mohammadi, M.; Monsef, K.; Mirhashemi, M.; Attaran Khorasani, A.; Mohtasham, N. Regenerative Application of Oral and Maxillofacial 3D Organoids Based on Dental Pulp Stem Cell. Tissue Cell 2024, 89, 102451. [Google Scholar] [CrossRef]
- Lancia, L.; Pulcini, F.; Mari, E.; Piccoli, L.; Biordi, L.A.; Mutti, L.; Festuccia, C.; Gravina, G.L.; Mattei, V.; Mauro, A.; et al. Dental Pulp Stem Cell-Derived Organoids: Advancing the Development of 3D Structures. Cells 2025, 14, 1603. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Al Madhoun, A.; Sindhu, S.; Haddad, D.; Atari, M.; Ahmad, R.; Al-Mulla, F. Dental Pulp Stem Cells Derived From Adult Human Third Molar Tooth: A Brief Review. Front. Cell Dev. Biol. 2021, 9, 717624. [Google Scholar] [CrossRef]
- Sasaki, J.-I.; Abe, G.L.; Li, A.; Matsumoto, T.; Imazato, S. Large Three-Dimensional Cell Constructs for Tissue Engineering. Sci. Technol. Adv. Mater. 2021, 22, 571–582. [Google Scholar] [CrossRef] [PubMed]
- Bédard, P.; Gauvin, S.; Ferland, K.; Caneparo, C.; Pellerin, È.; Chabaud, S.; Bolduc, S. Innovative Human Three-Dimensional Tissue-Engineered Models as an Alternative to Animal Testing. Bioengineering 2020, 7, 115. [Google Scholar] [CrossRef]
- Mierke, C.T. Bioprinting of Cells, Organoids and Organs-on-a-Chip Together with Hydrogels Improves Structural and Mechanical Cues. Cells 2024, 13, 1638. [Google Scholar] [CrossRef]
- França, C.M.; Tahayeri, A.; Rodrigues, N.S.; Ferdosian, S.; Puppin Rontani, R.M.; Sereda, G.; Ferracane, J.L.; Bertassoni, L.E. The Tooth On-a-Chip: A Microphysiologic Model System Mimicking the Biologic Interface of the Tooth with Biomaterials. Lab. Chip 2020, 20, 405–413. [Google Scholar] [CrossRef]
- Suzuki, T.; Lee, C.H.; Chen, M.; Zhao, W.; Fu, S.Y.; Qi, J.J.; Chotkowski, G.; Eisig, S.B.; Wong, A.; Mao, J.J. Induced Migration of Dental Pulp Stem Cells for in Vivo Pulp Regeneration. J. Dent. Res. 2011, 90, 1013–1018. [Google Scholar] [CrossRef]
- Xuan, K.; Li, B.; Guo, H.; Sun, W.; Kou, X.; He, X.; Zhang, Y.; Sun, J.; Liu, A.; Liao, L.; et al. Deciduous Autologous Tooth Stem Cells Regenerate Dental Pulp after Implantation into Injured Teeth. Sci. Transl. Med. 2018, 10, eaaf3227. [Google Scholar] [CrossRef]
- Decarli, M.C.; de Castro, M.V.; Nogueira, J.A.; Nagahara, M.H.T.; Westin, C.B.; de Oliveira, A.L.R.; da Silva, J.V.L.; Moroni, L.; Mota, C.; Moraes, Â.M. Development of a Device Useful to Reproducibly Produce Large Quantities of Viable and Uniform Stem Cell Spheroids with Controlled Diameters. Mater. Sci. Eng. C Mater. Biol. Appl. 2022, 135, 112685. [Google Scholar] [CrossRef]
- Niu, L.; Zhang, H.; Liu, Y.; Wang, Y.; Li, A.; Liu, R.; Zou, R.; Yang, Q. Microfluidic Chip for Odontoblasts in Vitro. ACS Biomater. Sci. Eng. 2019, 5, 4844–4851. [Google Scholar] [CrossRef]
- Zhang, H.; Li, L.; Wang, S.; Sun, X.; Luo, C.; Hou, B. Construction of Dentin-on-a-Chip Based on Microfluidic Technology and Tissue Engineering. J. Dent. 2024, 146, 105028. [Google Scholar] [CrossRef]
- Zhang, S.; Buttler-Buecher, P.; Denecke, B.; Arana-Chavez, V.E.; Apel, C. A Comprehensive Analysis of Human Dental Pulp Cell Spheroids in a Three-Dimensional Pellet Culture System. Arch. Oral. Biol. 2018, 91, 1–8. [Google Scholar] [CrossRef]
- de Oliveira Ribeiro, R.A.; Peruchi, V.; Soares, I.P.M.; Mon, F.K.W.; Soares, D.G.; Hebling, J.; de Souza Costa, C.A. Combined Catalytic Strategies Applied to In-Office Tooth Bleaching: Whitening Efficacy, Cytotoxicity, and Gene Expression of Human Dental Pulp Cells in a 3D Culture Model. Clin. Oral. Investig. 2024, 28, 669, Correction in Clin. Oral. Invest. 2025, 29, 427. https://doi.org/10.1007/s00784-025-06530-y. [Google Scholar] [CrossRef] [PubMed]
