The Endocannabinoid–Microbiota–Neuroimmune Super-System: A Unifying Feedback Architecture for Systems Resilience, Collapse Trajectories, and Precision Feedback Medicine
Abstract
1. Introduction: The Missing Neuro-Super-System
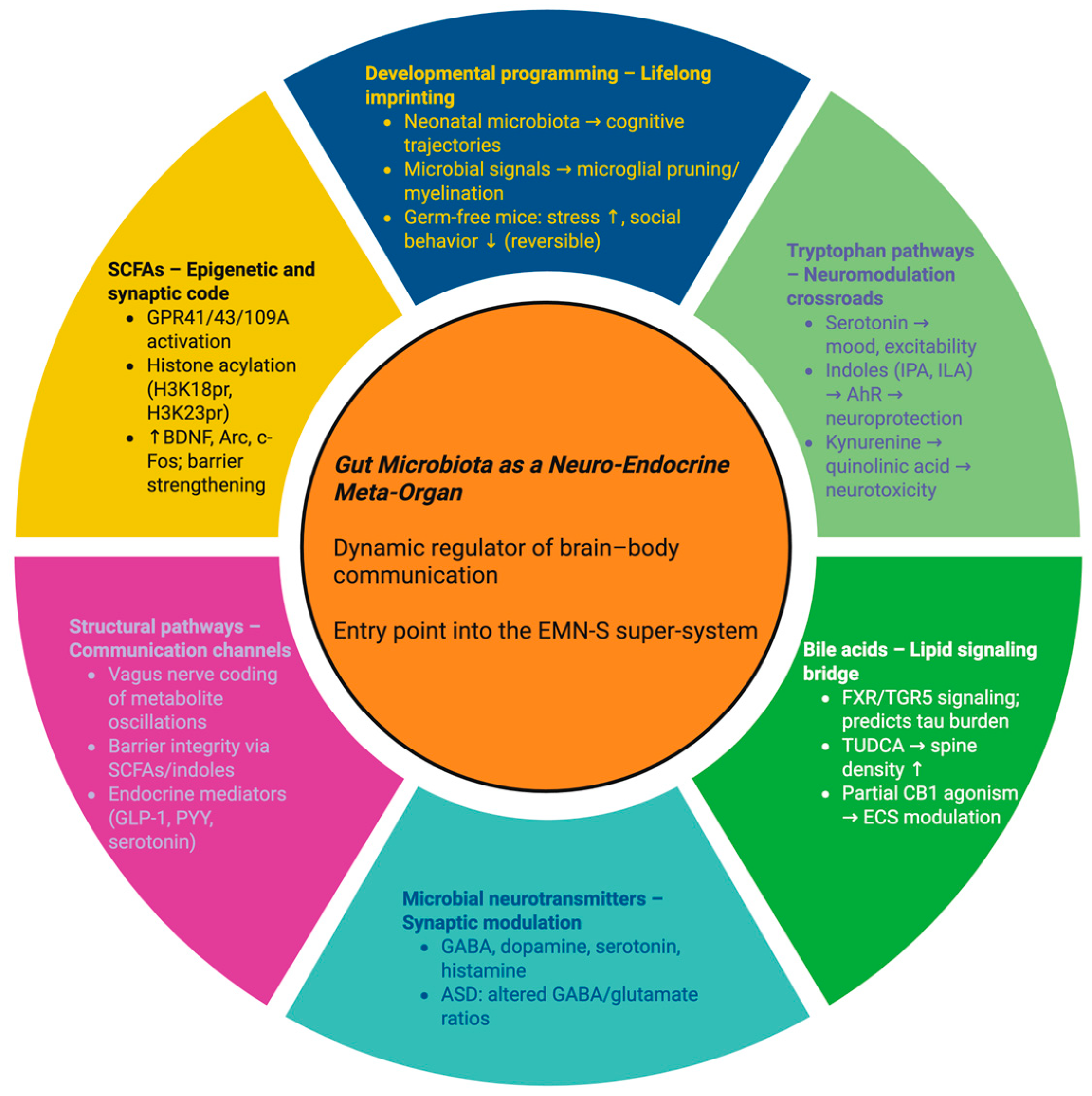
2. The Gut Microbiota as a Neuro-Endocrine Meta-Organ
2.1. Short-Chain Fatty Acids: Microbial Fermentation as an Epigenetic Code
2.2. Tryptophan Metabolism: Divergent Pathways into Mood, Immunity, and Excitotoxicity
2.3. Bile Acids: Lipid Messengers Shaped by Microbes
2.4. Microbial Neurotransmitters: The Hidden Neurochemical Reservoir
2.5. Structural Pathways: Vagus, Barriers, and Hormonal Signaling
2.6. Developmental Programming: Lifelong Imprints on the Brain
2.7. Disease-Specific Dysbiosis Patterns
2.8. Causality and Translational Levers
2.9. Integration into the EMN-S
3. The Endocannabinoidome: A Homeostatic Omninet
3.1. Canonical Receptors as Adaptive Anchors
3.2. Lipid Mediators: The Adaptive Vocabulary
3.3. Enzymatic Rheostats and Dynamic Tone
3.4. Systemic Integration Across Domains
3.5. Evolutionary and Systems Perspective
3.6. The eCBome as a Homeostatic Omninet in the EMN-S
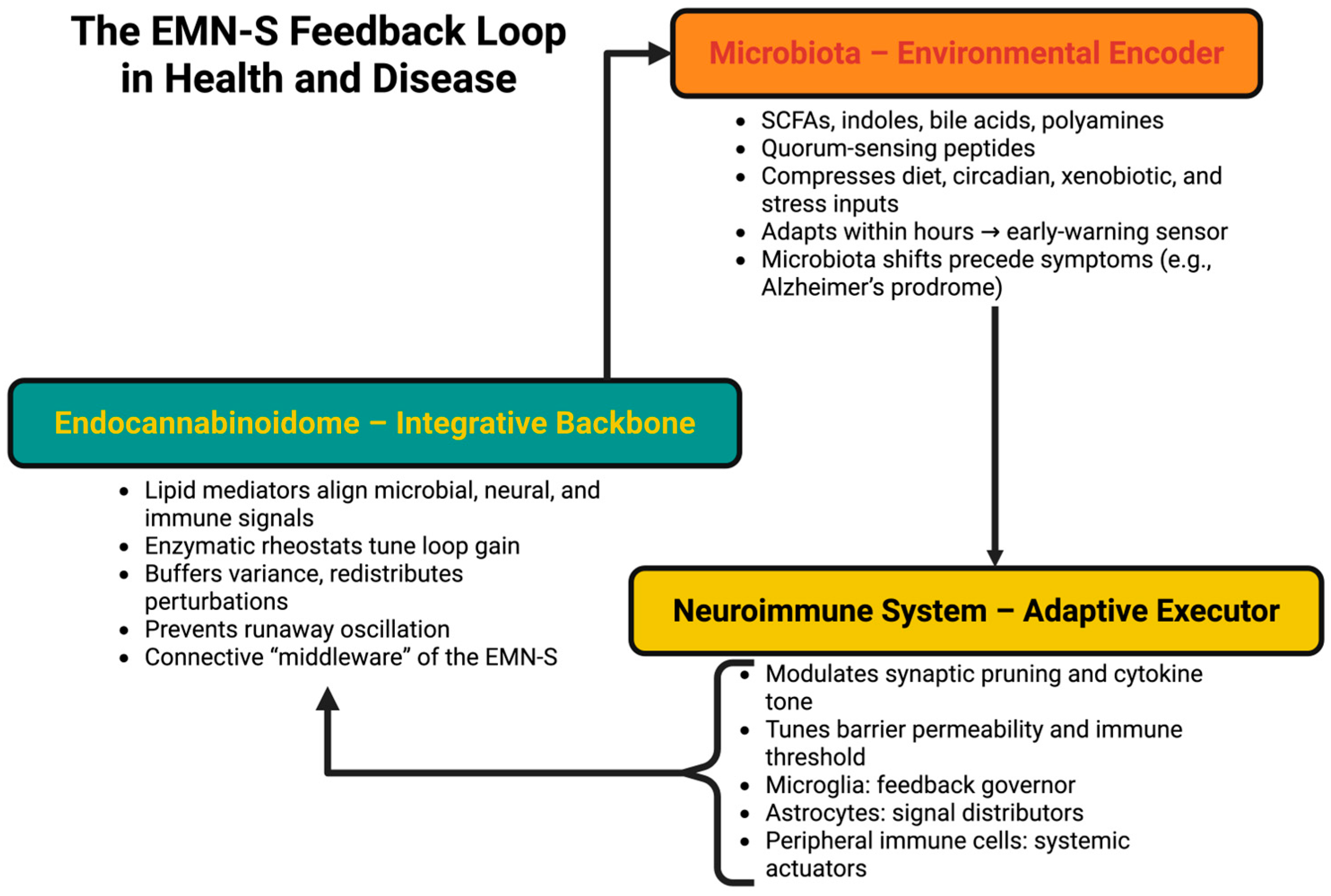
4. The EMN-S Loop: Tripartite Feedback Control
4.1. Microbiota as the Environmental Encoder
4.2. The eCBome as the Integrative Backbone
4.3. The Neuroimmune System as Adaptive Executor
4.4. Feedback Architecture and Temporal Layering
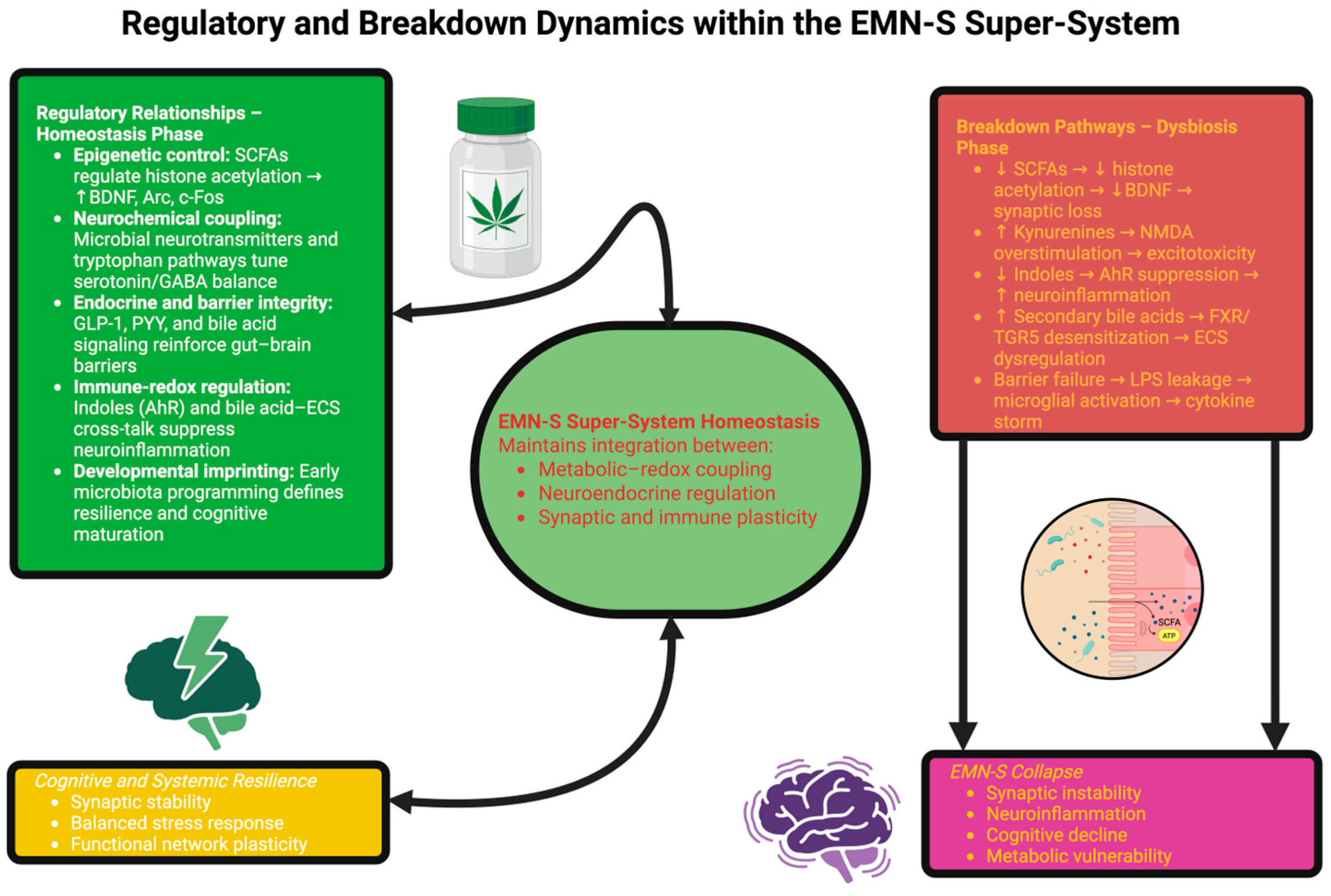
4.5. Collapse States: Dynamics of System Failure
- Slow collapse (for instance, Alzheimer’s disease), where decades of insidious feedback drift lessen resilience until clinical manifestation occurs [93].
- Rapid collapse (for instance, encephalopathy and sepsis), where overwhelming catastrophic perturbation leads to disintegration of loops in days [94].
- Oscillatory collapse (for instance, relapsing–remitting multiple sclerosis), where the system oscillates between partial (incomplete) recovery and cycled disintegration [95].
4.6. Early Alzheimer’s Onset as a Systemic Prodrome
- -
- Quantitative Criterial Tiers and Validations of Collapse Dynamics
- -
- Executor dimension—tau-PET trajectory
- -
- Integrator dimension—circadian rhythm variance of AEA
- -
- Prototypes of validation
4.7. EMN-S Signatures: Predictive Biomarkers of Collapse
4.8. Precision Feedback Medicine
4.9. Evolutionary Origins and Cross-Species Continuity
4.10. Conceptual Implications and Paradigm Shift
5. Disease Translation: EMN-S Collapse Trajectories Across Disorders
5.1. Neurodegeneration as Slow Collapse
5.1.1. Alzheimer’s Disease: Systemic Prodrome and Early-Onset Depth
5.1.2. Parkinson’s Disease: Gut-First Prodrome
5.1.3. Multiple Sclerosis: Oscillatory Collapse
5.2. Psychiatric Syndromes as Integrator-Centered Collapse
5.2.1. Major Depression
5.2.2. Anxiety
5.2.3. Autism Spectrum Disorder
5.3. Systemic Disorders as Whole-Body Collapse
5.3.1. Obesity and Metabolic Syndrome
5.3.2. Inflammatory Bowel Disease
5.3.3. Sepsis-Associated Encephalopathy
5.4. Collapse Taxonomy Across Disorders
- Prolonged collapse: AD, PD (decades of drift, prodromal signatures; hysteresis) [158].