- EzEldeen, M.; Loos, J.; Mousavi Nejad, Z.; Cristaldi, M.; Murgia, D.; Braem, A.; Jacobs, R. 3D-Printing-Assisted Fabrication of Chitosan Scaffolds from Different Sources and Cross-Linkers for Dental Tissue Engineering. Eur. Cell Mater. 2021, 41, 485–501. [Google Scholar] [CrossRef]
- Li, F.-C.; Shahin-Shamsabadi, A.; Selvaganapathy, P.R.; Kishen, A. Engineering a Novel Stem Cells from Apical Papilla-Macrophages Organoid for Regenerative Endodontics. J. Endod. 2022, 48, 741–748. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, T.C.; Rothermund, K.; Gabe, C.M.; Beniash, E.; Davidson, L.A.; Syed-Picard, F.N. Self-Assembly of Tooth Root Organoid from Postnatal Human Dental Stem Cells. Tissue Eng. Part. A 2024, 30, 404–414. [Google Scholar] [CrossRef] [PubMed]
- Perard, M.; Tricot-Doleux, S.; Pellen-Mussi, P.; Meary, F.; Pérez, F. Evaluation of the Cytotoxicity of Pulp Floor Perforation Filling Materials by Using in Parallel 2d and 3d Culture Models. Bull. Group. Int. Rech. Sci. Stomatol. Odontol. 2011, 50, 42–43. [Google Scholar]
- Riccio, M.; Resca, E.; Maraldi, T.; Pisciotta, A.; Ferrari, A.; Bruzzesi, G.; De Pol, A. Human Dental Pulp Stem Cells Produce Mineralized Matrix in 2D and 3D Cultures. Eur. J. Histochem. 2010, 54, e46. [Google Scholar] [CrossRef] [PubMed]
- Bu, N.-U.; Lee, H.-S.; Lee, B.-N.; Hwang, Y.-C.; Kim, S.-Y.; Chang, S.W.; Choi, K.-K.; Kim, D.-S.; Jang, J.-H. In Vitro Characterization of Dental Pulp Stem Cells Cultured in Two Microsphere-Forming Culture Plates. J. Clin. Med. 2020, 9, 242. [Google Scholar] [CrossRef]
- Kilic Bektas, C.; Zhang, W.; Mao, Y.; Wu, X.; Kohn, J.; Yelick, P.C. Self-Assembled Hydrogel Microparticle-Based Tooth-Germ Organoids. Bioengineering 2022, 9, 215. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, N.; Noda, S.; Yamamoto, M.; Okiji, T. Properties of Dental Pulp-Derived Mesenchymal Stem Cells and the Effects of Culture Conditions. J. Endod. 2017, 43, S31–S34. [Google Scholar] [CrossRef]
- Lancia, L.; Mauro, A.; Mattei, V.; Delle Monache, S.; Pulcini, F. Protocol for the Generation and Analysis of Organoids from Dental Pulp Stem Cells (DPSCs). Tissue Cell 2025, 96, 103010. [Google Scholar] [CrossRef] [PubMed]
- Raik, S.; Sharma, P.; Kumar, S.; Rattan, V.; Das, A.; Kumar, N.; Srinivasan, R.; Bhattacharyya, S. Three-Dimensional Spheroid Culture of Dental Pulp-Derived Stromal Cells Enhance Their Biological and Regenerative Properties for Potential Therapeutic Applications. Int. J. Biochem. Cell Biol. 2023, 160, 106422. [Google Scholar] [CrossRef]
- Zaccara, I.M.; Mestieri, L.B.; Moreira, M.S.; Grecca, F.S.; Martins, M.D.; Kopper, P.M.P. Photobiomodulation Therapy Improves Multilineage Differentiation of Dental Pulp Stem Cells in Three-Dimensional Culture Model. J. Biomed. Opt. 2018, 23, 095001. [Google Scholar] [CrossRef]
- Yamamoto, M.; Kawashima, N.; Takashino, N.; Koizumi, Y.; Takimoto, K.; Suzuki, N.; Saito, M.; Suda, H. Three-Dimensional Spheroid Culture Promotes Odonto/Osteoblastic Differentiation of Dental Pulp Cells. Arch. Oral. Biol. 2014, 59, 310–317. [Google Scholar] [CrossRef]
- Dissanayaka, W.L.; Zhan, X.; Zhang, C.; Hargreaves, K.M.; Jin, L.; Tong, E.H.Y. Coculture of Dental Pulp Stem Cells with Endothelial Cells Enhances Osteo-/Odontogenic and Angiogenic Potential in Vitro. J. Endod. 2012, 38, 454–463. [Google Scholar] [CrossRef]
- Seo, E.J.; Park, S.; Lee, E.; Huh, Y.H.; Ha, Y.E.; Tigyi, G.J.; Jeong, T.; Jang, I.H.; Shin, J. Establishing Three-Dimensional Explant Culture of Human Dental Pulp Tissue. Int. J. Stem Cells 2024, 17, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wan, M.; Cui, D.; Tian, Q.; Li, Y.; Yu, S.; Zheng, L.; Ye, L. DNMTi@ZIF-8 Enhances Biomimetic Pulp Regeneration via Epigenetic Regulation. J. Dent. Res. 2025, 104, 743–752. [Google Scholar] [CrossRef]
- Min, T.-J.; Kim, M.J.; Kang, K.-J.; Jeoung, Y.J.; Oh, S.H.; Jang, Y.-J. 3D Spheroid Formation Using BMP-Loaded Microparticles Enhances Odontoblastic Differentiation of Human Dental Pulp Stem Cells. Stem Cells Int. 2021, 2021, 9326298. [Google Scholar] [CrossRef]
- Feng, K.-C.; Li, J.; Wang, L.; Chuang, Y.-C.; Liu, H.; Pinkas-Sarafova, A.; Chang, C.-C.; Nam, C.-Y.; Simon, M.; Rafailovich, M. Combination of 3D Printing and ALD for Dentin Fabrication from Dental Pulp Stem Cell Culture. ACS Appl. Bio Mater. 2021, 4, 7422–7430. [Google Scholar] [CrossRef]
- Yuan, C.; Wang, P.; Zhu, S.; Zou, T.; Wang, S.; Xu, J.; Heng, B.C.; Diogenes, A.; Zhang, C. EphrinB2 Stabilizes Vascularlike Structures Generated by Endothelial Cells and Stem Cells from Apical Papilla. J. Endod. 2016, 42, 1362–1370. [Google Scholar] [CrossRef]
- Cavalcanti, B.N.; Zeitlin, B.D.; Nör, J.E. A Hydrogel Scaffold That Maintains Viability and Supports Differentiation of Dental Pulp Stem Cells. Dent. Mater. 2013, 29, 97–102. [Google Scholar] [CrossRef]
- Cao, S.; Han, J.; Sharma, N.; Msallem, B.; Jeong, W.; Son, J.; Kunz, C.; Kang, H.-W.; Thieringer, F.M. In Vitro Mechanical and Biological Properties of 3D Printed Polymer Composite and β-Tricalcium Phosphate Scaffold on Human Dental Pulp Stem Cells. Materials 2020, 13, 3057. [Google Scholar] [CrossRef]
- Huang, K.-H.; Chen, Y.-W.; Wang, C.-Y.; Lin, Y.-H.; Wu, Y.-H.A.; Shie, M.-Y.; Lin, C.-P. Enhanced Capability of Bone Morphogenetic Protein 2-Loaded Mesoporous Calcium Silicate Scaffolds to Induce Odontogenic Differentiation of Human Dental Pulp Cells. J. Endod. 2018, 44, 1677–1685. [Google Scholar] [CrossRef]
- Park, J.H.; Gillispie, G.J.; Copus, J.S.; Zhang, W.; Atala, A.; Yoo, J.J.; Yelick, P.C.; Lee, S.J. The Effect of BMP-Mimetic Peptide Tethering Bioinks on the Differentiation of Dental Pulp Stem Cells (DPSCs) in 3D Bioprinted Dental Constructs. Biofabrication 2020, 12, 035029. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.; Qiu, M.; Hwang, Y.-C.; Oh, W.-M.; Koh, J.-T.; Park, C.; Lee, B.-N. The Effects of 3-Dimensional Bioprinting Calcium Silicate Cement/Methacrylated Gelatin Scaffold on the Proliferation and Differentiation of Human Dental Pulp Stem Cells. Materials 2022, 15, 2170. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Cao, C.; Wang, A.; Zhao, Y.; Jin, M.; Wang, Y.; Chen, S.; Yu, M.; Yang, Z.; Qu, X.; et al. Injectable Double-Network Hydrogel-Based Three-Dimensional Cell Culture Systems for Regenerating Dental Pulp. ACS Appl. Mater. Interfaces 2023, 15, 7821–7832. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Lee, H.; Lee, G.-H.; Hoang, T.-H.; Kim, H.-R.; Kim, G.H. Fabrication of Bone-Derived Decellularized Extracellular Matrix/Ceramic-Based Biocomposites and Their Osteo/Odontogenic Differentiation Ability for Dentin Regeneration. Bioeng. Transl. Med. 2022, 7, e10317. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, Z.; Bi, F.; Tang, H.; Chen, J.; Huo, F.; Chen, J.; Lan, T.; Qiao, X.; Sima, X.; et al. Personalized 3D-Printed Scaffolds with Multiple Bioactivities for Bioroot Regeneration. Adv. Healthc. Mater. 2023, 12, e2300625. [Google Scholar] [CrossRef]