- Oscillatory collapse: MS (alternating gain and partial reconsolidation) [159].
- Fast collapse: Sepsis-associated encephalopathy (catastrophic breakdown within days) [160].
- Developmental setting: ASD (thresholds established early—stabilizing atypical attractors) [161].
- Integrator drift: Depression, anxiety (chronic low-grade amplification due to buffering loss) [162].
5.5. Translational Implications: From Nodes to Feedback
5.6. Evolutionary and Public-Health Framing
6. Therapeutic Horizons: Engineering EMN-S Resilience
6.1. Recalibrating the Encoder: Microbiota as the Therapeutic Sensor
6.2. Retuning the Integrator: The Endocannabinoidome as Systemic Gain Control
6.3. Reprogramming the Executor: Neuroimmune Circuits as Output Shapers
6.4. Restoring Loop Coherence Through Dual- and Triple-Module Therapies
6.5. Therapeutic Windows Across Collapse States
6.6. Dynamic Endpoints for Precision Feedback Trials
6.7. AI-Augmented Feedback Engineering and Digital Twins
6.8. Global Health and Evolutionary Reframing
6.9. Future Horizons in Feedback Medicine
6.10. Conceptual Implications
7. Predictive Signatures and Biomarker Integration
7.1. Encoder Biomarkers: Reading the Microbial Language of Stability
7.2. Integrator Biomarkers: Lipid Rhythms as Gain Indicators
7.3. Executor Biomarkers: Immune and Neural Echoes
7.4. Composite EMN-S Signatures: Multidomain Fingerprints
7.5. Early-Warning Metrics: From Ecology to Medicine
7.6. AI Integration and Digital Twins
7.7. Ethical and Translational Considerations
7.8. Conceptual Reflections: Toward Resiliomics
8. Integrative Systems Models and Control-Theory Frameworks for the EMN-S
8.1. Attractor Landscapes and Personalized Stability Basins
8.2. Lyapunov Stability and Collapse Velocity
8.3. Noise-Induced Transitions and Stochastic Vulnerability
8.4. Synchrony, Coupled Oscillators, and Phase Drift
8.5. Controllability and Intervention Thresholds
8.6. Entropy, Fractals, and Network Topology
8.7. Hybrid Models and Data Assimilation
8.8. Cross-Scale Analogies
8.9. Limitations and Open Questions
8.10. Conceptual Reflections
9. Translational Roadmap: From Concept to Clinic
9.1. Step I: Pilot Perturbation Studies
9.2. Step II: Biomarker Standardization and Infrastructure
9.3. Step III: Clinical Trials and Dynamic Endpoints
9.4. Phase IV: Implementation and Global Integration
9.5. Policy, Precision Medicine, and Preventive Healthcare
9.6. Economic and Societal Impact
9.7. Regulatory and Clinical Adoption Barriers
9.8. Digital Health Ecosystem
9.9. Global Equity and the Global South
9.10. Conceptual Reflections: A Medicine of Resilience
10. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Ricci, G.; Magosso, E.; Ursino, M. The Relationship between Oscillations in Brain Regions and Functional Connectivity: A Critical Analysis with the Aid of Neural Mass Models. Brain Sci. 2021, 11, 487. [Google Scholar] [CrossRef]
- Charitos, I.A.; Inchingolo, A.M.; Ferrante, L.; Inchingolo, F.; Inchingolo, A.D.; Castellaneta, F.; Cotoia, A.; Palermo, A.; Scacco, S.; Dipalma, G. The Gut Microbiota’s Role in Neurological, Psychiatric, and Neurodevelopmental Disorders. Nutrients 2024, 16, 4404. [Google Scholar] [CrossRef]
- Krishnamurthy, H.K.; Pereira, M.; Bosco, J.; George, J.; Jayaraman, V.; Krishna, K.; Wang, T.; Bei, K.; Rajasekaran, J.J. Gut commensals and their metabolites in health and disease. Front. Microbiol. 2023, 14, 1244293. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhu, N.; Su, X.; Gao, Y.; Yang, R. Gut-Microbiota-Derived Metabolites Maintain Gut and Systemic Immune Homeostasis. Cells 2023, 12, 793. [Google Scholar] [CrossRef] [PubMed]
- Ramadan, Y.N.; Alqifari, S.F.; Alshehri, K.; Alhowiti, A.; Mirghani, H.; Alrasheed, T.; Aljohani, F.; Alghamdi, A.; Hetta, H.F. Microbiome Gut-Brain-Axis: Impact on Brain Development and Mental Health. Mol. Neurobiol. 2025, 62, 10813–10833. [Google Scholar] [CrossRef]
- García-Domínguez, M. Role of the Endocannabinoid System in Fibromyalgia. Curr. Issues Mol. Biol. 2025, 47, 230. [Google Scholar] [CrossRef] [PubMed]
- Campanale, A.; Siniscalco, D.; Di Marzo, V. The endocannabinoidome–gut microbiome–brain axis as a novel therapeutic target for autism spectrum disorder. J. Biomed. Sci. 2025, 32, 60. [Google Scholar] [CrossRef]
- Rodrigues, R.J.; Marques, J.M.; Köfalvi, A. Cannabis, Endocannabinoids and Brain Development: From Embryogenesis to Adolescence. Cells 2024, 13, 1875. [Google Scholar] [CrossRef]
- Jagodic, A.; Krsek, A.; Schleicher, L.M.S.; Baticic, L. Microbiome Dysbiosis as a Driver of Neurodegeneration: Insights into Alzheimer’s and Parkinson’s Diseases. Gastrointest. Disord. 2025, 7, 28. [Google Scholar] [CrossRef]
- Al-Khazaleh, A.K.; Jaye, K.; Chang, D.; Münch, G.W.; Bhuyan, D.J. Buds and Bugs: A Fascinating Tale of Gut Microbiota and Cannabis in the Fight against Cancer. Int. J. Mol. Sci. 2024, 25, 872. [Google Scholar] [CrossRef]
- Camarda, L.; Mattioli, L.B.; Corazza, I.; Marzetti, C.; Budriesi, R. Targeting the Gut–Brain Axis with Plant-Derived Essential Oils: Phytocannabinoids and Beyond. Nutrients 2025, 17, 1578. [Google Scholar] [CrossRef] [PubMed]
- Urbani, G.; Rondini, E.; Distrutti, E.; Marchianò, S.; Biagioli, M.; Fiorucci, S. Phenotyping the Chemical Communications of the Intestinal Microbiota and the Host: Secondary Bile Acids as Postbiotics. Cells 2025, 14, 595. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; He, C.; An, Y.; Huang, Y.; Zhang, H.; Fu, W.; Wang, M.; Shan, Z.; Xie, J.; Yang, Y.; et al. The Role of Short Chain Fatty Acids in Inflammation and Body Health. Int. J. Mol. Sci. 2024, 25, 7379. [Google Scholar] [CrossRef] [PubMed]
- Werner, C.; Sauer, M.; Geis, C. Super-resolving Microscopy in Neuroscience. Chem. Rev. 2021, 121, 11971–12015. [Google Scholar] [CrossRef]
- Voicu, V.; Toader, C.; Șerban, M.; Covache-Busuioc, R.-A.; Ciurea, A.V. Systemic Neurodegeneration and Brain Aging: Multi-Omics Disintegration, Proteostatic Collapse, and Network Failure Across the CNS. Biomedicines 2025, 13, 2025. [Google Scholar] [CrossRef]
- Guo, X.; Liu, X.; Lin, J.; Huang, Z.; Lin, S.; Zhang, M.; Xu, Z.; Lin, X. Global, regional, and national burden of four major neurological diseases in women from 1990 to 2021. Front. Public Health 2025, 13, 1561216. [Google Scholar] [CrossRef]
- Aloisio Caruso, E.; De Nunzio, V.; Tutino, V.; Notarnicola, M. The Endocannabinoid System: Implications in Gastrointestinal Physiology and Pathology. Int. J. Mol. Sci. 2025, 26, 1306. [Google Scholar] [CrossRef]
- Yang, K.; Li, G.; Li, Q.; Wang, W.; Zhao, X.; Shao, N.; Qiu, H.; Liu, J.; Xu, L.; Zhao, J. Distribution of gut microbiota across intestinal segments and their impact on human physiological and pathological processes. Cell Biosci. 2025, 15, 47. [Google Scholar] [CrossRef]
- Li, J.; Zhou, S.; Fang, J.; Cai, Q.; Yang, Y.; Sun, Z.; Li, L.; Li, W. Integration of Transcriptomics and Metabolomics Provides Insight into the Growth-Promoting Functions of Solanum khasianum Endophyte in Medicago sativa. Agronomy 2025, 15, 251. [Google Scholar] [CrossRef]
- Kalkan, A.E.; BinMowyna, M.N.; Raposo, A.; Ahmad, M.F.; Ahmed, F.; Otayf, A.Y.; Carrascosa, C.; Saraiva, A.; Karav, S. Beyond the Gut: Unveiling Butyrate’s Global Health Impact Through Gut Health and Dysbiosis-Related Conditions: A Narrative Review. Nutrients 2025, 17, 1305. [Google Scholar] [CrossRef]
- Liebner, T.; Kilic, S.; Walter, J.; Aibara, H.; Narita, T.; Choudhary, C. Acetylation of histones and non-histone proteins is not a mere consequence of ongoing transcription. Nat. Commun. 2024, 15, 4962. [Google Scholar] [CrossRef]
- Alavian, F.; Safaeian, M. How the gut microbiome shapes learning and memory: A comprehensive review. IBRO Neurosci. Rep. 2025, 19, 491–506. [Google Scholar] [CrossRef]
- Barcutean, L.; Maier, S.; Burai-Patrascu, M.; Farczadi, L.; Balasa, R. The Immunomodulatory Potential of Short-Chain Fatty Acids in Multiple Sclerosis. Int. J. Mol. Sci. 2024, 25, 3198. [Google Scholar] [CrossRef]
- Roth, W.; Zadeh, K.; Vekariya, R.; Ge, Y.; Mohamadzadeh, M. Tryptophan Metabolism and Gut-Brain Homeostasis. Int. J. Mol. Sci. 2021, 22, 2973. [Google Scholar] [CrossRef] [PubMed]
- Li, S. Modulation of immunity by tryptophan microbial metabolites. Front. Nutr. 2023, 10, 1209613. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-H.; Han, C.; Shin, C. IUPHAR review: Microbiota-gut-brain axis and its role in neuropsychiatric disorders. Pharmacol. Res. 2025, 216, 107749. [Google Scholar] [CrossRef] [PubMed]
- Owe-Larsson, M.; Drobek, D.; Iwaniak, P.; Kloc, R.; Urbanska, E.M.; Chwil, M. Microbiota-Derived Tryptophan Metabolite Indole-3-Propionic Acid-Emerging Role in Neuroprotection. Molecules 2025, 30, 3628. [Google Scholar] [CrossRef]
- Chojnacki, C.; Konrad, P.; Błońska, A.; Medrek-Socha, M.; Przybylowska-Sygut, K.; Chojnacki, J.; Poplawski, T. Altered Tryptophan Metabolism on the Kynurenine Pathway in Depressive Patients with Small Intestinal Bacterial Overgrowth. Nutrients 2022, 14, 3217. [Google Scholar] [CrossRef]
- Lin, P.; Li, D.; Shi, Y.; Li, Q.; Guo, X.; Dong, K.; Chen, Q.; Lou, X.; Li, Z.; Li, P.; et al. Dysbiosis of the Gut Microbiota and Kynurenine (Kyn) Pathway Activity as Potential Biomarkers in Patients with Major Depressive Disorder. Nutrients 2023, 15, 1752. [Google Scholar] [CrossRef]
- Masse, K.E.; Lu, V.B. Short-chain fatty acids, secondary bile acids and indoles: Gut microbial metabolites with effects on enteroendocrine cell function and their potential as therapies for metabolic disease. Front. Endocrinol. 2023, 14, 1169624. [Google Scholar] [CrossRef]
- Singleton, E.H.; Mattsson-Carlgren, N.; Pichet Binette, A.; Stomrud, E.; Strandberg, O.; Palmqvist, S.; Ossenkoppele, R.; Hansson, O. Longitudinal tau aggregation, atrophy, and cognitive decline in Alzheimer’s disease. Alzheimers Dement. 2025, 21, e70435. [Google Scholar] [CrossRef] [PubMed]
- Zangerolamo, L.; Carvalho, M.; Barbosa, H.C.L. The Critical Role of the Bile Acid Receptor TGR5 in Energy Homeostasis: Insights into Physiology and Therapeutic Potential. Int. J. Mol. Sci. 2025, 26, 6547. [Google Scholar] [CrossRef] [PubMed]
- Forner-Piquer, I.; Giommi, C.; Sella, F.; Lombó, M.; Montik, N.; Dalla Valle, L.; Carnevali, O. Endocannabinoid System and Metabolism: The Influences of Sex. Int. J. Mol. Sci. 2024, 25, 11909. [Google Scholar] [CrossRef] [PubMed]
- Casertano, M.; Dekker, M.; Valentino, V.; De Filippis, F.; Fogliano, V.; Ercolini, D. Gaba-producing lactobacilli boost cognitive reactivity to negative mood without improving cognitive performance: A human Double-Blind Placebo-Controlled Cross-Over study. Brain. Behav. Immun. 2024, 122, 256–265. [Google Scholar] [CrossRef]
- Al Noman, A.; Alhudhaibi, A.M.; Afroza, M.; Tonni, S.D.; Shehab, H.M.; Jahan Iba, N.; Taha, T.H.; Abdallah, E.M. Neuroplasticity and the microbiome: How microorganisms influence brain change. Front. Microbiol. 2025, 16, 1629349. [Google Scholar] [CrossRef]
- O’Riordan, K.J.; Moloney, G.M.; Keane, L.; Clarke, G.; Cryan, J.F. The gut microbiota-immune-brain axis: Therapeutic implications. Cell Rep. Med. 2025, 6, 101982. [Google Scholar] [CrossRef]
- Siopi, E.; Galerne, M.; Rivagorda, M.; Saha, S.; Moigneu, C.; Moriceau, S.; Bigot, M.; Oury, F.; Lledo, P.-M. Gut microbiota changes require vagus nerve integrity to promote depressive-like behaviors in mice. Mol. Psychiatry 2023, 28, 3002–3012. [Google Scholar] [CrossRef]
- Kearns, R. Gut–Brain Axis and Neuroinflammation: The Role of Gut Permeability and the Kynurenine Pathway in Neurological Disorders. Cell. Mol. Neurobiol. 2024, 44, 64. [Google Scholar] [CrossRef]
- Hart, J.; Mansour, H.; Sawant, H.; Chicko, M.; Arthur, S.; Haynes, J.; Borthakur, A. Gut Microbial Metabolites of Tryptophan Augment Enteroendocrine Cell Differentiation in Human Colonic Organoids: Therapeutic Potential for Dysregulated GLP1 Secretion in Obesity. Int. J. Mol. Sci. 2025, 26, 7080. [Google Scholar] [CrossRef]