- Ha, M.; Athirasala, A.; Tahayeri, A.; Menezes, P.P.; Bertassoni, L.E. Micropatterned hydrogels and cell alignment enhance the odontogenic potential of stem cells from apical papilla in-vitro. Dent Mater. 2020, 36, 88–96. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, R.-S.; Hsu, S.-H.; Chang, H.-H.; Chen, M.-H. Challenge Tooth Regeneration in Adult Dogs with Dental Pulp Stem Cells on 3D-Printed Hydroxyapatite/Polylactic Acid Scaffolds. Cells 2021, 10, 3277. [Google Scholar] [CrossRef]
- Song, J.S.; Takimoto, K.; Jeon, M.; Vadakekalam, J.; Ruparel, N.B.; Diogenes, A. Decellularized Human Dental Pulp as a Scaffold for Regenerative Endodontics. J. Dent. Res. 2017, 96, 640–646. [Google Scholar] [CrossRef]
- Zheng, L.; Liu, Y.; Jiang, L.; Wang, X.; Chen, Y.; Li, L.; Song, M.; Zhang, H.; Zhang, Y.S.; Zhang, X. Injectable Decellularized Dental Pulp Matrix-Functionalized Hydrogel Microspheres for Endodontic Regeneration. Acta Biomater. 2023, 156, 37–48. [Google Scholar] [CrossRef]
- Itoh, Y.; Sasaki, J.I.; Hashimoto, M.; Katata, C.; Hayashi, M.; Imazato, S. Pulp Regeneration by 3-Dimensional Dental Pulp Stem Cell Constructs. J. Dent. Res. 2018, 97, 1137–1143. [Google Scholar] [CrossRef]
- Ding, J.; Sun, Z.; Ma, L.; Wang, L.; Liao, Z.; Liang, L.; Yang, H.; Mao, R. Microspheres of Stem Cells from Human Exfoliated Deciduous Teeth Exhibit Superior Pulp Regeneration Capacity. Dent. Mater. 2025, 41, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Wang, S.; Yuan, Z.; Wan, Z.; Zhang, L.; Song, R.; Ge, L.; Zhao, Y. Boosting the Angiogenesis Potential of Self-Assembled Mesenchymal Stem Cell Spheroids by Size Mediated Physiological Hypoxia for Vascularized Pulp Regeneration. Acta Biomater. 2025, 198, 102–114. [Google Scholar] [CrossRef] [PubMed]
- Dawood, R.M.; Mahdee, A.F. Fabrication and Characterization of 3D-Printed Polymeric-Based Scaffold Coated with Bioceramic and Naringin for a Potential Use in Dental Pulp Regeneration (in Vitro Study). Int. Endod. J. 2025, 58, 627–642. [Google Scholar] [CrossRef]
- Katata, C.; Sasaki, J.I.; Li, A.; Abe, G.L.; Nör, J.E.; Hayashi, M.; Imazato, S. Fabrication of Vascularized DPSC Constructs for Efficient Pulp Regeneration. J. Dent. Res. 2021, 100, 1351–1358. [Google Scholar] [CrossRef] [PubMed]
- Miao, G.; Liang, L.; Li, W.; Ma, C.; Pan, Y.; Zhao, H.; Zhang, Q.; Xiao, Y.; Yang, X. 3D Bioprinting of a Bioactive Composite Scaffold for Cell Delivery in Periodontal Tissue Regeneration. Biomolecules 2023, 13, 1062. [Google Scholar] [CrossRef]
- Pedano, M.S.; Li, X.; Jeanneau, C.; Ghosh, M.; Yoshihara, K.; Van Landuyt, K.; About, I.; Van Meerbeek, B. Survival of Human Dental Pulp Cells after 4-Week Culture in Human Tooth Model. J. Dent. 2019, 86, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Eap, S.; Ferrand, A.; Schiavi, J.; Keller, L.; Kokten, T.; Fioretti, F.; Mainard, D.; Ladam, G.; Benkirane-Jessel, N. Collagen Implants Equipped with ’Fish Scale’-like Nanoreservoirs of Growth Factors for Bone Regeneration. Nanomedicine 2014, 9, 1253–1261. [Google Scholar] [CrossRef] [PubMed]
- Schwinté, P.; Keller, L.; Lemoine, S.; Gottenberg, J.-E.; Benkirane-Jessel, N.; Vanleene, M. Nano-Engineered Scaffold for Osteoarticular Regenerative Medicine. J. Nanomed. Nanotechnol. 2015, 6, 1–9. [Google Scholar] [CrossRef]
- Yamada, S.; Malkmus, C.; Aasebø, E.; Mustafa, K.; Egusa, H.; Volponi, A.A. Production and Biobanking of Dental Stem Cells for Clinical Applications in Regenerative Dentistry: Current Practices and Future Perspectives—A Narrative Review. J. Dent. 2025, 161, 105934. [Google Scholar] [CrossRef]