- Frerichs, N.M.; de Meij, T.G.J.; Niemarkt, H.J. Microbiome and its impact on fetal and neonatal brain development: Current opinion in pediatrics. Curr. Opin. Clin. Nutr. Metab. Care 2024, 27, 297. [Google Scholar] [CrossRef]
- Buonfiglioli, A.; Kübler, R.; Missall, R.; De Jong, R.; Chan, S.; Haage, V.; Wendt, S.; Lin, A.J.; Mattei, D.; Graziani, M.; et al. A microglia-containing cerebral organoid model to study early life immune challenges. Brain. Behav. Immun. 2025, 123, 1127–1146. [Google Scholar] [CrossRef] [PubMed]
- Beghetti, I.; Barone, M.; Brigidi, P.; Sansavini, A.; Corvaglia, L.; Aceti, A.; Turroni, S. Early-life gut microbiota and neurodevelopment in preterm infants: A narrative review. Front. Nutr. 2023, 10, 1241303. [Google Scholar] [CrossRef] [PubMed]
- Ajongbolo, A.O.; Langhans, S.A. Brain Organoids and Assembloids—From Disease Modeling to Drug Discovery. Cells 2025, 14, 842. [Google Scholar] [CrossRef] [PubMed]
- Paraschiv, A.-C.; Vacaras, V.; Nistor, C.; Vacaras, C.; Strilciuc, S.; Muresanu, D.F. The effect of multiple sclerosis therapy on gut microbiota dysbiosis: A longitudinal prospective study. Microb. Cell 2024, 11, 106–115. [Google Scholar] [CrossRef]
- Młynarska, E.; Barszcz, E.; Budny, E.; Gajewska, A.; Kopeć, K.; Wasiak, J.; Rysz, J.; Franczyk, B. The Gut–Brain–Microbiota Connection and Its Role in Autism Spectrum Disorders. Nutrients 2025, 17, 1135. [Google Scholar] [CrossRef]
- D’Amato, A.; Di Cesare Mannelli, L.; Lucarini, E.; Man, A.L.; Le Gall, G.; Branca, J.J.V.; Ghelardini, C.; Amedei, A.; Bertelli, E.; Regoli, M.; et al. Faecal microbiota transplant from aged donor mice affects spatial learning and memory via modulating hippocampal synaptic plasticity- and neurotransmission-related proteins in young recipients. Microbiome 2020, 8, 140. [Google Scholar] [CrossRef]
- Cerna, C.; Vidal-Herrera, N.; Silva-Olivares, F.; Álvarez, D.; González-Arancibia, C.; Hidalgo, M.; Aguirre, P.; González-Urra, J.; Astudillo-Guerrero, C.; Jara, M.; et al. Fecal Microbiota Transplantation from Young-Trained Donors Improves Cognitive Function in Old Mice Through Modulation of the Gut-Brain Axis. Aging Dis. 2025, 16, 3649. [Google Scholar] [CrossRef]
- Fernández-Rodríguez, D.; Bravo, M.C.; Pizarro, M.; Vergara-Barra, P.; Hormazábal, M.J.; Leonario-Rodriguez, M. Efficacy of Lactobacillus spp. Interventions to Modulate Mood Symptoms: A Scoping Review of Clinical Trials. Int. J. Mol. Sci. 2025, 26, 8099. [Google Scholar] [CrossRef]
- Cao, Q.; Shen, M.; Li, R.; Liu, Y.; Zeng, Z.; Zhou, J.; Niu, D.; Zhang, Q.; Wang, R.; Yao, J.; et al. Elucidating the specific mechanisms of the gut-brain axis: The short-chain fatty acids-microglia pathway. J. Neuroinflamm. 2025, 22, 133. [Google Scholar] [CrossRef]
- Herrnreiter, C.J.; Murray, M.G.; Luck, M.; Ganesa, C.; Kuprys, P.V.; Li, X.; Choudhry, M.A. Bacterial dysbiosis and decrease in SCFA correlate with intestinal inflammation following alcohol intoxication and burn injury. eGastroenterology 2025, 3, e100145. [Google Scholar] [CrossRef]
- Paul, A.D.; Natarajan, H. From gut to brain: Exploring the impact of microbiota, dysbiosis, and neuroinflammation in neurodegenerative disorders. Future J. Pharm. Sci. 2025, 11, 105. [Google Scholar] [CrossRef]
- Stasiulewicz, A.; Znajdek, K.; Grudzień, M.; Pawiński, T.; Sulkowska, J.I. A Guide to Targeting the Endocannabinoid System in Drug Design. Int. J. Mol. Sci. 2020, 21, 2778. [Google Scholar] [CrossRef] [PubMed]
- Nampoothiri, S.; Nogueiras, R.; Schwaninger, M.; Prevot, V. Glial cells as integrators of peripheral and central signals in the regulation of energy homeostasis. Nat. Metab. 2022, 4, 813–825. [Google Scholar] [CrossRef] [PubMed]
- Rezende, B.; Alencar, A.K.N.; de Bem, G.F.; Fontes-Dantas, F.L.; Montes, G.C. Endocannabinoid System: Chemical Characteristics and Biological Activity. Pharmaceuticals 2023, 16, 148. [Google Scholar] [CrossRef] [PubMed]
- Dudok, B.; Soltesz, I. Imaging the endocannabinoid signaling system. J. Neurosci. Methods 2022, 367, 109451. [Google Scholar] [CrossRef]
- Ruiz de Martín Esteban, S.; Benito-Cuesta, I.; Terradillos, I.; Martínez-Relimpio, A.M.; Arnanz, M.A.; Ruiz-Pérez, G.; Korn, C.; Raposo, C.; Sarott, R.C.; Westphal, M.V.; et al. Cannabinoid CB2 Receptors Modulate Microglia Function and Amyloid Dynamics in a Mouse Model of Alzheimer’s Disease. Front. Pharmacol. 2022, 13, 841766. [Google Scholar] [CrossRef]
- Salum, K.C.R.; Miranda, G.B.A.; Dias, A.L.; Carneiro, J.R.I.; Bozza, P.T.; da Fonseca, A.C.P.; Silva, T. The endocannabinoid system in cancer biology: A mini-review of mechanisms and therapeutic potential. Oncol. Rev. 2025, 19, 1573797. [Google Scholar] [CrossRef]
- Öz-Arslan, D.; Yavuz, M.; Kan, B. Exploring orphan GPCRs in neurodegenerative diseases. Front. Pharmacol. 2024, 15, 1394516. [Google Scholar] [CrossRef]
- Simankowicz, P.; Stępniewska, J. The Role of Endocannabinoids in Physiological Processes and Disease Pathology: A Comprehensive Review. J. Clin. Med. 2025, 14, 2851. [Google Scholar] [CrossRef]
- Lau, B.K.; Drew, G.M.; Mitchell, V.A.; Vaughan, C.W. Endocannabinoid modulation by FAAH and monoacylglycerol lipase within the analgesic circuitry of the periaqueductal grey. Br. J. Pharmacol. 2014, 171, 5225–5236. [Google Scholar] [CrossRef]
- Guindon, J.; Lai, Y.; Takacs, S.M.; Bradshaw, H.B.; Hohmann, A.G. Alterations in endocannabinoid tone following chemotherapy-induced peripheral neuropathy: Effects of endocannabinoid deactivation inhibitors targeting fatty-acid amide hydrolase and monoacylglycerol lipase in comparison to reference analgesics following cisplatin treatment. Pharmacol. Res. 2013, 67, 94–109. [Google Scholar] [CrossRef]
- van Ackern, I.; Kuhla, A.; Kuhla, B. A Role for Peripheral Anandamide and 2-Arachidonoylglycerol in Short-Term Food Intake and Orexigenic Hypothalamic Responses in a Species with Continuous Nutrient Delivery. Nutrients 2021, 13, 3587. [Google Scholar] [CrossRef] [PubMed]
- Teichmann, T.; Pflüger-Müller, B.; Giménez, V.M.M.; Sailer, F.; Dirks, H.; Zehr, S.; Warwick, T.; Brettner, F.; Munoz-Tello, P.; Zimmer, A.; et al. The endocannabinoid anandamide mediates anti-inflammatory effects through activation of NR4A nuclear receptors. Br. J. Pharmacol. 2025, 182, 1164–1182. [Google Scholar] [CrossRef] [PubMed]
- Tyrtyshnaia, A.A.; Egorova, E.L.; Starinets, A.A.; Ponomarenko, A.I.; Ermolenko, E.V.; Manzhulo, I.V. N-Docosahexaenoylethanolamine Attenuates Neuroinflammation and Improves Hippocampal Neurogenesis in Rats with Sciatic Nerve Chronic Constriction Injury. Mar. Drugs 2020, 18, 516. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N.; Chiang, N.; Dalli, J. The resolution code of acute inflammation: Novel pro-resolving lipid mediators in resolution. Semin. Immunol. 2015, 27, 200–215. [Google Scholar] [CrossRef]
- Myers, M.N.; Chirivi, M.; Gandy, J.C.; Tam, J.; Zachut, M.; Contreras, G.A. Lipolysis pathways modulate lipid mediator release and endocannabinoid system signaling in dairy cows’ adipocytes. J. Anim. Sci. Biotechnol. 2024, 15, 103. [Google Scholar] [CrossRef]
- Sawai, A.; Shida, T. Impact of Acute Exercise Load on Clock Gene Expression: A Scoping Review of Human Studies with Implications for Female Physiology. Women 2025, 5, 15. [Google Scholar] [CrossRef]
- Drăgoi, C.M.; Nicolae, A.C.; Ungurianu, A.; Margină, D.M.; Grădinaru, D.; Dumitrescu, I.-B. Circadian Rhythms, Chrononutrition, Physical Training, and Redox Homeostasis—Molecular Mechanisms in Human Health. Cells 2024, 13, 138. [Google Scholar] [CrossRef]
- Vicente-Gutiérrez, C.; Jiménez-Blasco, D.; Quintana-Cabrera, R. Intertwined ROS and Metabolic Signaling at the Neuron-Astrocyte Interface. Neurochem. Res. 2021, 46, 23–33. [Google Scholar] [CrossRef]
- Cimmino, F.; Silvestri, C.; Trinchese, G.; Petrella, L.; Cavaliere, G.; Fogliano, C.; Piscitelli, F.; Cristino, L.; Avallone, B.; Banni, S.; et al. Anti-obesity effects of Oleoylethanolamide: Modulation of mitochondrial bioenergetics, endocannabinoidome and gut microbiome. Biomed. Pharmacother. 2025, 188, 118201. [Google Scholar] [CrossRef]
- Iannotti, F.A.; Di Marzo, V. The endocannabinoidomes: Pharmacological redundancy and promiscuity, and multi-kingdom variety of sources and molecular targets. Pharmacol. Rev. 2025, 77, 100070. [Google Scholar] [CrossRef]
- Oltrabella, F.; Melgoza, A.; Nguyen, B.; Guo, S. Role of the Endocannabinoid System in Vertebrates: Emphasis on the Zebrafish Model. Dev. Growth Differ. 2017, 59, 194–210. [Google Scholar] [CrossRef] [PubMed]
- Piscitelli, F.; Silvestri, C. Role of the Endocannabinoidome in Human and Mouse Atherosclerosis. Curr. Pharm. Des. 2019, 25, 3147–3164. [Google Scholar] [CrossRef] [PubMed]
- Dyndał, K.; Pańczyszyn-Trzewik, P.; Sowa-Kućma, M. Metabolic Modulators in Depression: Emerging Molecular Mechanisms and Therapeutic Opportunities. Int. J. Mol. Sci. 2025, 26, 8755. [Google Scholar] [CrossRef]
- Nabizadeh, F.; Valizadeh, P.; Fallahi, M.S. Alzheimer’s disease Neuroimaging Initiative Bile acid profile associated with CSF and PET biomarkers in Alzheimer’s disease. Aging Clin. Exp. Res. 2024, 36, 62. [Google Scholar] [CrossRef]
- Bedarf, J.R.; Romano, S.; Heinzmann, S.S.; Duncan, A.; Traka, M.H.; Ng, D.; Segovia-Lizano, D.; Simon, M.-C.; Narbad, A.; Wüllner, U.; et al. A prebiotic dietary pilot intervention restores faecal metabolites and may be neuroprotective in Parkinson’s Disease. NPJ Park. Dis. 2025, 11, 66. [Google Scholar] [CrossRef]
- Ladakis, D.C.; Harrison, K.L.; Smith, M.D.; Solem, K.; Gadani, S.; Jank, L.; Hwang, S.; Farhadi, F.; Dewey, B.E.; Fitzgerald, K.C.; et al. Bile acid metabolites predict multiple sclerosis progression and supplementation is safe in progressive disease. Med 2025, 6, 100522. [Google Scholar] [CrossRef]
- Amin, N.; Liu, J.; Bonnechere, B.; MahmoudianDehkordi, S.; Arnold, M.; Batra, R.; Chiou, Y.-J.; Fernandes, M.; Ikram, M.A.; Kraaij, R.; et al. Interplay of Metabolome and Gut Microbiome in Individuals With Major Depressive Disorder vs Control Individuals. JAMA Psychiatry 2023, 80, 597–609. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, X.; Li, Y.; Zeng, L.; Zhu, R.; Xin, Y.; Liu, L.; Hu, Z.; Huo, Y. Combined cerebral oxygen saturation and neuron-specific enolase evaluation for diagnosis and prognosis of sepsis-associated encephalopathy. Sci. Rep. 2025, 15, 15369. [Google Scholar] [CrossRef]
- Diab, J.; Al-Mahdi, R.; Gouveia-Figueira, S.; Hansen, T.; Jensen, E.; Goll, R.; Moritz, T.; Florholmen, J.; Forsdahl, G. A Quantitative Analysis of Colonic Mucosal Oxylipins and Endocannabinoids in Treatment-Naïve and Deep Remission Ulcerative Colitis Patients and the Potential Link With Cytokine Gene Expression. Inflamm. Bowel Dis. 2019, 25, 490–497. [Google Scholar] [CrossRef]
- Dipalma, G.; Marinelli, G.; Ferrante, L.; Di Noia, A.; Carone, C.; Colonna, V.; Marotti, P.; Inchingolo, F.; Palermo, A.; Tartaglia, G.M.; et al. Modulating the Gut Microbiota to Target Neuroinflammation, Cognition and Mood: A Systematic Review of Human Studies with Relevance to Fibromyalgia. Nutrients 2025, 17, 2261. [Google Scholar] [CrossRef]
- Al-Kabani, A.; Huda, B.; Haddad, J.; Yousuf, M.; Bhurka, F.; Ajaz, F.; Patnaik, R.; Jannati, S.; Banerjee, Y. Exploring Experimental Models of Colorectal Cancer: A Critical Appraisal from 2D Cell Systems to Organoids, Humanized Mouse Avatars, Organ-on-Chip, CRISPR Engineering, and AI-Driven Platforms—Challenges and Opportunities for Translational Precision Oncology. Cancers 2025, 17, 2163. [Google Scholar] [CrossRef]
- Singh, S.; Sharma, P.; Pal, N.; Kumawat, M.; Shubham, S.; Sarma, D.K.; Tiwari, R.R.; Kumar, M.; Nagpal, R. Impact of Environmental Pollutants on Gut Microbiome and Mental Health via the Gut–Brain Axis. Microorganisms 2022, 10, 1457. [Google Scholar] [CrossRef] [PubMed]
- Rainone, G.J.; Johansen, P.M.; Pressman, P.; Hayes, A.W. Putative Effects of Lead on the Endocannabinoid System: A Literature Review and Summary. Int. J. Mol. Sci. 2025, 26, 8994. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Guo, J.; Mao, Z.; Chen, Y. Symphony of the gut microbiota and endocannabinoidome: A molecular and functional perspective. Front. Cell. Infect. Microbiol. 2025, 15, 1566290. [Google Scholar] [CrossRef]
- Schiano Moriello, A.; Di Marzo, V.; Petrosino, S. Mutual Links between the Endocannabinoidome and the Gut Microbiome, with Special Reference to Companion Animals: A Nutritional Viewpoint. Animals 2022, 12, 348. [Google Scholar] [CrossRef]
- Monet, M.C.; Quan, N. Complex Neuroimmune Involvement in Neurodevelopment: A Mini-Review. J. Inflamm. Res. 2023, 16, 2979–2991. [Google Scholar] [CrossRef]
- Fiorin, F.D.S.; do Espírito Santo, C.C. The Potential Roles of Astrocytes and Microglia in the Spinal Cord and Brain After Spinal Cord Injury. Neuroglia 2025, 6, 12. [Google Scholar] [CrossRef]
- Lee, A.J.B.; Bi, S.; Ridgeway, E.; Al-Hussaini, I.; Deshpande, S.; Krueger, A.; Khatri, A.; Tsui, D.; Deng, J.; Mitchell, C.S. Restoring Homeostasis: Treating Amyotrophic Lateral Sclerosis by Resolving Dynamic Regulatory Instability. Int. J. Mol. Sci. 2025, 26, 872. [Google Scholar] [CrossRef]
- Watts, A.G.; Kanoski, S.E.; Sanchez-Watts, G.; Langhans, W. The physiological control of eating: Signals, neurons, and networks. Physiol. Rev. 2022, 102, 689–813. [Google Scholar] [CrossRef]
- Zannas, A.S.; West, A.E. Epigenetics and the regulation of stress vulnerability and resilience. Neuroscience 2014, 264, 157–170. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, D.A.; Deb, S.; Gal, G.; Thackeray, S.J.; Dutta, P.S.; Matsuzaki, S.S.; May, L.; Clements, C.F. Early warning signals have limited applicability to empirical lake data. Nat. Commun. 2023, 14, 7942. [Google Scholar] [CrossRef] [PubMed]
- Chew, C.S.; Lee, J.Y.; Ng, K.Y.; Koh, R.Y.; Chye, S.M. Resilience mechanisms underlying Alzheimer’s disease. Metab. Brain Dis. 2025, 40, 86. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.; Yao, R.; Zhang, H.; Feng, Y.; Yao, Y. Sepsis-associated encephalopathy: A vicious cycle of immunosuppression. J. Neuroinflamm. 2020, 17, 14. [Google Scholar] [CrossRef]
- Dogan, H.; Calak, S.; Dogan, H.; Calak, S. Evaluation of Response to Relapse Treatment in Multiple Sclerosis According to Relapse Characteristics. J. Mult. Scler. Res. 2025, 5, 18–22. [Google Scholar] [CrossRef]
- Gómez-Carrillo, A.; Kirmayer, L.J. A cultural-ecosocial systems view for psychiatry. Front. Psychiatry 2023, 14, 1031390. [Google Scholar] [CrossRef]
- Ossenkoppele, R.; Pichet Binette, A.; Groot, C.; Smith, R.; Strandberg, O.; Palmqvist, S.; Stomrud, E.; Tideman, P.; Ohlsson, T.; Jögi, J.; et al. Amyloid and tau PET-positive cognitively unimpaired individuals are at high risk for future cognitive decline. Nat. Med. 2022, 28, 2381–2387. [Google Scholar] [CrossRef]
- Malek, N.; Popiolek-Barczyk, K.; Mika, J.; Przewlocka, B.; Starowicz, K. Anandamide, Acting via CB2 Receptors, Alleviates LPS-Induced Neuroinflammation in Rat Primary Microglial Cultures. Neural Plast. 2015, 2015, 130639. [Google Scholar] [CrossRef]
- Valdez-Gaxiola, C.A.; Rosales-Leycegui, F.; Gaxiola-Rubio, A.; Moreno-Ortiz, J.M.; Figuera, L.E. Early- and Late-Onset Alzheimer’s Disease: Two Sides of the Same Coin? Diseases 2024, 12, 110. [Google Scholar] [CrossRef]
- Bahrami, M.; Donadi, S.; Ferialdi, L.; Bassi, A.; Curceanu, C.; Di Domenico, A.; Hiesmayr, B.C. Are collapse models testable with quantum oscillating systems? The case of neutrinos, kaons, chiral molecules. Sci. Rep. 2013, 3, 1952. [Google Scholar] [CrossRef]
- Švik, M.; Lukeš, P.; Lhotáková, Z.; Neuwirthová, E.; Albrechtová, J.; Campbell, P.E.; Homolová, L. Retrieving plant functional traits through time series analysis of satellite observations using machine learning methods. Int. J. Remote Sens. 2023, 44, 3083–3105. [Google Scholar] [CrossRef]
- Jack, C.R.; Bennett, D.A.; Blennow, K.; Carrillo, M.C.; Dunn, B.; Haeberlein, S.B.; Holtzman, D.M.; Jagust, W.; Jessen, F.; Karlawish, J.; et al. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement. J. Alzheimers Assoc. 2018, 14, 535–562. [Google Scholar] [CrossRef] [PubMed]
- Hanlon, E.C. Impact of circadian rhythmicity and sleep restriction on circulating endocannabinoid (eCB) N-arachidonoylethanolamine (anandamide). Psychoneuroendocrinology 2020, 111, 104471. [Google Scholar] [CrossRef] [PubMed]
- Mota, C.M.D.; Madden, C.J. Neural control of the spleen as an effector of immune responses to inflammation: Mechanisms and treatments. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2022, 323, R375–R384. [Google Scholar] [CrossRef] [PubMed]
- Rebolledo-Pérez, L.; Hernández-Bello, J.; Martínez-Ramos, A.; Castañeda-Arellano, R.; Fernández-Quezada, D.; Sandoval-García, F.; Aguilar-García, I.G. Substance Abuse and Cognitive Decline: The Critical Role of Tau Protein as a Potential Biomarker. Int. J. Mol. Sci. 2025, 26, 7638. [Google Scholar] [CrossRef]
- Morena, M.; Santori, A.; Campolongo, P. Circadian regulation of memory under stress: Endocannabinoids matter. Neurosci. Biobehav. Rev. 2022, 138, 104712. [Google Scholar] [CrossRef]
- Murillo-Rodríguez, E.; Budde, H.; Veras, A.B.; Rocha, N.B.; Telles-Correia, D.; Monteiro, D.; Cid, L.; Yamamoto, T.; Machado, S.; Torterolo, P. The Endocannabinoid System May Modulate Sleep Disorders in Aging. Curr. Neuropharmacol. 2020, 18, 97–108. [Google Scholar] [CrossRef]
- Thaiss, C.A.; Zeevi, D.; Levy, M.; Zilberman-Schapira, G.; Suez, J.; Tengeler, A.C.; Abramson, L.; Katz, M.N.; Korem, T.; Zmora, N.; et al. Transkingdom control of microbiota diurnal oscillations promotes metabolic homeostasis. Cell 2014, 159, 514–529. [Google Scholar] [CrossRef]
- Xie, L.; Das, S.R.; Wisse, L.E.M.; Ittyerah, R.; de Flores, R.; Shaw, L.M.; Yushkevich, P.A.; Wolk, D.A. Baseline structural MRI and plasma biomarkers predict longitudinal structural atrophy and cognitive decline in early Alzheimer’s disease. Alzheimers Res. Ther. 2023, 15, 79, Erratum in Alzheimers Res. Ther. 2024, 16, 11.. [Google Scholar] [CrossRef]
- Huys, Q.J.M.; Maia, T.V.; Frank, M.J. Computational psychiatry as a bridge from neuroscience to clinical applications. Nat. Neurosci. 2016, 19, 404–413. [Google Scholar] [CrossRef]
- Genin, K.; Grote, T.; Wolfers, T. Computational psychiatry and the evolving concept of a mental disorder. Synthese 2024, 204, 88. [Google Scholar] [CrossRef]
- Petersen, R.C.; Aisen, P.S.; Beckett, L.A.; Donohue, M.C.; Gamst, A.C.; Harvey, D.J.; Jack, C.R.; Jagust, W.J.; Shaw, L.M.; Toga, A.W.; et al. Alzheimer’s Disease Neuroimaging Initiative (ADNI): Clinical characterization. Neurology 2010, 74, 201–209. [Google Scholar] [CrossRef]
- Marek, K.; Chowdhury, S.; Siderowf, A.; Lasch, S.; Coffey, C.S.; Caspell-Garcia, C.; Simuni, T.; Jennings, D.; Tanner, C.M.; Trojanowski, J.Q.; et al. The Parkinson’s progression markers initiative (PPMI)—Establishing a PD biomarker cohort. Ann. Clin. Transl. Neurol. 2018, 5, 1460–1477. [Google Scholar] [CrossRef]
- Souchet, B.; Michaïl, A.; Heuillet, M.; Dupuy-Gayral, A.; Haudebourg, E.; Pech, C.; Berthemy, A.; Autelitano, F.; Billoir, B.; Domoto-Reilly, K.; et al. Multiomics Blood-Based Biomarkers Predict Alzheimer’s Predementia with High Specificity in a Multicentric Cohort Study. J. Prev. Alzheimers Dis. 2024, 11, 567–581, Erratum in J. Prev. Alzheimers Dis. 2025, 12, 100026. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Nie, Y.; Yang, J.; Yang, S.; Li, R.; Rao, V.; Hu, X.; Fang, C.; Li, S.; Song, D.; et al. Integrative multi-omic profiling of adult mouse brain endothelial cells and potential implications in Alzheimer’s disease. Cell Rep. 2023, 42, 113392. [Google Scholar] [CrossRef] [PubMed]
- Qiu, S.; Cai, Y.; Yao, H.; Lin, C.; Xie, Y.; Tang, S.; Zhang, A. Small molecule metabolites: Discovery of biomarkers and therapeutic targets. Signal Transduct. Target. Ther. 2023, 8, 132. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.B.; Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 2016, 7, 27–31. [Google Scholar] [CrossRef]
- Grabon, W.; Ruiz, A.; Gasmi, N.; Degletagne, C.; Georges, B.; Belmeguenai, A.; Bodennec, J.; Rheims, S.; Marcy, G.; Bezin, L. CB2 Expression in Mouse Brain: From Mapping to Regulation in Microglia under Inflammatory Conditions. J. Neuroinflamm. 2024, 21, 206. [Google Scholar] [CrossRef]
- Loryan, I.; Reichel, A.; Feng, B.; Bundgaard, C.; Shaffer, C.; Kalvass, C.; Bednarczyk, D.; Morrison, D.; Lesuisse, D.; Hoppe, E.; et al. Unbound Brain-to-Plasma Partition Coefficient, Kp,uu,brain—A Game Changing Parameter for CNS Drug Discovery and Development. Pharm. Res. 2022, 39, 1321–1341. [Google Scholar] [CrossRef]
- Ahn, J.-S.; Kang, M.-J.; Seo, Y.; Kim, H.-S. Intestinal organoids as advanced modeling platforms to study the role of host-microbiome interaction in homeostasis and disease. BMB Rep. 2023, 56, 15–23. [Google Scholar] [CrossRef]
- Marinelli, S.; Marrone, M.C.; Di Domenico, M.; Marinelli, S. Endocannabinoid signaling in microglia. Glia 2023, 71, 71–90. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.-W.; Shao, Q.-H.; Wang, X.-T.; Ma, K.-L.; Chen, N.-H.; Yuan, Y.-H. CB2 receptor activation inhibits the phagocytic function of microglia through activating ERK/AKT-Nurr1 signal pathways. Acta Pharmacol. Sin. 2022, 43, 2253–2266, Correction in Acta Pharmacol. Sin. 2024, 45, 2228. [Google Scholar] [CrossRef] [PubMed]
- Hoffmeister, L.; Diekmann, M.; Brand, K.; Huber, R. GSK3: A Kinase Balancing Promotion and Resolution of Inflammation. Cells 2020, 9, 820. [Google Scholar] [CrossRef]
- Dorrington, M.G.; Fraser, I.D.C. NF-κB Signaling in Macrophages: Dynamics, Crosstalk, and Signal Integration. Front. Immunol. 2019, 10, 705. [Google Scholar] [CrossRef]
- Mussbacher, M.; Derler, M.; Basílio, J.; Schmid, J.A. NF-κB in monocytes and macrophages—An inflammatory master regulator in multitalented immune cells. Front. Immunol. 2023, 14, 1134661. [Google Scholar] [CrossRef]
- Calvo, B.; Fernandez, M.; Rincon, M.; Tranque, P. GSK3β Inhibition by Phosphorylation at Ser389 Controls Neuroinflammation. Int. J. Mol. Sci. 2023, 24, 337. [Google Scholar] [CrossRef]
- Pradère, J.-P.; Hernandez, C.; Koppe, C.; Friedman, R.A.; Luedde, T.; Schwabe, R.F. Negative regulation of NF-κB p65 activity by serine 536 phosphorylation. Sci. Signal. 2016, 9, ra85. [Google Scholar] [CrossRef]
- Motolani, A.; Martin, M.; Sun, M.; Lu, T. Phosphorylation of the Regulators, a Complex Facet of NF-κB Signaling in Cancer. Biomolecules 2021, 11, 15. [Google Scholar] [CrossRef]
- Amenta, P.S.; Jallo, J.I.; Tuma, R.F.; Hooper, D.C.; Elliott, M.B. Cannabinoid receptor type-2 stimulation, blockade, and deletion alter the vascular inflammatory responses to traumatic brain injury. J. Neuroinflamm. 2014, 11, 191. [Google Scholar] [CrossRef]
- Petrosino, S.; Schiano Moriello, A. Palmitoylethanolamide: A Nutritional Approach to Keep Neuroinflammation within Physiological Boundaries—A Systematic Review. Int. J. Mol. Sci. 2020, 21, 9526. [Google Scholar] [CrossRef]
- Yang, S.; Ben-Shalom, R.; Ahn, M.; Liptak, A.T.; van Rijn, R.M.; Whistler, J.L.; Bender, K.J. β-arrestin-dependent dopaminergic regulation of calcium channel activity in the axon initial segment. Cell Rep. 2016, 16, 1518–1526. [Google Scholar] [CrossRef]
- Shi, H.; Wu, M.; Wu, X.; Liu, Z.; Jiang, S.; Li, G.; Yang, Y.; Fu, Y.; Wang, Q.; Zhang, G.; et al. Multi-omics integration reveals functional signatures of gut microbiome in atherosclerosis. Gut Microbes 2025, 17, 2542384. [Google Scholar] [CrossRef]