- Li, L.; Xu, J.; Ye, C.; Zhou, Y.; Yan, F.; Chen, Z.; Xiao, Y. Biomaterials-Based Strategy for Dental-Oral Tissue Regeneration: Current Clinical Application, Laboratory Development, and Future Direction. Biomaterials 2026, 326, 123714. [Google Scholar] [CrossRef]
- Zhao, X.; Li, N.; Zhang, Z.; Hong, J.; Zhang, X.; Hao, Y.; Wang, J.; Xie, Q.; Zhang, Y.; Li, H.; et al. Beyond Hype: Unveiling the Real Challenges in Clinical Translation of 3D Printed Bone Scaffolds and the Fresh Prospects of Bioprinted Organoids. J. Nanobiotechnol. 2024, 22, 500. [Google Scholar] [CrossRef]
- Salmikangas, P.; Schuessler-Lenz, M.; Ruiz, S.; Celis, P.; Reischl, I.; Menezes-Ferreira, M.; Flory, E.; Renner, M.; Ferry, N. Marketing Regulatory Oversight of Advanced Therapy Medicinal Products (ATMPs) in Europe: The EMA/CAT Perspective. In Regulatory Aspects of Gene Therapy and Cell Therapy Products: A Global Perspective; Galli, M.C., Serabian, M., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 103–130. ISBN 978-3-319-18618-4. [Google Scholar]
- Kakroodi, F.A.; Khodadoust, E.; Alizadeh, M.; Hayaei Tehrani, R.S.; Sarabi, P.A.; Rahmanian, M.; Vosough, M. Current Challenges and Future Directions of ATMPs in Regenerative Medicine. Regen. Ther. 2025, 30, 358–370. [Google Scholar] [CrossRef]
- Car, E.; Barbier, L.; Huys, I.; Simoens, S.; Vulto, A.G. Evolving Global Regulatory Landscape for Approval of Biosimilars: Current Challenges and Opportunities for Convergence. Expert. Opin. Biol. Ther. 2025, 25, 649–668. [Google Scholar] [CrossRef]
- Mirshafiei, M.; Rashedi, H.; Yazdian, F.; Rahdar, A.; Baino, F. Advancements in Tissue and Organ 3D Bioprinting: Current Techniques, Applications, and Future Perspectives. Mater. Des. 2024, 240, 112853. [Google Scholar] [CrossRef]
- Kim, I.H.; Jeon, M.; Cheon, K.; Kim, S.H.; Jung, H.S.; Shin, Y.; Kang, C.M.; Kim, S.O.; Choi, H.J.; Lee, H.S.; et al. In Vivo Evaluation of Decellularized Human Tooth Scaffold for Dental Tissue Regeneration. Appl. Sci. 2021, 11, 8472. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Diomede, F.; Fonticoli, L.; Marconi, G.D.; Della Rocca, Y.; Rajan, T.S.; Trubiani, O.; Murmura, G.; Pizzicannella, J. Decellularized Dental Pulp, Extracellular Vesicles, and 5-Azacytidine: A New Tool for Endodontic Regeneration. Biomedicines 2022, 10, 403. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jin, G.-Z.; Kim, H.-W. Co-Culture of Human Dental Pulp Stem Cells and Endothelial Cells Using Porous Biopolymer Microcarriers: A Feasibility Study for Bone Tissue Engineering. Tissue Eng. Regen. Med. 2017, 14, 393–401. [Google Scholar] [CrossRef] [PubMed]

| Feature | Advantages | Disadvantages |
|---|---|---|
| Injectability & Handling | Minimally invasive delivery Adapts to complex root canal geometry | Gelation and placement in confined spaces can be challenging |
| Cell support & Biocompatibility | Supports cell adhesion Proliferation and differentiation Biodegradable | Natural hydrogels may show batch variability Synthetic hydrogels require functionalization |
| Mechanical properties | Tunable stiffness Can be reinforced or crosslinked | Low intrinsic strength Rapid degradation limits long-term structural integrity |
| Bioactivity & Functionalization | Can incorporate growth factors (TGF-β, VEGF, BMPs) to enhance regeneration | Requires careful design to maintain stability and bioactivity |