- Abreu, A.C.; Navas, M.M.; Fernández, C.P.; Sánchez-Santed, F.; Fernández, I. NMR-Based Metabolomics Approach to Explore Brain Metabolic Changes Induced by Prenatal Exposure to Autism-Inducing Chemicals. ACS Chem. Biol. 2021, 16, 753–765. [Google Scholar] [CrossRef]
- Yaqub, M.O.; Jain, A.; Joseph, C.E.; Edison, L.K. Microbiome-Driven Therapeutics: From Gut Health to Precision Medicine. Gastrointest. Disord. 2025, 7, 7. [Google Scholar] [CrossRef]
- Ruan, W.; Yuan, X.; Eltzschig, H.K. Circadian rhythm as a therapeutic target. Nat. Rev. Drug Discov. 2021, 20, 287–307. [Google Scholar] [CrossRef] [PubMed]
- Montagnani, M.; Bottalico, L.; Potenza, M.A.; Charitos, I.A.; Topi, S.; Colella, M.; Santacroce, L. The Crosstalk between Gut Microbiota and Nervous System: A Bidirectional Interaction between Microorganisms and Metabolome. Int. J. Mol. Sci. 2023, 24, 10322. [Google Scholar] [CrossRef] [PubMed]
- Natterson-Horowitz, B.; Aktipis, A.; Fox, M.; Gluckman, P.D.; Low, F.M.; Mace, R.; Read, A.; Turner, P.E.; Blumstein, D.T. The future of evolutionary medicine: Sparking innovation in biomedicine and public health. Front. Sci. 2023, 1, 997136. [Google Scholar] [CrossRef]
- Gkintoni, E.; Vantarakis, A.; Gourzis, P. Neuroimaging Insights into the Public Health Burden of Neuropsychiatric Disorders: A Systematic Review of Electroencephalography-Based Cognitive Biomarkers. Medicina 2025, 61, 1003. [Google Scholar] [CrossRef]
- Dong, Y.; Wu, X.; Zhang, Y.; Hu, A.; Zhou, Q.; Yue, X.; Liu, Z.; Li, M. The Role of Probiotics in Modulating the Gut Microbiome in Alzheimer’s Disease: A Review. Foods 2025, 14, 1531. [Google Scholar] [CrossRef]
- Chen, T.; Wang, L.; Xie, G.; Kristal, B.S.; Zheng, X.; Sun, T.; Arnold, M.; Louie, G.; Li, M.; Wu, L.; et al. Serum Bile Acids Improve Prediction of Alzheimer’s Progression in a Sex-Dependent Manner. Adv. Sci. 2024, 11, e2306576. [Google Scholar] [CrossRef]
- Strobel, J.; Yousefzadeh-Nowshahr, E.; Deininger, K.; Bohn, K.P.; von Arnim, C.A.F.; Otto, M.; Solbach, C.; Anderl-Straub, S.; Polivka, D.; Fissler, P.; et al. Exploratory Tau PET/CT with [11C]PBB3 in Patients with Suspected Alzheimer’s Disease and Frontotemporal Lobar Degeneration: A Pilot Study on Correlation with PET Imaging and Cerebrospinal Fluid Biomarkers. Biomedicines 2024, 12, 1460. [Google Scholar] [CrossRef]
- Mallach, A.; Zielonka, M.; van Lieshout, V.; An, Y.; Khoo, J.H.; Vanheusden, M.; Chen, W.-T.; Moechars, D.; Arancibia-Carcamo, I.L.; Fiers, M.; et al. Microglia-astrocyte crosstalk in the amyloid plaque niche of an Alzheimer’s disease mouse model, as revealed by spatial transcriptomics. Cell Rep. 2024, 43, 114216. [Google Scholar] [CrossRef] [PubMed]
- Poniah, P.; Abdul Rashed, A.; Abdul Jalil, J.; Ali, E.Z. Clinical Significance of Early-Onset Alzheimer’s Mutations in Asian and Western Populations: A Scoping Review. Genes 2025, 16, 345. [Google Scholar] [CrossRef] [PubMed]
- Mumford, P.; Tosh, J.; Anderle, S.; Gkanatsiou Wikberg, E.; Lau, G.; Noy, S.; Cleverley, K.; Saito, T.; Saido, T.C.; Yu, E.; et al. Genetic Mapping of APP and Amyloid-β Biology Modulation by Trisomy 21. J. Neurosci. 2022, 42, 6453–6468. [Google Scholar] [CrossRef]
- Robinson, C.G.; Coleman, T.; Buciuc, M.; Singh, N.A.; Pham, N.T.T.; Machulda, M.M.; Graff-Radford, J.; Whitwell, J.L.; Josephs, K.A. Behavioral and Neuropsychiatric Differences Across Two Atypical Alzheimer’s Disease Variants: Logopenic Progressive Aphasia and Posterior Cortical Atrophy. J. Alzheimers Dis. JAD 2024, 97, 895–908. [Google Scholar] [CrossRef]
- Kurlyandchik, I.; Lauche, R.; Tiralongo, E.; Warne, L.N.; Schloss, J. Plasma and interstitial levels of endocannabinoids and N-acylethanolamines in patients with chronic widespread pain and fibromyalgia: A systematic review and meta-analysis. Pain Rep. 2022, 7, e1045. [Google Scholar] [CrossRef]
- Tenchov, R.; Sasso, J.M.; Wang, X.; Zhou, Q.A. Aging Hallmarks and Progression and Age-Related Diseases: A Landscape View of Research Advancement. ACS Chem. Neurosci. 2024, 15, 1–30. [Google Scholar] [CrossRef]
- Oliver, P.J.; Civitelli, L.; Hu, M.T. The gut–brain axis in early Parkinson’s disease: From prodrome to prevention. J. Neurol. 2025, 272, 413. [Google Scholar] [CrossRef]
- Vijay, A.; Kouraki, A.; Gohir, S.; Turnbull, J.; Kelly, A.; Chapman, V.; Barrett, D.A.; Bulsiewicz, W.J.; Valdes, A.M. The anti-inflammatory effect of bacterial short chain fatty acids is partially mediated by endocannabinoids. Gut Microbes 2021, 13, 1997559. [Google Scholar] [CrossRef]
- Cipriano, L.; Minino, R.; Liparoti, M.; Polverino, A.; Romano, A.; Bonavita, S.; Pirozzi, M.A.; Quarantelli, M.; Jirsa, V.; Sorrentino, G.; et al. Flexibility of brain dynamics is increased and predicts clinical impairment in relapsing–remitting but not in secondary progressive multiple sclerosis. Brain Commun. 2024, 6, fcae112. [Google Scholar] [CrossRef]
- Xia, X.; Ni, J.; Yin, S.; Yang, Z.; Jiang, H.; Wang, C.; Peng, J.; Wei, H.; Wang, X. Elevated Systemic and Intestinal Inflammatory Response Are Associated With Gut Microbiome Disorder After Cardiovascular Surgery. Front. Microbiol. 2021, 12, 686648. [Google Scholar] [CrossRef]
- Walther, A.; Eggenberger, L.; Debelak, R.; Kirschbaum, C.; Häberling, I.; Osuna, E.; Strumberger, M.; Walitza, S.; Baumgartner, J.; Herter-Aeberli, I.; et al. Major depressive disorder in children and adolescents is associated with reduced hair cortisol and anandamide (AEA): Cross-sectional and longitudinal evidence from a large randomized clinical trial. Transl. Psychiatry 2025, 15, 183. [Google Scholar] [CrossRef] [PubMed]
- Tobias, M.R.; Ito, T.A. Anxiety Increases Sensitivity to Errors and Negative Feedback Over Time. Biol. Psychol. 2021, 162, 108092. [Google Scholar] [CrossRef] [PubMed]
- Jana, A.; Nath, A.; Sen, P.; Kundu, S.; Alghamdi, B.S.; Abujamel, T.S.; Saboor, M.; Woon-Khiong, C.; Alexiou, A.; Papadakis, M.; et al. Unraveling the Endocannabinoid System: Exploring Its Therapeutic Potential in Autism Spectrum Disorder. Neuromolecular Med. 2024, 26, 20. [Google Scholar] [CrossRef] [PubMed]
- Salas-Venegas, V.; Flores-Torres, R.P.; Rodríguez-Cortés, Y.M.; Rodríguez-Retana, D.; Ramírez-Carreto, R.J.; Concepción-Carrillo, L.E.; Pérez-Flores, L.J.; Alarcón-Aguilar, A.; López-Díazguerrero, N.E.; Gómez-González, B.; et al. The Obese Brain: Mechanisms of Systemic and Local Inflammation, and Interventions to Reverse the Cognitive Deficit. Front. Integr. Neurosci. 2022, 16, 798995. [Google Scholar] [CrossRef]
- Thapa, D.; Patil, M.; Warne, L.N.; Carlessi, R.; Falasca, M. Enhancing Tetrahydrocannabinol’s Therapeutic Efficacy in Inflammatory Bowel Disease: The Roles of Cannabidiol and the Cannabinoid 1 Receptor Allosteric Modulator ZCZ011. Pharmaceuticals 2025, 18, 148. [Google Scholar] [CrossRef]
- Makovec, M.; Skitek, M.; Šimnovec, L.; Jerin, A. Neuron-Specific Enolase and S100B as Biomarkers of Ischemic Brain Injury During Surgery. Clin. Pract. 2025, 15, 74. [Google Scholar] [CrossRef]
- Calderone, A.; Latella, D.; La Fauci, E.; Puleo, R.; Sergi, A.; De Francesco, M.; Mauro, M.; Foti, A.; Salemi, L.; Calabrò, R.S. Artificial Intelligence-Driven Neuromodulation in Neurodegenerative Disease: Precision in Chaos, Learning in Loss. Biomedicines 2025, 13, 2118. [Google Scholar] [CrossRef]
- Nicolas, J.; King, B.R.; Lévesque, D.; Lazzouni, L.; Leroux, G.; Wang, D.; Grossman, N.; Swinnen, S.P.; Doyon, J.; Carrier, J.; et al. Unraveling the neurophysiological correlates of phase-specific enhancement of motor memory consolidation via slow-wave closed-loop targeted memory reactivation. Nat. Commun. 2025, 16, 2644. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, Y.; Liu, J.; Liu, W.; Cao, Y.; Yan, R.; Yao, Y. Neuroimmune Regulation in Sepsis-Associated Encephalopathy: The Interaction Between the Brain and Peripheral Immunity. Front. Neurol. 2022, 13, 892480. [Google Scholar] [CrossRef]
- Mallory, C.; Keehn, B. Implications of Sensory Processing and Attentional Differences Associated With Autism in Academic Settings: An Integrative Review. Front. Psychiatry 2021, 12, 695825. [Google Scholar] [CrossRef] [PubMed]
- Peksa, J.; Mamchur, D. State-of-the-Art on Brain-Computer Interface Technology. Sensors 2023, 23, 6001. [Google Scholar] [CrossRef] [PubMed]
- Rector, J.L.; Kuranova, A.; Olde Rikkert, M.G.M.; van Goor, H.; Melis, R.J.F.; Bredie, S.J.H. Continuous Monitoring Enables Dynamic Biomarkers to Assess Resilience in Acute COVID-19 Patients. J. Clin. Med. 2025, 14, 951. [Google Scholar] [CrossRef] [PubMed]
- Tahmasebi, H.; Arjmand, N.; Monemi, M.; Babaeizad, A.; Alibabaei, F.; Alibabaei, N.; Bahar, A.; Oksenych, V.; Eslami, M. From Cure to Crisis: Understanding the Evolution of Antibiotic-Resistant Bacteria in Human Microbiota. Biomolecules 2025, 15, 93. [Google Scholar] [CrossRef]
- Hitch, T.C.A.; Hall, L.J.; Walsh, S.K.; Leventhal, G.E.; Slack, E.; de Wouters, T.; Walter, J.; Clavel, T. Microbiome-based interventions to modulate gut ecology and the immune system. Mucosal Immunol. 2022, 15, 1095–1113. [Google Scholar] [CrossRef]
- Yoon, S.J.; Yu, J.S.; Min, B.H.; Gupta, H.; Won, S.-M.; Park, H.J.; Han, S.H.; Kim, B.-Y.; Kim, K.H.; Kim, B.K.; et al. Bifidobacterium-derived short-chain fatty acids and indole compounds attenuate nonalcoholic fatty liver disease by modulating gut-liver axis. Front. Microbiol. 2023, 14, 1129904. [Google Scholar] [CrossRef]
- Rusch, J.A.; Layden, B.T.; Dugas, L.R. Signalling cognition: The gut microbiota and hypothalamic-pituitary-adrenal axis. Front. Endocrinol. 2023, 14, 1130689. [Google Scholar] [CrossRef]
- Grasa-Ciria, D.; Couto, S.; Samatán, E.; Martínez-Jarreta, B.; Cenit, M.D.C.; Iguacel, I. Disrupted Rhythms, Disrupted Microbes: A Systematic Review of Shift Work and Gut Microbiota Alterations. Nutrients 2025, 17, 2894. [Google Scholar] [CrossRef]
- Yoon, H.H.; Grimsey, N.L. Cannabinoid Receptor 2 (CB2) in Macrophages: A Promising Clinical Target for Immune Disorders. Int. J. Mol. Sci. 2025, 26, 8657. [Google Scholar] [CrossRef]
- Di Stefano, V.; Steardo, L.; D’Angelo, M.; Monaco, F.; Steardo, L. Palmitoylethanolamide: A Multifunctional Molecule for Neuroprotection, Chronic Pain, and Immune Modulation. Biomedicines 2025, 13, 1271. [Google Scholar] [CrossRef]
- Petrosino, S.; Di Marzo, V. The pharmacology of palmitoylethanolamide and first data on the therapeutic efficacy of some of its new formulations. Br. J. Pharmacol. 2017, 174, 1349–1365. [Google Scholar] [CrossRef]
- O’Reilly, M.L.; Tom, V.J. Neuroimmune System as a Driving Force for Plasticity Following CNS Injury. Front. Cell. Neurosci. 2020, 14, 187. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Zhu, Z.; Zheng, T.; Li, D.; Wei, Y.; Yang, H.; Yu, H.; Han, J.; Wang, Y.; Wang, L.; et al. Astrocytic YAP prevents the glutamate neurotoxicity by upregulation of EAAT2 expression and promotes the gain of stemness in astrocytes in ischemic stroke mice. Cell Death Dis. 2025, 16, 577. [Google Scholar] [CrossRef] [PubMed]
- Mitrović, M.; Stanković Popović, V.; Erceg, S.; Perišić Mitrović, M.; Dobrosavljević, A.; Stupar, A.; Vuković, P.; Zlatković, D.; Svorcan, P. Exploring the Potential of Oral Butyrate Supplementation in Metabolic Dysfunction-Associated Steatotic Liver Disease: Subgroup Insights from an Interventional Study. Int. J. Mol. Sci. 2025, 26, 5561. [Google Scholar] [CrossRef]
- Chmiel, J.; Kurpas, D. Burnout and the Brain—A Mechanistic Review of Magnetic Resonance Imaging (MRI) Studies. Int. J. Mol. Sci. 2025, 26, 8379. [Google Scholar] [CrossRef]
- Fuad, S.A.; Ginting, R.P.; Lee, M.-W. Chrononutrition: Potential, Challenges, and Application in Managing Obesity. Int. J. Mol. Sci. 2025, 26, 5116. [Google Scholar] [CrossRef]
- Godos, J.; Currenti, W.; Ferri, R.; Lanza, G.; Caraci, F.; Frias-Toral, E.; Guglielmetti, M.; Ferraris, C.; Lipari, V.; Carvajal Altamiranda, S.; et al. Chronotype and Cancer: Emerging Relation Between Chrononutrition and Oncology from Human Studies. Nutrients 2025, 17, 529. [Google Scholar] [CrossRef]