| Adaptability & Versatility | Suitable for preclinical models and regenerative applications | Large-volume constructs may be difficult to maintain without reinforcement |
| 3D CULTURE OF DENTAL PULP: TO ANALYZE | ||
|---|---|---|
| Reference | Description | Interest |
| Physiologic and Pathologic Pulp | ||
| [37] | Dentin–pulp-like organoids with stem/odontoblast features hDPSCs embedded in Matrigel | Mechanistic studies and testing pulp-capping biomaterials (Biodentine) |
| [6] | Tissue engineering-based Dentin/Pulp tissue analogue | Advanced biocompatibility evaluation tool of dental restorative materials |
| [38] | Pulp organoid with vessel-like structures, odontogenic & endothelial marker expression hDPCs + endothelial cells ± hDP-ECM | Biocompatibility/toxicity screening and vascularization modeling in pulp regeneration |
| [51] | Microfluidic chip mimicking dentin tubules Odontoblasts extend processes into channels | Odontoblast physiology, dentin–pulp interactions, and material screening |
| [52] | Microfluidic dentin-on-chip | High-throughput testing of dental materials and pulp–dentin interactions |
| [53] | Human dental pulp cells (hDPCs) spheroids showed ECM gene upregulation and collagen-rich matrix formation | ECM organization and deposition in pulp |
| [54] | DPCs in 3D culture model | Combined catalytic strategies applied to in-office tooth bleaching |
| [55] | Chitosan-based scaffold (animal vs fungal sources) co-polymerized with gelatin and crosslinked with GPTMS or genipin | Assessment of chitosan sources and biocompatibility with human DPSCs |
| [56] | Scaffold-free, collagen self-assembly with (SCAPs) and macrophages Forms cap-shaped apical papilla-like organoid | Periapical biology, disease environment, and therapeutic testing |
| [57] | Tooth root organoid Co-culture DPSCs + (PDLSCs) | Tooth root and pulp regeneration |
| [22] | 3D dental pulp cell microtissues and S. mutans | Model of pathologic pulp |
| [58] | Cytotoxicity of filling materials on 3D pulp model | Model of pulp floor perforation |
| Stem Cell Differentiation | ||
| [59] | DPSCs in Matrigel and other hydrogels Formation of mineralized nodules osteo/odontogenic markers expressed | DPSC differentiation and mineralization in 3D matrices |
| [60] | DPSCs in microsphere-forming plates Multilineage differentiation capacity enhanced versus 2D culture | Accessible 3D platform to evaluate DPSC regenerative potential |
| [40] | Influence of the microenvironnement (ECM, growth factors) on odontogenic differenciation of MSC | Effet of ECM on differenciation MSC and formation of dentin-pulp |
| [61] | Organoïdes of dental germ made by microparticules and hydrogel, guiding cellular agregation | Dental developpement |
| [62] | DPSC-MSC spheroids: higher ALP, DSPP, osteocalcin expression versus 2D culture Rapid mineralized nodule formation | Differentiation potential of DPSC-MSC |
| [63] | Organoids Dental pulp stem cells (DPSCs) | Potential regenerative of DPSCs |
| [64] | 3D spheroid culture of dental pulp-derived stromal cells | Regenerative properties for therapeutic applications |
| [65] | Photobiomodulation therapy for DPSCs differenciation | Multilineage differentiation of DPSCs |
| Dentin Formation | ||
| [66] | Mouse dental papilla cell spheroids; expression of ALP, DSPP, DMP-1 Analyse of mineralized nodules | Model odontoblast differentiation and dentinogenesis in 3D spheroids. |
| [67] | DPSCs + HUVECs co-culture in matrigel; enhanced odontogenic differentiation and vascular-like structures | Show synergistic effects of DPSCs + ECs in angiogenesis and odontogenesis. |