- Gill, S.S.; Wu, H.; Patros, P.; Ottaviani, C.; Arora, P.; Pujol, V.C.; Haunschild, D.; Parlikad, A.K.; Cetinkaya, O.; Lutfiyya, H.; et al. Modern computing: Vision and challenges. Telemat. Inform. Rep. 2024, 13, 100116. [Google Scholar] [CrossRef]
- Jiang, G.; Xie, G.; Li, X.; Xiong, J. Cytoskeletal Proteins and Alzheimer’s Disease Pathogenesis: Focusing on the Interplay with Tau Pathology. Biomolecules 2025, 15, 831. [Google Scholar] [CrossRef]
- Xiao, D.; Wang, X.; Liang, W.; Yang, Y.; Du, Y.; Liu, C.; Xu, F.; Yang, Y.; Wei, M.; Yang, G. Convergence of sepsis-associated encephalopathy pathogenesis onto microglia. J. Transl. Med. 2025, 23, 622. [Google Scholar] [CrossRef]
- Pascoal, T.A.; Aguzzoli, C.S.; Lussier, F.Z.; Crivelli, L.; Suemoto, C.K.; Fortea, J.; Rosa-Neto, P.; Zimmer, E.R.; Ferreira, P.C.L.; Bellaver, B. Insights into the use of biomarkers in clinical trials in Alzheimer’s disease. eBioMedicine 2024, 108, 105322. [Google Scholar] [CrossRef]
- Sadée, C.; Testa, S.; Barba, T.; Hartmann, K.; Schuessler, M.; Thieme, A.; Church, G.M.; Okoye, I.; Hernandez-Boussard, T.; Hood, L.; et al. Medical digital twins: Enabling precision medicine and medical artificial intelligence. Lancet Digit. Health 2025, 7, 100864. [Google Scholar] [CrossRef] [PubMed]
- Saratkar, S.Y.; Langote, M.; Kumar, P.; Gote, P.; Weerarathna, I.N.; Mishra, G.V. Digital twin for personalized medicine development. Front. Digit. Health 2025, 7, 1583466. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Zhu, R.; Peng, I.; Xu, Z.; Jiang, Y. Wearable and implantable biosensors: Mechanisms and applications in closed-loop therapeutic systems. J. Mater. Chem. B 2024, 12, 8577–8604. [Google Scholar] [CrossRef] [PubMed]
- Ramsey, A.M.; Stowie, A.; Hill, A.; Ellis, I.; Rhodes, M.K.; Pollock, D.M.; Davidson, A.J. Long-term circadian disruption shortens life span and dampens blood pressure diurnal rhythms in stroke-prone spontaneously hypertensive rats. Am. J. Physiol.-Heart Circ. Physiol. 2023, 325, H106–H112. [Google Scholar] [CrossRef]
- Vitali, E.; Cattane, N.; D’Aprile, I.; Petrillo, G.; Cattaneo, A. Systemic Inflammation at the Crossroad of Major Depressive Disorder and Comorbidities: A Narrative Review. Int. J. Mol. Sci. 2025, 26, 9382. [Google Scholar] [CrossRef]
- Nazir, A.; Hussain, F.H.N.; Raza, A. Advancing microbiota therapeutics: The role of synthetic biology in engineering microbial communities for precision medicine. Front. Bioeng. Biotechnol. 2024, 12, 1511149. [Google Scholar] [CrossRef]
- Dudeja, C.; Mishra, A.; Ali, A.; Singh, P.P.; Jaiswal, A.K. Microbial Genome Editing with CRISPR–Cas9: Recent Advances and Emerging Applications Across Sectors. Fermentation 2025, 11, 410. [Google Scholar] [CrossRef]
- An, L.; Liu, Y.; Liu, Y. Organ-on-a-Chip Applications in Microfluidic Platforms. Micromachines 2025, 16, 201. [Google Scholar] [CrossRef]
- Alsadaan, N.; Ramadan, O.M.E. Barriers and Facilitators in Implementing Evidence-Based Practice: A Parallel Cross-Sectional Mixed Methods Study among Nursing Administrators. BMC Nurs. 2025, 24, 403. [Google Scholar] [CrossRef]
- Duan, D.; Wang, M.; Han, J.; Li, M.; Wang, Z.; Zhou, S.; Xin, W.; Li, X. Advances in Multi-Omics Integrated Analy-sis Methods Based on the Gut Microbiome and Their Applications. Front. Microbiol. 2025, 15. [Google Scholar] [CrossRef] [PubMed]
- del Carmen Sánchez-González, M.; Sarmiento-Suárez, R.; Cellai, L.L.; Moreau, C.; Bartoszewicz, M.; Fiedor, P.; Taruscio, D.; de Santis, M.; Posada de la Paz, M.; Julkowska, D.; et al. Integrative Research and Innovation Strategy for Rare Diseases. Insights from the 5-Year European Joint Programme on Rare Diseases, Including Analysis to Inform Recommendations for Future Actions. Health Res. Policy Syst. 2025, 23, 137. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, J.C.; Isom, D.C.; Evans, D.D.; Page, K.J. Digital Innovation in Medicinal Product Regulatory Submis-sion, Review, and Approvals to Create a Dynamic Regulatory Ecosystem—Are We Ready for a Revolution? Front. Med. 2021, 8, 660808. [Google Scholar] [CrossRef]
- Zhang, Z.; Xue, P.; Bendlin, B.B.; Zetterberg, H.; De Felice, F.; Tan, X.; Benedict, C. Melatonin: A potential nighttime guardian against Alzheimer’s. Mol. Psychiatry 2025, 30, 237–250. [Google Scholar] [CrossRef]
- Collins, L.M.; Nahum-Shani, I.; Guastaferro, K.; Strayhorn, J.C.; Vanness, D.J.; Murphy, S.A. Intervention optimization: A paradigm shift and its potential implications for clinical psychology. Annu. Rev. Clin. Psychol. 2024, 20, 21–47. [Google Scholar] [CrossRef]
- Li, L.; Yang, C.; Jia, M.; Wang, Y.; Zhao, Y.; Li, Q.; Gong, J.; He, Y.; Xu, K.; Liu, X.; et al. Synbiotic therapy with Clostridium sporogenes and xylan promotes gut-derived indole-3-propionic acid and improves cognitive impairments in an Alzheimer’s disease mouse model. Food Funct. 2024, 15, 7865–7882. [Google Scholar] [CrossRef]
- Kim, H.; Lee, E.; Park, M.; Min, K.; Diep, Y.N.; Kim, J.; Ahn, H.; Lee, E.; Kim, S.; Kim, Y.; et al. Microbiome-derived indole-3-lactic acid reduces amyloidopathy through aryl-hydrocarbon receptor activation. Brain. Behav. Immun. 2024, 122, 568–582, Erratum in Brain. Behav. Immun. 2025, 127, 423. [Google Scholar] [CrossRef]
- Shivyari, F.T.; Pakniat, H.; Nooshabadi, M.R.; Rostami, S.; Haghighian, H.K.; Shiri-Shahsavari, M.R. Examining the oleoylethanolamide supplement effects on glycemic status, oxidative stress, inflammation, and anti-mullerian hormone in polycystic ovary syndrome. J. Ovarian Res. 2024, 17, 111. [Google Scholar] [CrossRef]
- Long, H.; Simmons, A.; Mayorga, A.; Burgess, B.; Nguyen, T.; Budda, B.; Rychkova, A.; Rhinn, H.; Tassi, I.; Ward, M.; et al. Preclinical and first-in-human evaluation of AL002, a novel TREM2 agonistic antibody for Alzheimer’s disease. Alzheimers Res. Ther. 2024, 16, 235. [Google Scholar] [CrossRef]
- Stoll, A.C.; Kemp, C.J.; Patterson, J.R.; Kubik, M.; Kuhn, N.; Benskey, M.; Duffy, M.F.; Luk, K.C.; Sortwell, C.E. Alpha-synuclein inclusion responsive microglia are resistant to CSF1R inhibition. J. Neuroinflamm. 2024, 21, 108. [Google Scholar] [CrossRef]
- Kim, P.; Sanchez, A.M.; Penke, T.J.R.; Tuson, H.H.; Kime, J.C.; McKee, R.W.; Slone, W.L.; Conley, N.R.; McMillan, L.J.; Prybol, C.J.; et al. Safety, pharmacokinetics, and pharmacodynamics of LBP-EC01, a CRISPR-Cas3-enhanced bacteriophage cocktail, in uncomplicated urinary tract infections due to Escherichia coli (ELIMINATE): The randomised, open-label, first part of a two-part phase 2 trial. Lancet Infect. Dis. 2024, 24, 1319–1332. [Google Scholar] [CrossRef]
- Chen, T.; Hutchison, R.M.; Rubel, C.; Murphy, J.; Xie, J.; Montenigro, P.; Cheng, W.; Fraser, K.; Dent, G.; Hendrix, S.; et al. A Statistical Framework for Assessing the Relationship between Biomarkers and Clinical Endpoints in Alzheimer’s Disease. J. Prev. Alzheimers Dis. 2024, 11, 1228–1240. [Google Scholar] [CrossRef]
- Cai, J.; Rimal, B.; Jiang, C.; Chiang, J.Y.L.; Patterson, A.D. Bile acid metabolism and signaling, the microbiota, and metabolic disease. Pharmacol. Ther. 2022, 237, 108238. [Google Scholar] [CrossRef] [PubMed]
- McGuinness, A.J.; Stinson, L.F.; Snelson, M.; Loughman, A.; Stringer, A.; Hannan, A.J.; Cowan, C.S.M.; Jama, H.A.; Caparros-Martin, J.A.; West, M.L.; et al. From hype to hope: Considerations in conducting robust microbiome science. Brain. Behav. Immun. 2024, 115, 120–130. [Google Scholar] [CrossRef] [PubMed]
- Chmiel, J.; Nadobnik, J.; Smerdel, S.; Niedzielska, M. Neural Correlates of Huntington’s Disease Based on Electroencephalography (EEG): A Mechanistic Review and Discussion of Excitation and Inhibition (E/I) Imbalance. J. Clin. Med. 2025, 14, 5010. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Cao, L. Quantitative phase imaging based on holography: Trends and new perspectives. Light Sci. Appl. 2024, 13, 145. [Google Scholar] [CrossRef]
- Kratz, D.; Thomas, D.; Gurke, R. Endocannabinoids as potential biomarkers: It‘s all about pre-analytics. J. Mass Spectrom. Adv. Clin. Lab 2021, 22, 56–63. [Google Scholar] [CrossRef]
- Aguirre-Alvarado, C.; Cortes-Vázquez, M.Á.; Pérez-González, Y.S.; Meza-Sánchez, D.E.; Nuñez-Enriquez, J.C.; Pinto-Cardoso, S.M.; Bekker-Méndez, V.C. Elevated IL-6 and IL-10 Levels as Prognostic Biomarkers in COVID-19 Pneumonia: A Comparative Study in Mexican Patients. Healthcare 2025, 13, 1245. [Google Scholar] [CrossRef]
- Zhou, R.; Ji, B.; Kong, Y.; Qin, L.; Ren, W.; Guan, Y.; Ni, R. PET Imaging of Neuroinflammation in Alzheimer’s Disease. Front. Immunol. 2021, 12, 739130. [Google Scholar] [CrossRef]
- Stoll, S.; Lorentz, L.; Binkofski, F.; Randerath, J. Apraxia: From Neuroanatomical Pathways to Clinical Manifestations. Curr. Neurol. Neurosci. Rep. 2025, 25, 1. [Google Scholar] [CrossRef]
- Wang, R.; Tian, C. Within-domain and across-domain compensation: A systematic review, integrative framework and future research agenda. BMC Psychol. 2025, 13, 46. [Google Scholar] [CrossRef]
- Lenton, T.M.; Livina, V.N.; Dakos, V.; Van Nes, E.H.; Scheffer, M. Early warning of climate tipping points from critical slowing down: Comparing methods to improve robustness. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2012, 370, 1185–1204. [Google Scholar] [CrossRef]
- Wang, X.; Cheng, Z. Cross-Sectional Studies: Strengths, Weaknesses, and Recommendations. Chest 2020, 158, S65–S71. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, I.; Verma, S.; Kumar, S.; Jere, A.; Anamika, K. Multi-omics Data Integration, Interpretation, and Its Application. Bioinform. Biol. Insights 2020, 14, 1177932219899051. [Google Scholar] [CrossRef] [PubMed]
- Nasef, D.; Nasef, D.; Sher, M.; Toma, M. A Standardized Validation Framework for Clinically Actionable Healthcare Machine Learning with Knee Osteoarthritis Grading as a Case Study. Algorithms 2025, 18, 343. [Google Scholar] [CrossRef]
- Struckell, E.; Ojha, D.; Patel, P.C.; Dhir, A. Strategic choice in times of stagnant growth and uncertainty: An institutional theory and organizational change perspective. Technol. Forecast. Soc. Change 2022, 182, 121839. [Google Scholar] [CrossRef]
- Liu, J.; Yang, L.; Liu, D.; Wu, Q.; Yu, Y.; Huang, X.; Li, J.; Liu, S. The role of multi-omics in biomarker discovery, diagnosis, prognosis, and therapeutic monitoring of tissue repair and regeneration processes. J. Orthop. Transl. 2025, 54, 131–151. [Google Scholar] [CrossRef]
- Fung, T.C.; Olson, C.A.; Hsiao, E.Y. Interactions between the microbiota, immune and nervous systems in health and disease. Nat. Neurosci. 2017, 20, 145–155. [Google Scholar] [CrossRef]
- Zhou, H.; Tang, L.; Fenton, K.A.; Song, X. Exploring and evaluating microbiome resilience in the gut. FEMS Microbiol. Ecol. 2025, 101, fiaf046. [Google Scholar] [CrossRef]
- Papa, D.; Ingenito, A.; von Gal, A.; De Pandis, M.F.; Piccardi, L. Relationship Between Depression and Neurodegeneration: Risk Factor, Prodrome, Consequence, or Something Else? A Scoping Review. Biomedicines 2025, 13, 1023. [Google Scholar] [CrossRef]
- Wiegmann, D.A.; Wood, L.J.; Cohen, T.N.; Shappell, S.A. Understanding the “Swiss Cheese Model” and Its Application to Patient Safety. J. Patient Saf. 2022, 18, 119–123. [Google Scholar] [CrossRef]
- Sisto, A.; Vicinanza, F.; Campanozzi, L.L.; Ricci, G.; Tartaglini, D.; Tambone, V. Towards a Transversal Definition of Psychological Resilience: A Literature Review. Medicina 2019, 55, 745. [Google Scholar] [CrossRef]
- Méndez-Barrio, C.; Medina-Rodríguez, M.; Mendoza-Vázquez, G.; García-Roldán, E.; Rodrigo-Herrero, S.; Luque-Tirado, A.; Almodóvar-Sierra, Á.; Franco-Macías, E. Prodromal Alzheimer’s Disease: Global Cognition, Cue Efficiency, and Cerebrospinal Fluid Neurofilament Light Values Predict Short-Term Conversion to Dementia. J. Alzheimers Dis. JAD 2024, 101, 877–887. [Google Scholar] [CrossRef] [PubMed]