| [68] | 3D explant culture of human dental pulp tissue in matrigel | Physiology of Pre-odontoblast |
| [69] | DPSC spheroids loaded with ZIF-8 nanoplatform releasing DNA methyltransferases inhibitors | Enhance odontogenesis through sustained epigenetic modulation |
| [70] | 3D Spheroid Formation Using BMP-Loaded Microparticle Human–Differenciation DPSCs | Odontoblastic Differentiation |
| [71] | Combination of 3D Printing and ALD with DPSC | Dentin Fabrication |
| Angiogenesis and Vascularisation | ||
| [34] | DPSC -HUVEC co-culture spheroids in Matrigel with growth factor supplementation | Study prevascularization and vascular network formation for pulp regeneration |
| [36] | Spheroid organoids of hDPSCs + ECs under hypoxia; characterized by scRNA-seq | Model angiogenesis and odontoblastic differentiation pathways |
| [72] | SCAPs + ECs under hypoxia Formed stable vascular-like networks EphrinB2 signaling involved | Model angiogenesis & vascular stabilization for pulp revascularization strategies. |
| [52] | Neovascularization by DPSC-ECs in a Tube Model | Model of Neovascularization |
| 3D CULTURE OF DENTAL PULP: TO REGENERATE | ||
|---|---|---|
| Reference | Description | Interest |
| Implantable Adequate Scaffold | ||
| [73] | Puramatrix™ self-assembling peptide hydrogel, DPSCs Viability and differentiation maintained | A synthetic peptide hydrogel as a 3D injectable scaffold for pulp regeneration |
| [15] | 0.2% collagen type I + 0.5% agarose; inkjet bioprinted with human DPSCs and HUVECs | Formation of vascularized pulp-like networks ex vivo |
| [74] | Beta-TCP/PLGA (75:25) composite scaffold | Supporting osteo/odontogenic differentiation of DPSCs |
| [75] | Calcium silicate + PCL scaffold | Inducing odontogenic differentiation of DPSCs |
| [76] | GelMA conjugated with BMP-peptide, 3D bioprinted with DPSCs | Enhancing odontogenic differentiation and mineralization |
| [77] | GelMA with mineral trioxide aggregate (ProRoot MTA, Endosem Zr) | Promoting odontogenic differentiation of DPSCs |
| [78] | Injectable Double-Network Hydrogel | 3D injectable scaffold for pulp regeneration |
| [79] | ECM from bone combined with β-TCP scaffold | Promoting osteo/odontogenic differentiation of DPSCs |
| [80] | Treated dentin matrix (TDM) + 30% PCL composite | Supporting odontogenic differentiation of dental follicle stem cells |
| [33,81] | Demineralized dentin matrix (DDM) mixed 1:1 with alginate and soluble dentin proteins | Inducing SCAPs differentiation into dentin-like structures |
| [82] | PLA/HA scaffold seeded with DPSCs | Promoting dentin-pulp-like tissue mineralization |
| [83] | Decellularized dental pulp extracellular matrix reseeded with SCAPs | Natural ECM scaffold for pulp tissue regeneration |
| [84] | Injectable decellularized dental pulp matrix-functionalized hydrogel microspheres | Scaffold for pulp regeneration |
| Implantable Dental Pulp Tissue | ||
| [85] | Scaffold-free DPSC sheet/aggregate constructs, implanted into tooth root canals Pulp-like tissue with vasculature formed in vivo | Regenerate vascularized dental pulp tissue in vivo |
| [38] | Prevascularized pulp organoids formed from hDPSCs and HUVECs in Matrigel | Develop a physiologically relevant vascularized pulp organoid for regeneration |
| [86] | Microspheres of stem cells from human exfoliated deciduous teeth | Pulp regeneration capacity |
| [87] | Angiogenesis potential of self-assembled mesenchymal stem cell spheroids by size mediated physiological hypoxia | For vascularized pulp regeneration |