- Ilan, Y. The Relationship Between Biological Noise and Its Application: Understanding System Failures and Suggesting a Method to Enhance Functionality Based on the Constrained Disorder Principle. Biology 2025, 14, 349. [Google Scholar] [CrossRef] [PubMed]
- Zeydalinejad, N. An overview of the methods for evaluating the resilience of groundwater systems. MethodsX 2023, 10, 102134. [Google Scholar] [CrossRef] [PubMed]
- Parkar, S.G.; Kalsbeek, A.; Cheeseman, J.F. Potential Role for the Gut Microbiota in Modulating Host Circadian Rhythms and Metabolic Health. Microorganisms 2019, 7, 41. [Google Scholar] [CrossRef]
- Medaglia, J.D. Clarifying Cognitive Control and the Controllable Connectome. Wiley Interdiscip. Rev. Cogn. Sci. 2019, 10, e1471. [Google Scholar] [CrossRef]
- Ristori, S.; Bertoni, G.; Bientinesi, E.; Monti, D. The Role of Nutraceuticals and Functional Foods in Mitigating Cellular Senescence and Its Related Aspects: A Key Strategy for Delaying or Preventing Aging and Neurodegenerative Disorders. Nutrients 2025, 17, 1837. [Google Scholar] [CrossRef]
- Delgado-Bonal, A.; Marshak, A. Approximate Entropy and Sample Entropy: A Comprehensive Tutorial. Entropy 2019, 21, 541. [Google Scholar] [CrossRef]
- Saarela, M.; Podgorelec, V. Recent Applications of Explainable AI (XAI): A Systematic Literature Review. Appl. Sci. 2024, 14, 8884. [Google Scholar] [CrossRef]
- Noordijk, B.; Garcia Gomez, M.L.; ten Tusscher, K.H.W.J.; de Ridder, D.; van Dijk, A.D.J.; Smith, R.W. The rise of scientific machine learning: A perspective on combining mechanistic modelling with machine learning for systems biology. Front. Syst. Biol. 2024, 4, 1407994. [Google Scholar] [CrossRef]
- Yang, T.; Yuan, X.; Ji, P.; Zhu, E. The Impacts of Satellite Data Quality Control and Meteorological Forcings on Snow Data Assimilation over the Sanjiangyuan Region. Water 2025, 17, 1078. [Google Scholar] [CrossRef]
- Lane, P.A. Using Relational Biology with Loop Analysis to Study the North Atlantic Biological Carbon Pump in a ‘Hybrid’ Non-Algorithmic Manner. Mathematics 2024, 12, 3972. [Google Scholar] [CrossRef]
- Dries, C.; McDowell, M.; Schneider, C.R.; Rebitschek, F.G. The effect of uncertainty communication on public trust depends on belief–evidence consistency. Proc. Natl. Acad. Sci. USA 2025, 4, pgaf071. [Google Scholar] [CrossRef]
- Jaeger, J.; Riedl, A.; Djedovic, A.; Vervaeke, J.; Walsh, D. Naturalizing relevance realization: Why agency and cognition are fundamentally not computational. Front. Psychol. 2024, 15, 1362658. [Google Scholar] [CrossRef]
- Lee, E.C.; Whitehead, A.L.; Jacques, R.M.; Julious, S.A. The statistical interpretation of pilot trials: Should significance thresholds be reconsidered? BMC Med. Res. Methodol. 2014, 14, 41. [Google Scholar] [CrossRef]
- Hoseyni, S.M.; Cordiner, J. A novel framework for quantitative resilience assessment in complex engineering systems during early and late design stages. Process Saf. Environ. Prot. 2024, 189, 612–627. [Google Scholar] [CrossRef]
- Sasso, J.M.; Ammar, R.M.; Tenchov, R.; Lemmel, S.; Kelber, O.; Grieswelle, M.; Zhou, Q.A. Gut Microbiome–Brain Alliance: A Landscape View into Mental and Gastrointestinal Health and Disorders. ACS Chem. Neurosci. 2023, 14, 1717–1763. [Google Scholar] [CrossRef]
- Chen, C.; Wang, J.; Pan, D.; Wang, X.; Xu, Y.; Yan, J.; Wang, L.; Yang, X.; Yang, M.; Liu, G. Applications of multi-omics analysis in human diseases. MedComm 2023, 4, e315. [Google Scholar] [CrossRef]
- Pepić, A.; Stark, M.; Friede, T.; Kopp-Schneider, A.; Calderazzo, S.; Reichert, M.; Wolf, M.; Wirth, U.; Schopf, S.; Zapf, A. A diagnostic phase III/IV seamless design to investigate the diagnostic accuracy and clinical effectiveness using the example of HEDOS and HEDOS II. Stat. Methods Med. Res. 2024, 33, 433–448. [Google Scholar] [CrossRef]
- Lin, Y.; Weng, R.; Pan, H.; Hou, Y.; Sun, Y.; Wen, J. Synbiotics in Alzheimer’s disease: Mechanisms, clinical evidence, and therapeutic prospects. J. Transl. Med. 2025, 23, 1009. [Google Scholar] [CrossRef] [PubMed]
- Bertha, A.; Alaj, R.; Bousnina, I.; Doyle, M.K.; Friend, D.; Kalamegham, R.; Oliva, L.; Knezevic, I.; Kramer, F.; Podhaisky, H.-P.; et al. Incorporating digitally derived endpoints within clinical development programs by leveraging prior work. NPJ Digit. Med. 2023, 6, 139. [Google Scholar] [CrossRef] [PubMed]
- Lalu, M.M.; Montroy, J.; Begley, C.G.; Bubela, T.; Hunniford, V.; Ripsman, D.; Wesch, N.; Kimmelman, J.; Macleod, M.; Moher, D.; et al. Identifying and understanding factors that affect the translation of therapies from the laboratory to patients: A study protocol. F1000Research 2020, 9, 485. [Google Scholar] [CrossRef]
- Gubin, D.; Weinert, D.; Stefani, O.; Otsuka, K.; Borisenkov, M.; Cornelissen, G. Wearables in Chronomedicine and Interpretation of Circadian Health. Diagnostics 2025, 15, 327. [Google Scholar] [CrossRef] [PubMed]
- Mo, D.; Zhang, P.; Zhang, M.; Dai, H.; Guan, J. Cholesterol, high-density lipoprotein, and glucose index versus triglyceride–glucose index in predicting cardiovascular disease risk: A cohort study. Cardiovasc. Diabetol. 2025, 24, 116. [Google Scholar] [CrossRef]
- Balcázar, J.L. Wastewater-Based Epidemiology as a Complementary Tool for Antimicrobial Resistance Surveillance: Overcoming Barriers to Integration. Bioessays 2025, 47, e70034. [Google Scholar] [CrossRef]
- Ahmed, Z. Multi-omics strategies for personalized and predictive medicine: Past, current, and future translational opportunities. Emerg. Top. Life Sci. 2022, 6, 215–225. [Google Scholar] [CrossRef]
- Sommese, F. Nature-Based Solutions to Enhance Urban Resilience in the Climate Change and Post-Pandemic Era: A Taxonomy for the Built Environment. Buildings 2024, 14, 2190. [Google Scholar] [CrossRef]
- Lee, Y.-S.; Youn, H.; Jeong, H.-G.; Lee, T.-J.; Han, J.W.; Park, J.H.; Kim, K.W. Cost-effectiveness of using amyloid positron emission tomography in individuals with mild cognitive impairment. Cost Eff. Resour. Alloc. 2021, 19, 50. [Google Scholar] [CrossRef]
- McKee, M.; Wouters, O.J. The Challenges of Regulating Artificial Intelligence in Healthcare. Int. J. Health Policy Manag. 2022, 12, 7261. [Google Scholar] [CrossRef]
- Chopra, H.; Annu; Shin, D.K.; Munjal, K.; Priyanka; Dhama, K.; Emran, T.B. Revolutionizing clinical trials: The role of AI in accelerating medical breakthroughs. Int. J. Surg. 2023, 109, 4211–4220. [Google Scholar] [CrossRef]
- Hirten, R.P.; Suprun, M.; Danieletto, M.; Zweig, M.; Golden, E.; Pyzik, R.; Kaur, S.; Helmus, D.; Biello, A.; Landell, K.; et al. A machine learning approach to determine resilience utilizing wearable device data: Analysis of an observational cohort. JAMIA Open 2023, 6, ooad029. [Google Scholar] [CrossRef]
- Pammi, M.; Shah, P.S.; Yang, L.K.; Hagan, J.; Aghaeepour, N.; Neu, J. Digital twins, synthetic patient data, and in-silico trials: Can they empower paediatric clinical trials? Lancet Digit. Health 2025, 7, 100851. [Google Scholar] [CrossRef]
- Lotti, S.; Dinu, M.; Colombini, B.; Amedei, A.; Sofi, F. Circadian rhythms, gut microbiota, and diet: Possible implications for health. Nutr. Metab. Cardiovasc. Dis. NMCD 2023, 33, 1490–1500. [Google Scholar] [CrossRef]
| Indication | Encoder Drift (Microbiota/ Metabolites) | Integrator Imbalance (Endocannabinoidome) | Executor Overdrive (Immune/Neural) | EMN-S Collapse Trajectory (Inference) | References |
|---|---|---|---|---|---|
| Alzheimer’s disease (AD) | Peripheral bile acid (BA) signatures align with CSF Aβ/tau and PET amyloid/tau, indicating long-horizon encoder drift; microbial aromatic–tryptophan outputs act as stabilizers: indole-3-lactic acid (ILA) reduces Aβ via AhR signaling, and indole-3-propionic acid (IPA) (from C. sporogenes synbiotics) improves cognition in AD models. | Human eCB tone drift is emerging in prodromal spectra and plausibly co-moves with lipid/BA axes; paired lipidomics–metabolomics (serum–stool–CSF) is recommended to capture integrator buffering loss. | Tau-PET positivity and slope are the strongest near-term predictors of clinical transition vs. amyloid; ML on large tau-PET cohorts improves risk stratification and subpopulation discovery. | Slow-drift collapse: years-long encoder shifts (BA, indoles) erode integrator homeostasis; executor acceleration is indexed by tau-PET kinetics toward symptomatic conversion. | Nabizadeh et al., 2024; [75] |
| Parkinson’s disease (PD) | Multiple cohorts show SCFA depletion and microbial pathway reprogramming; spouse-controlled prebiotic pilots (dietary fiber + lactulose) elevate fecal SCFAs and partially normalize metabolomes, supporting encoder rescue feasibility. | CB2 upregulation on microglia is seen across neuroinflammatory states; PD-relevant studies and reviews support CB2-targeted modulation to attenuate microglial activation and dopaminergic injury—an integrator gain-drift/compensation axis. | Microglial activation and nigral degeneration across temporal windows; glial crosstalk and disease-associated microglia states track progression and inflammatory tone. | Gut-first oscillatory collapse: encoder SCFA loss → integrator CB2 tone drift → executor neuroinflammation, producing a motor prodrome years pre-diagnosis; combined encoder–integrator rescue is predicted to damp executor outputs. | Bedarf et al., 2025; [76] |
| Multiple sclerosis (MS) | Primary BA metabolites at baseline predict slower brain and retinal atrophy; TUDCA RCT in progressive MS is safe and shifts immune and gut features—human interventional anchor for encoder modulation. | Oscillatory N-acylethanolamide/eCBome tone is plausible; BA–lipid crosstalk and circadian sampling are recommended, given serum–CSF lipid decoupling and weak cross-compartment correlations. | CSF lipid modules correlate with cytokines, disability, and MRI activity, offering executor-state readouts that align with metabolomic encodings. | Relapsing–remitting oscillatory collapse: episodic encoder–executor coupling with seasonal or flare-linked imbalances; bile acid augmentation (e.g., TUDCA) is a rational stabilizer candidate. | Ladakis et al., 2025; [77] |
| Major depressive disorder (MDD) | Diet/sleep and stressors modulate microbiome outputs, but prospective recent human encoder metabolites tied to trajectories remain scarce—priority for perturbation trials with dense sampling. | Endocannabinoid tone: plasma AEA/2-AG alterations replicate; pediatric RCT cohort shows lower hair AEA as a longitudinal marker; in severe MDD, ECT courses track with plasma eCB shifts (and historical CSF AEA rises post-ECT). | Low-grade inflammatory variance intersects with the eCBome; multi-timepoint designs are needed to parse trait vs. state. | Bidirectional drift: stressor-tuned encoder noise + integrator amplitude loss → executor dysregulation; quantify recovery half-life as a target for trials. | Amin et al., 2023; [78] |
| Sepsis-associated encephalopathy (SAE) | Acute dysbiosis is likely but under-sampled in ICU windows (narrow encoder capture); methodologically, the earliest feasible encodings may be plasma metabolome ± stool if available. | Rapid NAE/eCBome swings are plausible integrator shock responses; prospective ICU lipidomics are needed to define amplitude and timing relative to organ failure. | Serum neuron-specific enolase (NSE) rises in SAE and improves diagnosis/prognosis; a recent cohort shows NSE + cerebral oximetry (rSO2) enhances identification and outcome prediction; multiple meta-analyses link higher NSE to mortality and adverse neurological outcomes. | Catastrophic rapid collapse: executor storm dominates early; composite NSE + physiologic monitoring provides an actionable signal while encoder/integrator streams lag. | Zhang et al., 2025; [79] |
| Inflammatory bowel disease (IBD) (gut–immune EMN-S comparator) | Disease–activity-linked bile acid remodeling is repeatedly observed; multi-omics studies show altered primary vs. secondary BA balance and microbial BA-metabolizing taxa shifts; interventional nutrition/phytochemicals (e.g., berberine) re-align BA–microbiome networks. | Human tissue shows altered CB1/CB2 expression with nociceptin (NOP) correlation; mucosal eCBs and oxylipins are elevated and track cytokine expression—an integrator remodeling axis in active disease. | Cytokine fields and innate immune cells (e.g., neutrophils) define executor burden and flare dynamics; linking mucosal eCB shifts to systemic immune variance is a near-term translational aim. | Regional oscillatory collapse: gut-centric encoder–integrator remodeling with executor flares; instructive analog for brain-directed EMN-S hypotheses. | Diab et al., 2019; [80] |
| Compound/Class | Targeted EMN-S Axis | Mechanistic Role | Dose Range (Human-Equivalent) | Dosing Frequency | Therapeutic Window (Effective Interval) | Combination Sequence/Notes | Key PK Parameters (Approx.) | Preliminary PD Effects |
|---|---|---|---|---|---|---|---|---|
| Palmitoylethanolamide (PEA) | Integrator (eCBome) | Endogenous lipid amide; PPAR-α and CB2 co-modulator; stabilizes lipid-immune tone and reduces glial excitability | 300–1200 mg day−1 (oral) | Once or twice daily | 6–12 h | May be co-administered within 4 h after CB2 agonist or ILA to extend feedback coherence | T1/2 = 6–8 h; Cmax = 0.2–0.5 µM; oral F ≈ 0.4 | ↓ cytokine variance, ↑ circadian AEA amplitude, restored integrator gain |
| CB2-Selective Agonists (e.g., JWH-133, HU-910, β-caryophyllene) | Executor (Neuroimmune) | Microglial state-reprogrammer; reinforces anti-inflammatory tone and restores executor homeostasis | 0.05–0.2 mg kg−1 eq. (oral/parenteral) | Every 24–48 h | 2–8 h post-dose (peak 2 h) | Administer 12 h after ILA preconditioning; co-administer PEA within 4 h | T1/2 = 4–6 h; Kp,uu,brain ≈ 0.25–0.35; RO = 50–70% | ↓ microglial activation, ↓ NF-κB, ↑ negative-feedback recovery |
| Indole-Lactic Acid (ILA) | Encoder–Integrator Interface | Microbial AhR agonist; modulates tryptophan metabolism and SCFA coupling | 50–150 mg kg−1 eq. (oral) | Once daily | 3–6 h | Administer 12 h before the CB2 agonist to prime microglial sensitivity | T1/2 ≈ 3 h; portal Cmax = 5–15 µM; high first-pass | ↑ microbial synchrony, ↑ intestinal barrier tone, entrains circadian microbial-lipid phase |
| Combined Regimen (ILA + CB2 Agonist ± PEA) | Multi-Axis (Encoder + Integrator + Executor) | Sequential loop recalibration restores encoder precision, integrator amplitude, and executor stability | Derived from above | Cyclic protocol: 36 h interval | 6–24 h composite | ILA → 12 h → CB2 Agonist → 4 h → PEA | Composite AUC match within ±25% across axes; steady-state after 3 cycles | Reduced variance inflation, normalized cross-modal coupling, and increased resilience index |
| Intervention Class | Primary EMN-S Targets | Mechanistic Pathway | Experimental Model/Cohort | Recent Evidence | Molecular/Cellular Outcomes | Clinical or Translational Signal | Development Stage | EMN-S Leverage/Strategic role | References |
|---|---|---|---|---|---|---|---|---|---|
| Indole-boosting synbiotics (ILA/IPA) | Encoder (microbiota → aromatic Trp catabolites) → Executor (glial AhR) | Targeted synbiotics and Trp co-substrates elevate indole-3-propionic acid (IPA) and indole-3-lactic acid (ILA). These ligands engage AhR in microglia/astroglia, suppress NF-κB/NLRP3, reduce amyloidogenic stress, and support synaptic/cognitive rescue. | (i) Clostridium sporogenes + xylan synbiotic in 5xFAD; (ii) Trp + ILA supplementation; (iii) Bifidobacterium strains in mouse + human biomarker studies. | Synbiotic raises IPA and improves cognition in AD mice (Li et al., 2024, Food and Function, preclinical). ILA reduces soluble Aβ via AhR signaling (Kim et al., 2024, BBI). Human/mouse work shows systemic ILA increases with psychobiotic regimens (translational). | ↓ Soluble Aβ; microglial shift to reparative states; astroglial antioxidant responses; synaptic integrity preserved. | Serum ILA/IPA increase in humans on targeted psychobiotics; plausible encoder-level repair with paired stool/serum monitoring. | Preclinical + human translational (biomarker) | Recalibrates encoder signal quality; dampens executor amplification via AhR-mediated neuroimmune modulation. | Li et al., 2024; [196]; Kim et al., 2024 [197]; |
| Bile acid augmentation (e.g., TUDCA) | Encoder Integrator interface (FXR/TGR5; gut–brain immune crosstalk) | Restores primary BA profiles linked to neuroprotection; activates FXR/TGR5 on immune/glial compartments; modulates cytokine tone and reshapes gut taxa; and aligns upstream encoder outputs with integrator stability. | Prospective MS cohort with serum metabolomics + MRI/OCT; randomized TUDCA trial in progressive MS assessing safety + immune/microbiome shifts. | Primary BAs at baseline predict slower brain/retinal atrophy; TUDCA is safe with immune + microbiome changes (Ladakis et al., 2025, Med; plus protocol/preprint). | BA modules show slower CNS atrophy; immune cell signatures and microbial composition shift with TUDCA. | Prognostic BA panel and feasible augmentation strategy support encoder stabilization and integrator re-biasing. | Prospective human + RCT (Phase 2–like safety) | Stabilizes encoder outputs and resets integrator–executor set points; seasonality-aware designs encouraged. | Ladakis et al., 2025; [77] |
| CB2-biased modulation | Integrator (eCBome) → Executor (microglia) | Selective CB2 agonism biases microglia toward reparative, phagocytic phenotypes with minimal CB1-linked psychotropic liability; reduces pro-inflammatory cytokines; and modulates α-syn aggregation/clearance. | (i) Brain cell-type mapping shows CB2 predominance in microglia; (ii) α-syn rodent models with CB2 ligands; (iii) human tissue/proteomic confirmation. | CB2 activation attenuates neuroinflammation and dopaminergic injury in PD-relevant models. Single-cell/cell-sort mapping refines CB2 distribution. | ↓ Microglial activation; ↑ homeostatic/reparative microglia; ↓ α-syn pathology. | Rationale for executor-sparing anti-inflammatories without CNS side effects: combinable with encoder BA/SCFA repair. | Preclinical + human tissue mapping | Tunes integrator gain and mitigates executor overdrive; strong synergy with microbiome-directed strategies. | Grabon et al., 2024; [118] |
| PEA/OEA (nutraceutical NAEs) | Integrator (PPARα-linked lipid tone; NAE buffering) | Oral OEA elevates N-acylethanolamide pools and activates PPARα, attenuating inflammation/oxidative stress and improving metabolic indices; good safety/tolerability; PEA often co-formulated clinically. | (i) Double-blind RCT in PCOS (n ≈ 60–100) tracking inflammatory/oxidative markers and glycemic control; (ii) 2025 meta-analysis of RCTs on cardiometabolic endpoints. | OEA improves glycemic indices and reduces inflammatory/oxidative markers (Taghizadeh-Shivyari et al., 2024). | ↓ CRP, TNF-α, IL-6; improved HOMA-IR and lipid profile (population-dependent). | Low-AE nutraceutical strategy with cross-domain relevance to neuro-immune settings as an integrator buffer. | Human RCTs + meta-analysis | Increases integrator buffering capacity; natural adjunct in multimodal EMN-S programs. | Taghizadeh-Shivyari et al., 2024; [198] |
| Microglial reprogramming (TREM2 agonism) | Executor (microglia) | AL002 (TREM2 agonist mAb) engages central microglial repair programs (phagocytosis, metabolic support). Dose-dependent ↓ CSF sTREM2 and ↑ microglia-recruitment markers show target engagement; however, Phase 2 INVOKE-2 was negative on clinical and biomarker outcomes. | Phase 1 (healthy volunteers/early AD biomarker PD); Phase 2 INVOKE-2 (early AD). | Phase 1: central target engagement and tolerability (Long et al., 2024). Phase 2: did not meet endpoints. | On-target biomarker shifts without clinical efficacy in Phase 2. | Mechanism validated; efficacy may depend on stage/combination/earlier windows. | Phase 1 completed; Phase 2 negative | Shapes executor waveform; likely needs pairing with amyloid/tau or encoder-level repair. | Long et al., 2024; [199] |
| CSF1R pathway modulation (e.g., pexidartinib/PLX3397) | Executor (microglial population dynamics) | CSF1R inhibition transiently depletes microglia with subsequent repopulation/reprogramming. Effects on α-syn pathology and neurodegeneration are model-, sex-, and timing-dependent (benefit or harm possible). | α-syn mouse/rat models; PLX3397/PLX5622 regimens; supportive mechanistic work across neuro-oncology/ALS. | α-syn inclusion-responsive microglia resist depletion by CSF1R blockade; sex-dependent microglial response to PLX3397. PLX3397 alters pathology trajectory in synucleinopathy model. | Microglial depletion/remodeling with variable α-syn outcomes and motor effects highlights precision timing. | Mechanistic tool with cautious translation; requires biomarker-guided dosing (e.g., TSPO/TREM2 panels). | Preclinical; early oncology neuro-applications | Executor “gain” reset; can reduce noise amplification if precisely timed; not disease-agnostic. | Stoll et al., 2024; [200] |
| CRISPR-enhanced phage/precision microbiota editing | Encoder (targeted bacterial deletion) | CRISPR-armed bacteriophages selectively debulk pathobionts via lytic killing + targeted DNA degradation, converting the microbiota into a programmable encoder with pathogen control and downstream metabolite re-balancing. | ELIMINATE Phase 2, Part 1 (LBP-EC01) in acute uncomplicated E. coli UTI; randomized open-label PK/PD lead-in with antibiotic arm; Part 2 blinded ongoing. | Part 1: Rapid, durable bacterial load reduction and acceptable safety with LBP-EC01 + TMP-SMX vs. antibiotic alone. Platform generalizable to gut targets; Part 2 ongoing. | ↓ Pathobiont load; validated PK/PD; favorable safety. | Proof-of-concept clinical efficacy for programmable microbiota control; strong template for gut-directed encoder engineering. | Early clinical (Phase 2 Part 1 complete) | Transforms encoder from static to programmable; pairs naturally with metabolite and immune readouts. | Kim et al., 2024; [201] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aliuș, C.; Breazu, A.; Pantu, C.; Toader, C.; Șerban, M.; Covache-Busuioc, R.-A.; Munteanu, O.; Dumitru, A.V. The Endocannabinoid–Microbiota–Neuroimmune Super-System: A Unifying Feedback Architecture for Systems Resilience, Collapse Trajectories, and Precision Feedback Medicine. Int. J. Mol. Sci. 2025, 26, 10959. https://doi.org/10.3390/ijms262210959
Aliuș C, Breazu A, Pantu C, Toader C, Șerban M, Covache-Busuioc R-A, Munteanu O, Dumitru AV. The Endocannabinoid–Microbiota–Neuroimmune Super-System: A Unifying Feedback Architecture for Systems Resilience, Collapse Trajectories, and Precision Feedback Medicine. International Journal of Molecular Sciences. 2025; 26(22):10959. https://doi.org/10.3390/ijms262210959
Chicago/Turabian StyleAliuș, Cătălin, Alexandru Breazu, Cosmin Pantu, Corneliu Toader, Matei Șerban, Răzvan-Adrian Covache-Busuioc, Octavian Munteanu, and Adrian Vasile Dumitru. 2025. "The Endocannabinoid–Microbiota–Neuroimmune Super-System: A Unifying Feedback Architecture for Systems Resilience, Collapse Trajectories, and Precision Feedback Medicine" International Journal of Molecular Sciences 26, no. 22: 10959. https://doi.org/10.3390/ijms262210959
APA StyleAliuș, C., Breazu, A., Pantu, C., Toader, C., Șerban, M., Covache-Busuioc, R.-A., Munteanu, O., & Dumitru, A. V. (2025). The Endocannabinoid–Microbiota–Neuroimmune Super-System: A Unifying Feedback Architecture for Systems Resilience, Collapse Trajectories, and Precision Feedback Medicine. International Journal of Molecular Sciences, 26(22), 10959. https://doi.org/10.3390/ijms262210959