| [54] | Repopulation of a 3D simulated periapical lesion cavity with dental pulp stem cell spheroids with triggered osteoblastic differentiation | Regeneration of periapical lesion |
| [88] | Fabrication and characterization of 3D-printed polymeric-based scaffold coated with bioceramic, naringin and nHA with hDPSCs | Potential use in dental pulp regeneration |
| [89] | Vascularized DPSC constructs by inducing endothelial differentiation or co-culture strategies; constructs show perfusable/vessel-like structures in vitro | Provide prevascularized constructs to improve graft survival and accelerate in vivo angiogenesis after transplantation for pulp/bone applications |
| [90] | GelMA–alginate–bioactive glass microsphere bioink + stem cells for 3D bioprinting of pulp constructs | Scalable 3D bioprinting of pulp/periodontal regenerative constructs |
| Feature | Cell-Free Biologics | Implantable Medical Devices | ATMPs | Combined ATMPs |
|---|---|---|---|---|
| Composition | Extracellular vesicles Secretome | Scaffold materials 3D-printed constructs | Living cells (stem/progenitor) | ATMPs integrated with implantable medical devices |
| Regulatory complexity | Moderate Less stringent than ATMPs | High Must meet high standards (biocompatibility, sterilization, etc.) | High Full ATMP regulatory requirements | Very high Must meet both ATMP and medical device regulations |
| Safety considerations | Low tumorigenicity risk Lower immunogenicity | Device-related safety (mechanical stability, biocompatibility, degradation) | Potential tumorigenicity Immune rejection risk | Combination of both |
| Manufacturing | Scalable Standardized | Scalable fabrication Sterilization Quality control of scaffold/device | Complex Batch-to-batch variability | Complex Reproducibility of both biological and device components |
| Storage Logistics | Easier Stable formulations | Generally stable Storage depends on material type and sterilization requirements | Cryopreservation required Limited shelf-life | Depends on both ATMP and device May require specialized storage |
| Functional integration | Paracrine effects Immunomodulation | Structural support Guiding tissue regeneration Facilitating delivery for biologics/ cells | Cellular and paracrine activity for enhanced tissue repair Differentiation | Structural support Cellular and paracrine activity for enhanced tissue repair |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smaida, R.; Hua, G.; Benkirane-Jessel, N.; Fioretti, F. Three-Dimensional Models of the Dental Pulp: Bridging Fundamental Biology and Regenerative Therapy. Int. J. Mol. Sci. 2025, 26, 10960. https://doi.org/10.3390/ijms262210960
Smaida R, Hua G, Benkirane-Jessel N, Fioretti F. Three-Dimensional Models of the Dental Pulp: Bridging Fundamental Biology and Regenerative Therapy. International Journal of Molecular Sciences. 2025; 26(22):10960. https://doi.org/10.3390/ijms262210960
Chicago/Turabian StyleSmaida, Rana, Guoqiang Hua, Nadia Benkirane-Jessel, and Florence Fioretti. 2025. "Three-Dimensional Models of the Dental Pulp: Bridging Fundamental Biology and Regenerative Therapy" International Journal of Molecular Sciences 26, no. 22: 10960. https://doi.org/10.3390/ijms262210960
APA StyleSmaida, R., Hua, G., Benkirane-Jessel, N., & Fioretti, F. (2025). Three-Dimensional Models of the Dental Pulp: Bridging Fundamental Biology and Regenerative Therapy. International Journal of Molecular Sciences, 26(22), 10960. https://doi.org/10.3390/ijms262210960






